Abstract
Introduction
We assessed patterns of illicit drug use using mobile health (mHealth) methods and subsequent health care indicators among drug users in Baltimore, MD
Methods
Participants of the EXposure Assessment in Current Time (EXACT) study were provided a mobile device for assessment of their daily drug use (heroin, cocaine or both), mood and social context for 30 days from November, 2008 through May, 2013. Real-time, self-reported drug use events were summed for individuals by day. Drug use risk was assessed through growth mixture modeling. Latent class regression examined the association of mHealth-defined risk groups with indicators of healthcare access and utilization.
Results
109 participants were a median of 48.5 years old, 90% African American, 52% male and 59% HIV-infected. Growth mixture modeling identified three distinct classes: low intensity drug use (25%), moderate intensity drug use (65%) and high intensity drug use (10%). Compared to low intensity drug users, high intensity users were younger, injected greater than once per day, and shared needles. At the subsequent study visit, high intensity drug users were nine times less likely to be medically insured (adjusted OR: 0.10, 95%CI: 0.01-0.88) and at greater risk for failing to attend any outpatient appointments (aOR: 0.13, 95%CI: 0.02-0.85) relative to low intensity drug users.
Conclusions
Real-time assessment of drug use and novel methods of describing sub-classes of drug users uncovered individuals with higher-risk behavior who were poorly utilizing healthcare services. mHealth holds promise for identifying individuals engaging in high-risk behaviors and delivering real-time interventions to improve care outcomes.
Keywords: mHealth, Ecological Momentary Assessment, illicit drug use, HIV, growth mixture models
1. INTRODUCTION
In epidemiologic studies, ascertainment of illicit drug use is commonly by self-report, which lends the data susceptible to substantial recall bias, particularly when captured within broad time periods (e.g., “any drug use in the past year”; Schroeder et al., 2006; Bell et al., 2010; Knowlton et al., 2010). Further, details regarding the intensity and patterns of drug use are rarely captured (Paganini-Hill and Ross, 1982; Grimes and Schulz, 2002). Additionally, these recall methods are limited in their ability to identify periods of daily intense or intermittent use and fail to capture the context of an individual's drug using experience.
Ecological Momentary Assessment (EMA) methods are able to collect individual-level data in real time as well as facilitate responsive communication between clinic and patient utilizing smart phones or hand-held devices. These mobile health (mHealth) methods have been utilized in smoking cession studies (Shiffman et al., 2007; Hedeker et al., 2009; Shiffman et al., 2009; Epstein et al., 2010; Bedi et al., 2011; Minami et al., 2011) and in methadone-maintained outpatient drug users to examine activities associated with cocaine and heroin use (Epstein et al., 2009; Preston et al., 2009; Epstein et al., 2010; Epstein and Preston, 2010; Preston and Epstein, 2011; Epstein and Preston, 2012). Detailed, longitudinal EMA data can provide information at varying time intervals (e.g., hourly, daily, weekly) of the changes and patterns of behaviors that are often not static over longer periods of time. By assessing participants in real-time, EMA studies can reduce recall bias and distinguish behavioral nuances that are not captured at periodic study visits in traditional cohort studies.
For many health-related conditions, longitudinal assessments are necessary to derive meaningful associations between exposures and outcomes as well as to describe heterogeneous patterns of exposure. Analytic methods, such as growth mixture modeling, have been previously used to identify distinct trajectories of drug using behavior over extended periods of time (Galai et al., 2003; Hser et al., 2007; Genberg et al., 2011) but these methods have yet to be widely applied to EMA data.
Optimal health care outcomes are achieved when individuals access available health care services, including regularly attending outpatient appointments rather than utilizing the emergency department for primary care needs (Knowlton et al., 2001; Center For Advancing Health: Evidence. Engagement. Equity., 2010). The HIV provider community has embraced the concept of engagement in care as part of the HIV Care Continuum. Limited engagement in care has been associated with poorer HIV treatment outcomes and reduced survival (Gardner et al., 2011). This framework may also be relevant for identifying barriers and optimizing care delivery for individuals who do not suffer from HIV. People who inject drugs (PWID) often have poor healthcare outcomes due to multiple contributing factors of prolonged substance abuse, mental health disorders, HIV and hepatitis C virus (HCV) infection, unstable housing, violence, poverty and incarceration (Compton et al., 2007; Bell et al., 2010; Westergaard et al., 2013). Identifying high-risk drug users who do not have appropriate access (e.g., lack health insurance) or do not utilize available healthcare services (e.g., not having regular primary outpatient care) could allow for targeted and tailored interventions to foster engagement and prevent needless morbidity, mortality, and inefficient use of health care resources.
In the current analysis, we utilize EMA methods to ascertain the amount and frequency of drug use over a 30-day period among a sample of drug users in Baltimore, MD. We then performed mixed effects modeling to identify distinct trajectories of drug use followed by an assessment of the sociodemographic and behavioral correlates of these drug-using trajectories. Lastly, we evaluated whether the EMA-derived drug using trajectories were associated with differences in health care access and utilization.
2. METHODS
2.1 EXACT study participants
Exposure Assessment in Current Time (EXACT) study participants were recruited from the AIDS Linked to the IntraVenous Experience (ALIVE) study, an on-going, community-recruited, observational cohort of over 3,000 persons with a history of injecting drugs (i.e., current and former injection drug users) in Baltimore, MD (Vlahov et al., 1991). The ALIVE cohort is community-based rather than clinic-based, thereby avoiding selection bias toward persons seeking or accessing care. While the ALIVE study examines the association between drug use and HIV at semi-annual clinic visits, the EXACT study was conceived as a feasibility study designed for near real-time characterization of illicit drug use in users’ natural environments. Details of the EXACT study have been previously described (Kirk et al., 2013b), and included four successive trials conducted from November, 2008 through May, 2013. Each trial was planned to follow 30 participants each for 30 days.
