Abstract
Background
Despite low HIV prevalence in the South Caucasus region, transmission is volatile. Little data are available from this region about addiction and infectious diseases among prisoners who transition back to communities.
Methods
A nation-wide randomly sampled biobehavioral health survey was conducted in 13 non-specialty Azerbaijani prisons among soon-to-be-released prisoners. After informed consent, participants underwent standardized health assessment surveys and testing for HIV, hepatitis B and C, and syphilis.
Results
Of the 510 participants (mean age = 38.2 years), 11.4% were female, and 31.9% reported pre-incarceration drug injection, primarily of heroin. Prevalence of HCV (38.2%), HIV (3.7%), syphilis (3.7%), and HBV (2.7%) was high. Among the 19 HIV-infected inmates, 14 (73.7%) were aware of their HIV status, 12 (63.2%) were receiving antiretroviral therapy (ART), and 5 (26.3%) had CD4 < 350 cells/mL (4 of these were on ART). While drug injection was the most significant independent correlate of HCV (AOR = 12.9; p = 0.001) and a significant correlate of HIV (AOR = 8.2; p = 0.001), both unprotected sex (AOR = 3.31; p = 0.049) and working in Russia/Ukraine (AOR = 4.58; p = 0.008) were also correlated with HIV.
Conclusion
HIV and HCV epidemics are concentrated among people who inject drugs (PWIDs) in Azerbaijan, and magnified among prisoners. A transitioning HIV epidemic is emerging from migration from high endemic countries and heterosexual risk. The high diagnostic rate and ART coverage among Azerbaijani prisoners provides new evidence that HIV treatment as prevention in former Soviet Union (FSU) countries is attainable, and provides new insights for HCV diagnosis and treatment as new medications become available. Within prison evidence-based addiction treatments with linkage to community care are urgently needed.
Keywords: Prisons, Azerbaijan, Substance abuse, HIV/AIDS, Hepatitis C virus, Opioid substitution therapy
1. Introduction
The Southern Caucuses, countries of the former Soviet Union (FSU) that includes Azerbaijan, Armenia, and Georgia, have low HIV prevalence (<0.3%), with HIV concentrated among most-at-risk groups (Joint United Nations Programme on HIV/AIDS (UNAIDS), 2014). Whereas HIV incidence is decreasing globally, the prevalence has increased 35-fold since 2000 in the Southern Caucuses (574–19,100 estimated cases in the region; (UNAIDS, 2012; Kvitsinadze et al., 2010). In Azerbaijan, adult HIV prevalence is 0.2%, primarily concentrated among people who inject drugs (PWIDs; Kvitsinadze et al., 2010). HIV prevalence among PWIDs ranges from 19% to 24% (Cook and Kanaef, 2008). Recent data, however, suggest emerging new cases among commercial sex workers (CSWs), heterosexual men and women, and migratory populations, indicating bridges to the general population through sexual contact (Ministry of Health of the Republic of Azerbaijan, 2014). Many of the vestiges of FSU infrastructure and drug-related policies persist in Azerbaijan, leaving it vulnerable to a volatile HIV epidemic in the absence of adequately scaled HIV prevention.
Prisons have long been sentinel sites for identifying emerging HIV epidemics because the individual and structural factors associated with HIV transmission (e.g., PWIDs, sexually transmitted infections (STIs), mental illness, homelessness, proscriptive drug policies) are concentrated among individuals who cycle through the criminal justice system (CJS; Altice et al., 2005, 1998; Flanigan et al., 2010; Springer and Altice, 2005). In Azerbaijan, 72% of people living with HIV/AIDS (PLWHA) have a history of imprisonment (United Nations Office on Drugs and Crime, 2010). Azerbaijan’s CJS, however, has not been harnessed to implement evidence-based interventions (EBIs) like opioid substitution therapy (OST) for treating substance use disorders (SUDs). OST prevents HIV transmission for at-risk individuals (Gowing et al., 2008) and improves HIV treatment outcomes for PLWHA (Altice et al., 2010b; Dutta et al., 2013; Kerr et al., 2004). OST, however, is highly restricted in the general population and unavailable in prisons (Vagenas et al., 2013), although the scale up has been under consideration for more than five years (United Nations Office on Drugs and Crime, 2010). We therefore conducted an extensive, nationally representative bio-behavioral survey of soon-to-be-released prisoners in Azerbaijan in order to identify their burden of disease, with a specific focus on inter-related factors that might foreshadow an emerging epidemic in a low HIV prevalence FSU country.
2. Methods
A nationally representative, bio-behavioral survey assessing health status, addiction and infectious diseases (HIV, hepatitis C, hepatitis B, and syphilis) was conducted in 13 prisons in Azerbaijan from February to May 2014.
2.1. Setting
Azerbaijan ranks 17th (413 prisoners per 100,000 population) globally in incarceration rate (Walmsley, 2014). Azerbaijan’s Penitentiary Service and its Prison Health Department (both under the Ministry of Justice) oversee the CJS and prisoner health in Azerbaijan, respectively. Pretrial detention and prison facilities are concentrated primarily in the capital, Baku. Inmates are housed in facilities of varying security levels, based on the severity of the crime committed, and women are housed separately from men. The Penitentiary Service includes 24 facilities housing ~18,000 inmates including 15 high-, medium-, and -mixed security prisons, two specialty prisons, three pre-trial detention centers (SIZO), one prison for women, one prison for juveniles, and two inpatient treatment units.
2.2. Selection of prisons and recruitment of participants
We sampled adult prisoners (18 years or older) within six months of release in the 16 non-specialized facilities that comprise 83.4% of Azerbaijan’s prisoners using a stratified random sampling scheme (Hunt and Tyrell, 2001), previously devised for FSU countries (Azbel et al., 2013); hospital and specialty prisons were excluded, as were juvenile and SIZO facilities (did not meet eligibility criteria of ≥18 years or being soon-to-be-released, respectively). Three medium security facilities were excluded (one of them was because it opened just before the study was completed).
