Abstract
Bioprosthetic heart valves (BHV) fabricated from glutaraldehyde pretreated heterograft materials, porcine aortic valves or bovine pericardium (BP), are widely used in cardiac surgery. BHV progressively fail in clinical use due to structural degeneration. Previously we reported that dityrosine, an oxidized amino acid, was present in failed clinical BP-BHV explants; unimplanted BP had no detectable dityrosine. In the same studies BP were demonstrated in vitro to be susceptible to oxidative damage, that could be mitigated with BP covalently modified with the antioxidant, 3-(4-hydroxy-3,5-di-tert-butylphenyl)propyl amine (DBP). The present studies compared in rat subdermal implants glutaraldehyde pretreated BP to BP modified with either DBP or the chemical reactions used to link DBP. All BP explants regardless of DBP demonstrated reduced hydroxyproline and increased digestibility by collagenase. However, the DBP-BP explants showed significant inhibition of reduced explant shrink temperatures (an index of crosslinking) compared to control BP. Significant mitigation of calcification was observed in both the BP-DBP and chemically modified explants compared to BP. Dityrosine was not detectable in the 90 day explants. It is concluded that rat subdermal BP implants undergo both calcific and non-calcific structural degeneration, but without the formation of dityrosine, unlike clinical BP explants.
Introduction
Bioprosthetic heart valves (BHV) are used annually in approximately 300,000 valve replacement procedures worldwide [1]. These devices, which are fabricated from glutaraldehyde-fixed heterograft tissues such as bovine pericardium (BP), have the advantage over the alternative mechanical valves in that they have a low risk of device-associated thrombosis [2] and some BHV can now be catheter-deployed, thereby providing a minimally invasive alternative to cardiac surgery [3]. Unfortunately, the clinical use of BHV is limited by poor durability due to structural deterioration, which results in a clinical failure rate of 30% after only 10 years of implantation [4]. The development of a more durable BHV would address the significant clinical need for longer-lasting BHV and a better alternative to the thrombogenic mechanical valves.
The poor durability of BHV results from primary leaflet degeneration which can be caused by mechanical stress [5], calcification [6], and as described in a recent report by our group, oxidation [7]. Failed clinical BHV explants have an elevation of the tyrosine oxidation product dityrosine [7], thereby indicating that these materials are susceptible to oxidation. Oxidation of the BHV material glutaraldehyde-fixed BP in an in vitro model of accelerated biomaterial oxidative damage results in a breakdown of glutaraldehyde cross-linking, an increase in susceptibility to degradation by collagenase, and overall structural degeneration [7].
Since it has been shown that oxidation can lead to BHV structural damage, it may be possible to mitigate this mechanism of degradation through the use of an antioxidant material modification. In our previous report we showed in vitro that oxidation-mediated material damage of BP can be mitigated through covalent attachment of the oxidant scavenger 3-(4-hydroxy-3,5-di-tert-butylphenyl)propyl amine (DBP) to the BHV material glutaraldehyde-fixed BP [7]. In this in vitro model using exposure to H2O2 and FeSO4, oxidation related damage was assessed with endpoints including loss of glutaraldehyde cross-linking, morphology changes, and collagenase susceptibility. In the present studies we use the juvenile rat subdermal implant model to further investigate DBP modified BP in vivo. This model system has been valuable for assessing calcification of BHV materials due to the acceleration of this process through the use of juvenile rats [8]. In addition to calcification, which is an important mechanism of BHV degradation [6], we assessed the effects of the DBP modification on the overall structural degeneration of BP including the formation of dityrosine as well as collagen and material cross-link stability.
Materials and Methods
Bovine pericardium preparation
Fresh BP obtained from an abattoir was treated with 0.625% glutaraldehyde/HEPES (pH 7.4) for 7 days at room temperature with gentle shaking. Unmodified (control) BP was exposed to 100% ethanol for 24 hours for calcification studies (EtOH) [9]. All BP samples were stored in 0.2% glutaraldehyde (Polysciences Inc., Warrington, PA) [8].
DBP modification
BP was modified with DBP through a carbodiimide-driven reaction as previously reported [7]. Briefly, the DBP modification reaction was prepared as two solutions: 42 mM DBP in 100% ethanol and 43 mM N-hydroxysuccinimide (SuOH) (Sigma Aldrich, St. Louis, MO) in deionized H2O. Immediately before adding BP, the two solutions were combined and 65 mM 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC) (Sigma Aldrich, St. Louis, MO) was added. The reaction proceeded for 24 hours at room temperature with gentle shaking. DBP modified BP was rinsed for 10 minutes in 100% ethanol to remove precipitate formed during the reaction. As a control for the effects of the carbodiimide-driven chemistry, BP was treated with the carbodiimide reaction components with the exclusion of DBP (chemistry). All BP samples were stored in 0.2% glutaraldehyde.
Uniaxial tensile testing
DBP modified and unmodified BP were subjected to uniaxial tensile testing to determine the effects of the DBP modification on material flexibility and strength. Samples were cut to dimensions of 8 mm width and 25 mm length. Semi-circular sections were cut from the middle of the sample to obtain a bone-like shape. Using an Instron Model TT (Instron, Norwood, MA), the samples were placed in pneumatic grips and stretched at a crosshead speed of 100 mm/min until failure. Stress and strain were calculated to generate stress-strain curves.