Eligibility criteria for the EXACT study included current enrollment in ALIVE and the ability to understand and follow directions on a personal digital assistant (PDA) or mobile phone. Convenience sampling was utilized to identify individuals for participation in EXACT. The specific inclusion criteria regarding drug use and HIV status were varied slightly between study trials to ensure a diverse sample; both injection and non-injection drug users were included. Individuals were excluded if they had any medical conditions that would prevent them from operating the hand-held device (e.g., vision impairment) or failed to attend the screening appointment where they were trained on device use.
The Johns Hopkins School of Public Health Institutional Review Board approved the study protocol. All participants provided written informed consent and were informed that involvement (or non-involvement) in EXACT would in no way affect their participation in ALIVE.
2.2 Data
On the hand-held devices provided, participants were asked to self-initiate a survey and self-report each time they used heroin or cocaine (or both) in any manner (smoked, snorted or injected); these responses represent event-contingent entries. [One in 8 adults in Baltimore are heroin dependent (ONDC, 2000) and the city is ranked first in the nation for heroin and crack-related emergency room visits (ONDC., 2000)]. All data used in the present analyses are from these self-reported event-contingent entries. Heroin only and cocaine only reports incorporated any reports of heroin or cocaine use (including those jointly used with another drug).
For each event, participants answered questions concerning their drug use, current mood, social, physical and activity environment, using survey instruments adapted from previous EMA studies (Epstein et al., 2009; Preston et al., 2009; Epstein and Preston, 2010; Preston and Epstein, 2011; Epstein and Preston, 2012). To ensure responses to event-contingent surveys were recorded in real-time, participants were required to indicate that the craving or use had occurred within the prior 30 minutes.
Participants were initially provided personal digital assistants (PDA, Palm Z22, Palm, Inc., Sunnyvale, CA, USA) running applications developed using Satellite Forms software (http://www.satelliteforms.net/) to complete data collection. When this PDA model became obsolete, data collection transitioned to Android Smartphones (Motorola Droid X2), running an application developed using the electronic mobile comprehensive health application (eMOCHA) platform, created at Johns Hopkins School of Medicine (Tumwebaze et al., 2012) and modified specifically for this study.
Baseline data were obtained from audio-computer assisted self interviews (ACASI) completed at enrollment into EXACT or from the existing ALIVE database and included sociodemographic, behavioral, and clinical characteristics obtained during biannual study visits. Sociodemographic variables included age, sex, race, education, marital status, employment, income, homelessness and health insurance status as well as self-reported alcohol, tobacco and illicit drug use (as assessed via the 28-item Drug Abuse Screening Test (DAST); Skinner, 1982), and depressive symptoms (as assessed via the Center for Epidemiologic Studies- Depression Scale (CES-D) ≥23) in the prior 6 months (Kohout et al., 1993; Golub et al., 2004).
2.3 Statistical Analyses
The outcome of this analysis was number of self-reported heroin and/or cocaine use events per day over 30 consecutive days. The outcome was modeled using semi-parametric latent class growth mixture models (Nagin and Tremblay, 2001; Jones and Nagin, 2007), specifically the zero-inflated Poisson model (ZIP; the outcome was a count with an excess of zero totals). This approach classified participants into different groups, each representing different subpopulations with unique longitudinal patterns. Although the number of groups can be hypothesized a priori, one aim of this method is to determine the number of meaningful groups that exist in the population. Selecting the number of groups involved fitting a series of iterative models, varying the number of groups up to 4. Models were compared using the Bayesian Information Criterion (BIC), the average posterior probabilities of group membership and the usefulness of the number of groups in practice (Muthen and Muthen, 2000; Nylund et al., 2007). Group membership was assigned by maximum posterior probability and groups were labeled based on trajectory characteristics. Backward selection of the parameters representing time (e.g., linear, quadratic, cubic) was used to determine trajectory shapes and parameters were removed on the basis of statistical significance (P ≤ 0.05). Baseline sociodemographic and behavioral characteristics were included as time-fixed covariates in bivariate analyses to describe the increase in relative odds of being in a trajectory group (relative to the lowest risk group) per unit increase in the risk factor.
Logistic regression methods were utilized to examine if EMA trajectories could predict key indicators of healthcare access at the next ALIVE visit (5 months after EXACT was completed). Outcomes included having any medical insurance or attending outpatient physician visits. All baseline sociodemographic and behavioral characteristics were examined as predictors of engagement in care and final multivariable models included significant variables from bivariate analyses (p-value<0.1) and known confounders; variables were entered simultaneously. Analyses were performed using STATA 12 (Stata Statistical Software, College Station, Texas) and SAS 9.2 (Proc Traj; SAS Institute Inc., Cary, North Carolina).
3. RESULTS
Table 1 provides data on baseline characteristics for the 109 EXACT participants. The median age was 48.5 years (inter-quartile range [IQR]: 43-53 years), 90% were African American, 52% were male and 59% were HIV-infected.
Table 1.
Baseline characteristics of EXACT participants*
| Characteristic | N=109 | % |
|---|---|---|
| Median age, IQR | 48.5 (43-53) | - |
| Male | 58 | 52 |
| African American | 98 | 90 |
| Never married | 66 | 61 |
| High school education or equivalent | 44 | 40 |
| Income<$5,000 | 83 | 77 |
| Homeless | 9 | 8 |
| Alcohol use | 71 | 65 |
| Cigarette use | 91 | 83 |
| <1/2 Pack cigarettes per daya | 20 | 18 |
| ≥ 1/2 Pack cigarettes per day | 71 | 65 |
| CES-D>23 | 26 | 23 |
| Drug Abuse Screening Test, DAST>16 | 6 | 18 |
| Emergency room visit (ER) | 28 | 26 |
| Methadone treatment | 26 | 24 |
| Marijuana use | 27 | 24 |
| Speedball useb | 25 | 23 |
| Heroin use (any route) | 49 | 46 |
| Cocaine use (any route) | 50 | 46 |
| Hepatitis C positivec | 94 | 86 |
| HIV positive | 64 | 59 |
| Any antiretroviral therapy | 42 | 65 |
| Median CD4 (IQR)+ | 360.5 (239-529) | - |
| Viral load>500+ | 35 | 55 |
All baseline characteristics represent behavior within the 6 months prior to the start of EXACT
Cigarette packs per day were assessed for those who reported smoking at baseline
Speedball is defined as the simultaneous injection of a mixture of cocaine and heroin
Diagnosis via HCV antibody testing
CD4 & viral load testing on HIV-infected participants only.
