Abstract
Objective
To investigate the association of hippocampal sclerosis (HS) with TAR-DNA binding protein of 43 kDa (TDP-43) and other common age-related pathologies, dementia, probable Alzheimer’s disease (AD), mild cognitive impairment (MCI) and cognitive domains in community-dwelling older subjects.
Methods
Diagnoses of dementia, probable AD and MCI in 636 autopsied subjects from the Religious Order Study and the Rush Memory and Aging Project were based on clinical evaluation and cognitive performance tests. HS was defined as severe neuronal loss and gliosis in the hippocampal CA1and/or subiculum. The severity and distribution of TDP-43 was assessed and other age-related pathologies were also documented.
Results
HS was more common in those aged > 90 years (18.0%) compared to younger subjects (9.2%). HS cases commonly coexisted with TDP-43 pathology (86%), which was more severe (p < 0.001) in HS cases. Although, HS also commonly coexisted with AD and Lewy body (LB) pathology; only TDP-43 pathology increased the odds of HS (OR=2.63; 95% CI 2.07-3.34). In logistic regression models accounting for age, TDP-43 and other common age-related pathologies; HS cases had higher odds of dementia (OR=3.71; 95% CI=1.93-7.16), MCI and probable AD (OR=3.75; 95% CI=2.01-7.02). In linear regression models, including an interaction term for HS and TDP-43 pathology; HS with coexisting TDP-43 was associated with lower function in multiple cognitive domains while HS without TDP-43 did not have statistically significant associations. TDP-43 without HS was separately related to lower episodic memory.
Interpretation
The combined role of hippocampal sclerosis and TDP-43 pathology are significant factors underlying global cognitive impairment and probable AD in older subjects.
Hippocampal sclerosis (HS) refers to the pathology of severe neuronal loss and astrogliosis of CA1 and/or subiculum. HS was first noted on macroscopic examination of brain of 8 epilepsy cases in 18251, but has been increasingly recognized in cases of dementia where it is often linked with neurodegenerative diseases particularly Alzheimer’s disease (AD), 2-4 and frontotemporal lobar degeneration (FTLD)3,5 as well as vascular diseases.3,4,6-8 Occasionally, HS is detected in the absence of apparent degenerative or vascular disease,2,4,9,10 in which case it has been variably called pure HS 11 or HS dementia.12 HS is also increasingly recognized in aging particularly the oldest old, with or without AD, and these cases have been described as HS of aging.13-15
Most data in regards to HS and dementia in aging are obtained from clinic studies which suggest that HS is associated with dementia.4,9,16 There are little data about the independent contribution of HS to the likelihood of dementia and a diagnosis of mild cognitive impairment (MCI) or clinical AD in community dwelling subjects. Moreover, the cognitive profile of those with HS compared to AD and other diseases is ill-defined, with limited neuropsychological testing data that do not provide information on the extent of involvement of specific cognitive domains.14,17 Recently, the TAR-DNA binding protein of 43 kDa (TDP-43) has been reported in 89%-90% of HS cases;14,18 however there are limited data on the severity, distribution and role of TDP-43 pathology in HS.
In this study, the hypothesis that HS is related to dementia, a clinical diagnosis of probable AD or MCI, and to impaired episodic memory was tested in 636 autopsied subjects (358 <90 and 278 ≥ 90 years) from the Religious Order Study (ROS) and the Rush Memory and Aging Project (MAP). The relation between HS and other age-related pathologies and the role of TDP-43 pathology were also investigated.
Methods
Participants
Data were from 2 clinical-pathological cohort studies of aging and dementia: ROS (n=296) 19 and Rush MAP (n=340) 20 which were approved by the institutional review board of Rush University Medical Center. Each subject signed an informed consent for annual examination and an Anatomic Gift Act for organ donation at the time of death. The ante-mortem and post-mortem data collection was similar in both studies allowing combined analyses of the cohorts.
Clinical evaluation and assessment of cognition
Each subject underwent a baseline uniform structured clinical evaluation, which included a medical history, neuropsychological testing (see below), and a neurological examination. Annual follow-up clinical evaluations were identical to the baseline evaluations in all essential details. Cognitive function was evaluated at baseline and each follow-up evaluation using a standardized battery of 20 cognitive performance tests. All neuropsychological data were reviewed by a neuropsychologist blinded to previously collected data. The Mini-Mental State Examination (MMSE) was used for descriptive purposes and Complex Ideational Material was only used for diagnostic classification. The remaining 18 tests were selected to assess five domains of cognitive function including episodic, semantic, and working memory, perceptual speed and visuospatial ability as described previously.21 Measurement error was minimized by using composite measures derived by standardization of the raw scores of the individual tests to z scores using the baseline mean and standard deviation of the entire pooled cohorts. The z scores of the 18 tests were averaged to compute the global cognitive function score while summary scores of the five specific cognitive domains were derived similarly by averaging the z scores from tests in a specific domain (Table 1).
Table 1.
