Abstract
Environmental organoarsenicals are produced by microorganisms and are introduced anthropogenically as herbicides and antimicrobial growth promoters for poultry and swine. Nearly every prokaryote has an ars (arsenic resistance) operon, and some have an arsH gene encoding an atypical flavodoxin. The role of ArsH in arsenic resistance has been unclear. Here we demonstrate that ArsH is an organoarsenical oxidase that detoxifies trivalent methylated and aromatic arsenicals by oxidation to pentavalent species. Escherichia coli, which does not have an arsH gene, is very sensitive to the trivalent forms of the herbicide monosodium methylarsenate (MSMA or MAs(V)) and antimicrobial growth promoter roxarsone (Rox(V)), as well as to phenylarsenite (PhAs(III), also called phenylarsine oxide or PAO). Pseudomonas putida has two chromosomally-encoded arsH genes and is highly resistant to the trivalent forms of these organoarsenicals. A derivative of P. putida with both arsH genes deleted is sensitive to MAs(III), PhAs(III) or Rox(III). P. putida arsH expressed in E. coli conferred resistance to each trivalent organoarsenical. Cells expressing PpArsH oxidized the trivalent organoarsenicals. PpArsH was purified, and the enzyme in vitro similarly oxidized the trivalent organoarsenicals. These results suggest that ArsH catalyzes a novel biotransformation that confers resistance to environmental methylated and aromatic arsenicals.
Introduction
The metalloid arsenic is the most prevalent environmental toxin (Zhu et al., 2014). This Group 1 human carcinogen ranks first on the Agency for Toxic Substances and Disease Registry (ATSDR) Priority List of Hazardous Substances (http://www.atsdr.cdc.gov/SPL/index.html). The bioavailability and toxicological properties of arsenic are highly dependant on its chemical form and oxidation state, which are dictated by microbial arsenic biotransformations. Trivalent arsenicals are more toxic than the pentavalent species. There is no single target of trivalent arsenicals, which react with thiol groups in proteins to inhibit numerous biochemical pathways and with small molecule thiols such as reduced glutathione, which results in formation of reactive oxygen species (Jomova et al., 2011). It seems likely that life evolved in an anoxic environment rich in trivalent inorganic As(III). Consequently, early organisms must have developed arsenic resistance pathways, and nearly every extant organism has one or more arsenic detoxification pathways (Zhu et al., 2014). In bacteria these are organized in arsenic resistance (ars) operons. At a minimum, cells have an arsR gene for an As(III)-responsive transcriptional repressor that controls expression of the operon, a gene, either arsB or acr3, that encodes an As(III) efflux permease, and arsC gene for reduction of inorganic arsenate to As(III) (Yang et al., 2012). As described below, there are additional ars genes for arsenical biotransformations, all of which are transcriptionally controlled by arsR.
The global arsenic biogeocycle is created by the intersection of redox and methylation cycles. The redox state of inorganic arsenic cycles between the relatively innocuous pentavalent arsenate and the considerably more toxic and carcinogenic trivalent arsenite (Stolz et al., 2006). Other microbes have cycles of methylation and demethylation. Cells with an arsM gene encoding an As(III) S-adenosylmethionine methyltransferase methylate As(III) to mono-, di- and trimethylated species (Qin et al., 2009, Qin et al., 2006). Trivalent MAs(III) and DMAs(III) are more toxic and carcinogenic than inorganic As(III) and may account for the majority of arsenic-related disease (Engstrom et al., 2011, Thomas & Rosen, 2013). Other microbes have an arsI gene encoding an C-As lyase and transform organoarsenicals back to As(III) (Yoshinaga & Rosen, 2014).
A third cycle of arsenic biotransformations has only recently been recognized: the organoarsenical redox cycle. Pentavalent herbicides and antimicrobial growth promoters are in themselves not especially toxic but are transformed into their active forms by microbial reduction (Chen et al., 2014). Organoarsenical reduction takes place environmentally by microbial communities (Yoshinaga et al., 2011), but the pathways of reduction have not been characterized at the molecular level. ArsH was first identified as a gene of unknown function in the ars operon of Yersinia species (Neyt et al., 1997). The presence of an arsH gene was reported to provide a slight increase in resistance to both inorganic arsenate and arsenite in Yersiniae (Neyt et al., 1997) and Serratia marcescens (Ryan & Colleran, 2002). In contrast, neither mutation nor overexpression of arsH in Thiobacillus ferrooxidans (Butcher et al., 2000) and the cyanobacterium Synechocystis sp. strain PCC 6803 (Lopez-Maury et al., 2003) affected resistance to inorganic arsenic. We similarly observed that deletion of arsH from Sinorhizobium meliloti had a modest effect on resistance to both arsenate and arsenite (Yang et al., 2005). There are currently nearly 9000 ArsH-related sequences in the NCBI database. Most are found in bacterial, primarily gammaproteobacteria, but there are closely related sequences in members of every kingdom including fungi such as Aspergillus fumigatus (Accession # XP_753154; 58% identity, 73% positives), archaea such as Halogranum salarium (Accession # WP_009367405; 28% identity, 49% positives), plants such as the eukaryotic green alga Coccomyxa subellipsoidea C-169 (Accession # EIE24473; 68% identity, 79% positives) and animals such as the Yangtze River freshwater dolphin Lipotes vexillifer (Accession # XP_007470985; 80% identity, 90% positives) and domestic cow Bos taurus DAA33786 71% identity, 80% positives). ArsH does not appear to be widely distributed in animals, so the significance of the few deposited mammalian sequences is unclear.
The ars operon of S. meliloti has four genes: arsR, aqpS (encoding an As(III)-conducting aquaglyceroporin), arsC and arsH (Yang et al., 2005). ArsH is an NADPH-dependent FMN reductase that reduces azo dyes and O2 to H2O2 (Ye et al., 2007), and Synechocystis ArsH reduces metals such as chromate and ferric iron (Xue et al., 2014). The kinetics of Synechocystis ArsH as a quinone reductase has been studied in detail, but no arsenic-related activity was observed (Hervas et al., 2012). ArsH purifies as a tetramer, and the crystal structure of S. meliloti ArsH shows that ArsH is a dimer of dimers (Ye et al., 2007). However, no arsenic-related activity was observed with the purified protein. That fact, coupled with the very modest observed increase in resistance to both oxidized and reduced forms of inorganic arsenic led us to suspect that ArsH has a different physiological function other than resistance to inorganic arsenic. Here we report that ArsH confers resistance to the activated trivalent form of the organoarsenical herbicide MSMA and the aromatic arsenical PhAs(III) and not to inorganic species. Pseudomonas putida has two chromosomally-encoded arsRBCH operons and is highly resistant to the trivalent forms of these organoarsenicals (Canovas et al., 2003), while a derivative with both arsH genes deleted is sensitive to MAs(III), PhAs(III) or Rox(III). The amino acid sequences of both PpArsH1 and PpArsH2 are similar to the ArsHs from other organisms (Fig. S1). The gene for PpArsH from the P. putida ars1 operon (accession number AAN67544.1) was cloned and expressed in the arsenic hypersensitive E. coli strain AW3110 (Δars) (Carlin et al., 1995), where it confers high-level resistance to trivalent MAs(III), PhAs(III) and Rox(III). Purified PpArsH oxidizes these trivalent organical arsenicals to pentavalent species in the order PhAs(III)>Rox(III)>MAs(III). This is the first identification of an organoarsenical oxidase and provides a role for ArsH in the arsenic biogeocycle.
