Abstract
Vitamin D is associated with skeletal muscle physiology and function and may play a role in intramuscular inflammation, possibly via the vitamin D receptor (VDR). We conducted two studies to examine (1) whether serum 25-hydroxyvitamin D (25OHD) and/or intramuscular VDR protein concentrations are associated with intramuscular interleukin-6 (IL-6) and/or tumor necrosis factor-α (TNFα); and (2) whether 16-week supplementation with vitamin D3 alters intramuscular IL-6 and/or TNFα. Potential-related signaling pathways were also examined. Muscle biopsies of 30 older, mobility-limited adults were obtained at baseline. A subset of 12 women were supplemented with either 4,000 IU/day of vitamin D3 (N = 5) or placebo (N = 7), and biopsies were repeated at 16 weeks. Serum 25OHD was measured, and intramuscular VDR, IL-6, and TNFα gene expressions and protein concentrations were analyzed. Baseline serum 25OHD was not associated with intramuscular IL-6 or TNFα gene expression or protein concentration. Baseline intramuscular VDR protein concentration, adjusted for baseline serum 25OHD, was positively associated with intramuscular IL-6 gene expression (n = 28; p = 0.04), but negatively associated with intramuscular IL-6 protein (n = 18; p = 0.03). Neither intramuscular IL-6 nor TNFα gene expression was different between placebo (n = 7) or vitamin D3 supplementation groups (n = 5) after 16 weeks (p = 0.57, p = 0.11, respectively). These data suggest that VDR is a better predictor than serum 25OHD concentration of intramuscular IL-6 gene and protein expressions. A similar relationship was not observed for TNFα expression. Further, supplementation with 4,000 IU vitamin D3 per day does not appear to affect intramuscular IL-6 or TNFα gene expression after 16 weeks.
Keywords: Vitamin D, Vitamin D receptor, Skeletal muscle, Inflammation
Introduction
Intramuscular inflammation has been demonstrated in a variety of skeletal muscle disorders [1, 2] and contributes to muscle loss and dysfunction in systemic inflammatory disorders, such as insulin resistance, type 2 diabetes mellitus, and cardiovascular disease [3].
An inverse relationship between serum 25-hydroxyvitamin-D (25OHD) and markers of inflammation has been shown in obesity [4], type 2 diabetes mellitus [5], and cardiovascular disease [6]. Further, vitamin D3 supplementation has been shown to result in a decreased innate immune response [7], although results from other studies have been mixed [8, 9].
Emerging evidence suggests that vitamin D may play a direct role in mediating inflammation within skeletal muscle [10, 11]. As skeletal muscle has been shown to produce and secrete proinflammatory cytokines, such as IL-6 [12], an interaction between vitamin D and intramuscular inflammatory pathways is of particular interest. Although the literature examining this interaction is limited, the data indicate that increased concentrations of circulating 25OHD aid muscular recovery from injury [13], and vitamin D3 supplementation may reduce exercise-induced intramuscular inflammation [10] and directly suppress intramuscular pathways of inflammation, particularly after exercise [11]. However, the mechanism by which vitamin D may directly impact human muscle-mediated inflammation is yet unclear.
Studies in other tissues, such as fibroblasts and breast cancer cells, suggest that vitamin D receptor (VDR) activation may regulate intracellular inflammation [14–16]. VDR may also be implicated in skeletal muscle-mediated inflammation as has been noted in other tissues, such as vascular endothelial cells [17]. Although there is some controversy [18] possibly resulting from differences between experimental protocols [19], VDR has been shown to be expressed in skeletal muscle tissue [19–21]; it appears to decrease with age [22], and increase with vitamin D3 supplementation [11, 19, 21]. VDR downstream signaling cascades in skeletal muscle have yet to be well characterized; however, recent animal model data suggest that inflammation pathways are altered after the administration of vitamin D. An investigation examining rats supplemented with vitamin D and exposed to high-intensity exercise demonstrated increased intramuscular VDR with concurrent reductions in signaling molecules p38 MAPK and components of the NFkB cascade which ultimately resulted in attenuation of intramuscular TNF-α and IL-6 [11]. While it has been posited that chronically decreased expression of VDR in aging skeletal muscle may play a role in decreased strength and functional ability, this emerging research suggests it may also have a role in intramuscular inflammation, possibly through non-genomic signaling events.
We hypothesized that serum 25OHD and intramuscular VDR gene expression and protein concentrations would be inversely related to inflammatory markers, IL-6 and TNFα. To investigate this hypothesis, we conducted a study to examine (1) the baseline association between serum 25OHD and intramuscular IL-6 or TNFα gene and protein concentrations, (2) the baseline association between intramuscular VDR gene expression or protein concentration with intramuscular IL-6 and TNFα gene and protein contents in older, mobility-limited adults. We also conducted a second study to assess changes in intramuscular IL-6 and TNFα after a 16-week supplementation of vitamin D3 in older, mobility-limited women. As a secondary analysis, we examined p38 MAPK and NFkB phosphorylation cascades that have been implicated in interactions between serum 25OHD, intramuscular VDR, and inflammatory markers, IL-6 and TNFα.
