Abstract
Background
Diffusion tensor imaging (DTI) is a useful technique for non-invasively investigating the microstructural organization of white matter (WM), and the most consistent DTI finding regarding cocaine-related WM alterations is in the corpus callosum (CC). WM injury has also been observed in subjects with traumatic brain injury (TBI), including in the CC.
Methods
We used DTI to test if the WM microstructure is relatively more impaired in cocaine-dependent subjects who had suffered a mild TBI (mTBI). Fractional anisotropy (FA), which reflects the degree of alignment of cellular structures within fiber tracts and their structural integrity, was compared across cocaine-dependent subjects with mTBI (COCTBI group, n=9), matched cocaine-dependent subjects without TBI (COC group, n=12), and matched healthy controls (CTL group, n=12).
Results
The COCTBI group had significantly lower FA in the genu, body, and splenium of CC, than the CTL group whenever the education was controlled or not. The COC group had significantly lower FA in the left and right anterior corona radiata than the CTL group only when the education was controlled. There was no significant difference in FA between the COC and COCTBI groups.
Conclusion
Cocaine dependence (or mTBI) related WM impairments in the CC were not detectable in this small subject sample. The significant finding in the CC suggests that the concurrence of cocaine dependence and mTBI might result in more severe damage to the CC, which could even be detected in small sample size.
Keywords: Diffusion tensor imaging, cocaine dependence, traumatic brain injury, TBSS
1. INTRODUCTION
Cocaine dependence is associated with white matter (WM) impairment (Moeller et al., 2005; Lim et al., 2008; Ma et al., 2009; Lane et al., 2010; Xu et al., 2010; Bell et al., 2011; Hanlon et al., 2013), which may compromise cognitive functions. Diffusion tensor imaging (DTI), which exploits the directionality of diffusion of water molecules in tissues, is a powerful technique for non-invasively investigating the microstructural organization of WM (Taber et al., 2002). Cocaine-induced alterations in WM are often observed in two of the DTI measures, i.e., fractional anisotropy (FA) (Moeller et al., 2005; Lim et al., 2008; Ma et al., 2009; Lane et al., 2010; Bell et al., 2011) and radial diffusivity (Moeller et al., 2007; Ma et al., 2009; Lane et al., 2010). FA measures deviation from isotropy and reflects the degree of alignment of cellular structures within fiber tracts as well as their structural integrity (Cercignani et al., 2001). Higher radial diffusivity is indicative of greater diffusion perpendicular to the fiber tracts and may be possibly associated with impairment in myelin (Song et al., 2002, 2003; Klawiter et al., 2011).
Previous DTI studies have reproducibly shown that cocaine dependence is associated with significant WM deficits. Moeller et al. (2005) reported significantly lower FA in the genu and rostral body of the corpus callosum (CC) in cocaine-dependent subjects compared to controls and an association between FA in the anterior CC and impulsivity within the cocaine-dependent subjects. Lim et al. (2008) found lower FA in the inferior frontal WM in cocaine-dependent subjects compared to control subjects. Ma et al. (2009) observed that cocaine-dependent subjects had significantly higher radial diffusivity in the rostral body and isthmus of the CC than non-drug-using controls. Lane et al. (2010) found lower FA and higher radial diffusivity in the CC, frontal and parietal WM regions in cocaine-dependent subjects compared to non-drug-using controls and an association between the DTI measures in these regions and decision-making. Xu et al. (2010) noted that in cocaine-dependent subjects, worse treatment outcomes were negatively-correlated with FA values and positively-correlated with radial diffusivity across several brain regions including the CC, frontal, parietal, temporal, and occipital lobes, and cerebellum. Bell et al. (2011) found that the cocaine-abstinent subjects had lower FA in genu of the CC, superior longitudinal fasciculus, and the superior corona radiata when compared against non-drug-using controls. So far, the most consistent finding regarding cocaine dependence related WM alterations is in the CC (Moeller et al., 2005, 2007; Ma et al., 2009; Lane et al., 2010; Xu et al., 2010; Bell et al., 2011). These findings have been replicated in controlled, animal studies (Narayana et al., 2009, 2014) using DTI.
There is a close association between substance use disorders (SUD) and traumatic brain injury (TBI). It has been well documented that individuals with SUD are more prone to TBI through motor vehicle accidents, violence, or falls (Taylor et al., 2003). Thus SUD can increase the risk of TBI (Cherpitel, 2007; Taylor et al., 2003). Preliminary results from our group have shown that subjects with cocaine dependence have a higher incidence of TBI compared to non-drug using controls and occurrence of TBI preceded initiation of cocaine use (Ramesh et al., 2015). In addition, previous DTI studies have consistently found that patients with TBI showed impaired WM microstructure than controls, especially in the CC (see Hulkower et al., 2013 for review). However, it is yet to be determined if subjects with co-morbid cocaine dependence and TBI have additional impairments in the WM structure. In this study, we used DTI to test if the WM microstructure is relatively more impaired in cocaine-dependent subjects with mild TBI (mTBI).
2. METHODS
2.1 Subjects
The present study was approved by the local university Committee for the Protection of Human Subjects (CPHS) and was performed in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki). Informed consent was obtained from each subject before including in this study.
Cocaine-dependent subjects and healthy controls were recruited via newspaper advertisements, and were initially screened by a brief telephone interview. Following the phone screen, eligible subjects attended an in-person intake assessment session, in which they were screened for psychiatric disorders using the structured clinical interview for DSM-IV (SCID-IV-TR; First et al., 2002), and completed a medical history and physical examination. Information about each participant's demographic and drug use history was also collected at the intake interview. For all subjects, the Addiction Severity Index (McLellan et al., 1992) was obtained in order to document lifetime drug and alcohol use. Immediately prior to MRI scanning, a sample of urine was obtained from each subject using integrated E–Z split key cup II (Innovacon Company, San Diego, CA, USA) in order to screen for tetrahydrocannabinol, opiates, cocaine, amphetamines, benzodiazepines, and pregnancy (for females only); each subject was also screened for recent alcohol use, using an Intoximeter Alcosensor III breathalyzer (Intoximeters, Inc., St Louis, MO).