EXACT participants were followed for a median of 28 days (IQR 26-29), during which time 98 (90%) participants reported using heroin or cocaine at least once, while 11 (10%) did not report any drug use. The median number of self-reported drug using events was 4 (IQR, 1-10). Of 844 total drug use events, 351 (41.6%) were exclusively heroin, 289 (34.2%) were exclusively cocaine and 201 (23.8%) were reports of concurrently using both heroin and cocaine.
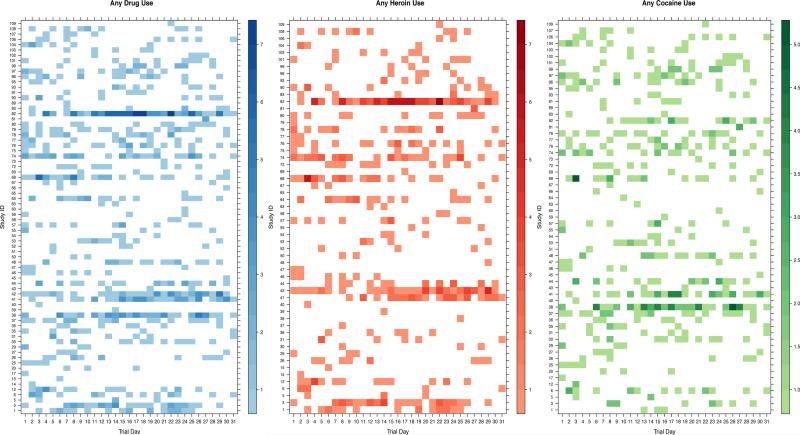
Figure 1 displays lattice plots (also known as heat maps) of the daily intensity of any self-reported drug use (Panel A, blue), heroin use (Panel B, red) and cocaine use (Panel C, green) of EXACT participants over the month of follow-up. Only participants reporting drug use were included in these figures. Among individuals self-reporting any drug use (Figure 1, Panel A), the mean number of drug-using days during study follow-up was 7.7 (standard deviation [SD] +/−6.7 days), with a range of 1-28 days. Over the 30-day follow-up period, the mean number of drug use events was 11 (SD +/−16) with a range of 1-105 reports.
Figure 1. Lattice plots of drug use intensity by day and drug type.
Panel A: Any heroin or cocaine use intensity by trial day. B. Any heroin use intensity by trial day. C. Any cocaine use intensity by trial day. Individuals are represented on the y-axis and the intensity of the color indicates the intensity of self-reported drug use for any given day (darker colors represent more reports of drug use).
The mean number of heroin-using days over follow-up was 6.6 days (SD+/− 6.5days) (Figure 1, Panel B). Over the 30-day follow-up period, the mean number of heroin use events was 10 (SD +/−15.1); the median number of heroin use events was 4.5 (IQR 2.0-10.5), and a maximum of 93 heroin use events were reported over follow-up.
On average, cocaine use was reported on 6.2 days (SD +/−5.3 days) during follow-up (Figure 1, Panel C). Among all participants reporting cocaine use, an average of 8 (SD+/− 9.5) cocaine use events, a median of 4 (IQR 3-10) cocaine use events, and a maximum of 61 cocaine use events were reported during the study period.
3.1 EMA-defined drug using risk groups
Mean drug use per day was examined using semi-parametric growth mixture models with 2, 3, and 4 groups (Table 2). The 3-group unadjusted model was chosen as the final model for the observed data (BIC 2-group= −1241.9, BIC 3-group= −1182.8, BIC 4-group= −1162.1) as it defined three interpretable and relevant subgroups with a low BIC. The groups were labeled for convenience based on their profiles of response as: low intensity (Group 1), moderate intensity (Group 2) and high intensity (multiple uses per day, Group 3) drug use. The average probability of most likely group membership was between 0.86 and 0.96 indicating a high degree of classification accuracy and exceeding the suggested threshold of 0.70 for these methods (Nagin and Odgers, 2010).
Table 2.
Growth mixture model indices for 2, 3, 4 drug using groups
| Model | Trajectory fit | Group membership N (%) | Standard Error | p-value | Average posterior probability of group membership | BIC (N=98) | AIC (N=98) |
|---|---|---|---|---|---|---|---|
| 2-groups | |||||||
| 1 | Linear | 86 (86) | 3.9 | <0.001 | 0.98 | −1241.8 | −1234.1 |
| 2 | Quadratic | 12 (14) | 3.9 | <0.001 | 0.98 | ||
| 3-groups | |||||||
| 1 | Linear | 24 (25) | 5.4 | <0.001 | 0.86 | −1182.8 | −1172.4 |
| 2 | Linear | 65 (65) | 5.8 | <0.001 | 0.92 | ||
| 3 | Linear | 9 (10) | 3.5 | <0.001 | 0.96 | ||
| 4-groups | |||||||
| 1 | Linear | 21 (21) | 4.9 | <0.001 | 0.86 | −1162.1 | −1146.5 |
| 2 | Linear | 67 (66) | 5.8 | <0.001 | 0.91 | ||
| 3 | Linear | 9 (12) | 3.9 | 0.002 | 0.97 | ||
| 4 | Quadratic | 1 (1) | 1.1 | 0.317 | 1.0 |
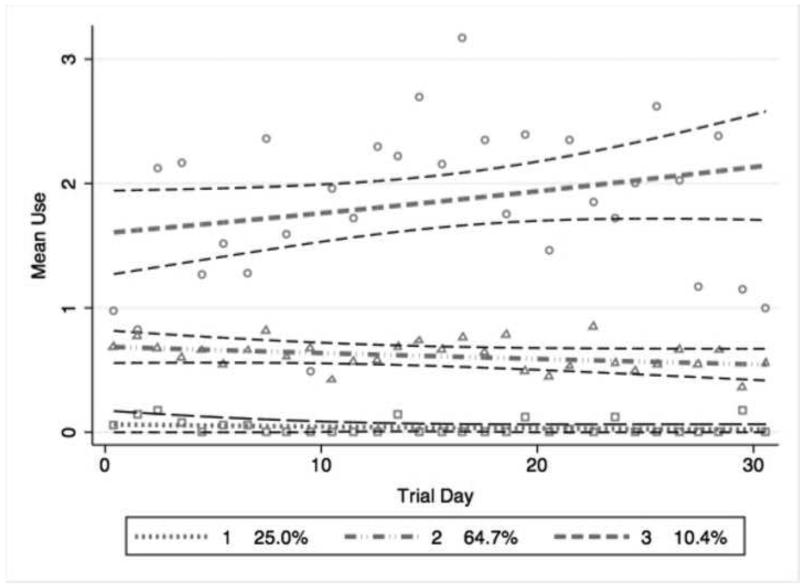
Figure 2 displays the trajectories of drug use over time based on the 3-group model. Although cubic and quadratic fits were explored, all three groups were best fit with linear trajectories. Group 1 represented 25.0% of the participants with a mean of 0 drug use events per day (SD +/− 0.04). The moderate intensity drug-using group (Group 2) comprised approximately 65.0% of participants and was marked by an overall average of less than 1 drug use event per day (SD +/−0.19). Group 3 represented individuals using drugs multiple times daily (10.5% of participants) and was marked by an overall average of 1.5 drug use events per day (SD +/− 0.85).