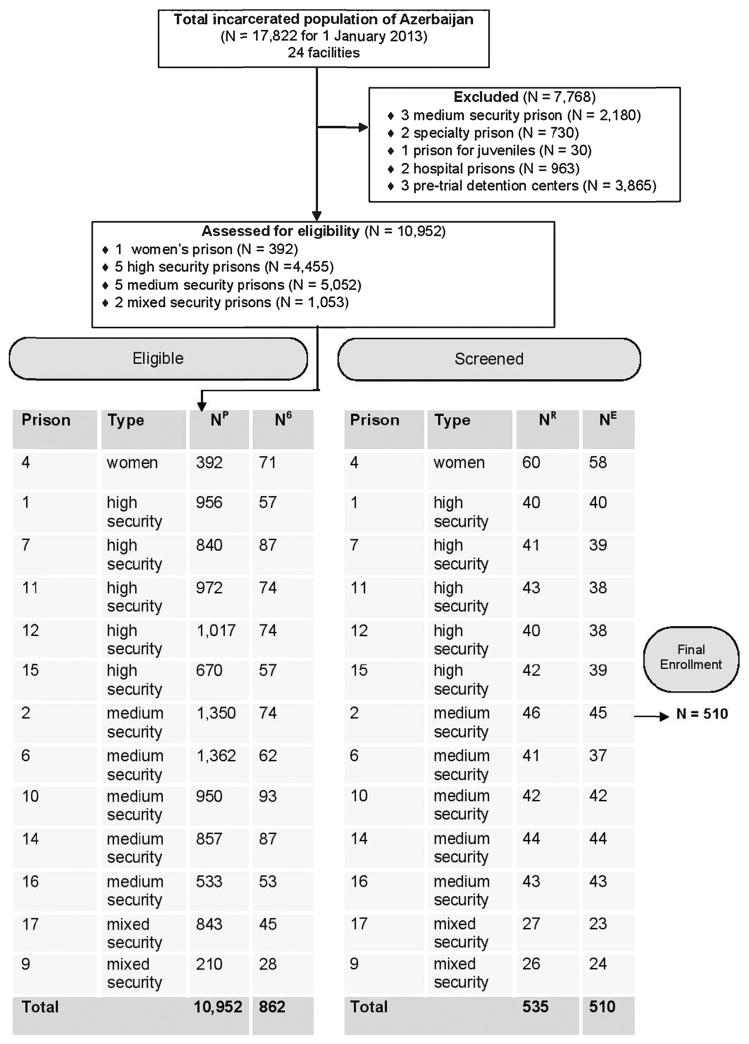
From an estimated 1,037 inmates in non-specialized facilities meeting eligibility criteria, we aimed to enroll 50% for our study, proportional to the number of prisoners within six months of release in each facility. Since women comprise only 2.2% of the overall prisoner population, they were oversampled to ensure adequate comparisons. The number enrolled from each facility is outlined in Fig. 1 and reflects the estimated proportion of prisoners in that type of facility six months pre-release based on the total number of prisoners in each facility.
Fig. 1.
Key: NP = number in prison population; N6 = number released in 6 months (18 months for women); NR = randomly chosen for consent; NE = number that gave consent; ♂ = male; ♀ = female. Reasons for non-participation: Already knew their health status (N = 7); cannot give blood (N = 7); did not provide a reason (N = 5); transferred to high security isolation unit (N = 4); felt too sick (N = 2).
The Ministry of Justice provided lists of all eligible prisoners in the selected facilities. Experienced research assistants (RAs) from a local NGO that works with prisoners underwent extensive training on study methods and confidentiality procedures. RAs used a random assignment chart to select participants who were informed by prison staff that they were randomly chosen for a voluntary and anonymous health study. Meetings between RAs and prisoners occurred in a private room on a one-by-one basis, in the absence of prison staff, where study details were discussed, followed by pre-test counseling for consented participants. Prisoners were informed that participation or continuation in the study would remain confidential and not influence status in any way. Prison staff was not informed about study participation or study results. After signed informed consent, she/he was assigned an anonymous personal identification number linked to her/his biobehavioral data. If not, her/his reason for non-participation was recorded without personal identifiers. Overall, 25 (4.7%) refused participation, with reasons provided in Fig. 1.
2.3. Data collection
Data collection occurred over three days using a previously described protocol (Azbel et al., 2013). Participants completed a self-administered behavioral interview using Audio-Computer-Assisted Self Survey Instruments (ACASI) on touch-screen laptop computers. ACASI enables anonymity, minimizes social desirability and reporting bias (Ghanem et al., 2005) and facilitates ethical principles of conducting research with prisoners. Participants were given the option to choose between English, Russian, or Azerbaijani versions of the instruments that had been translated and back-translated, according to standardized methods (Ware et al., 1995). Although adult literacy rate in Azerbaijan is 99.8%, survey questions were provided in written form and read aloud in a private room for those who did not have sufficient reading comprehension. All laptops included only the survey and no additional programs, files, or internet capability.
Participants then underwent phlebotomy, with specimen labeling including only the participant’s anonymous identification number. Serological tests were performed using commercially available quantitative chemiluminescent microparticle immunoassay (CMIA) Architect™ Combo for HIV-1/HIV-2 (specificity: 99.50%; sensitivity: 100%; Abbott Laboratories Tokyo, Japan), for hepatitis B surface antigen (specificity: 99.94%; sensitivity: 99.80%), for hepatitis C antibody (specificity: 99.60%; sensitivity: 99.10%), and for active syphilis Treponema pallidum antibody (specificity: 99.50%; sensitivity: 99.0). Initially positive HIV tests were confirmed with the Bio-Rad New Lav Blot 1 Western Blot (Bio-rad Laboratories, France). If both HIV tests were seropositive, reflex CD4T lymphocyte count assessment using the HumaCount CD4 was performed.
On the third day, participants reviewed their confidential HIV (including CD4), hepatitis B and C, and syphilis test results and underwent post-test counseling. They were offered voluntary referral to prison medical staff, received post-release referrals to care in the community, and were provided with a package of post-release resources. Though each participant was offered hygienic products totaling $10 USD for participation, most indicated their primary reason for participation was to receive the health assessment.
