Abstract
Objective
Assessing qualitative patterns of amplitude integrated EEG (aEEG) maturation of preterm infants requires personnel with training in interpretation and an investment of time. Quantitative algorithms provide a method for rapidly and reproducibly assessing an aEEG recording independent of provider skill level. Although there are several qualitative and quantitative normative datasets in the literature, this study provides the broadest array of quantitative aEEG measures in a carefully selected and followed cohort of preterm infants with mild or no visible injury on term equivalent MRI and subsequently normal neurodevelopment at 2 and 7 years of age.
Study Design
A two-channel aEEG recording was obtained on days 4,7,14, and 28 of life for infants born ≤30 weeks EGA. Measures of amplitude and continuity, spectral edge frequency, percentage of trace in interburst interval, interburst interval length, and frequency counts of smooth delta waves, delta brushes and theta bursts were obtained. MRI was obtained at term-equivalent age (TEA) and neurodevelopmental testing was conducted at 2 and 7 years of corrected age.
Result
Correlations were found between increasing post-menstrual age (PMA) and decreasing maximum amplitude (R=−0.23, p=0.05), increasing minimum amplitude (R=0.46, p=0.002), and increasing spectral edge frequency (R=0.78, p=4.17x10−14). Negative correlations were noted between increasing PMA and counts of smooth delta waves (R=−0.39, p=0.001), delta brushes (R=−0.37, p=0.003), and theta bursts (R=−0.61, p=5.66x10−8). Increasing PMA was also associated with a decreased amount of time spent in the interburst interval (R=−0.38, p=0.001) and a shorter length of the maximum IBI (R=−0.27, p=0.03).
Conclusion
This analysis supports a strong correlation between quantitatively determined aEEG measures and PMA, in a cohort of preterm infants with normal TEA neuroimaging and neurodevelopmental outcomes at 7 years of age, which is both predictable and reproducible. These “normative” quantitative values support the pattern of maturation previously identified by qualitative analysis.
Keywords: spectral edge frequency, Bayesian inference, developmental outcomes, quantitative aEEG
Introduction
Burdjalov et al. developed systematic amplitude integrated EEG (aEEG) interpretation guidelines for the term and preterm neonatal population. This approach uses a subjective review of the aEEG trace to determine the continuity of cerebral activity (i.e. the proportion of activity above a set amplitude threshold), the presence or absence of sleep-wake cycling, the minimum amplitude or “baseline” of the trace, as well as the difference between the minimum and maximum amplitudes (1). In addition, other work has demonstrated that there is a clear qualitative pattern of maturation in the aEEG traces of premature infants without neurodevelopmental impairment which parallels that of their overall development (2,3) and that this analysis can also be used to predict outcomes (4).
Digitally recorded aEEG traces allow for computation of a broad array of signal characteristics that can be readily compared across a cohort. The simplest measures, minimum, maximum, and mean amplitude, allow for assessment of continuity. Fast Fourier transformation, which decomposes a complex signal into individual frequencies, enables a more granular view into the exact distribution of activity within a defined frequency band. Pattern recognition algorithms can identify characteristic electrographic signals that evolve in parallel with overall development, such as the length of time between bursts (the interburst interval or IBI) or specific wave patterns such as delta waves, whose appearance in a neonatal aEEG is developmentally regulated. The value of an automated quantitative approach to aEEG interpretation has been discussed in several different forums (4–6) and has been applied largely to automated seizure detection algorithms with some remarkable success (7–11).
A remaining challenge, which must be overcome for widespread adoption of quantitative aEEG techniques, is the development of a ‘normative’ dataset for comparison. Thornberg and Thiringer published one of the earliest datasets (12); however, they did not record any infants born before 30 weeks EGA, had no long term neurodevelopmental data, and reported only the minimum and maximum voltages. Natalucci et al. published data (13) demonstrating predictable changes in amplitude characteristics over a broader range of gestational ages and included term-equivalent imaging as the measure of outcome. Nevertheless, these data are confounded by collection only during the first 96 hours of life, a limited number of quantitative measures, and no long-term neurodevelopmental data. Niemarkt et al. described an even broader array of amplitude (5) and spectral characteristics (14) which was collected longitudinally in a cohort of 18 preterm infants who subsequently had developmental testing scores within the normal range at 1 and 2 years of corrected age (CA), respectively. However there is emerging concern (15) that neurodevelopmental testing at 2 years of age does not accurately predict outcomes at school-age or beyond, and no further follow-up data is available.