Clinical pathologic characteristics and TDP-43 stages by presence of hippocampal sclerosis
| Hipocampal Sclerosis | |||
|---|---|---|---|
|
Not present n=553 |
Present n= 83 |
p- Vallue | |
| Age at death, y, mean (SD) | 88.3 (6.6) | 90.9 (6.0) | < 0.001 |
| Female, n (%) | 370 (85.9) | 61 (14.2) | .231 |
| Education, mean (SD) | 16.1 (3.6) | 16.0 (3.6) | .854 |
| Clinical Characteristics | |||
| No cognitive impairment, n (%) | 179 (35.1) | 7 (9.0) | < 0.001 |
| Mild cognitive impairment, n (%) | 149 (29.2) | 10 (12.8) | |
| Probable AD, n (%) | 182 (35.7) | 61 (78.2) | |
| Dementia, n (%) | 221 (40.3) | 64 (79.0) | < 0.001 |
| Cognitive function tests proximate to death, mean (SD) | |||
| MMSE score | 21.3 (8.9) | 13.8 (8.9) | < 0.001 |
| Global Cognition | −0.82 (1.1) | −1.72 (1.1) | |
| Episodic memory | −0.79 (1.3) | −2.03 (1.3) | |
| Semantic memory | −0.75 (1.2) | −1.90 (1.8) | |
| Working memory | −0.56 (1.0) | −0.91 (1.1) | |
| Visuospatial ability | −0.65 (1.1) | −1.22 (1.3) | |
| Perceptual speed | −1.18 (1.2) | −1.85 (1.1) | |
| Pathologic Characteristics | |||
| AD (NIA-Reagan), n (%) | 326 (59.0) | 66 (79.5) | < 0.001 |
| Lewy bodies, n (%) | 117 (21.2) | 27 (32.5) | 0.021 |
| Macroinfarcts, n (%) | 199 (36.0) | 35 (42.2) | 0.276 |
| Microinfarcts, n (%) | 151 (27.3) | 29 (34.9) | 0.150 |
| Arteriolosclerosis, n (%) | 420 (76.4) | 67 (80.7) | 0.380 |
| Atherosclerosis, n (%) | 475 (86.1) | 70 (84.3) | 0.677 |
| TDP-43 pathology, Stages | |||
| Stage 0, n (%) | 289 (52.3) | 12 (14.5) | < 0.001 |
| Stage 1, n (%) | 99 (17.9) | 7 (8.4) | |
| Stage 2, n (%) | 109 (19.7) | 23 (27.7) | |
| Stage 3, n (%) | 56 (10.1) | 41 (49.4) | |
t-test or chi-square test as appropriate
The diagnoses of dementia and AD were based on the recommendation of the joint working group of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association.22 Following death, a board-certified neurologist, blinded to pathologic data, reviewed all clinical information to render a classification of dementia proximate-to-death. The diagnosis of probable AD required a history of cognitive decline and evidence of impairment in episodic memory and at least one additional cognitive domain. MCI referred to those subjects rated as cognitively impaired by the neuropsychologist but not demented by the examining neurologist as described previously.23
Post-mortem evaluation
Completed post-mortem data on HS were available for 1052 subjects. The frequency of HS in these cases was 7.9%. Due to the low frequency of HS cases, all HS cases (n=83), and a consecutive series of cases without HS (n=553) but with complete TDP-43 data analyses were used in this study. The average post-mortem interval was 8.4 hrs (SD=7.4). A complete neuropathological evaluation including macroscopic examination of the brain, block selection, and microscopy was performed as described previously.24,25 The age, volume, and anatomic location of all macroscopic infarcts were documented. Sections (6 μm) were stained with hematoxylin and eosin and assessed by a neuropathologist blinded to age and all clinical data. The location and age of all microscopic infarcts were documented and only chronic macro and microinfarcts were included in the analyses as dichotomous variables. Arteriolosclerosis was assessed in the basal ganglia and atherosclerosis in vessels at the base of the brain using a semiquantitative grading system from 0 (none) to 6 (severe) as described previously.26 HS was evaluated unilaterally in a coronal section of the mid-hippocampus at the level of the lateral geniculate body, and graded as absent or present based on severe neuronal loss and gliosis in CA1and/or subiculum. Involvement of other sectors was also documented.
AD pathology was defined by the NIA-Reagan criteria 27 with intermediate and high likelihood cases indicating a pathologic diagnosis of AD as described previously.25 Modified Bielschowsky silver stain was used to assess AD pathology and manual counts of neuritic and diffuse plaques and neurofibrillary tangles were used to create a summary measure of AD pathology 24 for use in analyses.
Immunohistochemical studies
The Bond™ Polymer Refine Detection Kit and a Leica-Bond Max autostainer (Leica Microsystems Inc., New Buffalo, IL) were used. TDP-43 immunohistochemistry was performed on sections of the amygdala, hippocampus (CA1 and dentate), midfrontal, midtemporal and entorhinal cortices using a rat phosphorylated monoclonal TAR5P-1D3 (pS409/410; 1:100, Ascenion, Munich, Germany) TDP-43 antibody 28. Three stages of TDP-43 distribution were recognized (Stage 1 – localized to amygdala; Stage 2 extension to hippocampus and /or entorhinal cortex; Stage 3 – extension to the neocortex) and the severity of the TDP-43 cytoplasmic inclusions in neurons and glia were rated on a 6-point scale (Table 2) as described previously.29 In a subset of HS cases that did not have a pathologic diagnosis of AD (n=18), immunostaining was also done for phosphorylated tau, ubiquitin, fused in sarcoma protein and TDP-43 for detection of FTLD. Lewy bodies (LB) were detected in 6 regions as described previously 29 and recorded and analyzed as a dichotomous variable.
Table 2.
