Abstract
Background
Prescription opioid (PO) abuse has become an urgent public health issue in the United States. Detoxification is one important treatment option, yet relatively little is known about the time course and severity of opioid withdrawal during buprenorphine detoxification.
Methods
This is a secondary analysis of data from a randomized, placebo-controlled, double-blind evaluation of 1, 2, and 4-week outpatient buprenorphine tapers among primary prescription opioid (PO) abusers. The aim is to characterize the time course and severity of buprenorphine withdrawal under rigorous, double-blind conditions, across multiple taper durations, and using multiple withdrawal-related measures (i.e., self-report and observer ratings, pupil diameter, ancillary medication utilization). Participants were PO-dependent adults undergoing buprenorphine detoxification and biochemically-verified to be continuously abstinent from opioids during their taper (N=28).
Results
Participants randomly assigned to the 4-week taper regimen experienced a relatively mild and stable course of withdrawal, with few peaks in severity. In contrast, the 1- and 2-week taper groups experienced stark increases in withdrawal severity during the week following the last buprenorphine dose, followed by declines in withdrawal severity thereafter. The 4-week taper group also reported significantly fewer disruptions in sleep compared to the other experimental groups. When predictors of withdrawal were examined, baseline ratings of “Expected Withdrawal Severity” was the most robust predictor of withdrawal experienced during the taper.
Conclusion
Data from this trial may inform clinicians about the expected time course, magnitude, and pattern of buprenorphine withdrawal and aid efforts to identify patients who may need additional clinical support during outpatient buprenorphine detoxification.
Keywords: buprenorphine, detoxification, taper, withdrawal, prescription opioid
1. INTRODUCTION
In 2013, over 13 million people had abused a prescription opioid (PO) and approximately 2 million people required treatment (Substance Abuse and Mental Health Services Administration (SAMHSA), 2014). POs are now the second most commonly used drug in the United States, and annual treatment admissions for PO abuse have increased 32% in the past 10 years. PO abuse costs society over $56 billion annually in premature death, criminality, and other societal consequences (Centers for Disease Control, 2012; SAMHSA, 2013). Opioid-related overdose, which is largely driven by PO abuse, has increased by 114% between 2002 and 2012 (Warner et al., 2014). Finally, PO abuse has now been independently related to risky drug use behaviors and acquisition of HIV and hepatitis C (Havens et al., 2011; Bruneau et al., 2012; Havens et al., 2013).
While maintenance treatment with opioid agonist medications (i.e., methadone, buprenorphine) is widely-used and effective for many opioid-dependent patients, detoxification remains an important treatment option for several reasons. First, many PO abusers may be unwilling or unable to enter maintenance, perhaps due to stigma associated with methadone or because less severe opioid histories make them ineligible (Zacny et al., 2003; Appel et al., 2004; Kleber, 2007; Pinto et al., 2010). Second, access to maintenance can also be limited, particularly in rural areas where PO abuse is often most prevalent (Rounsaville and Kosten, 2000; Cicero et al., 2007; Rosenblum et al., 2011; Sigmon, 2014). Third, primary PO abusers may respond favorably to a detoxification treatment approach. For example, previously-reported comparisons of primary PO and heroin abusers have noted that PO abusers experienced less severe withdrawal during buprenorphine induction, provided more opioid-negative urine samples during a buprenorphine taper, were more likely to be retained in a buprenorphine taper, and were more likely to be opioid-negative at the end of a buprenorphine taper, compared to primary heroin abusers (Nielsen et al., 2012, 2013, 2014). Finally, the recent increases in adolescent opioid abuse warrant exploring potential alternatives to long-term maintenance (Zacny et al., 2003; McLellan and Turner, 2008; Volkow and McLellan, 2011), particularly given evidence that long-term opioid maintenance may alter cognitive functions (Gruber et al., 2007).
When considering the available pharmacotherapies for use in opioid detoxification, the partial mu-opioid agonist buprenorphine has a pharmacological profile that lends toward its use in taper regimens. Buprenorphine has a long plasma half-life and slow dissociation from the receptor (Hambrook and Rance, 1976; Jasinski et al., 1978; Bullingham et al., 1980; Bickel et al., 1988a; Fudala et al., 1990), which results in a more limited withdrawal syndrome when compared to other full opioid agonists (e.g., methadone; Jasinski et al., 1978; Bickel et al., 1988b; Fudala et al., 1990) and adrenergic agonists (e.g., clonidine; Janiri et al., 1994; O’Connor et al., 1997; Lintzeris et al., 2002; Ling et al., 2005; Marsch et al., 2005; Oreskovich et al., 2005). However, despite the data suggesting a favorable withdrawal profile for buprenorphine over other medications, few studies have directly evaluated the time course and severity of withdrawal during buprenorphine detoxification, and the existing studies on this topic have generally failed to account for ongoing illicit opioid and ancillary medication use that can confound assessments of opioid withdrawal (Dunn et al., 2011). Considering the association between opioid withdrawal severity and patient outcomes (Best et al., 1999), a thorough understanding of buprenorphine withdrawal across multiple taper durations could enhance efforts to develop more effective detoxification regimens.
We recently conducted a randomized, double-blind, placebo-controlled parametric comparison of three outpatient buprenorphine taper durations (i.e., 1-, 2-, or 4-week) in PO-dependent individuals (Sigmon et al., 2013). In the present analyses, we sought to characterize the time course and severity of withdrawal under rigorous, double-blind conditions, across multiple taper durations, and using multiple withdrawal-related measures (i.e., self-report and observer ratings, pupil diameter, ancillary medication utilization).
2. MATERIALS AND METHODS
2.1 Study Design
As a full description of the study methods has been reported elsewhere (Sigmon et al., 2013), only a brief summary of relevant details is presented here. Participants were 18 years old, met DSM-IV-TR criteria for opioid dependence, provided an opioid-positive urine sample, were seeking or willing to accept opioid detoxification, reported a PO as their primary drug of abuse, and reported using the PO illicitly (e.g., without a valid prescription). Those who required ongoing opioids for pain, were pregnant or nursing, or had a significant and unstable psychiatric or medical illness that could interfere with consent or participation were excluded.
Following a brief period of buprenorphine stabilization, 70 PO-dependent adults were randomized into a 12-week study in which they received either a 1-, 2-, or 4-week buprenorphine taper, followed by oral naltrexone therapy for those who successfully tapered without relapse to opioid use. Participants and research staff remained blind to participants’ buprenorphine dose, their assigned taper duration, and the timing of their naltrexone initiation. Participants visited the clinic daily during Phase 1 (Weeks 1–5 after randomization) and three times weekly during Phase 2 (Weeks 6–12). They received behavioral therapy based on the Community Reinforcement Approach (see Sigmon et al., 2013) and thrice-weekly urine toxicology testing throughout the trial. The present analyses focused on Phase 1, the 5-week period during which participants were undergoing their assigned double-blind taper regimen and visiting the clinic daily for assessments of withdrawal and illicit drug use.
2.2 Participants
The primary study enrolled 70 participants. Data from those participants were examined and only those who were biochemically-verified to be continuously abstinent from opioids throughout their assigned taper duration plus one additional week post-taper (N=28) were included in these analyses (40% of the original sample). This criterion was chosen to permit a thorough evaluation of withdrawal unconfounded by illicit opioid use. The additional post-taper week was included to permit examination of withdrawal in the days following the final buprenorphine dose, since several previous studies have reported a potential delayed onset of withdrawal following buprenorphine taper (see Dunn et al., 2011).