To address these issues, we designed a study that outlines a full array of quantitative aEEG characteristics, including amplitude, spectral, and wave pattern features, in a carefully selected population of preterm infants with favorable neuroimaging at term equivalent age and neurodevelopmental outcomes at 2 and 7 years of age. We aim to establish a “normative” range of aEEG measures for this population and hypothesize that healthy preterm infants will demonstrate a predictable pattern of maturation evident by quantitative analysis.
Materials and Methods
Subjects
The subjects in this analysis are a carefully chosen subset of a larger cohort recruited in a prospective monitoring and imaging study conducted by the Victorian Infant Brain Study (VIBeS) group at the Murdoch Children’s Research Institute in Victoria, Australia. Subjects were premature infants born at ≤30 completed weeks of gestation and admitted to the Royal Women’s Hospital in Melbourne, Australia between July 2001 and December 2003. Parents of eligible infants were approached either before delivery or as soon as possible after delivery for informed consent.
Inclusion criteria
Other aspects of this cohort have been described elsewhere in the literature (16–18). For the purposes of this study, subjects were included in the analysis if they were determined to meet strictly defined outcome parameters in terms of both radiographic injury and subsequent neurodevelopmental testing. Favorable injury outcome was defined as no greater than grade I IVH on cranial ultrasound and no greater than mild white matter injury on term equivalent MRI (15). Favorable developmental outcome was assessed at two age points, 2 years (Bayley Scales of Infant Development, 2nd Edition [The Psychological Corporation, San Antonio, TX, USA] with both mental developmental index and psychomotor developmental index sub-scores ≥85) and at 7 years (Wechsler Abbreviated Scale of Intelligence [The Psychological Corporation, San Antonio, TX, USA] with a score ≥85).
Study design
Information regarding the sex, gestational age, birth weight, and Apgar scores were obtained from the subject’s medical chart. Hydrospot electrodes [Physiometrix Inc, North Billerica, MA, USA] were applied in the conventional C3-P3, C4-P4 configuration and traces were recorded using the BRM2 monitor [Natus Medical Incorporated, San Carlos, CA, USA]. Each recording was a minimum of 120 minutes in length and was conducted on days 4,7,14 and 28 of life, regardless of gestational age at birth. All quantitative analysis was performed using Analyze [Natus Medical Incorporated, San Carlos, CA], an offline aEEG review software package.
Cranial ultrasounds were obtained four times during the hospital course: during the first 72 hours, day 7–10, day 28, and prior to discharge as per routine clinical practice. All infants underwent an MRI scan at term equivalent age without sedation. Infants were fed, swaddled, outfitted with earmuffs, and placed in a vacuum fixation beanbag. Sleeping infants were scanned in a 1.5T Signa LX Echospeed MRI System [General Electric, Milwaukee, WI, USA].
Basic Analysis
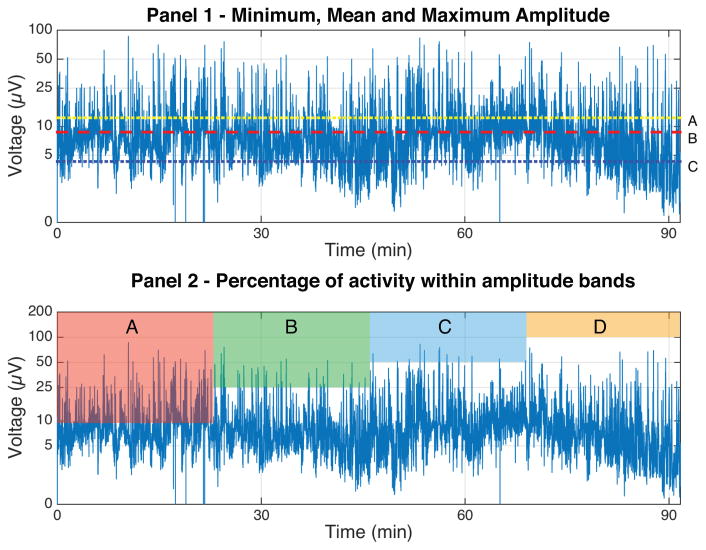
The recorded aEEG data were visually inspected, and regions of the recording with electrode impedance exceeding 15kΩ were discarded. The middle 60-minutes of each 120-minute recording was used for analysis, with the aim of avoiding artifacts sometimes found at the beginning and end of aEEG recordings. This one-hour segment was then visually reviewed to ensure that it was artifact and seizure-free. The minimum, maximum, and mean amplitude of each trace was then calculated. A sample tracing with quantitative measures highlighted is shown in the top panel of Figure 1.
