Abstract
Background
Despite harmful consequences of drug addiction, it is common for individuals with substance use disorders to deny having problems with drugs. Emerging evidence suggests that some drug users lack insight into their behavior due to neurocognitive dysfunction, but little research has examined potential neurocognitive contributions to denial.
Methods
This study explored the relationship between denial, cognitive performance and functional connectivity in brain. The participants were 58 non-treatment-seeking, methamphetamine-dependent participants who completed the URICA Precontemplation scale, a self-report measure of denial of drug problems warranting change, as well as a cognitive test battery. A subset of participants (N=21) had functional MRI scans assessing resting-state functional connectivity. Given literature indicating roles of the rostral anterior cingulate (rACC), anterior insula and precuneus in self-awareness, relationships between denial and resting-state connectivity were tested using seeds placed in these regions.
Results
The results revealed a negative relationship between denial and an overall cognitive battery score (p=0.001), the effect being driven particularly by performance on tests of memory and executive function. Denial was negatively associated with strength of connectivity between the rACC and regions of the frontal lobe (precentral gyri, left ventromedial prefrontal cortex, left orbitofrontal cortex), limbic system (left amygdala, left hippocampus and left parahippocampal gyrus), occipital lobes and cerebellum; and between the precuneus and the midbrain and cerebellum. Anterior insula connectivity was unrelated to denial.
Conclusions
These findings suggest that denial by methamphetamine users is linked with a cognitive and neural phenotype that may impede the development of insight into their behavior.
Keywords: cognition, insight, denial, awareness, substance abuse, connectivity
1. INTRODUCTION
Despite the negative impact on one’s health and quality of life, individuals who have drug use disorders may deny having a problem with drugs. Indeed, traditional models of addiction treatment, such as that embraced by Alcoholics Anonymous, conceptualize denial as a hallmark of the addictive process (Kurtz, 1982). More contemporary models of behavior change in addiction, such as the Transtheoretical Model (DiClemente et al., 2004; Prochaska et al., 1992), likewise suggest that individuals who abuse substances may deny or fail to recognize the need to change their behavior, and that only through insight into the problem and intrinsic motivation can true behavioral change take place. This view is consistent with evidence suggesting that, among those who meet criteria for methamphetamine dependence, lack of perceived need for treatment is one of the most problematic barriers to treatment utilization (Kenny et al., 2011).
An emerging literature suggests that abnormalities in cognition and brain function may reduce the degree to which drug users can reflect upon and have insight into the nature of their substance abuse (see Goldstein et al., 2009). The relevant evidence has raised the possibility that denial by substance users may reflect a deficit in self-awareness and/or interoceptive monitoring, rather than, or in addition to, a conscious or subconscious attempt by the drug user to minimize his or her symptoms (Goldstein et al., 2009). Limited research, however, has examined potential neurocognitive contributions to denial in addiction. A preliminary study of individuals in treatment for alcohol dependence found that participants who were judged by clinicians to have more denial regarding their addiction exhibited worse performance on tests of executive functioning, memory, and processing speed than their counterparts who were thought to have less denial (Rinn et al., 2002). However, other studies of cognitive or neural contributions to denial in substance users have not been conducted.
Research has indicated that individuals who abuse drugs may have less awareness of their behavior than those who do not abuse drugs. When compared to healthy control subjects, cocaine users have shown less awareness of their choices when viewing emotional and cocaine-related images (Moeller et al., 2010), and of errors they made when performing an inhibitory control task (Hester et al., 2007). In alcohol-abusing college students, reduced awareness of drinking problems was associated with worse performance on memory tests (Blume et al., 2000). Marijuana users completing a go/no-go task while undergoing functional magnetic resonance imaging (fMRI) had less awareness of errors and less error-associated BOLD activation in the anterior cingulate cortex (ACC) and right insula than healthy control subjects (Hester et al., 2009). Similarly, cocaine users with limited insight into their choices for emotional and drug-related images exhibited less activation of the rostral ACC (rACC) during errors on an fMRI Stroop Task, and had less gray matter volume in the rACC, than participants with intact insight (Moeller et al., 2014).
Evidence from error-monitoring studies in healthy subjects has likewise suggested that the rACC and insula contribute to one’s awareness of having made errors during task performance (Klein et al., 2007; Simoes-Franklin et al., 2010; Taylor et al., 2007). The anterior portion of the insula, in particular, is considered to be important in the integration of interoceptive states with environmental conditions necessary for self-awareness (Craig, 2009). In the resting state, the anterior insula and ACC act as a “salience network” that is sensitive to detecting inconsistencies in oneself or the environment, and serves to activate other brain regions for further processing of personally relevant information (Menon and Uddin, 2010).
There is also evidence that, in addition to the rACC and anterior insula, the precuneus is involved in self-awareness (see Cavanna and Trimble, 2006). In healthy participants, the level of activation of the precuneus is positively associated with the degree to which subjects are aware of internal thoughts and feelings compared to stimuli in the external environment (Vanhaudenhuyse et al., 2010). Further, transcranial magnetic stimulation of the precuneus inhibits the retrieval of judgments about oneself but not about others (Lou et al., 2004). Schizophrenic patients with low insight into their psychosis exhibit hypoperfusion of the precuneus compared with patients who have intact insight (Faget-Agius et al., 2012). Schizophrenic patients with poor insight into their condition also show less resting-state functional connectivity (RSFC) between the default mode network and the precuneus and ACC than those with greater insight (Liemburg et al., 2012). Notably, research participants who meet DSM-IV criteria for methamphetamine dependence also exhibit gray-matter deficits and task-related abnormalities in the precuneus, ACC and insula (London et al., 2005; Morales et al., 2012; Nestor et al., 2011; Thompson et al., 2004); however, the relationship between these abnormalities and denial or insight regarding their addiction has not been evaluated.
Given evidence of neurocognitive contributions to self-awareness in substance users, we evaluated whether denial of substance use problems is related to cognitive performance and RSFC of regions previously associated with self-awareness (i.e., rACC, anterior insula and precuneus) in a sample of individuals who met criteria for current methamphetamine dependence according to DSM-IV criteria (58 participants with cognitive tests, 21 of whom also had fMRI scans). Denial was assessed with the University of Rhode Island Change Assessment (URICA) Precontemplation subscale. This subscale is a measure of the degree to which participants deny having problems they wish to change, and has been conceptualized as assessing denial of problems (e.g., Dare and Derigne, 2010; Peteet et al., 1998; Zimmerman et al., 2000). Consistent with the aforementioned literature, the hypotheses tested were that denial of problems would be negatively associated with overall cognitive performance and with the strength of connectivity of the rACC, anterior insula and precuneus with regions important for decision-making and affect (e.g., prefrontal cortex, limbic regions).