2.3 Study Medications
Each day throughout the study, participants received 5.5 active and color-matched placebo sublingual buprenorphine tablets, as well as three gelatin capsules that contained either naltrexone or placebo. All study medication was ingested daily onsite under nurse observation. Five ancillary nonopioid medications (i.e., clonidine, hydroxyzine, ibuprofen, loperamide, promethazine) were available but not required and were dispensed according to a protocol based on Clinical Institute Narcotic Assessment (CINA; Peachey and Lei, 1988) scores. Participants ingested ancillary medications in the clinic and also received take-home doses of medications as needed. Take-home ancillary medication packages were returned at the next visit and logged for documentation.
2.4 Measures
At intake, participants completed a questionnaire to assess their history of opioid and other drug use, the Beck Depression Inventory (Beck et al., 1961), and the Brief Pain Inventory (Cleeland and Ryan, 1994). They also completed the following two Visual Analog Scale (VAS) ratings on a scale of 0 (None) -100 (Worst ever): “In general, how severe do you expect withdrawal from opioids to be?” (Expected Withdrawal Severity) and “How would you rate your average craving for opioids during the past month?” (Past 30-Day Cravings). The intake assessment also included other self-report and experimenter-administered measures that are not relevant to withdrawal and described in full elsewhere (Sigmon et al., 2013).
Withdrawal was assessed via self-report ratings, observer ratings, and pupil diameter measurements at each study visit. A research nurse blinded to taper group assignment and buprenorphine dose conducted all withdrawal assessments prior to dosing. The self-report ratings included five VAS items, each rated on a scale of 0 (Not at all) to 100 (Extremely): “Do you feel any withdrawal discomfort right now?” (Withdrawal), “How high are you?” (High), “Do you like the way you feel?” (Like The Way I Feel), “Do you feel sick?” (Sick), and “Do you crave opiates right now?” (Crave). Participants also rated the number of hours they slept the preceding night (Sleep Quantity) and the quality of their sleep (Sleep Quality) on a scale of 0 (Poor) to 10 (High). Observer ratings were conducted using the CINA, a widely-used 12-item clinical instrument that yields a withdrawal severity score from a combination of self-report and observer ratings. Finally, pupil diameter (millimeters (mm)), a physiological measure of withdrawal, was assessed in constant ambient lighting using a pupillometer (NeurOptics, San Clemente, CA), a handheld device that uses infrared technology to estimate pupil diameter over a 3-second period.
2.5 Data Analyses
Demographic (i.e., age, race), drug use (i.e., route of administration, duration of use), and study characteristics (i.e., number stabilization days, missed visits) that were hypothesized a priori to influence withdrawal were compared across the three taper duration groups using chi-square and one-way analysis of variance (ANOVA) tests. No significant differences were found.
Between-group (taper group) comparisons of mean peak ratings over the 5-week evaluation period were conducted, permitting an evaluation of withdrawal time course. Self-report VAS, CINA, and sleep measures were converted into maximum peak values (VAS, CINA) or lowest mean values (sleep measures) for each participant each week, representing the peak level of impairment and discomfort, and were averaged across taper duration for Weeks 1–5. Scores from both the individual items and the total overall CINA score were examined. CINA scores are presented using the severity score (Severity; range 0–31) as well as a symptom incidence score (Incidence; range 0–11), with incidence operationalized as an endorsement of a symptom at any level of severity. Pupil diameter (mm) was analyzed using final stabilization buprenorphine dose as a covariate. Ancillary medication utilization, collapsed across medications, was evaluated as the mean number of different medications taken per day (range 0–5). In order to evaluate between-group effects of taper group, within-subject effects of study week, and taper group x study week interactions, mean peak values were compared across taper durations using SAS Proc Mixed models for continuous measures and SAS Generalized Estimating Equations for dichotomous measures, with follow-up Tukey’s posthoc tests to identify specific group differences.
In addition, between-group comparisons regarding the time to first report of CINA symptoms and first ancillary medication use were evaluated using SAS Proc Mixed analyses, to determine whether the onset of symptoms (independent of symptom severity) or utilization of ancillary medications differed across taper durations. Cohen’s D effect sizes for between-group effects were calculated and are provided in Tables 2 and 3.
Table 2.
Mean Peak CINA Total and Individual Symptom Ratings
| Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | Within Subject | Between Subject | Effect Sizesb | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Week (df 4,96) | Week × Taper (df 8,96) | Taper Group (df 2,25) | ||||||||||||
|
|
|
|||||||||||||
| CINAa | Mean Peak Rating Per Study Week | F | p-value | F | p-value | F | p-value | 1 vs. 2 | 2 vs. 4 | 1 vs. 4 | ||||
|
|
|
|
|
|||||||||||
| Total Severity Score (0–31) | 4.79 | <.01 | 2.19 | 0.04 | 0.32 | 0.73 | 0.18 | 0.03 | 0.21 | |||||
| 1 week taper | 6.8 | 7.3 | 6.8 | 3.6 | 3.6 | |||||||||
| 2 week taper | 5.1 | 6.0 | 6.7 | 4.9 | 3.6 | |||||||||
| 4 week taper | 5.1 | 7.0 | 5.3 | 6.1 | 5.7 | |||||||||
| Abdominal Changes (0–2) | 2.76 | 0.03 | 1.39 | 0.21 | 1.42 | 0.26 | 0.36 | 0.44 | 0.08 | |||||
| 1 week taper | 1.0 | 1.4 | 0.9 | 0.1 | 0.0 | |||||||||
| 2 week taper | 0.9 | 1.4 | 1.3 | 1.0 | 0.7 | |||||||||
| 4 week taper | 1.0 | 1.1 | 1.1 | 1.1 | 1.3 | |||||||||
| Body Temperature (0–2) | 1.43 | 0.23 | 1.58 | 0.14 | 1.73 | 0.20 | 0.46 | 0.13 | 0.34 | |||||
| 1 week taper | 0.3 | 0.7 | 0.6 | 0.1 | 0.3 | |||||||||
| 2 week taper | 0.7 | 0.6 | 0.7 | 0.6 | 0.6 | |||||||||
| 4 week taper | 0.7 | 0.6 | 0.4 | 0.6 | 0.1 | |||||||||