Frequency of TDP scores (0-5) in subjects with TDP-43 pathology by presence of hippocampal sclerosis
| Brain Regions | Frequency of TDP-43 cytoplasmic inclusions in scores 0-5 /brain region | p-value | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No Hippocampal Sclerosis (n=244) | Hippocampal Sclerosis present (n=71) | ||||||||||||
| 0 | 1 | 2 | 3 | 4 | 5 | 0 | 1 | 2 | 3 | 4 | 5 | ||
| Amygdala, n, % | 10 4.5 |
64 29.0 |
44 19.9 |
43 19.5 |
22 10.0 |
38 17.2 |
0.0 0.0 |
5 7.1 |
4 5.7 |
10 14.3 |
12 17.1 |
39 55.7 |
< 0.001 |
| Entorhinal Cortex, n, % | 117 48.3 |
38 15.7 |
26 10.7 |
26 10.7 |
17 7.0 |
18 7.4 |
9 12.5 |
1 1.4 |
17 23.6 |
22 30.6 |
10 13.9 |
13 18.1 |
< 0.001 |
| Hippocampus,CA1, n, % | 122 50.2 |
37 15.2 |
31 12.7 |
42 17.3 |
6 2.5 |
5 2.1 |
8 11.0 |
5 6.9 |
21 28.8 |
33 45.2 |
3 4.1 |
3 4.1 |
< 0.001 |
| Hippocampus, dentate Gyrus, n, % |
153 63.5 |
44 18.3 |
17 7.1 |
18 7.5 |
6 2.5 |
3 1.2 |
10 13.7 |
6 8.2 |
8 11.0 |
21 28.8 |
13 17.8 |
15 20.6 |
< 0.001 |
| Middle frontal gyrus, n, % | 220 90.5 |
17 7.0 |
5 2.1 |
1 0.4 |
0 0.0 |
0 0.0 |
47 64.4 |
15 20.6 |
5 6.9 |
5 6.9 |
0 0.0 |
1 1.37 |
< 0.001 |
| Middle temporal Gyrus, n, % |
194 79.5 |
27 11.1 |
10 4.1 |
6 2.5 |
3 1.2 |
4 1.6 |
31 42.5 |
11 15.1 |
9 12.3 |
11 15.1 |
6 8.2 |
5 6.9 |
< 0.001 |
Cytoplasmic inclusions in Score 1=1-2, Score 2=3-5, Score 3=6-12, Score 4=13-19 and Score 5=>20; p-values based on the Wilcoxon rank sum test.
Statistical analysis
Chi-square or t-statistics were used to compare demographics, clinical characteristics and age-related pathologies including AD, LB, and TDP-43 pathologies, macro and microinfarcts, arteriolosclerosis and atherosclerosis between subjects without and with HS.
Multiple logistic regression analyses were used to evaluate (1) the association of common age-related pathologies (AD and LB pathology, arteriolosclerosis, atherosclerosis, macro and microinfarcts) with HS as outcome and, (2) the association of HS with dementia. Ordinal logistic regression was used with MCI and probable AD as outcomes to evaluate the association of HS with these conditions after adjusting for demographics and other age-related pathologies. Linear regression analyses were used to evaluate the association of HS with global cognitive function and separately the outcome measures of episodic, semantic, and working memory, perceptual speed and visuospatial skills. Another set of analyses were run adding an interaction term for HS by TDP-43 pathology. All models controlled for age, sex, and education.
All analyses were carried out using SAS software, version 9.3, of the SAS ® system for Linux. Model assumptions were examined graphically and analytically and were adequately met. A nominal threshold of p < 0.05 was used for statistical significance throughout.
Results
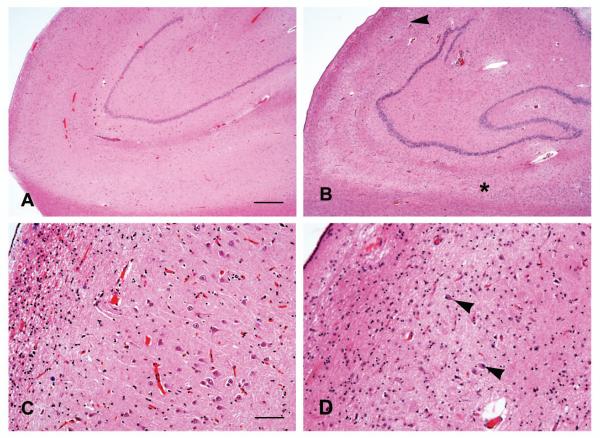
HS was present in 83 (13%) of the 636 subjects (Table 1) and involved both CA1 and/or subiculum (Fig 1) with additional involvement of CA2 and 3 in 4 cases and CA4 in another 4 cases. Overall, subjects with HS were older at the time of death (Table 1) and HS was twice as common in those aged at least 90 years (18.0%) compared to those aged <90 (9.2%).
Figure 1.
Photomicrographs showing the CA1 and subiculum in cases without (A) and with (B) HS in which sclerosis involves CA1 (starting below the arrowhead) and the subiculum (right of the *) in continuity. Higher magnification shows preservation of pyramidal neurons in the CA1 in the absence of sclerosis (C) and astrogliosis with few residual neurons (arrowheads) in the case with HS (D). Hematoxylin-eosin stain, Scale bar = 500 μm (A,B) and 200 μm (C, D).
HS, TDP-43 and other age-related pathologies
Compared to subjects without HS, HS subjects more frequently had neurodegenerative pathologies with over ¾ fulfilling diagnostic criteria for a neuropathologic diagnosis of AD and almost 1/3 with LB pathology (Table 1). The frequency of macro and microinfarcts and vessel disease was not significantly different in subjects without and with HS (Table 1).
TDP-43 pathology was identified in 86% of the HS subjects. The percentage of subjects with advanced TDP-43 stages was higher in subjects with HS with about ½ having extension of TDP-43 outside of the hippocampal region (Table 1). Also, the pathologic burden of TDP-43 inclusions was greater not only in the hippocampus and dentate gyrus but also in the amygdala, entorhinal and neocortices in HS subjects compared to those without HS (p <.001) (Table 2). In the small number of HS cases without TDP-43 pathology, there was a trend for lesser concurrent neurodegenerative diseases (AD and LB disease) compared to HS subjects with TDP-43 pathology; but differences between macro and microinfarcts and vessel disease in both groups were not significant (Table 3).
Table 3.
