Abstract
A new species of the group of black aspergilli, Aspergillus vadensis, was analyzed for its potential as a host for homologous and heterologous protein production. Unlike the other black aspergilli, this strain does not acidify the culture medium when nitrate is the nitrogen source and only produces very low levels of extracellular proteases, mainly serine metalloproteases. The stability of A. tubingensis feruloyl esterase A (FaeA) was compared upon production in wild-type A. vadensis, A. tubingensis, and an A. niger strain in which the three main protease-encoding genes were disrupted. The production of FaeA in A. vadensis resulted in larger amounts of intact protein than production in A. tubingensis and was similar to production in an A. niger protease disruptant, confirming in vivo the low proteolytic activity of A. vadensis. The protoplast formation and transformation efficiencies of A. vadensis were much higher than those of A. niger. These characteristics make A. vadensis a very promising candidate for homologous, and possibly heterologous, protein production.
The black aspergilli (Aspergillus section Nigri) form a subgroup of the genus Aspergillus. We recently identified a novel member of this subgroup and demonstrated that this species, Aspergillus vadensis, differs from the other black aspergilli by using sequence, morphological, metabolite, and restriction fragment length polymorphism analyses (3; R. P. de Vries, J. C. Frisvad, P. J. I. van de Vondervoort, K. Burgers, A. F. A. Kuijpers, R. A. Samson, and J. Visser, submitted for publication).
Several black aspergilli are commonly used for industrial applications, such as the production of metabolites (e.g., organic acids) and extracellular proteins (e.g., plant-cell-wall-degrading enzymes). The production of homologous proteins by A. niger resulted in up to 30 g/liter (for example, see reference 5) and started an interest in these fungi as production hosts for heterologous proteins. An additional advantage, besides the high secretory potential, is that fungi can perform all of the posttranslational modifications (e.g., glycosylation and disulfide bridge formation) that are required for the correct production of proteins from higher eukaryotes (8). Examples of the production of heterologous proteins in A. niger are hen egg white lysozyme (80 to 160 mg/liter) (18), bovine enterokinase (1.9 to 5 mg/liter) (13), chymosin (0.3 to 1.2 g/liter) (4), human interleukin-6 (200 to 300 mg/liter) (10), and Phanerochaete chrysosporium lignin peroxidase H8 (100 mg/liter) (1). With the exception of chymosin, none of these enzymes are produced on a gram-per-liter scale. One reason for the reduced level of heterologous protein production compared to homologous protein production in A. niger is the high level of secreted protease activity that efficiently degrades heterologous proteins (14). A second factor is the acidification of the medium during the growth of A. niger and other black aspergilli by the production of organic acids. This not only stimulates the production of proteases in A. niger (11, 16) but may also reduce the stability of heterologous proteins. Many studies have therefore aimed at constructing protease-deficient strains for protein production (15, 16). This paper describes the potential of an A. vadensis strain for homologous and possibly heterologous protein production and demonstrates that fungal strains with better characteristics with respect to protein production already exist and could be a better starting point for strain improvement strategies than the strains that are currently used.
MATERIALS AND METHODS
Strains and libraries.
The strains used for this study are listed in Table 1. For the construction of a genomic library, strain CBS 113365 was grown for 24 h in minimal medium (MM) with 0.1% yeast extract and 4% d-glucose, after which the mycelium was harvested and frozen in liquid nitrogen. The chromosomal DNA was isolated from the mycelium, partially digested with Sau3A, and separated by agarose gel electrophoresis. DNA fragments with a size of about 10 kb were isolated from the gel and ligated into BamHI-digested phage λ EMBL4.
TABLE 1.
Strains used for this study
| Species | Strain | Alternative number or description | Reference |
|---|---|---|---|
| A. foetidus | CBS 115.52 | ATCC 11358 | 9 |
| A. japonicus | CBS 114.51 | 9 | |
| A. niger | CBS 120.49 | N400, ATCC 9029, IMI 041876, NRRL 3 | 9 |
| N593 | cspA1 pyrA6 mutant | ||
| A. oryzae | ATCC 20836 | ||
| A. sojae | ATCC 20387 | ||
| A. tubingensis | NW756 | 9 | |
| CBS 643.92 | |||
| A. vadensis | CBS 113365 | CBS 102787, IMI 142717, IBT 24658 | 3; de Vries et al., submitted |
| CBS 113226 | NW282, pyrA5 mutant | This study | |
| NW282::pIM3208.3 | faeA transformant | This study | |
| NW282::pIM3208.6 | faeA transformant | This study | |
| A. tubingensis | NW241::pIM3208.6 | faeA transformant | 2 |
| A. niger | NW154::pIM3208.5 | faeA transformant, prtF28 mutant | 2 |
| NW196 | fwnA6 argB + dpepA | 15; this study | |
| argB + dpepB pyrA6 | |||
| argB + dpepE prtF28 | |||
| NW228 | bioA1 prtF28 | 16 |
Media and culture conditions.