Figure 2. 3-Class unadjusted growth mixture model of mean daily drug use in EXACT.
Trajectories of mean drug use per day among 109 illicit drug users in the Exposure Assessment in Current Time (EXACT) study. The y-axis represents the mean number of drug use events per day; the x-axis represents each day of the trial. The dashed grey lines represent the predicted mean use per day given group membership with their respective 95% confidence intervals shown as dashed black lines. The hallow shapes represent the observed mean use per day given group membership. The 3 groups (and proportion of total population within each group) represent: low intensity drug use (25.0%); moderate intensity drug use (64.7%); high intensity drug use (10.4%). All trajectories were modeled linearly.
3.2 Sociodemographic and behavioral characteristics of drug using risk groups
Factors associated with membership in the EMA-defined drug-using risk groups were examined by separately comparing Group 2 (moderate intensity users) and Group 3 (high intensity users) to the Group 1 (low intensity users) as the referent group (Table 3). Moderate intensity drug users (Group 2) had lower odds of being married (log Odds Ratio (log OR): −1.48, p-value=0.036) and increased odds of being a current injector at baseline (log OR injecting ≥1/day: 2.17, p-value=0.049), injecting heroin at baseline (log OR: 2.26, p-value=0.007) and using crack at baseline (log OR: 2.60, p-value=0.015) relative to Group 1. High intensity drug users (Group 3) were more likely to be younger (log OR: −2.11, p-value=0.068), share needles (log OR: 2.70, p-value=0.0296) inject cocaine at baseline (log OR: 2.34, p-value=0.028), inject heroin at baseline (log OR: 3.31, p-value=0.003), use crack (log OR: 3.25, p-value=0.009) and use speedball at baseline (log OR: 2.70, p-value=0.043) relative to those in Group 1.
Table 3.
Risk factors associated with drug use risk group membership relative to low intensity drug using group*
| EXACT EMA Drug Use Risk Groups | ||||||
|---|---|---|---|---|---|---|
| Risk factor | Group 2-Moderate intensity drug use | Group 3-High intensity drug use | ||||
| Log Odds | SE | p-value | Log Odds | SE | p-value | |
| Demographics | ||||||
| Age≥50 | −0.61 | 0.58 | 0.289 | −2.11 | 1.16 | 0.069 |
| Female | −0.17 | 0.55 | 0.755 | 0.60 | 1.04 | 0.564 |
| Black | −0.23 | 1.02 | 0.825 | −1.34 | 1.21 | 0.270 |
| Never married | −1.48 | 0.71 | 0.036 | −1.28 | 0.93 | 0.171 |
| High school educated | 0.24 | 0.59 | 0.684 | −1.41 | 1.16 | 0.223 |
| Income<$5,000 | 0.99 | 0.64 | 0.124 | 1.33 | 1.18 | 0.262 |
| Homeless | −1.06 | 0.84 | 0.207 | −0.72 | 1.39 | 0.604 |
| Any alcohol use | 0.29 | 0.62 | 0.644 | 0.72 | 0.95 | 0.448 |
| Any cigarette use | 0.62 | 0.71 | 0.382 | 17.13 | 3211.04 | 0.996 |
| Clinical Characteristics | ||||||
| CES-D≥23a | 0.17 | 0.66 | 0.798 | 1.39 | 0.88 | 0.116 |
| HIV | −0.58 | 0.60 | 0.340 | −1.94 | 0.95 | 0.042 |
| Highly active antiretroviral therapy (HAART) | −12.55 | 454.98 | 0.978 | −14.92 | 454.99 | 0.974 |
| Hepatitis C positiveb | 0.07 | 0.83 | 0.931 | −1.60 | 1.02 | 0.118 |
| Sexually transmitted infections (excluding chlamydia) | −17.31 | 2593.91 | 0.990 | −10.84 | 229.97 | 0.962 |
| Drug use characteristics | ||||||
| Frequency of daily injection | ||||||
| <1/day | 1.92 | 0.74 | 0.009 | −14.38 | 2798.93 | 0.996 |
| ≥1/day | 2.17 | 1.10 | 0.049 | 3.75 | 1.36 | 0.006 |
| Shared needles | 2.02 | 1.08 | 0.063 | 2.70 | 1.24 | 0.030 |
| Current injector | 1.92 | 0.66 | 0.004 | 2.75 | 1.00 | 0.006 |
| Marijuana use | 0.82 | 0.65 | 0.209 | −0.11 | 1.19 | 0.926 |
| Snort cocaine | 16.03 | 2805.64 | 0.995 | 16.84 | 2805.64 | 0.995 |
| Snort heroin | 18.07 | 2727.37 | 0.995 | 17.10 | 2727.37 | 0.995 |
| Inject cocaine | 1.17 | 0.91 | 0.202 | 2.34 | 1.07 | 0.028 |
| Inject heroin | 2.26 | 0.84 | 0.007 | 3.31 | 1.13 | 0.004 |
| Crack use | 2.60 | 1.07 | 0.015 | 3.25 | 1.24 | 0.009 |
| Speedball usec | 2.02 | 1.21 | 0.094 | 2.70 | 1.33 | 0.043 |
| Attended a detox program | 17.14 | 2808.83 | 0.995 | 16.75 | 2808.83 | 0.995 |
| Methadone treatment | −0.29 | 0.64 | 0.645 | −1.57 | 1.51 | 0.299 |
| Any other drug treatment program | −0.83 | 0.78 | 0.289 | −16.99 | 3135.07 | 0.996 |
| Attended a drug alcohol treatment program | −0.02 | 0.58 | 0.968 | −1.34 | 0.95 | 0.158 |
| DAST ≥16d | 0.49 | 1.33 | 0.715 | 0.82 | 1.68 | 0.627 |
All characteristics represent self-reported behavior within the 6 months prior to the start of EXACT
Bold values indicate p-value<0.05
Center for Epidemiologic Studies- Depression Scale
Diagnosis via HCV antibody testing
Speedball is defined as the simultaneous injection of a mixture of cocaine and heroin
Drug Abuse Screening Test
Additionally, compared to individuals reporting low intensity drug use, high intensity drug users had lower odds of being HIV positive (log OR: −1.94, p-value=0.042).