Subject inclusion criteria were: (1) 18 to 55 years old; (2) free of alcohol at the time of MRI scanning; (3) cocaine-dependent subjects met criteria for current cocaine dependence as determined by Structured Clinical Interview for DSM-IV (SCID; First et al., 1996); and (4) non-drug using controls had no current or lifetime history of any DSM-IV substance use or psychiatric disorder. Exclusion criteria were: (1) met current or past DSM-IV Axis I disorder other than substance abuse or dependence; (2) taking medication or having disorders that could affect the central nervous system; (3) reported claustrophobia during MRI simulator sessions; (4) having any definite or suspected clinically-significant brain abnormalities on the Fluid-Attenuated Inversion Recovery (FLAIR) MRI scans, as read by a board-certified radiologist; (5) positive urine drug screen (for controls only); and (6) positive pregnancy test result (for females only).
2.2 Closed head injury scale
The closed head injury (CHI) scale is a 13-item self-report measure (See Supplementary Materials for entire questionnaire1) designed by one of the co-authors (FGM). The scale was used to determine if the subjects had suffered any type of mTBI in their lifetime. The CHI scale was developed to assist in patient recall of prior instances of head trauma, including number of head trauma instances, duration of loss of consciousness/confusion, post-traumatic amnesia, age of first injury, hospitalizations related to the injury, etc. In the present study, a subject was considered to have mTBI if he/she answered “yes” to the question “Have you ever been hit in the head so hard, either by another person or in an accident, that it knocked you out or made you confused?”.
Nine cocaine-dependent subjects (1 female, all right handed) reported having had a mTBI (COCTBI group). Please see Table 1 for number of head trauma instances, longest duration of loss of consciousness/confusion, and years since TBI for all of these 9 COCTBI subjects. As shown in Table 1, six of the COCTBI subjects were each hit in the head one time, one of the COCTBI subjects was hit two times, one of the COCTBI subjects was hit four times, and one of the COCTBI subjects was hit six times. The COCTBI subjects were 41.5 ± 5.3 years old (mean ± standard deviation), ranging from 35.9 years to 52.1 years. Based on age and sex, 12 cocaine-dependent subjects without TBI (COC group, 2 females, all right handed), and 12 controls (CTL group, 2 females, 2 left handed and 10 right handed) were selected from our subject pool (137 subjects) to match the COCTBI subjects. This resulted in that 104 subjects were excluded from the study. The COC subjects were 41.4 ± 6.0 years old, ranging from 33.1 years to 54.8 years. The CTL subjects were 41.0 ± 6.0 years old, ranging from 35.5 years to 55.0 years. There was no significant difference in age across groups (COCTBI vs. COC: 2-tail p=0.9662, degree of freedome [Dof]=19, t=0.0429; COCTBI vs. CTL: 2-tail p=0.8544, DoF=19, t=0.1860; COC vs. CTL: 2-tail p=0.8857, DoF=22, t=0.1454). Fisher’s exact tests revealed that there was no significant difference in proportion of female and male subjects across groups (COCTBI vs. COC: 2-tail p>0.999; COCTBI vs. CTL: 2-tail p>0.999; COC vs. CTL: 2-tail p=1). Please see Table 2 for information about demographic (age, sex, race, and education), lifetime alcohol use, and alcohol diagnosis of the subjects in the three groups. Only five of the 33 subjects had intelligence quotient (IQ) measure, and therefore IQ was not reported in the present study.
Table 1.
Times of head injury, the longest period of time that the subject was knocked out or confused, year of TBI, and year of lifetime cocaine use, for all COCTBI subjects.
| Subject # | Number of head trauma instances |
Longest period of time |
Years since TBI | Years of cocaine use |
|---|---|---|---|---|
| 1 | 4 | < 30 min | 0.2 | 11 |
| 2 | 1 | NP | 19 | 15 |
| 3 | 1 | NP | 28 | 10 |
| 4 | 2 | 30 min – 1 day | 33 | 8 |
| 5 | 6 | 30 min – 1day | 26 | 15 |
| 6 | 1 | < 30 min | NP | NP |
| 7 | 1 | < 30 min | 27 | 8 |
| 8 | 1 | 30 min – 1 day | 29 | 25 |
| 9 | 1 | NP | 31 | 25 |
NP=Not provided by the subject.
Table 2.
Demographic, and lifetime alcohol use (kg) of the subjects in the three groups.
| Parameter | COCTBI (n=9) | COC (n=12) | CTL (n=12) |
|---|---|---|---|
| Age (years) | 41.5 ± 5.3 (35.9 – 52.1) | 41.4 ± 6.0 (33.1 – 54.8) | 41.0 ± 6.0 (35.5 – 55.0) |
| Sex | 1 F, 8 M | 2 F, 10 M | 2 F, 10 M |
| Race | 4 AA, 4 C, 1 H | 6 AA, 6 C | 9 AA, 2 C, 1 A |
| Education (years) | 13.7 ± 2.4 (9 – 16) | 12.2 ± 1.3 (11 – 14) | 14.9 ± 2.8 (11 – 18) |
| Lifetime alcohol use (kg) | 135.6 ± 141.7 (0 – 310.6) | 263.5 ± 320.2 (8.1 – 1130.0) | 11.1 ± 15.7 (0 – 40.6) |
| Alcohol diagnosis | 1 PA, 8 N | 4 PD, 2 PA, 1 CA, 5 N | 12 N |
F=female, M=male, AA=American African, C=Caucasian, H=Hispanic, A=Asian, PA=past alcohol abuse, PD=past alcohol dependence, CA=current alcohol abuse, and N=none.