MM contained the following (per liter): 6.0 g of NaNO3, 1.5 g of KH2PO4, 0.5 g of KCl, 0.5 g of MgSO4, 200 μl of trace elements (10 g of EDTA/liter, 4.4 g of ZnSO4 · 7H2O/liter, 1.01 g of MnCl2 · 4H2O/liter, 0.32 g of CoCl2 · 6H2O/liter, 0.315 g of CuSO4 · 5H2O/liter, 0.22 g of (NH4)6Mo7O24 · 4H2O/liter, 1.47 g of CaCl2 · 2H2O/liter, and 1.0 g of FeSO4 · 7H2O/liter [modified from reference 17]), and 1% (wt/vol) glucose as a carbon source, unless otherwise indicated. For complete medium, MM was supplemented with 0.2% (wt/vol) tryptone, 0.1% (wt/vol) yeast extract, 0.1% (wt/vol) Casamino Acids, and 0.05% (wt/vol) yeast RNAs. Liquid cultures were inoculated with 106 spores/ml and incubated at 30°C in an orbital shaker at 250 rpm. Agar was added at 1.5% (wt/vol) for solid medium. For the growth of strains with auxotrophic mutations, the necessary supplements were added to the medium. Precultures for protoplast formation were grown overnight at 30°C in MM with 0.5% (wt/vol) yeast extract, 0.2% (wt/vol) Casamino Acids, and 2% (wt/vol) d-glucose after the inoculation of 5 × 106 spores/ml.
To test medium acidification, we grew strains in MM and 0.3× MM at 30°C. We added 2% (wt/vol) d-fructose and 0.05% (wt/vol) yeast extract to both media. After 16, 24, and 40 h of growth, the pH of the culture fluid was measured. For plate tests using different protein substrates, all strains were grown on MM and MM in which 6 g of NaNO3/liter was replaced with 4.5 g of NH4Cl/liter. For the screening of FaeA production, transformants were grown on MM containing 1% (wt/vol) beechwood xylan and 0.03% (wt/vol) ferulic acid for 3 days.
Chemicals.
d-Xylose, d-glucose, d-fructose, d-galactose, d-mannose, and lactose were obtained from Merck (Darmstadt, Germany). d-Glucuronic and d-galacturonic acid were from Fluka (Buchs, Switzerland). Mellibiose, raffinose, stachyose, casein, casein hydrolysate, gelatin, l-arabinose, Glucanex, and beech wood xylan were purchased from Sigma (St. Louis, Mo.). Protifar is a protein-rich (95.6% protein) preparation from Nutricia Nahrungmittel GmbH & Co. (Vienna, Austria). Taq polymerase was purchased from Gibco BRL (Breda, The Netherlands). Novozyme 234 was obtained from Novo Industries (Bagsvaerd, Denmark).
Molecular biology methods.
Standard methods were used for DNA manipulations, subcloning, DNA digestion reactions, and DNA isolations (12).
Determination of extracellular protease activity.
Strains were grown for 3 days in MM containing 1% (wt/vol) wheat bran, 1% (wt/vol) glucose, and 0.05% (wt/vol) yeast extract. Culture filtrate samples were harvested, frozen in liquid nitrogen, and stored at −70°C. Protease activities were measured by an internally quenched method based on the work of Jones et al. (6). A mixture of 50 μl of culture filtrate diluted five times, 10 μl of Bodipy FL casein (EnzChel protease assay kit; Molecular Probes, Eugene, Oreg.), and 640 μl of buffer (see below) was incubated for 2 h at 30°C. Fluorescence (with excitation and emission wavelengths of 502 and 511 nm, respectively) was measured with an F-4500 fluorimeter (Hitachi, Tokyo, Japan). The buffers used for the assays were 0.1 mM sodium acetate (pH 4), 0.1 mM sodium phosphate (pH 6), and 0.1 mM Tris-HCl (pH 8). Protease assays were also performed in the presence of three protease inhibitors (16), specifically pepstatin (acidic protease inhibitor) at pH 4, phenylmethylsulfonyl fluoride (PMSF) (serine protease inhibitor) at pH 6, and EDTA (metalloprotease inhibitor) at pHs 6 and 8.
Enzyme assays.