| Nausea/Vomiting (0–6) | 1.20 | 0.32 | 1.44 | 0.19 | 0.41 | 0.67 | 0.12 | 0.13 | 0.25 | |||||
| 1 week taper | 2.4 | 1.3 | 1.1 | 0.8 | 0.0 | |||||||||
| 2 week taper | 1.0 | 1.6 | 1.8 | 1.6 | 0.9 | |||||||||
| 4 week taper | 0.7 | 1.6 | 0.7 | 1.1 | 0.9 | |||||||||
| Muscle Aches/Pains (0–2) | 2.66 | 0.04 | 1.67 | 0.17 | 0.94 | 0.41 | 0.08 | 0.41 | 0.33 | |||||
| 1 week taper | 1.4 | 1.7 | 1.4 | 0.8 | 0.7 | |||||||||
| 2 week taper | 1.0 | 1.5 | 1.5 | 0.9 | 1.0 | |||||||||
| 4 week taper | 0.9 | 0.9 | 0.9 | 1.1 | 1.0 | |||||||||
| Gooseflesh (0–3) | 1.72 | 0.15 | 1.41 | 0.20 | 2.49 | 0.10 | 0.51 | 0.78 | 0.28 | |||||
| 1 week taper | 1.1 | 1.4 | 0.7 | 1.0 | 0.9 | |||||||||
| 2 week taper | 0.8 | 0.6 | 0.7 | 0.4 | 0.4 | |||||||||
| 4 week taper | 0.1 | 0.7 | 0.2 | 0.3 | 0.4 | |||||||||
| Nasal Congestion (0–2) | 2.32 | 0.06 | 1.37 | 0.22 | 1.50 | 0.24 | 0.55 | 0.25 | 0.3 | |||||
| 1 week taper | 1.0 | 1.1 | 1.2 | 0.8 | 1.0 | |||||||||
| 2 week taper | 0.8 | 0.9 | 1.0 | 0.6 | 0.6 | |||||||||
| 4 week taper | 1.0 | 1.0 | 0.8 | 0.9 | 0.9 | |||||||||
| Restlessness (0–3) | 0.98 | 0.42 | 1.05 | 0.41 | 0.03 | 0.97 | 0.04 | 0.08 | 0.04 | |||||
| 1 week taper | 0.7 | 0.8 | 0.9 | 0.8 | 0.6 | |||||||||
| 2 week taper | 0.7 | 0.7 | 1.0 | 0.5 | 0.7 | |||||||||
| 4 week taper | 0.6 | 0.9 | 0.7 | 0.8 | 0.6 | |||||||||
| Tremor (0–3) | 3.10 | 0.02 | 0.56 | 0.81 | 3.22 | 0.062,4 | 0.29 | 0.42 | 0.71 | |||||
| 1 week taper | 0.3 | 0.8 | 0.9 | 0.5 | 0.7 | |||||||||
| 2 week taper | 0.5 | 0.7 | 0.7 | 0.2 | 0.2 | |||||||||
| 4 week taper | 0.8 | 1.0 | 1.0 | 0.9 | 0.8 | |||||||||
| Lacrimation (0–2) | 1.22 | 0.31 | 0.23 | 0.94 | 0.02 | 0.99 | 0.02 | 0.02 | 0.00 | |||||
| 1 week taper | 0.3 | 0.3 | 0.2 | 0.1 | 0.1 | |||||||||
| 2 week taper | 0.3 | 0.4 | 0.3 | 0.2 | 0.0 | |||||||||
| 4 week taper | 0.3 | 0.3 | 0.2 | 0.1 | 0.2 | |||||||||
| Sweating (0–3) | 0.39 | 0.82 | 0.86 | 0.55 | 1.79 | 0.19 | 0.09 | 0.34 | 0.49 | |||||
| 1 week taper | 0.7 | 0.9 | 0.8 | 0.6 | 0.6 | |||||||||
| 2 week taper | 0.7 | 0.7 | 0.6 | 0.8 | 0.6 | |||||||||
| 4 week taper | 0.9 | 0.8 | 1.0 | 0.9 | 0.9 | |||||||||
| Yawning (0–2) | 0.29 | 0.89 | 1.29 | 0.26 | 1.51 | 0.24 | 0.42 | 0.02 | 0.39 | |||||
| 1 week taper | 0.7 | 0.4 | 0.6 | 0.4 | 0.6 | |||||||||
| 2 week taper | 0.4 | 0.3 | 0.2 | 0.3 | 0.3 | |||||||||
| 4 week taper | 0.3 | 0.4 | 0.6 | 0.9 | 0.3 | |||||||||
| Total Incidence (1–11) | 6.76 | <.001 | 1.97 | 0.06 | 1.20 | 0.32 | 0.29 | 0.17 | 0.47 | |||||
| 1 week taper | 4.4 | 5.6 | 4.9 | 3.3 | 3.3 | |||||||||
| 2 week taper | 4.1 | 4.3 | 4.6 | 3.6 | 2.9 | |||||||||
| 4 week taper | 4.3 | 5.3 | 4.4 | 4.6 | 4.4 | |||||||||
Superscript numbers next to p-values convey taper groups who were significantly different (Tukey’s posthoc). Only significant differences are reported.
Score ranges presented in parentheses
Effects sizes reported as Cohen’s D
Table 3.
Mean Peak Self-Report Ratings, Pupil Diameter, and Ancillary Medication Utilization
| Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | Within Subject | Between Subject | Effect Sizesd | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Week | Week × Taper | Taper Group | Taper Groups | |||||||||||
|
|
|
|
|
|||||||||||
| Visual Analog Scale (0–100) | Mean Peak Ratinga | F (df) | p-value | F (df) | p-value | F (df) | p-value | 1 vs. 2 | 1 vs. 4 | 2 vs. 4 | ||||
|
|
|
|
|
|||||||||||
| High | 1.62 (4,96) | 0.18 | 1.13 (8,96) | 0.35 | 0.91 (2,25) | 0.42 | 0.45 | 0.09 | 0.54 | |||||
| 1 week taper | 1.2 | 1.6 | 4.3 | 2.6 | 11.6 | |||||||||
| 2 week taper | 8.1 | 11.6 | 10.2 | 7.4 | 10.7 | |||||||||
| 4 week taper | 5.9 | 3.2 | 2.4 | 1.2 | 1.2 | |||||||||
| Sick | 2.70 (4,96) | 0.04 | 1.98 (8,96) | 0.06 | 1.36 (2,25) | 0.28 | 0.45 | 0.10 | 0.34 | |||||
| 1 week taper | 61.4 | 50.8 | 40.8 | 7.8 | 6.0 | |||||||||
| 2 week taper | 23.3 | 23.7 | 27.7 | 22.5 | 12.8 | |||||||||
| 4 week taper | 33.3 | 35.7 | 27.1 | 31.0 | 34.0 | |||||||||
| Withdrawal | 3.38 (4,96) | 0.01 | 1.37 (8,96) | 0.22 | 0.28 (2,25) | 0.75 | 0.25 | 0.19 | 0.05 | |||||
| 1 week taper | 57.9 | 53.7 | 36.2 | 14.9 | 12.0 | |||||||||
| 2 week taper | 29.6 | 39.5 | 37.8 | 18.5 | 20.8 | |||||||||
| 4 week taper | 30.0 | 34.6 | 30.0 | 31.2 | 29.0 | |||||||||
| Crave | 1.20 (4,96) | 0.32 | 0.38 (8,96) | 0.93 | 0.29 (2,25) | 0.75 | 0.18 | 0.35 | 0.16 | |||||
| 1 week taper | 38.4 | 31.0 | 25.1 | 16.9 | 24.6 | |||||||||
| 2 week taper | 37.2 | 34.8 | 36.7 | 34.4 | 24.6 | |||||||||
| 4 week taper | 52.4 | 45.6 | 35.7 | 33.7 | 28.6 | |||||||||
| Like the Way I Feel | 1.98 (4,96) | 0.10 | 0.92 (8,96) | 0.50 | 0.98 (2,25) | 0.39 | 0.35 | 0.21 | 0.56 | |||||
| 1 week taper | 56.6 | 64.8 | 63.8 | 69.3 | 75.1 | |||||||||
| 2 week taper | 52.9 | 47.9 | 58.8 | 61.9 | 64.0 | |||||||||
| 4 week taper | 69.7 | 64.9 | 69.9 | 72.1 | 75.3 | |||||||||
| Pupil Diameter (mm)b | 5.57 (4,86) | <0.01 | 1.64 (8,86) | 0.13 | 0.10 (2,22) | 0.91 | 0.26 | 0.26 | 0.00 | |||||
| 1 week taper | 6.1 | 6.5 | 6.3 | 6.0 | 5.9 | |||||||||
| 2 week taper | 6.3 | 6.4 | 6.5 | 6.4 | 6.2 | |||||||||
| 4 week taper | 6.0 | 6.5 | 6.4 | 6.5 | 6.4 | |||||||||
| Sleep | Lowest Mean Ratingc
|
|||||||||||||
| Quantity (min hrs) | 0.30 (4,96) | 0.88 | 0.64 (8,96) | 0.74 | 3.90 (2,25) | 0.041,4 | 0.15 | 0.65 | 0.50 | |||||
| 1 week taper | 4.2 | 3.6 | 4.8 | 4.6 | 4.5 | |||||||||
| 2 week taper | 4.8 | 4.7 | 4.3 | 4.1 | 5.1 | |||||||||
| 4 week taper | 5.2 | 5.7 | 5.5 | 5.2 | 5.3 | |||||||||
| Quality (min quality, 0–10) | 0.28 (4,96) | 0.89 | 1.13 (9,96) | 0.25 | 0.44 (2,25) | 0.65 | 0.13 | 0.23 | 0.15 | |||||
| 1 week taper | 4.2 | 2.9 | 4.2 | 5.4 | 4.4 | |||||||||
| 2 week taper | 4.8 | 4.8 | 3.5 | 4.6 | 4.8 | |||||||||
| 4 week taper | 5.2 | 5.6 | 5.0 | 4.0 | 4.3 | |||||||||
| Medication Utilization | Mean Peak Utilization
|
|||||||||||||
| Medications Used (1–5) | 3.53 (4,100) | 0.01 | 2.87 (8,100) | <0.001 | 0.16 (2,25) | 0.86 | 0.03 | 0.19 | 0.16 | |||||
| 1 week taper | 1.2 | 2.2 | 1.6 | 1.2 | 0.9 | |||||||||
| 2 week taper | 1.2 | 1.6 | 2.2 | 1.7 | 0.6 | |||||||||
| 4 week taper | 1.2 | 1.3 | 1.9 | 1.9 | 2.1 | |||||||||
Superscript numbers next to p-values convey taper groups who were significantly different (Tukey’s posthoc). Only significant differences are reported.