2.4. Variables and data analysis
Self-reported survey included: (1) socio-demographic characteristics; (2) imprisonment and detention history; (3) health-related quality of life (HRQoL) measured continuously using the Medical Outcomes Survey SF-36 (Ware and Sherbourne, 1992); (4) major depression symptoms defined dichotomously with a score ≥11 on the 10-item Clinical Epidemiological Survey of Depression CES-D 10 (Saunders et al., 1993) (5) alcohol use disorders defined by a score of ≥8 (men) or ≥4 (women) on the Alcohol Use Disorders Inventory Test (WHO AUDIT; Saunders et al., 1993); (6) sexual and drug risk behaviors; (7) anxiety measured using the Zung anxiety scale with a score ≥45 meeting criteria (Zung, 1971); (8) past and current history of chronic illnesses; (9) social support measured continuously using a standardized scale (Eaton et al., 2004); (10) substance use history assessed using items adapted from the Addiction Severity Index (McLellan et al., 2006, 1992); and goals for re-entry and likelihood of recidivism.
To avoid response bias and repercussions from prison officials, substance use and sexual risk behaviors were limited exclusively to six months prior to participants’ arrest. Substance use was defined as having used one or more of the following substances before arrest: barbiturates, illegal opioids, sedatives, cocaine, hallucinogens, or amphetamines. Polysubstance use was defined as having used two or more of the aforementioned substances in the same period. Participants were asked about the route of administration of each substance. Sexual risk behaviors included vaginal or anal intercourse without a condom with men and/or women in the six months prior to incarceration.
Statistical analyses were performed using SPSS (version 19.0). The t-test and χ2 for categorical and continuous variables were used. Bivariate and multivariate logistic regression analyses were performed to determine the independent correlates of HIV and hepatitis C infection. If correlates were significant at the bivariate level (p < 0.05), they were included in the multivariate logistic regression. Variables in the final model were checked for multicollinearity using the Variation Inflation Factor (VIF). Tolerance values in the final model were high (>0.90) and all VIF values were low (<1.30). Drug injection and sex without a condom were included in lieu of other sex and drug risk variables because they are most directly associated with HIV transmission and resulted in best goodness-of-fit. Variables that remained in the model were tested for interactions with each other. Goodness-of-fit for the final logistic regression was measured using the Aikake Information Criterion (AIC). To account for the stratified sampling design, and thereby ensure that study results were reflective of the entire soon-to-be-released prison population at the targeted facilities in Azerbaijan, model estimates were adjusted using weights calculated using the number of prisoners within six months of release in each facility (N6 in Fig. 1).
2.5. Ethics statement
Institutional Review Boards at the Ukrainian Institute on Public Health Policy and Yale University approved the study. Further safety assurances were provided by the Office for Human Research Protections (OHRP) in accordance with 45 CFR 46.305(c) “Prisoner Research Certification” requirements.
3. Results
3.1. Sociodemographic characteristics
The final sample (N = 510) represents 59% of all Azerbaijan’s soon-to-be-released prisoners in the selected facilities and 49% of all soon-to-be-released prisoners. As planned per our sampling strategy, high and medium security prisons each represent about 40% and mixed security prisons represent 10% of the 862 soon-to-be-released prisoners in the selected facilities. Similarly, women were over-sampled and account for about 10% of the sample (N = 58). About a third (N = 171) of the total sample were recidivists (i.e., previously imprisoned).
Table 1 presents the basic characteristics of the sample. The mean age was 38.2 years and the sample had been incarcerated, on average, for 3.3 years. More than half earned less than $5 USD daily (the international poverty rate) prior to their current incarceration, compared to 16% in the general population (The World Bank, 2014).
Table 1.
Characteristics of the study sample of prisoners (N = 510).
| N (%)a | |
|---|---|
| Demographics | |
| Mean age, years (range) | 38.2 (21–63) |
| Gender | |
| Male | 452 (88.6) |
| Female | 58 (11.4) |
| Azerbaijani ethnicity | 462 (90.6) |
| Criminal justice history | |
| Recidivists | 171 (33.5) |
| Mean number of previous incarcerations for recidivists (S.D.) | 1.6 (0.7) |
| Mean current incarceration duration, years (S.D.) | 3.3 (2.6) |
| Mean time before community release, months (S.D.) | 4.0 (2.4) |
| Socioeconomic indicators | |
| In a relationship | |
| Yes | 278 (54.5) |
| No | 220 (43.1) |
| Completed high school | 188 (36.9) |
| Below poverty line | 270 (52.9) |
| Mental health | |
| Mean CES-D score (S.D.) | 7.16 (3.73) |
| Major depression | 128 (25.1) |
| Mild to severe anxiety levels | 24 (4.7) |
| Social support scale score (S.D.) | 3.1 (1.2) |
| Health-related Quality of Life (SF-36) | |
| Mean physical composite score (S.D.) | 49.0 (4.3) |
| Mean mental composite score (S.D.) | 35.9 (6.2) |
| HIV | |
| HIV infected | 19 (3.7) |
| Mean CD4 count, cells/mL (N = 19) | 392.5 |
| CD4 > 350 | 14 (73.7) |
| CD4 ≤350 | 5 (26.3) |
| Currently prescribed antiretroviral therapy | 12 (63.2) |
Key: S.D. = standard deviation; CES-D = 10-item Clinical Epidemiological Scale for Depression.
Percent of those reporting.
3.2. Prevalence of infectious diseases and chronic illness
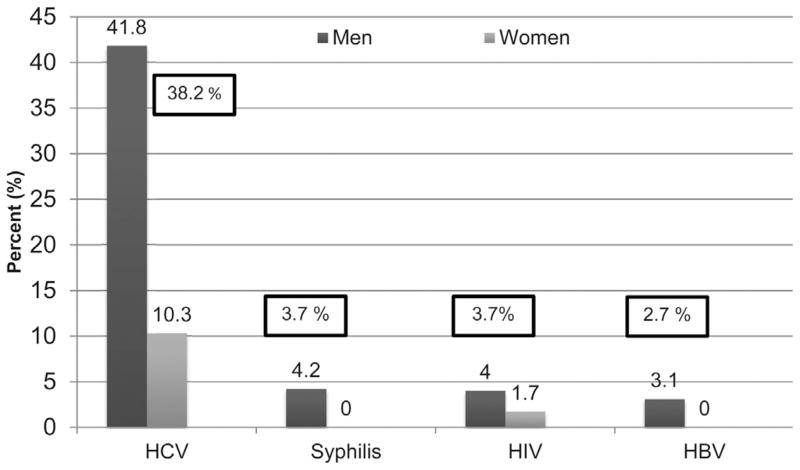
Fig. 2 shows the results of serological testing stratified by gender. Hepatitis C (N = 195, 38.2%) was most prevalent, followed by HIV (N = 19, 3.7%) and syphilis (N = 19, 3.7%), and hepatitis B (N = 14, 2.7%). All infectious diseases were significantly more prevalent among men. Nearly three-quarters (14 of 19) of the HIV-infected participants were aware of their HIV status prior to this study. The mean CD4 count was 392.5 cells/mL and 12 HIV-infected participants (63.2%) were currently prescribed antiretroviral therapy (ART). The mean CD4 count of those already aware and unaware of being HIV-infected was not significant (390.7 vs 397.6 cells/mL), but most aware patients were on ART.