Rat subdermal implants
Three week old male, Sprague-Dawley rats (60-90 grams) were used for subdermal implantation studies [8]. Surgical procedures were performed according to guidelines from the Institutional Animal Use and Care Committee at the Children's Hospital of Philadelphia. Rats were anesthetized with isofluorane and shaved in preparation of the surgical procedure. Two incisions were made on the dorsal surface to prepare subdermal pouches for the BP samples. BP samples were implanted in the subdermal pouches at 2-3 samples per animal. The BP samples were explanted at 7, 21, or 90 days.
Histology analysis
Explants that had been fixed in 10% neutral buffered formalin were dehydrated and embedded in paraffin. Hematoxylin & eosin stains were performed on 6 μm paraffin sections. Sections were imaged at 100× magnification.
Shrink temperature determination
Cross-linking of non-implanted glutaraldehyde fixed BP and 90 day rat subdermal BP explants was measured by differential scanning calorimetry (DSC) on a Perkin Elmer DSC 7 as previously described [11]. Briefly, samples that were hermetically sealed in aluminum pans were placed in the DSC where sample temperature was ramped from 25°C to 100° C until the endothermic peak corresponding to shrink temperature was observed.
Hydroxyproline quantification
Hydroxyproline content of rat subdermal explants was quantified with 1H-NMR, performed at 400 MHz on a Bruker Avance III™ 400 wide-bore spectrometer by Suzanne Werhli of the NMR core at the Children's Hospital of Philadelphia. Acid hydrolysates of 90 day explants (0.6 mL) were introduced in a 5 mm NMR tube. An external standard made of a sealed capillary containing a solution of trimethylsilylpropionic acid in D2O was used as chemical shift reference and quantification standard. Fully relaxed proton spectra were acquired with a 5 mm BBO probe. Standard acquisition conditions were as follows: PW 45o, TR 8s, water saturation during the relaxation delay, SW 6775 Hz, TD 64k and 64 scans.
Collagenase susceptibility
Collagenase digestion was performed on lyophilized non-implanted or 90 day rat subdermal explants of BP. Collagenase from Clostridium histolyticum (Sigma Aldrich, St. Louis, MO) was added to BP samples at 600 U/mL and incubated for 24 hours at 37°C. Digestion by collagenase was measured as a loss of weight following collagenase treatment.
Calcification analysis
BP explants from 21 and 90 day studies were hydrolyzed in 6 N HCl at 100 °C and fully dried under air flow. The hydrolysates were reconstituted in 0.01 N HCl. Calcium quantification was done using a Perkin Elmer 2380 atomic absorption spectrophotometer [10].
Dityrosine quantification
Dityrosine was quantified by established stable isotope dilution liquid chromatography tandem mass spectrometry (LC MS/MS) methods [12] at the Cleveland Clinic on an AB SCIEX API 5000 triple quadrupole mass spectrometer interfaced with an Aria LX Series HPLC multiplexing system (Cohesive Technologies Inc., Franklin, MA). Briefly, paraffin embedded BP explants were prepared from 7, 21, and 90 day rat subdermal studies. All paraffin sections were deparaffinized by xylene. [13C6]-labeled oxidized amino acid standards and universal labeled precursor amino acids ([13C9,15N1]tyrosine and [13C9,15N1]phenylalanine) were added to samples after protein delipidation and desalting with a single phase mixture of H2O/methanol/H2O-saturated diethyl ether (1:3:8 v/v/v). Proteins were hydrolyzed under argon gas in methane sulfonic acid and passed through C18 solid-phase extraction column (Discovery – DSC18 minicolumn, 3 ml, Supelco, Bellefone, PA) prior to MS analysis. Dityrosine and its precursors were monitored by characteristic parent to product ion transitions unique for each isotopologue monitored. Results are expressed relative to the content of the precursor tyrosine.
Statistical analysis
Results are shown as the mean ± standard error for the mean. Single ANOVA with Tukey's test or a two tailed t-test were used to determine significance, which was defined as a p value less than 0.05.
Results
Tensile testing
To assess whether the DBP modification affects the material properties of BP, uniaxial tensile testing was performed on unmodified (control) and DBP modified BP. A representative stress-strain curve is shown for both groups (Fig. 1). Ultimate tensile strength (UTS), maximum strain, and Young's elastic modulus were calculated for both control and DBP modified BP. UTS (control 21.1 ± 0.7 vs DBP 17.5 ± 1.7 MPa), maximum elongation (control 328.9% ± 0.4 vs. DBP 339.8% ± 5.7), and Young's elastic modulus (control 127 ± 14.8 vs DBP 117.8 ± 6.5 kPa) were not significantly different between the control and DBP modified BP groups. These results demonstrate that the DBP modification does not affect the tensile properties of BP.
Figure 1.