Mean peak increase (representing greater levels of withdrawal severity) is used to evaluate VAS outcome severity
Derived from pupillometer assessment; covaried for buprenorphine stabilization dose
Lowest mean rating (representing less sleep and lower sleep quality) is used to evaluate sleep-related outcome severity
Effects sizes reported as Cohen’s D
Finally, multiple linear regressions were conducted to evaluate whether any baseline demographic or drug use characteristics predicted withdrawal outcomes. Independent variables included VAS rating of Expected Withdrawal Severity and Past 30-Day Cravings, as well as sex, age, duration (years) of regular opioid use prior to the study, primary route of opioid administration, presence of pain (assessed via the BPI), cigarette smoker status, and mean BDI score. Missing data was minimal (only 6% of scheduled visits missed), therefore no statistical corrections were made. Statistical significance was defined as p<.05, and analyses were conducted on SPSS 21 and SAS 9.3.
3. RESULTS
3.1 Participants
Participant characteristics are presented in Table 1. There were no significant differences between the three taper duration groups on demographic, drug use, or parent study characteristics.
Table 1.
Demographic, Drug Use, and Parent Study Characteristics
| 1 Week Taper (n=9) | 2 Week Taper (n=10) | 4 Week Taper (n=9) | p-valuea | |
|---|---|---|---|---|
| Demographic Characteristics | ||||
| Caucasian (%) | 88.9 | 88.9 | 100 | 0.58 |
| Male (%) | 44.4 | 70.0 | 77.8 | 0.30 |
| Age (yrs) | 26.8 ± 7.6 | 28.2 ± 8.1 | 24.9 ± 2.5 | 0.59 |
| Opioid Use Characteristics | ||||
| Primary oxycodone abuse (%) | 77.8 | 60.0 | 66.7 | 0.71 |
| Mean number other PO abused | 2.4 ± 1.7 | 2.4 ± 1.6 | 2.3 ± 1.5 | 0.99 |
| Primary intranasal abuse (%) | 100.0 | 80.0 | 77.8 | 0.33 |
| Ever used opioids IV (%) | 33.3 | 50.0 | 33.3 | 0.69 |
| Ever used heroin (%) | 44.4 | 50.0 | 44.4 | 0.96 |
| Ever overdose on opioids (%) | 33.3 | 10.0 | 0.0 | 0.12 |
| Mean days used per week (days) | 6.7 ± 0.4 | 6.0 ± 1.3 | 6.0 ± 1.3 | 0.28 |
| Mean opioid craving past month (VASb) | 72.4 ± 15.2 | 68.1 ± 11.8 | 70.8 ± 19.2 | 0.84 |
| Mean expected withdrawal severity (VASb) | 75.6 ± 20.9 | 66.9 ± 24.0 | 71.8 ± 12.2 | 0.65 |
| Mean age began regular opioid abuse (yrs) | 20.8 ± 6.3 | 21.9 ± 7.2 | 20.0 ± 2.6 | 0.77 |
| Mean length of regular use (yrs) | 3.7 ± 2.2 | 5.5 ± 4.3 | 3.9 ± 1.9 | 0.37 |
| Pain or medical issues (%) | 33.3 | 40.0 | 22.2 | 0.71 |
| Smoke cigarettes (%) | 88.9 | 70.0 | 88.9 | 0.46 |
| Study Characteristics | ||||
| Number stabilization days (days) | 15 ± 2.5 | 13 ± 3.6 | 12.3 ± 3.6 | 0.78 |
| Final stabilization dose (mgs) | 11.3 ± 5.1 | 7.6 ± 5.0 | 9.8 ± 5.0 | 0.28 |
| Missed visits (%) | 11.1 | 5.7 | 2.2 | 0.25 |
Values represent Mean ± SD unless otherwise indicated
Values based on One-way Analysis of Variance comparisons across groups for continuous variables and chi-square analyses for dichotomous variables
VAS scales range 0–100
3.2 Primary Withdrawal Outcomes
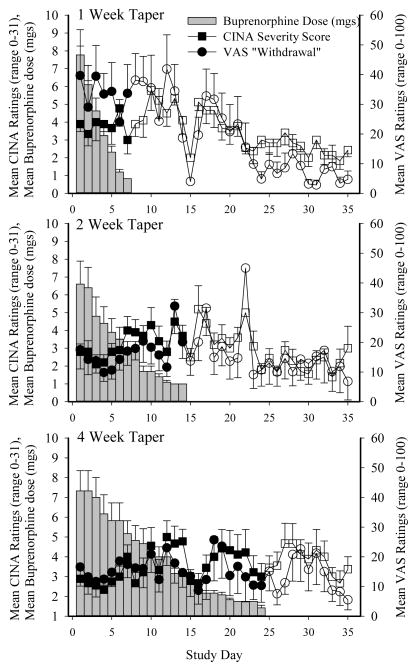
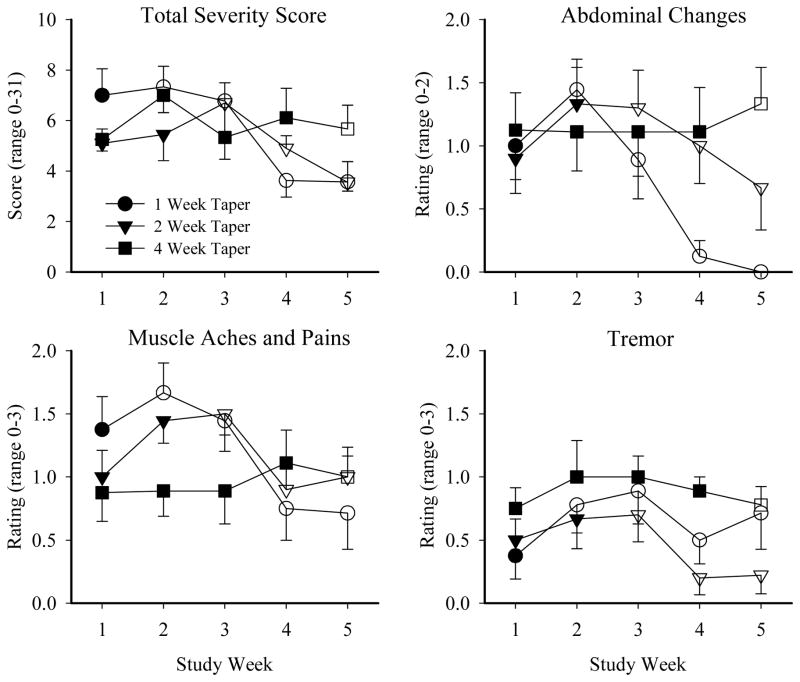
Withdrawal outcomes (i.e., CINA total score, VAS Withdrawal) are presented in Figure 1 as a function of study day for the 1, 2, and 4-week taper groups, with participants’ mean buprenorphine dose (SEM) also shown in order to aid interpretation. Visual inspection of these data suggest that the 4-week taper group experienced a relatively mild and stable course of withdrawal with fewer peaks in severity, compared to the 1- and 2-week taper groups who reported increases in withdrawal severity the week following the last buprenorphine dose that decreased thereafter. This general pattern was seen for almost every individual symptom examined. There were no significant effects of taper group on the CINA Total Severity and individual item scores, though the tremor item approached significance (p=.06; Table 2). Several CINA scores did reveal main effects of study week, including the CINA Total Severity score, as well as individual items of abdominal changes, muscle aches and pains, and tremor (Table 2; Figure 2). There was also a significant taper group x study week interaction for the CINA Total Severity score (p =.04). Finally, the peak number of CINA items endorsed (a measure of symptom incidence) varied significantly as a function of study week (p <.001), and the taper group x study week interaction approached significance (p =.06).