Figure 1.
The top panel depicts a 90-minute aEEG recording. Line A represents the median maximum value (12.3 μV), line B the mean value (8.8 μV) and line C the median minimum value (4.3 μV). The bottom panel depicts the 4 amplitude bands that were assessed in order to determine the content of the trace. Band A represents the percentage of 2 second epochs with a mean value greater than 10 μV, Band B represents those with a mean value greater than 25 μV, Band C represents those with a mean value greater than 50 μV and Band D those greater than 100 μV.
Continuity
Hellström-Westas and de Vries standardized (2) the terminology used to describe aEEG backgrounds with a five-category system consisting of continuous normal voltage, discontinuous normal voltage, continuous low voltage, burst suppression, and flat tracing. Each category is defined by minimum and maximum voltage parameters. Continuity can be evaluated quantitatively by determining the average baseline of a trace as compared to a predetermined threshold (19) or by determining the percentage of the trace contained within certain voltage thresholds (20). In this dataset, the percentage of two-second intervals where the amplitude of the recorded signal was above four different thresholds (10 μV, 25 μV, 50 μV, 100 μV) was calculated, thus allowing for background categorization. For example, using the criteria outlined by Hellström-Westas and de Vries, a continuous normal voltage pattern should have nearly 100% of samples with amplitudes above 10 μV and below 50 μV. A visual representation of this analysis is depicted in the bottom panel of Figure 1.
Sleep-wake cycling
The trace was inspected for evidence of sleep-wake cycling (SWC), defined as rhythmic sinusoidal elevations of the baseline lasting longer than 20 minutes (21). The SWC pattern was deemed “mature” if it was the predominant pattern during the recording period, “intermediate” if cycling was present but not predominant, and “absent” if SWC was not present.
Spectral edge frequency (SEF) analysis
SEF has been used by researchers to determine the frequency distribution in aEEG traces in healthy (14,20) and injured (22) preterm infants. Fast Fourier transformation was used to determine SEF 90, defined as the frequency between 2 and 20 Hz, below which 90% of the power was present in the same 60-minute epoch.
Bayesian probability analysis
A software algorithm developed by Mitchell et al. (23), using a broader array of the same infants in this cohort, takes a Bayesian inference approach to identify delta waves (including separate components of smooth delta waves and delta brushes) and theta bursts. In addition, it can calculate the percentage of the trace that is spent in the interburst interval (IBI) and the length of the maximum IBI.
Smooth delta waves were defined as a wave pattern at least 100μV in amplitude with a frequency between 0.5 Hz and 1.5 Hz. Delta brushes are superimposed waves of higher frequency (8–22 Hz) and lower amplitude (10–75μV). Theta bursts were defined as activity greater than 100μV in amplitude and between 4 and 6 Hz in frequency. Interburst interval was defined as a period of time greater than five seconds with minimal activity (<10μV). Absolute counts of each wave type (delta brush, smooth delta wave and theta burst) as well as the IBI data were measured for the same 60-minute epoch as the previous measurements.
Radiographic analysis
MRI images were analyzed qualitatively for white matter injury by a single investigator (TI) blinded to the infant’s clinical history. WMI was graded from 1–4 where grade 1 was normal, grade 2 was mild non-cystic abnormality, grade 3 was moderate-severe non-cystic abnormality and grade 4 was severe cystic abnormality (22).
Statistical analysis
As the sample was drawn from a group of infants with a wide range of gestational ages at birth and was collected across an even wider PMA, the recordings were divided into four groups based on the PMA at the time of aEEG recording (< 27 weeks, 27–29 weeks, 29–31 weeks and > 31 weeks) for some of the analysis. Due to variations in gestational age at birth, some infants had consecutive studies in the same grouping. Within each group, the mean, minimum, and maximum amplitude were calculated as well as the mean SEF 90 and continuity measures. Associations were made between the exact PMA at the time of each recording (rather than group) and each quantitative variable using the Pearson product-moment correlation coefficient. All statistical analysis was conducted using R version 3.1.1 [R Foundation for Statistical Computing, Vienna, Austria] and MATLAB 8.1 [The MathWorks, Inc., Natick, MA, USA].