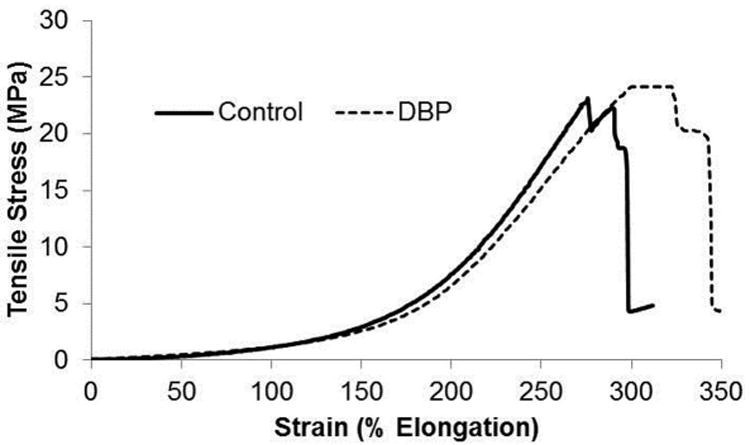
Uniaxial tensile testing stress-strain response. Tensile testing of glutaraldehyde fixed BP with and without the DBP modification. Representative curves shown for n=5.
Histology
Hematoxlyin & eosin staining was performed on 90 day explants to assess the differences in morphology and the extent of degree of inflammatory cell activity in proximity to the BP implants. The cellular staining for control, chemistry, and DBP BP explants was largely localized to the edges or inflammatory capsule of the explants rather than as infiltrate in the BP samples (Fig. 2A-C). There were no observable differences in either the extracellular matrix structure or the inflammatory recruitment to control, chemistry, or DBP modified BP. Thus, these results demonstrate no qualitative differences in morphology in the 90 day explants comparing control BP, DBP-modified, and BP modified with only the DBP linking chemistry.
Figure 2.
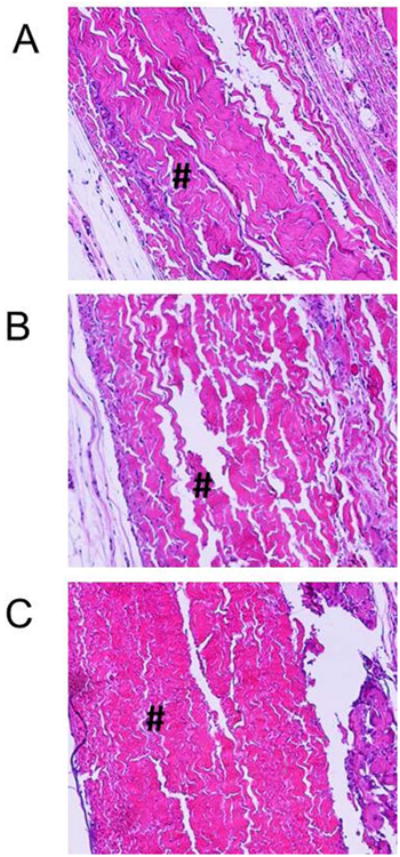
Histology of 90 day BP rat subdermal explants. Hematoxylin & eosin staining of 90 day BP explants (A) control, (B) chemistry and (C) DBP modified, 100× magnification. # indicates the BP explant. Control: glutaraldehyde fixed BP, Chemistry: glutaraldehyde fixed BP reacted with EDC and SuOH, DBP: glutaraldehyde fixed BP modified with DBP using EDC and SuOH.
Cross-link stability
Rat subdermal explants were analyzed by DSC to quantify shrink temperature, a measure of overall material cross-linking, to determine whether rat subdermal implantation reduces material cross-linking. Both the control (p=0.031) and chemistry (p=0.0012) groups displayed a significant decrease in shrink temperature in the 90 day explants as compared to the non-implanted tissues (Fig. 3). The shrink temperature for the DBP explants was not significantly less than non-implanted DBP modified BP (Fig. 3, p=0.647), thereby indicating that the DBP modification mitigates the reduction in material cross-linking following rat subdermal implantation that was observed for the control and chemistry BP explants.
Figure 3.
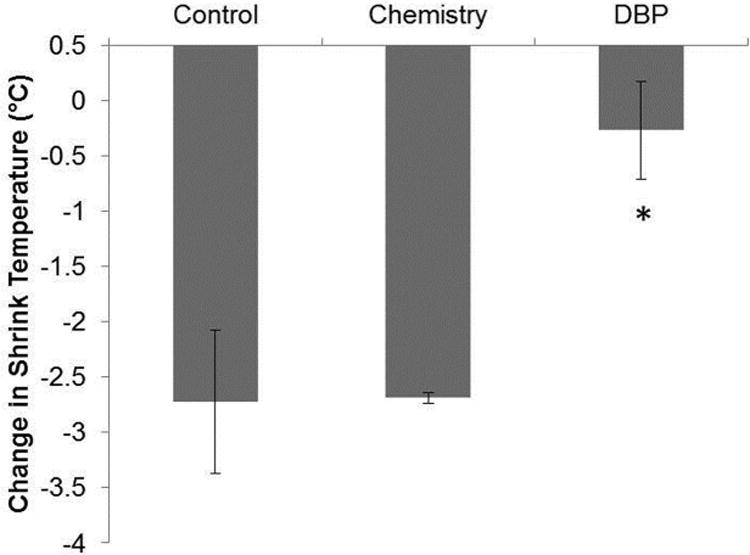
Shrink temperature change with rat subdermal implantation. The change in shrink temperature (decrease) in 90 day rat subdermal explants as compared to non-implanted materials. n=3. *p<0.05 vs non-implanted. Control: glutaraldehyde fixed BP, Chemistry: glutaraldehyde fixed BP reacted with EDC and SuOH, DBP: glutaraldehyde fixed BP modified with DBP using EDC and SuOH.