All the subjects were recruited from the Houston, Texas, area. About 50% of our cocaine-dependent subjects were from treatment studies, and all scans were conducted prior to the treatment phase for these subjects. The age range in the present study is consistent with the average age of cocaine-dependent subjects who were seeking treatment in Houston (see Schmitz et al., 2012 for example). The age range in the present study is also consistent with our other research (e.g., Moeller et al., 2010), and previous studies (e.g., Xu et al., 2010) published by other groups.
2.3 MRI Data Acquisition
MRI data were acquired on a Philips 3.0 T Intera system with a six channel receive head coil (Philips Medical Systems, Best, Netherlands). Whole brain diffusion-weighted images (DWI) were acquired in the transverse plane using a single shot diffusion sensitized spin echo echo-planar imaging (EPI) sequence, with the following parameters: b-factor = 1000 s/mm2, repetition time (TR) = 6100 ms, echo time (TE) = 84 ms, 44 contiguous axial slices, field-of-view (FOV) = 240 mm × 240 mm, 112 × 112 acquisition matrix, 256 × 256 reconstructed matrix, 0.9375 mm × 0.9375 mm reconstructed in-plane resolution, slice thickness = 3 mm, and zero interslice gap. The diffusion tensor encoding scheme was based on the uniformly distributed and balanced rotationally invariant Icosa21 (21 gradients) tensor-encoding set (Hasan and Narayana, 2003). A SENSE acceleration factor of 2 was used for the DWI acquistion. The diffusion-encoded volumes were acquired with fat suppression. The DTI acquisition time was approximately 7 min. FLAIR scan and T2-weighted spin-echo scans were acquired and were read by a board-certified radiologist in order to rule-out any incidental brain pathology.
2.4 DTI data preprocessing
The DTI images were processed using the FMRIB Software Library (www.fmrib.ox.ac.uk/fsl, version 5.04) (Jenkinson et al., 2012). For each scan, the DWI images were corrected for eddy current distortions and head motion (Jenkinson and Smith, 2001) after converting the Philips DICOM files into NIfTI format using dcm2nii as implemented in MRIcron (http://www.mccauslandcenter.sc.edu/mricro/mricron/). Next, brain was extracted from the images using FSL's Brain Extraction Tool (BET; Smith, 2002). After these preprocessing steps, the FMRIB’s Diffusion Toolbox (FDT/FSL; Behrens et al., 2003) was used to fit the data to extract the DTI parameters for each voxel. The DTI parameters included fractional anisotropy (FA), mean diffusivity (MD), the three eigenvalues (L1, L2, and L3) and the three eigenvectors (V1, V2, and V3). Among these DTI measures, FA is most commonly used. Axial diffusivity, radial diffusivity, and MD are the other three commonly used DTI measures. The axial diffusivity is the same as the first eigenvalue (L1), and the radial diffusivity is calculated as the mean of the second (L2) and third (L3) eigenvalues. It is commonly accepted that FA is a general measure of WM integrity, and axial and radial diffusivities may be more pathologically specific compared to FA (Hasan and Narayana, 2006).
2.5 Tract-Based Spatial Statistics (TBSS) voxelwise statistcal analysis
Whole brain voxelwise statistical analysis of the DTI data was carried out using Tract-Based Spatial Statistics (TBSS; Smith et al., 2006), part of the FSL software. The FA images were aligned to the standard MNI (Montreal Neurological Institute) space using the FSL's nonlinear registration with the FMRIB58_FA template image. Next, the mean FA image was created and thinned to create a mean FA skeleton representing the centers of all tracts common to all subjects. The FA threshold was set to be FA = 0.20. Each subject’s aligned FA data that was a local maximum within a short-radius plane normal to each point on the skeleton was then projected onto that point of the skeleton. TBSS was also applied to the other DTI measures (MD, axial diffusivity, and radial diffusivity).
The group differences in FA (or other DTI measures) were tested voxelwise over the whole-brain WM skeleton using the FSL nonparametric program, Randomise, with a Threshold-Free Cluster Enhancement (TFCE) option and 10000 random permutations. TFCE (Smith and Nichols, 2009; Salimi-Khorshidi et al., 2011) is a robust cluster-based thresholding approach, without a need of arbitrary initial cluster-forming threshold. There were three groups in the Randomise analysis: COCTBI, COC, and CTL. The group comparisons include: COCTBI greater than (>) CTL, COCTBI less than (<) CTL, COC > CTL, COC > CTL, COCTBI < COC, and COCTBI < COC. The TBSS randomise procedure automatically uses family-wise error (FWE) correction for multiple comparisons. In the present study, statistical significance of TBSS Randomise analysis was defined as 1-tail P < 0.025 (FWE corrected). The JHU DTI-based ICBM-DTI-81 WM labeled atlas (Mori et al., 2005; Wakana et al., 2007; Hua et al., 2008) was used to determine labels for brain regions that showed group differences.