Results
ArsH confers resistance to trivalent organoarsenicals in P. putida and S. meliloti
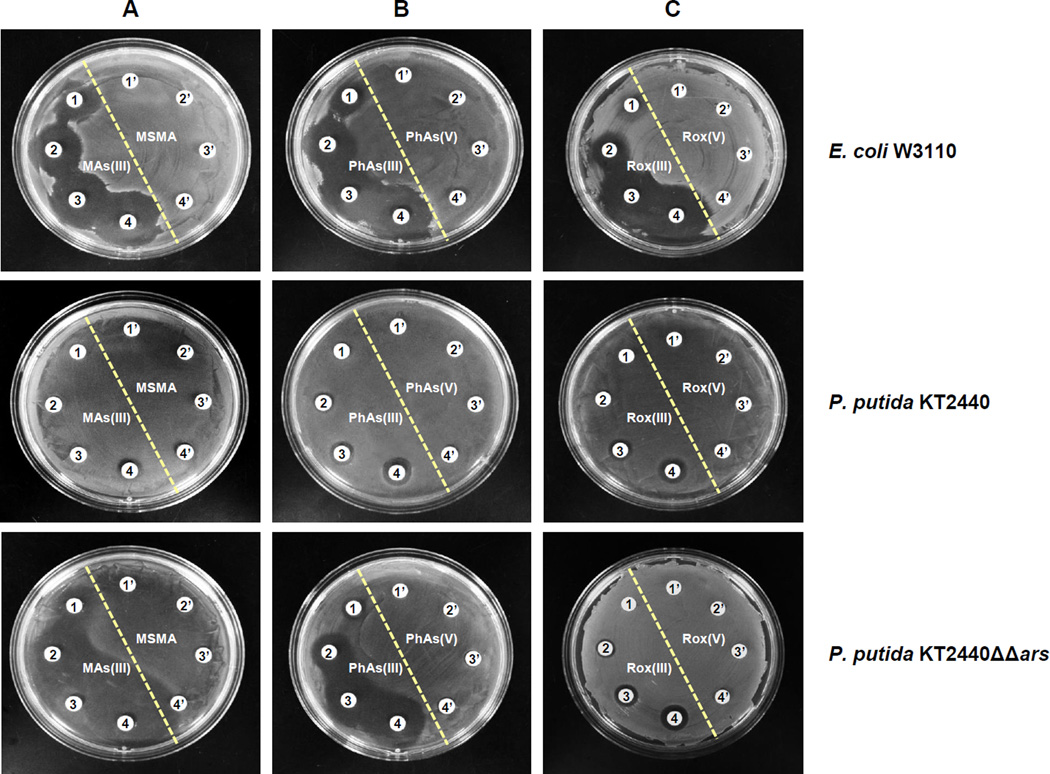
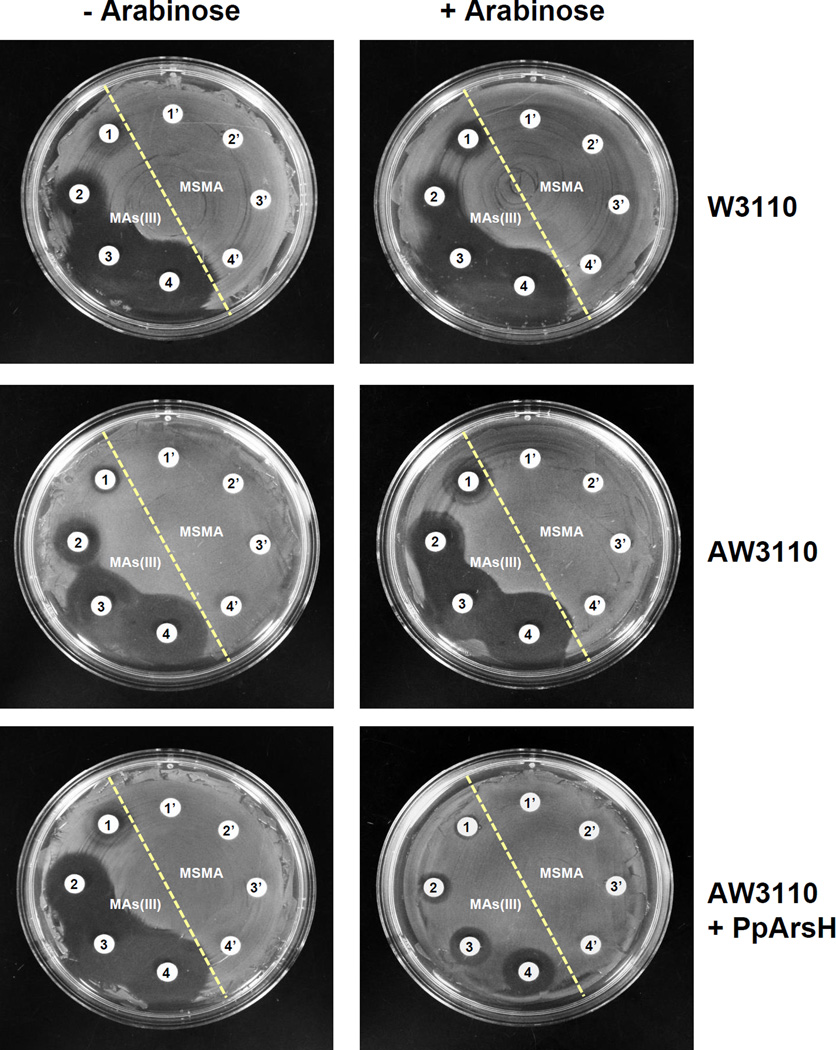
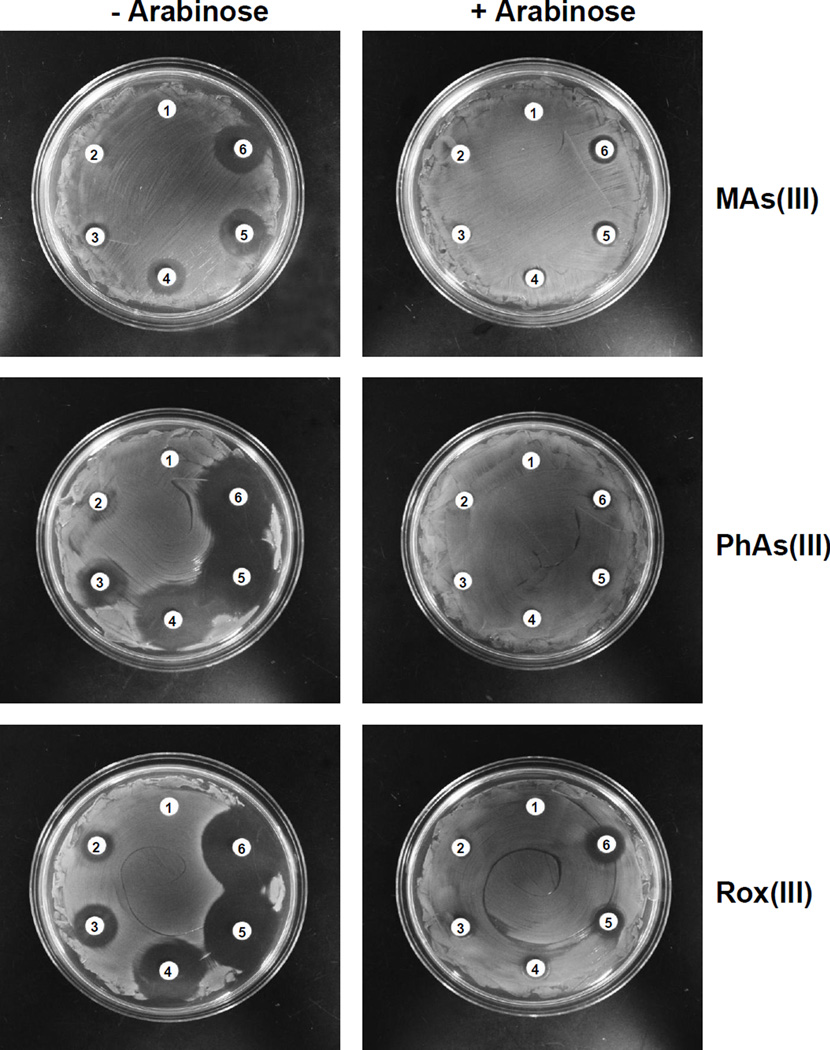
The effect of organoarsenicals on E. coli W3110, P. putida KT2440 and S. meliloti Rm1021 was examined. All three were resistant to pentavalent MAs(V), PhAs(V) and Rox(V) (Fig. 1 shows the results for E. coli and P. putida). E. coli exhibited sensitivity to as little as 30 µM MAs(III), 2 µM PhAs(III) and 5 µM Rox(III) (Fig. 1, top), while P. putida was approximately 10-fold more resistant to those organoarsenicals (Fig. 1, middle). P. putida has two ars operons, ars1 and ars2, both of which have arsRBCH genes (Paez-Espino et al., 2014). ars1 has three other genes of unknown function. Deletion of both operons (P. putida KT2440 (ΔΔars)) resulted in sensitivity to MAs(III) and PhAs(III), but not to Rox(III), on both solid (Fig. 1, bottom) and liquid medium (Fig. S2). Similar results were obtained with S. meliloti, which is relatively resistant to organoarsenicals (Fig. S3, top). The ars operon of S. meliloti has arsR, arsC, arsH and aqpS (Galibert et al., 2001, Yang et al., 2005), and its deletion (strain Rm310) rendered S. meliloti sensitive to the three trivalent organoarsenicals (Fig. S3, middle). Cells of S. meliloti SmK958, with deletion of only arsH, had the same phenotype (Fig. S3, bottom), suggesting that ArsH confers resistance to organoarsenicals. The P. putida arsH gene from ars1 was cloned and expressed in E. coli strain AW3110 (Δars) (Carlin et al., 1995) under control of the araC gene. E. coli W3110 (Fig. 2, top) and AW3110 (Fig. 2, middle) are equally sensitive to MAs(III), showing that the E. coli arsRBC operon does not confer resistance to MAs(III). In the absence of the inducer arabinose, E. coli AW3110 expressing PparsH remained sensitive to MAs(III) but became resistant in the presence of inducer on solid (Fig. 2, bottom and Fig. 3, top) and liquid (Fig. S4, top) medium. Expression of PparsH in E. coli AW3110 similarly conferred resistance to PhAs(III) (Fig. 3, middle, Fig. S4, bottom) and Rox(III) (Fig. 3, bottom and Fig. S4 bottom). Since the ars operon of E. coli AW3110 is deleted, arsH expression alone is sufficient to confer resistance to the active trivalent forms of the herbicide MSMA and the poultry antimicrobial growth promoter roxarsone.
Fig. 1. Resistance to MAs(III), PhAs(III) or Rox(III) of E. coli (top), P. putida wild type (middle) and P. putida ΔΔars (bottom).