Fig. 2.
Prevalence of infectious diseases. (N = 510).
Respondents were asked if a doctor had ever informed them that they had certain chronic conditions. Most commonly, they had been told they had depression (33%), hypertension (26%), and a heart condition (22%).
3.3. Substance use disorders and mental illness
One-quarter met symptomatic criteria for major depression and 4.7% met criteria for moderate to severe anxiety. On a social support scale ranging from 1 to 5, with 5 representing maximum social support, the mean score was 3.1.
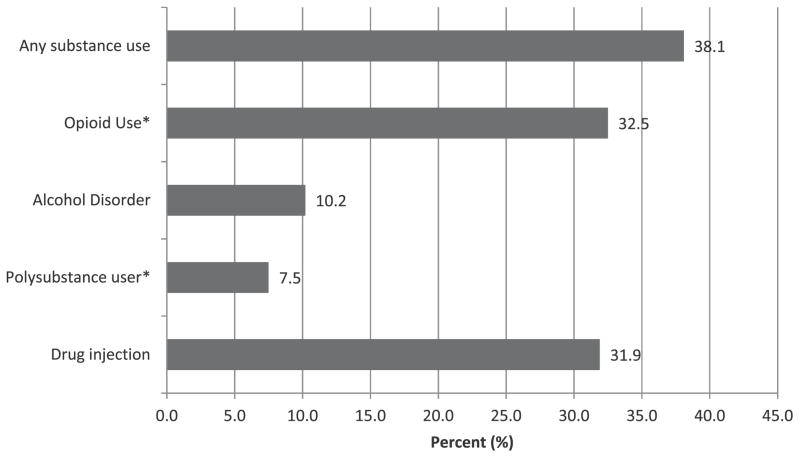
Fig. 3 depicts the prevalence of SUDs with drug administration route for the 30 days before incarceration. Overall, 38.1% met criteria for having a SUD, highest being opioid (32.5%) and lowest being alcohol use disorders (10.2%); 7.5% had used at least two substances, meeting criteria for polysubstance use. The overwhelming majority (76%) of those who had used any substance had used only one substance—all but two of whom had used opioids. There was evidence of concurrent lifetime dependencies: 60% of opioid users had an alcohol use disorder and 58% of opioid users had used amphetamines.
Fig. 3.
Prevalence of substance use disorders (N = 510).
3.4. Sex and drug risk behaviors
Nearly one-third of individuals (31.9%) reported having ever injected drugs. In the 30 days prior to their arrest and incarceration, 39.8% (N = 64) of injectors reported reusing injection equipment (a needle, syringe, or container) that had been used by someone else. Men were much more likely to have ever injected drugs (153 men vs 8 women) and 5.6% of those who had ever injected had used a syringe surrogate (e.g., ballpoint pen). Only 12 injectors (7.4%) reported receiving needles from a needle/syringe program (NSP) and only two injectors (one male and one female, both of whom stated they had been “satisfied” with the program) had ever been enrolled in community-based OST; however, 25% of PWIDs stated that they would like to be enrolled in OST, but only 13.4% agreed that OST should be available in the prison setting.
More than a third of the respondents (N = 166, 36.7%) reported having had sex without a condom in the 30 days prior to their arrest and incarceration. Of the male respondents, 17 had had unprotected anal sex with other men (approximately 10% of the total sampled males who had had sex without a condom); and two out of these 17 males were HIV positive. Four of the male respondents reported that they had engaged in unprotected sex with a partner they knew was HIV-positive. Women respondents were significantly (22% vs 4%; p < 0.001) more likely to engage in transactional sex than men.
3.5. Correlates of HIV and hepatitis C infection
Table 2 presents the bivariate and multivariate analyses of the correlates of HIV infection. The final model found previous STI diagnosis (sexual risk) and drug injection as the most significant correlates of HIV infection among prisoners. Additional sexual risks that were independently associated with HIV included unprotected anal or vaginal sex. Two other factors were associated with HIV, including meeting criteria for an anxiety disorder and having worked in Russia or Ukraine.
Table 2.
Correlates of HIV infection among soon-to-be-released prisoners in Azerbaijan (N = 510).