3.3 Associations of drug using risk groups with subsequent health care indicators
We next examined the association of EMA-classified drug-using risk groups with subsequent indicators of healthcare utilization as determined through the ALIVE study follow-up. At the first ALIVE study visit following EXACT study completion, 84% of EXACT participants reported having medical insurance of any kind (private or public) and 72% reported attending an outpatient medical appointment.
In unadjusted analyses (Table 4), high intensity drug users (Group 3) were 79% less likely to report attending outpatient appointments relative to low intensity drug users (OR: 0.19; 95% confidence interval [CI], 0.03-1.04). Additionally, compared to low intensity drug users, high intensity drug users had lower odds of having medical insurance (OR: 0.19; 95% CI, 0.03-1.04)
Table 4.
Unadjusted associations of drug use risk and engagement in care at subsequent ALIVE visit*
| At subsequent ALIVE Visit, did Participant report.... | Medical insurance status | Attended outpatient appointments | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| EMA drug use risk groups | ||||||
| Low intensity drug use | Ref | Ref | Ref | Ref | ||
| Moderate intensity drug use | 0.64 | 0.13-3.19 | 0.558 | 0.64 | 0.19-2.16 | 0.476 |
| High intensity drug use | 0.13 | 0.02-0.94 | 0.043 | 0.19 | 0.03-1.04 | 0.055 |
Bold values indicate p-values<0.05
In adjusted analyses (Table 5), the final model for health care utilization included EMA-defined drug use intensity risk group, being older, female gender, homelessness and recent methadone treatment. Adjusting for these covariates, individuals in the high intensity drug-using group reported an 88% reduced likelihood (aOR 0.12; 95% CI, 0.02-0.85) of attending any outpatient visit relative to low intensity users. Compared to low intensity users, high intensity drug users were notably less likely to have medical insurance (aOR 0.10; 95% CI, 0.01-0.88).
Table 5.
| At subsequent ALIVE Visit, did participant report.... | Medical insurance status | Attended outpatient appointments | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| EMA drug use risk groups | ||||||
| Low intensity drug use | Ref | Ref | Ref | Ref | Ref | Ref |
| Moderate intensity drug use | 0.50 | 0.09-2.89 | 0.486 | 0.52 | 0.14-1.89 | 0.322 |
| High intensity drug use | 0.10 | 0.01-0.88 | 0.040 | 0.13 | 0.02-0.85 | 0.033 |
| Female | 0.69 | 0.21-2.33 | 0.554 | 2.93 | 1.07-8.04 | 0.037 |
| Age≥50 | 1.24 | 0.32-4.84 | 0.757 | 0.68 | 0.25-1.88 | 0.460 |
| Homeless | 0.62 | 0.07-5.28 | 0.663 | 0.42 | 0.07-2.58 | 0.350 |
| Methadone Treatment | 0.74 | 0.23-2.35 | 0.604 | |||
| Same doctor for at least 2 years | 5.57 | 1.63-19.05 | 0.006 | |||
Models adjusted for variables with listed values
Bold values indicate p-values<0.05
4. DISCUSSION
Our results demonstrated that collecting self-reported drug use data utilizing Ecological Momentary Assessment (EMA) methods allowed for an in-depth understanding of the heterogeneous patterns of drug use and associated behaviors. Growth mixture modeling of real-time reports of daily heroin or cocaine use over a 30 day study period distinguished three drug using risk groups, represented as low, moderate and high intensity drug use. Our analysis demonstrated distinct behavioral profiles for each risk group with the high intensity users comprised of younger, polysubstance users who more likely to share needles but not to be HIV infected. Importantly, during subsequent follow-up the drug using groups identified were predictive of reductions in being medically insured and attending outpatient appointments.
The three drug using risk groups defined in this analysis represent individuals with increasing intensity of heroin or cocaine use. A quarter of study participants were in the low intensity drug-using group as they reported little heroin or cocaine use over the 30-day follow-up. This is an expected finding based on the eligibility criteria which aimed to recruit participants with variable intensities of drug use. Even among our population of persons with a self-identified history of injecting drugs, many participants had ceased injecting and were infrequent users of non-injection drugs. This low intensity drug-using group provided a baseline for comparison with groups with greater intensities of heroin and cocaine use. Further, this highlights the advantage of community-based samples as low intensity users may rarely be seen in drug treatment studies.
