Abstract
Major depressive disorder (MDD) is one of the most disabling diseases worldwide and is a significant public health threat. Current treatments for MDD primarily consist of monoamine-targeting agents and have limited efficacy. However, the glutamate neurotransmitter system has recently come into focus as a promising alternative for novel antidepressant treatments. We review the current data on the glutamate NMDA receptor antagonist ketamine, which has been shown in clinical trials to act as a rapid antidepressant in MDD. We also examine ketamine efficacy on dimensions of psychopathology, including anhedonia, cognition, and suicidality, consistent with the NIMH Research Domain Criteria (RDoC) initiative. Other aspects of ketamine reviewed in this paper include safety and efficacy, different administration methods, and the risks of misuse of ketamine outside of medical settings. Finally, we conclude with a discussion of other glutamatergic agents other than ketamine currently being tested as novel antidepressants.
Keywords: major depressive disorder, bipolar disorder, ketamine, antidepressant, treatment-resistant
Introduction
Major depressive disorder (MDD) is a significant public health threat, accounting for 65.5 million disability-adjusted life years and ranking third among the leading causes of global disease burden.1 Current state-of-the-art antidepressant treatments primarily work on the monoamine system, providing relief for 54% of patients, according to a meta-analysis of controlled trials.2 Novel treatments for depression have the potential to ameliorate both individual suffering and a public health cost burden. Recent research has highlighted the glutamate system as a potential novel antidepressant target, particularly for those suffering from treatment-resistant depression (TRD) and who are even less likely to benefit from additional monoamine treatments.
Ketamine is a high-affinity, non-competitive N-methyl-D-aspartate (NMDA) receptor antagonist. It is currently approved by the U.S. Food and Drug Administration (FDA) as an anesthetic agent and is additionally used off-label to help manage chronic pain.3 In the past ten years, evidence has emerged showing that sub-anesthetic doses of ketamine (0.5mg/kg) administered over a 40-min infusion period can have a fast-acting antidepressant effects on both treatment-naive and TRD patients.4–6 In the following section, we discuss the effects of ketamine in single- and multiple-infusion studies. Less attention has been dedicated so far to the ability of ketamine to ameliorate specific NIMH Research Domain Criteria (RDoC)3,7 symptom clusters, including anhedonia, cognition, and suicidality; we summarize these data in our review. We also review the existing limitations of ketamine use, including safety concerns and the risks of ketamine misuse. The limitations of ketamine have led to an active search for improved, ketamine-like glutamatergic agents; we briefly review several such agents currently being tested as novel antidepressants.
The efficacy of ketamine in mood disorders
Single infusion
The rapid antidepressant effect and safety of a single intravenous (IV) infusion of ketamine has been examined in at least five randomized controlled trials (RCTs) in unipolar MDD (Table 1). A single IV infusion of ketamine resulted in a 50–70% response rate in patients with MDD, with a response duration of 72 h post-infusion.8–10 Response was defined as a 50% or greater reduction from baseline in depression severity (e.g., Montgomery–Asberg Depression Rating Scale (MADRS), Hamilton Depression Rating Scale (HAM-D). Ketamine effects were seen as soon as 40 min after IV infusion and typically lasted 3–7 days, with some patients experiencing more prolonged mood improvement.10 In an RCT of the effects of ketamine versus an anesthetic control condition, midazolam, in TRD patients (n = 72), response rates were 64.8% and 28% in the ketamine and control conditions, respectively, after a single IV infusion over 40 minutes.6
Table 1.
Studies of single-dose ketamine administration in patients with MDD
| Study | Route of administration |
#
of patients (n) |
Design | # of patients receiving ketamine or placebo |
Treatment responsea (%) |
Duration of response |
|---|---|---|---|---|---|---|
| Berman et al.9 | Intravenous | 7 | Cross over | Ket: n = 7 Placebo: n = 7 |
50% | 72 h |
| Zarate et al.10 | Intravenous | 17 | Cross over | Ket: n = 17 Placebo: n = 17 |
71% | 72 h |
| Lapidus et al.13 | Intranasal | 20 | Cross over | Ket: n = 20 Placebo: n = 20 |
44% | 48 h |
| Mathew et al.8 | Intravenous | 26 | Open label | Ket: n = 26 Placebo: n = 0 |
66% | 72 h |
| Ibrahim et al.48 | Intravenous | 42 | Open label | Ket: n = 26 Placebo: n = 0 |
62% | 13 days |
| Murrough et al.16 | Intravenous | 73 | Double blind | Ket: n = 47 Placebo: n = 25 |
64% | 72 h |
Response is defined as ≥ 50% reduction in depressive symptoms (e.g., HAM-D or MADRS score) 24 h following IV ketamine.
Diazgrandos tested the effects of a single ketamine infusion on patients with bipolar depression in a crossover, placebo-controlled trial and found rapid (24 h) decreases in depressive symptoms, as measured by MADRS, including suicidal ideation, starting at 40 min post-infusion and continuing for at least the next three days.9 Seventy-one percent of patients responded to ketamine. This finding was replicated in another study that found a 79% response rate.10 The results of these studies support the hypothesis that ketamine could be an effective and safe treatment for bipolar as well as unipolar depression.11
Repeated infusion
Relapse of depressive symptoms after a single IV dose of ketamine often occurs within one week, although this time period is highly variable between subjects. Our group has recently examined a method to extend the period of response to ketamine through repeated infusions (Table 2). The effects of repeated infusions of ketamine (up to six IV infusions over two weeks) were examined in an open-label study by Murrough et al., and the response rate at the end of the study was 70.8%. Among responders, the average time to relapse after the last infusion was 18 days.6 An additional trial by Diamond et al. involved three or six repeated infusions of ketamine over a three-week period and found a lower response rate of 29%.12 In contrast to Murrough et al., Diamond and colleagues enrolled both unipolar and bipolar subjects, which were split into two groups: treatment with three or six infusions over 3 weeks. These differences in study design may account for the different results.
Table 2.