Results
Descriptive statistics
224/348 (64%) eligible infants born during the recruitment period were successfully enrolled in the initial cohort. 20/224 (9%) met the previously outlined inclusion criteria and had normal neurodevelopmental scores at 2 years of age, but only 18/20 (90%) had normal neurodevelopmental scores at 7 years of age. The characteristics of the included sample are provided in Table 1 and a comparison between those included and excluded is provided in Table 2.
Table 1.
Sample Perinatal and Clinical Characteristics
| Characteristic | n=18 |
|---|---|
| Gestational age at birth, m1 ± SD, weeks | 27.3 ± 1.9 |
| Male, n (%) | 9 (50) |
| Birth weight, m ± SD, g | 976 ± 173 |
| Intraventricular hemorrhage | |
| None, n (%) | 16 (89) |
| Grade I, n (%) | 2 (11) |
| White matter injury | |
| None, n (%) | 10 (56) |
| Mild non-cystic, n (%) | 8 (44) |
| Need for inotropic agents, n (%) | 4 (22) |
| Need for sedative agents, n (%) | 0 (0) |
| Antenatal steroids, n (%) | 17 (94) |
| Culture-proven sepsis, n (%) | 3 (17) |
| BPD2, n (%)c | 6 (33) |
| BSID-II | |
| MDI3, m ± SD | 98 ± 9 |
| PDI4, m ± SD | 99 ± 9 |
| WASI FSIQ, m ± SD | 104 ± 15 |
m denotes mean, M denotes median.
Defined as need for supplemental oxygen at 36 weeks PMA.
Mental Development Index.
Psychomotor Development Index.
Table 2.
Comparison of perinatal factors between included and excluded subjects
| Included (n=18) | Excluded (n=206) | P value | |
|---|---|---|---|
| Gestational age at birth, m1 ± SD, weeks | 27.3 ± 1.9 | 27.6 ± 1.9 | 0.521 |
| Male, n (%) | 9 (50) | 105 (51) | 0.992 |
| Birth weight, m ± SD, g | 976 ± 173 | 927.6 ± 197 | 0.308 |
| Antenatal steroids, n (%) | 17 (94) | 181 (88) | 0.260 |
| Intrauterine growth restriction, n (%) | 2 (11) | 16 (8) | 0.937 |
Post-menstrual age.
Amplitude measures
Both the minimum and maximum amplitudes were strongly correlated with PMA, with increasing minimum amplitude associated with increasing PMA (R=0.46, p=0.002) and a decreasing maximum amplitude with increasing PMA (R=−0.23, p=0.05). Mean amplitude was not significantly associated with PMA. There was also a strong correlation with an increase in the SEF 90 and advancing PMA (R=0.78, p=4.17x10−14), going from 7.9 to 10.1 Hz over the measured period.
Our data confirm the previously described qualitative measures of aEEG background maturation, demonstrating a decrease in the high frequency activity associated with high voltage bursts (> 100 μV) and a simultaneous increase in the percentage of the trace associated with continuous normal voltages (25–50 μV). Table 3 provides a summary of this data.
Table 3.
Amplitude characteristics by PMA1
| Group 1 <27 weeks (n=8) |
Group 2 27–29 weeks (n=16) |
Group 3 29–31 weeks (n=21) |
Group 4 >31 weeks (n=19) |
|
|---|---|---|---|---|
| Min Amplitude, M2 (range) | 1.7 (1.3–2.8) | 2.1 (1.5–2.8) | 2.4 (1.1–3.7) | 3.1 (2.0–3.5) |
| Mean Amplitude, M (range) | 6.2 (5.6–8.6) | 5.8 (4.4–8.3) | 6.2 (3.9–8.4) | 6.3 (4.7–8.8) |
| Max Amplitude, M (range) | 12.9 (8.6–15.7) | 10.7 (5.1–15.9) | 10.4 (5.4–15.7) | 9.7 (5.5–15.5) |
| SEF 953, M (range) | 7.9 (7.4–8.8) | 8.3 (7.3–9.5) | 9.4 (8.6–10.7) | 10.1 (9.0–11.2) |
| > 10μV, M (range)4 | 100 (97–100) | 100 (94–100) | 100 (88–100) | 100 (100–100) |
| > 25μV, M (range)4 | 76 (64–89) | 78 (55–89) | 80 (56–93) | 87 (71–100) |
| > 50μV, M (range)4 | 46 (40–59) | 44 (30–64) | 47 (24–60) | 39 (13–69) |
| > 100μV, M (range)4 | 22 (16–29) | 17 (4–28) | 12 (1–23) | 5 (0–16) |
PMA denotes post-menstrual age.
