Abstract
Astrocytes are highly ramified glial cells found throughout the central nervous system (CNS). They express a variety of neurotransmitter receptors that can induce widespread chemical excitation, placing these cells in an optimal position to exert global effects on brain physiology. However, the activity patterns of only a small fraction of astrocytes have been examined and techniques to manipulate their behavior are limited. As a result, little is known about how astrocytes modulate CNS function on synaptic, microcircuit, or systems levels. Here, we review current and emerging approaches for visualizing and manipulating astrocyte activity in vivo. Deciphering how astrocyte network activity is controlled in different physiological and pathological contexts is critical for defining their roles in the healthy and diseased CNS.
Introduction
The adult human brain contains roughly equal numbers of neurons and glial cells [1,2]. Historically, it was believed that there was a clear division of labor among these two cell classes, with glia relegated to performing supportive roles to ensure that neuronal activity can be sustained. In the last few decades, our knowledge about the diverse roles played by different glial cell types has expanded dramatically, and it is now clear that they can exert a profound influence on neuronal synaptic plasticity, excitability, and behavior. Among glial cells, astrocytes are in a unique position to modulate brain activity. They are ubiquitous in all gray and white matter regions [3], they express receptors for neurotransmitters, and they extend highly ramified processes that interact with synapses, nodes of Ranvier, blood vessels, and many other CNS elements. Astrocytes also exhibit structural and functional dynamics on spatial and temporal scales that span several orders of magnitude (from micrometers to millimeters and from milliseconds to weeks). Measuring their dynamics and relating these events to distinct CNS functions remains a significant challenge, requiring development of a wide range of techniques to monitor and manipulate their local and global activity patterns in vivo in both physiological and pathological contexts.
In this review, we focus on current and emerging approaches for measuring the activity of astrocytes at the synaptic, microcircuit, and systems levels. Although most of our insight into the physiology and function of astrocytes has come from in vitro studies (primary cultures, acute brain slices), emphasis here has been placed on techniques that enable visualization of their dynamics in the intact CNS of live animals, and insights that have been obtained from these studies. We conclude by discussing current technical challenges that need to be overcome to obtain a mechanistic understanding of the many roles of astrocytes in brain function.
Astrocytes in neural circuits
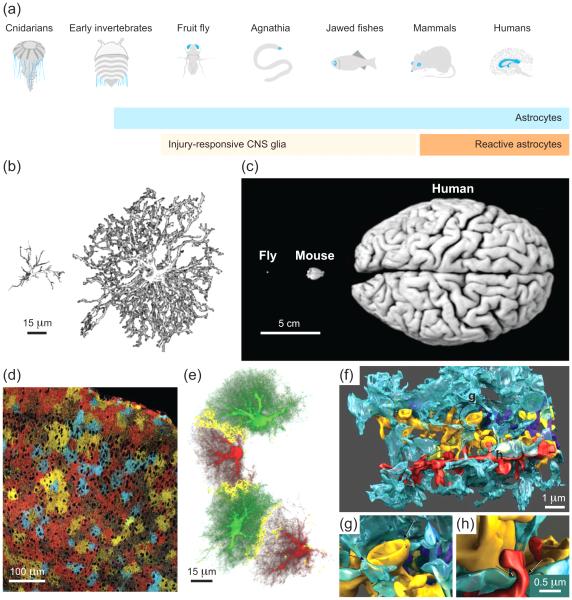
Astrocytes in different regions of the CNS share a number of common features – they have a high resting conductance to potassium and low membrane resistance, they are electrically unexcitable and lack synaptic specializations and long-range projections, they are extensively coupled to each other through gap junctions, they express a high density of glutamate transporters, they form end feet specializations on blood vessels, and they express G-protein coupled receptors that liberate intracellular calcium. Individual astrocytes also establish and maintain distinct territories, defined by their numerous, highly ramified processes, with adjacent cells occupying largely non-overlapping domains in rodents [4-6] (Figure 1d-e). Thin lamellae extend from their processes to wrap neuronal and non-neuronal structures [7], giving rise to their extraordinarily complex morphology (Figure 1f-h). At the tips of their processes they connect to each other through gap junctions; as a result, astrocytes form a vast network of interconnected cells, providing nearly complete coverage of the CNS.
Figure 1.
Astrocyte complexity across spatial scales and species. (a) Astrocytes are found in both vertebrate and invertebrate species, and their size and complexity increases with phylogeny (b). (b) Computer drawings show three-dimensional (3D) reconstructions of mouse (left) and human (right) cortical astrocytes based on glial fibrillary acidic protein (GFAP) immunostaining. Note that GFAP-positive filaments are restricted to the cell body and main processes of astrocytes, representing only a small fraction (~15%) of the cell’s actual volume. Cortical astrocytes with different morphologies are present in the human brain (not shown), suggesting that greater diversification has occurred with evolution. (c) Brain size changes with phylogeny. Because scattering and absorption restrict fluorescence imaging depth, a smaller proportion of astrocytes can be visualized in the larger brains of higher organisms in vivo. (d) Fluorescence image shows cross-section through the cortex of a “Brainbow” mouse, in which three different fluorophores were expressed stochastically in astrocytes. Dark round areas represent primarily neuronal cell bodies, illustrating the extraordinary coverage of the CNS by astrocytes. In a given brain region, astrocytes are extensively coupled through gap junctions. (e) Image shows 3D reconstruction of four dye-filled astrocytes in mouse dentate gyrus. Within gap junction-coupled networks, individual astrocytes (green or red) occupy distinct territories that exhibit minimal overlap (yellow) with those of neighboring astrocytes. (f) Image shows 3D reconstruction from electron microscopy data of four dendrites (red, yellow, gold, purple) and protrusions of a nearby astrocytic process (blue) in rat hippocampus. Processes of individual astrocytes exhibit highly intricate lamellar protrusions. (g-h) Astrocytic protrusions contact some but not all neuronal boutons or spines. Images are blow-ups of regions indicated in (f). (d-h) Note that images are snapshots taken at a particular point in the animal’s life. Astrocytes remain structurally and functionally plastic in the adult brain. See text for more details. (a-b), (f-h) adapted from [107,130,131] with permission from Elsevier, and John Wiley and Sons. (c) courtesy of Dr. Frank Hirth, King's College London. (d) courtesy of Jean Livet, Joshua Sanes and Jeff Lichtman, Harvard University, and adapted from [5] with permission from Nature Publishing Group. (e) reprinted from [132], copyright (2006) National Academy of Sciences, U.S.A.
Despite these shared characteristics, astrocytes are not homogenous. For example, fibrous astrocytes in white matter have processes that are more polarized and less complex than protoplasmic astrocytes, their gray matter counterparts, and astrocyte density varies between CNS regions and cell layers [8]. In addition, the complement of receptors and transporters expressed [9] and the extent of gap junction coupling varies between different regions of the CNS [10], suggesting that they can adapt to the unique requirements of their local environment. Some physiological features, such as gap junction coupling [11], glutamate transporter expression [12], and synapse ensheathment [13] can be modulated on rapid time scales by neuronal activity, while aging and pathological conditions can induce slower but more dramatic phenotypic changes (e.g., reactive astrocytosis) [14]. These structural and functional alterations at the synaptic, microcircuit, and systems level [15-18], are believed to help organisms adapt to new environmental demands, and conversely, disturbances in this homeostatic adaptation are likely to contribute to CNS disease [19].
Different scales of astrocyte functional dynamics
Astrocyte networks are particularly well positioned to integrate both neuronal and non-neuronal signals to regulate diverse CNS functions, such as neural network excitability and metabolism, on various spatial and temporal scales [20,21]. In particular, astrocytes express a rich repertoire of G-protein coupled receptors for neurotransmitters, and in some regions, ligand-gated ion channels (NMDA and AMPA receptors), providing a means to modulate their physiology in response to local neural activity and global shifts in brain states. However, little is known about the types of information that astrocytes extract from these events or how astrocytes use this information to modify their behavior.
Although astrocytes express ligand- and voltage-gated ion channels, receptors, and electrogenic transporters, they do not exhibit large deviations in membrane potential in response to neuronal stimulation [22], due to their low resistance and high conductance to potassium, which effectively clamps their membrane potential at the potassium equilibrium potential. As a result, astrocyte activity has been largely invisible to electrophysiological methods, such as extracellular unit recording, that are used to monitor neuronal activity in vivo. The development of fluorescent indicators for calcium that can be loaded into cells led to the discovery that astrocytes exhibit dynamic changes in intracellular calcium [23,24] that are markedly enhanced by neurotransmitters. Distinct forms of calcium signaling have been described that involve activation of calcium-permeable plasma membrane channels and/or release of calcium from internal stores [25,26]. These astrocyte calcium transients are widely considered to be a form of glial excitability, but they do not exhibit a stereotyped waveform like action potentials, and thus the functional outcomes of this activity are likely to be more diverse and nuanced. While it is certain that elevations in calcium are not the only form of activity exhibited by astrocytes, the ability to detect these changes has provided key insight into astrocyte dynamics and neuron-astrocyte interactions in vivo.
