Abstract
The inactivation of Listeria innocua BGA 3532 at subzero temperatures and pressures up to 400 MPa in buffer solution was studied to examine the impact of high-pressure treatments on bacteria in frozen matrices. The state of aggregation of water was taken into account. The inactivation was progressing rapidly during pressure holding under liquid conditions, whereas in the ice phases, extended pressure holding times had comparatively little effect. The transient phase change of ice I to other ice polymorphs (ice II or ice III) during pressure cycles above 200 MPa resulted in an inactivation of about 3 log cycles, probably due to the mechanical stress associated with the phase transition. This effect was independent of the applied pressure holding time. Flow cytometric analyses supported the assumption of different mechanisms of inactivation of L. innocua in the liquid phase and ice I (large fraction of sublethally damaged cells due to pressure inactivation) in contrast to cells subjected to ice I-to-ice III phase transitions (complete inactivation due to cell rupture). Possible applications of high-pressure-induced phase transitions include cell disintegration for the recovery of intracellular components and inactivation of microorganisms in frozen food.
Water under high pressure.
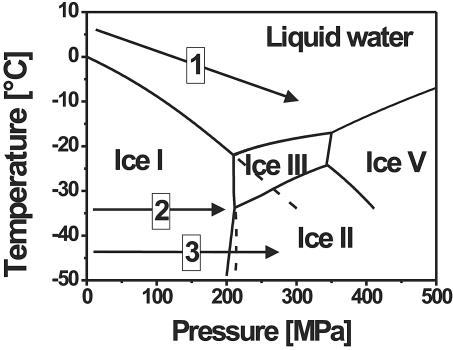
Under high pressure, water shows an unusual freezing point depression to −22°C at 210 MPa. In addition to the conventional ice I, various solid phases with a higher density than liquid water (ice II to ice V) exist in the pressure range between 210 and 500 MPa, as visualized in Fig. 1 (2). Since the early works of Tammann (33) and Bridgman (2), various other solid phases have been discovered under more extreme conditions (10), yet comparatively little is known about the kinetics of the phase transitions or metastable states.
FIG. 1.
Phase diagram of water under pressure and possible high-pressure-low-temperature treatments (arrows). The phase transition lines are based on the data reported by Bridgman (2). Prolonged transition lines are frequently observed due to metastable states, as in the case indicated for the ice I-to-liquid and ice I-to-ice III phase transitions. Treatment 1 consisted of high-pressure treatment in the liquid state of water at subzero temperatures (cooling during pressurization). Treatment 2 consisted of high-pressure treatment of ice I without phase transition (maximum pressure of about 210 MPa). Treatment 3 consisted of high-pressure treatment of ice including phase transitions between the ice polymorphs (pressure higher than 210 MPa).
When pressurizing ice I to pressures above 210 MPa in the temperature range of −22 to −35°C, i.e., beyond the phase transition line of ice I to ice III, ice I does not transform instantly to ice III (2). Although Bridgman described this phase transition to be of explosive rapidity at temperatures above −30°C in cases where it occurs, it was also observed that ice I melts as soon as it approaches the prolongation of the ice I-to-liquid line (2, 18). Ice I melts, since a solid can never exist in the domain of the liquid, even though the liquid is relatively unstable with respect to ice III. The volume change during the phase transition is about 18% during the direct phase transition from ice I to ice III at −30°C and 212 MPa within a second and vice versa. When pressurizing ice I at temperatures below −35°C (triple point), it is possible to form ice II or ice III, since both phase transition lines were detected (2). Ice II and ice III have similar phase transition lines to ice I, and they do not vary much in specific volume (Δvol < 3% at −34°C and 214 MPa) (2).
High-pressure inactivation of microorganisms.
It was shown frequently that high hydrostatic pressure is a powerful method for inactivating microorganisms in food matrices and other biomaterials without applying thermal treatments to the sensitive material. Even though it is already applied in food preservation, the mechanism of microbial inactivation by high pressure is not fully understood. Possible reasons for pressure inactivation include perturbations (rupture) of the cellular membranes, denaturation of nucleic acids, denaturation of ribosomes, events related to the maintenance of intracellular pH, and denaturation of various cellular enzymes (14, 23, 30).
The transition of proteins (enzymes) to the denatured state, as well as the plots of the rate constants of microbial inactivation, often show a similar elliptical shape in the pressure-temperature diagram (14). This behavior of proteins was frequently verified, also at high pressure-subzero temperature combinations (15); nevertheless, the data under these conditions concerning microbial inactivation are limited (8, 31). However, it is important to take into account that other effects, like membrane phase transitions (35), influence the pressure inactivation of microorganisms. High-pressure-low-temperature treatments in the liquid phase (Fig. 1) are a promising approach for increasing the efficacy of microbial inactivation at pressures lower than 400 MPa derived from this relationship. The reduced availability of liquid water as a reaction medium has to be considered when examining the impact of pressure on frozen aqueous systems (Fig. 1). The impact of phase transitions on microorganisms has to be studied when applying higher pressures to ice matrices (Fig. 1).
Inactivation of microorganisms under high pressure and low temperatures.
Although the effect of high pressure on various microorganisms has been studied extensively at temperatures above 0°C, little is known about the pressure inactivation of microorganisms below 0°C. Within this temperature range, various examinations of food treatments have been carried out in the field of pressure-supported phase transitions (3, 4). However, most of the work was focused on microstructure or engineering aspects, and few of the studies (6, 7, 17) extended the applied pressure beyond the area of stability of ice I. According to the complex phase behavior of water, special attention has to be paid to the control and detection of phase transitions during pressure treatments at temperatures below 0°C. This was not always the case in reports published so far.
Concerning microbial inactivation, it has to be considered that the water activity is lowered as soon as water solidifies because low water activities achieved by other means reduced the impact of pressure treatments on microorganisms (22, 24, 26). A second critical point is the stress caused by the phase transitions of water, which potentially influences the inactivation performance of these treatments. It is very likely that phase transitions between ice I and ice III cause considerable inactivation. Edebo and Hedén (5) found a disruption of Escherichia coli cells subjected to such phase transitions. Nevertheless, in the reported experiments, there was a lack of accurate temperature measurements inside the sample; thus, it was not possible to detect phase transitions. Magnusson and others reported a disruption of Saccharomyces cerevisiae by forcing the frozen cell suspension through an orifice at pressures in the range of 200 MPa (18, 19, 20, 21); however, this extrusion process is quite different from pressure treatments in a closed vessel.
Objectives.