Materials and methods
Subjects
Data for these studies were pooled from the baseline measurements of two larger randomized controlled studies for which inclusion and exclusion criteria are presented elsewhere [21, 23]. Specific characteristics of study subjects are presented in Table 1. Only those subjects who provided a muscle biopsy (N = 30; male = 7; female = 23) were included in the analysis. Participants were mobility limited as determined by the short physical performance battery (SPPB <10) [24] and were not obese (BMI <30). In the longitudinal study, a subset of 12 women were either supplemented with 4,000 IU/day of vitamin D3 (N = 5) or placebo (N = 7), and biopsies were repeated at 16 weeks. Both clinical studies were approved by the Institutional Review Board of the Tufts University Health Sciences Campus (Boston, MA).
Table 1. Participant descriptive statistics (mean ± SD).
| Subset 1 | Subset 2 | p value | |
|---|---|---|---|
| Age (years) | 78.5 ± 4.79 | 77.9 ± 4.05 | 0.71 |
| Weight (kg) | 67.2 ± 14.2 | 73.97 ± 9.11 | 0.08 |
| Height (cm) | 157.5 ± 9.43 | 165.28 ± 7.82 | 0.007* |
| BMI | 27.0 ± 5.5 | 27.06 ± 2.94 | 0.88 |
| Creatinine (mg/dL) | 0.84 ± 0.17 | 0.89 ± 0.27 | 0.56 |
| eGFR (mL/min/1.73 m2) | 70.83 ± 19.15 | 76.65 ± 20.39 | 0.34 |
| SPPB | 7.9 ± 1.55 | 8.65 ± 1.23 | 0.09 |
| Gender (f) | 100 % | 63 % | |
| N | 12 | 18 |
Biochemical measures
Archived fasting blood samples were assessed at baseline and 16-week periods. Serum 25OHD was analyzed utilizing Diasorin, LIAISON® 25 OH vitamin D total assay. Vitamin D deficiency was defined as 25OHD serum concentrations below 12 ng/mL, insufficiency as 12–19 ng/mL, and sufficiency ≥20 ng/mL [25].
Muscle biopsy
Muscle biopsies were obtained from the vastus lateralis at the level of the mid-thigh under local anesthesia (1 % lidocaine). The specimens were flash frozen in liquid nitrogen and stored in liquid nitrogen until analysis.
Western blotting analysis
Immunoblotting was utilized to examine intramuscular protein concentrations of VDR, IL-6, and TNFα in the vastus lateralis muscle as previously reported (Pojednic et al., under review). Membranes were incubated overnight at 4 °C with primary antibodies specific for VDR, IL-6, and TNFα (1:1,000 in 5 % bovine serum albumin and TBS-Tween; VDRNR 1|1 Perseus Proteomics via R&D Systems, Minneapolis, MN; IL6 AbCam ab6672, Cambridge, MA; TNFα D5G9 Cell Signaling, Danvers MA; phospho-p38 MAPK (Thr180/Tyr182) New England Biolabs Inc, Ipswich, MA; phospho-p65 (ser468) Cell Signaling, Danvers MA). Membranes were rinsed three times for 10 min in TBS-Tween and incubated at room temperature for VDR with secondary goat-anti mouse IgG2Aa HRP conjugate antibody (1:2,000 in 5 % nonfat dry milk and TBS-Tween; Invitrogen, Frederick, MD) and for IL-6, TNFα, phospho-p38, phospho-p65, and GAPDH with anti-rabbit IgG AP-linked antibody (1:1,000 in 5 % nonfat dry milk and TBS-Tween; Cell Signaling, Danvers, MA). Membranes were again rinsed three times for 10 min in TBS-Tween, and the immunoreactive proteins were detected with Supersignal Chemiluminescent Substrate (Thermo Scientific, Rockford, IL) and quantified by optical density (Image Lab 3.0.1; Bio-Rad Laboratories, Hercules, CA). Changes in optical density were calculated relative to values from glyceraldehyde-3-phosphate dehydrogenase (GAPDH, Cell Signaling, Danvers MA), and data are presented in arbitrary units.
mRNA preparation
Vastus lateralis muscle was prepared for mRNA analysis as reported previously (Pojednic et al., 2014, under review). mRNA extraction was completed utilizing Aurum Total RNA Fatty and Fibrous Tissue RNA Extraction Kit (Bio-Rad Laboratories, Hercules, CA). cDNA conversion was performed utilizing a commercially available reaction mixture (iScript Reverse Transcription SuperMix for RT-qPCR Bio-Rad Laboratories, Hercules, CA) on a T100 Thermal Cycler (Bio-Rad Laboratories, Hercules, CA).