Microdomain activity
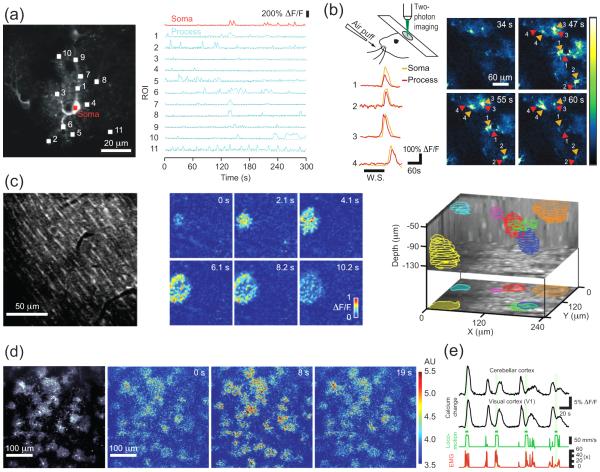
Astrocytic processes exhibit spatially confined (less than a few micrometers in diameter) calcium transients with an average duration of a few seconds [27,28] (Figure 2a-b); in vivo, this activity may be confined due to structurally isolated regions within the astrocyte arbor, termed microdomains. Microdomain activity has been observed in both anesthetized and awake animals and persists when neural activity and most, if not all, neurotransmitter receptors have been blocked, and is therefore thought to be intrinsically generated [29-31]. Moreover, activity in different microdomains is largely uncorrelated in the absence of stimulation [27,30], but can be synchronized when surface receptors are activated. However, because only a small region of the astrocyte, and thus only a tiny fraction of microdomains in a given cell, can be measured within the focal plane of a two-photon microscope, which is the predominant tool for monitoring astrocyte calcium signaling in vivo, a complete picture of their diversity and spatiotemporal evolution (i.e., in the z-direction) is lacking. Additionally, given the limited spatial resolution of two-photon microscopy much of the fine-scale dynamics of microdomain activity have not been resolved.
Figure 2.
Representative forms of astrocytic calcium activity in the adult brain. (a) Spontaneous calcium activity in astrocytic processes and soma. Left, image shows a GCaMP3-expressing astrocyte in stratum lucidum of a mouse hippocampal slice. Right, calcium transients detected in the numbered regions of interest (ROIs) shown at the left. (b) Sensory-evoked calcium activity in astrocytic processes and somata in vivo. Top left, schematic of experimental setup. Right, time-lapse images showing GCaMP5G-expressing astrocytes in layer 2/3 of barrel cortex from an anesthetized mouse. Time after onset of whisker stimulation (W.S.; 5 Hz, 30 s duration) is shown in top right corners. Pseudocolor scale on right indicates changes in GCaMP5G fluorescence over baseline (ΔF/F0). Bottom left, time course comparison of evoked calcium transients in astrocytic processes (red) and somata (orange) from regions indicated on the right. (c) Spontaneous, localized multi-cellular calcium waves/bursts in portions of few neighboring cortical cerebellar astrocytes (Bergmann glia) in vivo. Left, fluorescence image showing optical cross-section through processes from many different astrocytes in the molecular layer of cerebellar cortex from an anesthetized rat stained with synthetic calcium indicator Fluo-5F. Center, time-lapse images showing spatiotemporal evolution of a multi-cellular calcium wave, displayed as ΔF/F0, at a select imaging depth. Time after event onset is indicated in top right corners. Right, volumetric profiles of individual calcium waves/bursts (shown in the same color) detected by 3D two-photon microscopy during an 8.2 min imaging period. (d) Locomotion-evoked calcium transients in astrocytes of the visual cortex (V1) in vivo. Black and white image, fluorescence image of layer 1 astrocytes in primary visual cortex (V1) of a GLAST-CreER;R26-lsl-GCaMP3 mouse. Color images, spatiotemporal evolution of concerted astrocytic calcium activity. Time after onset of walking on a linear treadmill is indicated in top right corners. (e) Locomotion-evoked large-scale calcium activity in populations of astrocytes from two distinct brain regions, measured simultaneously using dual fiber-optic photometry. Black traces show ΔF/F0 in GCaMP3 bulk fluorescence from astrocytes in indicated brain regions. Green shows locomotor activity and rest as detected by an optical encoder coupled to the linear treadmill; periods of enforced locomotion are highlighted by the horizontal green bars and vertical green-shaded areas. Red trace shows simultaneously recorded electromyography (EMG; given as fold increase in 200 Hz – 1 kHz EMG power). (a-e) Note that kinetics, amplitude and frequency of detected calcium transients may depend on employed fluorescence indicator or staining method. See text for more details. (a-e) adapted from [31,34,35,38,40] with permission from Elsevier and National Academy of Sciences.
Somatic activity
In addition to spatially localized microdomain activity, astrocytes exhibit pronounced somatic calcium transients, particularly when animals experience strong sensory input or when astrocytes are directly stimulated with neuromodulators (Figure 2a-b, d). These somatic events result from IP3-mediated release of calcium from internal stores [32,33], are less frequent and exhibit somewhat slower kinetics than microdomain events [34,35]. Both neuronal activity-dependent and -independent transients have been described, with the frequency and degree of temporal correlation depending on brain region [30,36]. Although it is tempting to speculate that activity in microdomains, like synaptic events in neurons, summate to produce somatic events if a threshold is exceeded, the precise functional relationship between microdomain activity and somatic events has not yet been established. Sensory-evoked activity in astrocytic somata tends to be sparse, variable, and stimulus-dependent [37-39]. Due to tissue scattering and absorption, a lack of efficient long-wavelength calcium indicators, and technical challenges associated with imaging large tissue volumes, optical recording of large-scale astrocyte (somatic) activity is currently limited to a few hundred micrometers beneath the pial surface and relatively low (a few Hz) sampling rates. Thus, the relationship of these local events to neuronal and glial cell activity in other brain regions remains largely unexplored.
Localized multi-cellular waves of activity
Cortical cerebellar astrocytes (termed Bergmann glia) exhibit radially-expanding, ATP-dependent calcium waves that engage groups of astrocytic microdomains within an ellipsoid volume tens of micrometers in diameter around their focal site of origin [31,40] (Figure 2c). This activity is reminiscent of the intercellular “calcium waves” that can be induced in cultured astrocytes upon focal application of glutamate or mechanical stimulation [23,24,41]. These calcium waves depend on calcium release from internal stores, have an average duration of only a few seconds, appear to be independent of sensory input, and occur in both anesthetized and awake animals. Their frequency is low (tens to hundreds of mHz/mm2) but may increase with age and reductions in tissue oxygen tension [42]. Differences in tissue preparation may contribute to the variable incidence of these events, as less invasive procedures show seemingly fewer and spatially less clustered events (Nimmerjahn and Bergles, unpublished observations). Hence, the mechanisms that induce the release of ATP from astrocytes and the functional role of these multi-cellular calcium waves remains to be determined. Notably, this activity is rarely observed in the cerebral cortex [30,38,43] (but see [44]). In vitro, calcium wave propagation requires repetitive release of ATP and sequential activation of P2Y purinergic receptors as the wave spreads outward [45,46]; although gap junctions can modulate the speed and extent of propagation, they are not required. The smaller spread of these waves in vivo may reflect a higher abundance of extracellular nucleotidases, enzymes which hydrolyze ATP to adenosine.
Large-scale concerted activity
Two-photon imaging in primary somatosensory and anterior cerebellar cortex of head-restrained, mobile mice revealed that astrocytes show behavioral state- and neuronal activity-dependent concerted calcium increases across areas several hundred micrometers in diameter with an average duration of many seconds [31,44] (Figure 2d-e). These concerted transients, which involve most, but perhaps not all astrocytes or astrocytic processes, also appear to depend on calcium release from internal stores. Their amplitude and probability of occurrence depends on inter-stimulus interval [31,38], perhaps resulting from receptor desensitization or calcium store depletion. This large-scale calcium activity in astrocytes can be initiated by aversive stimuli [38,43], locomotion [31,38], or direct stimulation of the nucleus basalis [32] where cholinergic neurons are located, or locus coeruleus [47], where noradrenergic neurons are located. Using fiber photometry, which allows bulk but not cellular-level measurements, [38], it was shown that concerted astrocytic calcium increases were synchronized across anterior cerebellar and primary (V1) visual cortices (Figure 2e). Thus, it appears that different neuromodulatory inputs can trigger widespread activation of astrocytes, in keeping with the diffuse, highly ramified nature of these projections. This study also reported that while visual stimulation alone was ineffective in evoking concerted astrocytic calcium responses in V1 of awake, resting mice, calcium transients in astrocytes were enhanced beyond that produced by locomotion alone when visual stimuli were applied during locomotion, suggesting that norepinephrine can alter the responsiveness of astrocytes to local circuit activity. Visual stimuli-independent but locomotion-enhanced activity has also been described for cortical neurons (e.g., vasoactive intestinal peptide-positive V1 neurons, though in their case nicotinic inputs from basal forebrain were identified as the anatomical source of neuromodulation [48]). Although these studies suggest that widespread activation of astrocyte networks occurs when neuromodulators are released, the full three-dimensional extent of large-scale concerted astrocytic activity and its relationship to neuromodulatory projections and local neural network activity remains unknown. Approaches providing increased fields of view or simultaneous multi-site imaging, greater depth penetration, and fast volume sampling, would help define the patterns of astrocyte activity induced by neuromodulators in different behavioral contexts.