The moderate intensity drug-using trajectory had the largest membership and was associated with baseline injecting of heroin and smoking crack relative to the low intensity drug-using group. Despite polyroute and polysubstance use, these individuals reported less than daily drug use over follow-up and no higher risk behaviors associated with HIV risk such as sharing syringes. Prior research has indicated that reduced intensity of drug use may be associated with increased likelihood of sustaining cessation (Bruneau et al., 2004; Shah et al., 2006). Interestingly, at baseline, injecting more than once a day (not drug specific) was associated with membership in this group. This could be a result of recall bias; participants were asked to recall their injection drug using behavior in the prior 6-months and these behaviors did not uniformly match what was reported in real-time (this difference was not seen among high intensity drug users). This discrepancy in reporting may provide insight into how drug addiction is characterized and quantified by users themselves. Overall, the behavioral characteristics of this group suggest members who are stable but chronic drug users and that remain at risk for drug-related comorbidities and overdose.
Membership in the high intensity drug-using group was associated with younger age and self-report at baseline of recently injecting drugs greater than once daily. Relative to the low intensity drug users, sharing needles and the use of a variety of drugs, including heroin and cocaine separately, injecting both together as speedball, and smoking crack were associated with group membership. High frequency of poly-drug use and needle sharing is not only associated with longer time to cessation (Shah et al., 2006) but puts individuals at risk for the medical consequences of chronic substance abuse including HIV transmission or acquisition and poor treatment outcomes (Nelson et al., 2002).
Members of the high drug-using group were also less likely to be infected with HIV. Our analysis demonstrated that individuals who are high-risk users are young, intense drug users who remain at risk for HIV acquisition. In ALIVE, the risk of acquiring HIV from injection drug use has declined in recent years (Mehta et al., 2006). As a result, there is less circulating HIV and the risk for transmission from a single injection event has reduced. The paradoxical reduced HIV prevalence among high intensity users may represent a cohort effect of younger more intense users with distinct social networks from older users (Fuller et al., 2005) who, by nature of declining HIV prevalence have been spared from acquiring HIV. However, as we have seen with hepatitis C infection, HIV risk may simply be deferred rather than obviated (Mehta et al., 2011). Our results suggest that EMA methods could be used in drug treatment trials to calculate daily intensity of use and to capture how intensity relates to other drug- related behaviors (e.g., participation in shooting galleries or sharing needles).
Drug-seeking behavior often conflicts with the health promoting goals of safer sex, safer injecting, and adherence to recommended treatments. Our analysis demonstrated that the odds of attending outpatient appointments and having medical insurance and were reduced for those classified as high intense drug users relative to low intensity drug users even after adjustment for potential confounders. As evidenced by their ability to predict healthcare access and utilization, these EMA-defined risk groups displayed clinical relevance; moving forward, these risk groups could potentially help with targeting of resources for those who have difficulties of remaining engaged in care.
In addition to relatively small sample size, we were limited in this analysis by examination of only time-fixed baseline factors and behavioral variables as predictors of class membership. The latter was done intentionally to determine what stable factors predicted drug use, as inclusion of time-varying covariates would have only affected the shape of the trajectory rather than membership in the groups (Nagin and Tremblay, 2001). Our small sample size also limited our power to distinguish differences between engagement in care practices of HIV infected and uninfected individuals. In sensitivity analyses, stratification by HIV status resulted in no differences in engagement in care between HIV infected and uninfected individuals. Additionally, there may be a concern that our study population consists of mostly older individuals who may be maturing out of their drug use and/or beginning to transition to use of prescription drugs rather than injection of street drugs. While this may be true, our study population is representative of the aging population of drug-users in Baltimore, MD and many other cities nationally (e.g., Philadelphia, Newark, Detroit) (Murrill et al., 2002).
Our analysis described drug use from EMA self-reports and employed growth mixture models to describe the meaningful groups of drug users that existed in the population. To our knowledge, this is one of the first mHealth analyses to use semi-parametric growth mixture models with EMA data to examine sub-populations of heroin and cocaine users. While growth mixture modeling has been previously used to describe trajectories of drug use and cessation, these analyses have been generally utilized traditional cohort data collected in longer intervals rather than proximate EMA methods collected in near real-time (Hser et al., 2007; Genberg et al., 2011) This EMA data provided more refined assessments of drug using behavior, including daily intense or intermittent use. As a result, our analyses were able to demonstrate the presence of non-uniformity in drug using risk that varied by sociodemographics, drug type, and frequency of daily use.
As previously reported, the EXACT study demonstrates the ability to efficiently and effectively collect high-quality, real-time EMA data in a challenging study population of impoverished urban drug users (Kirk et al., 2013b). In contrast to the broad range of possibilities of mHealth applications among HIV and substance users, the current EMA analysis should be considered as an initial application (Kirk et al., 2013a). EMA methods can improve data collection techniques as well as expand approaches to delivering interventions, such as providing the ability to capture the environmental context of an individual's drug using experience (Linas et al., 2014). In the near future and building on the current analysis, novel real-time mHealth intervention strategies aimed at redirecting an individuals away from areas previously designated as a relapse triggers or places where the individual previously bought and/or used drugs and improving medication adherence to improve HIV treatment outcomes could be tailored as individualized ecologic momentary interventions.
HIGHLIGHTS.
Ecological Momentary Assessment (EMA) data modeled using latent class growth mixture models
Low, medium and high intensity drug using groups had distinct behavioral profiles
High intensity drug users had reduced odds of attending outpatient appointments
High intensity drug users were less likely to be medically insured
EMA captured non-uniformity of drug use and users at risk for poor healthcare access and utilization
Acknowledgements
The authors acknowledge the substantial commitment of EXACT study participants and staff.
Contributors: All listed authors have seen, approved, and contributed significantly to the manuscript. Larry W. Chang is supported by the National Institutes of Mental Health at the National Institutes of Health (K23MH086338). Ryan P. Westergaard is supported by the National Institutes of Health/National Institute on Drug Abuse (K23DA032306).