| Covariate | N (%)a | Bivariate analysis
|
Multivariate analysis
|
||||
|---|---|---|---|---|---|---|---|
| Unadjusted Odds Ratio | 95% C.I. | p-Value | Adjusted Odds Ratio | 95% C.I. | p-value | ||
| >36 years old | 279 (54.8) | 1.31 | 0.50–3.43 | 0.590 | – | – | – |
| ≤36 years old | 230 (45.2) | Ref | – | – | |||
| Recidivist | 339 (66.5) | 1.46 | 0.58–3.71 | 0.422 | – | – | – |
| First time offender | 171 (33.5) | Ref | – | – | |||
| Completed high school | 188 (36.9) | 3.36 | 0.97–11.68 | 0.057 | – | – | – |
| Income below poverty line | 269 (52.7) | 0.53 | 0.20–1.39 | 0.196 | – | – | – |
| Major depression | 129 (26.0) | 1.35 | 0.50–3.63 | 0.551 | – | – | – |
| Anxiety disorder | 24 (4.8) | 4.35 | 1.17–16.20 | 0.028* | 4.62* | 1.1–19.9* | 0.040* |
| Hazardous drinking | 52 (10.4) | 2.46 | 0.79–7.73 | 0.122 | – | – | – |
| Have worked abroad (in Russia or Ukraine) | 64 (12.9) | 3.62 | 1.31–10.01 | 0.013* | 4.58* | 1.5–14.1* | 0.008* |
| Told by a doctor they had a sexually transmitted infection | 30 (6.0) | 4.59 | 1.23–17.15 | 0.023* | 10.42* | 2.1–51.4* | 0.004* |
| Has partner | 278 (54.5) | 2.85 | 1.06–7.62 | 0.037* | – | – | – |
| Use in 30 days before incarceration | |||||||
| Any substance use | 154 (31.0) | 2.63 | 1.05–6.61 | 0.040* | – | – | – |
| Amphetamine use | 2 (0.4) | 0.00 | 0.00 | 0.999 | – | – | – |
| Opioid use | 151 (30.0) | 3.05 | 1.18–7.89 | 0.021* | – | – | – |
| Sedatives use | 13 (2.6) | 0.00 | 0.00 | 0.999 | – | – | – |
| Multiple-substance use | 38 (7.5) | 1.40 | 0.18–10.8 | 0.750 | – | – | – |
| Lifetime use | |||||||
| Injected drugs | 161 (31.9) | 5.94 | 2.08–17.0 | 0.001* | 8.24* | 2.4–28.4* | 0.001* |
| Any substance use | 169 (35.7) | 4.15 | 1.55–11.14 | 0.005* | – | – | – |
| Risk behavior | |||||||
| Reused syringe, container or needle | 65 (13.1) | 4.71 | 1.76–12.64 | 0.002* | – | – | – |
| Sex without condom | 50 (9.8) | 3.76 | 0.51–6.78 | 0.009* | 3.77* | 1.1–12.5* | 0.031* |
| Given money or drugs for sex | 27 (5.4) | 5.21 | 1.60–17.00 | 0.006* | 1.80 | 0.5–7.0 | 0.080 |
| Sex without condom under influence of drugs | 50 (9.8) | 3.39 | 1.02–11.26 | 0.047* | – | – | – |
| Tattoo from non-professional | 138 (73.8) | 1.37 | 0.53–3.53 | 0.514 | – | – | – |
| Akaike Information Criterion (AIC) | 277.8 | ||||||
Percent of those reporting.
Significant difference, defined as p ≤0.05.
Table 3 presents the correlates of hepatitis C infection. Similar to the correlates for HIV, a history of injecting drugs was a significant independent correlate of HCV infection (p < 0.001), associated with >12-fold odds of infection. Consistent with this, sharing injection equipment portends nearly a five-fold increased likelihood (p = 0.006) of having HCV infection. When controlling for other variables, meeting criteria for an alcohol use disorder or major depression, and female gender all emerge as independently associated with HCV; receiving a tattoo from a non-professional and prison recidivism did not emerge as significant in the final model.
Table 3.
Correlates of HCV infection among soon-to-be-released Azerbaijani prisoners (N = 510).
| Covariate | N (%)a | Bivariate analysis
|
Multivariate analysis
|
||||
|---|---|---|---|---|---|---|---|
| Unadjusted Odds Ratio | 95% C.I. | p-value | Adjusted Odds Ratio | 95% C.I. | p-value | ||
| Male | 452 (88.6) | 6.23 | 2.62–14.80 | <0.001* | 9.78* | 2.4–39.7* | 0.001* |
| Female | 58 (11.4) | Ref | – | – | Ref | – | – |
| Recidivist | 339 (66.5) | 1.84 | 1.27–2.68 | 0.001* | 0.76 | 0.44–1.31 | 0.315 |
| First time offender | 171 (33.5) | Ref | – | – | Ref | – | – |
| Completed high school | 188 (36.9) | 0.529 | 0.82–1.73 | 0.196 | – | – | – |
| Have worked abroad (in Russia or Ukraine) | 64 (12.9) | 1.10 | 0.64–1.87 | 0.736 | |||
| Income below poverty line | 269 (52.7) | 0.881 | 0.61–1.27 | 0.493 | – | – | – |
| Major depression | 129 (26.0) | 2.47 | 1.75–4.00 | <0.001* | 2.04* | 1.15–3.60* | 0.014* |
| Anxiety disorder | 24 (4.8) | 2.33 | 1.02–5.37 | 0.046* | 0.49 | 0.14–1.69 | 0.257 |
| Alcohol use disorder | 2 (10.2) | 4.42 | 2.35–8.32 | <0.001* | 3.33* | 1.46–7.59* | 0.004* |
| Use in 30 days before incarceration | |||||||
| Any substance use | 154 (31.0) | 13.91 | 8.75–22.11 | <0.001* | – | – | – |
| Amphetamine use | 2 (0.4) | 1.60 | 0.10–25.75 | 0.740 | – | – | – |
| Opioid use | 151 (30.0) | 15.69 | 9.74–25.26 | <0.001* | – | – | – |
| Sedatives use | 13 (2.6) | 3.72 | 1.13–12.26 | 0.031* | – | – | – |
| Reused syringe, container or needle | 65 (13.1) | 26.62 | 10.44–67.83 | <0.001* | 4.79* | 1.57–14.61* | 0.006* |
| Multiple-substance use | 38 (7.5) | 0.38 | 0.19–0.74 | 0.004* | – | – | – |
| Lifetime use | |||||||
| Injected drugs | 161 (31.9) | 20.63 | 12.68–33.57 | <0.001* | 12.85* | 7.09–23.29* | <0.001* |
| Any substance use | 169 (35.7) | 16.96 | 10.61–27.13 | <0.001* | – | – | – |
| Tattoo from non-professional | 138 (73.8) | 1.97 | 1.36–2.86 | <0.001* | 1.33 | 0.81–2.18 | 0.264 |
| Akaike Information Criterion (AIC) | 436.6 | ||||||
Significant difference, defined as p ≤0.05.
Percent of those reporting.
4. Discussion
This prison-based biosurveillance study is the first in Azerbaijan—a country with a low HIV prevalence concentrated among PWIDs. The prevalence of infectious diseases, HIV risk behaviors, and SUDs in our sample is high. For example, HIV prevalence is 18.5-fold greater among prisoners than found in the community (3.7% vs 0.2%). Our results are consistent with a concentrated HIV epidemic among PWIDs in Azerbaijani prisoners, similar to those in other FSU countries. At the same time, these data provide early evidence of the independent influence of sexual risks on HIV infection. Additionally, there are insights that other drivers of HIV in Azerbaijan are contributing to a growing epidemic, including migration to and from high HIV endemic countries (i.e., Russia and Ukraine) as well as having underlying mental illness.