There is increasing appreciation that astrocytes in different regions of the CNS exhibit different functional characteristics [49]; for example, astrocytes in the cortex have been shown to express NMDA receptors [50], while those in the hippocampus do not appear to [22]. However, the pattern of activity exhibited by astrocytes in vivo in regions other than the superficial cortical layers remains largely unexplored. Long-wavelength multi-photon microscopy [51-53], which experiences less light attenuation, in combination with red-shifted calcium indicators, sparse tissue labeling, and adaptive optics approaches, which correct for tissue-induced wavefront distortions, will allow minimally invasive optical recordings from deeper brain regions. However, imaging depth is ultimately limited by fluorophore brightness, out-of-focus background fluorescence generation, the objective/detector’s limited light collection angle, and other factors [54]. Optical recording from brain regions beyond the imaging depth limit has been achieved by aspirating overlying tissue, implanting a biocompatible tissue-stabilizing transparent gel or glass window-bearing guide tube, and imaging with a long working distance objective or gradient index (GRIN) lens [55-57]. Alternatively, optical components such as microprisms can be implanted directly into the brain to deliver and capture light [58]. Although these approaches have yielded new insight into the relationship between neural activity and behavior [55,56,59], they may be ill-suited for the study of normal astrocyte activity. Astrocytes are highly sensitive to tissue damage, and exhibit widespread and prolonged structural and functional changes following CNS injury [14,60]. Approaches that induce less inflammatory responses or can image far beyond the glial scar are needed.
Another largely unexplored issue is whether astrocyte activity patterns change over the course of hours, days, or weeks in response to changes in life experience. Long-term imaging studies in cortical and deep brain regions of behaving mice have revealed complex learning-related changes in neuronal activity patterns, such as enhanced temporal correlation of activity among neurons that respond to similar aspects of a learned task [61], increases in task-related population activity despite variable single-neuron responses [62], and neuron type- and layer-dependent changes in ensemble activity across trials [63,64]. Given the close interrelationship between astrocytic and neuronal activity, changes in the spatiotemporal excitation patterns of astrocytes may also occur following training, as a consequence of the change in activity patterns of nearby neurons or as a result of intrinsic adaptive changes. Indeed, recent studies suggest that secretion from astrocytes can alter ensemble neuronal network activity such as gamma oscillations [65] and influence cyclical behaviors such as sleep [66,67] (but see [68,69]). The development of new transgenic mouse lines in which genetically encoded calcium indicators (GECIs) can be expressed stably in astrocytes will help to define astrocyte network activity on more prolonged time scales [35,38,70,71], and enable simultaneous optical monitoring of astrocyte and neuronal activity through cell-specific expression of different-colored GECIs [72,73].
Although we do not yet know if or how learning alters astrocyte activity patterns, it is clear that calcium signaling in astrocytes is not static. Evolving changes in the spatiotemporal activity patterns of astrocytes have been observed in many diseases and following traumatic injury to the CNS [74]. For example, astrocytes in mouse models of Alzheimer’s disease exhibit higher resting calcium levels, a higher frequency of calcium transients, and intercellular waves that propagate outward from amyloid deposits [75]. In ischemic stroke, astrocytes at and near the lesion site show larger amplitude, higher frequency, and more synchronized, calcium wave-like activity during the acute phase [76]; changes in astrocytic calcium responses were also seen in the contralateral hemisphere [77], suggesting that their activity can be altered without direct injury. In addition, astrocytes show region-dependent structural changes during early and late post-ischemic phases [78,79]. Similarly complex structural and functional alterations are seen in other cell types [80-82]. However, most of these cellular-level changes in astrocytes have been recorded in cortical areas and represent between-animal comparisons, rather than longitudinal studies of the transformation of individual astrocytes. While long-wavelength and multi-site multi-photon imaging approaches are likely to increase the depth and area that can be monitored in the future, large parts of the affected tissue will remain inaccessible with these high-resolution techniques. One way to bridge this gap is to combine high-resolution imaging with lower-resolution, whole-tissue imaging approaches [82-84], allowing cellular level observations to be related to systems level changes and behavioral phenotypes.
Defining the role of astrocytes in neural networks
While fluorescent indicator imaging has revealed the remarkable diversity of astrocyte activity patterns in intact circuits, much remains to be learned about the pathways involved in generating various forms of astrocytic excitation, and their downstream functional consequences for astrocytes (e.g., acute metabolic regulation or long-term gene expression changes), and for surrounding neuronal and non-neuronal cells (e.g., neuronal excitability or blood flow changes).
To bridge this gap in our understanding, a variety of in vivo approaches are being employed to interrogate and manipulate astrocyte activity in healthy animals. For example, in vivo pharmacology has revealed that some forms of excitation, particularly spatially localized events within astrocytes, do not depend on neuronal activity, and for the ones that do, the signaling pathways that contribute to their generation [31,38,43]. In addition, transgenic approaches have shown that large-scale, correlated activity in astrocyte networks rely on IP3-mediated release of calcium from internal stores as a result of G-protein coupled receptor activation [32,33,85]. They have also provided evidence that calcium excitation can lead to local neuromodulatory effects and changes in animal behavior [65,67,70]. Transgenic manipulation is not always possible or practical; thus, development of approaches to acutely alter gene expression in astrocytes, such as viral infection [17] or in utero electroporation [86] provide additional opportunities for mechanistic studies of astrocyte functions in vivo.
Nevertheless, interrogating the behavior of astrocytes and defining the consequences of their activity in live animals remains challenging. For example, with in vivo pharmacological experiments it is difficult to control the concentration and sites of influence, limiting conclusions that can be made regarding the receptors and intracellular pathways involved in a response. Additionally, given our limited knowledge about in vivo drug actions [87] and the expression of receptors in other cell types [88,89], pharmacological interventions may lead to unexpected side effects (e.g., affect ion channel surface expression [90]). Exogenous fluorescent indicators can themselves influence optical recordings: insufficient indicator may lead to the erroneous conclusion of inactivity, while surplus indicator may compromise normal astrocytic function by chelating calcium [91]. In addition, transgenic approaches, particularly those involving constitutive expression of transgenes [92], may lead to whole-brain or whole-animal disturbances, making it difficult to relate local changes in astrocytic activity to altered microcircuit function or behavioral phenotypes [93,94]. Chemogenetic approaches, such as those involving DREADDS or optogenetic approaches, such as those which utilize channelrhodopsin or light-responsive G-protein receptors [95], have been effective at delineating the contribution of different classes of neurons and neural networks to behavior. At present, it is unclear whether the collection of genetically encoded effectors currently available will be similarly useful in defining the role of astrocytes or whether further modification or development of new effectors will be required; there is concern that these proteins may only partially engage endogenous signaling cascades (e.g., through pharmacological activation of foreign receptors expressed in astrocytes), cause inadvertent shifts in ion gradients (e.g., promote proton influx through channelrhodopsin) or insufficiently mimic normal spatiotemporal forms of astrocytic excitation (e.g., by recruiting pathways only active under high levels of calcium, or by inhibiting normal cell signaling in the aftermath of concerted calcium store depletion). The mode of gene delivery can also lead to problems. In particular, viral vectors can induce inflammatory responses that may affect astrocyte morphology or function directly or indirectly (e.g., through microglia-mediated chemokine release) [96], and all Cre-ER mouse lines used to manipulate gene expression in astrocytes (e.g., GLAST-CreER, GFAP-CreER, Cx30-CreER) also influence radial glial cells, leading to altered gene expression in neurons within the dentate gyrus and the olfactory bulb, which are continually generated from radial glia in adulthood. Finally, imaging itself can lead to phototoxic effects that may influence the frequency and form of astrocytic excitation [37], and surgical preparation, such as thinning or removing the skull overlying the imaging site, can induce reactive gliosis [97,98].
Similar challenges exist for studying the role of astrocytes in disease. For example, optogenetic manipulation of astrocyte activity following ischemic stroke induces ion and transmitter level alterations that reduce brain damage [99]. However, due to tissue scattering and absorption, such optical approaches are currently limited to comparatively small tissue volumes. In addition, optical accessibility may change over time due to tissue swelling, neovascularization, or glial scar formation. Likewise, astrocytic gene expression and morphology may change over time [100] potentially affecting the specificity of genetic targeting and pharmacological interventions. Development of red-shifted calcium indicators [72,101] and opsins [102,103], more efficient and less inflammatory viral vectors [96], more specific Cre/CreER mouse lines to manipulate distinct populations of astrocytes using information gained from gene expression analysis [89,104], will alleviate some of these issues and help resolve existing controversies in the field.