Collagen stability
Hydroxyproline loss
Only 90 day explants were studied for hydroxyproline content, that was normalized to the dry weight of the explant (Fig. 4A) or the dry weight adjusted for the weight of calcium and phosphate (Fig. 4B) to determine whether changes in hydroxyproline content occurred with calcification or independently. Hydroxyproline, a measure of collagen content [13], was found to be significantly reduced (p<0.001) in the 90 day control explants compared to non-implanted BP with both normalizations of hydroxyproline (Fig. 4A-B), thereby demonstrating deterioration of the collagen structure of BP in this model, potentially a significant factor in the overall degradation of these tissues. However, there were no significant differences in hydroxyproline content between the explanted groups, which indicate that the DBP modification and chemistry alone do not affect this index of collagen degradation.
Figure 4.
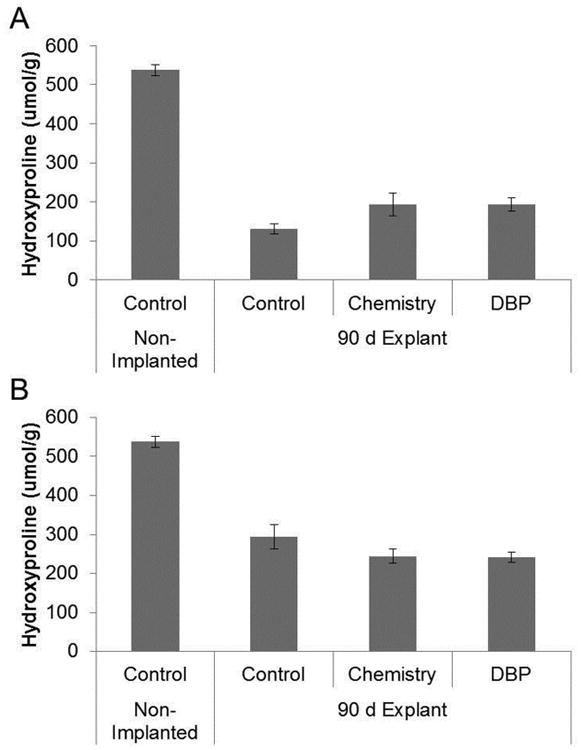
Hydroxyproline loss in rat subdermal explants. Quantification of hydroxyproline content by 1H-NMR in 90 day rat subdermal explants. (A) Hydroxyproline content reported/gram dry weight (B) hydroxyproline content/gram dry weight adjusted for calcium and phosphate weight. Control: glutaraldehyde fixed BP, Chemistry: glutaraldehyde fixed BP reacted with EDC and SuOH, DBP: glutaraldehyde fixed BP modified with DBP using EDC and SuOH. n=3-5.
Collagenase susceptibility
Studies with soluble collagen have demonstrated that oxidation results in an increase in susceptibility to collagenase or other proteolytic degradation [14-16]. The unmodified (control) 90 day BP explants were more susceptible to hydrolysis by collagenase as compared to non-implanted BP, but this increase was not statistically significant (Fig. 5). The DBP modified explants displayed less weight loss than the control and chemistry 90 day explants, but this difference also did not reach statistical significance. These results demonstrate a modest increase in collagenase susceptibility for BP following 90 day rat implantation that was not significantly affected by the DBP modification.
Figure 5.
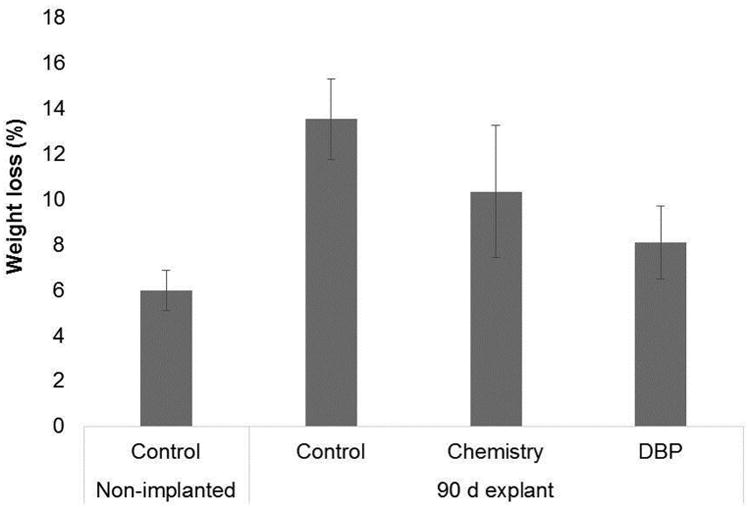
Rat subdermal explants susceptibility to collagenase digestion. Rat subdermal explants from longest time point (90 days) were treated with collagenase to determine change in susceptibility to proteolytic degradation, n=3. Control: glutaraldehyde fixed BP, Chemistry: glutaraldehyde fixed BP reacted with EDC and SuOH, DBP: glutaraldehyde fixed BP modified with DBP using EDC and SuOH.