Role of Funding Source: Funding for the study was provided through the National Institute on Drug Abuse (Grants U01-DA-04334; and R01-DA-12568).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests:
No conflict declared
REFERENCES
- Bedi G, Preston KL, Epstein DH, Heishman SJ, Marrone GF, Shaham Y, de Wit H. Incubation of cue-induced cigarette craving during abstinence in human smokers. Biol. Psychiatry. 2011;69:708–711. doi: 10.1016/j.biopsych.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell C, Metsch LR, Vogenthaler N, Cardenas G, Rodriguez A, Locascio V, Kuper T, Scharf E, Marquez A, Yohannan M, del Rio C. Never in care: characteristics of HIV-infected crack cocaine users in 2 us cities who have never been to outpatient HIV care. J. Acquir. Immune Defic. Syndr. 2010;54:376–380. doi: 10.1097/QAI.0b013e3181d01d31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruneau J, Brogly SB, Tyndall MW, Lamothe F, Franco EL. Intensity of drug injection as a determinant of sustained injection cessation among chronic drug users: the interface with social factors and service utilization. Addiction. 2004;99:727–737. doi: 10.1111/j.1360-0443.2004.00713.x. [DOI] [PubMed] [Google Scholar]
- Center For Advancing Health: Evidence. Engagement. Equity . A Snapshot of People's Engagement in Their Health Care. Washington, DC.: 2010. [Google Scholar]
- Compton WM, Thomas YF, Stinson FS, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV drug abuse and dependence in the United States: results from the national epidemiologic survey on alcohol and related conditions. Arch. Gen. Psychiatry. 2007;64:566–576. doi: 10.1001/archpsyc.64.5.566. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Marrone GF, Heishman SJ, Schmittner J, Preston KL. Tobacco, cocaine, and heroin: craving and use during daily life. Addict. Behav. 2010;35:318–324. doi: 10.1016/j.addbeh.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein DH, Preston KL. Daily life hour by hour, with and without cocaine: an ecological momentary assessment study. Psychopharmacology (Berl.) 2010;211:223–232. doi: 10.1007/s00213-010-1884-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein DH, Preston KL. TGI Monday?: drug-dependent outpatients report lower stress and more happiness at work than elsewhere. Am. J. Addict. 2012;21:189–198. doi: 10.1111/j.1521-0391.2012.00230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein DH, Willner-Reid J, Vahabzadeh M, Mezghanni M, Lin JL, Preston KL. Real-time electronic diary reports of cue exposure and mood in the hours before cocaine and heroin craving and use. Arch. Gen. Psychiatry. 2009;66:88–94. doi: 10.1001/archgenpsychiatry.2008.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller CM, Borrell LN, Latkin CA, Galea S, Ompad DC, Strathdee SA, Vlahov D. Effects of race, neighborhood, and social network on age at initiation of injection drug use. Am. J. Public Health. 2005;95:689–695. doi: 10.2105/AJPH.2003.02178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galai N, Safaeian M, Vlahov D, Bolotin A, Celentano DD. Longitudinal patterns of drug injection behavior in the ALIVE Study Cohort,1988–2000: description and determinants. Am. J. Epidemiol. 2003;158:695–704. doi: 10.1093/aje/kwg209. [DOI] [PubMed] [Google Scholar]
- Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin. Infect. Dis. 2011;52:793–800. doi: 10.1093/cid/ciq243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genberg BL, Gange SJ, Go VF, Celentano DD, Kirk GD, Mehta SH. Trajectories of injection drug use over 20 years (1988–2008) in Baltimore, Maryland. Am. J. Epidemiol. 2011;173:829–836. doi: 10.1093/aje/kwq441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub ET, Latka M, Hagan H, Havens JR, Hudson SM, Kapadia F, Campbell JV, Garfein RS, Thomas DL, Strathdee SA. Screening for depressive symptoms among HCV-infected injection drug users: examination of the utility of the CES-D and the Beck Depression Inventory. J. Urban Health. 2004;81:278–290. doi: 10.1093/jurban/jth114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes DA, Schulz KF. Bias and causal associations in observational research. Lancet. 2002;359:248–252. doi: 10.1016/S0140-6736(02)07451-2. [DOI] [PubMed] [Google Scholar]
- Hedeker D, Mermelstein RJ, Berbaum ML, Campbell RT. Modeling mood variation associated with smoking: an application of a heterogeneous mixed-effects model for analysis of ecological momentary assessment (EMA) data. Addiction. 2009;104:297–307. doi: 10.1111/j.1360-0443.2008.02435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hser YI, Huang D, Chou CP, Anglin MD. Trajectories of heroin addiction: growth mixture modeling results based on a 33-year follow-up study. Eval. Rev. 2007;31:548–563. doi: 10.1177/0193841X07307315. [DOI] [PubMed] [Google Scholar]
- Jones BL, Nagin DS. Advances in group-based trajectory modeling and an SAS procedure for estimating them. Sociol. Methods Res. 2007;35:542–571. [Google Scholar]
- Kirk GD, Himelhoch SS, Westergaard RP, Beckwith CG. Using mobile health technology to improve HIV care for persons living with HIV and substance abuse. AIDS Res. Treat. Epub ahead of print. 2013a doi: 10.1155/2013/194613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk GD, Linas BS, Westergaard RP, Piggott D, Bollinger RC, Chang LW, Genz A. The Exposure Assessment in Current Time Study: implementation, feasibility, and acceptability of real-time data collection in a community cohort of illicit drug users. AIDS Res. Treat. 2013b doi: 10.1155/2013/594671. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowlton AR, Arnsten JH, Eldred LJ, Wilkinson JD, Shade SB, Bohnert AS, Yang C, Wissow LS, Purcell DW. Antiretroviral use among active injection-drug users: the role of patient-provider engagement and structural factors. AIDS patient Care STDs. 2010;24:421–428. doi: 10.1089/apc.2009.0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowlton AR, Hoover DR, Chung SE, Celentano DD, Vlahov D, Latkin CA. Access to medical care and service utilization among injection drug users with HIV/AIDS. Drug Alcohol Depend. 2001;64:55–62. doi: 10.1016/s0376-8716(00)00228-3. [DOI] [PubMed] [Google Scholar]