The objective of this study was to examine the kinetics of inactivation of Listeria innocua in buffer solution as an indicator for the pathogen Listeria monocytogenes at subzero temperatures with respect to the different phases of water. These were the liquid phase, the ice I phase, and the phases of the high-pressure polymorphs ice II and ice III. The examination of the effect of the ice I-to-ice III phase transition was emphasized. Special attention was paid to the process control to assure the detection of all possible phase transitions. To cover that need, experiments were carried out to monitor the temperature in the thermal center of the sample.
MATERIALS AND METHODS
Sample preparation.
L. innocua BGA 3532 was obtained from the Federal Institute of Risk Management (Berlin, Germany). One bead from a deep-frozen culture was transferred into standard I nutrient broth (Merck, Darmstadt, Germany), which was incubated at 30°C for 24 h. This broth was used to inoculate the final broth with a cell count of about 103 CFU/ml, which was incubated again at 30°C for 24 h to obtain bacteria in the stationary growth phase with a cell count of about 109 CFU/ml. This culture was harvested by centrifugation at 5,200 × g for 15 min at room temperature and resuspended in phosphate-buffered saline (PBS) to the same volume. PBS containing 0.01 M K2HPO4 · 3H2O, 0.01 M KH2PO4, and 0.15 M NaCl at pH 7 was the suspension medium for the bacteria during all experiments.
The suspension was placed in 1-ml cryovials (type 375299; Nunc, Roskilde, Denmark) for all experiments, with the exception of the experiments involving solid-to-solid phase transitions. During the latter experiments, cracks were frequently observed in the cryovials. Hence, it was necessary to place the suspension in two layers of sealed bags. The interior bag consisted of a polyethylene film; the exterior one consisted of an aluminum-polyethylene composite film.
High-pressure equipment.
The samples were placed in a small-scale pressure vessel with a maximum pressure of 1 GPa in the temperature range −50 to 150°C as described previously (28) (U111 type; Unipress, Warsaw, Poland). The inner vessel volume was 3.7 ml at a diameter of 13 mm. Silicone oil (type 6165; Huber, Offenburg, Germany) was used as the pressure-transmitting medium. In preliminary experiments, the oil was checked to remain liquid down to the combined minimum temperature and maximum pressure of the experiments carried out. The pressure was generated by a hydraulic hand pump and a 20:1 pressure intensifier. The piston position of the intensifier was measured inductively. A plug with a variable feed-through was equipped with a type K thermocouple, which measured the temperature at the tip of the plug, and was used during the inactivation experiments. Alternatively, a longer thermocouple protruding 18 mm into the pressure chamber was used during the phase transition experiments. The temperature was controlled by immersing the complete pressure vessel in a tempering bath (RUK 50D; Lauda, Lauda, Germany), and the external temperature was measured by a type K thermocouple at a distance of about 20 mm from the outer vessel wall. All temperatures and the pressure were recorded by a personal computer with a data acquisition board at a frequency of 5 Hz during the inactivation experiments or 10 Hz during the phase transition experiments.
Experimental method.
The phase transition experiments were carried out by pricking a cryovial filled with bacterial suspension on the longer thermocouple in a way such that the tip of the thermocouple was located at the center of the vial. The sample was inserted in the pressure vessel along with the plug, which closed the vessel. After freezing to −25 or −45°C, the pressure was built up to 300 MPa. The pressure was kept constant for 5 min, and the pressure was released. To obtain analyzable pressure and temperature recordings, the pressure was released slower than in the inactivation experiments. This was achieved within about 1 min by carefully opening the pressure release valve specially designed for controlled pressure release. Phase transitions were identified by pressure changes which were not related to the piston movement of the pressure intensifier and by the corresponding exothermic or endothermic temperature peaks of the sample. With the experience of these recordings, it was possible later to identify phase transitions just by the pressure changes without measuring the temperature inside the sample during the inactivation experiments.
In the case of the inactivation experiments, the samples were put into the pressure vessel, which was closed. The pressure vessel was immersed in a bath that was set to the desired temperature, and temperature equalization was awaited. Pressure was built up, kept constant during the pressure holding time, and after that time, released within a few seconds by opening the respective valves. The sample was thawed in the pressure vessel and usually plated immediately or, in some cases, stored in a fridge at +4°C for a maximum of 2 h before being plated. Each experiment was performed at least in duplicate.
Plate count method.
The thawed samples were serially diluted in Ringer's solution (no. 15525; Merck) and drop plated in triplicate on NA agar (Oxoid, Basingstoke, United Kingdom). The colonies formed after 48 h of incubation at 37°C were counted. The inactivation result was expressed as the relative survivor fraction log(N/N0), where N is the number of CFU after treatment and N0 is the number of CFU of the untreated bacteria, which was determined in duplicate every day of the experiments.
Flow cytometric measurement.
Pressure-treated and thawed cells were initially incubated with 50 μM cFDA (carboxyfluorescein diacetate; Molecular Probes, Inc., Leiden, The Netherlands) at 37°C for 10 min to allow intracellular enzymatic conversion of cFDA into cF (carboxyfluorescein). The cells were then washed to remove excess cFDA. This step was followed by the addition of 30 μM PI (propidium iodide; Molecular Probes, Inc.) and by incubation in an ice bath for 10 min to allow labeling of membrane-compromised cells.
Analysis was performed on a Coulter-EPICS-XL-MCL flow cytometer (Beckman Coulter, Inc., Miami, Fla.) equipped with a 15-mW 488-nm air-cooled argon laser. Cells were delivered at the low flow rate, corresponding to 400 to 600 events per s. The forward scatter (FS), sideward scatter (SS), green, and red fluorescence of each single cell were measured, amplified, and converted into digital signals for further analysis. cF emits green fluorescence at 530 nm following excitation with laser light at 488 nm, whereas red fluorescence at 635 nm is emitted by PI-stained cells.
A set of band-pass filters of 525 nm (505 to 545 nm) and 620 nm (605 to 635 nm) was used to collect green fluorescence and red fluorescence, respectively. All registered signals were logarithmically amplified. A gate created in the dot plot of FS versus SS was preset on the basis of untreated cells to discriminate bacteria from artifacts. Hence, only cells with FS versus SS properties similar to untreated cells were analyzed after high-pressure treatments. In total, 20,000 cells, which were encountered within this gate, were selected for further fluorescence assessments. Data were analyzed with the software package Expo32 ADC (Beckman Coulter, Inc.). All detectors were calibrated with FlowCheck fluorospheres (Beckman Coulter, Inc.).
RESULTS
Phase transition experiments.