Astrocytes in different species
Astrocytes have evolved with phylogeny (Figure 1a). Human astrocytes, as defined by GFAP expression, are larger, structurally more complex, and contact many (around one order of magnitude) more synapses than their rodent counterparts [105] (Figure 1b). In addition, they show differences in calcium signaling. Humans and primates also exhibit types of astrocytes not found in rodents (e.g., interlaminar astrocytes whose processes traverse several cortical layers) [3,105]. Likewise, rodent astrocytes are larger, more complex, and functionally different from their fish, fly, or worm counterparts. Some of these differences appear to be cell autonomous, as human glial progenitors transplanted into the mouse brain develop into astrocytes and retain their larger size and more complex morphology [106]. Such xenographs may provide a means to study the unique characteristics of human astrocytes in an in vivo context. Despite the differences, many astrocyte signaling pathways appear conserved across species [107], similar to neural circuitries and adaptive behaviors [108]. In addition to their accessibility to genetic manipulations, organisms such as C. elegans, Drosophila, zebrafish, and mice have the distinct advantage of reduced size and complexity (Figure 1b-c), making them amenable to large-scale imaging of cellular networks. For example, recent studies in head-immobilized, optically transparent zebrafish larvae demonstrated the feasibility of whole-brain imaging with single-cell spatial and up to tens of Hertz temporal resolution using light sheet [109], or light field microscopy [110], and large-scale data analysis [111]. A similar analysis of astrocyte-like/astroglial cells and networks in the zebrafish brain [112] remains to be performed.
Conclusions
Astrocytes form a complex network of highly ramified, interconnected cells that are common to all regions of the CNS. Like neurons, they express many distinct classes of cell surface receptors and exhibit a form of excitability based on changes in intracellular calcium. Understanding how astrocytes detect and respond to changes in their environment, and ultimately influence other neuronal and glial cell populations, remains an ambitious, but achievable goal. Many approaches that have initially been developed to interrogate neuronal networks have been readily adopted by glial biologists, such as in vivo two-photon imaging and genetically encoded calcium indicators. However, the study of astrocytes presents many additional challenges – their activity is potently inhibited by anesthesia, they undergo dramatic morphological and physiological changes following CNS injury, and they do not exhibit stereotyped electrical activity akin to action potentials. Moreover, the simple addition of calcium indicators, which are themselves calcium buffers, and the act of illuminating tissue to visualize fluorescence changes, can markedly alter the state of astrocyte activity. Uncovering their full range of spatiotemporal activity patterns, particularly in behaving animals and animal models of human disease, will require the development and application of new imaging approaches that extend the depth of imaging, enhance the imaging area, report the activity of distinct signal transduction pathways, and enable visualization of astrocyte activity simultaneously with that of neurons, glia, and vascular cells. Relating their widely varying forms of activity to synaptic, microcircuit, and higher-order function will require combining large-scale imaging techniques with large-scale in vivo staining, manipulation, network anatomical, genomic, and computational approaches. While some of these complementary tools exist, others will need further or new development for use with astrocytes. For example, while an ever-growing list of optogenetic approaches enables precise manipulation of electrical activity and protein function in genetically defined neurons and their compartments [95,113-116], a comparable list of tools for precise spatiotemporal control of astrocyte function is largely lacking [117]. Likewise, optical approaches for three-dimensional control of cellular activity patterns are in their infancy [118-121]. There is perhaps undo emphasis being placed on calcium signaling at the present time, reflecting, in part, the tools available to monitor this behavior, but it is clear that astrocytes express receptors that couple to signaling pathways that do not directly alter intracellular calcium. Our knowledge about the role of these forms of signaling is very limited. Nevertheless, existing approaches have already begun to provide exciting new insight into how astrocytes participate in physiological functions such as sleep, breathing, feeding, and metabolism, and pathological processes associated with stroke, CNS trauma, and diseases such as epilepsy and Huntington’s disease [19,21,122,123].
Decoding how astrocytes integrate information from various cell types, adapt their behavior, and modulate brain physiology will further our understanding of how homeostasis and neuromodulation are achieved in the CNS, and reveal new therapeutic directions to treat complex diseases. Although much remains to be discovered about their diverse functions, it is clear that astrocytes are true stars in the complex CNS universe (Box 1).
Box 1. Selection of unresolved biological questions.
Synaptic level
How are frequency, duration, spatial and temporal pattern of synaptic activity encoded in the time course and spatial extent of astrocytic calcium activity [20]?
Do astrocytic microdomains contain functionally independent compartments, similar to dendritic spines [124]?
How do astrocytes functionally respond to diverse spatiotemporal inputs from different cell types (e.g., excitatory or inhibitory neurons, oligodendrocytes or microglia)?
To what extent do microdomain events summate to trigger somatic calcium transients?
Microcircuit level
How functionally independent are individual astrocytes in local gap junction-coupled networks?
What is the relationship between astrocytic calcium excitation and natural activity patterns in neuromodulatory projections from different anatomical sources in behaving animals?
How do the distinct forms of astrocytic calcium transients influence astrocytic gene expression, their physiological properties, gap junction coupling, or morphology [125]?
How are astrocytes’ spatiotemporal activity patterns linked to regional differences in gene expression profile [126-129]?
What role does astrocyte network activity play in regulating network function/homeostasis in healthy and diseased animals [21]?
Systems level
How do large-scale systemic changes (e.g., circadian rhythm/sleep or aging) influence astrocytic activity and effector function?
Highlights.
Astrocytes form large-scale, gap junction-coupled networks throughout the CNS.
Astrocytes exhibit cell-intrinsic and neurotransmitter-evoked calcium excitability.
The types of information encoded by their activity patterns remain largely unknown.
New imaging and manipulation techniques are required to decode astrocyte functions.
Understanding the roles of astrocytes will aid in treating human CNS diseases.
Acknowledgements
The authors thank members of their respective labs for their contributions to some of the work described in this review. They also wish to apologize to all colleagues whose important work was not directly cited due to topic, review period, or space limitations. This work was supported by grants from the Rita Allen Foundation, Whitehall Foundation, Brain Research Foundation, and the NIH (1DP2NS083038, 5R01NS085938) to A.N., and the NIH (5T32EY017203, 5P50MH100024, 5P30NS050274) to D.E.B.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bahney J, von Bartheld CS. Validation of the isotropic fractionator: comparison with unbiased stereology and DNA extraction for quantification of glial cells. J. Neurosci. Methods. 2014;222:165–174. doi: 10.1016/j.jneumeth.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Azevedo FA, Carvalho LR, Grinberg LT, Farfel JM, Ferretti RE, Leite RE, Jacob Filho W, Lent R, Herculano-Houzel S. Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J. Comp. Neurol. 2009;513:532–541. doi: 10.1002/cne.21974. [DOI] [PubMed] [Google Scholar]
- 3.Herculano-Houzel S. The glia/neuron ratio: how it varies uniformly across brain structures and species and what that means for brain physiology and evolution. Glia. 2014;62:1377–1391. doi: 10.1002/glia.22683. [DOI] [PubMed] [Google Scholar]
- 4.Kosaka T, Hama K. Three-dimensional structure of astrocytes in the rat dentate gyrus. J Comp Neurol. 1986;249:242–260. doi: 10.1002/cne.902490209. [DOI] [PubMed] [Google Scholar]
- 5.Livet J, Weissman TA, Kang H, Draft RW, Lu J, Bennis RA, Sanes JR, Lichtman JW. Transgenic strategies for combinatorial expression of fluorescent proteins in the nervous system. Nature. 2007;450:56–62. doi: 10.1038/nature06293. [DOI] [PubMed] [Google Scholar]
- 6.Bushong EA, Martone ME, Jones YZ, Ellisman MH. Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J Neurosci. 2002;22:183–192. doi: 10.1523/JNEUROSCI.22-01-00183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ventura R, Harris KM. Three-dimensional relationships between hippocampal synapses and astrocytes. J Neurosci. 1999;19:6897–6906. doi: 10.1523/JNEUROSCI.19-16-06897.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nimmerjahn A. Astrocytes going live: advances and challenges. J. Physiol. 2009;587:1639–1647. doi: 10.1113/jphysiol.2008.167171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Regan MR, Huang YH, Kim YS, Dykes-Hoberg MI, Jin L, Watkins AM, Bergles DE, Rothstein JD. Variations in promoter activity reveal a differential expression and physiology of glutamate transporters by glia in the developing and mature CNS. J Neurosci. 2007;27:6607–6619. doi: 10.1523/JNEUROSCI.0790-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee SH, Kim WT, Cornell-Bell AH, Sontheimer H. Astrocytes exhibit regional specificity in gap-junction coupling. Glia. 1994;11:315–325. doi: 10.1002/glia.440110404. [DOI] [PubMed] [Google Scholar]
- 11.Rouach N, Glowinski J, Giaume C. Activity-dependent neuronal control of gap-junctional communication in astrocytes. J. Cell Biol. 2000;149:1513–1526. doi: 10.1083/jcb.149.7.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Devaraju P, Sun MY, Myers TL, Lauderdale K, Fiacco TA. Astrocytic group I mGluR-dependent potentiation of astrocytic glutamate and potassium uptake. J Neurophysiol. 2013;109:2404–2414. doi: 10.1152/jn.00517.2012. [DOI] [PubMed] [Google Scholar]
- 13.Oliet SH, Piet R, Poulain DA. Control of glutamate clearance and synaptic efficacy by glial coverage of neurons. Science. 2001;292:923–926. doi: 10.1126/science.1059162. [DOI] [PubMed] [Google Scholar]
- 14.Anderson MA, Ao Y, Sofroniew MV. Heterogeneity of reactive astrocytes. Neurosci. Lett. 2014;565:23–29. doi: 10.1016/j.neulet.2013.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saab AS, Neumeyer A, Jahn HM, Cupido A, Simek AA, Boele HJ, Scheller A, Le Meur K, Götz M, Monyer H, et al. Bergmann glial AMPA receptors are required for fine motor coordination. Science. 2012;337:749–753. doi: 10.1126/science.1221140. [DOI] [PubMed] [Google Scholar]
- (**) This study reports that genetic deletion of AMPA-type glutamate receptors (GluA1/GluA4) from cerebellar astrocytes (Bergmann glia) results in retraction of astrocyte processes from neuronal synapses, leading to enhanced excitatory synaptic currents in Purkinje cells. These mice show impairments in fine motor coordination, as determined by locomotion conditioning on an Erasmus ladder, but no alteration of Pavlovian eyeblink conditioning.