2. METHODS AND MATERIALS
2.1 Participants
The participants were 58 currently methamphetamine-dependent subjects who were not seeking treatment, 21 of whom received an fMRI resting-state scan. Participants were recruited using Internet and local newspaper advertisements and completed procedures in return for monetary compensation. After receiving a detailed description of the protocol, they provided written informed consent, following the guidelines of the UCLA Office for Protection of Research Subjects. Forty-eight participants completed the study as inpatients at the UCLA General Clinical Research Center (GCRC), and 10 completed the study as outpatients after closure of the GCRC. All participants tested positive for methamphetamine in urinalysis at study entry, but negative for methamphetamine and other illicit substances (amphetamine, opiates, cocaine, benzodiazepines) during cognitive and fMRI assessments, following 4 to 12 days of abstinence (given the long duration in which marijuana can be detected through urinalysis, brief abstinence from marijuana for outpatients was verified through saliva testing (Oratect; Grapevine, Texas), with all participants endorsing at least 4 days of abstinence at testing. Abstinence for inpatients was supervised). Participants were originally recruited to complete studies of cognition and brain structure in methamphetamine dependence (e.g., Morales et al., 2012; Simon et al., 2010), and fMRI scanning in the resting state was added to this protocol in later years (e.g., Kohno et al., 2014). All participants were fluent in English and were administered the Structured Clinical Interview for the DSM-IV (SCID) for Axis I diagnosis (First et al., 1995). The exclusion criteria, based on interview and laboratory tests, were: neurological disease (e.g., stroke, head trauma with loss of consciousness > 30 min); frank structural brain abnormalities on MRI; systemic disease; cardiovascular disease; pulmonary disease; HIV infection (HIV1/HIV2 antibody screen); abnormal laboratory tests (hematocrit, plasma electrolytes, markers for hepatic and renal function); use of psychotropic medications; diagnosis of current abuse or dependence for any substance other than methamphetamine, marijuana or nicotine; and any current non-substance-induced Axis I psychiatric conditions (with the exception of one subject with current social phobia who did not receive an fMRI scan). Eight participants also met criteria for current marijuana dependence (N=6) or abuse (N=2).
2.2 Measures
2.2.1 Cognitive battery
The cognitive battery was identical to that used by Dean et al. (2012), and included measures of
2.2.2 Processing speed/attention
Trailmaking Part A (Reitan, 1958), Stroop Color and Word identification (Golden, 1978), and Digit Symbol Coding from the WAIS-R (Wechsler, 1981).
2.2.3 Learning/memory
Selective Reminding Test (Buschke, 1963) and Repeated Memory Test- Pictures (Simon et al., 2004; based on stimuli from Snodgrass and Vanderwart, 1980);
2.2.4 Working memory
Backward Digit Span (Woodcock and Johnson, 1977) and Missing Digit Span (patterned after Buschke, 1963; Simon et al., 2004).
2.2.5 Executive function
Stroop Color-Word Interference (Golden, 1978); Trailmaking Part B (Reitan, 1958), Controlled Oral Word Fluency (FAS, Borkowski et al., 1967), Logical Problems (Simon et al., 2004), and Discrimination Learning (Simon et al., 2004; similar to Concept Learning from Woodcock and Johnson, 1977).
As reported previously (Dean et al., 2012), an overall cognitive battery score was created by centering and scaling each of the test scores based on the mean and standard deviation of an age-matched control group (N=42), and then averaging the resulting standardized scores (tests on which lower scores indicated better performance were multiplied by -1 to keep the directionality of the measures consistent). Due to a change in procedures, the participants with fMRI scans (N=21) were not administered the full cognitive battery, but received only the Selective Reminding Test, Stroop Test, Trailmaking Test and Controlled Oral Word Fluency; therefore, their cognitive battery score was comprised of these tests alone. However, for participants receiving the full battery, composite scores comprised of only these tests were highly correlated with composite scores comprised of the full battery (r=0.91, p<0.0001).
2.2.6 Denial: University of Rhode Island Change Assessment-Precontemplation subscale: (URICA-P; McConnaughy, 1989)
The URICA is a measure of the motivation to change one’s behavior which is often applied to addictive disorders, based on the Transtheoretical Model (Prochaska et al., 1992). The model proposes that the motivation to change one’s behavior undergoes a series of stages, beginning with denial or a lack of recognition that a problem exists and needs to be changed (Precontemplation), followed by increased recognition and motivation to initiate change (Contemplation, Action, Maintenance). The Precontemplation subscale has been posited to assess denial (e.g., Dare and Derigne, 2010; Peteet et al., 1998; Zimmerman et al., 2000) by measuring the explicit denial of problems (e.g., “As far as I’m concerned, I don’t have any problems that need changing”; and “I’m not the problem one; it doesn’t make much sense for me to consider changing”). We used it to assess the denial of methamphetamine-related problems by instructing the participants to answer the questions as it pertained to their “methamphetamine use”. The scale consists of 8 items ranging from strongly disagree (1) to strongly agree (5), with higher total scores (8 to 40) indicating greater adamance that one does not have a problem that needs changing. Chronbach’s alpha reliability coefficient for the Precontemplation items was acceptable at a level of α = 0.801.
2.3 fMRI Resting-State Scanning
Imaging was performed at 3 Tesla on a Siemens Magnetom Trio MRI system. For the resting-state scan, participants were presented with a black screen for 5-min and were asked to keep their eyes open. A set of 152 functional, T2*-weighted, echoplanar images (EPI) was acquired (slice thickness=4 mm; 34 slices; repetition time (TR)=2 s; echo time (TE)=30 ms; flip angle=90°; matrix=64 × 64; field of view=200 mm). High-resolution, T2-weighted, matched-bandwidth and magnetization-prepared rapid-acquisition gradient echo (MPRAGE) scans were also acquired. The orientation for matched-bandwidth and EPI scans was oblique-axial to maximize brain coverage and to optimize signal from ventromedial prefrontal regions.