Studies of repeated-dose IV ketamine administration in patients with MDD
| Study | Method | # of patients (n) |
Design | Treatment responsea (%) |
|---|---|---|---|---|
| Aan Het Rot et al.49 | IV | 10 | Open label | 65% |
| Murrough et al.16 | IV | 24 | Open label | 70.8% |
| Diamond et al.12 | IV | 28 | Open label | 29% |
| Rasmussen et al.50 | IV | 10 | Open label | 80% |
| Segmiller et al.51 | IV | 6 | Open label (esketamine) | 50% |
Response is defined as ≥ 50% reduction in depressive symptoms (e.g., HAM-D or MADRS score) 24 h following IV ketamine.
A large RCT comparing the efficacy of ketamine given either twice or three times per week over a 4-week period was recently completed and results are pending (NCT01627782). An RCT investigating the efficacy of repeated self-administered intranasal S-ketamine administrations over a 2-week double-blind period is also currently ongoing (NCT01998958).
Intranasal ketamine administration
While IV ketamine has potential as an effective treatment for depression, it may not be the most feasible standardized administration for use as a widespread treatment, for patients and clinicians alike. To come up with a less invasive approach, other administrative methods have been considered; however, these efforts have had to take into account lower bioavailability of other methods. For example, oral administration has relatively low bioavailability (16–20%). Intranasal (IN) administration has a bioavailability of approximately 25–50% bioavailability13 and may represent an appropriate balance between feasibility and efficacy. Our group recently completed a pilot RCT of IN ketamine in TRD patients and found that IN ketamine was associated with a 44% response rate compared to a 6% response rate in the saline placebo condition.13 Patients were continued on their current (failed) antidepressant medications. Response to IN ketamine was sustained for 48 h post-administration.13 We found minimal psychotomimetic or dissociative effects, and ketamine was well tolerated by participants. The lower response rate for IN compared to IV ketamine administration may be consistent with the lower blood ketamine levels achieved in the IN study compared with levels previously reported after IV administration. In the IN sample, the mean ketamine blood level was 72 ng/mL at 20 min and 84 ng/ML at 40 minutes. In contrast, mean ketamine levels reported after IV infusion are approximately 150 ng/mL at 30 min and 200 ng/mL at 40 minutes.14,15 Further studies will be necessary to determine if and how equivalent bioavailability and efficacy can be obtained with IN administration. No RCTs as of yet have compared response rates between different administration methods.
The efficacy of ketamine on symptom clusters
A recent trend in NIMH-funded studies has strongly encouraged clinical trials to focus on RDoC (specific symptom clusters, defined on the basis of underlying neurobiology)7 instead of discrete Diagnostic and Statistical Manual of Mental Disorders (DSM) diagnoses (for more information, see http://www.nimh.nih.gov/research-priorities/rdoc/index.shtml). This effort aims to pinpoint basic dimensions of functioning across disorders and implement favorably matched treatments. Symptom clusters germane to mood disorders include anhedonia/negative valence, cognition, working memory, positive valence, arousal symptoms, and social processing. While no specific studies have been yet completed testing ketamine efficacy for specific RDoC symptom clusters, we present below secondary analyses from previous studies by our group that suggest that ketamine may be particularly effective at attenuating anhedonia and suicidal ideation. Potentially informative pilot data also exist regarding ketamine’s effect on cognition. Although suicidality is not classified as a specific RDoC category, it is nevertheless an important transdiagnostic clinical domain and a specific dimension of psychopathology within mood disorders. These data come from the largest parallel-arm, controlled clinical trial to date.16 While the data presented in this section are only preliminary, they highlight the potential efficacy of ketamine in RDoC-defined symptom clusters and the need for future studies specifically designed to answer these questions.
Anhedonia
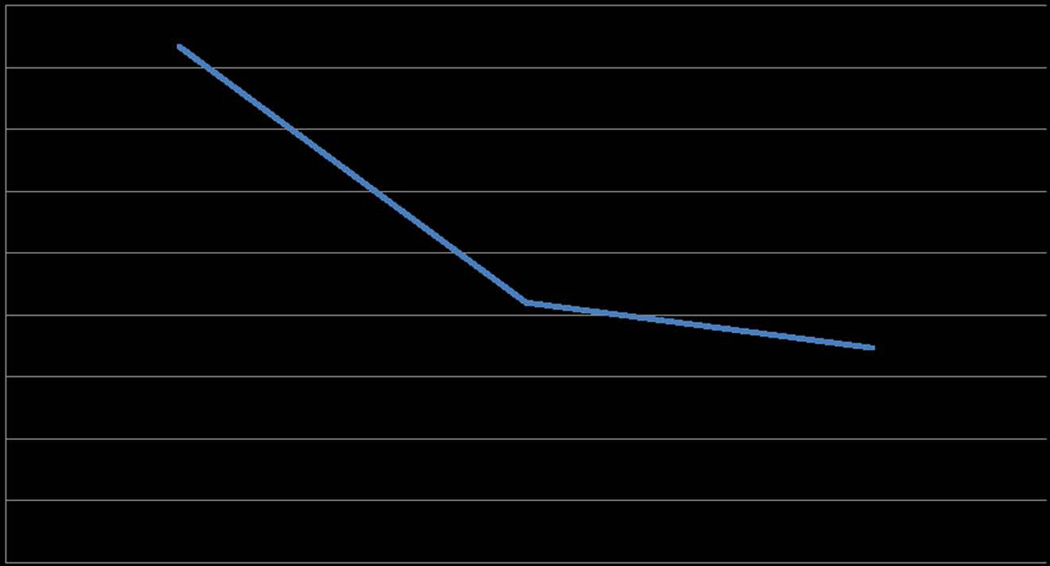
Anhedonia, reflecting deficits within the brain reward system, is a salient feature across mood disorders17 and has been associated with treatment resistance, illness chronicity,18–22 and suicidality.23 In a recent placebo-controlled, crossover study using positron emission tomography (PET) imaging to compare the effects of ketamine and saline, ketamine was suggested to have anti-anhedonic effects associated with regions involved in the reward circuit.24 A secondary analysis from a human ketamine study shows further promise for this avenue of research; an analysis of the MADRS, a 10-item clinician-rated scale of depression, from a previous study of IV ketamine conducted by our group16 showed that the scale’s individual item for anhedonia improved by 63% at 24 h compared to baseline following a single infusion of ketamine in patients with TRD (Fig. 1.). Our group proposes that ketamine directly affects the reward circuit across diagnostic groups, thus relieving anhedonia. There is a significant need for studies pinpointing the effects of ketamine on symptoms of anhedonia and its neurobiological mechanisms. Anhedonia, a reduced capacity to experience pleasure, reflects abnormal reward processing within specific brain circuitry and is a core deficit in a subpopulation of patients across a spectrum of mood, anxiety, and trauma-related disorders.