Polysaccharide hydrolase activities were determined by using p-nitrophenyl glycosides as substrates. Culture filtrate samples (10 μl) were incubated with 10 μl of a 0.1% (wt/vol) solution of the substrate, 50 μl of a 50 mM sodium acetate buffer (pH 5.0), and 30 μl of water for 2 h at 30°C in microtiter plates. The reaction was stopped by the addition of 100 μl of 0.25 M bisodium carbonate, and the optical density at 405 nm was measured on a model 550 microplate reader (Bio-Rad). Activities were expressed as nanomoles of p-nitrophenol liberated per minute per milligram of total protein in the culture filtrate.
UV mutagenesis.
Strain CBS 113365 (106 spores) was spread on a 9-cm-diameter plate containing 20 ml of complete medium with 1% (wt/vol) d-glucose, 5 mM uridine, and 20 mg of fluoroorotic acid. The plate was irradiated for 1 min at 2 J m−2 sec−1 with a Philips UV-A lamp. The plate was then incubated at 30°C. Fluoroorotic acid is converted into a toxic compound in strains carrying a functional pyrA gene, thus enabling selection for pyrA-negative mutants. Uridine is added to the plate because the pyrA gene product is needed for uridine biosynthesis. After 5 days of growth, colonies were picked from this plate, purified, and tested for growth on MM with 1% d-glucose in the presence and absence of 5 mM uridine.
Transformation of A. vadensis.
The formation of protoplasts by Aspergillus strains was based on the protocol of Kusters-van Someren et al. (7). Strains were grown for 16 h, after which the mycelia were gently harvested by use of a Büchner funnel and were washed with SMC (1.33 M sorbitol, 50 mM CaCl2, 20 mM morpholineethanesulfonic acid buffer, pH 5.8). Aliquots of 1 g (wet weight) were resuspended in 20 ml of SMC, and 200 mg of lysing enzyme (Novozyme 234 or Glucanex) was added. The mixture was incubated at 30°C in an orbital shaker for 1 to 2 h with gentle shaking (120 rpm). Protoplasts were separated from the mycelium by filtering over glass wool. The protoplasts were recovered by centrifugation in a swing-out rotor (10 min at 2,200 rpm) and were washed once with STC (1.33 M sorbitol, 50 mM CaCl2, 10 mM Tris-HCl, pH 7.5). Transformation was performed as described before (7), with 2 × 106 protoplasts, 0.5 μg of pGW635 (carrying the A. niger pyrA gene for selection), and 20 μg of pIM3208 (carrying the A. tubingensis faeA gene).
RESULTS
Acidification of growth media.
One of the problems of protein production in A. niger is the strong acidification of the culture medium by this species. The acidification of culture media by A. niger, A. tubingensis, and A. vadensis was compared by using MM and 0.3× MM, with sodium nitrate as the nitrogen source for both, and MM with ammonium chloride as the nitrogen source. All cultures contained 2% fructose and 0.05% yeast extract, and the starting pH was set at 6.0. The pH of the culture was measured after 16, 24, and 40 h and dry weights were measured after 24 and 40 h. When nitrate was used as a nitrogen source, acidification was observed in the A. niger cultures (Table 2), and to a lesser extent in the A. tubingensis cultures, while the pH of the A. vadensis cultures went up during cultivation. When ammonium was used as a nitrogen source, the acidification was strongest for A. niger, while A. tubingensis and A. vadensis acidified the medium to a lesser extent. The growth rate was higher for all species when ammonium was used as a nitrogen source, but the difference in growth between the species was minimal (Table 2).
TABLE 2.
Acidification of culture media by different aspergillia
| Species | Strain | Medium | pH at indicated time (h)
|
Dry weight (mg) at indicated time (h)
|
|||
|---|---|---|---|---|---|---|---|
| 16 | 24 | 40 | 24 | 40 | |||
| A. niger | CBS 120.49 | MM-nitrate | 3.8 | 3.6 | 3.3 | 51 | 138 |
| 0.3×MM-nitrate | 4.3 | 3.9 | 1.8 | ND | ND | ||
| MM-ammonium | 2.8 | 2.4 | 1.9 | 161 | 343 | ||
| A. tubingensis | NW756 | MM-nitrate | 6.1 | 5.2 | 5.0 | 47 | 132 |
| 0.3×MM-nitrate | 6.6 | 6.5 | 4.1 | ND | ND | ||
| MM-ammonium | 3.4 | 3.2 | 2.7 | 134 | 291 | ||
| A. vadensis | CBS 113365 | MM-nitrate | 6.5 | 7.0 | 6.7 | 48 | 129 |
| 0.3×MM-nitrate | 6.7 | 7.0 | 6.2 | ND | ND | ||
| MM-ammonium | 4.1 | 3.9 | 3.5 | 146 | 327 | ||
Samples were measured after 16, 24, and 40 h of growth. The total dry weight of the 50-ml cultures was determined after 24 and 40 h. ND, not determined.