2.4 Data Analysis
The relationship between demographic characteristics and Precontemplation and overall cognitive scores was examined using correlations or t-tests, as appropriate. Although the Precontemplation subscale is considered to be a measure of denial of having substance use problems, it is possible that those with the highest scores were not denying but actually had the fewest drug problems. To investigate this possibility, we also tested correlations between drug use and Precontemplation. The relationship between Precontemplation and cognitive scores was evaluated with multiple regression. For all primary cognitive and fMRI analyses, age, gender, years of education and inpatient status (yes/no) were included as nuisance covariates. Functional MRI image analysis was performed using the FMRIB Software Library (FSL 5.0.2.1; www.fmrib.ox.ac.uk/fsl). The image series from each participant were first realigned to compensate for small head movements (Jenkinson et al., 2002), and high-pass temporal filtering was applied (Gaussian-weighted least-squares straight-line fitting, with sigma=33s). Images were spatially smoothed using a 5-mm FWHM Gaussian kernel, and skull-stripping was performed using the FSL Brain Extraction Tool. Registration was conducted through a three-step procedure, whereby EPI images were first registered to the matched-bandwidth structural image, then to the high-resolution MPRAGE structural image, and finally into standard MNI space, using 12-parameter affine transformations. Registration of MPRAGE structural images to standard space was further refined using FNIRT nonlinear registration (Andersson et al., 2007). Bilateral volumes of interest (VOIs) for the rACC and precuneus were anatomically derived using the Desikan-Killiany Atlas (Desikan et al., 2006). The anterior portion of the insula was defined using information from a functional parcellation study (Deen et al., 2011). Statistical analyses for each VOI were performed separately using the general linear model (GLM) in each participant’s native space using FMRIB’s fMRI Expert Analysis Tool (FEAT), with the above-mentioned transformation to standard space carried out on resulting statistical maps. Nuisance regressors were added to each GLM to account for sources of noise, including activation measured in the cerebrospinal fluid and two variables accounting for motion artifact: frame-wise displacement (FD) and the root mean squared change in BOLD signal (DVARS; Power et al., 2011). The mean time series of the VOIs were calculated by averaging the time series of all voxels within each VOI; this average was included as a regressor for each GLM. The relationships between functional connectivity with each VOI and Precontemplation scores were examined in group-wise, whole-brain, voxel-wise regression analyses, using Precontemplation scores as a regressor in addition to the aforementioned covariates. Each whole-brain fMRI statistic was corrected for multiple comparisons using cluster-correction with a voxel height threshold of Z > 2.3 and a cluster-extent threshold of p < 0.016 to adjust (Bonferroni) for the three separate VOI analyses.
2.5 Post-hoc Analyses
Analyses revealed that Precontemplation was negatively associated with overall cognition (see below). To determine whether this relationship was driven by performance on one or more specific cognitive domains, we examined the relationship between Precontemplation and composite scores calculated from tests in the cognitive domains previously described (calculated and statistically analyzed in an identical fashion to the overall battery score, but comprised of domain-specific tests). Further, because Precontemplation was also negatively associated with RSFC of the rACC and precuneus (below), the extent to which overall cognition attenuated these relationships was examined by including it as a covariate in the aforementioned models. Because methamphetamine use may also contribute to a lack of insight, years of regular methamphetamine use (using methamphetamine three times per week, or twice weekly binges) was included as a covariate into the base RSFC models to determine whether it altered existing relationships.
Finally, as an exploratory analysis to assess specificity of the findings with the Precontemplation scale, we evaluated the relationship between RSFC of the VOIs and scores on the Contemplation scale of the URICA, which measures the tendency for respondents to consider having a problem that warrants making a change (McConnaughy, 1989). Of note, the Maintenance and Action subscales of the URICA were not evaluated because the participants were active methamphetamine users who were not seeking treatment.
3. RESULTS
3.1 Demographic analyses
When controlling for age, Precontemplation scores were negatively correlated with the age of onset of methamphetamine use (r=−0.36, p=0.007), indicating that higher Precontemplation scores were associated with a younger age of onset. A similar relationship was observed with respect to years of regular methamphetamine use, in which higher Precontemplation scores were associated with more years of methamphetamine use (r=0.26, p=0.052; as expected, years of regular MA use and onset were negatively correlated, r=−0.31, p=0.021). Precontemplation was not significantly related to days of methamphetamine use in the last 30 days (r=−0.03, p=0.835), times of use per day (r=0.03, p=0.815) or grams used in the week prior to study entry (r=0.06, p=0.676). Among participants who provided relevant self-report data (N=37), those with high Precontemplation scores (grouped by median split) had a significantly greater number of incidents of being arrested and charged for drug offenses, relative to those with low Precontemplation scores (t(34)=−2.09, p=0.045). These data suggested that, if anything, Precontemplation was associated with more, not fewer, drug problems, consistent with the presence of denial and/or a lack of awareness regarding one’s behavior.
Precontemplation was negatively correlated with years of education (r=−0.297, p=0.024), while overall cognition was significantly lower in male than female participants (t(56)=−3.19, p=0.002) and inpatient participants than outpatient participants (t(56)=2.04, p=0.046). Other demographic and drug use characteristics showed no significant associations with Precontemplation or cognition (e.g., ethnicity, days of marijuana use or abuse/dependence diagnosis, days of alcohol use, smoking status, p’s>.05). With respect to exploratory analyses, as expected, Precontemplation scores were negatively correlated with Contemplation scores (r=−0.29, p=0.027).
3.2 Cognition and Denial
Precontemplation scores were negatively related to overall cognitive function (β=−0.425, t(57)=−3.513, p=0.001, Figure 1), and this relationship was unchanged when additionally controlling for years of methamphetamine use (p=0.002; excluding the subject with social phobia also had no effect, p=.001). In post-hoc analyses, Precontemplation was significantly negatively related to learning/memory (β=−0.392, t(56)=−2.833, p=0.007) and executive functioning (β=−0.327, t(57)=−2.646, p=0.011), while it was negatively related to processing speed/attention at a trend level (β=−0.231, t(57)=−1.706, p=0.094). Precontemplation was unrelated to working memory (β =−0.155, t(36)=−0.846, p=0.404), although the sample size tested for working memory was relatively small (N = 37) because these tests were not administered in the portion of participants who received fMRI scans. In exploratory analyses, overall cognitive performance was not significantly related to the Contemplation subscale (β=−0.184, t(57)=−1.34, p=0.186).
Figure 1.
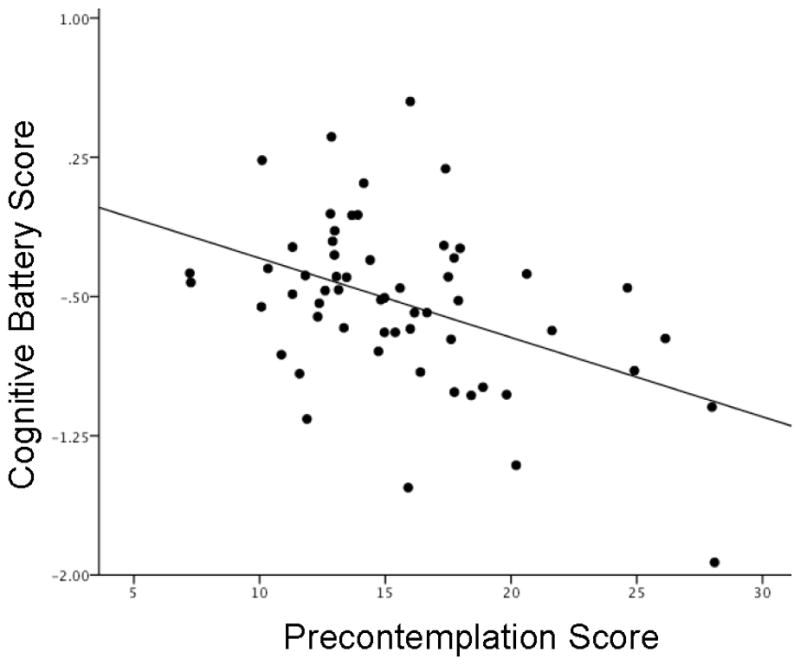
The relationship between Precontemplation scores and overall cognitive performance in methamphetamine-dependent participants.