Clinical pathologic characteristics by presence of hippocampal sclerosis and TDP-43 pathology
| Hippocampal Sclerosis | ||||||
|---|---|---|---|---|---|---|
| Not present | Present | |||||
|
TDP-43 absent n=308 |
TDP-43 present n=245 |
p-value |
TDP-43 absent n=12 |
TDP-43 present n=71 |
p-value | |
| Age at death, y, mean (SD) | 87.2, 6.9 | 89.5, 5.9 | < 0.001 | 88.2, 7.4 | 91.4, 5.7 | 0.085 |
| Female, n (%) | 190, 65.3 | 180, 68.7 | 0.395 | 9, 75.0 | 52, 73.2 | 0.898 |
| Education, mean (SD) | 16.1, 3.7 | 16.0, 3.4 | 0.843 | 16.3, 4.5 | 16.0, 3.5 | 0.797 |
| MMSE, mean (SD) | 22.6, 8.1 | 19.9, 9.6 | < 0.001 | 22.6, 8.6 | 12.3, 9.7 | < 0.001 |
| No Cognitive Impairment | 116, 40.6 | 63, 28.1 | 0.009 | 2, 18.1 | 5, 7.5 | 0.011 |
| Mild Cognitive Impairment | 82, 28.7 | 67, 29.9 | 4, 36.4 | 6, 9.0 | ||
| Probable AD | 88, 30.8 | 94, 42.0 | 5, 45.5 | 56, 83.6 | ||
| NIA-Reagan, score 1-2, n (%) | 148, 52.9 | 178,67.9 | < 0.001 | 7, 58.3 | 59, 83.1 | 0.063 |
| Macroinfarcts, n (%) | 96, 33.0 | 103, 39.3 | 0.122 | 5, 41.7 | 30, 42.3 | 0.970 |
| Microinfarcts, n (%) | 80, 27.5 | 71, 27.1 | 0.918 | 6, 50.0 | 23, 32.4 | 0.327 |
| Arteriolosclerosis, n (%) | 208, 71.7 | 212, 81.5 | 0.007 | 8, 66.7 | 59, 83.1 | 0.233 |
| Atherosclerosis, n (%) | 239, 82.4 | 236, 90.1 | 0.010 | 9, 75.0 | 61, 85.9 | 0.390 |
| Lewy bodies, n (%) | 60, 20.6 | 57, 21.8 | 0.744 | 1, 8.3 | 26, 36.6 | 0.092 |
Chi-square or Fisher exact test as appropriate
Although AD, LB and TDP-43 pathology are all common in HS subjects, it was not clear if each was independently related to increased odds of having HS. Therefore, multiple logistic regression models were performed controlling for age, sex and education. In these models, TDP-43 pathology, but not AD, LB, infarcts or arteriolosclerosis, was associated with increased odds of HS pathology (Table 4), with each stage increase of TDP-43 pathology increasing the odds of HS by over 2.5 fold.
Table 4.
Relation of HS to TDP-43 and age-related pathologies
| Model terms | Relation to HS | |
|---|---|---|
| OR (95% CI) | p-value | |
| AD Pathology | 1.47 (0.79, 2.72) | 0.225 |
| Macroinfarcts | 1.04 (0.73,1.47) | 0.835 |
| Microinfarcts | 1.10 (0.74,1.64) | 0.621 |
| Lewy bodies | 1.29 (0.72, 2.31) | 0.384 |
| Arteriolosclerosis | 0.92 (0.68,1.23) | 0.561 |
| TDP-43 pathology | 2.63 (2.07, 3.34) | < 0.001 |
Estimated from a single logistic regression model, adjusted for age at death, sex and education.
TDP-43 pathology is a common feature of FTLD. In the current study, only 4 of the 18 HS cases without a pathological diagnosis of AD were diagnosed with FTLD. Three of these cases were diagnosed to have FTLD-TDP while one case had the neurofibrillary tangle predominant form of senile dementia. The clinical diagnosis in all of these cases was probable AD and the MMSE scores of these cases varied from 0-25. Exclusion of these 4 cases did not alter results of these or subsequent analyses.
HS and TDP-43 pathologies in dementia, probable AD and MCI
Dementia, probable AD and MCI were more frequent and the MMSE score was lower in HS subjects compared to those without HS (Table 1). Over ¾ of HS subjects had dementia (Table 1) compared to about 10% of HS subjects who had no cognitive impairment (Table 5). The pattern appeared to differ depending on whether there was coexisting TDP-43 pathology. In the small number of HS cases without TDP-43, the MMSE scores were significantly higher, NCI and MCI were more common, and dementia less common compared to HS with TDP-43 pathology (Table 4).
Table 5.
Clinical characteristics of 83 subjects with hippocampal sclerosis by age and sex
| < 90 years | >=90 years | |||
|---|---|---|---|---|
| Males n=9 |
Females n=24 |
Males n=13 |
Females n=37 |
|
| Education, mean (SD) | 18.8, 4.0 | 15.8, 3.0 | 14.9, 10.9 | 16.2, 3.7 |
| MMSE, mean (SD) | 15.1, 11.8 | 14.9, 10.9 | 16.7, 2.7 | 16.1, 8.4 |
| NCI, n (%)* | 1, 12.5 | 4, 18.2 | 1, 8.3 | 1, 2.8 |
| MCI, n (%)* | 2, 25.0 | 1, 4.6 | 1, 8.3 | 6, 16.7 |
| Probable AD, n (%)* | 5, 62.5 | 17, 73.9 | 10, 76.9 | 29, 78.4 |
| Possible AD, n (%)* | 0 | 0 | 1, 7.7 | 1, 2.7 |
| Other dementias, n (%)* | 0 | 1, 4.4 | 0 | 0 |
| Parkinson’s Disease, n (%) | 0 | 0 | 1, 7.7 | 2, 5.4 |
| Stroke, n (%) | 1, 11.1 | 6, 25.0 | 2, 15.4 | 4, 10.8 |
data based on 81 subjects
The association of HS and dementia, investigated by logistic regression analysis controlling for demographics and other pathologies including TDP-43 pathology, showed a 3.7-fold increase in odds of dementia in HS subjects (Table 6). TDP-43 pathology was also independently associated with dementia in the same model. TDP-43 pathology did not modify the association of HS on the odds of dementia (p=0.146).
Table 6.