Figure 1.
Changes in anhedonia. MADRS item score #7 showing improvements in anhedonia during ketamine infusion (percentage change from baseline to 24 h = −0.58). A lower score on the MADRS #7 signifies improved anhedonia. Data from an RCT of 72 patients with TRD.16
Cognition
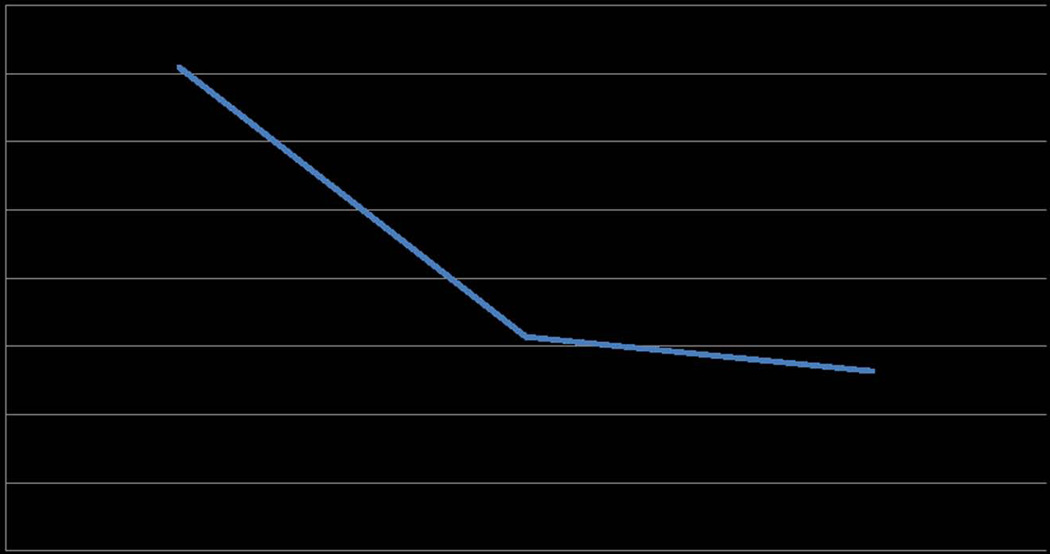
Recent studies have examined the cognitive effects of low dosages of ketamine in patients with depression.12,16 Ketamine administered in low dosages to treat depression was associated with mild, short-term cognitive deficits (40 min following a single IV ketamine infusion).16 An analysis of the MADRS items from previous studies of IV ketamine conducted by our group showed that concentration (item # 6) improved by 58.3% at 24 h compared to baseline after a single infusion of ketamine in patients with TRD (Fig. 2.). In addition, a study of the neurocognitive effects of a single 40-min IV infusion of ketamine in TRD patients found no deleterious short-term neurocognitive effects 7 days following treatment compared to an active control, midazolam.25 Furthermore, two studies have demonstrated that slower baseline processing speed predicted a rapid antidepressant response at 24 h following ketamine administration.25 While these studies address the short-term neurocognitive effects of ketamine, more research is required to establish the potential cognitive risks of longer-term repeated ketamine treatments for patients with treatment-resistant depression.
Figure 2.
Changes in concentration. MADRS item score #6 showing improvements in cognition pre/post–ketamine infusion (percentage change from baseline to 24 h = −0.63). A lower score on the MADRS #6 signifies improved cognition. Data from an RCT of 72 patients with TRD.16
Frequent ketamine abuse with high doses over long intervals can lead to deficits in several cognitive capacities (working memory, executive functions, visual and motor speed) and dissociative symptoms.26,27 More specifically, Morgan found that only daily ketamine abusers had the impairments in cognition and dissociative symptoms described previously, while infrequent and ex-users did not show any distinct cognitive impairment. Long-term cognitive effects of ketamine were examined in a recent paper in which patients with unipolar and bipolar depression received up to six infusions of ketamine over a 3-week period;12 in that setting, ketamine administration was not associated with negative cognitive effects. These studies suggest that low-dose ketamine administered over short periods to patients with unipolar and bipolar depression does not cause cognitive deficits, in contrast to studies of high-dose, long-term administration in frequent ketamine abusers. Ketamine is used as an adult anesthetic at doses of 1–3 mg/kg; sub-anesthetic doses of ketamine used to treat depression range from 0.1–1 mg/kg.28 Given the fact that other NMDA antagonists (such as memantine) have proven pro-cognitive effects, it would be important to design clinical trials to further study the effects of memantine and other glutamatergic agents on cognitive symptoms in patients with mood disorders.
Suicidal ideation
Finding an effective and rapid-acting treatment for acute suicidal ideation, a common symptom in severely depressed patients, is critical. Current monoamine antidepressants, often considered the first line of defense to treat depression and anxiety, are slow to act, although they may have enduring effects with consistent daily dosing.29 Contrastingly, ketamine has been shown to be effective at rapidly reducing suicidal ideation.15,30 While monoamine antidepressants take several weeks to take effect, ketamine’s rapid-acting effects could make it uniquely suited to treat acute suicidal ideation in hospitalized patients.
