Abstract
Background
Exercise influences drug craving and consumption in humans and drug self-administration in laboratory animals, but the effects can be variable. Improved understanding of how exercise affects drug intake or craving would enhance applications of exercise programs to human drug users attempting cessation.
Methods
Rats were trained in the intravenous self-administration (IVSA) of d-methamphetamine (METH; 0.05 mg/kg/inf), 3,4-methylenedioxymethamphetamine (MDMA; 0.5 mg/kg/inf) or methylone (0.5 mg/kg/inf). Once IVSA was established, the effect of ~22 hrs of wheel access in the home cage on subsequent drug taking was assessed in a two cohort crossover design.
Results
Provision of home cage wheel access during the day prior to IVSA sessions significantly decreased the self-administration of METH, MDMA and methylone. At the individual level, there was no correlation between the amount a rat used the wheel and the size of the individual’s decrease in drug intake.
Conclusions
Wheel access can reduce self-administration of a variety of psychomotor stimulants. It does so immediately, i.e., without a need for weeks of exercise prior to drug access. This study therefore indicates that future mechanistic investigations should focus on acute effects of exercise. In sum, the results predict that exercise programs can be used to decrease stimulant drug use in individuals even with no exercise history and an established drug taking pattern.
Keywords: bathsalts, self-administration, exercise, stimulant, reinforcement
1. INTRODUCTION
Physical exercise has been shown to produce transient and short-term protective effects against psychoactive drug use. For example, nicotine craving and smoking cue reactivity are decreased in human smokers immediately after workout sessions (Bock et al., 1999; Elibero et al., 2011; Taylor and Katomeri, 2007). The evidence for a sustained effect of exercise programs on smoking cessation is mixed (Ussher et al., 2008), potentially because of variability in the intensity and frequency of the exercise programs. Animal models can be used to better define the way in which exercise of different intensity, frequency, duration, and timing relative to drug access may suppress the urge to take that drug (or other alternatively available drugs).
An initial study found that 6 hrs of wheel access concurrent with cocaine access led to a decrease in cocaine intravenous self-administration (IVSA) in rats (Cosgrove et al., 2002). Miller and colleagues subsequently reported that introduction of concurrent wheel access during a 1 hr session of access to the IVSA of methamphetamine decreased drug intake if wheel access began at the start of IVSA training, but had no effect if it began 7 or 14 sessions after the start of training (Miller et al., 2012). In the Cosgrove study, animals were given fourteen 6-hour wheel access sessions prior to that start of IVSA (to stabilize wheel activity), but the rats in the Miller study had no significant wheel access prior to the start of IVSA training. Thus, prior experiences with either the wheel or drug IVSA appeared to interact with the effect of wheel access on IVSA in concurrent-access models. However, in a model that is perhaps more relevant to human cessation therapy, Smith and Witte (2012) showed that daylong access to a wheel for the ~22 hr outside of the cocaine IVSA session reduced cocaine intake. Moreover, in this study a prior history of wheel access (6 weeks of continual wheel access) appeared to play no additional role in changing drug intake (Smith and Witte, 2012).
This study was therefore designed to determine if the effect of ~22 hr of wheel access prior to the IVSA session on drug intake would generalize to three other psychostimulant drugs including methamphetamine, 3,4-methylenedioxymethamphetamine (MDMA, Ecstasy) and 3,4-methylenedioxymethcathinone (methylone). If the effects of wheel access extend across IVSA models which differ in specific methods than the inferences can be stronger. An additional goal was to merge design aspects of prior rat studies into a design with greater relevance to human cessation. To this end, the rats had no extensive exercise training prior to drug experience and IVSA was established in the absence of any concurrent opportunity for wheel exercise. In addition, a cross-over repeated-measures design was selected to better determine the stability and persistence of the effect of wheel access on drug intake.
2. MATERIALS AND METHODS
2.1 Subjects
Male (Wistar and Sprague Dawley, Charles River, New York) and female (Wistar, Charles River, New York) rats were used for these investigations. Animals were housed in a humidity and temperature-controlled (23±1 °C) vivarium on 12:12 hour light:dark cycles. Animals entered the laboratory at 11–12 weeks of age. Animals had ad libitum access to food and water in their home cages and all self-administration sessions were conducted in the dark cycle, under protocols approved by the Institutional Care and Use Committees of The Scripps Research Institute and consistent with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (Garber et al., 2011).