The relation of HS, AD and TDP-43 pathologies to dementia, MCI and clinical probable AD and cognitive outcomes
| Model terms | Relation to Dementia* |
Relation to MCI and Probable AD** |
Cognitive Outcomes*** | |||||
|---|---|---|---|---|---|---|---|---|
| Global Function |
Episodic Memory |
Semantic Memory |
Working Memory |
Perceptual Speed |
Visuospatial Abilities |
|||
| Model 1 | ||||||||
| Hippocampal sclerosis |
3.71 (1.93, 7.16) < 0.001 |
3.75 (2.01, 7.02) < 0.001 |
−0.51 (0.12) < 0.001 |
−0.74 (0.14) < 0.001 |
−0.80 (0.16) < 0.001 |
−0.16 (0.12) 0.177 |
−0.34 (0.14) 0.016 |
−0.38 (0.14) 0.008 |
| AD pathology | 3.53 (2.55, 4.88) < 0.001 |
3.68 (2.58, 5.2) < 0.001 |
−0.77 (0.06) < 0.001 |
−0.93 (0.07) < 0.001 |
−0.78 (0.08) < 0.001 |
−0.46 (0.06) < 0.001 |
−0.49 (0.07) < 0.001 |
−0.45 (0.07) < 0.001 |
| TDP-43 pathology |
1.26 (1.05, 1.51) 0.014 |
1.29 (1.09, 1.51) 0.003 |
−0.10 (0.04) 0.009 |
−0.17 (0.04) < 0.001 |
−0.07 (0.05) 0.171 |
−0.02 (0.04) 0.613 |
−0.08 (0.04) 0.077 |
0.02 (0.04) 0.650 |
| Model 2 (HS by TDP-43 interaction) | ||||||||
| Hippocampal sclerosis |
0.07 (0.23) 0.762 |
−0.19 (0.26) 0.461 |
0.07 (0.29) 0.817 |
0.07 (0.23) 0.742 |
0.06 (0.26) 0.817 |
0.22 (0.27) 0.410 |
||
| AD pathology | −0.78 (0.06) < 0.001 |
−0.95 (0.07) < 0.001 |
−0.81 (0.08) < 0.001 |
−0.47 (0.06) < 0.001 |
−0.51 (0.07) < 0.001 |
−0.41 (0.07) < 0.001 |
||
| TDP-43 pathology |
−0.05 (0.04) 0.200 |
−0.13 (0.05) 0.006 |
0.004 (0.05) 0.943 |
0.0002 (0.04) 0.995 |
−0.05 (0.05) 0.295 |
0.07 (0.04) 0.149 |
||
| HS × TDP-43 pathologies |
−0.31 (0.10) 0.003 |
−0.29 (0.12) 0.015 |
−0.46 (0.13) < 0.001 |
−0.13 (0.10) 0.221 |
−0.21 (0.12) 0.076 |
−0.32 (0.12) 0.008 |
||
Estimated from two logistic regressions adjusted for age at death, sex, education, Lewy body pathology, and macro and microinfarcts. Cell entries are odds ratio, (95% confidence interval), and p-value.
Estimated from an ordinal logistic regression model adjusted for age at death, sex, education, Lewy body pathology, macro and microinfarcts atherosclerosis and arteriolosclerosis.
Cognitive outcomes are estimated from 12 separate linear regressions, all adjusted for age at death, sex, education and LB pathology. Cell entries are estimate (Standard Error) and p-value.
Next, the association of HS with probable AD and MCI was investigated. All but 3 of the 64 subjects with HS and dementia were diagnosed with probable AD (Table 5). In the 17 subjects with HS and no dementia, over half had MCI (Table 5). Ordinal regression analyses with MCI and probable AD as outcomes showed that the odds of both MCI or probable AD was higher in HS subjects after controlling for other pathologies (Table 6). TDP-43 pathology was also independently associated with MCI and probable AD (Table 6). The interaction term for HS and TDP-43 pathology was not significant.
Overlap between AD, HS and TDP-43 pathologies and the diagnosis of probable AD
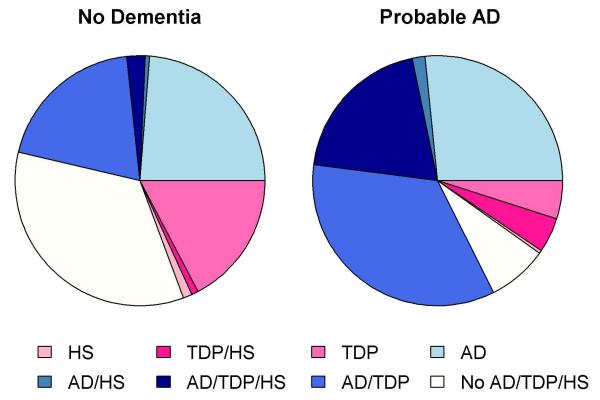
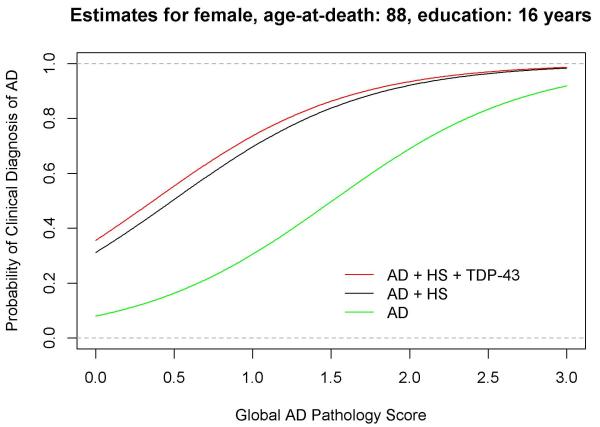
Though AD, HS and TDP-43 pathologies each separately increased the odds of probable AD, there was strong overlap in these pathologic diagnoses, and the majority of subjects with dementia, HS and TDP-43 pathology also had AD pathology (Fig 2). In subjects with probable AD, over half had AD pathology combined with HS, TDP-43 pathology or both. The additive influences of HS and TDP-43 pathologies on the odds of a clinical diagnosis of AD with increasing burden of AD pathology are illustrated in Fig 3.