Figure 1. Mean Withdrawal Scores During Buprenorphine Taper.
Withdrawal outcomes presented as a function of study day for the 1, 2, and 4-week taper groups, with participants’ mean buprenorphine dose (mg; grey bars) also shown to aid interpretation. Mean CINA Total Severity score (square symbols) and mean VAS ratings of Withdrawal (circle symbols) are shown for the 1-week (top panel), 2-week (middle panel), and 4-week (bottom panel) taper groups. Filled symbols represent the days on which participants received an active buprenorphine dose; open symbols represent days on which they received color-matched placebo. Error bars represent SEM.
Figure 2. Mean Peak CINA Scores.
Mean peak CINA total and individual item scores are presented across study week for the 1-week (circle), 2-week (triangle), and 4-week (square) taper groups. Filled symbols represent weeks in which participants received buprenorphine; open symbols represent weeks in which they received placebo. Error bars represent SEM. Only items with significant effects are presented. No taper group x study week posthoc test results reached significance.
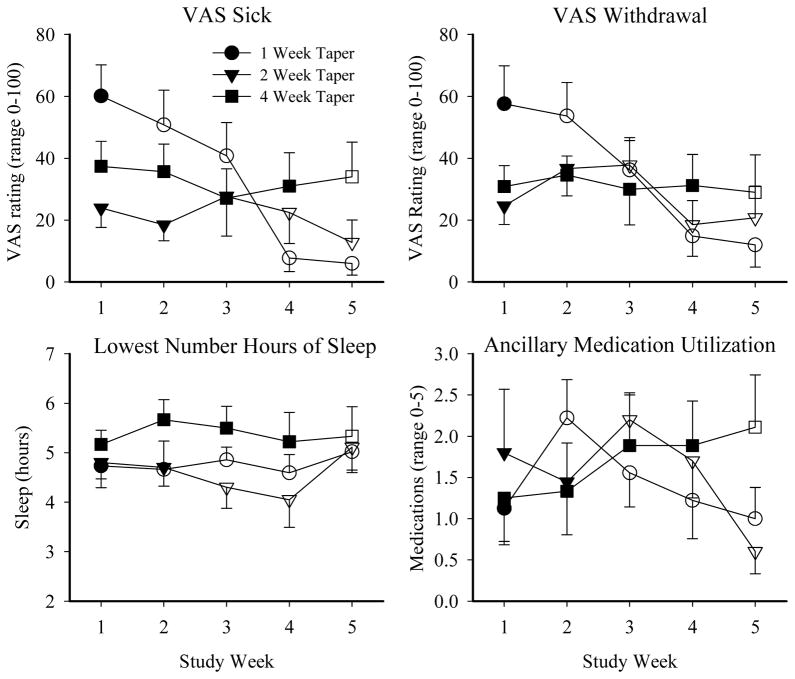
A similar pattern of withdrawal (marked increases and subsequent decreases in severity in the briefer taper durations vs. more steady levels of mild withdrawal in the 4-week group) was seen on the self-report, ancillary medication, and pupil diameter measures. A significant effect of taper group was evident on lowest mean hours of sleep, with the 4-week taper group reporting less loss of sleep compared to the 1- and 2-week groups (p =.04; Table 3; Figure 3). A significant effect of study week (p =.01) and interaction between taper group and study week (p <.001) were also observed for the number of ancillary medications used during the study (Table 3; Figure 3). No additional effects of taper group were found, though VAS ratings of Sick (p =.04), Withdrawal (p =.01), and mean pupil diameter (p =.001) (covaried for final buprenorphine stabilization dose) varied significantly as a function of study week (Table 3; Figure 3).
Figure 3. Mean Peak Self-Report Ratings and Ancillary Medication Utilization.
Mean peak self-report ratings of VAS items, Sleep Quantity, and ancillary medication utilization are presented across study week for the 1-week (circle), 2-week (triangle), and 4-week (square) taper groups. Filled symbols represent weeks in which participants received buprenorphine; open symbols represent weeks in which they received placebo. Error bars represent SEM. Only items with significant effects are presented. No taper group x study week posthoc test results reached significance.
3.3 Time to Onset of Withdrawal and Ancillary Medication Use
There were no between-group differences in time to onset of CINA symptoms, use of any ancillary medication (collapsed across medications), or use of specific individual ancillary medications (data not shown).
3.4 Predictors of Withdrawal
Participants’ baseline ratings of Expected Withdrawal Severity were significantly associated with several subsequent VAS withdrawal ratings, even after controlling for other potential predictors. More specifically, ratings of Expected Withdrawal Severity significantly predicted mean peak VAS ratings of Withdrawal (R2=.28; B=.65, t(26)=2.6, p =.02) and Feel Sick (R2=.39; B=.60, t(26)=2.5, p =.02), as well as CINA Total Severity (R2=.37; B=.74, t(26)=3.08, p <.01), with higher baseline ratings predicting greater subsequent withdrawal severity. In contrast, the other intake variables examined (i.e., sex, age, mean duration of regular opioid use, primary route of opioid administration, presence of pain, being a cigarette smoker, mean BDI score) were not significantly predictive of withdrawal. Total number of CINA items endorsed (i.e., CINA Incidence) was positively predicted (R2=.47) by Expected Withdrawal Severity (B=.57, t(26)=2.98, p <.01) and being male (B=−.54, t(26)=−2.17), p =.04). Finally, Experiencing Pain was significantly predictive of subsequent mean peak ratings of Like How I Feel (R2=.31; B=−.56, t(26)−2.26, p =.04).
4. DISCUSSION
In this study, we sought to characterize opioid withdrawal among opioid-dependent patients who successfully completed double-blind, outpatient buprenorphine detoxification. Participants randomly assigned to the 1- and 2-week taper regimens experienced increases in withdrawal in the week following their final buprenorphine dose, followed by a decline in symptom severity thereafter. In contrast, those randomized to the 4-week taper group generally experienced a steady but relatively mild level of withdrawal across all study weeks with no delayed emergence of withdrawal in the week following buprenorphine discontinuation. Only two other published reports have examined withdrawal during multiple buprenorphine taper durations (Amass et al., 1994; Ling et al., 2009), and only one of those evaluated how withdrawal changed over time (Amass et al., 1994). In that study, and similar to our results, a 36-day gradual taper was associated with a more continuous withdrawal syndrome without extreme peaks in severity over time, whereas the briefer 12-day taper produced a marked increase in self-reported withdrawal severity following discontinuation of buprenorphine. Our results build upon that prior finding by parametrically comparing three buprenorphine taper durations on multiple, frequent withdrawal measures and in participants biochemically-verified to be abstinent from additional opioid use. These data may inform both providers and patients about the time course, magnitude, and pattern of buprenorphine withdrawal that patients might experience during and in the weeks following different taper durations.