Note: N = 58. Higher Precontemplation scores reflect greater adamance that one does not have a problem with drug use that requires changing. Overall cognitive scores (z-scores) are normalized to an age-matched healthy control group (N = 42), in which higher scores (more positive) reflect better performance. Results and the figure have been adjusted for age, gender, inpatient status and years of education (p = 0.001).
3.3 RSFC and Denial
Relationships between Precontemplation scores and RSFC of the rACC and precuneus are presented in Table 2. Noteworthy negative relationships were exhibited between Precontemplation and strength of connectivity of the rACC with the precentral gyri, left middle temporal gyrus, ventromedial PFC (vmPFC), left orbitofrontal cortex (OFC), left amygydala, left hippocampus, left parahippocampal gyrus, occipital lobes, and cerebellum (p<0.016, whole-brain, cluster corrected; Figure 2). Strength of connectivity between the precuneus and the midbrain/brainstem and cerebellum was also negatively related to Precontemplation (p<0.016). Anterior insula connectivity was not significantly related to Precontemplation scores (p>0.016). No areas of positive relationship were found between the VOIs and Precontemplation.
Table 2.
Connectivity of brain regions related to Precontemplation scores
| Brain region | Cluster size (voxels) | Xa | y | z | Z statistic |
|---|---|---|---|---|---|
| Rostral Anterior Cingulate Seed | |||||
| Cluster #1b | 5843 | ||||
| † Precentral Gyrus (R) | 38 | −6 | 60 | 4.08 | |
| † Middle frontal Gyrus (R) | 38 | 12 | 28 | 2.61 | |
| Cluster #2 | 2396 | ||||
| Lingual Gyrus (R) | 14 | −58 | 0 | 4.11 | |
| † Occipital Cortex (R/L) | 58 | −64 | −4 | 3.83 | |
| † Middle Temporal Gyrus (L) | −52 | −48 | 0 | 3.02 | |
| Cluster #3 | 906 | ||||
| †† Precentral Gyrus (L) | −40 | −14 | 52 | 3.52 | |
| Postcentral Gyrus (L) | −36 | −32 | 44 | 3.16 | |
| Cluster #4 | 841 | ||||
| Amygdala (L) | −22 | −2 | −18 | 4.21 | |
| Hippocampus (L) | −14 | −10 | −26 | 3.57 | |
| Parahippocampal Gyrus | −14 | −34 | −6 | 3.24 | |
| Ventromedial PFC | −12 | 26 | −18 | 3.01 | |
| Orbital Frontal Cortex | −28 | 18 | −26 | 3.00 | |
| Cluster #5 | 603 | ||||
| †† Cerebellum | 28 | −64 | −54 | 3.28 | |
| Cluster #6 | 602 | ||||
| †† Anterior Cingulate Cortex | 12 | 16 | 34 | 3.35 | |
|
| |||||
| Precuneus Seed | |||||
| Cluster #1 | 3329 | ||||
| † Cerebellum | 16 | −46 | −24 | 3.95 | |
| Brainstem/Midbrain | 12 | −20 | −14 | 3.76 | |
| † Brainstem/Pons | 0 | −36 | −40 | 3.56 | |
Z-statistic maps were thresholded using cluster-corrected statistics with a height-threshold of Z > 2.3 and cluster-forming threshold of p < 0.016, covariates include age, sex, years of education and inpatient status.
x, y, z reflect coordinates for peak voxel or for other local maxima in MNI space.
Clusters are numbered and presented in order of decreasing size.
Regions that remained significant after controlling for years of regular methamphetamine use.
Regions extending beyond original cluster after controlling for years of methamphetamine use.
Figure 2.
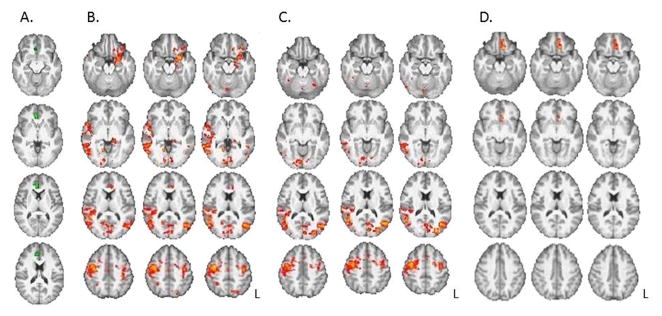
Relationships between resting-state connectivity strength of the rostral anterior cingulate cortex and Precontemplation scores (B, C) and years of regular methamphetamine use (D).
A. Rostral anterior cingulate cortex (rACC) seed (in green). B. Connectivity maps show negative relationships between Precontemplation scores and connectivity strength between rACC and amygdala, parahippocampal gyrus, medial and lateral orbital frontal cortex, bilateral precentral gyrus, temporal lobe, bilateral occipital cortex and cerebellum. C. After covarying for years of regular methamphetamine use, relationships between Precontemplation and connectivity of the rACC were attenuated, with remaining relationships in precentral cortex, occipital cortex and right middle frontal gyrus. D. The relationship between connectivity strength of the rACC with years of methamphetamine use, independent of Precontemplation. All results controlled for age, gender, inpatient status and years of education (p’s < 0.016, whole-brain cluster corrected). L = left hemisphere.
When controlling for cognitive battery scores, the aforementioned relationships between Precontemplation and RSFC of the rACC and precuneus were not attenuated. However, when controlling for years of methamphetamine use, the negative relationships between Precontemplation and connectivity strength of the rACC with limbic regions (parahippocampal gyrus, hippocampus, amygdala) and some frontal regions (vmPFC, OFC) were no longer significant, nor was the relationship between Precontemplation and RSFC between precuneus and midbrain (p’s>0.016). Table 2 denotes which clusters were altered by covarying for years of methamphetamine use (see † symbols). Demonstrating possible shared and independent relationships between RSFC of the VOIs, Precontemplation and years of methamphetamine use, Figures 2 and 3 show differences in results when controlling and not controlling for years of methamphetamine use, as well as the independent relationship between years of methamphetamine use and connectivity.