Real-time qPCR
Quantitative real-time PCR was performed utilizing a commercially available reaction mixture (SsoAdvanced SYBR Green Supermix; Bio-Rad Laboratories, Hercules, CA) on a CFX96 Real-Time System (Bio-Rad Laboratories, Hercules, CA). cDNA levels of VDR (QT01010170), IL-6 (QT00083720), and TNFα (QT01079561) were measured using commercially available primer mixtures (Quantitect Primer Assays: Qiagen) as previously reported (Pojednic et al., under review). Changes in target gene expression were calculated relative to values from glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (QT00199633). Efficiencies of each primer set were assessed using a standard curve, and analyzed using 0.0025–25 ng of control cDNA. Data are presented as fold change from baseline.
Statistical analysis
Statistical analysis was completed using SAS JMP software (v. 10.0, SAS Institute Inc, Cary, NC). Correlational analyses were utilized to determine associations between serum 25OHD and intramuscular IL-6 and TNFα gene expression and protein concentration. Multivariable linear regression was used to determine whether VDR, adjusted for serum 25OHD, accounts for the variability of IL-6 and TNFα gene or protein content in the cross-sectional analyses. The proportion of variation in IL-6 and TNFα accounted for by the main variables (intramuscular VDR and serum 25OHD) was calculated as partial R2 values. Body Mass Index (BMI) was originally included in each model, but determined to be non-significant (p > 0.05), so this variable was removed. In the longitudinal analysis, student's t test was used to analyze differences in intramuscular IL-6 and TNFα mRNA fold changes between placebo and supplemented groups. Secondary correlational analyses were used to assess associations between VDR or 25OHD with intramuscular inflammatory markers and signaling molecules in the cross-sectional study. Statistical significance was set at p ≤ 0.05 for all analyses. Variables were each examined for normal distribution, and were log-transformed, if necessary, to better approximate normal distribution.
Results
Subject characteristics
Subjects from the two studies were pooled for the baseline cross-sectional analysis. Mean age (±SD) of the baseline sample (N = 30) was 78.2 ± 4.4 years, mean BMI was 27.0 ± 3.5 kg/m2, and mean serum 25OHD was 22.5 ± 10.6 ng/mL (range 9–53 ng/mL). A comparison between subjects from the two studies is presented in Table 1.
Skeletal muscle IL-6 and TNFα gene expressions
Cross-sectional analysis
In the combined group at baseline, it should be noted that within subset 1, two subjects did not have their VDR protein concentration measured and were, thus, excluded from the cross-sectional analysis. Neither baseline 25OHD nor VDR gene expression (adjusted for baseline 25OHD) was associated with IL-6 gene expression (n = 28; p = 0.92 and p = 0.21, Fig. 1a, b respectively). Neither baseline 25OHD nor VDR gene expression (adjusted for baseline 25OHD) was associated with TNFα gene expression (p = 0.68 and p = 0.18, Fig. 1d, e respectively). However, in a multilinear regression model adjusted for serum 25OHD, intramuscular VDR protein concentration (log VDR) was positively associated with intramuscular IL-6 gene expression (Total Model R2 = 0.15; p = 0.04, Fig. 1c), but not with TNFα gene expression (p > 0.18, Fig. 1f).
Fig 1.
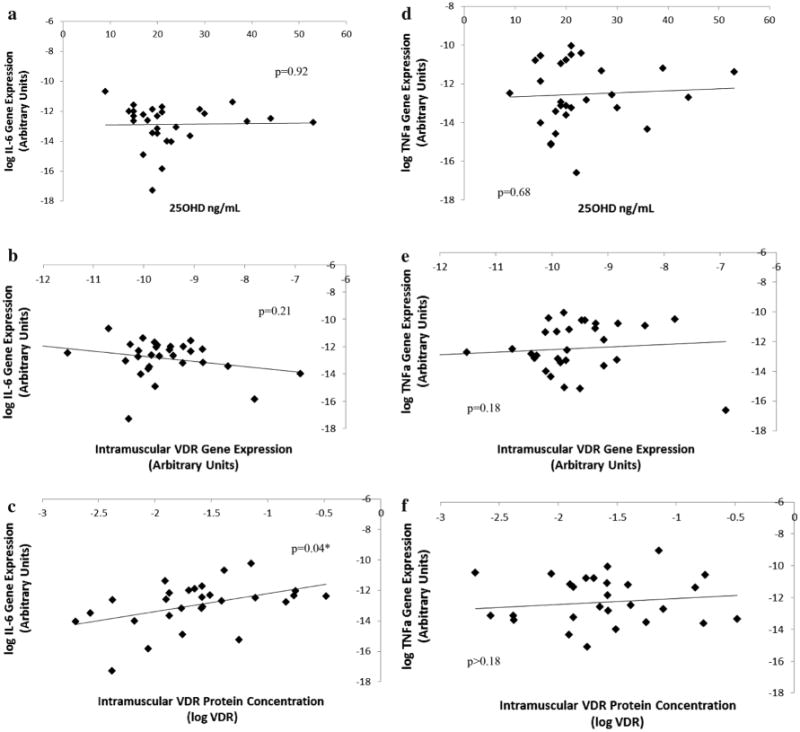
Cross-sectional association between IL-6 gene expression versus VDR protein and serum 25OHD. In a sample of older, mobility-limited adults (N = 28), neither baseline 25OHD nor VDR gene expression (adjusted for baseline 25OHD) were associated with IL-6 gene expression (p = 0.92 and p = 0.21; a, b, respectively) or TNFα gene expression (p = 0.68 and p = 0.18; d, e, respectively). Intramuscular VDR protein concentration (logVDR), adjusted for baseline 25OHD, was significantly associated with intramuscular IL-6 gene expression (p = 0.04*; c), but not with TNFα gene expression (p > 0.18; f). 25OHD was determined by serum assay, and RT-PCR analyses were carried out using VDR and anti-IL6 primers. Changes in Ct values were calculated relative to values from glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (*p < 0.05)
Longitudinal analysis
At baseline, older, mobility-limited women (subset 1, N = 12) were classified as either vitamin D insufficient or deficient. After 16 weeks, all vitamin D-supplemented subjects were classified as vitamin D sufficient, while placebo subjects did not reach sufficient 25OHD levels [21].