The only item to show a significant effect of taper duration was sleep quantity, with the 4-week taper group reporting greater sleep quantity (indicating less sleep impairment) compared to the 1- and 2-week groups. Although the role of sleep and sleep impairment in opioid detoxification has not been well characterized, at least one study has reported that self-reported sleeping problems was predictive of attrition from opioid detoxification (Dijkstra et al., 2008). A more recent study also reported that provision of sleep medication during the first two weeks of opioid detoxification was predictive of better treatment outcomes (Warden et al., 2012). These data suggest that that clinical support and perhaps medications aimed at managing sleep problems during opioid detoxification might be warranted.
Several measures revealed significant effects of study week, including changes in CINA Total Severity Scores, individual CINA items (i.e., abdominal changes, muscle aches and pain, tremor), the peak number of CINA items endorsed, and VAS ratings of Sick and Withdrawal. In all cases, participants assigned to the 1- and 2-week taper groups experienced a peak in withdrawal severity the week following buprenorphine termination and then a subsequent decline in withdrawal severity, compared to the 4-week taper group who reported a more steady and mild withdrawal time course with fewer peaks in severity. The general decline in severity ratings over time that was observed in the 1 and 2 week groups is an expected result following discontinuation of buprenorphine, and it is interesting that the 4-week taper duration did not show this same profile. Analysis of CINA symptoms and ancillary medication utilization showed that the onset of withdrawal symptoms did not vary as a function of taper duration.
Interestingly, participants’ baseline expectations of withdrawal severity were strong predictors of subsequent withdrawal experienced during and following the buprenorphine taper, independent of a variety of other covariates that were hypothesized a priori to be associated with withdrawal magnitude and/or self-report. This is consistent with a previous study that reported self-report of withdrawal is a robust predictor of opioid treatment outcome (Kosten et al., 1985), and with prior studies suggesting that fear of withdrawal is associated with poor detoxification outcomes (Hollonds et al., 1980; Phillips et al., 1986; Milby et al., 1987; Gentile and Milby, 1992). There may be value in evaluating whether a single-item question that asks patients to rate their expected withdrawal severity during the taper might aid clinician efforts to identify those at higher risk for experiencing withdrawal and thus failing treatment.
Strengths of this study include the randomization of participants to double-blind buprenorphine taper duration conditions, the frequent (i.e., daily) assessments of withdrawal, and the comprehensive set of withdrawal measures. An additional strength was the effort taken to minimize confounding of withdrawal data by any ongoing illicit opioid use. Several potential limitations should also be noted. First, as this was a secondary analysis of a previously-completed randomized trial, there was a limited sample size in which to investigate withdrawal. Several outcomes that approached but did not achieve statistical significance may have been underpowered to detect an effect. Future efforts to evaluate withdrawal outcomes in larger samples are important. Second, participants were primary PO (vs. heroin) abusers, which may limit generality to the larger opioid-dependent population. That said, 46% of these participants had a history of heroin use, and 85% of those participants reported having used heroin more than 5 times in their life. Third, withdrawal ratings may have been influenced by concurrent ancillary medication use during taper or naltrexone initiation following the taper. However, inspection of our data produced no evidence that naltrexone initiation in the days after the taper influenced withdrawal, and ancillary medications were protocolized and administered to participants in a uniform way across all taper groups. While our data do not permit us to isolate the effect of buprenorphine vs. ancillary medications on withdrawal outcomes, they do provide the most complete picture to date of the time course and severity of withdrawal that a patient might expect during outpatient buprenorphine detoxification. Fourth, although we controlled for several variables likely to contribute to withdrawal outcomes, other characteristics remain which may predict individual differences in withdrawal (e.g., prior experience with withdrawal, primary opioid of abuse, route of administration). We did not collect detailed information on participants’ prior withdrawal experiences, but doing so in future studies could aid interpretation of withdrawal data. Finally, we excluded participants who relapsed to illicit opioid use during the trial, and it is possible that those participants experienced a unique profile of withdrawal that is not captured here. Future studies should examine whether withdrawal severity and time course, particularly in the days before relapse, may be useful in identifying patients who need more intensive support to prevent resumption of illicit opioid use.
In summary, this study provides new information regarding the time course and severity of withdrawal during outpatient buprenorphine taper. These data may inform clinicians and patients alike about what to expect during detoxification, as prior data suggest that patients’ knowledge of the opioid withdrawal syndrome is associated with lower peak withdrawal score and symptomatology (Green and Gossop, 1988). They may also assist clinicians in identifying critical time points (e.g., in the week following the last buprenorphine dose rather than earlier in the taper) and areas (e.g., assistance with managing sleep-related problems) with which patients may need additional support for favorable outcomes. Taken together, an improved understanding of withdrawal during buprenorphine taper will aid efforts to develop more effective detoxification strategies as one possible treatment for opioid-dependent patients.
Highlights.
Evaluation of withdrawal following 1, 2, or 4-week outpatient buprenorphine taper.
Withdrawal assessed daily using self-reports, observer-ratings, and pupillometer measurements
4-week group showed a mild and stable course of withdrawal; 1- and 2-week groups showed marked increases in withdrawal severity that decreased thereafter
Single item Visual Analog Scale (VAS) for Expected Withdrawal Severity robustly predicted peak withdrawal ratings
Results can help inform providers and patients about the time course, magnitude, and pattern of withdrawal following different outpatient buprenorphine taper durations
Acknowledgments
Buprenorphine/naloxone and color-matched placebo sublingual tablets were provided by Reckitt Benckiser Pharmaceuticals, Inc. (Richmond, VA) through the National Institute on Drug Abuse (Rockville, MD). We thank John Brooklyn MD, Betsy Bahrenburg RN, Bruce Brown, LICSW, LADC, Allison Newth, BA, Matthew Scanlin B.S., Nancey Kinlin, R.N., and Susan Schmidt R.N., for their assistance conducting this study.
Funding Source: Preparation of this manuscript was supported in part by National Institutes of Health research (R01DA019989, Sigmon; R34DA037385, Sigmon; R01DA035246, Dunn; R21DA035327, Dunn) and training (T32DA007242) grants, as well as a National Institute of General Medical Sciences center grant (P20GM103644).
Footnotes
Contributors: Author Sigmon developed and obtained funding for this study. Authors Dunn, Saulsgiver, Patrick, and Sigmon conducted the study. Author Nuzzo conducted the study analyses. All authors have reviewed and contributed to the study manuscript.