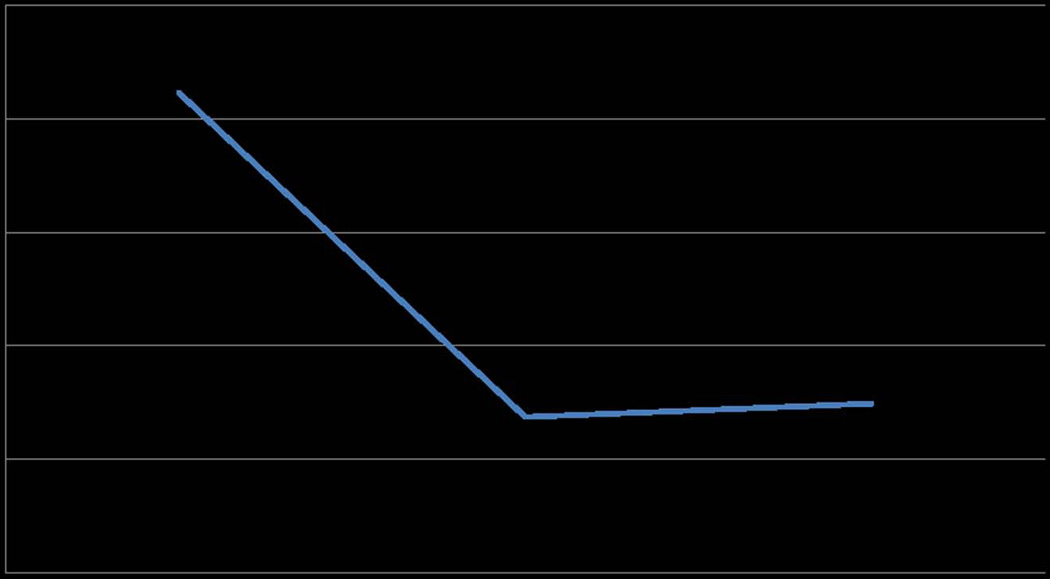
Price studied the effects of intravenous ketamine on suicidal ideation 2 h before and 24 h following a single IV infusion of ketamine (n = 26). Two primary measures were used: the Implicit Association Test (IAT), an implicit suicidality measure, and the suicidality item of the MADRS (MADRS-SI), an explicit suicidality measure. Price et al. found MADRS-SI scores reduced by 2.08 points on a 0–6 scale following ketamine infusion, as well as a decrease in implicit associations connecting the self to escape-related concepts.30 These open-label findings were confirmed in an RCT comparing ratings of suicidality on both implicit and explicit measures following either ketamine or midolazam infusion. Fifty-three percent of ketamine patients had no explicit suicidal ideation at 24 h, compared with 24% of placebo (P = 0.03). We replicated this finding in three previous studies of IV ketamine, which showed that the MADRS’s individual item for suicidal ideation improved by 64.7% at 24 h compared to baseline following a single infusion of ketamine in patients with TRD (Fig. 3).16 These findings suggest that ketamine produces rapid reductions in suicidal ideation as compared to placebo, although future research is necessary to further explore long-term effects.
Figure 3.
Changes in suicidality. MADRS item score #10 showing improvements in suicidal ideation pre/post–ketamine infusion (percentage change from baseline to 24 h = −0.65). A lower score on the MADRS #10 signifies improved suicidal ideation. Data from an RCT of 72 patients with TRD.16
Furthermore, a recent multiple-infusion open-label ketamine study showed decreased suicidal ideation in patients after the first infusion.12 Within 6 h of receiving a single ketamine infusion, 61% of bipolar or unipolar patients (n = 28) experienced a reduction in suicidal ideation, calibrated by the Beck Depression Inventory (BDI) and item three on the Hamilton Rating Scale of Depression. Ongoing research at the Icahn School of Medicine at Mount Sinai is investigating the anti-suicidal effects of ketamine over the course of 6 weeks following a single, double-blind IV infusion of ketamine or midazolam (NCT01507181).
In conclusion, emerging data suggest that ketamine may be potentially effective for several RDoC-defined symptom clusters. As these data were acquired in depressed (TRD) populations, we cannot distinguish a pseudo-specific effect (e.g., improvement in specific domains, such as anhedonia or suicidality, may be related to overall improvement of depression). But these data justify the need for specific studies evaluating the efficacy of ketamine in RDoC-defined domains and including subjects with a variety of DSM diagnoses.
Putative mechanisms
While a detailed discussion of ketamine mechanisms is beyond the scope of this clinical review, we would like to emphasize a series of recent studies highlighting important mechanisms of ketamine activity and potentially explaining the rapid clinical effects of ketamine in mood disorders. Glutamate is the most prevalent excitatory neurotransmitter in the brain and a critical modulator of neuroplasticity, learning, and memory. Recent research suggests that antidepressant agents targeting the glutamate system may provide a novel treatment for MDD.31 Glutamate has been found to play an essential role in regulating neuroplasticity but its activity can be influenced by environmental factors. Excessive levels of glutamate (caused by stress) in MDD patients have been suggested to decrease plasticity and brain volume; glutamate antagonists, including ketamine, can help to ameliorate these effects.32
Low-dose ketamine increases glutamate activity in prefrontal cortical regions, catalyzing synaptogenesis and enhanced synaptic functioning through a cascade of molecular events. A probable mechanism of action involves enhancing glutamate signaling through α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors (AMPARs).33,34 Several studies demonstrate that AMPA signaling is essential for the behavioral and molecular changes associated with ketamine to occur.35,36 Ketamine also affects other neurological pathways that contribute to its observed antidepressant effects, including signaling pathways such as the brain-derived neurotrophic factor (BDNF) tropomyosin-related kinase B (TrkB) pathway and the associated downstream phosphatidyl inositol-3 kinase (P13K)-Akt and the Ras/mitogen-activated protein kinase (MAPK) pathways, as well as glycogen synthase kinase-3 (GSK-3)–associated pathways, and the mammalian target of rapamycin (mTOR) pathway.33,34
Ketamine has also been shown to affect overall plasma BDNF levels as analyzed through blood draws at baseline and at 240 min following ketamine.37 In an RCT using midolazam as placebo, responders had higher plasma BDNF levels at 240 min following ketamine infusion as compared to non-responders. Additionally, elevated BDNF levels were negatively correlated with decreasing MADRS scores in the ketamine group. No correlations were found for BDNF levels in the midolazam group.