There was no significant difference between the fold changes of intramuscular IL-6 mRNA expression between the placebo (mean 0.45, 95 % CI 2.53, 3.43) and supplemented groups (mean 3.02, 95 % CI 7.21, 13.26; p = 0.57, Fig. 2a) after 16 weeks of vitamin D3 supplementation. With regard to intramuscular TNFα, after 16 weeks of vitamin D3 supplementation, there was no significant difference in the fold changes between the placebo (mean 2.46, 95 % CI 5.37–0.45) and supplemented groups (mean −6.83, 95 % CI −5.68 to 19.38; p = 0.11, Fig. 2b).
Fig 2.

Vitamin D supplementation does not significantly aAlter intramuscular IL-6 or TNFα gene expression In a cohort of older, mobility women (N = 12) supplemented with 4,000 IU Vitamin D3 for 16 weeks, and there was no significant difference between the fold changes of intramuscular IL-6 mRNA between the placebo (N = 7) and vitamin D3 supplemented groups (N = 5, p = 0.57, a) or was there a significant difference in TNFα (p = 0.11, b) as assessed by student's t test (p* < 0.05)
IL-6 and TNFa protein concentration and signaling pathways
Cross-sectional analysis
In subset 2 (Table 1; N = 18) for which intramuscular IL-6 and TNFα protein was available for analysis by Western Blot, baseline serum 25OHD was not associated with intramuscular IL-6 protein concentration (p = 0.13, Fig. 3a). Baseline VDR protein concentration (adjusted for serum 25OHD) was negatively associated with intramuscular IL-6 protein concentration (Total Model R2 = 0.35; p = 0.03, Fig. 3b). Neither serum 25OHD (p = 0.93, Fig. 3c) nor VDR protein (adjusted for serum 25OHD) was associated with intramuscular TNFα protein (Total Model R2 = 0.11; p = 0.40, Fig. 3d).
Fig 3.
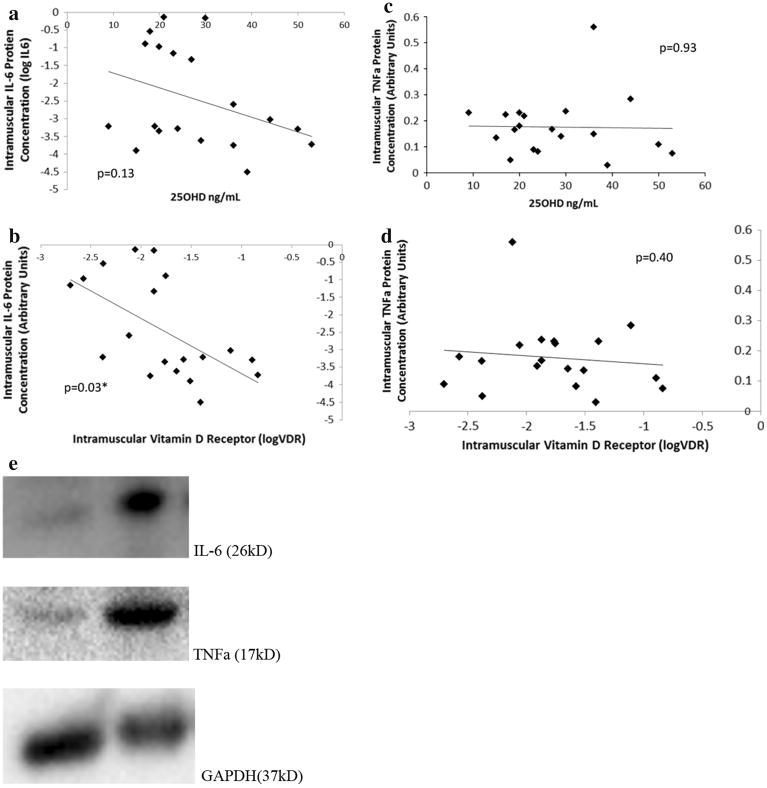
Cross-sectional association between intramuscular IL-6 protein expression versus Serum 25OHD and Intramuscular VDR Protein In a sample of older, mobility-limited adults (n = 18), 25OHD was not associated with intramuscular IL-6 protein expression (p = 0.13; a). However, in a multilinear regression model adjusted for serum 25OHD, intramuscular VDR protein concentration (log VDR) negatively predicted intramuscular IL-6 protein concentration (Total Model R2 = 0.35; p = 0.03; b). 25OHD was determined by serum assay, and western blot analyses were carried out using anti-VDR and anti-IL6 antibodies. Neither serum 25OHD (p = 0.93; d) nor VDR protein (adjusted for serum 25OHD) was associated with intramuscular TNFα protein (Total Model R2 = 0.11; p = 0.40; e). Changes in optical density were calculated relative to values from glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and data are presented in arbitrary units (*p < 0.05)
Further analysis was undertaken to determine potential signaling pathways responsible for the correlation between intramuscular VDR and IL-6 protein. Both intramuscular phosphorylated NFkB and p38 were examined. Intramuscular phosphorylated NFkB was not found to be significantly associated with serum 25OHD (p = 0.70), IL-6 protein (p = 0.99), or TNFα protein (p = 0.73) in this subset. NFkB was positively associated with intramuscular VDR protein concentration, but it was not statistically significant below the 0.05 level (p = 0.06). Intramuscular phosphorylated p38 was not significantly associated with serum 25OHD (p = 0.22), VDR (p = 0.41), or TNFα protein (p = 0.53). However, intramuscular phosphorylated p38 was positively associated with IL-6 protein (p = 0.002). Western blots are presented in Fig. 4.
Fig 4.

Baseline western blots of IL-6, TNFα, phosphorylated/total p38, and phosphorylated/total p65(NfKb) proteins are presented with vitamin D insufficient (18 ng/mL; left) and vitamin D sufficient (30 ng/mL; right) over corresponding GAPDH (p > 0.05)