M denotes median value.
Spectral edge frequency.
Percentage of one-minute epochs with average baseline exceeding given threshold.
Sleep-wake cycling
SWC was noted in increasing proportions as PMA advanced, similar in proportion to that noted in prior studies (21). An overview is provided in Table 4.
Table 4.
Sleep-wake cycling by PMA1
| Group 1 <27 weeks (n=8) |
Group 2 27–29 weeks (n=16) |
Group 3 29–31 weeks (n=21) |
Group 4 >31 weeks (n=19) |
|
|---|---|---|---|---|
| SWC2 absent | 2 | 2 | 0 | 0 |
| SWC2 intermediate | 6 | 14 | 16 | 6 |
| SWC2 mature | 0 | 0 | 5 | 13 |
PMA denotes post-menstrual age.
SWC denotes sleep-wake cycling.
Delta and Theta activity
A significant negative correlation was noted with increasing PMA and counts of all wave types including smooth delta wave (R=−0.39, p=0.001), delta brush (R=−0.37, p=0.003) and theta burst (R=−0.61, p=5.66x10−8). Median detected wave counts for each gestational age grouping are provided in Table 5.
Table 5.
Wave characteristics by PMA1
| Group1 <27 weeks (n=8) |
Group 2 27–29 weeks (n=16) |
Group 3 29–31 weeks (n=21) |
Group 4 >31 weeks (n=19) |
|
|---|---|---|---|---|
| SDW2, M3 (range) | 191 (112–315) | 167 (57–367) | 131 (24–350) | 101 (18–254) |
| DB4, M (range) | 61 (25–74) | 47 (5–94) | 34 (0–119) | 20 (3–70) |
| TB5, M (range) | 21 (9–36) | 7 (0–41) | 1 (0–9) | 1 (0–6) |
| IBI6 %, m (SD) | 38 (14) | 35 (9) | 33 (12) | 24 (9) |
| Max. IBI Length, seconds, m (SD) | 31 (17) | 41 (51) | 24 (10) | 17 (8) |
Post-menstrual age.
Smooth delta wave.
m denotes mean, M denotes median.
Delta brush.
Theta burst.
Percentage of recording time between bursts.
Interburst interval
The mean percentage of the recording spent in the interburst interval decreased as PMA increased, with a significant negative correlation (R=−0.38, p=0.001). The maximum length of the interburst interval decreased with increasing PMA, with a significant negative correlation (R=−0.27, p=0.03). Complete information about changes in the interburst interval at different PMAs is provided in Table 5.
Discussion
A significant limitation preventing the widespread adoption of routine EEG monitoring in neonatal intensive care units is the need for interpretation by skilled epileptologists, adding a significant time and cost burden to health care systems. aEEG has filled this gap in many centers around the world, using pattern recognition to provide information about brain function that can be readily interpreted by non-expert clinicians, enabling detection of electrographic seizures and assessment of background activity (24). Nevertheless, both conventional and aEEG methods rely on subjective interpretation which can be variable, time consuming, and requires a degree of experience.
This study uses quantitative methods on aEEG to confirm the maturational patterns that have been previously described in the literature using qualitative analysis. Our analysis of the content of these aEEG recordings supports a predictable transition that is strongly correlated with PMA. This developmental continuum transitions from the infrequent high amplitude bursts and long low-voltage quiescent periods of burst suppression and tracé discontinue, which predominate the earliest gestational ages, to the continuous, moderate voltage activity with infrequent quiet periods of tracé alternans associated with more developmentally mature infants.