Extracellular protease activity of A. vadensis.
Another major problem of protein production in A. niger and other aspergilli is the presence of extracellular proteases that reduce the levels of the desired protein. The extracellular protease activity of A. vadensis was compared to those of other Aspergillus species at pHs 4, 6, and 8 and in the absence and presence of protease inhibitors (Table 3). A. vadensis produced very little protease activity at all three pH values tested. A. niger, A. tubingensis, and A. sojae produced predominantly acidic and neutral proteases, whereas A. oryzae produced only neutral and alkaline proteases. The highest protease activities of A. japonicus and A. foetidus were detected at pH 6, but these species also produced significant amounts of acidic and alkaline proteases (Table 3). Pepstatin did not have a significant effect on the protease activity of A. vadensis at pH 4, but it did affect the protease activities of A. niger, A. tubingensis, A. foetidus, and A. sojae. PMSF, and to a lesser extent, EDTA both reduced the protease activities at pH 6 of A. vadensis, A. tubingensis, A. foetidus, and A. sojae. EDTA reduced the protease activity at pH 8 of A. niger, A. oryzae, and A. japonicus.
TABLE 3.
Relative extracellular protease activities of A. vadensis and other aspergillia
| Strain | Relative protease activity without inhibitors at indicated pH
|
Relative protease activity with inhibitor at indicated pH
|
|||||
|---|---|---|---|---|---|---|---|
| 4 | 6 | 8 | Pepstatin
|
PMSF
|
EDTA
|
EDTA
|
|
| 4 | 6 | 6 | 8 | ||||
| A. vadensis CBS 113365 | 2.1 | 1.6 | 0.0 | 2.0 | 0.0 | 0.1 | 0.0 |
| A. niger CBS 120.49 | 100.0 | 3.8 | 0.6 | 54.5 | 4.1 | 3.7 | 0.4 |
| A. tubingensis CBS 643.92 | 33.6 | 20.6 | 6.1 | 15.8 | 5.9 | 12.9 | 6.7 |
| A. foetidus CBS 115.52 | 12.0 | 27.5 | 12.1 | 3.8 | 3.4 | 19.3 | 13.1 |
| A. japonicus CBS 114.51 | 2.3 | 6.4 | 1.6 | 1.7 | 5.1 | 5.3 | 0.0 |
| A. oryzae ATCC 20836 | 0.0 | 16.2 | 19.4 | 0.0 | 0.0 | 14.1 | 12.0 |
| A. sojae ATCC 20387 | 19.3 | 23.1 | 3.1 | 13.7 | 1.2 | 16.5 | 2.8 |
The protease activity of A. niger at pH 4 was set at 100.
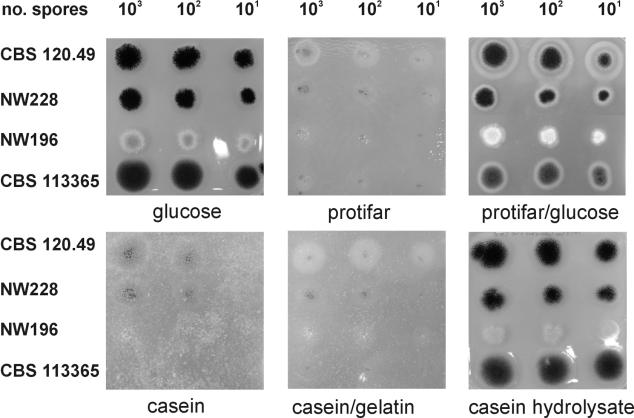
To determine the effects of the very low level of extracellular protease activity of A. vadensis on its growth on protein substrates, we grew wild-type strains of A. vadensis and A. niger as well as two A. niger mutant strains with reduced proteolytic activities and perturbations in oxalate production (Table 1) for 4 days on MM containing the carbon sources indicated in Fig. 1, with sodium nitrate or ammonium chloride used as the nitrogen source. Both wild types grew equally well on d-glucose, while the A. niger mutants grew a little slower. Growth on casein, casein plus gelatin, and Protifar (a protein-rich crude preparation) was poor in general, but growth and sporulation were best for the A. niger wild type (CBS 120.49), followed by the prtF mutant (NW228) and the prtF mutant with three disrupted proteases (NW196), while the growth of the A. vadensis wild type (CBS 113365) was similar to its growth on agar plates to which no carbon source had been added (data not shown), and no sporulation was observed for the A. vadensis strain. Growth on plates containing Protifar and glucose was similar to growth on glucose alone. For CBS 120.49 only, a halo was visible outside the paler nonsporulating part of the colony, indicating proteolytic degradation of the Protifar. Growth on casein hydrolysate was also similar to growth on glucose, indicating that the uptake of peptides and/or amino acids is similar for A. vadensis and A. niger. No difference was observed for either nitrate or ammonium being used as a nitrogen source.