Figure 3.
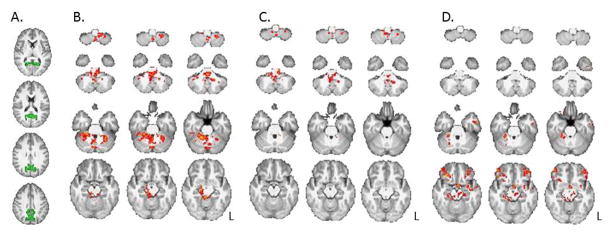
Relationships between resting-state connectivity strength of the precuneus and Precontemplation scores (B, C) and years of regular methamphetamine use (D).
A. Precuneus seed (in green). B. Negative relationships between Precontemplation scores and connectivity strength between the precuneus and midbrain, brainstem and cerebellum. C. After covarying for years of regular methamphetamine use, relationships between Precontemplation and connectivity of the precunues were attenuated, with remaining relationships in cerebellum and brainstem. D. The relationship between connectivity strength of the precuneus with years of methamphetamine use, independent of Precontemplation. All results controlled for age, gender, inpatient status and years of education (p’s < 0.016, whole-brain cluster corrected). L = left hemisphere.
In exploratory analyses, Contemplation scores were positively related to RSFC strength between the rACC and cerebellum (x=34, y= 29, z=23) and left superior frontal gyrus (x=54, y= 95, z=47). Similarly, Contemplation scores were positively related to RSFC between the Precuneus and ACC (x=44, y= 58, z= 58), left parietal operculum (x=69, y=46, z=44), and right middle frontal gyrus (x=60, y= 82, z= 51; p’s<.0.016); these results remained significant when controlling for methamphetamine use. Contemplation was unrelated to RSFC of the anterior insula (p>0.016). Of note, in contrast to Precontemplation, no significant negative relationships were found between Contemplation and connectivity of the VOIs.
4. DISCUSSION
The results obtained here suggest that denial in methamphetamine users is associated with a neurocognitive phenotype that may impede insight into one’s behavior. Specifically, in methamphetamine-dependent participants, denial was negatively associated with overall cognitive function, with the effect particularly driven by performance on tests of memory and executive function. Further, denial was negatively correlated with connectivity strength between the rACC and regions of the frontal lobe (precentral gyri, vmPFC, left OFC), limbic system (left temporal lobe, left amygdala, left hippocampus and left parahippocampal gyrus), occipital cortex and cerebellum; and between the precuneus and the midbrain and cerebellum. These relationships were specific to denial, as the tendency to consider one’s substance use potentially problematic and in need of change (Contemplation) was positively associated with connectivity between the rACC and precuneus with other cortical regions. Given previous evidence for a role of the rACC and precuneus in self-awareness (see Cavanna and Trimble, 2006; Goldstein et al., 2009), these findings support the hypothesis that some “denial” may reflect a deficit in self-awareness apart from, or in addition to, a conscious or subconscious attempt to minimize one’s symptoms (Goldstein et al., 2009).
The finding that denial is associated with a weakness in RSFC of the rACC in methamphetamine-dependent subjects is consistent with a report regarding cocaine users (Moeller et al., 2014), indicating that subjects with low insight into their preferences for emotional and drug-related images had less error-related activation of the rACC during performance of an inhibitory control task, and less gray matter volume in the rACC, than participants with intact insight. The current results suggest that stimulant users with low insight may also exhibit a general weakness in connectivity between the rACC and regions important for decision-making (vPFC, OFC; Mesulam, 2000), memory (hippocampus, parahippocampal gyrus; Mesulam, 2000) and emotional reactivity (amygdala; Mesulam, 2000). Given evidence implicating the role of the rACC in conflict-monitoring and evaluating the personal relevance of stimuli (Moeller and Goldstein, 2014; Nestor et al., 2011; Simoes-Franklin et al., 2010), judgments made by stimulant users with low insight may reflect poorer integration between neural substrates important for detecting inconsistencies and making value-laden decisions. This view supports conceptualizations of error awareness as a manifestation of neural networks working in concert, incorporating specialized modules for attention, motivation, conflict-monitoring and cognitive control (Taylor et al., 2007).
The present findings of a negative relationship between denial and precuneus connectivity with midbrain and cerebellum extend previous evidence for a link between precuneus activity and self-awareness (Cavanna and Trimble, 2006). It is unclear precisely how this connectivity contributes to awareness of substance abuse problems, but it is noteworthy that the midbrain and cerebellum have both been associated with learning about the difference between actual versus predicted outcomes. The cerebellum has been hypothesized to encode error signals when cognitive events differ from internal predictive models (similar to error detection in motor control; Popa et al., 2014), while dopaminergic neurons in the midbrain have been shown to track differences between predicted versus actual rewards (i.e., prediction error; Tobler et al., 2005). As such, reduced connectivity between the precuneus, cerebellum and midbrain may confer difficulties in adjusting behavior to information, which violates preconceived expectations.
Some of the relationships between denial and RSFC of the rACC and precuneus were attenuated when covarying for years of regular methamphetamine use. The relationships affected included RSFC between rACC and limbic and frontal regions (hippocampus, amygdala, vmPFC, OFC), as well as RSFC between precuneus and midbrain. Independent of denial, years of methamphetamine use was negatively related to strength of RSFC between rACC and the OFC, and between precuneus and midbrain (as well as ventrolateral PFC). It is therefore possible that relationships between denial and RSFC of these regions, in particular, were spurious. Alternatively, it is also possible that toxicity from methamphetamine use led to reduced RSFC of these regions and denial/poor insight, such that the effects are naturally confounded. Such a causal hypothesis has been supported in a study of rodents which found that cocaine administration impaired both function in the OFC and behavioral markers of insight, with insight restored by subsequent normalizing (optogenetic activation) of the OFC (Lucantonio et al., 2014).
RSFC in several regions was negatively related to denial irrespective of methamphetamine use. These relationships included RSFC of rACC with bilateral precentral gyri, bilateral occipital cortex, and right middle frontal gryus (rMFG); and RSFC of precuneus with regions of cerebellum and brainstem. The persistence of these relationships after controlling for methamphetamine use suggests that weak connectivity between these regions may predate drug abuse and promote denial of drug-related problems after addiction is established. While reduced connectivity with the rMFG may implicate some aspect of decision-making (Cao et al., 2015), it is less immediately clear how connectivity with regions important for vision (occipital lobes; Mesulam, 2000) and motor processing (precentral cortex; Mesulam, 2000) may play a role in denial. Additional research is needed to determine whether these patterns of connectivity play a causal role in denial or alternatively represent epiphenomena.