Limitations of ketamine use and its implications
Safety
Infusions of IV ketamine in patients with TRD were found to be generally safe and well tolerated in our RCT,16 which is consistent with the findings of a recent reanalysis of 205 IV ketamine infusions.28 This reanalysis examined ketamine infusions administered across three clinical trials (two trials used single IV infusions and one trial used up to six IV infusions over 2 weeks), conducted between 2006–2012 at two academic medical centers. It was found that ketamine induced transient changes in hemodynamic measures in almost 30% of all participants, significantly elevating blood pressure and/or heart rate. In addition, psychotomimetic symptoms, as measured by the BPRS+, and dissociative symptoms, as measured by the CADSS, showed small, but significant elevations during the ketamine infusions. These changes were all resolved within 4 h post-infusion. Out of 205 ketamine infusions, only four infusions (1.95%) had to be discontinued due to adverse events, none of which were life threatening. The most common side effects were drowsiness, dizziness, poor coordination, blurred vision, and feeling strange or unreal.28 Side effects only rarely led to termination of infusion. These studies suggest that low-dose ketamine could rapidly and safely reduce core symptoms of depression within 24–72 h of single and continuous infusions; however, adequate medical support and monitoring should be present to optimize patient safety.
Potential for abuse
Although ketamine has possible rapid antidepressant properties, this promising treatment must be discussed in the context of its possible global health abuse potential. Ketamine has been used an anesthetic since 1970, and although ketamine is not used very frequently in the United States for anesthesia, general medical practices in Africa rely on ketamine for anesthesia, according to the World Health Organization (WHO) Critical Review of Ketamine.38 Ketamine is an ideal anesthetic in countries with limited anesthesiologists, money, and equipment because it is inexpensive, has a wide margin of safety, and does not suppress the cardiorespiratory system.38
Despite ketamine’s importance as an anesthetic in resource-poor African nations, ketamine is becoming a frequently abused drug in various regions of the world. Forty-eight countries labeled it as a controlled substance in 2009 compared to 34 countries in 2008.38 Most notably, ketamine abuse has escalated in recent years in Southeast Asia. Ketamine was the third most used illicit substance in China in 2011,39 although it is frequently used in combination with other drugs. In Hong Kong, ketamine abuse accounted for 60.5–85.2% of reported abuse among substance abusers from 2005–2011, making ketamine the most prominent drug of abuse in that region.38; 39 Ketamine abuse may damage important bodily functions, including cardiovascular, respiratory, gastrointestinal, reproductive, genitourinary, and immune systems.39 Findings from research studies in Hong Kong suggest that habitual abuse of ketamine may result in significant urinary bladder dysfunction and renal impairment—ketamine cystitis—as well as the cognitive impairments discussed previously.39
In 2012, the United Nations decided that ketamine would remain classified as an unscheduled substance, leaving ketamine regulation under the domain of individual countries. The scheduling of ketamine would negatively impact the ability of resource-poor nations to use it for anesthesia. The WHO recommended that, “It is important for the international community to work in harmony to strike a balance between legitimate use of ketamine for medical purposes and prevention of trafficking in and abuse of ketamine.”38 Current research on ketamine’s rapid antidepressant effects should consider ketamine’s complex public health impact and how ketamine could be feasibly implemented as a standard treatment for depression on a global scale.
Other glutamatergic agents
Although the antidepressant effects of ketamine were discovered serendipitously, the exciting clinical observations supporting the rapid and robust clinical effects of ketamine have motivated industry-wide drug discovery efforts to uncover more potent glutamatergic agents with more user-friendly routes of administration. Ketamine has a high efficacy, but some safety risks, as detailed in other sections of this paper. Additionally, its transient psychotomimetic effects make ketamine potentially unsuitable for patients with a history of psychosis (due to theoretical concerns that it may restart or worsen a psychotic process). The limitations of ketamine are the impetus for pharmacological discovery of novel glutamatergic agents for patients with mood disorders. While other reviews40 have presented these compounds in more detail, we summarize here some of the glutamatergic agents tested so far (Table 3). In addition to ketamine, multiple compounds have been found to affect the complex glutamate system, binding to either ionotropic or metabotropic receptors, resulting in a wide range of mechanisms. The extensive molecular architecture involved in regulating and supporting glutamate systems allows for a variety of targets for glutamatergic drug development. Compounds currently being studied include ketamine, memantine, riluzole, lamotrigine, GLYX-13, CERC-301, and several others.
Table 3.
Clinical trials withother glutamatergic agents in patients with MDD
| Drug | Target | Major mechanism of action |
Treatment effect |
|---|---|---|---|
| AZD6765 | NMDAR | Low- to moderate-affinity open-channel antagonist, “low trapping” | Initial limited evidence for antidepressant efficacy; development halted after failed phase IIb study |
| memantine | NMDAR | Non-competitive antagonist | Limited controlled data does not support antidepressant efficacy. |
| CP-101,606 | NMDAR, GluN2B | GluN2B selective antagonist | Preliminary evidence for antidepressant efficacy; safety concerns |
| MK-0657 | GluN2B | GluN2B selective antagonist | Preliminary evidence for antidepressant efficacy |
| Org 26575 | AMPAR | PAM | No clinical evidence for antidepressant efficacy to date |
| acamprosate | Other | NMDA and mGluR5 antagonist | Controlled data does not support antidepressant efficacy. |
| lamotrigine | Other | Inhibits voltage-dependent channels to reduce glutamate release | Limited evidence for antidepressant efficacy |
| dextromethorphan and quinidine | NMDAR | Non-competitive antagonist | Ongoing trial in MDD (NCT01882829) |
| ketamine | NMDAR | Non-competitive antagonist | Good evidence for antidepressant efficacy |
| D-cycloserine | NMDAR Glycine | Partial agonist at glycine site | Limited evidence for antidepressant efficacy |
| riluzole | Other | Reduces extrasynaptic glutamate by inhibiting presynaptic release; enhances astroglial uptake | Preliminary evidence for antidepressant efficacy |
Abbreviations: NMDAR, N-methyl-D-aspartate-receptor; GluN2B, NMDA receptor subunit, glutamate-binding site; AMPAR, alpha-amino-3-hydroxy-5-methyl-4-isozazolepropionic acid receptor; mGluR5, metabotropic glutamate receptor 5.