FIG. 1.
Growth of A. vadensis, the A. niger wild type, and two A. niger protease mutants. Serial dilutions (1,000, 100, and 10 spores) were plated on agar plates containing the indicated carbon sources. CBS 120.49, wild-type A. niger; NW 228, A. niger prtF mutant; NW196, A. niger prtF mutant in which pepA, pepB, and pepE are disrupted; CBS 113365, wild-type A. vadensis.
Production of cell wall polysaccharide-degrading enzymes.
One of the most widely applied enzyme systems of Aspergillus is the polysaccharide-degrading enzyme system. To determine whether A. vadensis produces the same range of enzymes as A. niger or whether protein production in general is reduced in A. vadensis, we grew both species for 4 days in MM containing a 0.5% concentration of the polymeric compounds indicated in Table 4. In general, lower hydrolase activities were detected for A. vadensis than for A. niger (Table 4). Low or no hydrolase activities were detected during the growth of both strains on cellulose, whereas the highest activities were detected during growth on beech wood xylan and a crude xylan preparation.
TABLE 4.
Extracellular enzyme activities of A. vadensis and A. niger during growth on polymeric carbon sourcesa
| Strain and carbon source | Activity of enzyme (nmol of p-nitrophenol released/min/mg of extracellular protein)b
|
|||||
|---|---|---|---|---|---|---|
| ABF | AGL | LAC | BGL | MND | BXL | |
| AN lax | 59.9 ± 2.4 | 318.8 ± 7.4 | 310.2 ± 17.7 | 492.3 ± 2.6 | 49.9 ± 0.3 | 453.5 ± 15.5 |
| AV lax | 8.2 ± 1.0 | 77.6 ± 6.9 | 14.5 ± 4.5 | 396.0 ± 11.9 | 6.3 ± 0.5 | 63.4 ± 8.7 |
| AN awb | 7.5 ± 0.8 | 14.0 ± 0.1 | 9.0 ± 0.3 | 47.9 ± 6.7 | 13.2 ± 1.7 | 12.6 ± 1.5 |
| AV awb | 6.4 ± 0.2 | 7.3 ± 0.7 | 7.7 ± 0.2 | 34.0 ± 2.8 | 7.1 ± 0.5 | 18.2 ± 1.9 |
| AN gg | 9.8 ± 0.4 | 253.7 ± 1.3 | 148.2 ± 1.6 | 240.1 ± 5.1 | 132.3 ± 6.3 | 68.7 ± 2.8 |
| AV gg | ND | 53.0 ± 5.8 | 10.3 ± 0.5 | 225.0 ± 7.6 | 48.7 ± 4.8 | 5.6 ± 0.6 |
| AN cell | ND | 48.2 ± 3.3 | ND | 35.1 ± 3.9 | ND | 3.9 ± 0.4 |
| AV cell | ND | ND | ND | 26.0 ± 2.9 | ND | ND |
| AN bwx | 55.4 ± 3.5 | 507.2 ± 12.1 | 149.4 ± 4.2 | 331.0 ± 15.8 | 6.1 ± 0.3 | 441.2 ± 28.4 |
| AV bwx | 39.2 ± 2.8 | 140.0 ± 6.3 | 1.6 ± 0.2 | 238.8 ± 1.9 | 0.7 ± 0.2 | 289.0 ± 13.7 |
AN, A. niger N402; AV, A. vadensis CBS 113365; lax, crude xylan preparation; awb, alder wood bark; gg, guar gum; cell, cellulose; bwx, birchwood xylan; ABF, α-arabinofuranosidase; AGL, α-galactosidase; LAC, β-galactosidase; BGL, β-glucosidase; MND, β-mannosidase; BXL, β-xylosidase.
Data are means ± standard deviations. ND, not detected.
Construction of a transformation system for A. vadensis.
In order to use A. vadensis as a host for protein production, we needed to develop a transformation system for this species. We performed UV mutagenesis as described in Materials and Methods. After 5 days of growth, 20 colonies were selected, purified, and tested for growth on MM with 1% (wt/vol) d-glucose in the presence or absence of 5 mM uridine. Fourteen colonies required uridine, and after the transformation of six of these mutants with pGW635 (carrying the A. niger pyrA gene), three mutant strains were identified as pyrA mutants. One of these mutant strains (CBS 113226) was used for further experiments.
A. vadensis CBS 113226 and A. niger N593 were grown for 16 h, after which 1 g of wet mycelium was used to obtain protoplasts, as described in Materials and Methods. The protoplast numbers were determined after 1 and 2 h (Table 5). More than 10 times as many protoplasts were obtained for A. vadensis than for A. niger when we used Novozyme 234. When we used Glucanex, protoplasts were only obtained for A. vadensis.