Figure 2.
Blue shades represent the pathologic diagnosis of AD with or without coexisting HS and/or TDP-43 pathology, while the pink shades represent the pathologic diagnosis of HS and/or TDP without pathologic AD.
Figure 3.
The probability of a clinical diagnosis of AD (green line) with increasing burden of AD pathology and the additive influences of HS (black line) and TDP-43 pathology (red line) was predicted for a female aged 88 years with 16 years of education controlling for Lewy body pathology, macro and microinfarcts. The probability of a clinical diagnosis of AD was about 30 % per one unit of global AD pathology score (AD pathology score range 0-3.2 units). Concurrence of HS further increased the probability of the clinical diagnosis of AD to about 70 %, and the further addition of TDP-43 pathology increased the probability to about 75%.
HS and TDP-43 pathologies and cognitive domains
There are little data on the cognitive profiles associated with HS and/or TDP-43 pathologies. Using linear regression models, controlling for demographics, AD and other common age-related pathologies, HS and TDP-43 pathologies were each associated with lower levels of global cognitive function (Table 6, Model 1). HS was specifically associated with lower function in episodic and semantic memory, perceptual speed and visuospatial ability, however, in the same models, and unlike HS, TDP-43 pathology showed an association only with episodic memory.
In additional analyses, a term for an interaction between HS and TDP-43 pathologies was added to each of the linear regression models with global cognitive function and 5 separate cognitive domains as outcomes (Table 6, Model 2). These models showed that HS subjects with TDP-43 had greater impairment than those without TDP-43 pathology in global cognition and the domains of episodic and semantic memory, perceptual speed and visuospatial skills. In addition, TDP-43 without HS remained separately associated with episodic memory. In adjusted models, HS without TDP-43 pathology was not associated with lower levels of any of the cognitive domains, however, the number of cases in this group was small (Table 3).
Discussion
This clinical-pathologic study of 636 community-dwelling older subjects demonstrates that HS which has a limited footprint in the spectrum of clinical dementia diagnoses in older subjects has an important relationship with dementia, MCI and probable AD in aging. HS often coexists with TDP-43 pathology and each separately lowers the threshold for a diagnosis of dementia, and probable AD. There is often coexisting AD pathology as well, but HS does not have an independent relationship with AD or LB pathologies after accounting for TDP-43 pathology. Of interest is finding that HS with TDP-43 pathology is associated with lower function in multiple cognitive domains, whereas TDP-43 pathology without HS is associated only with lower episodic memory.
The current study shows a dramatic increase in the frequency of HS in the oldest old compared to old with HS being twice as common in the oldest old; having a higher frequency to that of neocortical-type LB disease which our group previously reported as about 10% in the oldest old.30 The prevalence of dementia is reported to rise in the oldest-old; however, our previous study and those of others 30,31 have reported that mixed pathologies continue to increase while the effect of AD pathology on the odds of dementia is attenuated in the oldest old.30,32 Given the strong association of HS with age, and the growth of the oldest old segment of the population,33 further study of HS in the oldest old is especially warranted.
HS often coexisted with either AD, TDP-43 pathology or both. The coexistence of HS with AD pathology has been long recognized.2-4 In the current study, HS was also associated with LB disease, however, in regression models including age and TDP-43 pathology, the relationship of HS with AD and LB pathologies was no longer significant and only TDP-43 pathology remained associated with HS. These data may suggest both common and discrepant risk factors and etiopathogenesis, which may create novel opportunities for prevention and treatment.
An ischemic basis to HS is often considered because of selective vulnerability of the hippocampus to ischemia. However, the frequency of both macro and microinfarcts did not differ significantly between subjects with and without HS, a finding similar to some 10,14,17 but not all previous studies.4,9,34 In addition, a recent large study reported that HS was related to widespread arteriolosclerosis.13 This finding was not replicated in the current study, however the assessment of arteriolosclerosis in the current study was limited to one region. HS cases without coexisting TDP-43 pathology are considered by some 6,7 to track with vascular disease and are less likely to be of degenerative etiology. In these analyses, HS subjects without TDP-43 did not have significantly more vascular disease however the number of cases in this group was very small. Further study of the role of vascular disease in HS with and without TDP-43 is warranted.
As reported previously,4,9 in the current study there was a striking increase in the likelihood of dementia in subjects with HS. Evaluation of TDP-43 pathology in a subset of cases showed that both HS and TDP-43 pathologies were independently associated with dementia and the clinical diagnoses of MCI and probable AD and having these pathologies increased the odds of dementia more than either pathology alone. Since most cases of HS have TDP-43 pathology, the finding that both pathologies are contributing is important to recognize.
Few community studies 14 have investigated the role of HS in the diagnosis of probable AD in older subjects while the association of clinical AD with TDP-43 pathology has been observed previously.29,35 In the current study, while a pathologic diagnosis of AD was confirmed in the majority of subjects with a clinical diagnosis of probable AD, a large number of cases also had both HS and TDP-43 pathologies. In addition, both HS and TDP-43 pathology further increased the odds of a diagnosis of probable AD. These observations have important implications and further emphasize the pathologic heterogeneity underlying the clinical diagnosis of AD. It also, suggests that HS and TDP-43 may be important and often unrecognized factors in decreasing cognitive reserve in subjects with preclinical AD. The common co-occurrence of these other pathologies with AD, suggests that research should focus on other pathways in addition to amyloid plaques and tau positive tangles.