Calcification
BP rat subdermal explants from 21 and 90 day studies were assessed for calcium accumulation to determine the effect of the DBP modification on BHV calcification. Ethanol was used as a positive anti-calcification control since ethanol pre-treatment is an established anti-calcification pretreatment used in clinical BHV [17], and ethanol is also used as a solvent for the DBP modification. Furthermore, the DBP modification involves carbodiimide-driven chemistry therefore an additional experimental group was included in this study to control for any effects of the DBP-conjugation chemistry. The mean calcium level in control BP explants was 59.4 ± 10.0 μg calcium/mg tissue for 21 day explants (Fig. 6A) and 334.6 ± 24.8 μg calcium/mg tissue for 90 day explants (Fig. 6B). Calcium levels were significantly lower than control explants for ethanol 21 day explants (p<0.05). Control 21 day explant calcium levels were higher than those of the DBP and chemistry groups (30.3 ± 1.5 μg calcium/mg tissue and 31.9 ± 8.9 μg calcium/mg tissue, respectively), but these differences were not statistically significant from each other. Thus, the results of the 21 day explants indicate that ethanol exposure provides more resistance to calcification than either the additional DBP modification or carbodiimide chemistry in this time frame.
Figure 6.
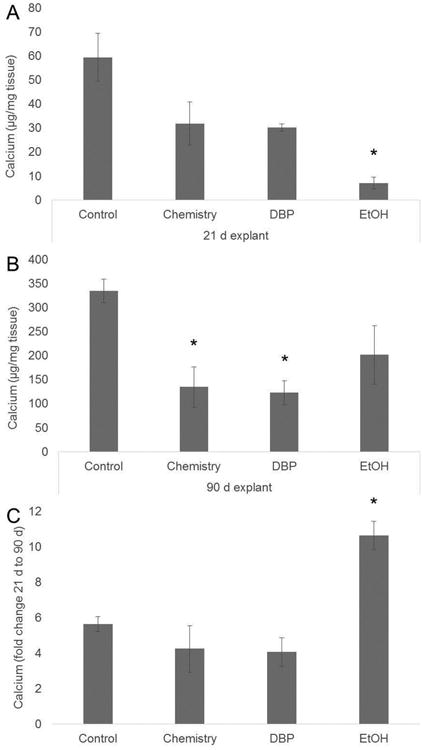
Calcification of rat subdermal explants. Calcium was quantified in both 21 day (A) and 90 day (B) explants to determine the effect of the DBP modification and chemistry on the calcium accumulation in BP. (C) Fold change in calcium accumulation from 21 d to 90 d, n=5. *p<0.05 vs control. Control: glutaraldehyde fixed BP, Chemistry: glutaraldehyde fixed BP reacted with EDC and SuOH, DBP: glutaraldehyde fixed BP modified with DBP using EDC and SuOH
Calcification was assessed in 90 day explants in addition to the 21 day time point since previous studies have demonstrated that although 21 days explants typically have a calcification level comparable to clinically failed valves, maximal amounts of calcification occur by 90 days in this animal model [11]. A significant increase in calcium accumulation after 90 days implantation was observed in all BP explant groups. However, the DBP and chemistry groups had significantly lower calcium levels than the control BP explants, and the ethanol pretreated explants. Furthermore, the fold increase in calcium levels in the 90 day explants as compared to the 21 day explants was significantly higher in the ethanol group as compared to all other treatment groups (Fig. 6C). Neither the DBP nor chemistry BP explants had the same fold increase in calcium over the 21 to 90 day period compared to the ethanol group, indicating that DBP and its linking chemistry may mitigate the rate of calcification after initial implantation of an ethanol pre-treated BHV. These results demonstrate that although calcium levels were reduced with the DBP modification, this effect could be due to the conjugation chemistry reactions, and not the presence of DBP, since calcium levels were also reduced in chemistry group.
Dityrosine formation
Since we previously determined that dityrosine is elevated in failed clinical BHV explants [7] and is undetectable in unimplanted BP, this marker was analyzed in rat subdermal explants to determine whether this model recapitulates this aspect of BHV oxidation. Three time points (7, 21, and 90 days) were assessed to determine the time course of dityrosine formation; the 21 and 90 day time points have been correlated with clinical calcium levels in failed bioprostheses [8, 18, 19]. Dityrosine was not detectable in the 7 or 90 day BP explants but was present in a subset of the 21 day explants (35.5 ±17.2 μmol/mol, control explants). In addition, there was no significant effect of the DBP modification on the levels of dityrosine in comparison to control samples.
Discussion
The prior report by our group of oxidative modifications of glutaraldehyde crosslinked bovine pericardial bioprosthetic cusps [7], while including data from clinical explants did not include results from animal models used to study bioprosthetic calcification and structural degeneration, such as rat subdermal implants [8]. The present results demonstrated significant structural degeneration occurred in the control, glutaraldehyde crossliked rat subdermal explants, including a loss of collagen per reduction in hydroxyproline, reduction in material cross-linking, and progressive calcification. The DBP anti-oxidant modification investigated in our prior in vitro studies did not have comparable effects in these in vivo studies as discussed below.