Group difference in DTI measures might be affected by other factors, e.g., group differences in education and lifetime alcohol use (see Results section). Thus the education level and the lifetime alcohol use may need to be controlled by including them as covariates in the DTI analysis. Miller and Chapman (2011) suggested that a meaningful covariate should be the one in which the group difference in the value of the covariate is due to random chance. In the present study, the group difference in education was probably due to random chance since there was no inclusion or exclusion criteria regarding level of education for either group. Thus, following Miller and Chapman (2001), education has been included as a covariate in the DTI analysis. On the other hand, according to the inclusion and exclusion criteria for this study, all the “controls” with past or current alcohol dependence or abuse were excluded; but the COCTBI and COC groups could include subjects with past alcohol dependence, or past or current alcohol abuse. Thus the COCTBI group and COC group could inherently have higher measures of lifetime alcohol use than the CTL group, and hence the group difference in lifetime alcohol use was not simply due to random chance. As shown in Table 1, five (about 42%) of the twelve COC subjects, eight (about 89%) of the nine COCTBI subjects, and all (100%) of the CTL subjects were non-drinkers. Therefore, when comparing the COC group to the CTL group and the COCTBI group, lifetime alcohol use was not included in the DTI analysis because in these group comparisons, group difference in the lifetime alcohol use was probably not due to random chance and thus it did not qualify as a meaningful covariate according to Miller and Chapman (2001). However, using above criterion suggested by Miller and Chapman (2001), it was not straightforward if lifetime alcohol use was a meaningful covariate when comparing the COCTBI group and the CTL group. Pocock et al. (2002, Page 2924) suggested that it is important to control a covariate only when this covariate shows strong correlation (for example r > 0.5) with the variate to be tested, and that it is unimportant to control a covariate when this covariate shows weak correlation (for example r < 0.3) with the variate to be tested. Based on the suggestion of Pocock et al. (2002), we conducted two post hoc analyses (see Supplementary Materials) in order to test the correlation between FA (variate to be tested) and lifetime alcohol use (covariate). Because there was significant difference in the lifetime alcohol use between the COCTBI group and the CTL group (see Results section), the these correlation analyses were separately conducted in the COCTBI group and the CTL group. These post hoc correlation analyses revealed that the lifetime alcohol use was weakly (r < 0.2 for all correlations) correlated to FA in both the COCTBI group and the CTL group (see Supplementary Materials2), suggesting that it was unimportant to control the liftime alcohol use when comparing the COCTBI group and the CTL group. Therefore lifetime alcohol use was not included in the DTI analysis in the present study.
3. RESULTS
3.1 Non-imaging measures
The education level (years) of the COC group was significantly shorter than that of the CTL group (p=0.008, t=2.94, DoF=22). There was no significant difference in education between the COCTBI group and CTL group (p=0.28, t=1.10, DoF=19), and between the COCTBI group and the COC group (p=0.11, t=1.66, DoF=19). The COC subjects (p=0.020, t=2.52, DoF=22) and the COCTBI subjects (p=0.030, t=2.34, DoF=19) consumed significantly more alcohol (kg) than CTL subjects. However, there was no significant difference in lifetime alcohol use between the COCTBI group and COC group (p=0.23, t=1.23, DoF=19). Eight of the nine COCTBI subjects and all of the CTL subjects were non-drinkers. Only two male subjects (COC subjects) consumed more than 500 kg alcohol lifetime; and only one male subject (COC subject) consumed more than 1000 kg alcohol lifetime (approximate mean value for the male alcoholics in Pfefferbaum et al., [2009]). Seven of the nine COCTBI subjects started using cocaine after the acquisition of mTBI, and one before the onset of mTBI. The years of lifetime cocaine use, and the years since the onset of TBI, are shown in Table 1 for all the COCTBI subjects.
3.2 TBSS DTI analysis
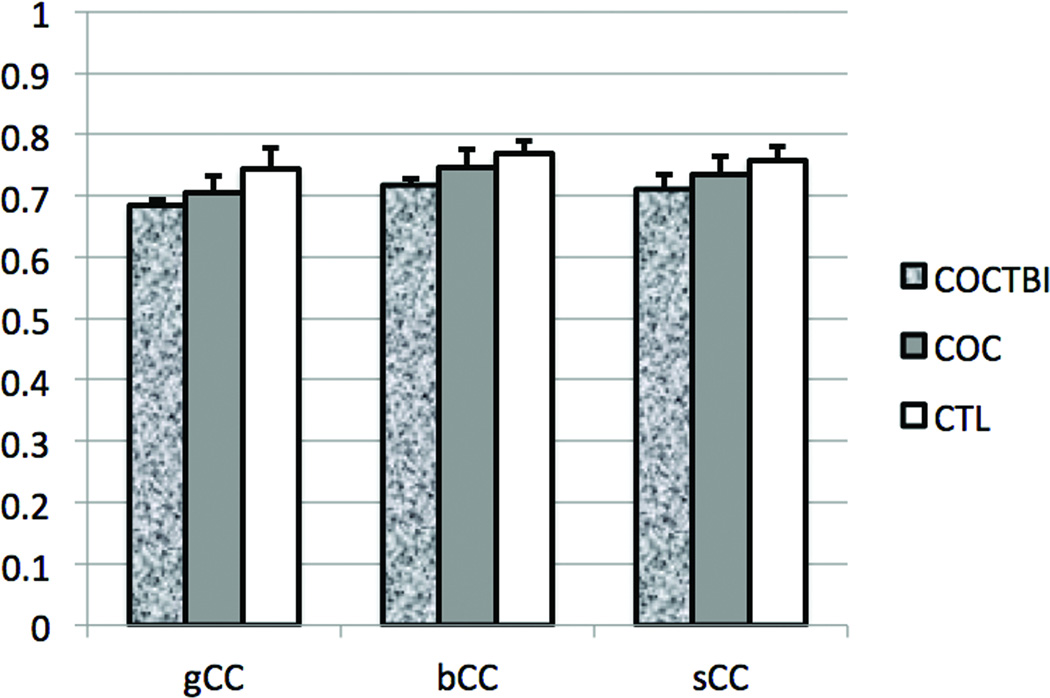
The TBSS Randomise analysis revealed that the COCTBI group had significantly (1-tail FEW corrected P<0.025) lower FA than the CTL group in several WM brain regions (Figure 1, red color). These WM regions were in genu (85 voxels), body (117 voxels), and splenium (320 voxels) of the CC (gCC, bCC, and sCC respectively). After controlling the education, the COCTBI group still showed significantly (1-tail FWE corrected P<0.025) lower FA than the CTL group in several WM brain regions (Figure 1, blue color). These WM regions were in gCC (297 voxels), bCC (223 voxels), and sCC (468 voxels), left anterior corona radiata (538 voxels), left superior corona radiata (141 voxels), and left external capsule (32 voxels). The WM regions found in the previous two TBSS analysis (controlling the education or not) had overlap in the gCC (85 voxels), bCC (79 voxels), and sCC (312 voxels). This result suggests that the COCTBI group had significantly lower FA in the gCC, bCC, and the sCC whenever the education was controlled or not. The mean and standard deviation (across subjects) of mean (across voxels) FA on these three CC regions (overlapped between TBSS results controlling the education and those without controlling the education) are shown in Figure 2 for the three subject groups. For all the three CC regions, mean FA of the COC group was between that of the other two groups: less than the CTL group, but greater than the COCTBI group, although these differences were not statistically significant.