Reduced connectivity of the rACC and precuneus has been observed in studies of other addictive disorders. For example, compared with healthy control subjects, cocaine users showed weaker RSFC between the rACC and the amygdala, hippocampus, parahippocampal gyrus, insula and temporal cortex (Gu et al., 2010). In another study, cocaine users showed weaker RSFC between the ACC and striatum than controls, and RSFC was negatively associated non-planning impulsivity (Wisner et al., 2013). In addition, subjects with a pathological gambling disorder displayed weaker RSFC between the precuneus and the default mode network than control subjects, with the strength of RSFC inversely associated with the severity of gambling symptoms (Jung et al., 2014).
A recent study found that Precontemplation scores were associated with larger gray matter volume of the left dorsomedial prefrontal cortex in treatment-seeking cocaine users (Moreno-Lopez et al., 2014). Given that these subjects were treatment-seeking, it would be useful to determine whether the results generalize to non-treatment-seeking individuals, in whom Precontemplation may be more indicative of denial. While few other studies have examined relationships between denial and the brain in addiction, the cortical and limbic regions identified in the current study have been variously associated with other abnormalities in methamphetamine users. Compared to healthy control subjects, methamphetamine-dependent participants have exhibited hypoactivity of the ACC associated with inhibitory control deficits (Nestor et al., 2011), increase in cerebral glucose metabolism in the precuneus during early abstinence (Berman et al., 2007), gray matter deficits in the ACC, OFC, precuneus, amygdala and hippocampus (Morales et al., 2012; Thompson et al., 2004), and aberrant relative glucose metabolism in the ACC, OFC and amygdala associated with inattention and mood disturbances (London et al., 2005; London et al., 2004). Future studies are needed to investigate mechanisms by which these abnormalities may, in isolation or combination, contribute to reduced insight/denial.
Significant relationships between anterior insula connectivity and denial were not identified in this study, despite previous research relating the anterior insula to awareness (Craig, 2009). Notably, parallel results have been obtained from cocaine-dependent participants, in which those with low insight into their behavior did not significantly differ in gray matter volume or error-related activation of the insula, compared with those with intact insight (Moeller et al., 2014). These results may suggest that insight into drug abuse, as measured here, is not largely attributable to insula activity. Alternatively, it is possible that insula activity is more critical for situational aspects of insight (rather than generalized at rest), such as the awareness of interoceptive and external craving cues (see Naqvi et al., 2014).
The results relating denial to cognition in this study extend previous research documenting memory and executive deficits in alcohol-abusing individuals with low insight/denial (Blume et al., 2000; Rinn et al., 2002). Given the relationships between these domains and functioning in multimodal association cortex (PFC, parietal cortex) and limbic regions (e.g., hippocampus) (Strauss, 2006), it is possible that function in some of the structures associated with denial also contribute to reduced cognitive function. However, when overall cognitive function was included as a covariate in analyses, it did not attenuate the relationships with rACC and precuneus connectivity. This suggests that potential neuronal commonalities of denial and cognition may not be evident in the resting state, although they might be evident when participants are placed under task demands.
Limitations of this research should be noted. This study is correlational and cannot determine whether the identified relationships between denial and connectivity are causal in nature, nor whether methamphetamine use has potentially caused poor insight. In addition, the sample size of the RSFC analyses was modest (N=21), and further studies are recommended to help establish the generalizability of the results, particularly as some of the null findings may be attributable to insufficient power (e.g., anterior insula). Nonetheless, the results suggest that denial in drug abuse may have important neurocognitive correlates, which could potentially influence the effectiveness of behavioral treatments intended to promote awareness and the desire to change drug use behavior.
Table 1.
Characteristics of Methamphetamine-Dependent Participants
| Entire sample | fMRI subsample | |
|---|---|---|
| Sample size | 58 | 21 |
| Age (years) | 34.14 ± 8.29 (21 – 52) | 36.19 ± 9.30 (22 – 52) |
| Gender (# male) | 37 | 13 |
| Education (years) | 12.57 ± 1.66 (7 – 18) | 12.67 ± 1.68 (10 – 18) |
| Ethnicity | ||
| Caucasian | 28 | 7 |
| African American | 2 | 1 |
| Hispanic | 18 | 8 |
| Asian American | 3 | 1 |
| Other | 7 | 4 |
| Days used alcohol last 30 days | 4.14 ± 6.65 (0 – 30) | 2.89 ± 4.24 (0 – 16) |
| Days used marijuana last 30 days | 3.58 ± 7.34 (0 – 30) | 3.94 ± 8.03 (0 – 30) |
| Tobacco use (# smokers) | 49 | 18 |
| Days used methamphetamine last 30 days | 22.05 ± 7.96 (2 – 30) | 23.00 ± 7.20 (5 – 30) |
| Grams methamphetamine used last week | 3.28 ± 4.25 (0.13 – 28) | 2.18 ± 1.89 (0.25 – 7) |
| Years of regular methamphetamine use | 6.62 ± 5.94 (1 – 28) | 6.52 ± 4.97 (1 – 18) |
Note: Data reflect mean ± standard deviation (range); Regular methamphetamine use = using at least 3 days per week, or twice weekly binges.
Highlights.
It is common for drug abusers to deny having problems with drugs
In methamphetamine abusers, denial is associated with cognitive dysfunction
Denial is linked to weaker brain connectivity in regions vital to self-awareness
The cognitive and neural phenotype of denial may impede insight development
Acknowledgments
Role of Funding Source
Research was supported by NIH grants K23 DA027734 (ACD), R21 DA034928 (ACD), DA 022539 (EDL), DA 020726 (EDL), DA 15179 (EDL), M01 RR00865 (UCLA GCRC), UL1TR000124 (UCLA CTSI) and endowments from the Thomas P. and Katherine K. Pike Chair in Addiction Studies (EDL) and the Marjorie M. Greene Trust. M. Kohno and A. Morales were supported by training grants T32 DA 024635, F31 DA033120-02 and F31 DA0331-17. The listed funding sources had no further role in study design, in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Contributors
AD had primary responsibility for writing the manuscript and developing its conceptual aims. MK conducted and described fMRI analyses and contributed to conceptual content. AM assisted with fMRI analyses and contributed to conceptual content. DG contributed to conceptual content. EL supervised all research procedures and contributed to conceptual content. All authors critically reviewed the manuscript and approved the final version for publication.