There are little data on the profile of cognitive impairment associated with HS. In subjects > 90 years, HS was associated with lower MMSE scores, higher verbal fluency scores and lower word list delayed recall scores,14 while in a small clinic series HS subjects compared to AD subjects showed more deficits in episodic memory and language and to a lesser extent, attention and executive function.9 The current study confirms and extends these findings to a large community cohort and suggests that in addition to the expected strong effects on episodic memory, HS (most commonly with coexisting TDP-43 pathology) is associated with widespread impairment in other cognitive domains including language, perceptual speed, and even visuospatial skills. These findings suggest that although HS is diagnosed by involvement of a single brain structure, it likely reflects a more global diffuse brain disease at least in cases with coexisting TDP-43 pathology. Indeed, the current study and others 2,14,18,36 have shown that subjects with HS have diffuse distribution of TDP-43 pathology. In subjects with TDP-43 pathology without HS, the severity and localization of TDP-43 pathology is less extensive and is associated with a relatively limited episodic memory deficit. Finally, there were too few HS subjects without TDP-43 pathology to make any definitive statements regarding the cognitive profile of these cases. Further study will be necessary to determine whether HS subjects without TDP-43 track with and have a differing relationship with cognition.
Strengths of this study include detailed, systematic, and uniform data collection of multiple neuropathologies on a large number of cases blinded to clinical data. In addition, this study capitalized on diagnostic data on dementia and probable AD as well as a detailed battery of neuropsychological tests which were available proximate to death. Finally, the cohorts comprised a large group of community-dwelling subjects with and without dementia, with both high follow-up and autopsy rates, providing internal validity of findings.
There are also limitations to this study. The subjects of both cohorts, but particularly ROS, may not be representative of the general population in terms of average dietary intake, access to health care and levels of education, all of which may affect the presence of dementia, AD and cognitive impairment. HS was evaluated unilaterally and in a single section of the midhippocampus; thus the frequency and relative importance of HS may be an underestimate. In addition, we may be detecting mostly cases with more severe disease, which may bias toward stronger effect sizes. Finally, pathologic evaluation for FTLD was only performed in HS subjects without AD and demented subjects without a pathological diagnosis of AD or other pathologies that could account for dementia. Thus the number of FTLD cases in this study and the significance of FTLD in hippocampal sclerosis in aging may be an underestimate.
ACKNOWLEDGMENTS
The authors thank the participants of the Religious Orders Study and the Memory and Aging Project, and the staff of the Rush Alzheimer’s Disease Center. Thanks are expressed to Manuela Neumann and Elisabeth Kremmer for providing the phosphorylation specific TDP-43, 1D3 antibody.
This study was supported by National Institute on Aging (R01AG17917, P30AG10161, R01AG15819, R01AG42210). The funding organization had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
Footnotes
Conflict of Interest Drs David A. Bennett and Julie A. Schneider received funding from the National Institute of Aging and the Illinois Department of Public Health as listed above.
Author Contributions
Drs Nag and Schneider had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Bennett, Schneider, Nag, Leurgans
Acquisition, analysis, or interpretation of data: Nag, Schneider
Drafting of manuscript:Nag
Critical revision of manuscript for important intellectual content: All authors
Statistical analysis and interpretation: all authors
Obtained funding: Bennett, Schneider
Adminstrative, technical, or material support: Bennett, Schneider
References
- 1.Bouchet C, Cazauvieilh M De. L ’epilepsie consideree dans ses rapports avec l’alienation mentale. Recherches sur la nature et la siege de ces deux maladies. Arch Gen Med. 1825;9:510–542. [Google Scholar]
- 2.Amador-Ortiz C, Lin WL, Ahmed Z, et al. TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer’s disease. Ann Neurol. 2007;61:435–445. doi: 10.1002/ana.21154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Attems J, Jellinger KA. Hippocampal sclerosis in Alzheimer disease and other dementias. Neurology. 2006;66:775. doi: 10.1212/01.wnl.0000200959.50898.26. [DOI] [PubMed] [Google Scholar]
- 4.Dickson DW, Davies P, Bevona C, et al. Hippocampal sclerosis: a common pathological feature of dementia in very old (> or = 80 years of age) humans. Acta Neuropathol. 1994;88:212–221. doi: 10.1007/BF00293396. [DOI] [PubMed] [Google Scholar]
- 5.Josephs KA, Dickson DW. Hippocampal sclerosis in tau-negative frontotemporal lobar degeneration. Neurobiol Aging. 2007;28:1718–1722. doi: 10.1016/j.neurobiolaging.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 6.Hatanpaa KJ, Raisanen JM, Herndon E, et al. Hippocampal sclerosis in dementia, epilepsy, and ischemic injury: differential vulnerability of hippocampal subfields. J Neuropathol Exp Neurol. 2014;73:136–142. doi: 10.1097/OPX.0000000000000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rauramaa T, Pikkarainen M, Englund E, et al. Consensus recommendations on pathologic changes in the hippocampus: a postmortem multicenter inter-rater study. J Neuropathol Exp Neurol. 2013;72:452–461. doi: 10.1097/NEN.0b013e318292492a. [DOI] [PubMed] [Google Scholar]
- 8.Vinters HV, Ellis WG, Zarow C, et al. Neuropathologic substrates of ischemic vascular dementia. J Neuropathol Exp Neurol. 2000;59:931–945. doi: 10.1093/jnen/59.11.931. [DOI] [PubMed] [Google Scholar]
- 9.Corey-Bloom J, Sabbagh MN, Bondi MW, et al. Hippocampal sclerosis contributes to dementia in the elderly. Neurology. 1997;48:154–160. doi: 10.1212/wnl.48.1.154. [DOI] [PubMed] [Google Scholar]