Figure 1.
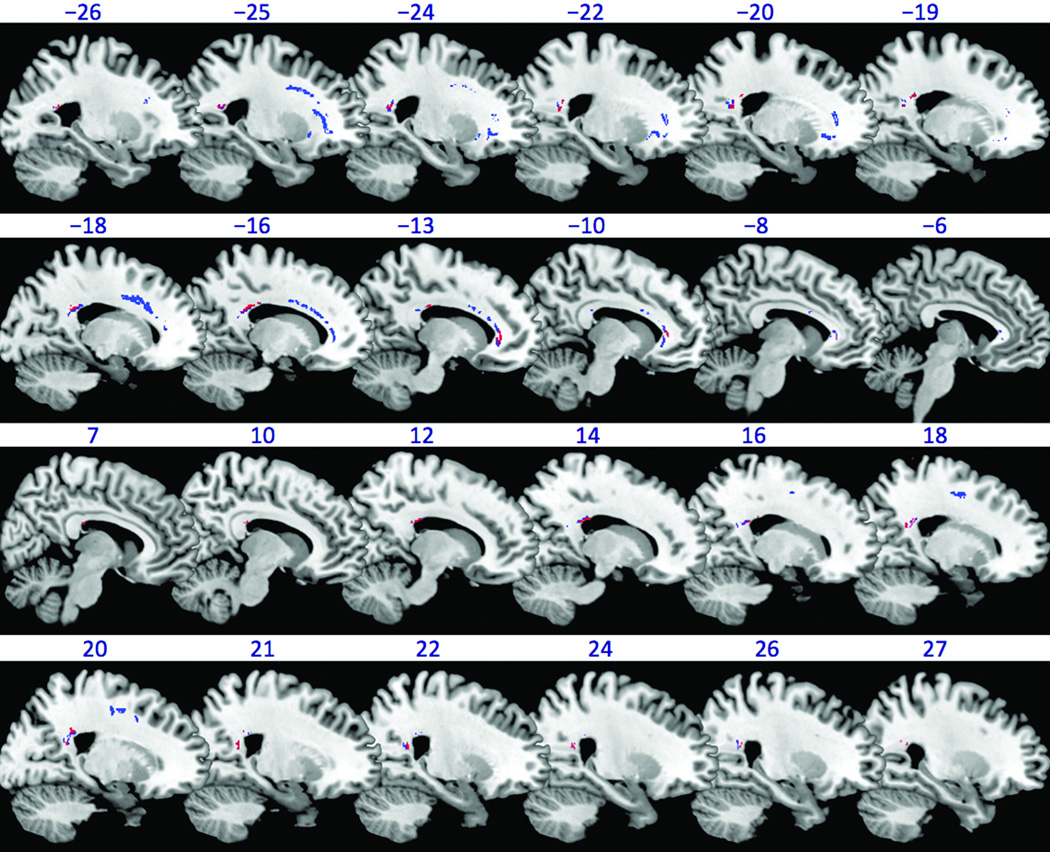
White matter brain regions showing significant (1-tail, FWE corrected P<0.025) group difference in FA as revealed by the TBSS randomise analysis. The white matter regions in which the COCTBI group had significantly lower (1-tail, FWE corrected P<0.025) FA than the CTL group is shown in red color (without controlling the education) or blue color (controlling the education). The background is an MNI brain template image which is available in the MRIcron software (http://www.mccauslandcenter.sc.edu/mricro/mricron/). The number above each slice indicates slice location (mm) of the MNI y coordinate. Negative (positive) y indicates left (right) hand side of brain.
Figure 2.
The mean and standard deviation (across subjects) of mean (across voxels) FA on the three CC regions (overlapped between TBSS results controlling the education and those without controlling the education) showing significant group difference (COCTBI < CTL), for the three subject groups. Error bar represents standard deviation.
The TBSS Randomise analysis also revealed that the COC group had a trend (1-tail TFCE corrected P<0.075) of lower FA than the CTL group in several WM regions. After the education was controlled, the COC group had signifcantly (1-tail FWE corrected P<0.025) lower FA than the CTL groups in left (180 voxels) and right (115 voxels) anterior corona radiata. No significant group difference was found for the other group comparisons on FA (COCTBI > CTL, COC > CTL, COCTBI < COC, COCTBI > COC) whenever the education was controlled or not.
The TBSS Randomise analysis further revealed that the COCTBI group showed a trend (1-tail TFCE corrected P<0.075) of higher radial diffusivity than the CTL group in several WM regions whenever the education was controlled or not. No significant (or trend) cluster was found for the other group comparisons on radial diffusivity (COCTBI < CTL, COC > CTL, COC < CTL, COCTBI < COC, COCTBI > COC) whenever the education was controlled or not.
No significant (or trend) group difference was found for the other DTI measures (MD, or axial diffusivity) whenever the education was controlled or not.