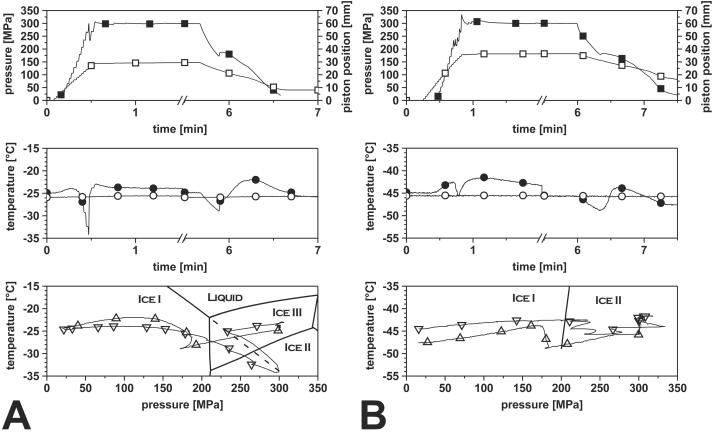
It was necessary to carry out separate experiments with accurate temperature measurement inside the sample to elucidate the phase transitions occurring during pressurization of ice I above 210 MPa. Figure 2 shows the recordings of a pressure treatment of frozen suspensions of L. innocua in PBS at 300 MPa for 5 min at −25°C (Fig. 2A) and −45°C (Fig. 2B).
FIG. 2.
Transient solid-to-solid phase transition of PBS inoculated with L. innocua at −25°C (A) and −45°C (B) during pressurization to 300 MPa for 5 min. ▪, pressure; □, piston position; •, sample core temperature; ○, cryostat temperature; ▿, sample core temperature during compression in pT plot; ▵, sample core temperature during decompression in pT plot. The pT plot, the plot of temperature versus pressure, was obtained by plotting the sample core temperature versus the respective system pressure, which visualizes the pressure and temperature combinations during the experiment in relation to the phase transition lines of water. At −25°C, the phase transition from ice I to ice III is accompanied by (partial) thawing, as indicated by the decreasing temperature when approaching the prolonged phase transition line of ice I to liquid (dashed line). At −45°C, thawing of ice I by pressurization is impossible and the direct transition to ice II or ice III occurs.
During pressurization at −25°C, the sample temperature rose to −24°C at 100 MPa due to compression heating, but during further pressure increase, the sample temperature decreased to a minimum temperature of −34°C, which was lower than the temperature outside the vessel (−26°C). The temperature shifted downwards along the phase transition line of ice I to liquid and approached it slowly as visualized in the plot of temperature versus pressure, a behavior that continued in the ice III stability area. At about 300 MPa and −34°C, a sudden pressure drop to 230 MPa occurred in less than 1 s, and the temperature rose from −34 to −25°C. During further pressure increase, no other pressure shifts were detected, and the temperature equalized within the system to −25°C.
Decompression was going along with a decrease in temperature, but after passing the phase boundary between ice I and ice III, an increase in pressure was observed while the piston was consistently moving back. This transition was concurrent with an instant increase in temperature from −29 to −26°C and up to −22°C during further decompression.
During pressurization at −45°C, no endothermic event comparable to the experiment at −25°C was detected. The heat of compression caused an increase in temperature to −42.6°C. In contrast to the experiment at −25°C, a pressure decrease was already detectable at 225 MPa, soon after passing the phase boundary between ice I and ice II. A decrease in pressure was detected after each stroke of the pressurizing hand pump, accompanied by a decrease in temperature, until the pressure was rising again constantly to 300 MPa. During the pressure holding time, the temperature equalized to −45.7°C. Similar to the previous experiment, the temperature dropped during decompression until an increase in sample volume was indicated by a period of constant pressure while the piston was moving backwards. During this time without pressure decrease, the temperature rose to −44°C.
Inactivation experiments.
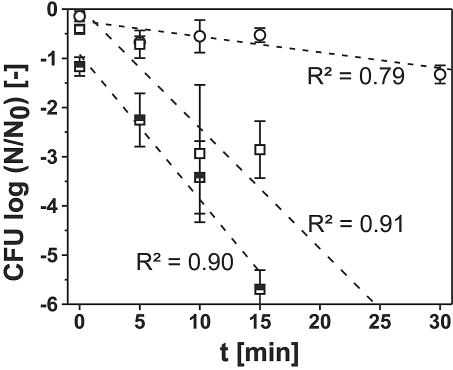
To examine the influence of high pressure on L. innocua in the area of stability of liquid water, the inactivation kinetics in the liquid phase of water at low temperatures were examined (Fig. 3). Due to the experimental method, freezing occurred during the pretempering and during the pressure release at 300 MPa and −10°C. Freezing to −45°C resulted in the inactivation of about 7% of the cells, and the data obtained from flow cytometric measurements also suggested an influence of freezing on a fraction of the cells (see Fig. 6c). Hence, an influence of partial freezing on the inactivation results cannot be neglected. During the experiments at 0°C, freezing was avoided by slow pressure release and warming during decompression. All inactivation kinetics shown in Fig. 3 could be approximated by linear regression curves with a high coefficient of correlation. The regression lines at 0°C ran approximately through the point of origin, i.e., there was no inactivation after 0 min of pressure holding time. Deviations can be explained by the action of pressure during the pressure buildup and release time during the 0-min experiments. However, there is a bigger deviation in the case of the experiments at 300 MPa and −10°C (intercept, −0.922).
FIG. 3.
Inactivation of L. innocua in buffer solution under high-pressure-low-temperature conditions in the liquid phase of water. Treatment parameters: ○, 200 MPa, 0°C; □, 300 MPa, 0°C; ⬒, 300 MPa, −10°C. The regression lines and their respective coefficients of determination (R2) were fitted on the basis of the results from the single experiments. Some experiments not shown here were included in the regression analysis.
FIG. 6.