Discussion
This study found that intramuscular IL-6, a marker of inflammation, was associated with intramuscular VDR in older, mobility-limited adults. Intramuscular VDR protein concentration was positively associated with intramuscular IL-6 gene expression, but negatively associated with intramuscular IL-6 protein concentration. There was no significant change in intramuscular IL-6 gene expression after 16 weeks of vitamin D3 supplementation. Because the sample size was small, it is premature to conclusively rule out possible contributions of serum 25OHD to intramuscular inflammation; however, we were able show noteworthy evidence that VDR may be associated with intramuscular IL-6 in human skeletal muscle.
Although traditionally assumed to be a marker of inflammation in serum, intramuscular IL-6 has also recently been termed a myokine and may be an emerging endocrine factor [26] shown to have several roles in skeletal muscle metabolism [27] in addition to participation in intramuscular inflammatory pathways [26, 28]. IL-6 is present in skeletal muscle at rest and becomes elevated with muscular contraction [29]. Of note, murine intramuscular IL-6 is attenuated by vitamin D supplementation, possibly via intramuscular VDR [11]. We hypothesized that IL-6 gene and protein expressions would be inversely associated with intramuscular VDR. However, our results demonstrated a contrasting relationship between VDR protein concentration and IL-6 gene expression versus protein concentration. One explanation could be that both VDR and IL-6 were measured intramuscularly, while 25OHD is measured from serum. Interestingly, however, a similar relationship has previously been reported with dietary glucose ingestion resulting in decreased intramuscular IL-6 protein release from exercising muscle with no change in IL-6 mRNA [30]. Further, other human myokines demonstrate differential effects between mRNA and protein expression upon stimulation [31, 32]. Finally, as VDR has been shown to have rapid non-genomic effects in skeletal muscle [33, 34], there may be a signaling or inhibitory effect through which VDR protein itself may attenuate translation of IL-6 protein (Fig. 5). It is possible that VDR protein decreases the present inflammatory response through a signaling process as noted in animal models [11] or affects other yet unidentified molecules which then initiate post-translational inhibitory effects as has been demonstrated by other myokines in skeletal muscle [35].
Fig 5.

Theoretical model of VDR interaction in attenuating intramuscular inflammation models are suggested via p38 MAPK and NFkB phosphorylation cascades, although further investigation must be completed in order to confirm, as our results demonstrated no association with these pathways despite prior-reported relationships
We did not observe a relationship between intramuscular TNFα, intramuscular VDR, or serum 25OHD. Our results are consistent with those reported for vascular endothelial cells in middle-aged and older adults [17]. These authors concluded there was no involvement in vitamin D-related effects on endothelial cell inflammation with regard to TNFα. This disparate response between IL-6 and TNFa has previously been established in skeletal muscle in response to contraction where intramuscular IL-6 is produced independently of TNFα [35]. Finally, the parallel relationship between inflammatory cytokines traditionally noted in serum may not hold true within muscle given the unique emerging roles of IL-6 within muscle.