4. DISCUSSION
This study investigated the potential role of mTBI in the altered WM microstructure in cocaine-dependent subjects, which has been consistently reported in the literature (Moeller et al., 2005; Lim et al., 2008; Ma et al., 2009; Lane et al., 2010; Xu et al., 2010; Bell et al., 2011; Li et al., 2013; Lebel et al., 2013). Using TBSS, we examined whether the WM microstructure was altered in cocaine-dependent subjects with mTBI by comparing their DTI measures with matched non-drug using controls and matched cocaine-dependent subjects without TBI, on a voxel-by-voxel basis in the whole brain. Results of the TBSS analysis showed that the COCTBI group had significantly lower FA in several sub-regions of the CC than the CTL group whenever the education was controlled or not. The COC group had significantly lower FA in left and right anterior corona radiata than controls only when the education was controlled. The regions (left and right anterior corona radiata) showing significant difference between the COC and CTL groups are consistent with the results found by Lane et al. (2010) in which a relatively bigger subject sample (15 COC subjects and 18 controls) was used. No significant difference in the FA was found between the COC group and COCTBI group whenever the education was controlled or not.
The COCTBI group had lower FA than the CTL group in genu, body, and splenium of the CC whenever the education was controlled or not. Although it is known that cocaine dependence can result in altered WM microstructure especially in the CC (Moeller et al., 2005; 2007; Ma et al., 2009; Lane et al., 2010; Xu et al., 2010; Bell et al., 2011), we did not find a significant difference in the CC between the COC group and the CTL group, suggesting that the cocaine dependence related WM impairments in CC were not detectable in this small sample size. It is also known that mTBI can result in altered WM microstructure in the CC (Wilde et al., 2006; Voelbel et al., 2012; Hulkower et al., 2013; Hasan et al., 2014; Narayana et al., 2015), but we did not find significant a group difference when we compared the COCTBI group and the COC group, suggesting that the mTBI related WM impairments were not detectable in this small sample size. Given the fact that both cocaine dependence and TBI can result in impaired WM microstructure in the CC, we speculate that the significant group difference found between the COCTBI group and the CTL group may reflect a combined effect of cocaine dependence and TBI that overcomes the limited sample size. Both the COCTBI subjects and COC subjects consumed significantly more alcohol (kg) than the CTL subjects. Thus, alcohol use might also have contributed to the significant difference found between the COCTBI and CTL groups. However, only one of the COCTBI subjects was diagnosed as past alcohol abuse and all the other eight COCTBI subjects were non-drinkers, suggesting that the alcohol use may not have been a major factor.
The relationship between the onset of mTBI and the onset of cocaine use could not be established in the present study, and thus the present study is not able to answer the question of whether people who have suffered a previous TBI are more vulnerable to addiction to drugs (Bjork and Grant, 2009). In the present study, seven of the nine COCTBI subjects initiated cocaine use after sustaining mTBI. This result is consistent with a previous study from our group (Ramesh et al., 2015), in which the mean age of mTBI was found to be significantly lower than the mean age of initiating cocaine use in 28 cocaine-dependent subjects with mTBI. A potential explanation of this result might be that a great deal of mTBI occurs in childhood whereas cocaine use onset is usually later. Another potential explanation might be that alcohol use preceeded, and might be implicated in, both the TBI and the cocaine use. However, the alcohol use in the COCTBI subjects might not have a major effect, as discussed above.
The screening process for research studies carried out by our research group includes two levels of screening. There is an initial phone screen to determine whether subjects meet general inclusion and exclusion criteria. Subjects that appear to meet these criteria are invited for an in-person interview, which includes physical examination, structured clinical interview for DSM disorders (SCID), and blood work. Exact numbers of subjects excluded at each step of the process are not available. However, approximately half of phone-screened subjects are scheduled for an interview, and approximately half of the subjects who are interviewed ultimately undergo scanning. Thus, the results of the present study are not characteristic of all cocaine-dependent subjects. In fact, the imaging findings are likely to be more pronounced in cocaine-dependent subjects in general, since subjects with a history of severe head trauma or neurologic findings on physical exam were excluded from this study prior to scanning.
Small sample size was a significant limitation of this study and may account for the lack of differences seen between the COCTBI and COC groups. Lifetime alcohol use has not been included in the DTI analysis as covariate because lifetime alcohol use could not qualify as a meaningful covariate according to Miller and Chapman (2001) and Pocock et al. (2002). This is also a major limitation of the present study. Another limitation is the lack of control subjects with TBI. Cocaine dependence (or mTBI) related WM impairments in the CC were not detectable in this small subject sample. The finding that the COCTBI group had significantly lower FA in the CC than the CTL group suggests that the concurrence of cocaine dependence and mTBI might result in more severe damage to the CC, which could even be detected in small sample size. These findings were from a preliminary study with small sample size, and one should be cautious when generalizing these findings.
Supplementary Material
Highlights.
We tested white matter impairment in cocaine-dependent subjects (CDs).
We conducted diffusion tensor imaging Tract-Based Spatial Statistics (TBSS) analysis.
CDs with mild traumatic brain injury (mTBI), CDs without mTBI, and controls were compared.
CDs with mTBI had lower fractional anisotropy (FA) on corpus callosum (CC) than controls.
The concurrence of cocaine dependence and mTBI might result in more severe damage to the CC.
Acknowledgements
This work is financially supported by National Institute on Drug Abuse (NIDA) Grants # R01 DA034131 (LM), U54 DA038999 (FGM/JLS), P50 DA009262 (FGM/JLS), and MCRR Shared Instrumentation Grant # 1 S10 RR019186-01 (PAN). We thank Zahra N. Kamdar, Vipulkumar S. (Vips) Patel, and Edward A. Zuniga for their excellent technical support. We also thank the anonymous reviewers for their constructive comments which have resulted in significant improvement of this article.