- 16.Kim JG, Suyama S, Koch M, Jin S, Argente-Arizon P, Argente J, Liu ZW, Zimmer MR, Jeong JK, Szigeti-Buck K, et al. Leptin signaling in astrocytes regulates hypothalamic neuronal circuits and feeding. Nat. Neurosci. 2014;17:908–910. doi: 10.1038/nn.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iino M, Goto K, Kakegawa W, Okado H, Sudo M, Ishiuchi S, Miwa A, Takayasu Y, Saito I, Tsuzuki K, et al. Glia-synapse interactions through Ca2+-permeable AMPA receptors in Bergmann glia. Science. 2001;292:926–929. doi: 10.1126/science.1058827. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Barres BA. Astrocyte heterogeneity: an underappreciated topic in neurobiology. Curr. Opin. Neurobiol. 2010;20:588–594. doi: 10.1016/j.conb.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 19.Verkhratsky A, Sofroniew MV, Messing A, deLanerolle NC, Rempe D, Rodriguez JJ, Nedergaard M. Neurological diseases as primary gliopathies: a reassessment of neurocentrism. ASN Neuro. 2012;4:e00082. doi: 10.1042/AN20120010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Volterra A, Liaudet N, Savtchouk I. Astrocyte Ca2+ signalling: an unexpected complexity. 15. 2014;5 doi: 10.1038/nrn3725. [DOI] [PubMed] [Google Scholar]
- 21.Araque A, Carmignoto G, Haydon PG, Oliet SH, Robitaille R, Volterra A. Gliotransmitters travel in time and space. Neuron. 2014;81:728–739. doi: 10.1016/j.neuron.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (**) This is an excellent in-depth review of the role of astrocytes in central nervous system function. In particular, the authors discuss how astrocytes in the brain can act as integrators of cellular activity to modulate network function on spatiotemporal scales distinct from neurons.
- 22.Bergles DE, Jahr CE. Synaptic activation of glutamate transporters in hippocampal astrocytes. Neuron. 1997;19:1297–1308. doi: 10.1016/s0896-6273(00)80420-1. [DOI] [PubMed] [Google Scholar]
- 23.Nedergaard M. Direct signaling from astrocytes to neurons in cultures of mammalian brain cells. Science. 1994;263:1768–1771. doi: 10.1126/science.8134839. [DOI] [PubMed] [Google Scholar]
- 24.Cornell-Bell AH, Finkbeiner SM, Cooper MS, Smith SJ. Glutamate induces calcium waves in cultured astrocytes: long-range glial signaling. Science. 1990;247:470–473. doi: 10.1126/science.1967852. [DOI] [PubMed] [Google Scholar]
- 25.Petravicz J, Fiacco TA, McCarthy KD. Loss of IP3 receptor-dependent Ca2+ increases in hippocampal astrocytes does not affect baseline CA1 pyramidal neuron synaptic activity. J. Neurosci. 2008;28:4967–4973. doi: 10.1523/JNEUROSCI.5572-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shigetomi E, Jackson-Weaver O, Huckstepp RT, O'Dell TJ, Khakh BS. TRPA1 channels are regulators of astrocyte basal calcium levels and long-term potentiation via constitutive D-serine release. J. Neurosci. 2013;33:10143–10153. doi: 10.1523/JNEUROSCI.5779-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shigetomi E, Kracun S, Sofroniew MV, Khakh BS. A genetically targeted optical sensor to monitor calcium signals in astrocyte processes. Nat. Neurosci. 2010;13:759–766. doi: 10.1038/nn.2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grosche J, Matyash V, Moller T, Verkhratsky A, Reichenbach A, Kettenmann H. Microdomains for neuron-glia interaction: parallel fiber signaling to Bergmann glial cells. Nat Neurosci. 1999;2:139–143. doi: 10.1038/5692. [DOI] [PubMed] [Google Scholar]
- 29.Nett WJ, Oloff SH, McCarthy KD. Hippocampal astrocytes in situ exhibit calcium oscillations that occur independent of neuronal activity. J Neurophysiol. 2002;87:528–537. doi: 10.1152/jn.00268.2001. [DOI] [PubMed] [Google Scholar]
- 30.Takata N, Hirase H. Cortical layer 1 and layer 2/3 astrocytes exhibit distinct calcium dynamics in vivo. PLoS One. 2008;3:e2525. doi: 10.1371/journal.pone.0002525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nimmerjahn A, Mukamel EA, Schnitzer MJ. Motor behavior activates Bergmann glial networks. Neuron. 2009;62:400–412. doi: 10.1016/j.neuron.2009.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takata N, Mishima T, Hisatsune C, Nagai T, Ebisui E, Mikoshiba K, Hirase H. Astrocyte calcium signaling transforms cholinergic modulation to cortical plasticity in vivo. J. Neurosci. 2011;31:18155–18165. doi: 10.1523/JNEUROSCI.5289-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (*) Together with Chen, N. et al. this paper demonstrates that astrocytes in anesthetized mice exhibit IP3-dependent calcium transients in barrel cortex following electrical stimulation of cholinergic neurons in the nucleus basalis of Meynert (NBM). The authors provide evidence that pairing whisker deflection with NBM stimulation potentiates excitatory synapses by inducing the release of D-serine from astrocytes.
- 33.Chen N, Sugihara H, Sharma J, Perea G, Petravicz J, Le C, Sur M. Nucleus basalis-enabled stimulus-specific plasticity in the visual cortex is mediated by astrocytes. Proc. Natl. Acad. Sci. USA. 2012;109:E2832–E2841. doi: 10.1073/pnas.1206557109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (*) Together with Takata, N. et al., this paper demonstrates that electrical stimulation of the nucleus basalis of Meynert (NBM), where cholinergic neuron cell bodies are located, triggers widespread calcium transients in cortical astrocytes in anesthetized mice. In IP3R2 conditional knockout mice, synaptic potentiation induced by pairing visual stimulation with NBM electrical stimulation was absent, suggesting that astrocyte calcium signaling plays a key role in potentiating stimulus-specific visual responses in excitatory neurons.
- 34.Haustein MD, Kracun S, Lu XH, Shih T, Jackson-Weaver O, Tong X, Xu J, Yang XW, O'Dell TJ, Marvin JS, et al. Conditions and constraints for astrocyte calcium signaling in the hippocampal mossy fiber pathway. Neuron. 2014;82:413–429. doi: 10.1016/j.neuron.2014.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gee JM, Smith NA, Fernandez FR, Economo MN, Brunert D, Rothermel M, Morris SC, Talbot A, Palumbos S, Ichida JM, et al. Imaging Activity in Neurons and Glia with a Polr2a-Based and Cre-Dependent GCaMP5G-IRES-tdTomato Reporter Mouse. Neuron. 2014;83:1058–1072. doi: 10.1016/j.neuron.2014.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (*) This paper introduces a conditional mouse line that enables expression of both GCaMP5G and tdTomato in the same cell when Cre or CreER is expressed, providing both morphological information and a readout of calcium dynamics. When bred to GFAP-CreER mice, the authors successfully detected sensory evoked calcium signals in the processes and somata of astrocytes in the barrel cortex of anesthetized mice.
- 36.Schipke CG, Haas B, Kettenmann H. Astrocytes discriminate and selectively respond to the activity of a subpopulation of neurons within the barrel cortex. Cereb. Cortex. 2008;18:2450–2459. doi: 10.1093/cercor/bhn009. [DOI] [PubMed] [Google Scholar]
- 37.Wang X, Lou N, Xu Q, Tian GF, Peng WG, Han X, Kang J, Takano T, Nedergaard M. Astrocytic Ca2+ signaling evoked by sensory stimulation in vivo. Nat. Neurosci. 2006;9:816–823. doi: 10.1038/nn1703. [DOI] [PubMed] [Google Scholar]
- 38.Paukert M, Agarwal A, Cha J, Doze VA, Kang JU, Bergles DE. Norepinephrine controls astroglial responsiveness to local circuit activity. Neuron. 2014;82:1263–1270. doi: 10.1016/j.neuron.2014.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (**) This paper reports generation of conditional GCaMP3 mice (R26-lsl-GCaMP3), which are used to show that locomotion induces calcium elevation in visual cortex (V1) astrocytes due to the release of norepinephrine and activation of α1-adrenergic receptors. The authors show that norepinephrine enhances the sensitivity of astrocytes to local increases in neural activity, and use dual-fiber photometry to demonstrate that locomotion triggers widespread activation of astrocyte networks in both the cerebellar and cerebral cortex.