The effects of pentavalent and trivalent organoarsenicals on cells grown on M9 agar plates overnight were compared. A: 1’) no addition; 2’) 40 µM MSMA; 3’) 50 µM MSMA; 4’) 60 µM MSMA; 1) 30 µM MAs(III); 2) 40 µM MAs(III); 3) 50 µM MAs(III); 4) 60 µM MAs(III). B: 1’) no addition; 2’) 5 µM PhAs(V); 3’) 10 µM PhAs(V); 4’) 15 µM PhAs(V); 1) 2 µM PhAs(III); 2) 5 µM PhAs(III); 3) 10 µM PhAs(III); 4) 15 µM PhAs(III). C: 1’) no addition; 2’) 10 µM Rox(V); 3’) 15 µM Rox(V); 4’) 20 µM Rox(V); 1) 5 µM Rox(III); 1) 10 µM Rox(III); 3) 15 µM Rox(III); 4) 20 µM Rox(III).
Fig. 2. PpArsH confers resistance to MAs(III) in E. coli.
The effects of MAs(III) on the growth of E. coli W3110 (wild type) (top), AW3110 Δars (middle) or AW3110 expressing the PparsH gene (bottom) were grown on M9 agar plates overnight with the indicated concentrations of methyl arsenicals at 37 °C. PparsH was under control of the ara promoter in vector plasmid pBAD, which was uninduced or induced by 0.2% arabinose. 1’) no addition; 2’) 40 µM MSMA; 3’) 50 µM MSMA; 4’) 60 µM MSMA; 1) 30 µM MAs(III); 2) 40 µM MAs(III); 3) 50 µM MAs(III); 4) 60 µM MAs(III).
Fig. 3. Heterologous expression of PparsH confers resistance to trivalent organoarsenicals.
Cells of E. coli AW3110 expressing the PparsH gene were grown on M9 agar plates with the indicated concentrations of organoarsenicals at 37 °C. The PparsH gene was either uninduced (left) or induced with 0.2% arabinose (right). Top: MAs(III) concentration 1) none, 2) 5 µM, 3) 15 µM, 4) 25 µM, 5) 35 µM, 6) 45 µM. Middle: PhAs(III) 1) none, 2) 2 µM; 3) 5 µM, 4) 10 µM, 5) 15 µM, 6) 20 µM. Bottom: Rox(III) concentration 1) none, 2) 5 µM, 3) 10 µM, 4) 15 µM, 5) 20 µM, 6) 25 µM.
PpArsH oxidizes MAs(III), PhAs(III) and Rox(III)
To further investigate the role of PpArsH in trivalent organic arsenic detoxification, the 241-residue P. putida ArsH from the ars1 operon (AAN67544.1) was purified as a C-terminally six-histidine tagged construct with a mass of 28.5 KDa. PpArsH is 81% identical to the 233-residue ArsH encoded by the ars2 operon, and is closely related to putative ArsH orthologs from other organisms (Fig. S1).
Purified PpArsH is yellow in appearance and exhibits a typical flavoprotein absorption spectrum, with absorption maxima at 373 and 455 nm (Fig. S5). However, purified enzyme contained only 0.17 moles of FMN/mole protein and exhibited significant NADPH:FMN oxidoreductase activity only with exogenously added FMN (Vmax of 83 µmol of NADPH oxidized/min/mg protein) (Fig. S6). Neither NADH nor FAD could replace NADPH or FMN. Purified S. meliloti ArsH is also yellow, but the occupancy is low, and no FMN density is observed in the crystal structure (Ye et al., 2007). Moreover, the structure exhibits no obvious binding site for arsenicals.