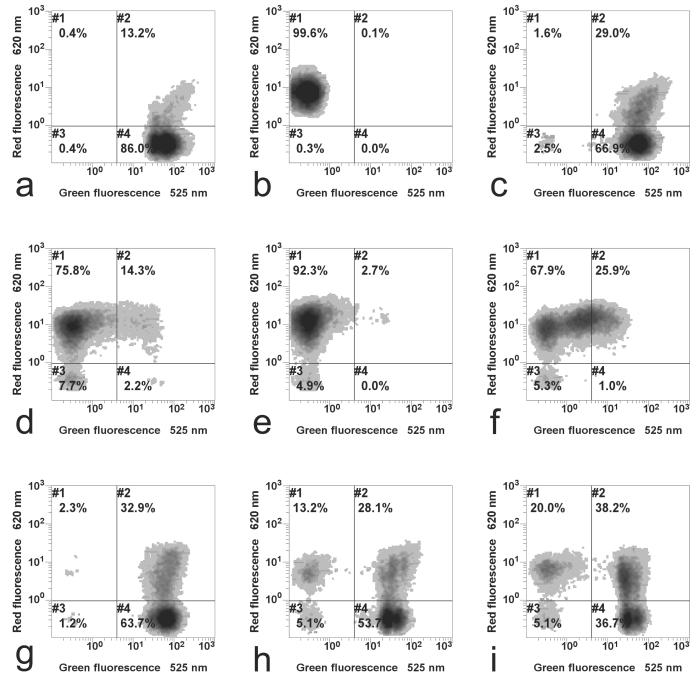
Assessment of cellular damage of L. innocua by flow cytometry after various high-pressure-low-temperature treatments. (a) Control, viable cells; (b) thermal treatment at 95°C, complete inactivation; (c) cells frozen to −40°C for 1 h without pressure treatment, inactivation according to results shown in Fig. 5; (d) treatment of ice at −45°C, 300 MPa, 0 min (phase transition to ice III), log (N/N0) = −2.9; (e) treatment of ice at −45°C, 300 MPa, 5 times for 0 min (5 times repeated phase transition to ice III), log (N/N0) = −4.2; (f) treatment of ice at −25°C, 300 MPa, 0 min (phase transition to ice III including partial thawing), log (N/N0) = −2.6; (g) treatment at 0°C, 200 MPa, 5 min (in liquid conditions), log (N/N0) = −0.6; (h) treatment at 0°C, 300 MPa, 15 min (in liquid conditions), log (N/N0) = −2.8; (i) treatment at −45°C, 200 MPa, 10 min (treatment of ice I without phase transition), log (N/N0) = −0.6. The inactivation results correspond to the inactivation determined for the specific sample which was used for flow cytometry.
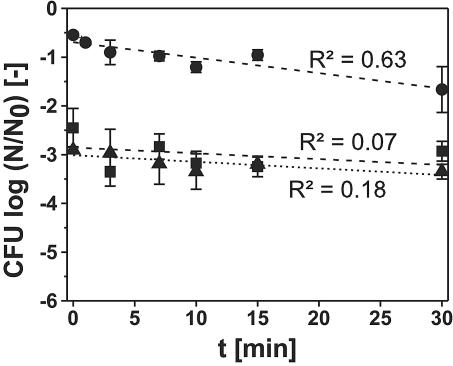
The inactivation kinetics of L. innocua under frozen conditions at −45°C and at pressures of 200 to 400 MPa were examined in comparison to the liquid phase (Fig. 4). The inactivation at 200 MPa and −45°C was about at the same low level as the inactivation at 200 MPa and 0°C. At 300 and 400 MPa, a phase transition to ice III during compression and back to ice I during decompression was detected in each experiment by the characteristic pressure changes. The general characteristics of the kinetics of inactivation differed considerably from the curves detected under liquid conditions. Already after pressure treatments without holding time, there was a logarithmic inactivation of 2.5 log cycles at 300 MPa and of 2.9 log cycles at 400 MPa. However, after longer pressure holding times under these conditions, the inactivation did not increase much, showing almost no dependence on the pressure holding time. As a result, the inactivation is higher at short pressurization times than inactivation in the liquid state of water, whereas it is considerably lower at longer pressure holding times. The linear regression curves of these experiments show that there is only a low correlation between the treatment time and the decrease in CFU (R2 is <0.2 for both).
FIG. 4.
Inactivation of L. innocua in buffer solution under high-pressure-low-temperature conditions in the frozen phase of water. Treatment parameters: •, 200 MPa, −45°C; ▪, 300 MPa, −45°C (dashed line); ▴, 400 MPa, −45°C (dotted line). Experiments at 300 and 400 MPa included one phase transition from ice I to ice III during compression and back to ice I during decompression. The regression lines and their respective coefficients of determination (R2) were fitted on the basis of the results from the single experiments. Some experiments not shown here were included in the regression analysis.
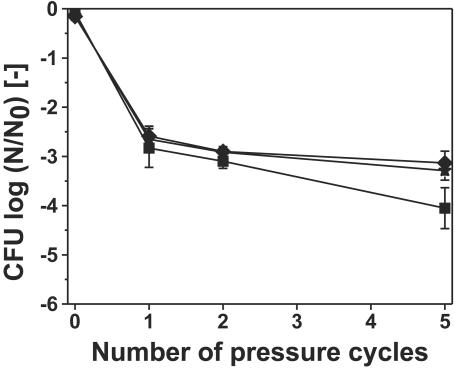
After repeated pressure cycles, which also means that the solid-to-solid phase transition of ice was acting on the sample repeatedly, the inactivation was not enhanced significantly (Fig. 5). Different temperature levels were applied, and the difference in the results was not very big. The data presented in Fig. 5 also show that freezing without pressure application (0 pressure cycles) for about 1 h did not cause much inactivation.
FIG. 5.
Inactivation of L. innocua in buffer solution due to multiple pressure cycles to 300 MPa without holding time at maximum pressure at various subzero temperatures. Treatment temperature: ★, −25°C; ⧫, −35°C; ▪, −45°C. The data presented for zero pressure cycles indicates the inactivation due to freezing of the bacterial suspension for 1 h at the respective temperature levels.
Flow cytometric analysis.
A density plot of green versus red fluorescence was applied to resolve the fluorescence properties of the population measured by the flow cytometer (Fig. 6). With this analysis mode, the population was graphically differentiated and gated according to their fluorescence behaviors. Each dot, which constitute the dot clouds depicted in the diagrams, represented one single cell, which was in turn represented as a coordinate of its green and red fluorescence values. The relative number of the cell population with a particular green and red fluorescence correlates positively with the darkness intensity of the cloud. The gates were set in such a way that unstained cells were located in gate 3, whereas stained cells treated at 95°C were located in gate 1, as discussed below.