- 39.Schummers J, Yu H, Sur M. Tuned responses of astrocytes and their influence on hemodynamic signals in the visual cortex. Science. 2008;320:1638–1643. doi: 10.1126/science.1156120. [DOI] [PubMed] [Google Scholar]
- 40.Hoogland TM, Kuhn B, Göbel W, Huang W, Nakai J, Helmchen F, Flint J, Wang SS. Radially expanding transglial calcium waves in the intact cerebellum. Proc. Natl. Acad. Sci. USA. 2009;106:3496–3501. doi: 10.1073/pnas.0809269106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Newman EA, Zahs KR. Calcium waves in retinal glial cells. Science. 1997;275:844–847. doi: 10.1126/science.275.5301.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mathiesen C, Brazhe A, Thomson K, Lauritzen M. Spontaneous calcium waves in Bergmann glia increase with age and hypoxia and may reduce tissue oxygen. J. Cereb. Blood Flow Metab. 2013;33:161–169. doi: 10.1038/jcbfm.2012.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ding F, O'Donnell J, Thrane AS, Zeppenfeld D, Kang H, Xie L, Wang F, Nedergaard M. Alpha1-adrenergic receptors mediate coordinated Ca2+ signaling of cortical astrocytes in awake, behaving mice. Cell Calcium. 2013;54:387–394. doi: 10.1016/j.ceca.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (*) Together with Paukert, M. et al. this paper demonstrates that sensory-evoked large-scale astrocytic calcium activity in the cortex of awake, head-restrained mice depends on noradrenergic receptor activation. Using in vivo pharmacology and whisker or air-puff stimulation, the authors link norepinephrine-evoked calcium changes in astrocytes to α1-adrenergic receptor activation.
- 44.Dombeck DA, Khabbaz AN, Collman F, Adelman TL, Tank DW. Imaging large-scale neural activity with cellular resolution in awake, mobile mice. Neuron. 2007;56:43–57. doi: 10.1016/j.neuron.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beierlein M, Regehr WG. Brief bursts of parallel fiber activity trigger calcium signals in Bergmann glia. J. Neurosci. 2006;26:6958–6967. doi: 10.1523/JNEUROSCI.0613-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Newman EA. Propagation of intercellular calcium waves in retinal astrocytes and Muller cells. J Neurosci. 2001;21:2215–2223. doi: 10.1523/JNEUROSCI.21-07-02215.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bekar LK, He W, Nedergaard M. Locus coeruleus alpha-adrenergic-mediated activation of cortical astrocytes in vivo. Cereb Cortex. 2008;18:2789–2795. doi: 10.1093/cercor/bhn040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fu Y, Tucciarone JM, Espinosa JS, Sheng N, Darcy DP, Nicoll RA, Huang ZJ, Stryker MP. A cortical circuit for gain control by behavioral state. Cell. 2014;156:1139–1152. doi: 10.1016/j.cell.2014.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matyash V, Kettenmann H. Heterogeneity in astrocyte morphology and physiology. Brain Res. Rev. 2010;63:2–10. doi: 10.1016/j.brainresrev.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 50.Verkhratsky A, Hoppe D, Kettenmann H. K+ channel properties in cultured mouse Schwann cells: dependence on extracellular K+ J Neurosci Res. 1991;28:210–216. doi: 10.1002/jnr.490280208. [DOI] [PubMed] [Google Scholar]
- 51.Horton NG, Wang K, Kobat D, Clark CG, Wise FW, Schaffer CB, Xu C. In vivo three-photon microscopy of subcortical structures within an intact mouse brain. Nat. Photonics. 2013;7:205–209. doi: 10.1038/nphoton.2012.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kobat D, Horton NG, Xu C. In vivo two-photon microscopy to 1.6-mm depth in mouse cortex. J. Biomed. Opt. 2011;16:106014. doi: 10.1117/1.3646209. [DOI] [PubMed] [Google Scholar]
- 53.Cheng LC, Horton NG, Wang K, Chen SJ, Xu C. Measurements of multiphoton action cross sections for multiphoton microscopy. Biomed. Opt. Express. 2014;5:3427–3433. doi: 10.1364/BOE.5.003427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Helmchen F, Denk W. Deep tissue two-photon microscopy. Nat. Methods. 2005;2:932–940. doi: 10.1038/nmeth818. [DOI] [PubMed] [Google Scholar]
- 55.Dombeck DA, Harvey CD, Tian L, Looger LL, Tank DW. Functional imaging of hippocampal place cells at cellular resolution during virtual navigation. Nat. Neurosci. 2010;13:1433–1440. doi: 10.1038/nn.2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Flusberg BA, Nimmerjahn A, Cocker ED, Mukamel EA, Barretto RP, Ko TH, Burns LD, Jung JC, Schnitzer MJ. High-speed, miniaturized fluorescence microscopy in freely moving mice. Nat. Methods. 2008;5:935–938. doi: 10.1038/nmeth.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kuga N, Sasaki T, Takahara Y, Matsuki N, Ikegaya Y. Large-scale calcium waves traveling through astrocytic networks in vivo. J. Neurosci. 2011;31:2607–2614. doi: 10.1523/JNEUROSCI.5319-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Andermann ML, Gilfoy NB, Goldey GJ, Sachdev RN, Wölfel M, McCormick DA, Reid RC, Levene MJ. Chronic cellular imaging of entire cortical columns in awake mice using microprisms. Neuron. 2013;80:900–913. doi: 10.1016/j.neuron.2013.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ziv Y, Burns LD, Cocker ED, Hamel EO, Ghosh KK, Kitch LJ, El Gamal A, Schnitzer MJ. Long-term dynamics of CA1 hippocampal place codes. Nat. Neurosci. 2013;16:264–266. doi: 10.1038/nn.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Burda JE, Sofroniew MV. Reactive gliosis and the multicellular response to CNS damage and disease. Neuron. 2014;81:229–248. doi: 10.1016/j.neuron.2013.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Komiyama T, Sato TR, O'Connor DH, Zhang YX, Huber D, Hooks BM, Gabitto M, Svoboda K. Learning-related fine-scale specificity imaged in motor cortex circuits of behaving mice. Nature. 2010;464:1182–1186. doi: 10.1038/nature08897. [DOI] [PubMed] [Google Scholar]
- 62.Huber D, Gutnisky DA, Peron S, O'Connor DH, Wiegert JS, Tian L, Oertner TG, Looger LL, Svoboda K. Multiple dynamic representations in the motor cortex during sensorimotor learning. Nature. 2012;484:473–478. doi: 10.1038/nature11039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Peters AJ, Chen SX, Komiyama T. Emergence of reproducible spatiotemporal activity during motor learning. Nature. 2014;510:263–267. doi: 10.1038/nature13235. [DOI] [PubMed] [Google Scholar]
- 64.Masamizu Y, Tanaka YR, Tanaka YH, Hira R, Ohkubo F, Kitamura K, Isomura Y, Okada T, Matsuzaki M. Two distinct layer-specific dynamics of cortical ensembles during learning of a motor task. Nat. Neurosci. 2014;17:987–994. doi: 10.1038/nn.3739. [DOI] [PubMed] [Google Scholar]
- 65.Lee HS, Ghetti A, Pinto-Duarte A, Wang X, Dziewczapolski G, Galimi F, Huitron-Resendiz S, Pina-Crespo JC, Roberts AJ, Verma IM, et al. Astrocytes contribute to gamma oscillations and recognition memory. Proc Natl Acad Sci U S A. 2014;111:E3343–E3352. doi: 10.1073/pnas.1410893111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (**) This paper shows that conditional expression of tetanus neurotoxin (TeNT) in astrocytes to inhibit exocytosis reduces cortical gamma oscillations in the 20-40 Hz range in awake mice. Mice with this manipulation also had impaired novel object recognition memory, but working memory and fear conditioning were unaffected. Synaptic function was also not affected by expressing TeNT in astrocytes.
- 66.Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, O'Donnell J, Christensen DJ, Nicholson C, Iliff JJ, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342:373–377. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Halassa MM, Florian C, Fellin T, Munoz JR, Lee SY, Abel T, Haydon PG, Frank MG. Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron. 2009;61:213–219. doi: 10.1016/j.neuron.2008.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Slezak M, Grosche A, Niemiec A, Tanimoto N, Pannicke T, Münch TA, Crocker B, Isope P, Härtig W, Beck SC, et al. Relevance of exocytic glutamate release from retinal glia. Neuron. 2012;74:504–516. doi: 10.1016/j.neuron.2012.03.027. [DOI] [PubMed] [Google Scholar]
- (*) This paper reports that blocking calcium-dependent exocytosis from astrocytes and Müller glia in the retina through conditional expression of clostridial botulinum neurotoxin serotype B light chain, impairs volume regulation by Müller glia, but does not alter retinal histology or visual processing.
- 69.Fujita T, Chen MJ, Li B, Smith NA, Peng W, Sun W, Toner MJ, Kress BT, Wang L, Benraiss A, et al. Neuronal transgene expression in dominant-negative SNARE mice. J. Neurosci. 2014;34:16594–16604. doi: 10.1523/JNEUROSCI.2585-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pfrieger FW, Slezak M. Genetic approaches to study glial cells in the rodent brain. Glia. 2012;60:681–701. doi: 10.1002/glia.22283. [DOI] [PubMed] [Google Scholar]
- (*) This review provides a comprehensive overview of available gene manipulation tools for astrocytes and other glial cells in the central nervous system.