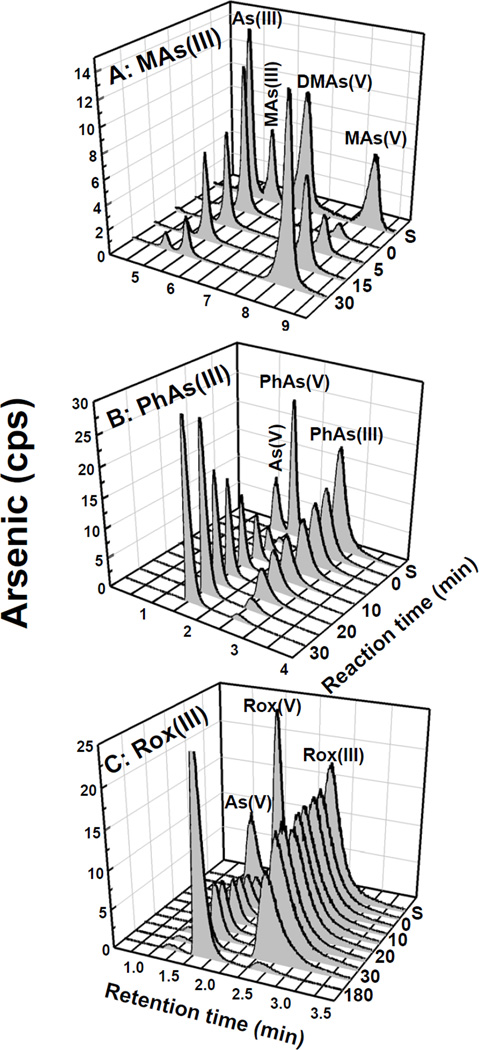
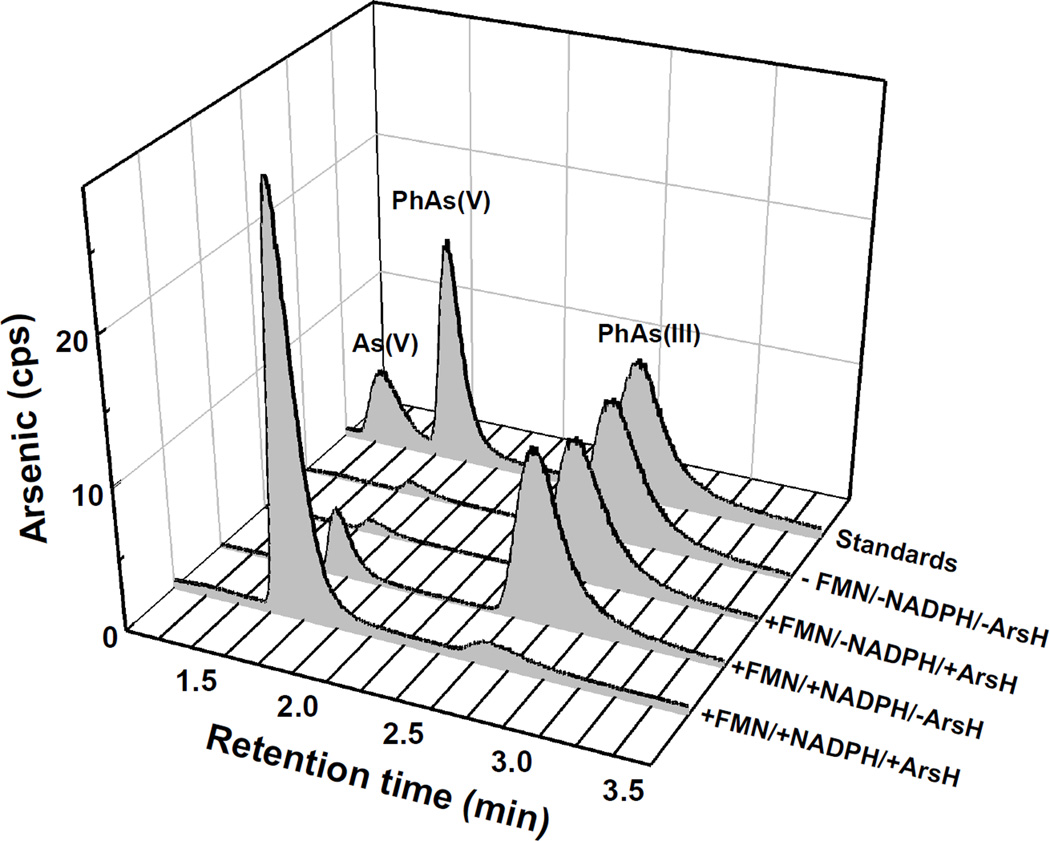
The Km for NADPH was approximately 74 µM, and the concentration of FMN required for half-maximal activation was 7.8 µM (Fig. S7). No activity was detected with NADH or FAD (Fig. S6). PpArsH exhibited NADPH-dependent FMN reductase activity, but reductase activity was not stimulated by inorganic arsenate or arsenite. PpArsH oxidized MAs(III), PhAs(III) and Rox(III) to the pentavalent arsenical species (Fig. 4), but no oxidation of As(III) was observed (Fig. S8). PpArsH oxidized MAs(III) (Fig. 4, top) and PhAs(III) (Fig. 4, middle) in 30–40 min. The apparent affinity for MAs(III) was approximately 5 µM (Fig. S7). Oxidation of Rox(III) was slower, requiring nearly 3 hrs for complete oxidation (Fig. 4, bottom). The oxidation reaction required both NADPH and FMN (Fig. 5). The reaction was faster with MAs(GS)2 compared with the unconjugated species, which is not unexpected since most trivalent arsenicals would be expected be in complex with reduced glutathione (GSH) in the cytosol of cells (Fig. S9). These in vitro results are consistent with the results of the in vivo resistance assays and demonstrate that ArsH functions as a trivalent organoarsenical oxidase.
Fig. 4. Time course of oxidation of trivalent organoarsenicals by PpArsH.
Oxidation of organoarsenicals by purified PpArsH was assayed as described in Material and Methods. A: 4 µM MAs(III) and 0.5 µM PpArsH; B: 4 µM PhAs(III) and 0.1 µM ArsH; C: 4 µM Rox(III) and 0.1 µM ArsH. Samples were separated by HPLC using either a C18 (MAs(III)) or C4 (PhAs(III) and Rox(III)) reverse phase column, and the amount of arsenic in relative counts per second (cps) was estimated by ICP-MS.
Fig. 5. NADPH and FMN are required for ArsH catalysis.
PhAs(III) oxidation by PpArsH was assayed with or without NADPH and/or FMN, as described in Materials and Methods. The reaction mixture contained 0.1 µM ArsH, 4 µM PhAs(III), 0.2 mM NADPH and 25 µM FMN. Samples were separated by HPLC using a C4 reverse phase column, and the amount of arsenic in relative counts per second (cps) was estimated by ICP-MS.