2.2 Drugs
Racemic 3,4-methylenedioxymethamphetamine (MDMA) HCl and D-methamphetamine HCl were provided by RTI International (Research Triangle Park, NC, USA), under contract to the National Institute on Drug Abuse. Racemic 3,4-methylenedioxymethcathinone (methylone) HCl was obtained from Cayman Chemical (Ann Arbor, MI, USA). All doses are expressed as the salt and were dissolved in physiological saline for injection.
2.3 Apparatus
For wheel-running conditions, activity wheels (Med Associates Model ENV-046; 35.6 cm diameter) were attached to the rats’ home cages in the vivarium. Each full wheel revolution was equivalent to 1.12 meters and each quarter-rotation of the wheel was recorded. Standard operant conditioning chambers (Med Associates, St. Albans, VT, USA; Model ENV-007) were used for self-administration sessions as described in (Aarde et al., 2013, 2014). Each chamber was enclosed in a sound-attenuating cubicle and equipped with 2 response levers, 2 stimulus lights, a pellet hopper, and a drug-infusion pump (Med Associates Model PHM-100-15). Responses to the left lever produced a single drug infusion (4 sec/infusion; 0.1 ml/infusion) and responses on the right lever were counted but produced no consequences. Equipment was controlled by MED-PC IV software.
2.4 Intravenous catheterization
Rats were anesthetized with an isoflurane/oxygen vapor mixture (isoflurane 5% induction, 1–3% maintenance) and prepared with chronic intravenous catheters as described previously (Aarde et al., 2013; Aarde et al., 2014; Miller et al., 2012). Briefly, the catheters consisted of an 14-cm length of polyurethane based tubing (Micro-Renathane®, Braintree Scientific, Inc, Braintree MA, USA) fitted to a guide cannula (Plastics One, Roanoke, VA) curved at an angle and encased in dental cement anchored to an ~3 cm circle of durable mesh. Catheter tubing was passed subcutaneously from the animal’s back to the right jugular vein. Catheter tubing was inserted into the vein and tied gently with suture thread. A liquid tissue adhesive was used to close the incisions (3M™ Vetbond™ Tissue Adhesive; 1469SB). A minimum of 4 days was allowed for surgical recovery prior to starting an experiment. For the first three days of the recovery period, an antibiotic (cephazolin) and an analgesic (flunixin) were administered daily. During testing and training, intravenous catheters were flushed with heparinized saline before sessions and heparinized saline containing cefazolan (100 mg/mL) after sessions. Catheter patency was assessed nearly once a week after the last session of the week via administration through the catheter of ~0.2 ml (10 mg/ml) of the ultra-short-acting barbiturate anesthetic Brevital sodium (1% methohexital sodium; Eli Lilly, Indianapolis, IN). Animals with patent catheters exhibit prominent signs of anesthesia (pronounced loss of muscle tone) within 3 sec after infusion. Animals that failed to display these signs were considered to have faulty catheters and were discontinued from the study. Data that was taken prior to failing this test and after the previous passing of this test were excluded from analysis.