In an effort to replicate the antidepressant effects of ketamine, several compounds that are also non-competitive glutamate antagonists at the NMDA receptors have been studied. AZD6765 (AstraZeneca), an NMDA receptor antagonist with low to moderate affinity, has been reported to provide short-lived attenuation of depressive symptoms without triggering dissociative symptoms,41, 42 although the compound’s development has been recently abandoned following a negative phase IIb RCT. Our group is currently investigating the combination of dextromethorphan, formerly used as a cough suppressant, with quinidine (called Neudexta) to study its efficacy as an NMDA receptor antagonist, although other molecular mechanisms have been invoked as well (NCT01882829). An additional mechanism of action involves the glycine-binding site of NMDA receptors. GLYX-13 has partial agonist activity at this site; the antidepressant effects are currently being studied in an RCT (NCT01684163).
Riluzole, a glutamate modulator that is currently FDA-approved for the treatment of amyotrophic lateral sclerosis (ALS), is currently under investigation as an antidepressant due to its ability to inhibit glutamate release, increase glial reuptake of glutamate, and increase AMPA receptor trafficking (NCT01204918). An open-label clinical trial of riluzole in unipolar and bipolar depression found significant antidepressant effects.43
G protein–coupled metabotropic glutamate receptors (mGluRs) have powerful modulatory effects on signal transduction and can affect synaptic plasticity. Researchers at Yale University are currently conducting an observational imaging study with MDD and posttraumatic stress disorder (PTSD) participants, looking at the effects of ketamine or n-acetyl cysteine on mGluR5 (NCT01691092). In addition, a number of protocols are investigating advanced imaging techniques studying mGluR subtypes in order to help understand the detailed molecular mechanisms involved and their therapeutic potential (e.g., NCT02230592, NCT01896843).
Future directions
The recent shift in focus away from discrete diagnoses and toward RDoC symptom clusters will affect the population of subjects participating in ketamine trials. The principle focus in past ketamine studies has been to treat unipolar TRD patients, with a few studies also focusing on bipolar and PTSD patients. Going forward, the focus on symptoms clusters defined by RDoC domains will result in the inclusion of patients with additional comorbidities and varying diagnoses, which could help enhance the external validity of these studies.
Ketamine has demonstrated efficacy and safety as a rapidly-acting antidepressant administered intravenously. However, the antidepressant effect is time limited, typically lasting between one week and one month. The critical next step in future research will be to determine the best strategy for maintenance of efficacy. This will require experimentation with varying multiple-infusion schedules and different methods of administering ketamine in an attempt to prolong the antidepressant effects and minimize dissociative or potential addictive side effects. A critical but unresolved research question, however, is how to maintain the antidepressant response to ketamine. Therefore, our team is currently conducting a placebo-controlled RCT to study the efficacy of lithium in TRD patients who exhibit an antidepressant response to ketamine on the basis of a mechanistic model of synergism between ketamine and lithium (NCT01880593). Inhibition of GSK3 is required for the antidepressant and neuroplasticity-related effects of ketamine in depression models,44,45 and lithium is a potent inhibitor of GSK3,46 suggesting an opportunity to capitalize on a mechanistic synergy between ketamine and lithium.45,47 GSK3 is critically involved in synaptic deconsolidation (also known as synaptic pruning)—the opposite of synaptogenesis—via regulation of glutamate receptor cycling, and hence blockade of GSK3 would be expected to enhance and extend the synaptogenic effects of ketamine. The successful execution of the proposed project has the potential to move the field closer toward the establishment of a novel and urgently needed therapy for treatment-resistant forms of depression.
Although ketamine does have significant abuse potential, the small doses used to treat bipolar and unipolar depression have not resulted in negative effects in study participants so far. This promising treatment, when used carefully and at low doses, has great potential as a rapidly acting antidepressant.
Conclusion
Antidepressant treatments that target the glutamate system hold high promise as novel, efficacious, and rapidly acting treatments for MDD. However, essential information on safety and efficacy in long-term use, necessary to justify these alternative drugs as viable clinical treatments, are insufficient for ketamine and completely lacking for other glutamatergic compounds. Further research is needed to help answer these questions.
Footnotes
Conflicts of interest
Ms. DeWilde and Ms. Levitch report no conflicts of interest. In the past three years, Dr. Murrough has served on advisory boards for Janssen Research and Development and Genentech, has provided consultation services for ProPhase, LLC and Impel Neuropharma and has received research support from Janssen and Avanir Pharmaceuticals; he is named on a patent pending for neuropeptide Y as a treatment for mood and anxiety disorders. Dr. Mathew has received consulting fees from Bristol-Myers Squibb, Cerecor, Genentech, and Naurex, and research support from AstraZeneca, Janssen Research and Development, and Otsuka. In the past three years, Dr. Iosifescu has consulted for Avanir, Axsome, CNS Response, INSYS Therapeutics, Lundbeck, Otsuka, Servier, and Sunovion and he has received research support through the Icahn School of Medicine at Mount Sinai from Alkermes, Astra Zeneca, Brainsway, Euthymics, Neosync, Roche, Shire. The Icahn School of Medicine at Mount Sinai and its Dean, Dr. Charney, have been named on a use patent on ketamine for the treatment of depression. The Icahn School of Medicine has entered into a licensing agreement for the use of ketamine as therapy for treatment-resistant depression. The Icahn School of Medicine at Mount Sinai could potentially benefit if ketamine were to gain approval for the treatment of depression.