The FMN binding site
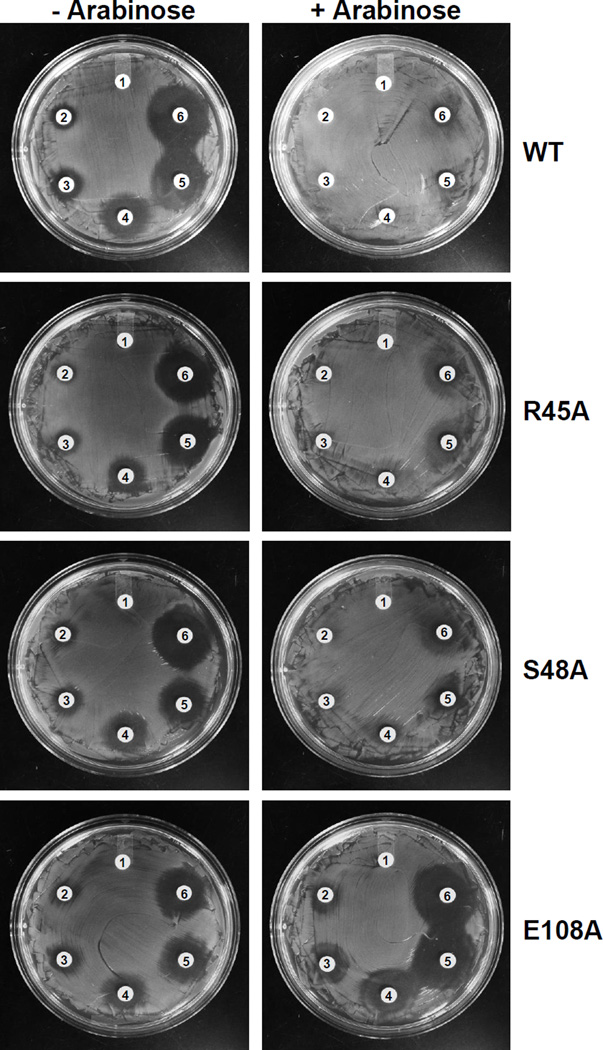
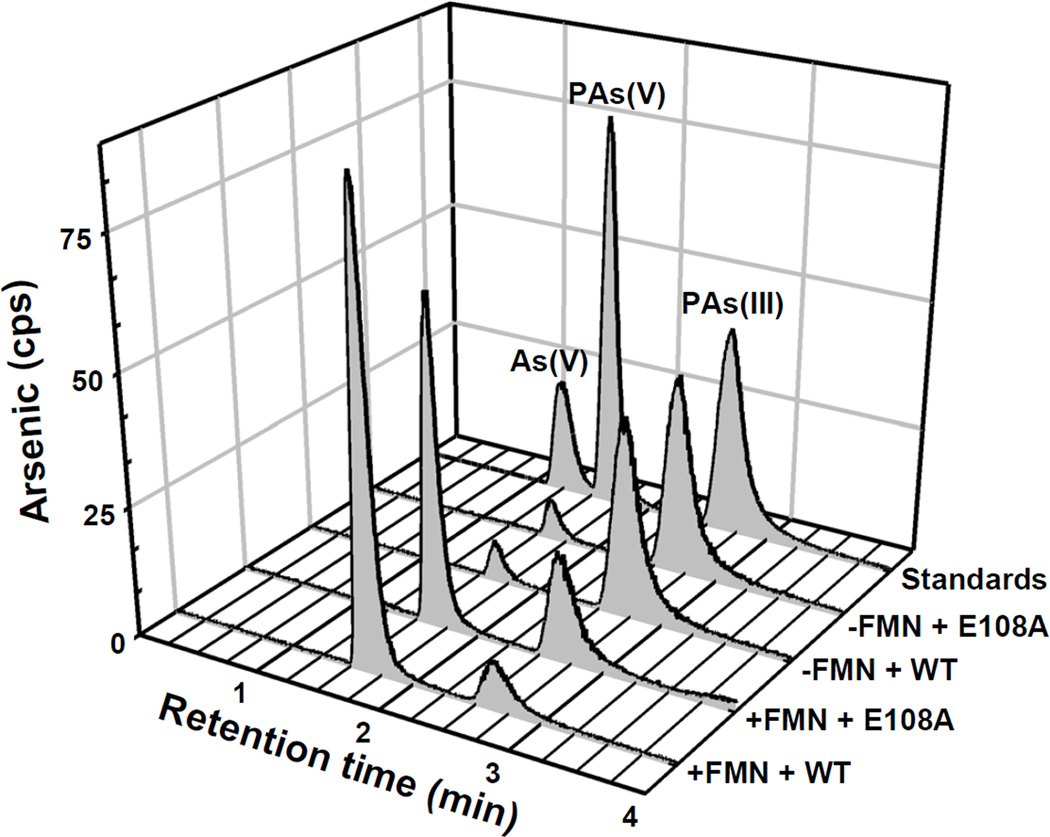
ArsH orthologs exhibit a high degree of sequence similarity (Fig. S1). The FMN binding site in S. meliloti ArsH is predicted to contain the sequence G15SLRTVSYS (Ye et al., 2007). In PpArsH, the corresponding sequence is G42STRERSFS. Alanine substitution of conserved amino acid residues Arg45 and Ser48 reduced resistance to PhAs(III) (Fig. 6). Conserved residue Glu108 is also predicted to contribute to binding of the FMN cofactor. An E108A mutant did not confer resistance to trivalent organoarsenicals (Fig. 6). In contrast to the yellow wild type PpArsH, purified E108A was colorless and did not have the typical FMN absorption maxima at 373 and 455 nm (Fig. S5). Addition of excess FMN restored 80% of wild type activity to the E108A mutant (Fig. 7) indicating that Glu108 contributes to FMN binding.
Fig. 6. PhAs(III) resistance of PpArsH mutants.
Cells of E. coli AW3110 expressing the wild type PparsH (top) or mutants R45A (2nd row), S48A (3rd row) or E108A (bottom) were grown on M9 agar plates with the indicated concentrations PhAs(III) overnight at 37 °C. Cells were uninduced (left) or induced with by 0.2% arabinose (right). PhAs(III) concentration: 1) none, 2) 2 µM, 3) 4 µM, 4) 6 µM, 5) 8 µM, 6) 10 µM.
Fig. 7. FMN restores PhAs(III) oxidation in an E108A mutant.
0.1 µM E108A ArsH was incubated with 4 µM PhAs(III), 0.2 mM NADPH and 75 µM FMN, and the reaction was monitored as described in Materials and Methods. Samples were separated by HPLC using a C4 reverse phase column, and the amount of arsenic in relative counts per second (cps) was estimated by ICP-MS.
The relationship of H2O2 formation to MAs(III) oxidation
The ability to oxidize rather than reduce its substrate makes ArsH unique. NADPH:FMN oxidoreductase homologs of ArsH are promiscuous in their enzymatic activity, but their physiological roles are not well defined. For example, closely related FerB from Paracoccus denitrificans reduces ferric iron and chromate, but no binding site for a putative substrate is observed in its structure, and its physiological function has not been identified (Sedlacek et al., 2014). Perhaps the reason is because ArsH homologs actually oxidize their true substrates, not reduce them. For example, YhdA from Bacillus subtilis oxidizes α,β-unsaturated carbonyl compounds, but this is believed to be the result of indirect and nonenzymatic oxidation by the strong oxidant H2O2 produced by YhdA (Mueller et al., 2009).
We considered the possibility that MAs(V) might be produced indirectly as the result of oxidation by H2O2. To test this possibility, catalase was added to the reaction to catalyze decomposition of H2O2 to H2O and O2. Catalase eliminated production of H2O2 but not MAs(III) oxidation (Fig. S10), and we conclude that ArsH directly oxidizes MAs(III). Importantly, addition of MAs(III), PhAs(III) or Rox(III) reduced H2O2 formation (Fig. S11), suggesting competition for electrons. These results are consistent with H2O2 formation being a side reaction and not directly responsible for the majority of MAs(III) oxidation.