These findings have significant biological relevance as there is compelling evidence that early cortical activity is important for brain growth (25), the absence or delay of which can be predicted by a persistent or transient arrest in electrographic maturation (26) and is known to be associated with adverse neurodevelopment (27). More specifically, an increased IBI (and thus overall decreased cortical activity) has been associated with adverse events both in the acute setting such as acidosis (28) or sedative administration such as morphine and phenobarbital (29), as well as long term with an increased likelihood of developmental handicap at 2 years CA (30).
Technical aspects of aEEG signal processing, as is the case with all quantitative aEEG or EEG analysis, limit this study. In order to produce the aEEG output, the raw signal is transformed, first by an asymmetric bandpass filter, which attenuates the signal between 2 and 15 Hz, followed by rectification and averaging over a moving two second time window. This design, while intentionally minimizing the unwanted signals introduced by muscle artifact, also removes the low frequency component of cerebral activity. As a result, the aEEG signal is not a 1:1 representation of a traditionally acquired EEG trace, nor is it intended to be. Nevertheless, the correlation between these quantitatively determined features and PMA is predictable, reproducible, and is remarkably similar to similar examinations made using conventional EEG.
Constructing a longitudinal cohort of former preterm infants with developmental outcomes indistinguishable from the general population is quite challenging. Every step along the way carries the potential risk of exclusion, largely due to death or disability. Indeed, given the NICHD Neonatal Research Network data, only 44% of the infants in this study’s cohort, with a mean EGA of 27 weeks, would be expected to survive without major morbidity at the time of hospital discharge (31), much less have a neurodevelopmental outcome within the normal range at school age. As described earlier, Niemarkt et al. constructed a similar longitudinal cohort which examined 4% (18/449) of the initially eligible infants, very similar to the 5% (18/348) discussed in this study, particularly given our collection of an additional time point of data at age 7. These additional data proved quite valuable, as a further testing revealed that 10% of the cohort that appeared to be developing normally at 2 years CA, had fallen behind by school age.
The results of this study have both clinical and research applications. Although this analysis is presented as group data, all 18 infants exhibited the same developmental maturation patterns along a similar trajectory. This reproducibility allows clinicians to use this information as an aid in identifying preterm infants with reassuring aEEG parameters, potentially assisting in the management of the patient or counseling the parents of premature infants. Similarly, these same parameters can be longitudinally tracked in future prospective studies in order to assess the potential impact of a number of frequently encountered clinical situations that have been associated with adverse neurodevelopmental outcomes such as sepsis, necrotizing enterocolitis, and medications such as morphine, dexmetatomidine, and general anesthesia.
Acknowledgments
Funding Source(s):
National Institutes of Health, NICHD (P30 HD062171 and R01 HD057098)
Doris Duke Distinguished Clinical Scientist Award.
The authors wish to thank Peter Anderson, PhD and the Victorian Infants Brain Study research group for their assistance in collection and analysis of the presented data.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Burdjalov VF, Baumgart S, Spitzer AR. Cerebral function monitoring: a new scoring system for the evaluation of brain maturation in neonates. Pediatrics. 2003 Oct;112(4):855–61. doi: 10.1542/peds.112.4.855. [DOI] [PubMed] [Google Scholar]