Conflict of Interest
All other authors declare that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersson J, Jenkinson M, Smith S. Non-linear registration, aka Spatial normalisation. FMRIB technical report 2007 [Google Scholar]
- Berman SM, Voytek B, Mandelkern MA, Hassid BD, Isaacson A, Monterosso J, Miotto K, Ling W, London ED. Changes in cerebral glucose metabolism during early abstinence from chronic methamphetamine abuse. Mol Psychiatry. 2007;13:897–908. doi: 10.1038/sj.mp.4002107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blume AW, Marlatt GA, Schmaling KB. Executive cognitive function and heavy drinking behavior among college students. Psychol Addict Behav. 2000;14:299–302. [PubMed] [Google Scholar]
- Borkowski J, Benton A, Spreen O. Word fluency and brain damage. Neuropsychologia. 1967;5:135–140. [Google Scholar]
- Buschke H. Retention in immediate memory estimated without retrieval. Science. 1963;140:56–57. doi: 10.1126/science.140.3562.56. [DOI] [PubMed] [Google Scholar]
- Cao J, Chen JM, Kuang L, Ai M, Fang WD, Gan Y, Wang W, Chen XR, Xu XM, Wang HG, Lv Z. Abnormal regional homogeneity in young adult suicide attempters with no diagnosable psychiatric disorder: a resting state functional magnetic imaging study. Psychiatry Res. 2015;231:95–102. doi: 10.1016/j.pscychresns.2014.10.011. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Dare PAS, Derigne L. Denial in alcohol and other drug use disorders: a critique of theory. Addict Res Theory. 2010;18:181–193. [Google Scholar]
- Dean AC, Hellemann G, Sugar CA, London ED. Educational attainment is not a good proxy for cognitive function in methamphetamine dependence. Drug Alcohol Depend. 2012;123:249–254. doi: 10.1016/j.drugalcdep.2011.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deen B, Pitskel NB, Pelphrey KA. Three systems of insular functional connectivity identified with cluster analysis. Cereb Cortex. 2011;21:1498–1506. doi: 10.1093/cercor/bhq186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- DiClemente CC, Schlundt D, Gemmell L. Readiness and stages of change in addiction treatment. Am J Addict. 2004;13:103–119. doi: 10.1080/10550490490435777. [DOI] [PubMed] [Google Scholar]
- Faget-Agius C, Boyer L, Padovani R, Richieri R, Mundler O, Lancon C, Guedj E. Schizophrenia with preserved insight is associated with increased perfusion of the precuneus. J Psychiatry Neurosci. 2012;37:297–304. doi: 10.1503/jpn.110125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. The Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-IP) American Psychiatric Press; Washington, D.C: 1995. [Google Scholar]
- Golden CJ. Stroop Color And Word Test: A Manual For Clinical And Experimental Uses. Stoelting Company; Wood Dale, IL: 1978. [Google Scholar]
- Goldstein RZ, Craig AD, Bechara A, Garavan H, Childress AR, Paulus MP, Volkow ND. The neurocircuitry of impaired insight in drug addiction. Trends Cogn Sci. 2009;13:372–380. doi: 10.1016/j.tics.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H, Salmeron BJ, Ross TJ, Geng X, Zhan W, Stein EA, Yang Y. Mesocorticolimbic circuits are impaired in chronic cocaine users as demonstrated by resting-state functional connectivity. Neuroimage. 2010;53:593–601. doi: 10.1016/j.neuroimage.2010.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester R, Nestor L, Garavan H. Impaired error awareness and anterior cingulate cortex hypoactivity in chronic cannabis users. Neuropsychopharmacology. 2009;34:2450–2458. doi: 10.1038/npp.2009.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester R, Simoes-Franklin C, Garavan H. Post-error behavior in active cocaine users: poor awareness of errors in the presence of intact performance adjustments. Neuropsychopharmacology. 2007;32:1974–1984. doi: 10.1038/sj.npp.1301326. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jung MH, Kim JH, Shin YC, Jung WH, Jang JH, Choi JS, Kang DH, Yi JS, Choi CH, Kwon JS. Decreased connectivity of the default mode network in pathological gambling: a resting state functional MRI study. Neurosci Lett. 2014;583:120–125. doi: 10.1016/j.neulet.2014.09.025. [DOI] [PubMed] [Google Scholar]
- Kenny P, Harney A, Lee NK, Pennay A. Treatment utilization and barriers to treatment: results of a survey of dependent methamphetamine users. Subst Abuse Treat Prev Policy. 2011;6:3. doi: 10.1186/1747-597X-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein TA, Endrass T, Kathmann N, Neumann J, von Cramon DY, Ullsperger M. Neural correlates of error awareness. Neuroimage. 2007;34:1774–1781. doi: 10.1016/j.neuroimage.2006.11.014. [DOI] [PubMed] [Google Scholar]
- Kohno M, Morales AM, Ghahremani DG, Hellemann G, London ED. Risky Decision making, prefrontal cortex, and mesocorticolimbic functional connectivity in methamphetamine dependence. JAMA Psychiatry. 2014;71:812–820. doi: 10.1001/jamapsychiatry.2014.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz E. Why A.A. works; the intellectual significance of Alcoholics Anonymous. J Stud Alcohol. 1982;43:38–80. doi: 10.15288/jsa.1982.43.38. [DOI] [PubMed] [Google Scholar]
- Liemburg EJ, van der Meer L, Swart M, Curcic-Blake B, Bruggeman R, Knegtering H, Aleman A. Reduced connectivity in the self-processing network of schizophrenia patients with poor insight. PLoS One. 2012;7:e42707. doi: 10.1371/journal.pone.0042707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London ED, Berman S, Voytek B, Simon SL, Monterosso J, Geaga JA, Hong M, Hayashi KM, Thompson P, Mandelkern MA, Brody AL, Rawson R, Ling W. Cerebral metabolic dysfunction and impaired vigilance in recently abstinent methamphetamine abusers. Biol Psychiatry. 2005;58:770–778. doi: 10.1016/j.biopsych.2005.04.039. [DOI] [PubMed] [Google Scholar]
- London ED, Simon SL, Berman SM, Mandelkern MA, Lichtman AM, Bramen J, Shinn AK, Miotto K, Learn J, Dong Y, Matochik JA, Kurian V, Newton T, Woods R, Rawson R, Ling W. Mood disturbances and regional cerebral metabolic abnormalities in recently abstinent methamphetamine abusers. Arch Gen Psychiatry. 2004;61:73–84. doi: 10.1001/archpsyc.61.1.73. [DOI] [PubMed] [Google Scholar]