- 71.Zariwala HA, Borghuis BG, Hoogland TM, Madisen L, Tian L, De Zeeuw CI, Zeng H, Looger LL, Svoboda K, Chen TW. A Cre-dependent GCaMP3 reporter mouse for neuronal imaging in vivo. J Neurosci. 2012;32:3131–3141. doi: 10.1523/JNEUROSCI.4469-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Akerboom J, Carreras Calderon N, Tian L, Wabnig S, Prigge M, Tolö J, Gordus A, Orger MB, Severi KE, Macklin JJ, et al. Genetically encoded calcium indicators for multi-color neural activity imaging and combination with optogenetics. Front. Mol. Neurosci. 2013;6:2. doi: 10.3389/fnmol.2013.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhao Y, Araki S, Wu J, Teramoto T, Chang YF, Nakano M, Abdelfattah AS, Fujiwara M, Ishihara T, Nagai T, et al. An expanded palette of genetically encoded Ca2+ indicators. Science. 2011;333:1888–1891. doi: 10.1126/science.1208592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nedergaard M, Rodriguez JJ, Verkhratsky A. Glial calcium and diseases of the nervous system. Cell Calcium. 2010;47:140–149. doi: 10.1016/j.ceca.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 75.Kuchibhotla KV, Lattarulo CR, Hyman BT, Bacskai BJ. Synchronous hyperactivity and intercellular calcium waves in astrocytes in Alzheimer mice. Science. 2009;323:1211–1215. doi: 10.1126/science.1169096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ding S, Wang T, Cui W, Haydon PG. Photothrombosis ischemia stimulates a sustained astrocytic Ca2+ signaling in vivo. Glia. 2009;57:767–776. doi: 10.1002/glia.20804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Takatsuru Y, Eto K, Kaneko R, Masuda H, Shimokawa N, Koibuchi N, Nabekura J. Critical role of the astrocyte for functional remodeling in contralateral hemisphere of somatosensory cortex after stroke. J. Neurosci. 2013;33:4683–4692. doi: 10.1523/JNEUROSCI.2657-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Masamoto K, Takuwa H, Seki C, Taniguchi J, Itoh Y, Tomita Y, Toriumi H, Unekawa M, Kawaguchi H, Ito H, et al. Microvascular sprouting, extension, and creation of new capillary connections with adaptation of the neighboring astrocytes in adult mouse cortex under chronic hypoxia. J. Cereb. Blood Flow Metab. 2014;34:325–331. doi: 10.1038/jcbfm.2013.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Knowland D, Arac A, Sekiguchi KJ, Hsu M, Lutz SE, Perrino J, Steinberg GK, Barres BA, Nimmerjahn A, Agalliu D. Stepwise recruitment of transcellular and paracellular pathways underlies blood-brain barrier breakdown in stroke. Neuron. 2014;82:603–617. doi: 10.1016/j.neuron.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sigler A, Murphy TH. In vivo 2-photon imaging of fine structure in the rodent brain: before, during, and after stroke. Stroke. 2010;41:S117–S123. doi: 10.1161/STROKEAHA.110.594648. [DOI] [PubMed] [Google Scholar]
- 81.Sandoval KE, Witt KA. Blood-brain barrier tight junction permeability and ischemic stroke. Neurobiol. Dis. 2008;32:200–219. doi: 10.1016/j.nbd.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 82.Kirchhoff F, Debarbieux F, Kronland-Martinet C, Cojocaru GR, Popa-Wagner A. Combined two-photon laser-scanning microscopy and spectral microCT X- ray imaging to characterize the cellular signature and evolution of microstroke foci. Rom. J. Morphol. Embryol. 2012;53:671–675. [PubMed] [Google Scholar]
- 83.Masamoto K, Tomita Y, Toriumi H, Aoki I, Unekawa M, Takuwa H, Itoh Y, Suzuki N, Kanno I. Repeated longitudinal in vivo imaging of neuro-glio-vascular unit at the peripheral boundary of ischemia in mouse cerebral cortex. Neuroscience. 2012;212:190–200. doi: 10.1016/j.neuroscience.2012.03.034. [DOI] [PubMed] [Google Scholar]
- 84.Schulz K, Sydekum E, Krueppel R, Engelbrecht CJ, Schlegel F, Schröter A, Rudin M, Helmchen F. Simultaneous BOLD fMRI and fiber-optic calcium recording in rat neocortex. Nat. Methods. 2012;9:597–602. doi: 10.1038/nmeth.2013. [DOI] [PubMed] [Google Scholar]
- (**) Using multi-modal imaging this paper reveals a potential connection between sensory-evoked, widespread astrocytic calcium activity, measured by fiber photometry, and BOLD fMRI signal components in primary somatosensory cortex of anesthetized rats.
- 85.Nizar K, Uhlirova H, Tian P, Saisan PA, Cheng Q, Reznichenko L, Weldy KL, Steed TC, Sridhar VB, MacDonald CL, et al. In vivo stimulus-induced vasodilation occurs without IP3 receptor activation and may precede astrocytic calcium increase. J. Neurosci. 2013;33:8411–8422. doi: 10.1523/JNEUROSCI.3285-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen F, LoTurco J. A method for stable transgenesis of radial glia lineage in rat neocortex by piggyBac mediated transposition. J Neurosci Methods. 2012;207:172–180. doi: 10.1016/j.jneumeth.2012.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Eroglu C, Allen NJ, Susman MW, O'Rourke NA, Park CY, Ozkan E, Chakraborty C, Mulinyawe SB, Annis DS, Huberman AD, et al. Gabapentin receptor alpha2delta-1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell. 2009;139:380–392. doi: 10.1016/j.cell.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sun W, McConnell E, Pare JF, Xu Q, Chen M, Peng W, Lovatt D, Han X, Smith Y, Nedergaard M. Glutamate-dependent neuroglial calcium signaling differs between young and adult brain. Science. 2013;339:197–200. doi: 10.1126/science.1226740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, et al. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci. 2008;28:264–278. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Woo DH, Han KS, Shim JW, Yoon BE, Kim E, Bae JY, Oh SJ, Hwang EM, Marmorstein AD, Bae YC, et al. TREK-1 and Best1 channels mediate fast and slow glutamate release in astrocytes upon GPCR activation. Cell. 2012;151:25–40. doi: 10.1016/j.cell.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 91.Nedergaard M, Verkhratsky A. Artifact versus reality - how astrocytes contribute to synaptic events. Glia. 2012;60:1013–1023. doi: 10.1002/glia.22288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sweger EJ, Casper KB, Scearce-Levie K, Conklin BR, McCarthy KD. Development of hydrocephalus in mice expressing the G(i)-coupled GPCR Ro1 RASSL receptor in astrocytes. J Neurosci. 2007;27:2309–2317. doi: 10.1523/JNEUROSCI.4565-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Agulhon C, Boyt KM, Xie AX, Friocourt F, Roth BL, McCarthy KD. Modulation of the autonomic nervous system and behaviour by acute glial cell Gq protein-coupled receptor activation in vivo. J. Physiol. 2013;591:5599–5609. doi: 10.1113/jphysiol.2013.261289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shao W, Zhang SZ, Tang M, Zhang XH, Zhou Z, Yin YQ, Zhou QB, Huang YY, Liu YJ, Wawrousek E, et al. Suppression of neuroinflammation by astrocytic dopamine D2 receptors via alphaB-crystallin. Nature. 2013;494:90–94. doi: 10.1038/nature11748. [DOI] [PubMed] [Google Scholar]
- (*) This paper indicates that astrocytic D2-dopamine receptors have immune modulatory functions. Astrocytes deficient in these receptors show reduced αB-crystallin (CRYAB) levels, become activated, and produce more proinflammatory mediators, causing neurons to be more vulnerable to inflammatory stimuli.
- 95.Airan RD, Thompson KR, Fenno LE, Bernstein H, Deisseroth K. Temporally precise in vivo control of intracellular signalling. Nature. 2009;458:1025–1029. doi: 10.1038/nature07926. [DOI] [PubMed] [Google Scholar]
- 96.Merienne N, Le Douce J, Faivre E, Deglon N, Bonvento G. Efficient gene delivery and selective transduction of astrocytes in the mammalian brain using viral vectors. Front. Cell. Neurosci. 2013;7:106. doi: 10.3389/fncel.2013.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xu HT, Pan F, Yang G, Gan WB. Choice of cranial window type for in vivo imaging affects dendritic spine turnover in the cortex. Nat Neurosci. 2007;10:549–551. doi: 10.1038/nn1883. [DOI] [PubMed] [Google Scholar]
- 98.Nimmerjahn A. Two-photon imaging of microglia in the mouse cortex in vivo. Cold Spring Harb. Protoc. 2012;2012:594–603. doi: 10.1101/pdb.prot069294. [DOI] [PubMed] [Google Scholar]
- 99.Beppu K, Sasaki T, Tanaka KF, Yamanaka A, Fukazawa Y, Shigemoto R, Matsui K. Optogenetic countering of glial acidosis suppresses glial glutamate release and ischemic brain damage. Neuron. 2014;81:314–320. doi: 10.1016/j.neuron.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 100.Zamanian JL, Xu L, Foo LC, Nouri N, Zhou L, Giffard RG, Barres BA. Genomic analysis of reactive astrogliosis. J. Neurosci. 2012;32:6391–6410. doi: 10.1523/JNEUROSCI.6221-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Inoue M, Takeuchi A, Horigane S, Ohkura M, Gengyo-Ando K, Fuji H, Kamijo S, Takemoto-Kimura S, Kano M, Nakai J, et al. Rational design of a high-affinity, fast, red calcium indicator R-CaMP2. Nat. Methods. 2015;12:64–70. doi: 10.1038/nmeth.3185. [DOI] [PubMed] [Google Scholar]
- 102.Lin JY, Knutsen PM, Muller A, Kleinfeld D, Tsien RY. ReaChR: a red-shifted variant of channelrhodopsin enables deep transcranial optogenetic excitation. Nat. Neurosci. 2013;16:1499–1508. doi: 10.1038/nn.3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chuong AS, Miri ML, Busskamp V, Matthews GA, Acker LC, Sorensen AT, Young A, Klapoetke NC, Henninger MA, Kodandaramaiah SB, et al. Noninvasive optical inhibition with a red-shifted microbial rhodopsin. Nat. Neurosci. 2014;17:1123–1129. doi: 10.1038/nn.3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O'Keeffe S, Phatnani HP, Guarnieri P, Caneda C, Ruderisch N, et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci. 2014;34:11929–11947. doi: 10.1523/JNEUROSCI.1860-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (**) This resource paper and associated website provide ready access to gene expression and RNA splicing information for neurons and glial cells in the cerebral cortex of juvenile mice.