- 2.Hellström-Westas L, De Vries LS, Rosén I. Atlas of amplitude-integrated EEGs in the newborn. London; Boca Raton, FL: Informa Healthcare_; Distributed in North and South America by Taylor & Francis; 2008. [Google Scholar]
- 3.Vecchierini M-F, André M, d’ Allest AM. Normal EEG of premature infants born between 24 and 30 weeks gestational age: terminology, definitions and maturation aspects. Neurophysiol Clin Clin Neurophysiol. 2007 Nov;37(5):311–23. doi: 10.1016/j.neucli.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 4.West CR, Harding JE, Williams CE, Nolan M, Battin MR. Cot-side electroencephalography for outcome prediction in preterm infants: observational study. Arch Dis Child Fetal Neonatal Ed. 2011 Mar;96(2):F108–13. doi: 10.1136/adc.2009.180539. [DOI] [PubMed] [Google Scholar]
- 5.Niemarkt HJ, Andriessen P, Peters CHL, Pasman JW, Blanco CE, Zimmermann LJ, et al. Quantitative analysis of amplitude-integrated electroencephalogram patterns in stable preterm infants, with normal neurological development at one year. Neonatology. 2010;97(2):175–82. doi: 10.1159/000252969. [DOI] [PubMed] [Google Scholar]
- 6.Palmu K, Wikström S, Hippeläinen E, Boylan G, Hellström-Westas L, Vanhatalo S. Detection of “EEG bursts” in the early preterm EEG: visual vs. automated detection. Clin Neurophysiol. 2010 Jul;121(7):1015–22. doi: 10.1016/j.clinph.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 7.Wilson SB. A neural network method for automatic and incremental learning applied to patient-dependent seizure detection. Clin Neurophysiol. 2005 Aug;116(8):1785–95. doi: 10.1016/j.clinph.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 8.Roessgen M, Zoubir AM, Boashash B. Seizure detection of newborn EEG using a model-based approach. IEEE Trans Biomed Eng. 1998 Jun;45(6):673–85. doi: 10.1109/10.678601. [DOI] [PubMed] [Google Scholar]
- 9.Liu A, Hahn JS, Heldt GP, Coen RW. Detection of neonatal seizures through computerized EEG analysis. Electroencephalogr Clin Neurophysiol. 1992 Jan;82(1):30–7. doi: 10.1016/0013-4694(92)90179-l. [DOI] [PubMed] [Google Scholar]
- 10.Gotman J, Flanagan D, Zhang J, Rosenblatt B. Automatic seizure detection in the newborn: methods and initial evaluation. Electroencephalogr Clin Neurophysiol. 1997 Sep;103(3):356–62. doi: 10.1016/s0013-4694(97)00003-9. [DOI] [PubMed] [Google Scholar]
- 11.Navakatikyan MA, Colditz PB, Burke CJ, Inder TE, Richmond J, Williams CE. Seizure detection algorithm for neonates based on wave-sequence analysis. Clin Neurophysiol. 2006 Jun;117(6):1190–203. doi: 10.1016/j.clinph.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 12.Thornberg E, Thiringer K. Normal pattern of the cerebral function monitor trace in term and preterm neonates. Acta Paediatr Scand. 1990 Jan;79(1):20–5. doi: 10.1111/j.1651-2227.1990.tb11324.x. [DOI] [PubMed] [Google Scholar]
- 13.Natalucci G, Hagmann C, Bernet V, Bucher H-U, Rousson V, Latal B. Impact of perinatal factors on continuous early monitoring of brain electrocortical activity in very preterm newborns by amplitude-integrated EEG. Pediatr Res. 2014 Jun;75(6):774–80. doi: 10.1038/pr.2014.32. [DOI] [PubMed] [Google Scholar]
- 14.Niemarkt HJ, Jennekens W, Pasman JW, Katgert T, Van Pul C, Gavilanes AWD, et al. Maturational changes in automated EEG spectral power analysis in preterm infants. Pediatr Res. 2011 Nov;70(5):529–34. doi: 10.1203/PDR.0b013e31822d748b. [DOI] [PubMed] [Google Scholar]
- 15.Hack M, Taylor HG, Drotar D, Schluchter M, Cartar L, Wilson-Costello D, et al. Poor predictive validity of the Bayley Scales of Infant Development for cognitive function of extremely low birth weight children at school age. Pediatrics. 2005 Aug;116(2):333–41. doi: 10.1542/peds.2005-0173. [DOI] [PubMed] [Google Scholar]