In order to assess possible signaling relationships between VDR and IL-6 gene expressions and protein concentration, we examined two key signaling pathways, p38 MAPK and NFκB. We did not find an association between serum 25OHD or intramuscular VDR with intramuscular NFκB or MAPK p38, although associations between NFκB and VDR did trend toward significance. While our sample size prohibits us from ruling out these pathways as a potential link between intramuscular inflammation and VDR, our results may suggest that any relationship may be outside this particular p38 MAPK pathway in human skeletal muscle. It is recommended that future studies with larger sample sizes re-examine these as well as alternative signaling pathways to determine the mechanistic relationship between intramuscular VDR and intramuscular IL-6. This is of interest due to the proposed non-inflammatory properties of IL-6 in skeletal muscle [26, 28] and the noted lack of association between serum 25OHD and IL-6 in our results.
Our study had several strengths including our ability to examine both a cross-sectional sample of human biopsied muscle and a longitudinal model following vitamin D3 supplementation versus placebo. Limitations of this study were that it had a small sample size and was a secondary analysis of data pooled from two studies. However, as our results demonstrate a significant association between human VDR and IL-6, there could be future clinical implications with regard to supplementation in vulnerable populations.
The results of our study lend evidence to the potential relationship between VDR and IL-6 in human skeletal muscle. We found that intramuscular VDR protein concentration is positively correlated with intramuscular IL-6 gene expression, but negatively associated with intramuscular IL-6 protein concentration in older, mobility-limited adults. Neither intramuscular VDR nor serum 25OHD appear to be associated with intramuscular TNFα in humans. These findings should be considered hypothesis generating and need to be confirmed in larger randomized trials specifically designed to examine intramuscular inflammation. Future studies are needed to examine the relationship between protein concentrations of VDR and IL-6 in skeletal muscle after supplementation, IL-6-related signaling pathways, and implications on muscle function.
Acknowledgments
This study is supported by the USDA Agricultural Research Service, under agreements No. 58-1950-7-707 (to BDH and LC) and No. 58-1950-0-014 (to RAF); The Dairy Research Institute (R.A.F.), Boston Claude D. Pepper Older Americans Independence Center (1P30AG031679 to R.A.F.); the Boston Nutrition/Obesity Research Center (DK046200 to R.A.F.); and an NHLBI pre-doctoral training Grant (T32HL69772 to RMP). [Any opinions, findings, conclusion, or recommendation expressed in this publication are those of the author(s) and do not necessarily reflect the views of the U.S. Department of Agriculture.]
Footnotes
Conflicts of interest: The authors declare no conflicts of interest.
Contributor Information
Rachele M. Pojednic, Email: rachele.pojednic@joslin.harvard.edu, Nutrition, Exercise Physiology and Sarcopenia Laboratory, Jean Mayer USDA Human Nutrition Research Center on Aging at Tufts University, 711 Washington St., Boston, MA 02111, USA.
Lisa Ceglia, Division of Endocrinology, Diabetes and Metabolism, Tufts Medical Center, Boston, MA, USA; Bone Metabolism Laboratory, Jean Mayer USDA Human Nutrition Research Center on Aging at Tufts University, Boston, MA, USA.
Alice H. Lichtenstein, Cardiovascular Nutrition Laboratory, Jean Mayer USDA Human Nutrition Research Center on Aging at Tufts University, Boston, MA, USA
Bess Dawson-Hughes, Bone Metabolism Laboratory, Jean Mayer USDA Human Nutrition Research Center on Aging at Tufts University, Boston, MA, USA.
Roger A. Fielding, Nutrition, Exercise Physiology and Sarcopenia Laboratory, Jean Mayer USDA Human Nutrition Research Center on Aging at Tufts University, 711 Washington St., Boston, MA 02111, USA
References
- 1.Schaap LA, Pluijm SM, Deeg DJ, Harris TB, Kritchevsky SB, Newman AB, Colbert LH, Pahor M, Rubin SM, Tylavsky FA, Visser M. Higher inflammatory marker levels in older persons: associations with 5-year change in muscle mass and muscle strength. J Gerontol A Biol Sci Med Sci. 2009;64:1183–1189. doi: 10.1093/gerona/glp097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Visser M, Pahor M, Taaffe DR, Goodpaster BH, Simonsick EM, Newman AB, Nevitt M, Harris TB. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: the Health ABC Study. J Gerontol A Biol Sci Med Sci. 2002;57:M326–M332. doi: 10.1093/gerona/57.5.m326. [DOI] [PubMed] [Google Scholar]