The presence of di-tyrosine in our prior series of clinically failed BP-bioprostheses was notable, since unimplanted BP had no detectable levels of di-tyrosine. In the present studies, a slight elevation in the levels of dityrosine in BP 21 days post implantation was observed. However the levels of dityrosine in the 21 day rat subdermal explants are an order of magnitude less than levels quantified in clinical BHV explants [7]. Furthermore, the 90 day rat BP explants had no detectable dityrosine. These results could be attributed to the lack of blood exposure in this model since both blood contact and flow stresses can contribute to an activation of inflammatory or endothelial cells, leading to oxidant production and oxidative modifications of the implants [24, 25]. The lack of robust dityrosine formation may indicate that BP juvenile rat subdermal implants may not fully recapitulate the oxidative processes involved in BHV structural degeneration observed clinically in the circulation.
Our previous studies utilized radio-labeled glutaraldehyde to document the in vitro destruction of glutaraldehyde crosslinks in BP samples under mild oxidative conditions. The loss of radio-labeled glutaradehyde in these studies was prevented through DBP modification of BP. In the present studies we utilized changes in thermal denaturation temperature, also termed shrink temperature to assess crosslink stability in our BP subdermal explants. The DBP modification, but not the carbodiimide-driven attachment chemistry, which also includes exposure to ethanol, prevented the reductions in shrink temperature observed in the rat subdermal explants. The reduction in cross-linking observed in the rat subdermal implant model could be attributed to several factors including collagen fragmentation due to oxidative stress, oxidation of the actual crosslink chemical bonds, and proteolytic degradation. The DBP explant group demonstrated significantly less reduction in shrink temperature, thus agreeing with our prior in vitro studies, and providing some evidence of oxidative crosslink damage, and its mitigation with DBP modified BP.
Our prior studies demonstrated that oxidative conditions increased the digestibility of BP by collagenase, and this was also reduced in these in vitro studies by DBP modification. Oxidation of collagen has been shown in related research to result in an increase in susceptibility to proteolytic degradation [14-16]. However, in the rat subdermal explants reported in this paper, there were no significant differences in collagenase digestibility between DBP modified and control groups. Nevertheless, the combined effects of oxidation and proteolytic degradation could result in significant material degradation due to both oxidative fragmentation [22] and digestion by enzymes such as matrix metalloproteinases [23]. Thus, the observation that the DBP modification did not significantly reduce collagenase susceptibility in the rat subdermal implant model may be due to the presence of metalloproteinase activity apart from the otherwise mild oxidative environment that led to collagen breakdown and a uniform increased susceptibility to collagenase in our present studies.
We further explored the possibility of collagen breakdown in vivo with hydroxyproline assays, since the heterograft tissues used in BHV fabrication are composed primarily of collagen. The significant loss of hydroxyproline found in the rat subdermal explants demonstrates the overall degradation in vivo of the collagen structure, apart from increased susceptibility to collagenase studied in our explant experiments. Collagen, which contains abundant amounts of hydroxyproline, could be degraded enzymatically or it may be susceptible to oxidative fragmentation since proline has been shown to be highly susceptible to specific oxidant attack [20, 21]. Nevertheless, the identification of hydroxyproline loss in the rat subdermal implant model occurred despite DBP modification and demonstrates significant deterioration of the collagen structure that may be attributed to mechanisms other than oxidative damage.
Calcification has been observed as a failure mechanism for bioprosthetic heart valves for more than thirty years [24]. As expected for the rat subdermal implant model, the control BP rat subdermal explants had significant calcium accumulation at both 21 days and 90 days. Previously these levels have been shown to be comparable to calcium levels seen in failed explanted clinical BHV [8]. DBP modified and carbodiimide-reacted BP rat subdermal explants were less calcified than control BP at both the 21 day and 90 day time points. Additionally, the clinically-used anti-calcification technology, pretreatment of BP with 24 hours ethanol exposure, did not have significantly lower calcium accumulation than control BP at 90 days; thereby demonstrating that the DBP modification, particularly the carbodiimide-driven chemistry, may have advantages for calcification mitigation compared to ethanol pretreatment in preventing long-term calcification in rat subdermal implants. In addition, the fold increase in calcium levels for the ethanol BP explants has previously not been reported, but highlights the breakthrough calcification that can occur with ethanol used alone as an anti-calcification strategy.