Discussion
The arsH gene is found in many arsenic resistance (ars) operons, implying a role in arsenic detoxification. Yet cells expressing an arsH gene exhibit only low-level resistance to either arsenate or arsenite (Xue et al., 2014, Ye et al., 2007). How, then, does ArsH participate in arsenic resistance? One possibility is that ArsH is a nonspecific reductase that protects cells from oxidative stress (Hervas et al., 2012). If so, why is the arsH gene nearly always in ars operons? In this study we began with the assumption that arsH would not be in an ars operon unless it conferred resistance to some form of arsenic. If ArsH does not catalyze a redox reaction with inorganic As(V) or As(III), then a logical conjecture is that the substrate could be an organic form of arsenic. For example, ArsH might confer resistance to the herbicide MAs(V) by reducing it to MAs(III). Yet we found that MAs(V) is not very toxic to bacteria, while MAs(III) is quite toxic, suggesting that the active form of the herbicide is the trivalent species (Chen et al., 2014). However, species with an arsH gene in their ars operon such as Pseudomonas putida KT2440 and Sinorhizobium meliloti are resistant to MAs(III), and deletion of the arsH gene in these microbes rendered them sensitive. Heterologous expression of an arsH gene in E. coli conferred resistance to MAs(III). In addition, arsH conferred resistance to the reduced forms of aromatic arsenicals such as phenylarsenite and reduced roxarsone, the poultry antimicrobial growth promoter. Interestingly, cells of P. putida KT2440 with deletion of both ars operons remained resistant to Rox(III). One possibility is that P. putida lacks an uptake system for Rox(III). Like all ars genes, arsH is ancient, evolving long before man and the anthropogenic use of herbicides, quite likely arose in response to the production of methylarsenicals by arsM-containing bacteria. Just as human use of antibiotics has caused the spread of antibiotic resistance genes, the recent use of methylarsenicals in agriculture may have triggered the spread of organoarsenical resistance genes.
ArsH is a member of the NADPH-dependent FMN oxidoreductase superfamily (Huijbers et al., 2014). It has previously been characterized as a reductase with various substrates, including reduction of azo dyes and O2 to H2O2 (Ye et al., 2007), reduction of Cr(V) to Cr(III) and Fe(III) to Fe(II) (Xue et al., 2014), and one- and two-electron reduction of quinones but not reduction of inorganic As(V) (Hervas et al., 2012). Since the arsH gene confers resistance to the trivalent forms of the organoarsenicals, it was more reasonable to consider that the purified enzyme would oxidize them to the relatively less toxic pentavalent species.
No oxidation was observed when NADP+ was supplied as an oxidant. In contrast, ArsH oxidized MAs(III), PhAs(III) and Rox(III) when the reductant NADPH was supplied. Mixed-function oxidases use the two atoms of oxygen in O2 differently, coupling FMN reduction with substrate oxidation (Huijbers et al., 2014). We hypothesize that, rather than being a strict reductase, ArsH catalyzes mixed oxidation-reduction, with reduction of O2 to H2O coupled to oxidation of trivalent organoarsenicals. We propose the following reaction scheme for ArsH-catalyzed oxidation of trivalent organoarsenicals, where R can be a methyl group (MAs(III)), phenyl group (PhAs(III)) or 3-nitro-4-hydroxyphenyl group (Rox(III)):
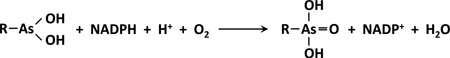 |
In this scheme, the reductive half-reaction consists of formation of an oxidized FMN-NADPH complex followed by a hydride transfer from NADPH to FMN, resulting in FMNH2 and release of NADP+. In the oxidative half-reaction part of the catalytic cycle, an organoarsenical molecule is positioned in proximity of the FMNH2. The reduced flavin is oxidized by O2, producing H2O, followed by oxidation of trivalent arsenical to its pentavalent form. In other words, two electrons from the flavin are transferred to one oxygen atom of dioxygen, reducing it to water, and two electrons from MAs(OH)2 (MAs(III)) are transferred to the other oxygen atom, forming MAsO(OH)2 (MAs(V)). ArsH may follow this pathway only when a trivalent organoarsenical substrate is present. In the absence of an organoarsenical substrate, ArsH nonspecifically reduces metals, quinones and azo dyes and can also produce H2O2. We consider these reactions to be in vitro artifacts that take place only in the absence of physiological substrate. These observations can be explained from the redox potential of the reactions. The mid-point redox potential of the FMN/FMNH2 couple is −0.27 V. In contrast, the mid-point redox potential of Fe3+/Fe2+ is +0.77 V, ubiquinone (ox/red) is 0.10 V, and O2/H2O is +0.82 V. In the absence of a physiological substrate, the reductive half-reaction of the ArsH catalytic cycle reduces FMN to FMNH2 (E’0 = −0.27 V), while NADPH is oxidized to NADP+ (E’0 = +0.32 V). Electrons then flow from FMNH2 to nonspecifically reduce the oxidized forms of dyes, metals or quinines, or generate H2O2.
Even though ArsH has the ability to reduce azo dyes and chromate, the fact that the ArsH gene is found only in ars operons points to an arsenic-related physiological function, and the only high-level activity observed in vivo is MAs(III) resistance. The combination of in vivo and in vitro results clearly demonstrate that the physiological function of ArsH is to confer resistance to trivalent organorsenicals by oxidizing them to pentavalent species. Various environmental bacterial species in microbial communities reduce MSMA (Yoshinaga et al., 2011), which most likely then become the active herbicide or, at the least, is toxic to other species in the communities (Chen et al., 2014). Bacterial species that carry an arsH gene would consequently be resistant to the toxic organoarsencials produced by their neighbors. The arsH lineage predates human invention of herbicides, so the gene must have arisen in response to other environmental pressures. Many members of microbial communities methylate inorganic arsenic to mono- and dimethylated species, which accumulate in the environment as the oxidized species (Ye et al., 2012). We hypothesize that microbial arsenic methylation was the driving force for ArsH evolution.
Materials and Methods
Chemicals
The herbicide MSMA Target 6.6® (sodium methanearsonate) was a generous gift from Luxembourg-Pamol (Houston, TX). Roxarsone (3-nitro-4-hydroxyphenylarsonic acid) was purchased from Acros Organics (Fair Lawn, NJ). Unless otherwise indicated, all other chemicals were obtained from Sigma-Aldrich (St. Louis, MO). Pentavalent arsenicals were reduced as described (Reay & Asher, 1977).
Strain, plasmids, medium, and reagents
Strains and plasmids used in this study are described in Table S1. E. coli cultures were grown aerobically in Luria-Bertani (LB) or M9 medium at 37 °C supplemented with the appropriate antibiotic (Sambrook et al., 1989). P. putida cells were grown aerobically at 30 °C in LB medium, without any antibiotics. For resistance assays, 0.1 ml of fresh cultures of E. coli strains (1 × 108 cells) were spread over the surface of M9 minimal agar plates supplemented with 0.5% glycerol (v/v) ± 0.2% arabinose (w/v) as inducer. Similarly, fresh cultures of P. putida strains (1 × 108 cells) were spread onto M9 minimal agar plates supplemented with 0.2% glucose (w/v). After the liquid dried, sterile filter paper disks were applied on to the plates. 10 µl of each organoarsenical was dispensed on the disks, and the plates were incubated overnight at either 30 °C or 37 °C. The growth rate of cultures of P. putida or E. coli was monitored from the A600nm. Cultures were grown in LB medium to A600nm of 0.6−0.8, harvested by centrifugation, suspended in M9 minimal medium and inoculated into fresh M9 medium to a final A600nm of 0.004−0.006. The growth of the cultures was monitored over 24 h with organoarsenicals at the indicated concentrations.