Role of Funding Source
Nothing declared
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Contributors
All authors reviewed and edited the manuscript. Liangsuo Ma analyzed all data, and wrote up the manuscript. Divya Ramesh and Lori Keyser-Marcus provided close head injury measures for all subjects. Liangsuo Ma, Joel L. Steinberg, Ponnada A. Narayana, and F. Gerard Moeller designed the experiment. F. Gerard Moeller designed the 13-item self-report measure of close head injury.
Conflict of Interest
No conflict declared
REFERENCES
- American Psychiatric Association. Diagnostic And Statistical Manual Of Mental Disorders, Fourth Edition, Text Revision. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- Bell RP, Foxe JJ, Nierenberg J, Hoptman MJ, Garavan H. Assessing white matter integrity as a function of abstinence duration in former cocaine-dependent individuals. Drug Alcohol Depend. 2011;114:159–168. doi: 10.1016/j.drugalcdep.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TE, Johansen-Berg H, Woolrich MW, Smith SM, Wheeler-Kingshott CA, Boulby PA, Barker GJ, Sillery EL, Sheehan K, Ciccarelli O, Thompson AJ, Brady JM, Matthewset PM. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat. Neurosci. 2003;6:750–757. doi: 10.1038/nn1075. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Grant SJ. Does traumatic brain injury increase risk for substance abuse? J. Neurotrauma. 2009;26:1077–1082. doi: 10.1089/neu.2008.0849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cercignani M, Inglese M, Pagani E, Comi G, Filippi M. Mean diffusivity and fractional anisotropy histograms of patients with multiple sclerosis. Am. J. Neuroradiol. 2001;22:952–958. [PMC free article] [PubMed] [Google Scholar]
- Cherpitel CJ. Alcohol and injuries: a review of international emergency room studies since 1995. Drug Alcohol Rev. 2007;26:201–214. doi: 10.1080/09595230601146686. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Biometrics Research Department. New York: New York State Psychiatric Institute; 1996. Structured Clinical Interview for DSM-IV Axis I Disorders--Patient Edition (SCID-I/P, Version 2.0) [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW. Biometrics Research. New York: New York State Psychiatric Institute; 2002. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. [Google Scholar]
- Hanlon CA, Beveridge TJ, Porrino LJ. Recovering from cocaine: insights from clinical and preclinical investigations. Neurosci. Biobehav. Rev. 2013;37:2037–2046. doi: 10.1016/j.neubiorev.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan KM, Narayana PA. Computation of the fractional anisotropy and mean diffusivity maps without tensor decoding and diagonalization: theoretical analysis and validation. Magn. Reson. Med. 2003;50:589–598. doi: 10.1002/mrm.10552. [DOI] [PubMed] [Google Scholar]
- Hasan KM, Narayana PA. Retrospective measurement of the diffusion tensor eigenvalues from diffusion anisotropy and mean diffusivity in DTI. Magn. Reson. Med. 2006;56:130–137. doi: 10.1002/mrm.20935. [DOI] [PubMed] [Google Scholar]
- Hasan KM, Wilde EA, Miller ER, Patel VK, Staewen TD, Frisby ML, Garza HM, McCarthy JJ, Hunter JV, Levin HS, Robertson CS, Narayana PA. Serial atlas-based diffusion tensor imaging study of uncomplicated mild traumatic brain injury in adults. J. Neurotrauma. 2014;31:466–475. doi: 10.1089/neu.2013.3085. [DOI] [PubMed] [Google Scholar]
- Hua K, Zhang J, Wakana S, Jiang H, Li X, Reich DS, Calabresi PA, Pekar JJ, van Zijl PC, Mori S. Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. Neuroimage. 2008;39:336–347. doi: 10.1016/j.neuroimage.2007.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulkower MB, Poliak DB, Rosenbaum SB, Zimmerman ME, Lipton ML. A decade of DTI in traumatic brain injury: 10 years and 100 articles later. Am J Neuroradiol. 2013;34:2064–2074. doi: 10.3174/ajnr.A3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med. Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. Fsl. Neuroimage. 2012;62:782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Klawiter EC, Schmidt RE, Trinkaus K, Liang HF, Budde MD, Naismith RT, Song SK, Cross AH, Benzinger TL. Radial diffusivity predicts demyelination in ex vivo multiple sclerosis spinal cords. Neuroimage. 2011;55:1454–1460. doi: 10.1016/j.neuroimage.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Santhanam P, Coles CD, Ellen Lynch M, Hamann S, Peltier S, Hu X. Prenatal cocaine exposure alters functional activation in the ventral prefrontal cortex and its structural connectivity with the amygdala. Psychiatry Res. 2013;213:47–55. doi: 10.1016/j.pscychresns.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim KO, Wozniak JR, Mueller BA, Franc DT, Specker SM, Rodriguez CP, Silverman AB, Rotrosen JP. Brain macrostructural and microstructural abnormalities in cocaine dependence. Drug Alcohol Depend. 2008;92:164–172. doi: 10.1016/j.drugalcdep.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane SD, Steinberg JL, Ma L, Hasan KM, Kramer LA, Zuniga EA, Narayana PA, Moeller FG. Diffusion tensor imaging and decision making in cocaine dependence. PLoS One. 2010;5:e11591. doi: 10.1371/journal.pone.0011591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Warner T, Colby J, Soderberg L, Roussotte F, Behnke M, Davis Eyler F, Sowell ER. White matter microstructure abnormalities and executive function in adolescents with prenatal cocaine exposure. Psychiatry Res. 2013;213:161–168. doi: 10.1016/j.pscychresns.