2.5 Wheel Access Conditions
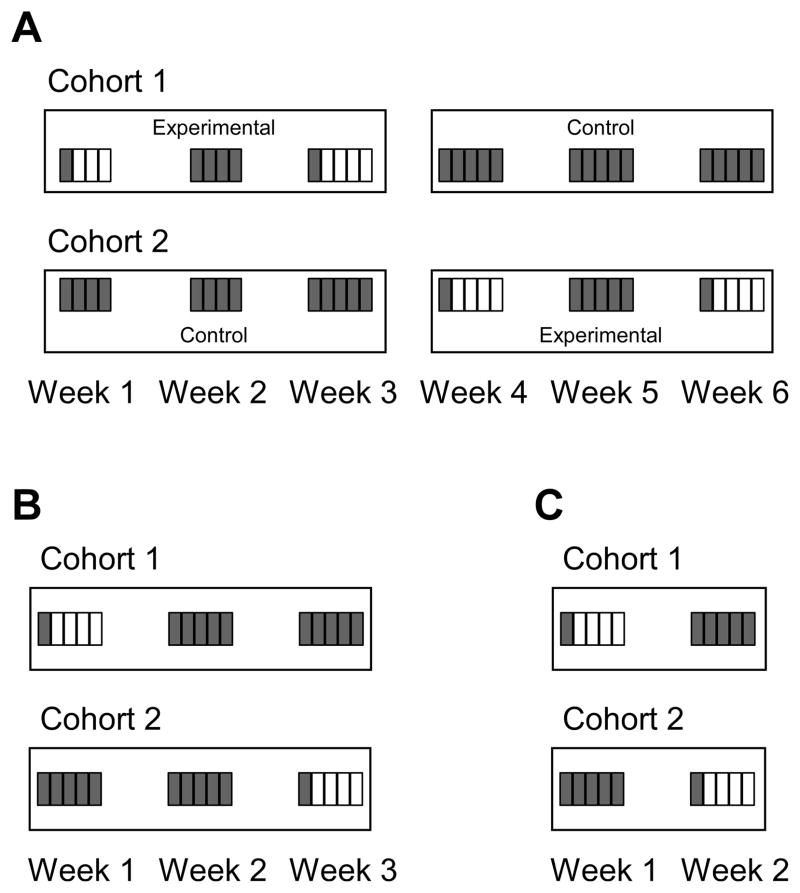
The experimental conditions were arranged by testing week (M-F). For a Wheel Access week, the rats were provided with access to an activity wheel in the vivarium starting upon return from the first IVSA session of that week. Thus, all subsequent sessions within that week were preceded by a day of wheel access. All individuals started operant sessions at a consistent time of day across their experimental conditions, thus, each individual had ~22 hr of wheel access prior to the IVSA session for each subsequent day within a week. Cohort-crossover designs were used in which wheel access was alternated week-to-week, but wheel access was never given to both cohorts on the same week (Figure 1).
Figure 1.
Schematic of the sequence of test sessions grouped by experimental week and differentiated to indicate whether wheel access was provided in the day prior to the session. The shaded rectangles indicate there was no prior wheel access and the open rectangles indicate there was wheel access prior to that session. A) Design for Methamphetamine Group 1. B) Design for Methamphetamine Group 2. C) Design for the MDMA Group and methylone males. The experimental week of Cohort 2 in A represents the treatment of the methylone female rats, except they had an additional control week after the second experimental week.
2.5.1 Experiment 1: Methamphetamine Group 1
Rationale
This experiment was conducted to determine if the introduction of a day of wheel access would alter methamphetamine self-administration on the following day. The goal was to determine if a prior effect with cocaine IVSA (Smith and Witte, 2012) generalized to methamphetamine and whether it could be observed within-subject.
IVSA training
A group of male Sprague Dawley rats (N=28; Charles River, New York; 12 weeks of age and ~350–400 g at the start of the study) were used in this experiment. Following surgical recovery animals were trained for 19 one hour sessions under a per-infusion dose of 0.1 mg/kg methamphetamine and a Fixed Ratio 1 reinforcer schedule. The following 7 IVSA acquisition training sessions were under a per-infusion dose of 0.05 mg/kg.
Wheel-access conditions
Rats were divided into two cohorts based on drug intake in the last 3 acquisition training sessions such that the means were similar for each cohort. A crossover design was used so as to allow comparisons to a no-wheel/no-intervention group with equivalent numbers of IVSA sessions and testing conditions (Figure 1A). Cohort 1 was subjected to an ABA pattern of weeks of wheel access (Wheel Access Week, No-Wheel Week, Wheel Access Week) while Cohort 2 received no wheel access interventions during those 3 weeks. This was followed by a second 3-week, ABA block of wheel access wherein the two cohorts’ wheel access conditions were swapped. The first two weeks of this study were limited to 4 sessions due to holidays, thus the analysis in the entire study focused on the first 4 days of each week, even if animals were run 5 days. Eight rats from Cohort 1 and eleven rats from Cohort 2 completed most of the study; one Cohort 2 rat did not remain patent for the final Wheel Access week thus only a single week is included for this individual.
2.5.2 Experiment 2: Methamphetamine Group 2
Rationale
A second methamphetamine experiment was conducted to control for a possible confound in the Group 1 methamphetamine study. In the Wheel-Access manipulations for Group 1, the animals were moved from their usual housing location to a different area of the (same) vivarium room that was used to hold the wheels, however, the no-wheel (control) sessions did not involve any similar change in cage and location within the room. We therefore conducted a study similar to the first experiment, however the animals were housed throughout in the same vivarium location, with cages always adjacent to the wheels. Thus, if the results from Group 1 were principally due to factors associated with housing location, rather than wheel activity, it would be expected that with this variable now controlled the general result would not be replicated. This study also used 2 h operant sessions and FR3 reinforcement contingency for more consistent drug intake and higher lever-discrimination ratios.