Untreated cells of L. innocua were able to convert cFDA into cF and retain this fluorescence product, thus conferring them a high green fluorescence intensity. Since no membrane damage occurred, these cells were not stained by PI and red fluorescence was low. This specific fluorescence property of untreated cells toward the applied dyes made it possible to locate them in gate 4 (Fig. 6a). However, a fraction of the population, which could not be separated from the rest of the population in the FS versus SS plot, was already stained by PI, shifting them to gate 2. After the cell membranes were damaged severely, which was achieved by exposing the cells to 95°C, the membrane-impermeant PI penetrated the cells and stained all cells with compromised membranes. This event shifted the population into gate 1 (Fig. 6b). Freezing the cells at atmospheric pressure did not result in a significant change compared to untreated cells (Fig. 6c). However, it was observed that more cells were encountered in gate 2. Cells framed in this gate were still able to retain cF, but apparently cell membranes were already damaged, which led to a penetration of PI into the cells. On the other hand, despite apparent membrane damage in the double-stained population, cF was retained. Consequently, the population in gate 2 showed high green and red fluorescence.
Pressurization of L. innocua, including phase transitions between ice I and ice III, resulted in pronounced membrane damage and loss of cF retention, irrespective of the temperature level applied (Fig. 6d and f). The main population of cells treated as such were stained by PI and framed in gate 1; however, this amount was slightly lower at −25°C (Fig. 6f). Repeating the pressurization to 300 MPa at −45°C up to five times without any holding period increased the fraction in gate 1 (Fig. 6e).
In contrast, when pressure treatment was applied without phase change in either the liquid or the solid phase, the damage to cellular membranes of L. innocua (Fig. 6g, h, and i) was less pronounced. This fact was documented in Fig. 6g and h (liquid conditions) and Fig. 6i (ice I conditions at 200 MPa), which showed that the major part of cells pressure treated in the liquid state was still able to retain cF; thus, they were gated in gates 2 and 4.
DISCUSSION
Phase transition experiments.
Little is known about the kinetic behavior of water and ice phase transitions under pressure. Combined temperature-pressure recordings of these phase transition phenomena were published by Magnusson regarding the extrusion of ice through an orifice in the pressure range examined here (18). Furthermore, only the experiments of Bridgman (2), which were carried out without temperature measurements inside the sample, are available to compare the data and conclusions reported here.
During the phase transition experiments, different mechanisms of pressure-induced phase transitions between the solid ice polymorphs ice I and ice III at different temperature levels were observed. When pressurizing ice or frozen aqueous systems at −25°C, partial thawing occurred, which was indicated by a transient decrease in sample temperature, suggesting the energy requirement of pressure-induced thawing. Due to the limitations of heat transfer, this transition did not take place instantly; hence, the temperature decreased along the phase transition line and approached it slowly as thawing progressed (28). Ice cannot be superheated with respect to the liquid (2), which implies that ice I cannot exist beyond the phase transition line of ice I to liquid, even though the line is extended into the area of stability of ice III.
When the pressure approached 300 MPa during thawing, ice I could no longer exist as a metastable phase in the stability range of ice II or ice III. The sudden pressure decrease indicated the conversion of ice I to ice III, which has a higher density than ice I (28). However, the phase transition from ice I to ice III is endothermic at this temperature level, although the enthalpy change is quite small (4.5 kJ/kg for pure water, all enthalpies for water are extrapolations based on the work of Bridgman [2]). The only possible explanation for the temperature increase was that, during the ice I-to-ice III phase transition of the solid parts of the sample, water froze exothermically to ice III (242.5 kJ/kg at 300 MPa) in the previously thawed outer regions of the sample.
During the transition and afterwards, the pressure was increased constantly to the maximum pressure, and during the pressure holding time, the temperature was equalizing within the system to −25°C. It should be noted that −25°C is only about 5°C below the melting point of aqueous biological systems, which was, e.g., −19.6°C at 300 MPa for potato tissue (O. Schlüter and D. Knorr, ASAE Annu. Int. Meet., CIGR XVth World Congr., abstr. no. 026024, 2002). Thus, it is likely that not all water was present as solid ice III and some was present as liquid water, as would be the case of samples at −6°C at atmospheric pressure. During decompression, ice III converted back to ice I, which was indicated by an increase in pressure. The exothermic nature of the transition was indicated by an instant increase in temperature to −26°C, but the later increase to −22°C might also be due to freezing of the mentioned liquid water.
During the experiment at −45°C, no partial thawing of the sample occurred. In contrast to the experiment at −25°C, the solid-to-solid phase transition to ice II or ice III began soon after passing the phase boundary of ice I to ice II. An increase in sample volume indicated a transition to ice I when the sample was decompressed after the pressure holding time. The ice III-to-ice I transition at −49°C is slightly endothermic (7.7 kJ/kg); however, a rise of temperature during this period of constant pressure was detected, which was likely to be an effect of equalizing temperatures, as the sample was colder than the surrounding pressure vessel. As ice II and ice III have about the same density and phase transition line with ice I, it could not be concluded from the temperature and pressure measurements whether ice II or ice III was formed at about −45°C. Despite that the ice II stability area borders the area of stability of ice I, ice III is usually formed (2) when pressurizing ice at about −45°C and above the transition pressure. To validate this fact, samples were warmed under pressure at 300 MPa above the ice II-to-ice III phase transition line (data not shown); however, no phase transition was observed. Based on the recordings of piston position and cryostat temperature, events other than the mentioned phase transitions can be ruled out as the reasons for the described pressure and temperature changes.
In similar experiments carried out at −35°C, thawing was detected, as in the case of the sample pressurized at −25°C (data not shown); however, the temperature decrease was smaller, indicating a lower extent of thawing. The complexity of the phase transitions at −25°C and the uncertain amount of frozen water during the pressure holding time were likely to cause problems in the reproducibility and interpretation of inactivation experiments. Hence, −45°C was chosen as the temperature level of the microbial experiments, as the major focus of this work was to evaluate the effect of solid-to-solid phase transitions on microbial inactivation.
Magnusson reported the same phase transition effects in frozen yeast suspensions during the development of a freeze press (18, 19). He also detected the melting of frozen samples upon pressurization at about −20°C and the direct phase transition to ice III at considerably lower temperatures. Hence, these reports support the findings described here; however, it has to be stressed that the processes are difficult to compare, as the extrusion of frozen material is not the same as the pressure treatment of a frozen sample in a sealed pressure vessel.
Inactivation experiments.
The inactivation behavior of L. innocua in liquid conditions was linear, as shown in Fig. 2. The general expectation that the inactivation should be higher at lower temperatures was met; however, it remains unclear whether the inactivation was higher due to lower temperature or due to partial freezing. The results are in substantial agreement with the inactivation of S. cerevisiae under these conditions (8). In this study, freezing during pressure release was reported in the case of subzero temperature treatments; nevertheless, an influence on the inactivation was ruled out, as the continuous character of the results did not show anomalies due to this event, a conclusion that cannot be drawn here.