- Lou HC, Luber B, Crupain M, Keenan JP, Nowak M, Kjaer TW, Sackeim HA, Lisanby SH. Parietal cortex and representation of the mental Self. Proc Natl Acad Sci USA. 2004;101:6827–6832. doi: 10.1073/pnas.0400049101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucantonio F, Takahashi YK, Hoffman AF, Chang CY, Bali-Chaudhary S, Shaham Y, Lupica CR, Schoenbaum G. Orbitofrontal activation restores insight lost after cocaine use. Nat Neurosci. 2014;17:1092–1099. doi: 10.1038/nn.3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnaughy EA, DiClemente CC, Prochaska JO, Velicer WF. Stages of change in psychotherapy: measurement and sample profiles. Psychothera Theory Res Pract. 1989;20:368–375. [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M. Behavioral neuroanatomy: large-scale networks, association cortex, frontal syndromes, the limbic system, and hemispheric specializations. In: Mesulam M, editor. Principles Of Behavioral And Cognitive Neurology. Oxford University Press; Oxford: 2000. pp. 1–120. [Google Scholar]
- Moeller SJ, Goldstein RZ. Impaired self-awareness in human addiction: deficient attribution of personal relevance. Trends Cogn Sci. 2014;18:635–641. doi: 10.1016/j.tics.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller SJ, Konova AB, Parvaz MA, Tomasi D, Lane RD, Fort C, Goldstein RZ. Functional, structural, and emotional correlates of impaired insight in cocaine addiction. JAMA Psychiatry. 2014;71:61–70. doi: 10.1001/jamapsychiatry.2013.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller SJ, Maloney T, Parvaz MA, Alia-Klein N, Woicik PA, Telang F, Wang GJ, Volkow ND, Goldstein RZ. Impaired insight in cocaine addiction: laboratory evidence and effects on cocaine-seeking behaviour. Brain. 2010;133:1484–1493. doi: 10.1093/brain/awq066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales AM, Lee B, Hellemann G, O’Neill J, London ED. Gray-matter volume in methamphetamine dependence: cigarette smoking and changes with abstinence from methamphetamine. Drug Alcohol Depend. 2012;125:230–238. doi: 10.1016/j.drugalcdep.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Lopez L, Albein-Urios N, Martinez-Gonzalez JM, Soriano-Mas C, Verdejo-Garcia A. Prefrontal gray matter and motivation for treatment in cocaine-dependent individuals with and without personality disorders. Front Psychiatry. 2014;5:52. doi: 10.3389/fpsyt.2014.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Gaznick N, Tranel D, Bechara A. The insula: a critical neural substrate for craving and drug seeking under conflict and risk. Ann N Y Acad Sci. 2014;1316:53–70. doi: 10.1111/nyas.12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestor LJ, Ghahremani DG, Monterosso J, London ED. Prefrontal hypoactivation during cognitive control in early abstinent methamphetamine-dependent subjects. Psychiatry Res. 2011;194:287–295. doi: 10.1016/j.pscychresns.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peteet JR, Brenner S, Curtiss D, Ferrigno M, Kauffman J. A stage of change approach to addiction in the medical setting. Gen Hosp Psychiatry. 1998;20:267–273. doi: 10.1016/s0163-8343(98)00033-4. [DOI] [PubMed] [Google Scholar]
- Popa LS, Hewitt AL, Ebner TJ. The cerebellum for jocks and nerds alike. Front Syst Neurosci. 2014;8:113. doi: 10.3389/fnsys.2014.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage. 2011;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochaska JO, DiClemente CC, Norcross JC. In search of how people change. Applications to addictive behaviors. Am Psychol. 1992;47:1102–1114. doi: 10.1037//0003-066x.47.9.1102. [DOI] [PubMed] [Google Scholar]
- Reitan RM. Validity of the trail making test as an indicator of organic brain damage. Percept Mot Skills. 1958;8:271–276. [Google Scholar]
- Rinn W, Desai N, Rosenblatt H, Gastfriend DR. Addiction denial and cognitive dysfunction: a preliminary investigation. J Neuropsychiatry Clin Neurosci. 2002;14:52–57. doi: 10.1176/jnp.14.1.52. [DOI] [PubMed] [Google Scholar]
- Simoes-Franklin C, Hester R, Shpaner M, Foxe JJ, Garavan H. Executive function and error detection: the effect of motivation on cingulate and ventral striatum activity. Hum Brain Mapp. 2010;31:458–469. doi: 10.1002/hbm.20879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon SL, Dacey J, Glynn S, Rawson R, Ling W. The effect of relapse on cognition in abstinent methamphetamine abusers. J Subst Abuse Treat. 2004;27:59–66. doi: 10.1016/j.jsat.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Simon SL, Dean AC, Cordova X, Monterosso JR, London ED. Methamphetamine dependence and neuropsychological functioning: evaluating change during early abstinence. J Stud Alcohol Drugs. 2010;71:335–344. doi: 10.15288/jsad.2010.71.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodgrass JG, Vanderwart M. A standardized set of 260 pictures: norms for name agreement, image agreement, familiarity, and visual complexity. J Exp Psychol Hum Learn. 1980;6:174–215. doi: 10.1037//0278-7393.6.2.174. [DOI] [PubMed] [Google Scholar]
- Strauss E, Sherman EMS, Spreen O. Administration, Norms, And Commentary. Oxford University Press; New York: 2006. A Compendium Of Neuropsychological Tests. [Google Scholar]
- Taylor SF, Stern ER, Gehring WJ. Neural systems for error monitoring: recent findings and theoretical perspectives. Neuroscientist. 2007;13:160–172. doi: 10.1177/1073858406298184. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Hayashi K, Simon SL, Geaga JA, Hong MS, Sui Y, Lee JY, Toga AW, Ling W, London ED. Structural abnormalities in the brains of human subjects who use methamphetamine. J Neurosci. 2004;24:6028–6036. doi: 10.1523/JNEUROSCI.0713-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobler PN, Fiorillo CD, Schultz W. Adaptive coding of reward value by dopamine neurons. Science. 2005;307:1642–1645. doi: 10.1126/science.1105370. [DOI] [PubMed] [Google Scholar]
- Vanhaudenhuyse A, Demertzi A, Schabus M, Noirhomme Q, Bredart S, Boly M, Phillips C, Soddu A, Luxen A, Moonen G, Laureys S. Two distinct neuronal networks mediate the awareness of environment and of self. J Cogn Neurosci. 2010;23:570–578. doi: 10.1162/jocn.2010.21488. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechsler Adult Intelligence Scale - Revised. The Psychological Corporation; New York: 1981. [Google Scholar]
- Wisner KM, Patzelt EH, Lim KO, MacDonald AW., 3rd An intrinsic connectivity network approach to insula-derived dysfunctions among cocaine users. Am J Drug Alcohol Abuse. 2013;39:403–413. doi: 10.3109/00952990.2013.848211. [DOI] [PubMed] [Google Scholar]
- Woodcock RW, Johnson MB. Woodcock-Johnson Psycho-Educational Battery. DLM Teaching Resources; Allen, TX: 1977. [Google Scholar]
- Zimmerman GL, Olsen CG, Bosworth MF. A ‘stages of change’ approach to helping patients change behavior. Am Fam Physician. 2000;61:1409–1416. [PubMed] [Google Scholar]