References
- 1.Mathers C, Fat DM, Boerma J. The Global Burden of Disease: 2004 Update. World Health Organization; 2008. [Google Scholar]
- 2.Undurraga J, Baldessarini RJ. Randomized, placebo-controlled trials of antidepressants for acute major depression: thirty-year meta-analytic review. Neuropsychopharmacology. 2011;37:851–864. doi: 10.1038/npp.2011.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mathew SJ, Shah A, Lapidus K, et al. Ketamine for Treatment-Resistant Unipolar Depression. CNS drugs. 2012;26:189–204. doi: 10.2165/11599770-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fond G, Loundou A, Rabu C, et al. Ketamine administration in depressive disorders: a systematic review and meta-analysis. Psychopharmacology (Berl.) 2014;231:3663–3676. doi: 10.1007/s00213-014-3664-5. [DOI] [PubMed] [Google Scholar]
- 5.McGirr A, Berlim M, Bond D, et al. A systematic review and meta-analysis of randomized, double-blind, placebo-controlled trials of ketamine in the rapid treatment of major depressive episodes. Psychol. Med. 2014:1–12. doi: 10.1017/S0033291714001603. [DOI] [PubMed] [Google Scholar]
- 6.Murrough JW, Iosifescu DV, Chang LC, et al. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am. J. Psychiatry. 2013;170:1134–1142. doi: 10.1176/appi.ajp.2013.13030392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Insel T, Cuthbert B, Garvey M, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am. J. Psychiatry. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- 8.Mathew SJ, Murrough JW, Collins KA, et al. Riluzole for relapse prevention following intravenous ketamine in treatment-resistant depression: a pilot randomized, placebo-controlled continuation trial. The International Journal of Neuropsychopharmacology. 2010;13:71–82. doi: 10.1017/S1461145709000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berman RM, Cappiello A, Anand A, et al. Antidepressant effects of ketamine in depressed patients. Biol. Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 10.Zarate CA, Singh JB, Carlson PJ, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch. Gen. Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 11.Permoda-Osip A, Skibińska M, Bartkowska A, et al. Factors connected with efficacy of single ketamine infusion in bipolar depression. Psychiatr. Pol. 2014;48:35–46. [PubMed] [Google Scholar]
- 12.Diamond PR, Farmery AD, Atkinson S, et al. Ketamine infusions for treatment resistant depression: a series of 28 patients treated weekly or twice weekly in an ECT clinic. J. Psychopharmacol. 2014;28:536–544. doi: 10.1177/0269881114527361. [DOI] [PubMed] [Google Scholar]
- 13.Lapidus KA, Levitch CF, Perez AM, et al. A Randomized Controlled Trial of Intranasal Ketamine in Major Depressive Disorder. Biol. Psychiatry. 2014 doi: 10.1016/j.biopsych.2014.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zarate CA, Jr, Brutsche N, Laje G, et al. Relationship of ketamine's plasma metabolites with response, diagnosis, and side effects in major depression. Biol. Psychiatry. 2012;72:331–338. doi: 10.1016/j.biopsych.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diazgranados N, Ibrahim L, Brutsche NE, et al. A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch. Gen. Psychiatry. 2010;67:793–802. doi: 10.1001/archgenpsychiatry.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murrough JW, Perez AM, Pillemer S, et al. Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression. Biol. Psychiatry. 2013;74:250–256. doi: 10.1016/j.biopsych.2012.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pizzagalli DA. Depression, stress, and anhedonia: toward a synthesis and integrated model. Annual review of clinical psychology. 2014;10:393–423. doi: 10.1146/annurev-clinpsy-050212-185606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moos RH, Cronkite RC. Symptom-based predictors of a 10-year chronic course of treated depression. J. Nerv. Ment. Dis. 1999;187:360–368. doi: 10.1097/00005053-199906000-00005. [DOI] [PubMed] [Google Scholar]
- 19.McMakin DL, Olino TM, Porta G, et al. Anhedonia Predicts Poorer Recovery Among Youth With Selective Serotonin Reuptake Inhibitor Treatment–Resistant Depression. Journal of the American Academy of Child & Adolescent Psychiatry. 2012;51:404–411. doi: 10.1016/j.jaac.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spijker J, Bijl R, De Graaf R, et al. Determinants of poor 1-year outcome of DSM-III-R major depression in the general population: results of the Netherlands Mental Health Survey and Incidence Study (NEMESIS) Acta Psychiatr. Scand. 2001;103:122–130. doi: 10.1034/j.1600-0447.2001.103002122.x. [DOI] [PubMed] [Google Scholar]
- 21.Vrieze E, Pizzagalli DA, Demyttenaere K, et al. Reduced reward learning predicts outcome in major depressive disorder. Biol. Psychiatry. 2013;73:639–645. doi: 10.1016/j.biopsych.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wardenaar KJ, Giltay EJ, van Veen T, et al. Symptom dimensions as predictors of the two-year course of depressive and anxiety disorders. J. Affect. Disord. 2012;136:1198–1203. doi: 10.1016/j.jad.2011.11.037. [DOI] [PubMed] [Google Scholar]
- 23.Spijker J, de Graaf R, ten Have M, et al. Predictors of suicidality in depressive spectrum disorders in the general population: results of the Netherlands Mental Health Survey and Incidence Study. Soc. Psychiatry Psychiatr. Epidemiol. 2010;45:513–521. doi: 10.1007/s00127-009-0093-6. [DOI] [PubMed] [Google Scholar]
- 24.Lally N, Nugent A, Luckenbaugh D, et al. Anti-anhedonic effect of ketamine and its neural correlates in treatment-resistant bipolar depression. Translational psychiatry. 2014;4:e469. doi: 10.1038/tp.2014.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murrough JW, Burdick KE, Levitch CF, et al. Neurocognitive Effects of Ketamine and Association with Antidepressant Response in Individuals with Treatment-Resistant Depression: A Randomized Controlled Trial. Neuropsychopharmacology. 2014 doi: 10.1038/npp.2014.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morgan CJ, Muetzelfeldt L, Curran HV. Ketamine use, cognition and psychological wellbeing: a comparison of frequent, infrequent and ex-users with polydrug and non-using controls. Addiction. 2009;104:77–87. doi: 10.1111/j.1360-0443.2008.02394.x. [DOI] [PubMed] [Google Scholar]