IVSA training
A group of male Sprague Dawley rats (N=27; Charles River, New York; 10 weeks of age and 300–350 g at the start of the study) participated in this experiment. Following surgical recovery animals were trained for 10 two-hour sessions under a fixed-ratio 1 schedule of reinforcement (FR1), 0.1 mg/kg per-infusion dose. The reinforcement schedule was then increased to FR2 (next two sessions) followed by FR3 (five sessions) to ensure high lever discrimination in this group. Additionally, the per-infusion dose was reduced to 0.05 mg/kg for the remainder of the study after the 1st FR3 session. Methamphetamine intake in the final three FR3 training sessions were used to balance the cohorts.
Wheel-access conditions
Wheel access within a Wheel-Access week was again manipulated as in the first methamphetamine group, using a shortened cohort crossover design (Figure 1B). For this experiment, a total of three weeks were included wherein the Wheel-Access intervention was given to Cohort 1 in the 1st week and Cohort 2 in the 3rd week; neither cohort had wheel access in the intervening 2nd week. Seven rats from Cohort 1 and six rats from Cohort 2 which completed the entire study with patent catheters were included.
2.5.3 Experiment 3: MDMA
Rationale
A third experiment was conducted to determine if the effects of the methamphetamine studies generalize to a structurally related stimulant, MDMA, which has proven less efficacious than what is observed for cocaine or methamphetamine in IVSA models (De La Garza et al., 2006; Schenk et al., 2012, 2007).
IVSA training
A group of male Wistar N=11 male rats were initial trained on MDMA (0.5 mg/kg/inf) IVSA for 14 two-hour sessions under FR1. These animals then participated in studies for which drug-type, dose and schedule of reinforcement were manipulated (MDMA, mephedrone and methylone at 0.125–2.5 mg/kg/inf under FR1 and at 0.125 and 1.0 mg/kg/inf under progressive-ratio), in procedures nearly identical to those recently described for female rats (Creehan et al., 2015). The rats were thereafter returned to the MDMA training dose under FR1 for one week before the Wheel-Access manipulations commenced.
Wheel-access conditions
Wheel access was again manipulated within a Wheel Access week as described for the first methamphetamine group, using a condensed cohort-crossover design (Figure 1C). The Wheel-Access intervention was given to Cohort 1 (N=6) in the 1st week and Cohort 2 (N=5) in the 2nd week; all 11 completed they study with patent catheters.
2.5.4 Experiment 4: Methylone
Rationale
The fourth experiment was conducted to determine if the effects of the MDMA studies generalize to Methylone, the cathinone parallel of MDMA. This compound was first reported to robustly support IVSA in male rats (Watterson et al., 2012) and subsequently reported similar to MDMA in female rats (Creehan et al., 2015).
IVSA training
A group of N=12 Wistar rats (5 F), trained on Methylone (0.5 mg/kg/inf) IVSA for 14 two-hour sessions under FR1, then in dose (0.125–2.5 mg/kg/inf) substitutions of MDMA, mephedrone and methylone under FR1 and finally two doses (0.125, 1.0 mg/kg/inf) of each of these three compounds in a PR procedure. Thereafter rats to the methylone training dose under FR1 and after a week the following experiment commenced. The prior studies in females have been reported elsewhere (Creehan et al., 2015) and there were no sex differences confirmed during initial acquisition, FR dose substitution or PR dose-substitution phases (unpublished observation). Thus for this study, the male and female animals were analyzed as one group.
Wheel-access conditions
Wheel access was again manipulated as in the Wheel-Access conditions described for the first methamphetamine group. The Wheel-Access intervention was given to the female rats twice in a single experimental cohort, with the No-Wheel weeks following each Wheel-Access week. The average of the two repetitions of each condition was used for analysis. The males were divided into two cohorts and evaluated on two sequential weeks. One male cohort (n=4) received the Wheel-Access week first and the No-Wheel second and the other cohort (N=3) completed the weeks in the opposite order. All 12 subjects completed all conditions with patent catheters.