Cloning of P. putida ArsH
The full-length P. putida KT2440 arsH (PpArsH; Genbank accession NP_744859) was cloned into the pBAD/myc-HisA vector (Life Technologies, Grand Island, NY) so that expression of arsH is controlled by the arabinose promoter. The arsH gene was amplified by PCR from a P. putida genomic DNA library (a gift from Víctor de Lorenzo, Madrid, Spain) to introduce an NcoI site at the 5’-end and an XhoI site at the 3’-end. The forward and reverse primers were 5'-CATGCCATGGAGACAGAAATGAACGAC-3' and 5'-CCGCTCGAGAATAGACCGAATATTGACTC-3', respectively. The amplified product was gel purified, digested with NcoI and XhoI, and inserted into the NcoI-SalI sites of pBAD/myc-HisA vector, in frame with the C-terminal six-histidine-residue tag.
Mutagenesis of the arsH gene
Mutations in P. putida arsH were introduced by site-directed mutagenesis using QuikChange II Site-Directed Mutagenesis Kit from Agilent Technologies (Santa Clara, CA). The mutagenic oligonucleotides used for both strands and the respective changes introduced (underlined) are as follows: R45A, 5'-CTGTACGGATCGACCGCCGAACGCTCATTCAG-3' and 5'-CTGAATGAGCGTTCGGCGGTCGATCCGTACAG-3'; S48A, 5'-GACCCGCGAACGCGCATTCAGCCGCTT-3' and 5'-AAGCGGCTGAATGCGCGTTCGCGGGTC-3'; E108A, 5'-GTGCTCCCCCGCGCGTCACGGCT-3' and 5'-AGCCGTGACGCGCGGGGGAGCAC-3'. Each mutation was confirmed by DNA sequencing using Sequetech DNA Sequencing Services (Mountain View, CA).
ArsH purification
E. coli TOP10 cells (Life Technologies) bearing PpArsH in vector plasmid pBAD/myc-HisA were grown in Luria-Bertani medium containing 100 µg/ml ampicillin with shaking at 37 °C. At an A600 nm of 0.6, L-arabinose was added as an inducer to a final concentration of 0.2% (w/v), and the culture was grown for an additional 4 h at 37 °C. The cells were harvested and suspended in 5 ml per gram of wet cells in buffer A (50 mM 4-morpholinepropanesulfonic acid, 20 mM imidazole, 0.5 M NaCl, 10 mM 2-mercaptoethanol and 20% glycerol (vol/vol), pH 7.5). The cells were broken by a single passage through a French pressure cell at 20,000 psi, and immediately treated with diisopropyl fluorophosphate (2.5 µl per gram wet cell). Membranes and unbroken cells were removed by centrifugation at 150,000 × g for 1 h, and the supernatant solution was loaded onto a Ni2+-nitrilotriacetic acid column (Qiagen, Valencia, CA) at a flow rate of 0.5 ml/min. The column was washed with more than 25 column volumes of buffer A. PpArsH was eluted with buffer A containing 0.2 M imidazole, and the purity was analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS PAGE). Protein concentrations were estimated from A280 nm (ε = 28,120 M−1 cm−1). PpArsH-containing fractions were divided into small portions, rapidly frozen, and stored at −80 °C until use.
Identification of the prosthetic group and assay of FMN reductase activity
The absorption spectrum of both wild type PpArsH and the E108A derivative were compared with a standard solution of 25 µM FMN. PpArsH was assayed for FMN reductase activity in reaction mixtures (1 ml) consisting of 25 mM Bis-Tris Propane (pH 7.0), 0.2 mM NADPH, 25 µM FMN with or without 20 nM ArsH at 37 °C. NADPH oxidation was monitored by the decrease in absorbance at 340 nm (ε = 6,220 M−1 cm−1). An Amplex® Red Hydrogen Peroxide/Peroxidase Assay Kit (Invitrogen) was used to determine H2O2 formation (Zhou et al., 1997), and catalase was purchased from Sigma-Aldrich.
Assay of PpArsH activity
P. putida ArsH was assayed for organoarsenical oxidation activity in 1 ml of 25 mM Tris, 25 mM BIS-Tris Propane (pH 7.0), 1 mM EDTA, 0.2 mM NADPH, 25 µM FMN, 4 µM organoarsenicals and 0.1 µM PpArsH at 37 °C. At the indicated times, a 0.1 ml portion was filtered through 3-kDa cutoff Amicon Ultrafilter (EMD Millipore, Billerica, MA). The filtrate was then speciated by high performance liquid chromatography (HPLC) (Series 2000; Perkin-Elmer, Waltham, MA) coupled to inductively coupled plasma mass spectroscopy (ICP-MS) (ELAN DRC-e; Perkin-Elmer) using either a Jupiter® 5 µm C18 300 Å reverse-phase column (250 mm × 4.6 mm; Phenomenex, Torrance, CA) eluted isocratically with a mobile phase consisting of 3 mM malonic acid, 5 mM tetrabutylammonium hydroxide, and 5% methanol (v/v), pH 5.6, with a flow rate of 1 ml/min at 25 °C or an Inertsil® 5 µm C4 150 Å reverse-phase column (150 mm × 2.1 mm; GL Sciences, Torrance, CA) eluted with 15% acetonitrile (v/v), 15% ethanol (v/v), 80% water (v/v), pH 1.5, with a flow rate of 0.8 ml/min at 60 °C.
Supplementary Material
Summary.
The arsH gene found in many arsenic resistance (ars) operons encodes an atypical flavodoxin, but the physiological role of ArsH in arsenic resistance has been unclear. Here we demonstrate that ArsH is an organoarsenical oxidase that detoxifies the trivalent forms of the herbicide sodium methylarsenate (MSMA) and the poultry antimicrobial growth promoter roxarsone by oxidation to pentavalent species. Our results suggest that ArsH catalyzes a novel biotransformation that confers resistance to environmental methylated and aromatic arsenicals.
Acknowledgements
This work was supported by NIH grant R37 GM55425.
Abbreviations
- MAs(III)
Methylarsenite
- MAs(GS)2
methylarsenite diglutathione
- MAs(V) or MSMA
methylarsenate
- DMAs(V)
dimethylarsenate
- PhAs(V)
phenylarsenate
- PhAs(III)
phenylarsenite
- Rox(V)
roxarsone 3-nitro-4-hydroxybenzenearsonic acid or
- Rox(III)
roxarsone with reduced As(III)
- (HPLC)
high pressure liquid chromatography
- (ICP-MS)
inductively coupled plasma mass spectroscopy
- FMN
flavin mononucleotide
- GSH
reduced glutathione.