TABLE 5.
Protoplasting efficiency of A. niger N593 and A. vadensis CBS 113226
| Species | No. of protoplasts in the incubation mixture (20 ml)a
|
|||
|---|---|---|---|---|
| Novozyme 234
|
Glucanex
|
|||
| 1 h | 2 h | 1 h | 2 h | |
| A. niger | 1.2 × 108 ± 0.3 × 108 | 2.3 × 108 ± 0.8 × 108 | ND | ND |
| A. vadensis | 23.5 × 108 ± 1.7 × 108 | 56.0 × 108 ± 4.3 × 108 | 0.4 × 108 ± 0.2 × 108 | 1.1 × 108 ± 0.5 × 108 |
The results are averages of two independent protoplasting experiments. ND, not detected.
Production of A. tubingensis FaeA in A. vadensis.
It was reported previously that the feruloyl esterase A from A. tubingensis is highly sensitive to proteolytic degradation when it is produced in A. niger or A. tubingensis (2), indicating that this protein was a suitable candidate for determining the effect of the low level of extracellular protease activity of A. vadensis on protein production. We cotransformed A. vadensis CBS 113226 with pGW635 and pIM3208 (carrying the A. tubingensis faeA gene) as described in Materials and Methods. More than 800 transformants were obtained, 10 of which were purified and analyzed for FaeA production. Two transformants were selected and compared to A. tubingensis NW241::pIM3208.6 and an A. niger mutant with a strongly reduced proteolytic activity (NW154::pIM3208.5) (2). A wild-type A. niger strain producing A. tubingensis FaeA was not included, as a previous study demonstrated that no intact FaeA was produced in culture filtrates of this class of transformants (2). These strains were grown in MM containing 1% beechwood xylan and 0.03% ferulic acid. After 3 days, culture filtrate samples were harvested, concentrated by a factor of 5, and analyzed by a Western blot analysis using antibodies against FaeA (2). The degradation of A. tubingensis FaeA in A. vadensis was strongly reduced compared to that in A. tubingensis (Fig. 2). A similar amount of the enzyme was present in its mature form, as was observed for the A. niger protease-deficient mutant.
FIG. 2.
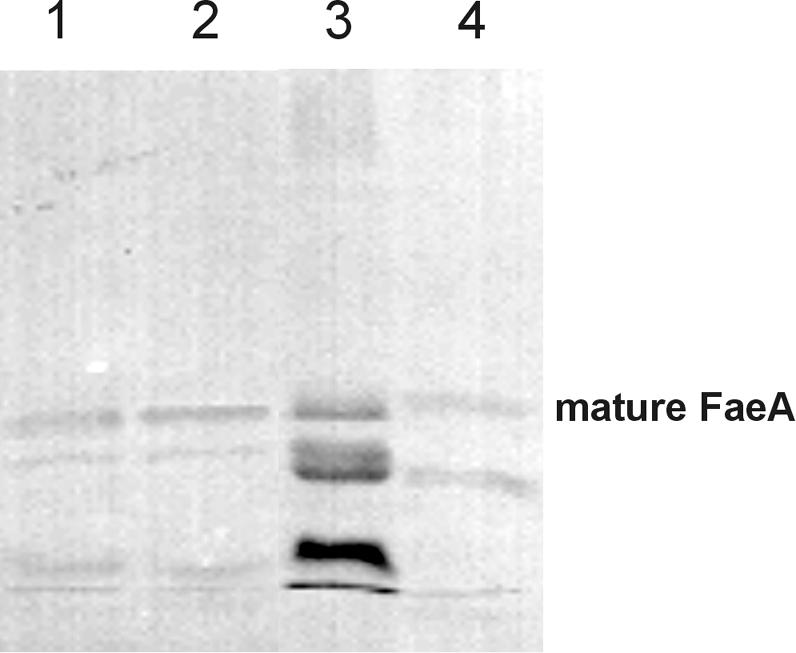
Degradation pattern of A. tubingensis FaeA after production in several aspergilli. Lanes: 1, A. vadensis NW282::pIM3208.3; 2, A. vadensis NW282::pIM3208.6; 3, A. tubingensis NW241::pIM3208.6; 4, A. niger NW154::pIM3208.5. The band corresponding to the intact mature form of FaeA is indicated.