In comparison to the previous experiments, a substantially different behavior was detected during high-pressure treatments of frozen microbial suspensions at 300 and 400 MPa (Fig. 4). It is obvious that the beginning of the regression line is shifted to higher inactivation results, and the shift is big enough to exclude the action of pressure during the compression and decompression time as a reason for this behavior. The shift is also higher than the usual standard deviation during these determinations. However, after this inactivation in the beginning, the inactivation progressed slowly. The inactivation kinetics at 200 MPa and −45°C are, in contrast, only slightly different from the inactivation at the same pressure at 0°C. The shift of the inactivation kinetics at pressures higher than 200 MPa without having an impact on the further kinetics of the treatment suggests that a single event during the treatment was responsible for this exceptional behavior. However, the only event that was different during these treatments at 300 and 400 MPa was the phase transition from ice I to ice III and back to ice I, which was detected in every experiment at 300 and 400 MPa at −45°C by the characteristic pressure drop. The sudden change in the surrounding crystal structure of the bacteria went along with a sudden decrease or increase in sample volume, leading probably to mechanical stress acting on the bacteria.
The second characteristic difference is the low progression after initially high inactivation. As in normal freezing preservation, it seems reasonable to assume that the low water activity due to the frozen conditions as well as the lowered reaction speed due to low temperatures reduce the impact of pressure on microorganisms, as it was frequently reported in the case of other systems under pressure with reduced availability of liquid water (22, 24, 26). This reduced impact of pressure led to much slower inactivation kinetics compared to liquid conditions, and there was also almost no difference in inactivation at 300 and 400 MPa. This low impact was statistically proven by the low coefficients of correlation. High pressure is transmitted uniformly through frozen samples (2, 13); hence, it cannot be assumed that the slow inactivation arose from the fact that pressure was not acting on bacteria suspended in the solid ice matrix.
After repeated pressure cycles, which means also that the solid-to-solid phase transition of ice was acting on the sample repeatedly, the inactivation was slightly enhanced (Fig. 5). Different temperature levels were applied, and the difference in the results was not very big, despite partial thawing at −25 and −35°C. However, the inactivation after five pressure cycles was a little higher at −45°C. It is not certain whether the combined application of high pressure at low temperature in liquid conditions (at a higher water activity) during partial thawing might be responsible for the inactivation at the higher temperature levels. These experiments show that there is a small fraction of the initial bacterial population which is not affected by repeated solid-to-solid phase transitions. This fraction might survive due to an inhomogeneity during the phase transition, possibly related to the vessel wall or due to the fact that a certain amount of bacteria was in a state of higher resistance. Although the pressure is homogeneous throughout the vessel, it seems reasonable to assume that bacteria at the outer edge of the sample, which are not completely surrounded by ice, are not exposed to the same mechanic stress as bacteria in the middle of the sample. The data presented in Fig. 5 also show that freezing without pressure application (0 pressure cycles) for less than 1 h did not cause much inactivation.
These inactivation results are in excellent agreement with the experiments reported by Edebo and Hedén (5). They used a pressure cycling method to disrupt E. coli in frozen conditions suspended in buffer solution. Repeated passing of the phase transition line from ice I to ice III at −25°C produced mechanic stress and yielded the best cell disintegration compared to pressure cycling at other pressures, including other phase transitions of water. However, there was also no temperature measurement inside the sample; hence, it is doubtful that a direct solid-to-solid phase transition of ice I to ice III took place at this temperature level, due to the close phase boundary of ice I to liquid. Nevertheless, the results give no reason to cast doubt on them, but as the method was developed to disintegrate cells, the disruption was not detected by cell counts but by measuring the release of intracellular components via extinction and by analysis of the released protein. The experiments reported by Magnusson and Edebo (20, 21) during the development of the so-called X-Press are difficult to compare with the results shown here for a number of reasons. Magnusson himself stated the cell disintegration effect he found was probably the consequence of extrusion effects (shear and internal friction) and not the ice I-to-III phase transition (20). The results he obtained were always given as a percentage of disintegrated cells detected with a microscope, which may not be equated with the inactivation results provided here. Furthermore, the sample composition is quite different, as yeast cells and bacteria differ considerably in cell size, and the yeast cell pastes he used had a dry mass content of up to 270 mg/g, which is a dimensional difference compared to the aqueous suspensions examined here.
A decrease of 1.6 log cycles of vegetative cells of Bacillus subtilis in frozen nutrient broth was caused by a phase transition of ice III to ice I after freezing to ice III under pressure at −25°C (34). Nevertheless, the temperature during the experiment was not measured, and hence, it is not certain whether freezing under pressure really occurred, due to the high supercooling that was frequently observed (17, 27). In some cases, other microbial experiments were reported with frozen conditions under pressure which were usually focused on other aspects and were usually limited to the stability range of ice I (9, 16) Hence, it is not possible to compare the results. In other cases, little attention was paid to the detection of phase transition, so it is not clear which phase transitions occurred (25, 32, 36).
Flow cytometric analysis.
Sublethal damage of microorganisms was frequently reported after high-pressure treatments of bacteria (11, 12, 29), a fact that points out the technological importance of characterizing this damage more precisely in comparison to a simple live-dead assay, e.g., by flow cytometric studies.
As already discussed above, imposed changes in the ice modification exert mechanical stress on bacteria, and apparently, cellular membranes were highly sensitive to these dynamic conditions. On the other hand, exposure to a similar pressure level in a static phase condition resulted in the loss of culturability as well. According to plate count results, pressure treatment at 300 MPa in the liquid state led to a bacterial reduction in the magnitude of 3 log cycles. A fluorescence profile of cells treated as such is shown in Fig. 6h. Since most of the L. innocua cells pressure treated in the liquid state were found to not have severe membrane damage, it could be speculated that the mechanism leading to cell death by exposure to high pressure in the liquid state was not straightforwardly related to membrane rupture. Minimal damage to the membranes of cells treated as such indicated that the damage to other crucial components may be responsible for their inability to recover on agar. This latter finding is in accordance with previous data compiled in the pressure inactivation work on Lactobacillus rhamnosus GG, where the loss of capacity to form visible colonies was not necessarily associated with the occurrence of membrane damage. Instead, it was most likely related to the malfunction of a dye extrusion system (1). Moreover, cells of L. innocua inactivated by pressure treatment in the liquid state still contained active enzymes, indicated by the capability of enzymatically converting cFDA into cF and retaining this fluorescent dye.