The mechanism responsible for the mitigation of calcification of BP in the 90 day explants, especially compared to the clinically used ethanol pretreatment, is not clear from the present results. 90 day ethanol subdermal implant results for ethanol have not been published by our group or others; all of the previous subdermal published results concerning ethanol pretreatment have been for shorter durations, typically 21 days [9,10]. Our 21 day subdermal explant results in the present studies for ethanol pretreatment show significant inhibition of calcification as previously. The 90 day “breakthrough” calcification noted in the present rat subdermal studies indicate a limited benefit of ethanol pretreatment. However, despite these results, published sheep mitral valve replacement studies using ethanol pretreatment show persistent inhibition of calcification in explants of up to 150 days [9, 25, 26]. The mechanism responsible for the inhibition of calcification in the 90 day rat subdermal explants in the present study by the DBP-linking chemistry reactions could be due to inactivation of intrinsic alkaline phosphatase by the DBP linking chemistry. Prior research has shown that cellular oriented alkaline phosphatase activity is present in non-implanted BP even after glutaraldehyde fixation, and these foci of BP alkaline phosphatase activity have been hypothesized to be one of the sites of initiation of BP calcification [27]. The DBP-linking chemistry could be hypothesized to inactivate this instrinsic alkaline phosphatase activity. However, a complete histochemistry and enzymology study involving BP alkaline phosphatase activity under the conditions of interest was beyond the scope of the present paper; this will be the subject of future research.
In conclusion, the present studies reporting the first in vivo experimental studies of BP oxidative modification indicate the rat subdermal BP implant model may be suboptimal for investigating the susceptibility of BP to oxidative damage. Our prior clinical observation of high levels of the oxidized amino acid, di-tyrosine, in clinical BP explants, was not duplicated in these subdermal studies where di-tyrosine was undetectable in 90 day explants. Anti-oxidant modification with DBP prevented the reduction in thermal denaturation temperature indicating possibile protection of glutaraldehyde crosslinks from oxidative damage in agreement with our prior in vitro studies. The DBP-linking chemistry, which involves the use of ethanol, a known anti-calcification pretreatment, conferred significantly more effective long term mitigation than ethanol pretreatment in our subdermal studies. Thus, oxidative stress as a potential failure mechanism for bioprostheses should be further investigated in circulatory models that better reproduce the clinical environment, where oxidative modifications have already been definitively demonstrated.
Acknowledgments
This research was supported by the following grants from the National Institutes of Health: 5T32GM008076-29 (AJC predoctoral training grant in pharmacology), HL054926 (HI). This research was also supported by the following: A grant from the Cardiac Center of The Children's Hospital of Philadelphia (AJC, RJL), The William J. Rashkind Endowment of the Children's Hospital of Philadelphia (RJL), The Erin's Fund Endowment of the Children's Hospital of Philadelphia (RJL), a grant from the Kibel Foundation (RJL), a grant from The Children's Heart Foundation (RJL, AC), and The Gisela and Dennis Alter Research Professor of Pediatrics (HI). The authors would like to thank Stanley Hazen and Hongqiao Lin (Cleveland Clinic) for providing mass spectrometry analysis and valuable scientific discussion. The authors would also like to thank Susan Kerns (The Children's Hospital of Philadelphia) for her assistance in submitting the manuscript.
References
- 1.Pibarot P, Dumesnil JG. Prosthetic heart valves: selection of the optimal prosthesis and long-term management. Circulation. 2009;119:1034–48. doi: 10.1161/CIRCULATIONAHA.108.778886. [DOI] [PubMed] [Google Scholar]
- 2.Siddiqui RF, Abraham JR, Butany J. Bioprosthetic heart valves: modes of failure. Histopathology. 2009;55:135–44. doi: 10.1111/j.1365-2559.2008.03190.x. [DOI] [PubMed] [Google Scholar]
- 3.Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–607. doi: 10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]
- 4.Otto C, Bonow R. Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine. Philadelphia, PA: Elseiver; 2012. Valvular Heart Disease; pp. 1468–539. [Google Scholar]
- 5.Sacks MS, Schoen FJ. Collagen fiber disruption occurs independent of calcification in clinically explanted bioprosthetic heart valves. J Biomed Mater Res. 2002;62:359–71. doi: 10.1002/jbm.10293. [DOI] [PubMed] [Google Scholar]
- 6.Schoen FJ, Levy RJ. Calcification of tissue heart valve substitutes: progress toward understanding and prevention. Ann Thorac Surg. 2005;79:1072–80. doi: 10.1016/j.athoracsur.2004.06.033. [DOI] [PubMed] [Google Scholar]