DISCUSSION
One of the major problems of industrial protein production using Aspergillus is the production of extracellular proteases which degrade the protein of interest (14). All of the aspergilli that are commonly used for protein production produce significant levels of proteases. A. niger produces predominantly acidic proteases, which is consistent with the low pH of the medium after the growth of A. niger when the pH is not controlled. A. tubingensis acidifies the medium to a lesser extent and produces both acidic and neutral proteases. Since A. vadensis does not acidify the medium when nitrate is present as a nitrogen source, we expected that this species would produce neutral and alkaline proteases, as was observed for A. oryzae. However, only a very small amount of neutral protease activity and no alkaline protease activity was detected in this species, indicating that the overall protease production of A. vadensis is very low. This does not seem to affect the growth of this species, as germination, biomass formation, and spore formation were similar to those of A. niger and A. tubingensis (data not shown). Pepstatin had no significant effect on the residual acidic protease activity of A. vadensis, indicating that this activity is not caused by aspartic proteases. PMSF completely abolished the protease activity at pH 6, suggesting that the residual proteases are serine proteases. EDTA also abolished most of the protease activity of A. vadensis at pH 6, indicating that the majority of the proteases produced by A. vadensis are serine metalloproteases. This is not the case for A. niger, since the protease activity at pH 6 of this species was not affected by PMSF or EDTA. This demonstrates that the proteolytic spectrum of A. vadensis is very different from the well-studied proteolytic spectrum of A. niger (14). The low level of extracellular protease activity of A. vadensis is in agreement with the inability of this species to grow on plates with protein as the only carbon source. Growth on protein carbon sources was strongly reduced when either nitrate or ammonium was the nitrogen source, indicating that the low level of extracellular protease activity was not the result of altered nitrogen regulation. This also indicates that the low level of protease activity was not caused by the absence of acidification, since growth in a medium with ammonium as a nitrogen source did result in acidification of the medium. The ratio of intact and degraded A. tubingensis FaeA proteins after production in A. vadensis was much higher than that after the production of this enzyme in A. tubingensis or wild-type A. niger (2) and was similar to that observed after production in an A. niger strain in which the three main protease-encoding genes had been disrupted. The residual protease activity was <6% that of the A. niger wild type, indicating that the lower protease activity demonstrated in vitro for A. vadensis also results in higher amounts of intact FaeA in vivo.
A. vadensis produces a similar range of polysaccharide hydrolases as does A. niger during growth on crude and pure plant cell wall components, but at lower levels. However, significant levels of the enzymes were produced, indicating that the absence of extracellular proteases in A. vadensis is not an indication of an overall low level of protein secretion. The reason for the lower production of the polysaccharide hydrolases is not clear at this point. One reason could be that the expression of the genes encoding these enzymes is more tightly controlled, but more study is required to elucidate whether this is true. Alternatively, it is possible that A. vadensis grows somewhat slower on this carbon source. Since the crude arabinoxylan itself is not soluble, this is not easy to monitor during incubation. However, the range of hydrolases produced by A. vadensis indicates that it can also be used for the overproduction of homologous enzymes for industrial applications. For the production of specific enzymes, the lower level of wild-type production may even be an advantage, as it might simplify the purification of the introduced enzyme.
The protoplast formation and transformation frequencies of A. vadensis were significantly higher than those of A. niger. When we used Novozyme 234, about 20 times more protoplasts were released from the mycelium of A. vadensis than from that of A. niger. When we used Glucanex, no protoplasts were obtained for A. niger, whereas for A. vadensis the number of protoplasts was about one-third to one-half the number of A. niger protoplasts obtained with Novozyme 234. The latter enzyme preparation is no longer available from Novozyme, whereas Glucanex is available from Sigma.
Considering the differences between A. vadensis and other aspergilli, this species is a very promising candidate as a host for protein production. It has a very high transformation frequency, which is convenient for the high-throughput screening of transformants. It does not acidify the growth medium and produces very low levels of extracellular protease activity, both of which contribute to an increased stability of the protein of choice in the culture broth of this fungal species. In this paper, we have demonstrated that A. vadensis produces very small amounts of extracellular proteases and that this feature results in the improved production of an Aspergillus protein (FaeA). The absence of acidification and the very low level of extracellular protease activity also suggest that A. vadensis is a promising host for the production of heterologous proteins.
Acknowledgments
We thank H. J. Wagteveld of Avebe Latenstein BV (Nijmegen, The Netherlands) for the crude xylan preparation, Demian Snel for technical assistance, and K. Swart for strain N593.