Similarly, when pressure treatment was conducted in the frozen state without any imposed change of ice modification during the pressurization and decompression step (200 MPa, −45°C), most of the population measured by flow cytometry was still able to retain cF (in gates 2 and 4). It was evident from the data shown in Fig. 6i that this procedure of treatment (freezing and applying pressure in the solid state) merely led to an increased occurrence of cells with ruptured membranes but not to a drastic shift towards cells solely stained by PI. As soon as additional mechanical stress was applied by means of a further increase in pressure above the ice I-to-ice III phase transition line, a pronounced detrimental effect on the membrane was observed (Fig. 6d, e, and f).
The extent of cellular damage was influenced mainly by imposed changes in the ice modifications, even when no holding time under pressure was applied. Exposure of cells to 300 MPa at −45°C resulted in a predominant occurrence of cells with severe membrane damage, which were in turn stained solely by PI, when the sample underwent a change in ice modifications during pressurization at −45°C (Fig. 6d). Repetition of the pressurization step up to five times without any holding period intensified the perturbation of membranes slightly, which was already on a high level (Fig. 6e). The exposure to ice I-to-ice III phase transitions at −25°C led to a slightly lower amount of cells encountered in gate 1. As discussed above, this lower damage of the cell population compared to the treatment at −45°C (Fig. 6d) may be associated with a lower amount of frozen water at −25°C.
Surprisingly, the two pressure treatments at 300 MPa and 0°C (liquid) and −45°C (change of ice modification between ice I and ice III), respectively, resulted in an identical magnitude of cell inactivation (approximately 3 log cycles). In conjunction with the flow cytometric profiles discussed above, these observations suggested that the mechanisms by which bacteria were inactivated (i.e., lost the ability to form visible colonies on agar) differed in these treatments. It could be that mechanical stress on the cell membrane, as indicated by PI staining, led to the inactivation of L. innocua at −45°C.
However, since a disruption of the cells during the ice I-to-ice III phase transitions most likely occurs, the cause of the loss of cF retention was not able to be identified. It remained unclear whether this deficiency was associated with the inactivation of intracellular esterase, making cF formation impossible, or with complete cell disintegration, making the retention of cF inside the cells impossible.
Conclusions.
Subjecting L. innocua to phase transitions between ice I and ice III by pressurizing frozen aqueous systems above 200 MPa appears to be an effective way to reduce bacterial contamination, since with this strategy, 3 log cycles of reduction can be achieved instantaneously. In contrast, a holding time of 15 min is required to obtain the identical degree of bacterial decontamination in the liquid state. It was pointed out that the cellular damage is probably associated with the (mechanical) stress during the phase transition and not with the impact of pressure itself. Differences in the impact of high pressure in the liquid state and in the impact of solid-to-solid phase transitions were shown by flow cytometry. The necessity of monitoring the sample temperature inside the sample was demonstrated, as transient phase transition phenomena (in this case, partial thawing at −25°C and 300 MPa) cannot be detected just by pressure measurements.
Further investigations potentially make applications of ice I-to-ice III phase transitions possible, such as the inactivation of microorganisms in frozen foods. This technique would provide a unique tool for achieving a reduction of the microbial load in the frozen state by applying short time treatment cycles at a rather low pressure level. However, it has to be considered that the impact of the solid-to-solid phase transitions has an impact on structured matrices, like cellular foods (17). Another possibility would be to disintegrate microorganisms without thermally affecting heat-sensitive intracellular components (5).
The next steps should be the validation of these findings for other microorganisms and more complex (food) systems. To improve the applicability, it should be clarified whether the application of phase transitions at higher temperatures (−25°C) leads to the same inactivation effects, including computer modeling of the kinetics of the phase transitions.
Acknowledgments
Parts of this work were supported by the German Ministry for Education and Research (BMBF grant no. 0330089).
The skillful assistance of Irene Hemmerich is gratefully acknowledged.
REFERENCES
- 1.Ananta, E., V. Heinz, and D. Knorr. 2004. Assessment of high pressure induced damage on Lactobacillus rhamnosus GG by flow cytometry. Food Microbiol. 21:567-577.
- 2.Bridgman, P. W. 1911. Water, in the liquid and five solid forms, under pressure. Proc. Am. Acad. Arts Sci. 47:441-558. [Google Scholar]
- 3.Cheftel, J. C., M. Thiebaud, and E. Dumay. 2002. Pressure-assisted freezing and thawing of foods: a review of recent studies. High Press. Res. 22:601-611. [Google Scholar]
- 4.Cheftel, J.-C., J. Levy, and E. Dumay. 2000. Pressure-assisted freezing and thawing: principles and potential applications. Food Rev. Int. 16:453-483. [Google Scholar]
- 5.Edebo, L., and C.-G. Hedén. 1960. Disruption of frozen bacteria as a consequence of changes in the crystal structure of ice. J. Biochem. Microbiol. Technol. Eng. 2:113-120. [Google Scholar]
- 6.Fuchigami, M., N. Ogawa, and A. Teramoto. 2002. Trehalose and hydrostatic pressure effects on the structure and sensory properties of frozen tofu (soybean curd). Innov. Food Sci. Emerg. Technol. 3:139-147. [Google Scholar]
- 7.Fuchigami, M., and A. Teramoto. 2003. Changes in temperature and structure of agar gel as affected by sucrose during high-pressure freezing. J. Food Sci. 68:528-533. [Google Scholar]
- 8.Hashizume, C., K. Kimura, and R. Hayashi. 1995. Kinetic analysis of yeast inactivation by high pressure treatment at low temperatures. Biosci. Biotech. Biochem. 59:1455-1458. [DOI] [PubMed] [Google Scholar]
- 9.Hayakawa, K., Y. Ueno, S. Kawamura, T. Kato, and R. Hayashi. 1998. Microorganism inactivation using high-pressure generation in sealed vessels under sub-zero temperature. Appl. Microbiol. Biotechnol. 50:415-418. [DOI] [PubMed] [Google Scholar]
- 10.Hobbs, P. V. 1974. Ice physics. Clarendon Press, Oxford, United Kingdom.
- 11.Jordan, S. L., C. Pascual, E. Bracey, and B. M. Mackey. 2001. Inactivation and injury of pressure-resistant strains of Escherichia coli O157 and Listeria monocytogenes in fruit juices. J. Appl. Microbiol. 91:463-469. [DOI] [PubMed] [Google Scholar]
- 12.Kalchayanand, N., A. Sikes, C. P. Dunne, and B. Ray. 1998. Factors influencing death and injury of foodborne pathogens by hydrostatic pressure-pasteurization. Food Microbiol. 15:207-214. [Google Scholar]
- 13.Kanda, T., K. Kitagawa, and K. Fujinuma. 1993. Behavior of ice under pressure, p. 24-26. In R. Hayashi (ed.), High pressure bioscience and food science. San-Ei Suppan Co., Kyoto, Japan.