- 16.Spittle AJ, Cheong J, Doyle LW, Roberts G, Lee KJ, Lim J, et al. Neonatal white matter abnormality predicts childhood motor impairment in very preterm children. Dev Med Child Neurol. 2011 Nov;53(11):1000–6. doi: 10.1111/j.1469-8749.2011.04095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Filan PM, Hunt RW, Anderson PJ, Doyle LW, Inder TE. Neurologic outcomes in very preterm infants undergoing surgery. J Pediatr. 2012 Mar;160(3):409–14. doi: 10.1016/j.jpeds.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 18.Shah DK, Doyle LW, Anderson PJ, Bear M, Daley AJ, Hunt RW, et al. Adverse neurodevelopment in preterm infants with postnatal sepsis or necrotizing enterocolitis is mediated by white matter abnormalities on magnetic resonance imaging at term. J Pediatr. 2008 Aug;153(2):170–5. 175.e1. doi: 10.1016/j.jpeds.2008.02.033. [DOI] [PubMed] [Google Scholar]
- 19.West CR, Harding JE, Williams CE, Gunning MI, Battin MR. Quantitative electroencephalographic patterns in normal preterm infants over the first week after birth. Early Hum Dev. 2006 Jan;82(1):43–51. doi: 10.1016/j.earlhumdev.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 20.Bell AH, McClure BG, McCullagh PJ, McClelland RJ. Spectral edge frequency of the EEG in healthy neonates and variation with behavioural state. Biol Neonate. 1991;60(2):69–74. doi: 10.1159/000243390. [DOI] [PubMed] [Google Scholar]
- 21.Sisman J, Campbell DE, Brion LP. Amplitude-integrated EEG in preterm infants: maturation of background pattern and amplitude voltage with postmenstrual age and gestational age. J Perinatol. 2005 Jun;25(6):391–6. doi: 10.1038/sj.jp.7211291. [DOI] [PubMed] [Google Scholar]
- 22.Inder TE, Buckland L, Williams CE, Spencer C, Gunning MI, Darlow BA, et al. Lowered electroencephalographic spectral edge frequency predicts the presence of cerebral white matter injury in premature infants. Pediatrics. 2003 Jan;111(1):27–33. doi: 10.1542/peds.111.1.27. [DOI] [PubMed] [Google Scholar]
- 23.Mitchell TJ, Neil JJ, Zempel JM, Thio LL, Inder TE, Bretthorst GL. Automating the analysis of EEG recordings from prematurely-born infants: a Bayesian approach. Clin Neurophysiol Off J Int Fed Clin Neurophysiol. 2013 Mar;124(3):452–61. doi: 10.1016/j.clinph.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hellstrom-Westas L, Rosen I, de Vries LS, Greisen G. Amplitude-integrated EEG Classification and Interpretation in Preterm and Term Infants. NeoReviews. 2006 Feb 1;7(2):e76–87. [Google Scholar]
- 25.Benders MJ, Palmu K, Menache C, Borradori-Tolsa C, Lazeyras F, Sizonenko S, et al. Early Brain Activity Relates to Subsequent Brain Growth in Premature Infants. Cereb Cortex [Internet] 2014 May 27; doi: 10.1093/cercor/bhu097. [cited 2014 Nov 7]; Available from: http://www.cercor.oxfordjournals.org/cgi/doi/10.1093/cercor/bhu097. [DOI] [PubMed]
- 26.Hayakawa F, Okumura A, Kato T, Kuno K, Watanabe K. Dysmature EEG pattern in EEGs of preterm infants with cognitive impairment: maturation arrest caused by prolonged mild CNS depression. Brain Dev. 1997 Mar;19(2):122–5. doi: 10.1016/s0387-7604(96)00491-3. [DOI] [PubMed] [Google Scholar]
- 27.Kidokoro H, Anderson PJ, Doyle LW, Woodward LJ, Neil JJ, Inder TE. Brain injury and altered brain growth in preterm infants: predictors and prognosis. Pediatrics. 2014 Aug;134(2):e444–53. doi: 10.1542/peds.2013-2336. [DOI] [PubMed] [Google Scholar]
- 28.Eaton DG, Wertheim D, Oozeer R, Dubowitz LM, Dubowitz V. Reversible changes in cerebral activity associated with acidosis in preterm neonates. Acta Paediatr 1992. 1994 May;83(5):486–92. doi: 10.1111/j.1651-2227.1994.tb13064.x. [DOI] [PubMed] [Google Scholar]
- 29.Bell AH, Greisen G, Pryds O. Comparison of the effects of phenobarbitone and morphine administration on EEG activity in preterm babies. Acta Paediatr 1992. 1993 Jan;82(1):35–9. doi: 10.1111/j.1651-2227.1993.tb12511.x. [DOI] [PubMed] [Google Scholar]
- 30.Wikström S, Ley D, Hansen-Pupp I, Rosén I, Hellström-Westas L. Early amplitude-integrated EEG correlates with cord TNF-alpha and brain injury in very preterm infants. Acta Paediatr 1992. 2008 Jul;97(7):915–9. doi: 10.1111/j.1651-2227.2008.00787.x. [DOI] [PubMed] [Google Scholar]
- 31.Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010 Sep;126(3):443–56. doi: 10.1542/peds.2009-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]