- 3.Fernandez-Real JM, Ricart W. Insulin resistance and chronic cardiovascular inflammatory syndrome. Endocr Rev. 2003;24:278–301. doi: 10.1210/er.2002-0010. [DOI] [PubMed] [Google Scholar]
- 4.Bellia A, Garcovich C, D'Adamo M, Lombardo M, Tesauro M, Donadel G, Gentileschi P, Lauro D, Federici M, Lauro R, Sbraccia P. Serum 25-hydroxyvitamin D levels are inversely associated with systemic inflammation in severe obese subjects. Intern Emerg Med. 2013;8:33–40. doi: 10.1007/s11739-011-0559-x. [DOI] [PubMed] [Google Scholar]
- 5.Chagas CE, Borges MC, Martini LA, Rogero MM. Focus on vitamin D, inflammation and type 2 diabetes. Nutrients. 2012;4:52–67. doi: 10.3390/nu4010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Norman PE, Powell JT. Vitamin D and cardiovascular disease. Circ Res. 2014;114:379–393. doi: 10.1161/CIRCRESAHA.113.301241. [DOI] [PubMed] [Google Scholar]
- 7.Bischoff-Ferrari HA, Dawson-Hughes B, Stocklin E, Sidelnikov E, Willett WC, Orav EJ, Stahelin HB, Wolfram S, Jetter A, Schwager J, Henschkowski J, von Eckardstein A, Egli A. Oral supplementation with 25(OH)D(3) versus vitamin D(3): effects on 25(OH)D levels, lower extremity function, blood pressure and markers of innate immunity. J Bone Miner Res. 2011;27(1):160–169. doi: 10.1002/jbmr.551. [DOI] [PubMed] [Google Scholar]
- 8.Wamberg L, Cullberg KB, Rejnmark L, Richelsen B, Pedersen SB. Investigations of the anti-inflammatory effects of vitamin D in adipose tissue: results from an in vitro study and a randomized controlled trial. Horm Metab Res. 2013;45:456, 462. doi: 10.1055/s-0032-1331746. [DOI] [PubMed] [Google Scholar]
- 9.Sokol SI, Srinivas V, Crandall JP, Kim M, Tellides G, Lebastchi AH, Yu Y, Gupta AK, Alderman MH. The effects of vitamin D repletion on endothelial function and inflammation in patients with coronary artery disease. Vasc Med. 2012;17:394–404. doi: 10.1177/1358863X12466709. [DOI] [PubMed] [Google Scholar]
- 10.Barker T, Henriksen VT, Martins TB, Hill HR, Kjeldsberg CR, Schneider ED, Dixon BM, Weaver LK. Higher serum 25-hydroxyvitamin D concentrations associate with a faster recovery of skeletal muscle strength after muscular injury. Nutrients. 2013;5:1253–1275. doi: 10.3390/nu5041253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi M, Park H, Cho S, Lee M. Vitamin D3 supplementation modulates inflammatory responses from the muscle damage induced by high-intensity exercise in SD rats. Cytokine. 2013;63:27–35. doi: 10.1016/j.cyto.2013.03.018. [DOI] [PubMed] [Google Scholar]
- 12.Steensberg A, van Hall G, Osada T, Sacchetti M, Saltin B, Klarlund Pedersen B. Production of interleukin-6 in contracting human skeletal muscles can account for the exercise-induced increase in plasma interleukin-6. J Physiol. 2000;529(Pt 1):237–242. doi: 10.1111/j.1469-7793.2000.00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stratos I, Li Z, Herlyn P, Rotter R, Behrendt AK, Mittlmeier T, Vollmar B. Vitamin D increases cellular turnover and functionally restores the skeletal muscle after crush injury in rats. Am J Pathol. 2013;182:895–904. doi: 10.1016/j.ajpath.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 14.Sun J, Kong J, Duan Y, Szeto FL, Liao A, Madara JL, Li YC. Increased NF-kappaB activity in fibroblasts lacking the vitamin D receptor. American Journal of Physiology Endocrinol Metab. 2006;291:E315–E322. doi: 10.1152/ajpendo.00590.2005. [DOI] [PubMed] [Google Scholar]
- 15.Szeto FL, Sun J, Kong J, Duan Y, Liao A, Madara JL, Li YC. Involvement of the vitamin D receptor in the regulation of NF-kappaB activity in fibroblasts. J Steroid Biochem Mol Biol. 2007;103:563–566. doi: 10.1016/j.jsbmb.2006.12.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tse AK, Zhu GY, Wan CK, Shen XL, Yu ZL, Fong WF. 1alpha,25-Dihydroxyvitamin D3 inhibits transcriptional potential of nuclear factor kappa B in breast cancer cells. Mol Immunol. 2010;47:1728–1738. doi: 10.1016/j.molimm.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 17.Jablonski KL, Chonchol M, Pierce GL, Walker AE, Seals DR. 25-Hydroxyvitamin D deficiency is associated with inflammation-linked vascular endothelial dysfunction in middle-aged and older adults. Hypertension. 2011;57:63–69. doi: 10.1161/HYPERTENSIONAHA.110.160929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, DeLuca HF. Is the vitamin d receptor found in muscle? Endocrinology. 2011;152:354–363. doi: 10.1210/en.2010-1109. [DOI] [PubMed] [Google Scholar]