- 10.Jellinger KA. Hippocampal sclerosis: a common pathological feature of dementia in very old humans. Acta Neuropathol. 1994;88:599. doi: 10.1007/BF00296500. [DOI] [PubMed] [Google Scholar]
- 11.Ala TA, Beh GO, Frey WH. Pure hippocampal sclerosis: a rare cause of dementia mimicking Alzheimer’s disease. Neurology. 2000;54:843–848. doi: 10.1212/wnl.54.4.843. [DOI] [PubMed] [Google Scholar]
- 12.Hatanpaa KJ, Blass DM, Pletnikova O, et al. Most cases of dementia with hippocampal sclerosis may represent frontotemporal dementia. Neurology. 2004;63:538–542. doi: 10.1212/01.wnl.0000129543.46734.c0. [DOI] [PubMed] [Google Scholar]
- 13.Neltner JH, Abner EL, Baker S, et al. Arteriolosclerosis that affects multiple brain regions is linked to hippocampal sclerosis of ageing. Brain. 2014;137:255–267. doi: 10.1093/brain/awt318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nelson PT, Schmitt FA, Lin Y, et al. Hippocampal sclerosis in advanced age: clinical and pathological features. Brain. 2011;134:1506–1518. doi: 10.1093/brain/awr053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nelson PT, Smith CD, Abner EL, et al. Hippocampal sclerosis of aging, a prevalent and high-morbidity brain disease. Acta Neuropathol. 2013;126:161–177. doi: 10.1007/s00401-013-1154-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beach TG, Sue L, Scott S, et al. Hippocampal sclerosis dementia with tauopathy. Brain Pathol. 2003;13:263–278. doi: 10.1111/j.1750-3639.2003.tb00027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leverenz JB, Agustin CM, Tsuang D, et al. Clinical and neuropathological characteristics of hippocampal sclerosis: a community-based study. Arch Neurol. 2002;59:1099–1106. doi: 10.1001/archneur.59.7.1099. [DOI] [PubMed] [Google Scholar]
- 18.Pao WC, Dickson DW, Crook JE, et al. Hippocampal sclerosis in the elderly: genetic and pathologic findings, some mimicking Alzheimer disease clinically. Alzheimer Dis Assoc Disord. 2011;25:364–368. doi: 10.1097/WAD.0b013e31820f8f50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bennett DA, Schneider JA, Arvanitakis Z, et al. Overview and findings from the religious orders study. Curr Alzheimer Res. 2012;9:628–645. doi: 10.2174/156720512801322573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bennett DA, Schneider JA, Buchman AS, et al. Overview and Findings From the Rush Memory and Aging Project. Curr Alzheimer Res. 2012;9:646–663. doi: 10.2174/156720512801322663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bennett DA, Schneider JA, Arvanitakis Z, et al. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 2006;66:1837–1844. doi: 10.1212/01.wnl.0000219668.47116.e6. [DOI] [PubMed] [Google Scholar]
- 22.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 23.Bennett DA, Wilson RS, Schneider JA, et al. Natural history of mild cognitive impairment in older persons. Neurology. 2002;59:198–205. doi: 10.1212/wnl.59.2.198. [DOI] [PubMed] [Google Scholar]
- 24.Schneider JA, Wilson RS, Bienias JL, et al. Cerebral infarctions and the likelihood of dementia from Alzheimer disease pathology. Neurology. 2004;62:1148–1155. doi: 10.1212/01.wnl.0000118211.78503.f5. [DOI] [PubMed] [Google Scholar]
- 25.Schneider JA, Arvanitakis Z, Leurgans SE, et al. The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann Neurol. 2009;66:200–208. doi: 10.1002/ana.21706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schneider JA, Bienias JL, Wilson RS, et al. The apolipoprotein E epsilon4 allele increases the odds of chronic cerebral infarction [corrected] detected at autopsy in older persons. Stroke. 2005;36:954–959. doi: 10.1161/01.STR.0000160747.27470.2a. [DOI] [PubMed] [Google Scholar]
- 27.Hyman BT, Trojanowski JQ. Consensus recommendations for the postmortem diagnosis of Alzheimer disease from the National Institute on Aging and the Reagan Institute Working Group on diagnostic criteria for the neuropathological assessment of Alzheimer disease. J Neuropathol Exp Neurol. 1997;56:1095–1097. doi: 10.1097/00005072-199710000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Neumann M, Kwong LK, Lee EB, et al. Phosphorylation of S409/410 of TDP-43 is a consistent feature in all sporadic and familial forms of TDP-43 proteinopathies. Acta Neuropathol. 2009;117:137–149. doi: 10.1007/s00401-008-0477-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilson RS, Yu L, Trojanowski JQ, et al. TDP-43 pathology, cognitive decline, and dementia in old age. JAMA Neurol. 2013;70:1418–1424. doi: 10.1001/jamaneurol.2013.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.James BD, Bennett DA, Boyle PA, et al. Dementia from Alzheimer disease and mixed pathologies in the oldest old. JAMA. 2012;307:1798–1800. doi: 10.1001/jama.2012.3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jellinger KA, Attems J. Prevalence and pathology of vascular dementia in the oldest-old. J Alzheimers Dis. 2010;21:1283–1293. doi: 10.3233/jad-2010-100603. [DOI] [PubMed] [Google Scholar]
- 32.Savva GM, Wharton SB, Ince PG, et al. Age, neuropathology, and dementia. N Engl J Med. 2009;360:2302–2309. doi: 10.1056/NEJMoa0806142. [DOI] [PubMed] [Google Scholar]
- 33.Wetle TF. The oldest old: missed public health opportunities. Am J Public Health. 2008;98:1159. doi: 10.2105/AJPH.2008.141440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kril JJ, Patel S, Harding AJ, et al. Patients with vascular dementia due to microvascular pathology have significant hippocampal neuronal loss. J Neurol Neurosurg Psychiatry. 2002;72:747–751. doi: 10.1136/jnnp.72.6.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Josephs KA, Whitwell JL, Weigand SD, et al. TDP-43 is a key player in the clinical features associated with Alzheimer’s disease. Acta Neuropathol. 2014;127:811–824. doi: 10.1007/s00401-014-1269-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zarow C, Weiner MW, Ellis WG, et al. Prevalence, laterality, and comorbidity of hippocampal sclerosis in an autopsy sample. Brain Behav. 2012;2:435–442. doi: 10.1002/brb3.66. [DOI] [PMC free article] [PubMed] [Google Scholar]