References
- Butcher BG, Deane SM, Rawlings DE. The chromosomal arsenic resistance genes of Thiobacillus ferrooxidans have an unusual arrangement and confer increased arsenic and antimony resistance to Escherichia coli. Appl Environ Microbiol. 2000;66:1826–1833. doi: 10.1128/aem.66.5.1826-1833.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canovas D, Cases I, de Lorenzo V. Heavy metal tolerance and metal homeostasis in Pseudomonas putida as revealed by complete genome analysis. Environ Microbiol. 2003;5:1242–1256. doi: 10.1111/j.1462-2920.2003.00463.x. [DOI] [PubMed] [Google Scholar]
- Carlin A, Shi W, Dey S, Rosen BP. The ars operon of Escherichia coli confers arsenical and antimonial resistance. J Bacteriol. 1995;177:981–986. doi: 10.1128/jb.177.4.981-986.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Sun S, Li CZ, Zhu YG, Rosen BP. Biosensor for organoarsenical herbicides and growth promoters. Environ Sci Technol. 2014;48:1141–1147. doi: 10.1021/es4038319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engstrom K, Vahter M, Mlakar SJ, Concha G, Nermell B, Raqib R, Cardozo A, Broberg K. Polymorphisms in arsenic(+III oxidation state) methyltransferase (AS3MT) predict gene expression of AS3MT as well as arsenic metabolism. Environ Health Perspect. 2011;119:182–188. doi: 10.1289/ehp.1002471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galibert F, Finan TM, Long SR, Puhler A, Abola P, Ampe F, Barloy-Hubler F, Barnett MJ, Becker A, Boistard P, Bothe G, Boutry M, Bowser L, Buhrmester J, Cadieu E, Capela D, Chain P, Cowie A, Davis RW, Dreano S, Federspiel NA, Fisher RF, Gloux S, Godrie T, Goffeau A, Golding B, Gouzy J, Gurjal M, Hernandez-Lucas I, Hong A, Huizar L, Hyman RW, Jones T, Kahn D, Kahn ML, Kalman S, Keating DH, Kiss E, Komp C, Lelaure V, Masuy D, Palm C, Peck MC, Pohl TM, Portetelle D, Purnelle B, Ramsperger U, Surzycki R, Thebault P, Vandenbol M, Vorholter FJ, Weidner S, Wells DH, Wong K, Yeh KC, Batut J. The composite genome of the legume symbiont Sinorhizobium meliloti. Science. 2001;293:668–672. doi: 10.1126/science.1060966. [DOI] [PubMed] [Google Scholar]
- Hervas M, Lopez-Maury L, Leon P, Sanchez-Riego AM, Florencio FJ, Navarro JA. ArsH from the cyanobacterium Synechocystis sp. PCC 6803 is an efficient NADPH-dependent quinone reductase. Biochemistry. 2012;51:1178–1187. doi: 10.1021/bi201904p. [DOI] [PubMed] [Google Scholar]
- Huijbers MM, Montersino S, Westphal AH, Tischler D, van Berkel WJ. Flavin dependent monooxygenases. Arch Biochem Biophys. 2014;544:2–17. doi: 10.1016/j.abb.2013.12.005. [DOI] [PubMed] [Google Scholar]
- Jomova K, Jenisova Z, Feszterova M, Baros S, Liska J, Hudecova D, Rhodes CJ, Valko M. Arsenic: toxicity, oxidative stress and human disease. J Appl Toxicol. 2011;31:95–107. doi: 10.1002/jat.1649. [DOI] [PubMed] [Google Scholar]
- Lopez-Maury L, Florencio FJ, Reyes JC. Arsenic sensing and resistance system in the cyanobacterium Synechocystis sp. strain PCC 6803. J Bacteriol. 2003;185:5363–5371. doi: 10.1128/JB.185.18.5363-5371.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller NJ, Stueckler C, Hall M, Macheroux P, Faber K. Epoxidation of conjugated C=C-bonds and sulfur-oxidation of thioethers mediated by NADH:FMN-dependent oxidoreductases. Org Biomol Chem. 2009;7:1115–1119. doi: 10.1039/b819057g. [DOI] [PubMed] [Google Scholar]
- Neyt C, Iriarte M, Thi VH, Cornelis GR. Virulence and arsenic resistance in Yersiniae. J Bacteriol. 1997;179:612–619. doi: 10.1128/jb.179.3.612-619.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paez-Espino AD, Durante-Rodriguez G, de Lorenzo V. Functional coexistence of twin arsenic resistance systems in Pseudomonas putida KT2440. Environ Microbiol. 2014 doi: 10.1111/1462-2920.12464. [DOI] [PubMed] [Google Scholar]
- Qin J, Lehr CR, Yuan C, Le XC, McDermott TR, Rosen BP. Biotransformation of arsenic by a Yellowstone thermoacidophilic eukaryotic alga. Proc Natl Acad Sci U S A. 2009;106:5213–5217. doi: 10.1073/pnas.0900238106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J, Rosen BP, Zhang Y, Wang G, Franke S, Rensing C. Arsenic detoxification and evolution of trimethylarsine gas by a microbial arsenite S-adenosylmethionine methyltransferase. Proc Natl Acad Sci U S A. 2006;103:2075–2080. doi: 10.1073/pnas.0506836103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reay PF, Asher CJ. Preparation and purification of 74As-labeled arsenate and arsenite for use in biological experiments. Analytical biochemistry. 1977;78:557–560. doi: 10.1016/0003-2697(77)90117-8. [DOI] [PubMed] [Google Scholar]
- Ryan D, Colleran E. Arsenical resistance in the IncHI2 plasmids. Plasmid. 2002;47:234–240. doi: 10.1016/s0147-619x(02)00012-4. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular cloning, a laboratory manual. New York: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- Sedlacek V, Klumpler T, Marek J, Kucera I. The structural and functional basis of catalysis mediated by NAD(P)H:acceptor oxidoreductase (FerB) of Paracoccus denitrificans. PLoS One. 2014;9:e96262. doi: 10.1371/journal.pone.0096262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolz JF, Basu P, Santini JM, Oremland RS. Arsenic and selenium in microbial metabolism. Annu Rev Microbiol. 2006;60:107–130. doi: 10.1146/annurev.micro.60.080805.142053. [DOI] [PubMed] [Google Scholar]
- Thomas DJ, Rosen BP. Arsenic methyltransferases. In: Kretsinger RH, Uversky VN, Permyakov EA, editors. Encyclopedia of Metalloproteins. New York: Springer New York; 2013. pp. 140–145. [Google Scholar]
- Xue XM, Yan Y, Xu HJ, Wang N, Zhang X, Ye J. ArsH from Synechocystis sp. PCC 6803 reduces chromate and ferric iron. FEMS Microbiol Lett. 2014;356:105–112. doi: 10.1111/1574-6968.12481. [DOI] [PubMed] [Google Scholar]
- Yang HC, Cheng J, Finan TM, Rosen BP, Bhattacharjee H. Novel pathway for arsenic detoxification in the legume symbiont Sinorhizobium meliloti. J Bacteriol. 2005;187:6991–6997. doi: 10.1128/JB.187.20.6991-6997.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HC, Fu HL, Lin YF, Rosen BP. Pathways of arsenic uptake and efflux. Curr Top Membr. 2012;69:325–358. doi: 10.1016/B978-0-12-394390-3.00012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J, Rensing C, Rosen BP, Zhu YG. Arsenic biomethylation by photosynthetic organisms. Trends in plant science. 2012;17:155–162. doi: 10.1016/j.tplants.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J, Yang HC, Rosen BP, Bhattacharjee H. Crystal structure of the flavoprotein ArsH from Sinorhizobium meliloti. FEBS Lett. 2007;581:3996–4000. doi: 10.1016/j.febslet.2007.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshinaga M, Cai Y, Rosen BP. Demethylation of methylarsonic acid by a microbial community. Environ Microbiol. 2011;13:1205–1215. doi: 10.1111/j.1462-2920.2010.02420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshinaga M, Rosen BP. A CAs lyase for degradation of environmental organoarsenical herbicides and animal husbandry growth promoters. Proc Natl Acad Sci U S A. 2014;111:7701–7706. doi: 10.1073/pnas.1403057111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Diwu Z, Panchuk-Voloshina N, Haugland RP. A stable nonfluorescent derivative of resorufin for the fluorometric determination of trace hydrogen peroxide: applications in detecting the activity of phagocyte NADPH oxidase and other oxidases. Analytical biochemistry. 1997;253:162–168. doi: 10.1006/abio.1997.2391. [DOI] [PubMed] [Google Scholar]
- Zhu YG, Yoshinaga M, Zhao FJ, Rosen BP. Earth abides arsenic biotransformations. Annu Rev Earth and Planet Sci. 2014;42:443–467. doi: 10.1146/annurev-earth-060313-054942. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.