Conflict of Interest: No authors have a conflict of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amass L, Bickel WK, Higgins ST, Hughes JR. A preliminary investigation of outcome following gradual or rapid buprenorphine detoxification. J Addict Dis. 1994;13:33–45. doi: 10.1300/j069v13n03_04. [DOI] [PubMed] [Google Scholar]
- Appel PW, Ellison AA, Jansky HK, Oldak R. Barriers to enrollment in drug abuse treatment and suggestions for reducing them: opinions of drug injecting street outreach clients and other system stakeholders. Am J Drug Alcohol Abuse. 2004;30:129–153. doi: 10.1081/ada-120029870. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Becker AB, Strain EC, Bigelow GE, Stitzer ML, Johnson RE. Gradual dose taper following chronic buprenorphine. Am J Addict. 2001;10:111–121. doi: 10.1080/105504901750227778. [DOI] [PubMed] [Google Scholar]
- Best D, Gossop M, Stewart D, Marsden J, Lehmann P, Strang J. Continued heroin use during methadone treatment: relationships between frequency of use and reasons reported for heroin use. Drug Alcohol Depend. 1999;53:191–195. doi: 10.1016/s0376-8716(98)00132-x. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Stitzer ML, Bigelow GE, Liebson IA, Jasinski DR, Johnson RE. Buprenorphine: dose-related blockade of opioid challenge effects in opioid dependent humans. J Pharmacol Exp Ther. 1988a;247:47–53. [PubMed] [Google Scholar]
- Bickel WK, Stitzer ML, Bigelow GE, Liebson IA, Jasinski DR, Johnson RE. A clinical trial of buprenorphine: comparison with methadone in the detoxification of heroin addicts. Clin Pharmacol Ther. 1988b;43:72–78. doi: 10.1038/clpt.1988.13. [DOI] [PubMed] [Google Scholar]
- Bruneau J, Roy E, Arruda N, Zang G, Jutras-Aswad D. The rising prevalence of prescription opioid injection and its association with hepatitis C incidence among street-drug users. Addiction. 2012;107:1318–1327. doi: 10.1111/j.1360-0443.2012.03803.x. [DOI] [PubMed] [Google Scholar]
- Bullingham RE, McQuay HJ, Moore A, Bennett MR. Buprenorphine kinetics. Clin Pharmacol Ther. 1980;28:667–672. doi: 10.1038/clpt.1980.219. [DOI] [PubMed] [Google Scholar]
- Cameron D, Allen D, Galway K. A pilot study of the effectiveness of buprenorphine and methadone as detoxification agents when choice is given to the consumer. J Subst Use. 2001;6:101–109. [Google Scholar]
- Centers for Disease Control. CDC grand rounds: Prescription drug overdoses- a U.S. epidemic. MMWR. 2012;61:10–13. [PubMed] [Google Scholar]
- Cheskin LJ, Fudala PJ, Johnson RE. A controlled comparison of buprenorphine and clonidine for acute detoxification from opioids. Drug Alcohol Depend. 1994;36:115–121. doi: 10.1016/0376-8716(94)90093-0. [DOI] [PubMed] [Google Scholar]
- Cicero TJ, Surratt H, Inciardi JA, Munoz A. Relationship between therapeutic use and abuse of opioid analgesics in rural, suburban, and urban locations in the United States. Pharmacoepidemiol Drug Saf. 2007;16:827–840. doi: 10.1002/pds.1452. [DOI] [PubMed] [Google Scholar]
- Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23:129–138. [PubMed] [Google Scholar]
- Correia CJ, Walsh SL, Bigelow GE, Strain EC. Effects associated with double-blind omission of buprenorphine/naloxone over a 98-h period. Psychopharmacology (Berl) 2006;189:297–306. doi: 10.1007/s00213-006-0571-4. [DOI] [PubMed] [Google Scholar]
- Dijkstra BA, Krabbe PF, De Jong CA, van der Staak CP. Prediction of withdrawal symptoms during opioid detoxification. J Opioid Manag. 2008;4:311–319. doi: 10.5055/jom.2008.0035. [DOI] [PubMed] [Google Scholar]
- Dunn KE, Sigmon SC, Strain EC, Heil SH, Higgins ST. The association between outpatient buprenorphine detoxification duration and clinical treatment outcomes: a review. Drug Alcohol Depend. 2011;119:1–9. doi: 10.1016/j.drugalcdep.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fudala PJ, Jaffe JH, Dax EM, Johnson RE. Use of buprenorphine in the treatment of opioid addiction. II Physiologic and behavioral effects of daily and alternate-day administration and abrupt withdrawal. Clin Pharmacol Ther. 1990;47:525–534. doi: 10.1038/clpt.1990.67. [DOI] [PubMed] [Google Scholar]
- Gentile MA, Milby JB. Methadone maintenance detoxification fear: a study of its components. J Clin Psychol. 1992;48:797–807. doi: 10.1002/1097-4679(199211)48:6<797::aid-jclp2270480614>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Green L, Gossop M. Effects of information on the opiate withdrawal syndrome. Br J Addict. 1988;83:305–309. doi: 10.1111/j.1360-0443.1988.tb00472.x. [DOI] [PubMed] [Google Scholar]
- Gruber SA, Silveri MM, Yurgelun-Todd DA. Neuropsychological consequences of opiate use. Neuropsychol Rev. 2007;17:299–315. doi: 10.1007/s11065-007-9041-y. [DOI] [PubMed] [Google Scholar]
- Hambrook JM, Rance MJ. The interaction of buprenorphine with the opiate receptor: lipophilicity as a determining factor in drug-receptor kinetics. In: Kosterlitz HW, editor. Opiates and Endogenous Opioid Peptides. Biomedical Press; Amsterdam: 1976. pp. 295–301. [Google Scholar]
- Havens JR, Lofwall MR, Frost SD, Oser CB, Leukefeld CG, Crosby RA. Individual and network factors associated with prevalent hepatitis C infection among rural Appalachian injection drug users. Am J Public Health. 2013;103:e44–52. doi: 10.2105/AJPH.2012.300874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havens JR, Oser CB, Leukefeld CG. Injection risk behaviors among rural drug users: implications for HIV prevention. AIDS Care. 2011;23:638–645. doi: 10.1080/09540121.2010.516346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillhouse M, Domier CP, Chim D, Ling W. Provision of ancillary medications during buprenorphine detoxification does not improve treatment outcomes. J Addict Dis. 2010;29:23–29. doi: 10.1080/10550880903438925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollonds GB, Oei TP, Turecek LR. An evaluation of a behaviour therapy programme as an intervention treatment for the fear of withdrawal with heroin-dependent persons. Drug Alcohol Depend. 1980;5:153–160. doi: 10.1016/0376-8716(80)90193-3. [DOI] [PubMed] [Google Scholar]
- Janiri L, Mannelli P, Persico AM, Serretti A, Tempesta E. Opiate detoxification of methadone maintenance patients using lefetamine, clonidine and buprenorphine. Drug Alcohol Depend. 1994;36:139–145. doi: 10.1016/0376-8716(94)90096-5. [DOI] [PubMed] [Google Scholar]
- Jasinski DR, Pevnick JS, Griffith JD. Human pharmacology and abuse potential of the analgesic buprenorphine: a potential agent for treating narcotic addiction. Arch Gen Psychiatry. 1978;35:501–516. doi: 10.1001/archpsyc.1978.01770280111012. [DOI] [PubMed] [Google Scholar]
- Kleber HD. Pharmacologic treatments for opioid dependence: detoxification and maintenance options. Dialogues Clin Neurosci. 2007;9:455–470. doi: 10.31887/DCNS.2007.9.2/hkleber. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TR, Rounsaville BJ, Kleber HD. Comparison of clinician ratings to self reports of withdrawal during clonidine detoxification of opiate addicts. Am J Drug Alcohol Abuse. 1985;11:1–10. doi: 10.3109/00952998509016845. [DOI] [PubMed] [Google Scholar]