- 14.Knorr, D., and V. Heinz. 2001. Development of nonthermal methods for microbial control, p. 853-877. In S. S. Block (ed.), Disinfection, sterilization and preservation. Lippincott, Williams, & Wilkins, Philadelphia, Pa.
- 15.Kunugi, S., and N. Tanaka. 2002. Cold denaturation of proteins under high pressure. Biochim. Biophys. Acta 1595:329-344. [DOI] [PubMed] [Google Scholar]
- 16.LeBail, A., D. Mussa, J. Rouillé, H. S. Ramaswamy, N. Chapleau, M. Anton, M. Hayert, L. Boillereaux, and D. Chevalier. 2002. High pressure thawing: application to selected sea-foods, p. 563-570. In R. Hayashi (ed.), Trends in high pressure bioscience and biotechnology. Elsevier, Amsterdam, The Netherlands.
- 17.Luscher, C., O. Schlüter, and D. Knorr. High pressure-low temperature processing of foods: impact on cell membranes, texture, color and visual appearance of potato tissue. Innov. Food Sci. Emerg. Technol., in press.
- 18.Magnusson, K. E. 1977. The continuation of the phase-boundary between ice I and liquid into the region of ice III and II and its relation to freeze-pressing of biological material. Cryobiology 14:68-77. [DOI] [PubMed] [Google Scholar]
- 19.Magnusson, K. E. 1977. A physical description of freeze-pressing of biological material with the X-Press. Cryobiology 14:78-86. [DOI] [PubMed] [Google Scholar]
- 20.Magnusson, K. E., and L. Edebo. 1976. Influence of cell concentration, temperature, and press performance on flow characteristics and disintegration in the freeze-pressing of Saccharomyces cerevisiae with the X-Press. Biotechnol. Bioeng. 18:865-883. [DOI] [PubMed] [Google Scholar]
- 21.Magnusson, K. E., and L. Edebo. 1976. Influence of salts and gelatin on disintegration of Saccharomyces cerevisiae by freeze-pressing. Biotechnol. Bioeng. 18:449-463. [DOI] [PubMed] [Google Scholar]
- 22.Oxen, P., and D. Knorr. 1993. Baroprotective effects of high solute concentrations against inactivation of Rhodotorula rubra. Lebensm. Wiss. Technol. 26:220-223. [Google Scholar]
- 23.Palou, E., A. López-Malo, G. V. Barbosa-Cánovas, and B. G. Swanson. 1999. High-pressure treatment in food preservation, p. 533-576. In M. S. Rahman (ed.), Handbook of food preservation. Marcel Dekker, New York, N.Y.
- 24.Palou, E., A. Lopez-Malo, G. V. Barbosa-Canovas, J. Welti-Chanes, and B. G. Swanson. 1997. Effect of water activity on high hydrostatic pressure inhibition of Zygosaccharomyces bailii. Lett. Appl. Microbiol. 24:417-420. [Google Scholar]
- 25.Ponce, E., R. Pla, M. Mor-Mur, R. Gervilla, and B. Guamis. 1998. Inactivation of Listeria innocua inoculated in liquid whole egg by high hydrostatic pressure. J. Food Prot. 61:119-122. [DOI] [PubMed] [Google Scholar]
- 26.Raso, J., M. M. Góngora-Nieto, G. V. Barbosa-Cánovas, and B. G. Swanson. 1998. Influence of several environmental factors on the initiation of germination and inactivation of Bacillus cereus by high hydrostatic pressure. Int. J. Food Microbiol. 44:125-132. [DOI] [PubMed] [Google Scholar]
- 27.Schlüter, O., V. Heinz, and D. Knorr. 2002. Freezing kinetics due to ice III formation in potato tissue, p. 425-429. In R. Winter (ed.), Advances in high pressure bioscience and biotechnology II. Springer, Berlin, Germany.
- 28.Schlüter, O., G. Urrutia Benet, V. Heinz, and D. Knorr. Metastable states of water and ice during pressure-supported freezing of potato tissue. Biotechnol. Prog., in press. [DOI] [PubMed]
- 29.Simpson, R. K., and A. Gilmour. 1997. The effect of high hydrostatic pressure on the activity of intracellular enzymes of Listeria monocytogenes. Lett. Appl. Microbiol. 25:48-53. [DOI] [PubMed] [Google Scholar]
- 30.Smelt, J. P., J. C. Hellemons, and M. Patterson. 2002. Effects of high pressure on vegetative microorganisms, p. 55-76. In M. E. G. Hendrickx and D. Knorr (ed.), Ultra high pressure treatments of foods. Kluwer Academic/Plenum Publishers, New York, N.Y.
- 31.Sonoike, K., T. Setoyama, Y. Kuma, and S. Kobayashi. 1992. Effect of pressure and temperature on the death rates of L. casei and E. coli, p. 297-300. In C. Balny, R. Hayaski, K. Heremans, and P. Masson (ed.), High pressure and biotechnology. Colloques INSERM/John Libbey Eurotext, Montrouge, France.
- 32.Takahashi, K. 1992. Sterilisation of microorganisms by hydrostatic pressure at low temperature, p. 303-307. In C. Balny, R. Hayashi, K. Heremans, and P. Masson (ed.), High pressure and biotechnology. Colloques INSERM/John Libbey Eurotext, Montrouge, France.
- 33.Tammann, G. 1903. Kristallisieren und Schmelzen. Barth, Leipzig, Germany.
- 34.Timson, W. J., and A. J. Short. 1965. Resistance of microorganisms to hydrostatic pressure. Biotechnol. Bioeng. 7:139-159. [Google Scholar]
- 35.Ulmer, H. M., H. Herberhold, S. Fahsel, M. G. Gaenzle, R. Winter, and R. F. Vogel. 2002. Effects of pressure-induced membrane phase transitions on inactivation of HorA, an ATP-dependent multidrug resistance transporter, in Lactobacillus plantarum. Appl. Environ. Microbiol. 68:1088-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yuste, J., R. Pla, E. Beltran, and M. Mor-Mur. 2002. High pressure processing at subzero temperature: effect on spoilage microbiota of poultry. High Press. Res. 22:673-676. [Google Scholar]