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Hasan KM, Steinberg JL, Narayana PA, Lane SD, Zuniga EA, Kramer LA, Moeller FG. Diffusion tensor imaging in cocaine dependence: regional effects of cocaine on corpus callosum and effect of cocaine administration route. Drug Alcohol Depend. 2009;104:262–267. doi: 10.1016/j.drugalcdep.2009.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M. The Fifth Edition of the Addiction Severity Index. J. Subst. Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- Miller GA, Chapman JP. Misunderstanding analysis of covariance. J. Abnorm. Psychol. 2001;110:40–48. doi: 10.1037//0021-843x.110.1.40. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Hasan KM, Steinberg JL, Kramer LA, Dougherty DM, Santos RM, Valdes I, Swann AC, Barratt ES, Narayana PA. Reduced anterior corpus callosum white matter integrity is related to increased impulsivity and reduced discriminability in cocaine-dependent subjects: diffusion tensor imaging. Neuropsychopharmacology. 2005;30:610–617. doi: 10.1038/sj.npp.1300617. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Hasan KM, Steinberg JL, Kramer LA, Valdes I, Lai LY, Swann AC, Narayana PA. Diffusion tensor imaging eigenvalues: preliminary evidence for altered myelin in cocaine dependence. Psychiatry Res. 2007;154:253–258. doi: 10.1016/j.pscychresns.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Steinberg JL, Schmitz JL, Ma L, Liu S, Kjome KL, Rathnayaka N, Kramer LA, Narayana PA. Working memory fMRI activation in cocaine dependent subjects: association with treatment response. Psychiatry Res. Neuroimag. 2010;181:174–182. doi: 10.1016/j.pscychresns.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Wakana S, Van Zijl PCM. MRI Atlas Of Human White Matter. Elsevier Science; 2005. [Google Scholar]
- Narayana PA, Ahobila-Vajjula P, Ramu J, Herrera J, Steinberg JL, Moeller FG. Diffusion tensor imaging of cocaine-treated rodents. Psychiatry Res. 2009;71:242–251. doi: 10.1016/j.pscychresns.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayana PA, Herrera JJ, Bockhorst KH, Esparza-Coss E, Xia Y, Steinberg JL, Moeller FG. Chronic cocaine administration causes extensive white matter damage in brain: diffusion tensor imaging and immunohistochemistry studies. Psychiatry Res. 2014;221:220–230. doi: 10.1016/j.pscychresns.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayana PA, Yu X, Hasan KM, Wilde EA, Levin HS, Hunter JV, Miller ER, Patel VK, Robertson CS, McCarthy JJ. Multi-modal MRI of mild traumatic brain injury. Neuroimage Clin. 2015;7:87–97. doi: 10.1016/j.nicl.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom M, Rohlfing T, Sullivan EV. Degradation of association and projection white matter systems in alcoholism detected with quantitative fiber tracking. Biol. Psychiatry. 2009;65:680–690. doi: 10.1016/j.biopsych.2008.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocock SJ, Assmann SE, Enos LE, Kasten LE. Subgroup analysis, covariate adjustment and baseline comparisons in clinical trial reporting: current practice and problems. Stat. Med. 2002;21:2917–2930. doi: 10.1002/sim.1296. [DOI] [PubMed] [Google Scholar]
- Ramesh D, Keyser-Marcus LA, Ma L, Schmitz JM, Lane SD, Marwitz JH, Kreutzer JS, Moeller FG. Prevalence of traumatic brain injury in cocaine-dependent research volunteers. Am. J. Addict. 2015 Feb 6; doi: 10.1111/ajad.12192. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salimi-Khorshidi G, Smith SM, Nichols TE. Adjusting the effect of nonstationarity in cluster-based and TFCE inference. Neuroimage. 2011;54:2006–2019. doi: 10.1016/j.neuroimage.2010.09.088. [DOI] [PubMed] [Google Scholar]
- Schmitz JM, Rathnayaka N, Green CE, Moeller FG, Dougherty AE, Grabowski J. Combination of modafinil and d-amphetamine for the treatment of cocaine dependence: a preliminary investigation. Front. Psychiatry. 2012;3:77. doi: 10.3389/fpsyt.2012.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum. Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17:1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage. 2003;20:1714–1722. doi: 10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Taber KH, Pierpaoli C, Rose SE, Rugg-Gunn FJ, Chalk JB, Jones DK, Hurley RA. The future for diffusion tensor imaging in neuropsychiatry. J. Neuropsychiatry Clin. Neurosci. 2002;14:1–5. doi: 10.1176/jnp.14.1.1. [DOI] [PubMed] [Google Scholar]
- Taylor LA, Kreutzer JS, Demm SR, Meade MA. Traumatic brain injury and substance abuse: a review and analysis of the literature. Neuropsychol. Rehabil. 2003;13:165–188. doi: 10.1080/09602010244000336. [DOI] [PubMed] [Google Scholar]
- Voelbel GT, Genova HM, Chiaravalotti ND, Hoptman MJ. Diffusion tensor imaging of traumatic brain injury review: implications for neurorehabilitation. NeuroRehabilitation. 2012;31:281–293. doi: 10.3233/NRE-2012-0796. [DOI] [PubMed] [Google Scholar]
- Wakana S, Caprihan A, Panzenboeck MM, Fallon JH, Perry M, Gollub RL, Hua K, Zhang J, Jiang H, Dubey P, Blitz A, van Zijl P, Mori S. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage. 2007;36:630–644. doi: 10.1016/j.neuroimage.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde EA, Chu Z, Bigler ED, Hunter JV, Fearing MA, Hanten G, Newsome MR, Scheibel RS, Li X, Levin HS. Diffusion tensor imaging in the corpus callosum in children after moderate to severe traumatic brain injury. J. Neurotrauma. 2006;23:1412–1426. doi: 10.1089/neu.2006.23.1412. [DOI] [PubMed] [Google Scholar]
- Xu J, DeVito EE, Worhunsky PD, Carroll KM, Rounsaville BJ, Potenza MN. White matter integrity is associated with treatment outcome measures in cocaine dependence. Neuropsychopharmacology. 2010;35:1541–1549. doi: 10.1038/npp.2010.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.