- Ling W, Amass L, Shoptaw S, Annon JJ, Hillhouse M, Babcock D, Brigham G, Harrer J, Reid M, Muir J, Buchan B, Orr D, Woody G, Krejci J, Ziedonis D Buprenorphine Study Protocol Group. A multi-center randomized trial of buprenorphine-naloxone versus clonidine for opioid detoxification: findings from the National Institute on Drug Abuse Clinical Trials Network. Addiction. 2005;100:1090–1100. doi: 10.1111/j.1360-0443.2005.01154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling W, Hillhouse M, Domier C, Doraimani G, Hunter J, Thomas C, Jenkins J, Hasson A, Annon J, Saxon A, Selzer J, Boverman J, Bilangi R. Buprenorphine tapering schedule and illicit opioid use. Addiction. 2009;104:256–265. doi: 10.1111/j.1360-0443.2008.02455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lintzeris N, Bell J, Bammer G, Jolley DJ, Rushworth L. A randomized controlled trial of buprenorphine in the management of short-term ambulatory heroin withdrawal. Addiction. 2002;97:1395–1404. doi: 10.1046/j.1360-0443.2002.00215.x. [DOI] [PubMed] [Google Scholar]
- Marsch LA, Bickel WK, Badger GJ, Stothart ME, Quesnel KJ, Stanger C, Brooklyn J. Comparison of pharmacological treatments for opioid-dependent adolescents: a randomized controlled trial. Arch Gen Psychiatry. 2005;62:1157–1164. doi: 10.1001/archpsyc.62.10.1157. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Turner B. Prescription opioids, overdose deaths, and physician responsibility. JAMA. 2008;300:2672–2673. doi: 10.1001/jama.2008.793. [DOI] [PubMed] [Google Scholar]
- Milby JB, Gurwitch RH, Hohmann AA, Wiebe DJ, Ling W, McLellan AT, Woody GE. Assessing pathological detoxification fear among methadone maintenance patients: the DFSS. J Clin Psychol. 1987;43:528–538. doi: 10.1002/1097-4679(198709)43:5<528::aid-jclp2270430517>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Nielsen S, Hillhouse M, Mooney L, Ang A, Ling W. Buprenorphine pharmacotherapy and behavioral treatment: Comparison of outcomes among prescription opioid users, heroin users and combination users. J Subst Abuse Treat. 2014 doi: 10.1016/j.jsat.2014.06.006. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen S, Hillhouse M, Mooney L, Fahey J, Ling W. Comparing buprenorphine induction experience with heroin and prescription opioid users. J Subst Abuse Treat. 2012;43:285–290. doi: 10.1016/j.jsat.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen S, Hillhouse M, Thomas C, Hasson A, Ling W. A comparison of buprenorphine taper outcomes between prescription opioid and heroin users. J Addict Med. 2013;7:33–38. doi: 10.1097/ADM.0b013e318277e92e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor PG, Carroll KM, Shi JM, Schottenfeld RS, Kosten TR, Rounsaville BJ. Three methods of opioid detoxification in a primary care setting. A randomized trial. Ann Intern Med. 1997;127:526–530. doi: 10.7326/0003-4819-127-7-199710010-00004. [DOI] [PubMed] [Google Scholar]
- Oreskovich MR, Saxon AJ, Ellis ML, Malte CA, Reoux JP, Knox PC. A double-blind, double-dummy, randomized, prospective pilot study of the partial mu opiate agonist, buprenorphine, for acute detoxification from heroin. Drug Alcohol Depend. 2005;77:71–79. doi: 10.1016/j.drugalcdep.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Peachey JE, Lei H. Assessment of opioid dependence with naloxone. Br J Addict. 1988;83:193–201. doi: 10.1111/j.1360-0443.1988.tb03981.x. [DOI] [PubMed] [Google Scholar]
- Phillips GT, Gossop M, Bradley B. The influence of psychological factors on the opiate withdrawal syndrome. Br J Psychiatry. 1986;149:235–238. doi: 10.1192/bjp.149.2.235. [DOI] [PubMed] [Google Scholar]
- Pinto H, Maskrey V, Swift L, Rumball D, Wagle A, Holland R. The SUMMIT trial: a field comparison of buprenorphine versus methadone maintenance treatment. J Subst Abuse Treat. 2010;39:340–352. doi: 10.1016/j.jsat.2010.07.009. [DOI] [PubMed] [Google Scholar]
- Raistrick D, West D, Finnegan O, Thistlethwaite G, Brearley R, Banbery J. A comparison of buprenorphine and lofexidine for community opiate detoxification: results from a randomized controlled trial. Addiction. 2005;100:1860–1867. doi: 10.1111/j.1360-0443.2005.01273.x. [DOI] [PubMed] [Google Scholar]
- Reed LJ, Glasper A, de Wet CJ, Bearn J, Gossop M. Comparison of buprenorphine and methadone in the treatment of opiate withdrawal: possible advantages of buprenorphine for the treatment of opiate-benzodiazepine codependent patients? J Clin Psychopharmacol. 2007;27:188–192. doi: 10.1097/JCP.0b013e318032ec2a. [DOI] [PubMed] [Google Scholar]
- Rosenblum A, Cleland CM, Fong C, Kayman DJ, Tempalski B, Parrino M. Distance traveled and cross-state commuting to opioid treatment programs in the United States. J Environ Public Health. 2011;2011:948789. doi: 10.1155/2011/948789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rounsaville BJ, Kosten TR. Treatment for opioid dependence: quality and access. JAMA. 2000;283:1337–1339. doi: 10.1001/jama.283.10.1337. [DOI] [PubMed] [Google Scholar]
- San L, Cami J, Fernandez T, Olle JM, Peri JM, Torrens M. Assessment and management of opioid withdrawal symptoms in buprenorphine-dependent subjects. Br J Addict. 1992;87:55–62. doi: 10.1111/j.1360-0443.1992.tb01900.x. [DOI] [PubMed] [Google Scholar]
- Sigmon SC. Access to treatment for opioid dependence in rural America: challenges and future directions. JAMA Psychiatry. 2014;71:359–360. doi: 10.1001/jamapsychiatry.2013.4450. [DOI] [PubMed] [Google Scholar]
- Sigmon SC, Dunn KE, Badger GJ, Heil SH, Higgins ST. Brief buprenorphine detoxification for the treatment of prescription opioid dependence: a pilot study. Addict Behav. 2009;34:304–311. doi: 10.1016/j.addbeh.2008.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigmon SC, Dunn KE, Saulsgiver K, Patrick ME, Badger GJ, Heil SH, Brooklyn JR, Higgins ST. A randomized, double-blind evaluation of buprenorphine taper duration in primary prescription opioid abusers. JAMA Psychiatry. 2013;70:1347–1354. doi: 10.1001/jamapsychiatry.2013.2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA) Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings. 2013. NSDUH Series H-46. [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA) Results from the 2013 National Survey on Drug use and Health: Summary of National Findings. HHS; Rockville, MD: 2014. [PubMed] [Google Scholar]
- Tripathi BM, Hemaraj P, Dhar NK. Buprenorphine withdrawal syndrome. Indian J Psychiatry. 1995;37:23–25. [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, McLellan TA. Curtailing diversion and abuse of opioid analgesics without jeopardizing pain treatment. JAMA. 2011;305:1346–1347. doi: 10.1001/jama.2011.369. [DOI] [PubMed] [Google Scholar]
- Warden D, Subramaniam GA, Carmody T, Woody GE, Minhajuddin A, Poole SA, Potter J, Fishman M, Bogenschutz M, Patkar A, Trivedi MH. Predictors of attrition with buprenorphine/naloxone treatment in opioid dependent youth. Addict Behav. 2012;37:1046–1053. doi: 10.1016/j.addbeh.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner M, Hedegaard H, Chen L. Trends in Drug Poisoning Deaths Involving Opioid Analgesics and Heroin: United States, 1999–2012. Centers for Disease Control and Prevention National Vitals Statistics System; Atlanta: 2014. [Google Scholar]
- White R, Alcorn R, Feinmann C. Two methods of community detoxification from opiates: an open-label comparison of lofexidine and buprenorphine. Drug Alcohol Depend. 2001;65:77–83. doi: 10.1016/s0376-8716(01)00149-1. [DOI] [PubMed] [Google Scholar]
- Zacny J, Bigelow G, Compton P, Foley K, Iguchi M, Sannerud C. College on Problems of Drug Dependence taskforce on prescription opioid non-medical use and abuse: position statement. Drug Alcohol Depend. 2003;69:215–232. doi: 10.1016/s0376-8716(03)00003-6. [DOI] [PubMed] [Google Scholar]