- 105.Oberheim NA, Goldman SA, Nedergaard M. Heterogeneity of astrocytic form and function. Methods Mol. Biol. 2012;814:23–45. doi: 10.1007/978-1-61779-452-0_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Han X, Chen M, Wang F, Windrem M, Wang S, Shanz S, Xu Q, Oberheim NA, Bekar L, Betstadt S, et al. Forebrain engraftment by human glial progenitor cells enhances synaptic plasticity and learning in adult mice. Cell Stem Cell. 2013;12:342–353. doi: 10.1016/j.stem.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Freeman MR, Rowitch DH. Evolving concepts of gliogenesis: a look way back and ahead to the next 25 years. Neuron. 2013;80:613–623. doi: 10.1016/j.neuron.2013.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Strausfeld NJ, Hirth F. Deep homology of arthropod central complex and vertebrate basal ganglia. Science. 2013;340:157–161. doi: 10.1126/science.1231828. [DOI] [PubMed] [Google Scholar]
- 109.Ahrens MB, Orger MB, Robson DN, Li JM, Keller PJ. Whole-brain functional imaging at cellular resolution using light-sheet microscopy. Nat. Methods. 2013;10:413–420. doi: 10.1038/nmeth.2434. [DOI] [PubMed] [Google Scholar]
- 110.Prevedel R, Yoon YG, Hoffmann M, Pak N, Wetzstein G, Kato S, Schrödel T, Raskar R, Zimmer M, Boyden ES, et al. Simultaneous whole-animal 3D imaging of neuronal activity using light-field microscopy. Nat. Methods. 2014;11:727–730. doi: 10.1038/nmeth.2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Freeman J, Vladimirov N, Kawashima T, Mu Y, Sofroniew NJ, Bennett DV, Rosen J, Yang CT, Looger LL, Ahrens MB. Mapping brain activity at scale with cluster computing. Nat. Methods. 2014;11:941–950. doi: 10.1038/nmeth.3041. [DOI] [PubMed] [Google Scholar]
- 112.Grupp L, Wolburg H, Mack AF. Astroglial structures in the zebrafish brain. J. Comp. Neurol. 2010;518:4277–4287. doi: 10.1002/cne.22481. [DOI] [PubMed] [Google Scholar]
- 113.Fenno LE, Mattis J, Ramakrishnan C, Hyun M, Lee SY, He M, Tucciarone J, Selimbeyoglu A, Berndt A, Grosenick L, et al. Targeting cells with single vectors using multiple-feature Boolean logic. Nat. Methods. 2014;11:763–772. doi: 10.1038/nmeth.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yizhar O, Fenno LE, Davidson TJ, Mogri M, Deisseroth K. Optogenetics in neural systems. Neuron. 2011;71:9–34. doi: 10.1016/j.neuron.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 115.Sandoz G, Levitz J, Kramer RH, Isacoff EY. Optical control of endogenous proteins with a photoswitchable conditional subunit reveals a role for TREK1 in GABA(B) signaling. Neuron. 2012;74:1005–1014. doi: 10.1016/j.neuron.2012.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Levitz J, Pantoja C, Gaub B, Janovjak H, Reiner A, Hoagland A, Schoppik D, Kane B, Stawski P, Schier AF, et al. Optical control of metabotropic glutamate receptors. Nat. Neurosci. 2013;16:507–516. doi: 10.1038/nn.3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Figueiredo M, Lane S, Stout RFJ, Liu B, Parpura V, Teschemacher AG, Kasparov S. Comparative analysis of optogenetic actuators in cultured astrocytes. Cell Calcium. 2014;56:208–214. doi: 10.1016/j.ceca.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pisanello F, Sileo L, Oldenburg IA, Pisanello M, Martiradonna L, Assad JA, Sabatini BL, De Vittorio M. Multipoint-emitting optical fibers for spatially addressable in vivo optogenetics. Neuron. 2014;82:1245–1254. doi: 10.1016/j.neuron.2014.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Prakash R, Yizhar O, Grewe B, Ramakrishnan C, Wang N, Goshen I, Packer AM, Peterka DS, Yuste R, Schnitzer MJ, et al. Two-photon optogenetic toolbox for fast inhibition, excitation and bistable modulation. Nat. Methods. 2012;9:1171–1179. doi: 10.1038/nmeth.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Packer AM, Russell LE, Dalgleish HW, Häusser M. Simultaneous all-optical manipulation and recording of neural circuit activity with cellular resolution in vivo. Nat. Methods. 2014 doi: 10.1038/nmeth.3217. doi: 10.1038/nmeth.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Szabo V, Ventalon C, De Sars V, Bradley J, Emiliani V. Spatially selective holographic photoactivation and functional fluorescence imaging in freely behaving mice with a fiberscope. Neuron. 2014;84:1157–1169. doi: 10.1016/j.neuron.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 122.Verkhratsky A, Nedergaard M, Hertz L. Why are astrocytes important? Neurochem. Res. 2014 doi: 10.1007/s11064-014-1403-2. doi: 10.1007/s11064-11014-11403-11062. [DOI] [PubMed] [Google Scholar]
- 123.Tong X, Ao Y, Faas GC, Nwaobi SE, Xu J, Haustein MD, Anderson MA, Mody I, Olsen ML, Sofroniew MV, et al. Astrocyte Kir4.1 ion channel deficits contribute to neuronal dysfunction in Huntington's disease model mice. Nat. Neurosci. 2014;17:694–703. doi: 10.1038/nn.3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chen Y, Sabatini BL. Signaling in dendritic spines and spine microdomains. Curr. Opin. Neurobiol. 2012;22:389–396. doi: 10.1016/j.conb.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pirttimaki TM, Parri HR. Astrocyte plasticity: implications for synaptic and neuronal activity. Neuroscientist. 2013;19:604–615. doi: 10.1177/1073858413504999. [DOI] [PubMed] [Google Scholar]
- 126.Houades V, Koulakoff A, Ezan P, Seif I, Giaume C. Gap junction-mediated astrocytic networks in the mouse barrel cortex. J. Neurosci. 2008;28:5207–5217. doi: 10.1523/JNEUROSCI.5100-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ko Y, Ament SA, Eddy JA, Caballero J, Earls JC, Hood L, Price ND. Cell type-specific genes show striking and distinct patterns of spatial expression in the mouse brain. Proc. Natl. Acad. Sci. USA. 2013;110:3095–3100. doi: 10.1073/pnas.1222897110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Molofsky AV, Kelley KW, Tsai HH, Redmond SA, Chang SM, Madireddy L, Chan JR, Baranzini SE, Ullian EM, Rowitch DH. Astrocyte-encoded positional cues maintain sensorimotor circuit integrity. Nature. 2014;509:189–194. doi: 10.1038/nature13161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhou H, Lin Z, Voges K, Ju C, Gao Z, Bosman LW, Ruigrok TJ, Hoebeek FE, De Zeeuw CI, Schonewille M. Cerebellar modules operate at different frequencies. Elife. 2014;3:e02536. doi: 10.7554/eLife.02536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Oberheim NA, Wang X, Goldman S, Nedergaard M. Astrocytic complexity distinguishes the human brain. Trends Neurosci. 2006;29:547–553. doi: 10.1016/j.tins.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 131.Witcher MR, Kirov SA, Harris KM. Plasticity of perisynaptic astroglia during synaptogenesis in the mature rat hippocampus. Glia. 2007;55:13–23. doi: 10.1002/glia.20415. [DOI] [PubMed] [Google Scholar]
- 132.Wilhelmsson U, Bushong EA, Price DL, Smarr BL, Phung V, Terada M, Ellisman MH, Pekny M. Redefining the concept of reactive astrocytes as cells that remain within their unique domains upon reaction to injury. Proc. Natl. Acad. Sci. USA. 2006;103:17513–17518. doi: 10.1073/pnas.0602841103. [DOI] [PMC free article] [PubMed] [Google Scholar]