- 7.Christian AJ, Lin H, Alferiev IS, Connolly JM, Ferrari G, Hazen SL, et al. The susceptibility of bioprosthetic heart valve leaflets to oxidation. Biomaterials. 2014;35:2097–102. doi: 10.1016/j.biomaterials.2013.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levy RJ, Schoen FJ, Levy JT, Nelson AC, Howard SL, Oshry LJ. Biologic determinants of dystrophic calcification and osteocalcin deposition in glutaraldehyde-preserved porcine aortic valve leaflets implanted subcutaneously in rats. Am J Pathol. 1983;113:143–55. [PMC free article] [PubMed] [Google Scholar]
- 9.Vyavahare N, Hirsch D, Lerner E, Baskin JZ, Schoen FJ, Bianco R, et al. Prevention of bioprosthetic heart valve calcification by ethanol preincubation. Efficacy and mechanisms. Circulation. 1997;95:479–88. doi: 10.1161/01.cir.95.2.479. [DOI] [PubMed] [Google Scholar]
- 10.Vyavahare NR, Jones PL, Hirsch D, Schoen FJ, Levy RJ. Prevention of glutaraldehyde-fixed bioprosthetic heart valve calcification by alcohol pretreatment: further mechanistic studies. J Heart Valve Dis. 2000;9:561–6. [PubMed] [Google Scholar]
- 11.Connolly JM, Bakay MA, Alferiev IS, Gorman RC, Gorman JH, 3rd, Kruth HS, et al. Triglycidyl amine crosslinking combined with ethanol inhibits bioprosthetic heart valve calcification. Ann Thorac Surg. 2011;92:858–65. doi: 10.1016/j.athoracsur.2011.04.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Citardi MJ, Song W, Batra PS, Lanza DC, Hazen SL. Characterization of oxidative pathways in chronic rhinosinusitis and sinonasal polyposis. Am J Rhinol. 2006;20:353–9. doi: 10.2500/ajr.2006.20.2858. [DOI] [PubMed] [Google Scholar]
- 13.Woessner JF. The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961;93:440–7. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- 14.Au V, Madison SA. Effects of singlet oxygen on the extracellular matrix protein collagen: oxidation of the collagen crosslink histidinohydroxylysinonorleucine and histidine. Arch Biochem Biophys. 2000;384:133–42. doi: 10.1006/abbi.2000.2070. [DOI] [PubMed] [Google Scholar]
- 15.Monboisse JC, Borel JP. Oxidative damage to collagen. EXS. 1992;62:323–7. doi: 10.1007/978-3-0348-7460-1_32. [DOI] [PubMed] [Google Scholar]
- 16.Henrotin Y, Deberg M, Mathy-Hartert M, Deby-Dupont G. Biochemical biomarkers of oxidative collagen damage. Adv Clin Chem. 2009;49:31–55. doi: 10.1016/s0065-2423(09)49002-4. [DOI] [PubMed] [Google Scholar]
- 17.Jamieson WR, Lewis CT, Sakwa MP, Cooley DA, Kshettry VR, Jones KW, et al. St Jude Medical Epic porcine bioprosthesis: results of the regulatory evaluation. J Thorac Cardiovasc Surg. 2011;141:1449–54. doi: 10.1016/j.jtcvs.2010.05.055. [DOI] [PubMed] [Google Scholar]
- 18.Schoen FJ, Tsao JW, Levy RJ. Calcification of bovine pericardium used in cardiac valve bioprostheses. Am J Pathol. 1986;123:134–45. [PMC free article] [PubMed] [Google Scholar]
- 19.Schoen FJ, Kujovich JL, Webb CL, Levy RJ. Chemically determined mineral content of explanted porcine aortic valve bioprostheses: correlation with radiographic assessment of calcification and clinical data. Circulation. 1987;76:1061–6. doi: 10.1161/01.cir.76.5.1061. [DOI] [PubMed] [Google Scholar]
- 20.Kato Y, Uchida K, Kawakishi S. Oxidative-Degradation of Collagen and Its Model Peptide by Ultraviolet-Irradiation. J Agric Food Chem. 1992;40:373–9. [Google Scholar]
- 21.Kato Y, Uchida K, Kawakishi S. Oxidative fragmenation of collagen and prolyl peptide by Cu(II)/H2O2. J Biol Chem. 1992;267:23646–51. [PubMed] [Google Scholar]
- 22.Monboisse J, Braquet P, Randoux A, Borel J. Non-enyzmatic degradation of acid-soluble calf skin collagen by superoxide ion: protective effect of flavonoids. Biochem Pharmacol. 1983;32:53–8. doi: 10.1016/0006-2952(83)90651-2. [DOI] [PubMed] [Google Scholar]
- 23.Simionescu A, Simionescu DT, Deac R. Biochemical pathways of tissue degeneration in bioprosthetic cardiac valves. ASAIO J. 1996;42:M561–7. doi: 10.1097/00002480-199609000-00049. [DOI] [PubMed] [Google Scholar]
- 24.Sanders SP, Levy RJ, Freed MD, Norwood WI, Castaneda AR. Use of Hancock porcine xenografts in children and adolescents. Am J Cardiol. 1980;46:429–38. doi: 10.1016/0002-9149(80)90012-0. [DOI] [PubMed] [Google Scholar]
- 25.Ogle MF, Kelly SJ, Bianco RW, Levy RJ. Calcification resistance with aluminum-ethanol treated porcine aortic valve bioprostheses in juvenile sheep. Ann Thorac Surg. 2003;75:1267–73. doi: 10.1016/s0003-4975(02)04489-2. [DOI] [PubMed] [Google Scholar]
- 26.Connolly JM, Alferiev I, Kronsteiner A, Lu Z, Levy RJ. Ethanol inhibition of porcine bioprosthetic heart valve cusp calcification is enhanced by reduction with sodium borohydride. J Heart Valve Dis. 2004;13:487–93. [PubMed] [Google Scholar]
- 27.Maranto AR, Schoen FJ. Alkaline phosphatase activity of glutaraldehyde-treated bovine pericardium used in bioprosthetic cardiac valves. Circ Res. 1988;63:844–8. doi: 10.1161/01.res.63.4.844. [DOI] [PubMed] [Google Scholar]