J. C. Frisvad thanks the Danish Technical Research Council for financial support.
REFERENCES
- 1.Conesa, A., C. A. van den Hondel, and P. J. Punt. 2000. Studies on the production of fungal peroxidases in Aspergillus niger. Appl. Environ. Microbiol. 66:3016-3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Vries, R. P., B. Michelsen, C. H. Poulsen, P. A. Kroon, R. H. H. van den Heuvel, C. B. Faulds, G. Williamson, J. P. T. W. van den Hombergh, and J. Visser. 1997. The faeA genes from Aspergillus niger and Aspergillus tubingensis encode ferulic acid esterases involved in the degradation of complex cell wall polysaccharides. Appl. Environ. Microbiol. 63:4638-4644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Vries, R. P., P. J. I. van de Vondervoort, and J. Visser. 2002. Method for the production of proteins and polypeptides using fungal cells, in particular Aspergillus vadensis. Patent WO 02/02825 A1.
- 4.Dunn-Coleman, N. S., P. Bloebaum, R. M. Berka, E. Bodie, N. Robinson, G. Armstrong, M. Ward, M. Przetak, G. L. Carter, R. LaCost, et al. 1991. Commercial levels of chymosin production by Aspergillus. J. Biotechnol. 9:976-981. [DOI] [PubMed] [Google Scholar]
- 5.Finkelstein, D. B. 1987. Improvement of enzyme production in Aspergillus. Antonie Leeuwenhoek 53:349-352. [DOI] [PubMed] [Google Scholar]
- 6.Jones, L. J., R. H. Upson, R. P. Haugland, N. Panchuk-Voloshina, M. Zhou, and R. P. Haugland. 1997. Quenched BODIPY dye-labeled casein substrates for the assay of protease activity by direct fluorescence measurement. Anal. Biochem. 251:144-152. [DOI] [PubMed] [Google Scholar]
- 7.Kusters-van Someren, M. A., J. A. M. Harmsen, H. C. M. Kester, and J. Visser. 1991. The structure of the Aspergillus niger pelA gene and its expression in Aspergillus niger and Aspergillus nidulans. Curr. Genet. 20:293-299. [DOI] [PubMed] [Google Scholar]
- 8.MacKenzie, D. A., D. J. Jeenes, N. J. Belshaw, and D. B. Archer. 1993. Regulation of secreted protein production by filamentous fungi: recent developments and perspectives. J. Gen. Microbiol. 139:2295-2307. [DOI] [PubMed] [Google Scholar]
- 9.Parenicová, L., J. A. E. Benen, R. A. Samson, and J. Visser. 1997. Evaluation of RFLP analysis of the classification of selected black aspergilli. Mycol. Res. 101:810-814. [Google Scholar]
- 10.Punt, P. J., N. van Biezen, A. Conesa, A. Albers, J. Mangnus, and C. van den Hondel. 2002. Filamentous fungi as cell factories for heterologous protein production. Trends Biotechnol. 20:200-206. [DOI] [PubMed] [Google Scholar]
- 11.Ruijter, G. J. G., P. J. I. van de Vondervoort, and J. Visser. 1999. Oxalic acid production by Aspergillus niger: an oxalate-non-producing mutant produces citric acid at pH 5 and in the presence of manganese. Microbiology 145:2569-2576. [DOI] [PubMed] [Google Scholar]
- 12.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 13.Svetina, M., N. Krasevic, V. Gaberc-Porekar, and R. Komel. 2000. Expression of catalytic subunit of bovine enterokinase in the filamentous fungus Aspergillus niger. J. Biotechnol. 76:245-251. [DOI] [PubMed] [Google Scholar]
- 14.van den Hombergh, J. P., P. J. van de Vondervoort, L. Fraissinet-Tachet, and J. Visser. 1997. Aspergillus as a host for heterologous protein production: the problem of proteases. Trends Biotechnol. 15:256-263. [DOI] [PubMed] [Google Scholar]
- 15.van den Hombergh, J. P. T. W., M. D. Sollewijn Gelpke, P. J. I. van de Vondervoort, F. P. Buxton, and J. Visser. 1997. Disruption of three acid proteases in Aspergillus niger—effects on protease spectrum, intracellular proteolysis, and degradation of target proteins. Eur. J. Biochem. 247:605-613. [DOI] [PubMed] [Google Scholar]
- 16.van den Hombergh, J. P. T. W., P. J. I. van de Vondervoort, N. C. B. A. van der Heijden, and J. Visser. 1995. New protease mutants in Aspergillus niger result in strongly reduced in vitro degradation of target proteins; genetic and biochemical characterization of seven complementation groups. Curr. Genet. 28:299-308. [DOI] [PubMed] [Google Scholar]
- 17.Vishniac, W., and M. Santer. 1957. The thiobacilli. Bacteriol. Rev. 21:195-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wongwicharn, A., B. McNeil, and L. M. Harvey. 1999. Effect of oxygen enrichment on morphology, growth, and heterologous protein production in chemostat cultures of Aspergillus niger B1-D. Biotechnol. Bioeng. 20:416-424. [DOI] [PubMed] [Google Scholar]