The data presented in this study, showing an HIV prevalence 50% greater than previously reported (WHO/UNAIDS/UNICEF, 2010), portends concerns of a more volatile, transitioning HIV epidemic. Indeed, the findings correspond closely with the burgeoning heterosexual HIV transmission pathway in Azerbaijan through which almost a quarter of new HIV cases are contracted (Kvitsinadze et al., 2010). For example, a previous diagnosis of a STI raised the odds of HIV infection by a factor of 10 in our sample, and 14 of the 19 cases of diagnosed syphilis were unaware of being infected. Prior unprotected sex was a strong correlate of HIV infection, and almost a quarter of women (21.6%) engaged in unprotected transactional sex, potentially facilitating the spread of HIV and other STIs. These findings are especially alarming given the major STI epidemic currently underway in FSU countries (Borisenko et al., 1999; Uuskula et al., 2010).
While systematic approaches to address the co-occurring HIV and substance abuse epidemics in Central Asia are currently limited (Vagenas et al., 2013), these data from Azerbaijan provide some of the first evidence that HIV Treatment as Prevention (TasP) goals are attainable in prison settings within a FSU country. Unlike in Ukraine where <50% knew their HIV status and almost none were on ART (Azbel et al., 2014), nearly three-quarters of HIV-infected prisoners t knew their status and 63.2% were receiving ART. This performance was likely facilitated by a 2006 scale-up of ART in prisons, and is corroborated by an evaluation which found prisoners comprised 25.3% (N = 238) of the 941 PLWHA receiving ART in Azerbaijan in 2011 (Gadirova and Alakbarov, 2011). Despite this optimism, there is still room for improved HIV detection, linkage to care, and deployment of evidence-based continuity to care interventions post-release (Thompson et al., 2012).
While ART coverage is relatively high in Azerbaijan, scale-up of effective HIV prevention strategies like OST and NSPs is far below even low target levels (Vickerman et al., 2014). In our sample, only two and 12 inmates had ever utilized OST or NSPs, respectively. One potential solution to enhance HIV prevention and treatment is to introduce OST for prisoners with opioid use disorders and maintain it post-release (Haig, 2003; Kinlock et al., 2009; Wickersham et al., 2013a, 2013b). Despite OST having been introduced in Azerbaijan as a pilot project in 2004, the program has not been expanded and is not available within prisons with only 137 people receiving OST in 2013 (Ministry of Health of the Republic of Azerbaijan, 2014). Recent modeling from FSU countries like Azerbaijan where the HIV prevalence among PWIDs is low suggest that OST and NSP coverage can remain as low as 23–34% in order to curtail the HIV epidemic (Vickerman et al., 2014).
The extraordinarily high HCV prevalence in prisoners, primarily associated with drug injection, has important implications for HCV treatment. The first critical step of the HCV treatment cascade, diagnosis, is relatively poor (30.2%) and will require expanded HCV testing. The long duration of incarceration is certainly sufficient to treat patients with interferon-based therapies, but these treatments are unduly long, are associated with numerous adverse side effects, and have relatively low cure rates (Berenguer et al., 2009). Newer well-tolerated, highly efficacious direct-acting antivirals are currently inaccessible in Azerbaijan, as in many low- and middle-income countries due to price constraints. Evidence of significantly reduced pricing is emerging, however, including availability of generic medications (Hill et al., 2014). Moreover, these data suggest that there are over 7,000 prisoners infected with HCV and treatment would require monumental efforts, but could potentially be achieved within the structured prison setting. Without treatment, 330 HCV-infected prisoners would be released without treatment within the next six months and, in the absence of evidence-based harm reduction programs, will continue to fuel HCV transmission.
Our data also provide a glimpse into the role of migration between Azerbaijan and FSU countries of Russia and Ukraine, with our finding of migration as an independent contributor to the Azerbaijani HIV epidemic. Earlier data suggest that international long distance truck drivers, many of whom inject drugs, have high HIV prevalence (Botros et al., 2009). Their drug use persists when in Azerbaijan, resulting in incarceration. An estimated 500,000 Azerbaijanis seeking employment opportunities in Russia (Paul, 2014), which has one of the world’s fastest growing HIV epidemics (Degenhardt et al., 2013; UNAIDS, 2012; Kazatchkine, 2014; Zabransky et al., 2014). This migration from high to lower endemic countries, in the setting of drug injection, presents a potential bridge that fuels community transmission and should present an opportunity for targeted interventions.
Despite the many important findings from this study, the cross-sectional study design has inherent limitations. Although our study successfully describes correlates of infectious diseases, it provides no insight as to the temporal or causal nature of the observed relationships. In addition, observational studies are vulnerable to confounding such that some of the correlations reported herein cannot be substantiated (i.e., correlation between HIV and anxiety or hepatitis C and depression) and best elucidated with longitudinal designs. Moreover, recall bias from remote pre-incarceration events and/or under reporting bias about potentially stigmatizing conditions and HIV risk behaviors limit interpretation. Clearly, further analyses to disentangle possible mediating and moderating relationship among the variables, as well as longitudinal designs that would allow establishing causality are warranted.
The dearth of evidence-based harm reduction programs (OST and NSPs) in Azerbaijani communities and prisons generates a high-risk injection environment furthering HIV transmission, which is compounded in the setting of high-risk sex, drug use, and the presence of STIs. Though incarceration itself should be replaced where possible with non-custodial alternatives like community-based evidence-based treatment with OST, prisons still present a seminal platform for the prevention, detection, and treatment of diseases in this vulnerable population, which is otherwise largely missed by existing community treatment and prevention services. The HIV epidemic in Azerbaijan is still low enough that scaling up ART, OST and NSPs to as low as 25–35% would be sufficient to contain and even reduce HIV incidence. Despite this, the prison setting in Azerbaijan has not been fully harnessed to implement evidence-based interventions, which would significantly improve the health, not only of inmates, but also of their community contacts. Since the transition to the community is critical to ending the cycle of relapse and recidivism, efforts must be concentrated on the time before release to establish comprehensive harm reduction and treatment programs with linkage to community care.
Acknowledgments
Role of funding source
This research received funding from the National Institute on Drug Abuse for research (R01 DA029910, Altice, PI and R01 DA033679), career development (K24 DA017072 for Altice and K01 DA038529 for Wickersham). This work was also supported in part by the Doris Duke Charitable Foundation through a grant supporting the Doris Duke International Clinical Research Fellows Program at Yale University School of Medicine. Wegman is a Doris Duke International Clinical Research Fellow.