- 27.Tang W, Liang H, Lau C, et al. Relationship between cognitive impairment and depressive symptoms in current ketamine users. Journal of studies on alcohol and drugs. 2013;74:460. doi: 10.15288/jsad.2013.74.460. [DOI] [PubMed] [Google Scholar]
- 28.Wan L, Levitch CF, Perez AM, et al. Ketamine Safety and Tolerability in Clinical Trials for Treatment-Resistant Depression. Journal of Clinical Psychiatry. 2014 doi: 10.4088/JCP.13m08852. [DOI] [PubMed] [Google Scholar]
- 29.Niciu MJ, Henter ID, Luckenbaugh DA, et al. Glutamate receptor antagonists as fast-acting therapeutic alternatives for the treatment of depression: ketamine and other compounds. Annu. Rev. Pharmacol. Toxicol. 2014;54:119–139. doi: 10.1146/annurev-pharmtox-011613-135950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Price RB, Nock MK, Charney DS, et al. Effects of intravenous ketamine on explicit and implicit measures of suicidality in treatment-resistant depression. Biol. Psychiatry. 2009;66:522–526. doi: 10.1016/j.biopsych.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murrough JW, Charney DS. Is there anything really novel on the antidepressant horizon? Curr. Psychiatry Rep. 2012;14:643–649. doi: 10.1007/s11920-012-0321-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arnone D, McIntosh A, Ebmeier K, et al. Magnetic resonance imaging studies in unipolar depression: systematic review and meta-regression analyses. European Neuropsychopharmacology. 2012;22:1–16. doi: 10.1016/j.euroneuro.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 33.Li N, Liu R, Dwyer JM, et al. Glutamate<i>N</i>-methyl-D-aspartate Receptor Antagonists Rapidly Reverse Behavioral and Synaptic Deficits Caused by Chronic Stress Exposure. Biol. Psychiatry. 2011;69:754–761. doi: 10.1016/j.biopsych.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li N, Lee B, Liu RJ, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maeng S, Zarate CA, Jr, Du J, et al. Cellular mechanisms underlying the antidepressant effects of ketamine: role of α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol. Psychiatry. 2008;63:349–352. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 36.Autry AE, Adachi M, Nosyreva E, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haile C, Murrough J, Iosifescu D, et al. Plasma brain derived neurotrophic factor (BDNF) and response to ketamine in treatment-resistant depression. The International Journal of Neuropsychopharmacology. 2014;17:331–336. doi: 10.1017/S1461145713001119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.World Health Organization. Thirty-Fifth Meeting of the Expert Committee on Drug Depedence. 2012;35 In. [Google Scholar]
- 39.Li J, Vicknasingam B, Cheung Y, et al. To use or not to use: an update on licit and illicit ketamine use. Substance abuse and rehabilitation. 2011;2:11. doi: 10.2147/SAR.S15458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lapidus KA, Soleimani L, Murrough JW. Novel glutamatergic drugs for the treatment of mood disorders. Neuropsychiatric disease and treatment. 2013;9:1101. doi: 10.2147/NDT.S36689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanacora G, Smith M, Pathak S, et al. Lanicemine: a low-trapping NMDA channel blocker produces sustained antidepressant efficacy with minimal psychotomimetic adverse effects. Mol. Psychiatry. 2013;19:978–985. doi: 10.1038/mp.2013.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zarate CA, Jr, Mathews D, Ibrahim L, et al. A Randomized Trial of a Low-Trapping Nonselective< i>N</i>-Methyl-D-Aspartate Channel Blocker in Major Depression. Biol. Psychiatry. 2013;74:257–264. doi: 10.1016/j.biopsych.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zarate CA, Jr, Quiroz JA, Singh JB, et al. An open-label trial of the glutamate-modulating agent riluzole in combination with lithium for the treatment of bipolar depression. Biol. Psychiatry. 2005;57:430–432. doi: 10.1016/j.biopsych.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 44.Duman RS, Aghajanian GK. Synaptic dysfunction in depression: potential therapeutic targets. Science. 2012;338:68–72. doi: 10.1126/science.1222939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beurel E, Song L, Jope RS. Inhibition of glycogen synthase kinase-3 is necessary for the rapid antidepressant effect of ketamine in mice. Mol. Psychiatry. 2011;16:1068–1070. doi: 10.1038/mp.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gould TD, Manji HK. Glycogen synthase kinase-3: a putative molecular target for lithium mimetic drugs. Neuropsychopharmacology. 2005;30:1223–1237. doi: 10.1038/sj.npp.1300731. [DOI] [PubMed] [Google Scholar]
- 47.Ghasemi M, Raza M, Dehpour AR. NMDA receptor antagonists augment antidepressant-like effects of lithium in the mouse forced swimming test. J. Psychopharmacol. 2010;24:585–594. doi: 10.1177/0269881109104845. [DOI] [PubMed] [Google Scholar]
- 48.Ibrahim L, et al. Course of improvement in depressive symptoms to a single intravenous infusion of ketamine vs add-on riluzole: results from a 4-week, double-blind, placebo-controlled study. Neuropsychopharmacology. 2012;37:1526–1533. doi: 10.1038/npp.2011.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.aan het Rot M, et al. Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol. Psychiatry. 2010;67:139–145. doi: 10.1016/j.biopsych.2009.08.038. [DOI] [PubMed] [Google Scholar]
- 50.Rasmussen KG, et al. Serial infusions of low-dose ketamine for major depression. J Psychopharmacol. 2013;27:444–450. doi: 10.1177/0269881113478283. [DOI] [PubMed] [Google Scholar]
- 51.Segmiller F, et al. Repeated S-ketamine infusions in therapy resistant depression: a case series. J. Clin. Pharmacol. 2013;53:996–998. doi: 10.1002/jcph.122. [DOI] [PubMed] [Google Scholar]