- Kohout FJ, Berkman LF, Evans DA, Cornoni-Huntley J. Two shorter forms of the CES-D (Center for Epidemiological Studies Depression) depression symptoms index. J. Aging Health. 1993;5:179–193. doi: 10.1177/089826439300500202. [DOI] [PubMed] [Google Scholar]
- Linas BS, Latkin C, Westergaard RP, Chang LW, Bollinger RC, Genz A, Kirk GD. Capturing illicit drug use where and when it happens: an ecological momentary assessment of the social, physical and activity environment of using versus craving illicit drugs. Addiction. 2014;110:315–25. doi: 10.1111/add.12768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta SH, Astemborski J, Kirk GD, Strathdee SA, Nelson KE, Vlahov D, Thomas DL. Changes in blood-borne infection risk among injection drug users. J. Infect. Dis. 2011;203:587–594. doi: 10.1093/infdis/jiq112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta SH, Galai N, Astemborski J, Celentano DD, Strathdee SA, Vlahov D, Nelson KE. HIV incidence among injection drug users in Baltimore, Maryland (1988-2004). J. Acquir. Immune Defic. Syndr. 2006;43:368–372. doi: 10.1097/01.qai.0000243050.27580.1a. [DOI] [PubMed] [Google Scholar]
- Minami H, McCarthy DE, Jorenby DE, Baker TB. An Ecological Momentary Assessment analysis of relations among coping, affect and smoking during a quit attempt. Addiction. 2011;106:641–650. doi: 10.1111/j.1360-0443.2010.03243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrill CS, Weeks H, Castrucci BC, Weinstock HS, Bell BP, Spruill C, Gwinn M. Age-specific seroprevalence of HIV, hepatitis B virus, and hepatitis C virus infection among injection drug users admitted to drug treatment in 6 US cities. Am. J. Public Health. 2002;92:385–387. doi: 10.2105/ajph.92.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthen B, Muthen LK. Integrating person-centered and variable-centered analyses: growth mixture modeling with latent trajectory classes. Alcohol. Clin. Exp. Res. 2000;24:882–891. [PubMed] [Google Scholar]
- Nagin DS, Odgers CL. Group-based trajectory modeling in clinical research. Annu. Rev. Clin. Psychol. 2010;6:109–138. doi: 10.1146/annurev.clinpsy.121208.131413. [DOI] [PubMed] [Google Scholar]
- Nagin DS, Tremblay RE. Analyzing developmental trajectories of distinct but related behaviors: a group-based method. Psychol. Methods. 2001;6:18–34. doi: 10.1037/1082-989x.6.1.18. [DOI] [PubMed] [Google Scholar]
- Nelson KE, Galai N, Safaeian M, Strathdee SA, Celentano DD, Vlahov D. Temporal trends in the incidence of human immunodeficiency virus infection and risk behavior among injection drug users in Baltimore, Maryland, 1988-1998. Am. J. Epidemiol. 2002;156:641–653. doi: 10.1093/aje/kwf086. [DOI] [PubMed] [Google Scholar]
- Nylund KL, Asparoutiov T, Muthen BO. Deciding on the number of classes in latent class analysis and growth mixture modeling: a Monte Carlo simulation study. Struct. Equ. Modeling. 2007;14:535–569. [Google Scholar]
- ONDC . Smart Steps: Treating Baltimore's Drug Problem. Washington, DC.: 2000. [Google Scholar]
- Paganini-Hill A, Ross RK. Reliability of recall of drug usage and other health-related information. Am. J. Epidemiol. 1982;116:114–122. doi: 10.1093/oxfordjournals.aje.a113386. [DOI] [PubMed] [Google Scholar]
- Preston KL, Epstein DH. Stress in the daily lives of cocaine and heroin users: relationship to mood, craving, relapse triggers, and cocaine use. Psychopharmacology (Berl.) 2011;218:29–37. doi: 10.1007/s00213-011-2183-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston KL, Vahabzadeh M, Schmittner J, Lin JL, Gorelick DA, Epstein DH. Cocaine craving and use during daily life. Psychopharmacology (Berl.) 2009;207:291–301. doi: 10.1007/s00213-009-1655-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JR, Epstein DH, Umbricht A, Preston KL. Changes in HIV risk behaviors among patients receiving combined pharmacological and behavioral interventions for heroin and cocaine dependence. Addict. Behav. 2006;31:868–879. doi: 10.1016/j.addbeh.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Shah NG, Galai N, Celentano DD, Vlahov D, Strathdee SA. Longitudinal predictors of injection cessation and subsequent relapse among a cohort of injection drug users in Baltimore, MD, 1988-2000. Drug Alcohol Depend. 2006;83:147–156. doi: 10.1016/j.drugalcdep.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Balabanis MH, Gwaltney CJ, Paty JA, Gnys M, Kassel JD, Hickcox M, Paton SM. Prediction of lapse from associations between smoking and situational antecedents assessed by ecological momentary assessment. Drug Alcohol Depend. 2007;91:159–168. doi: 10.1016/j.drugalcdep.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Kirchner TR, Ferguson SG, Scharf DM. Patterns of intermittent smoking: an analysis using Ecological Momentary Assessment. Addict. Behav. 2009;34:514–519. doi: 10.1016/j.addbeh.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner HA. The drug abuse screening test. Addict. Behav. 1982;7:363–371. doi: 10.1016/0306-4603(82)90005-3. [DOI] [PubMed] [Google Scholar]
- Tumwebaze H, Tumwesigye E, Baeten JM, Kurth AE, Revall J, Murnane PM, Chang LW, Celum C. Household-based HIV counseling and testing as a platform for referral to HIV care and medical male circumcision in Uganda: a pilot evaluation. PLoS One. 2012;7:e51620. doi: 10.1371/journal.pone.0051620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlahov D, Anthony JC, Munoz A, Margolick J, Nelson KE, Celentano DD, Solomon L, Polk BF. The ALIVE study, a longitudinal study of HIV-1 infection in intravenous drug users: description of methods and characteristics of participants. NIDA Res. Monogr. 1991;109:75–100. [PubMed] [Google Scholar]
- Westergaard RP, Hess T, Astemborski J, Mehta SH, Kirk GD. Longitudinal changes in engagement in care and viral suppression for HIV-infected injection drug users. AIDS. 2013;27:2559–2566. doi: 10.1097/QAD.0b013e328363bff2. [DOI] [PMC free article] [PubMed] [Google Scholar]