Footnotes
Contributors
FLA and JAW conceived and designed the study along with SD and LA, analyzed the data with LA and revised the manuscript in association with SD, MP, and MW. LA was responsible for performing the experiments along with JAW and wrote the manuscript together with MW and MP.
Conflict of interest
No conflict declared.
References
- Altice FL, Kamarulzaman A, Soriano VV, Schechter M, Friedland GH. Treatment of medical, psychiatric, and substance-use comorbidities in people infected with HIV who use drugs. Lancet. 2010b;376:367–387. doi: 10.1016/S0140-6736(10)60829-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altice FL, Marinovich A, Khoshnood K, Blankenship KM, Springer SA, Selwyn PA. Correlates of HIV infection among incarcerated women: implications for improving detection of HIV infection. J Urban Health. 2005;82:312–326. doi: 10.1093/jurban/jti055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altice FL, Mostashari F, Selwyn PA, Checko PJ, Singh R, Tanguay S, Blanchette EA. Predictors of HIV infection among newly sentenced male prisoners. J Acquir Immune Defic Syndr. 1998;18:444–453. doi: 10.1097/00042560-199808150-00005. [DOI] [PubMed] [Google Scholar]
- Azbel L, Wickersham JA, Grishaev Y, Dvoryak S, Altice FL. Burden of infectious diseases, substance use disorders, and mental illness among Ukrainian prisoners transitioning to the community. PLOS ONE. 2013;8:e59643. doi: 10.1371/journal.pone.0059643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azbel L, Wickersham JA, Grishaev Y, Dvoryak S, Altice FL. Correlates of HIV infection and being unaware of HIV status among soon-to-be-released Ukrainian prisoners. J Int AIDS Soc. 2014;17:19005. doi: 10.7448/IAS.17.1.19005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenguer J, Alvarez-Pellicer J, Martin PM, Lopez-Aldeguer J, Von-Wichmann MA, Quereda C, Mallolas J, Sanz J, Tural C, Bellon JM, Gonzalez-Garcia J GESIDA3603 /5607 Study Group. Sustained virological response to interferon plus ribavirin reduces liver-related complications and mortality in patients coinfected with human immunodeficiency virus and hepatitis C virus. Hepatology. 2009;50:407–413. doi: 10.1002/hep.23020. [DOI] [PubMed] [Google Scholar]
- Borisenko KK, Tichonova LI, Renton AM. Syphilis and other sexually transmitted infections in the Russian Federation. Int J STD AIDS. 1999;10:665–668. doi: 10.1258/0956462991913240. [DOI] [PubMed] [Google Scholar]
- Botros BA, Aliyev QM, Saad MD, Michael AA, Sanchez JL, Carr JK, Earhart KC. HIV infection and associated risk factors among long-distance truck drivers travelling through Azerbaijan. Int J STD AIDS. 2009;20:477–482. doi: 10.1258/ijsa.2008.008396. [DOI] [PubMed] [Google Scholar]
- Cook C, Kanaef N. Global State of Harm Reduction 2008: Mapping the Response to Drug-related HIV and Hepatitis C Epidemics. International Harm Reduction Program; London: 2008. [Google Scholar]
- Degenhardt L, Whiteford HA, Ferrari AJ, Baxter AJ, Charlson FJ, Hall WD, Freedman G, Burstein R, Johns N, Engell RE, Flaxman A, Murray CJ, Vos T. Global burden of disease attributable to illicit drug use and dependence: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382:1564–1574. doi: 10.1016/S0140-6736(13)61530-5. [DOI] [PubMed] [Google Scholar]
- Dutta A, Wirtz A, Stanciole A, Oelrichs R, Semini I, Baral S, Pretorius C, Haworth C, Hader S, Beyrer C, Cleghorn F. The Global HIV Epidemics among People Who Inject Drugs. World Bank; Washington, DC: 2013. http://dx.doi.org/10.1596/1978-1590-8213-9776-1593. [Google Scholar]
- Eaton WW, Muntaner C, Smith C, Tien A, Ybarra M. The Use of Psychological Testing for Treatment Planning and Outcomes Assessment. Mawah, NJ: 2004. [Google Scholar]
- Flanigan T, Zaller N, Beckwith CG, Bazerman LB, Rana A, Gardner A, Wohl DA, Altice FL. Testing for HIV, sexually transmitted infections, and viral hepatitis in jails: still a missed opportunity for public health and HIV prevention. J Acquir Immune Defic Syndr. 2010;55:S78–S83. doi: 10.1097/QAI.0b013e3181fbc94f. [DOI] [PubMed] [Google Scholar]
- Gadirova HAE, Alakbarov F. Antiretroviral Therapy in Penitentiary System of Azerbaijan. Poster at IAS Conference on HIV Pathogenesis.2011. [Google Scholar]
- Ghanem KG, Hutton HE, Zenilman JM, Zimba R, Erbelding EJ. Audio computer assisted self interview and face to face interview modes in assessing response bias among STD clinic patients. Sex Transm Infect. 2005;81:421–425. doi: 10.1136/sti.2004.013193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowing L, Farrell M, Bornemann R, Sullivan L, Ali R. Substitution treatment of injecting opioid users for prevention of HIV infection. Cochrane Database Syst Rev. 2008:CD004145. doi: 10.1002/14651858.CD004145.pub3. [DOI] [PubMed] [Google Scholar]
- Haig T. Randomized controlled trial proves effectiveness of methadone maintenance treatment in prison. Can HIV AIDS Policy Law Rev. 2003;8:48. [PubMed] [Google Scholar]
- Hill A, Khoo S, Fortunak J, Simmons B, Ford N. Minimum costs for producing hepatitis C direct-acting antivirals for use in large-scale treatment access programs in developing countries. Clin Infect Dis. 2014;58:928–936. doi: 10.1093/cid/ciu012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt N, Tyrell S. [accessed 04.10.12];Stratified Sampling. 2001 http://www.coventry.ac.uk/ec/~nhunt/meths/strati.html.