- 19.Girgis CM, Mokbel N, Cha KM, Houweling PJ, Abboud M, Fraser DR, Mason RS, Clifton-Bligh RJ, Gunton JE. The vitamin D receptor (VDR) is expressed in skeletal muscle of male mice and modulates 25-hydroxyvitamin D (25OHD) uptake in myofibers. Endocrinology. 2014;155:3227–3237. doi: 10.1210/en.2014-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ceglia L, da Silva Morais M, Park LK, Morris E, Harris SS, Bischoff-Ferrari HA, Fielding RA, Dawson-Hughes B. Multistep immunofluorescent analysis of vitamin D receptor loci and myosin heavy chain isoforms in human skeletal muscle. J Mol Histol. 2010;41:137–142. doi: 10.1007/s10735-010-9270-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ceglia L, Niramitmahapanya S, Morais MD, Rivas DA, Harris SS, Bischoff-Ferrari H, Fielding RA, Dawson-Hughes B. A randomized study on the effect of vitamin D3 supplementation on skeletal muscle morphology and vitamin D receptor concentration in older women. J Clin Endocrinol Metab. 2013;98(12):E1927–E1935. doi: 10.1210/jc.2013-2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bischoff HA, Borchers M, Gudat F, Duermueller U, Theiler R, Stahelin HB, Dick W. In situ detection of 1,25-dihydroxyvitamin D3 receptor in human skeletal muscle tissue. Histochem J. 2001;33:19–24. doi: 10.1023/a:1017535728844. [DOI] [PubMed] [Google Scholar]
- 23.Chale A, Cloutier GJ, Hau C, Phillips EM, Dallal GE, Fielding RA. Efficacy of whey protein supplementation on resistance exercise-induced changes in lean mass, muscle strength, and physical function in mobility-limited older adults. J Gerontol A Biol Sci Med Sci. 2013;68:682–690. doi: 10.1093/gerona/gls221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Scherr PA, Wallace RB. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 25.The National Academies Press; 2011. Dietary Reference Intakes for Calcium and Vitamin D. http://www.nap.edu/openbook.php?record_id=13050. [PubMed] [Google Scholar]
- 26.Pedersen BK, Febbraio MA. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev. 2008;88:1379–1406. doi: 10.1152/physrev.90100.2007. [DOI] [PubMed] [Google Scholar]
- 27.Munoz-Canoves P, Scheele C, Pedersen BK, Serrano AL. Interleukin-6 myokine signaling in skeletal muscle: a double-edged sword? FEBS J. 2013;280:4131–4148. doi: 10.1111/febs.12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Febbraio MA, Hiscock N, Sacchetti M, Sacchetti M, Fischer CP, Pedersen BK. Interleukin-6 is a novel factor mediating glucose homeostasis during skeletal muscle contraction. Diabetes. 2004;53(7):1643–1648. doi: 10.2337/diabetes.53.7.1643. [DOI] [PubMed] [Google Scholar]
- 29.Lauritzen HP, Brandauer J, Schjerling P, Koh HJ, Treebak JT, Hirshman MF, Galbo H. Goodyear, Contraction and AICAR stimulate IL-6 vesicle depletion from skeletal muscle fibers in vivo. Diabetes. 2013;62:3081–3092. doi: 10.2337/db12-1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Febbraio MA, Steensberg A, Keller C, Starkie RL, Nielsen HB, Krustrup P, Ott P, Secher NH, Pedersen BK. Glucose ingestion attenuates interleukin-6 release from contracting skeletal muscle in humans. J Physiol. 2003;549:607–612. doi: 10.1113/jphysiol.2003.042374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rinnov A, Yfanti C, Nielsen S, Akerstrom TC, Peijs L, Zankari A, Fischer CP. Pedersen, Endurance training enhances skeletal muscle interleukin-15 in human male subjects. Endocrine. 2014;45:271–278. doi: 10.1007/s12020-013-9969-z. [DOI] [PubMed] [Google Scholar]
- 32.Nielsen AR, Mounier R, Plomgaard P, Mortensen OH, Penkowa M, Speerschneider T, Pilegaard H, Pedersen BK. Expression of interleukin-15 in human skeletal muscle effect of exercise and muscle fibre type composition. J Physiol. 2007;584:305–312. doi: 10.1113/jphysiol.2007.139618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Capiati D, Benassati S, Boland RL. 1,25(OH)2-vitamin D3 induces translocation of the vitamin D receptor (VDR) to the plasma membrane in skeletal muscle cells. J Cell Biochem. 2002;86:128–135. doi: 10.1002/jcb.10191. [DOI] [PubMed] [Google Scholar]
- 34.Buitrago C, Pardo VG, Boland R. Role of VDR in 1alpha,25-dihydroxyvitamin D-dependent non-genomic activation of MAPKs, Src and Akt in skeletal muscle cells. J Steroid Biochem Mol Biol. 2013;136:125–130. doi: 10.1016/j.jsbmb.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 35.Molanouri Shamsi M, Hassan ZH, Gharakhanlou R, Quinn LS, Azadmanesh K, Baghersad L, Isanejad A, Mahdavi M. Expression of interleukin-15 and inflammatory cytokines in skeletal muscles of STZ-induced diabetic rats: effect of resistance exercise training. Endocrine. 2014;46:60–69. doi: 10.1007/s12020-013-0038-4. [DOI] [PubMed] [Google Scholar]