- Joint United Nations Programme on HIV/AIDS (UNAIDS) The Gap Report. Geneva, Switzerland: 2014. [accessed 24.08.14]. Available at: http://www.unaids.org/en/media/unaids/contentassets/documents/unaidspublication/2014/unaidsgapreporten.pdf. [Google Scholar]
- Kazatchkine M. Drug use, HIV, HCV and TB: major interlinked challenges in Eastern Europe and Central Asia. J Int AIDS Soc. 2014;17:19501. doi: 10.7448/IAS.17.4.19501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr T, Wodak A, Elliott R, Montaner JS, Wood E. Opioid substitution and HIV/AIDS treatment and prevention. Lancet. 2004;364:1918–1919. doi: 10.1016/S0140-6736(04)17490-4. [DOI] [PubMed] [Google Scholar]
- Kinlock TW, Gordon MS, Schwartz RP, Fitzgerald TT, O’Grady KE. A randomized clinical trial of methadone maintenance for prisoners: results at 12 months postrelease. J Subst Abuse Treat. 2009;37:277–285. doi: 10.1016/j.jsat.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvitsinadze L, Tvildiani D, Pkhakadze G. HIV/AIDS prevalence in the Southern Caucasus. Georgian Med News. 2010:26–36. [PubMed] [Google Scholar]
- McLellan AT, Cacciola JC, Alterman AI, Rikoon SH, Carise D. The Addiction Severity Index at 25: origins, contributions and transitions. Am J Addict. 2006;15:113–124. doi: 10.1080/10550490500528316. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M. The fifth edition of the addiction severity index. J Subst Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- Ministry of Health of the Republic of Azerbaijan. Reporting Period; National Report on Monitoring Progress towards the UNGASS Declaration of Commitment on HIV/AIDS; January 2012–December 2013; Baku. 2014. [Google Scholar]
- Paul A. Azerbaijan and the Two EUs. Today’s Zaman; Istanbul, Turkey: 2014. [accessed 05.09.14]. Available at: http://www.todayszaman.com/columnist/amanda-paul/azerbaijan-and-the-two-eus352182.html. [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption – II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- Springer SA, Altice FL. Managing HIV/AIDS in correctional settings. Curr HIV/AIDS Rep. 2005;2:165–170. doi: 10.1007/s11904-005-0011-9. [DOI] [PubMed] [Google Scholar]
- The World Bank. Azerbaijan Partnership Program Snapshot. Geneva: 2014. [Google Scholar]
- Thompson MA, Mugavero MJ, Amico KR, Cargill VA, Chang LW, Gross R, Orrell C, Altice FL, Bangsberg DR, Bartlett JG, Beckwith CG, Dowshen N, Gordon CM, Horn T, Kumar P, Scott JD, Stirratt MJ, Remien RH, Simoni JM, Nachega JB. Guidelines for improving entry into and retention in care and antiretroviral adherence for persons with HIV: evidence-based recommendations from an International Association of Physicians in AIDS Care panel. Ann Intern Med. 2012;156:817–833. doi: 10.7326/0003-4819-156-11-201206050-00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNAIDS. Otchetnost’ o dostupnosti progresse v osushchestvlenii global’nykh mer v otvet na SPID: 2010–2011 2012 [Google Scholar]
- United Nations Office on Drugs and Crime. Accessibility of HIV Prevention, Treatment and Care Services for People who Use Drugs and Incarcerated People in Azerbaijan, Kazakhstan, Kyrgyzstan, Tajikistan, Turkmenistan and Uzbekistan: Legislative and Policy Analysis and Recommendations for Reform. United Nations Office on Drugs and Crime, Regional Office for Central Asia; 2010. [Google Scholar]
- Uuskula A, Puur A, Toompere K, DeHovitz J. Trends in the epidemiology of bacterial sexually transmitted infections in eastern Europe, 1995–2005. Sex Transm Infect. 2010;86:6–14. doi: 10.1136/sti.2009.037044. [DOI] [PubMed] [Google Scholar]
- Vagenas P, Azbel L, Polonsky M, Kerimi N, Mamyrov M, Dvoryak S, Altice FL. A review of medical and substance use co-morbidities in Central Asian prisons: implications for HIV prevention and treatment. Drug Alcohol Depend. 2013;132(Suppl 1):S25–S31. doi: 10.1016/j.drugalcdep.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickerman P, Platt L, Jolley E, Rhodes T, Kazatchkine MD, Latypov A. Controlling HIV among people who inject drugs in Eastern Europe and Central Asia: insights from modelling. Int J Drug Policy. 2014;25:1163–1173. doi: 10.1016/j.drugpo.2014.09.013. [DOI] [PubMed] [Google Scholar]
- Walmsley R. World Prison Population List. 10. University of Essex; London, England: 2014. [accessed 14.07.14]. Available at: http://www.prisonstudies.org/sites/prisonstudies.org/files/resources/downloads/wppl2010.pdf. [Google Scholar]
- Ware JE, Jr, Keller SD, Gandek B, Brazier JE, Sullivan M. Evaluating translations of health status questionnaires, methods from the IQOLA project. International Quality of Life Assessment. Int J Technol Assess Health Care. 1995;11:525–551. doi: 10.1017/s0266462300008710. [DOI] [PubMed] [Google Scholar]
- Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- WHO/UNAIDS/UNICEF. Towards Universal Access: Scaling Up Priority HIV/AIDS Interventions in the Health Sector. Geneva: 2010. [Google Scholar]
- Wickersham JA, Marcus R, Kamarulzaman A, Zahari MM, Altice FL. Implementing methadone maintenance treatment in prisons in Malaysia. Bull World Health Organ. 2013a;91:124–129. doi: 10.2471/BLT.12.109132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickersham JA, Zahari MM, Azar MM, Kamarulzaman A, Altice FL. Methadone dose at the time of release from prison significantly influences retention in treatment: implications from a pilot study of HIV-infected prisoners transitioning to the community in Malaysia. Drug Alcohol Depend. 2013b;132:378–382. doi: 10.1016/j.drugalcdep.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabransky T, Mravcik V, Talu A, Jasaitis E. Post-Soviet Central Asia: a summary of the drug situation. Int J Drug Policy. 2014;25:1186–1194. doi: 10.1016/j.drugpo.2014.05.004. [DOI] [PubMed] [Google Scholar]
- Zung WW. A rating instrument for anxiety disorders. Psychosomatics. 1971;12:371–379. doi: 10.1016/S0033-3182(71)71479-0. [DOI] [PubMed] [Google Scholar]