2.6 Data Analysis
The analysis of the self-administration data included the number of infusions obtained per session and the discrimination ratio (drug associated lever responses/all lever responses) as dependent variables. Any cases of undefined discrimination ratio due to zero infusions being obtained were defined as 0 for the analysis. The IVSA data were analyzed with repeated-measures Analysis of Variance (rmANOVA) with Wheel Access condition and day of the week as within-subjects factors. Wheel quarter rotations were analyzed with one-factor (day of the week) rmANOVA. Any significant rmANOVA effects were followed with post-hoc analysis using Sidak’s correction for all possible comparisons. Correlational analysis was conducted using Fisher’s r-to-z test. The criterion for significant results was set at P < 0.05. Analyses were conducted using Prism 6 for Windows (v. 6.02; GraphPad Software, Inc, San Diego CA) and Statview (SAS Institute, Inc.). Graphs were generated with Excel (Microsoft, Redmond WA) and Statview and figures were created in Canvas (v.12; ACD Systems of America, Inc, Seattle, WA).
3. RESULTS
3.1 Experiment 1: Methamphetamine Group 1
Preliminary analysis of the first methamphetamine group found no significant differences in drug intake between the three baseline sessions used to divide the cohorts. Additional preliminary analyses on the un-pooled data within wheel-access condition did not confirm main effects of week number (i.e., replications) nor a week number by day of the week interaction. Thus, pooled data (collapsed across replications within the wheel-access conditions) were analyzed.
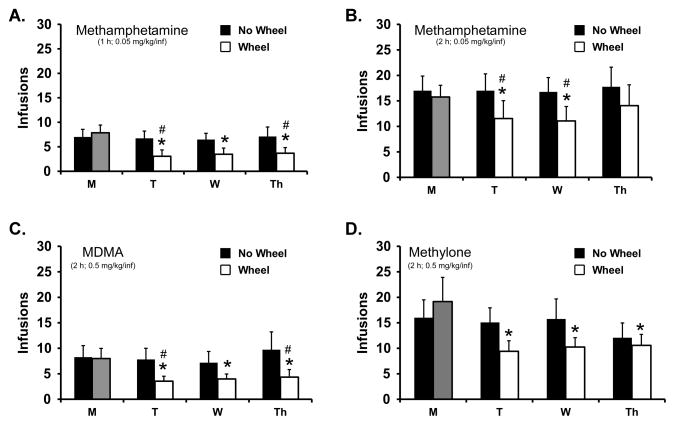
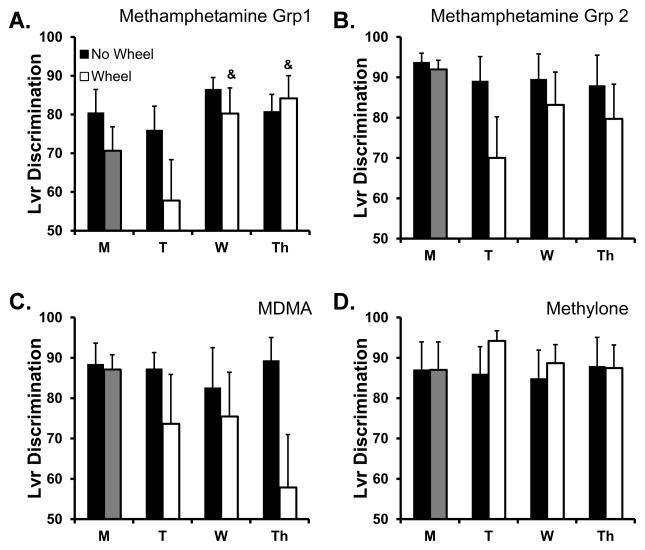
The number of infusions of methamphetamine self-administered was reduced by the availability of an activity wheel in the ~22 h prior to the session as is illustrated in Figure 2A. The statistical analysis confirmed a significant effect of wheel access condition [F (1, 17) = 8.00; P < 0.05], day of the week [F (3, 51) = 6.41; P < 0.001] and also of the interaction of factors [F (3, 51) = 10.00; P < 0.0001]. The post hoc test confirmed that the number of infusions was significantly lower on Tuesday (T), Wednesday (W) and Thursday (Th) of the Wheel-Access week compared with the first day of that week and also compared with the corresponding days on the No-Wheel week. There was a significant effect of both wheel-access condition [F (1, 17) = 4.91; P < 0.05] and day of the week [F (3, 51) = 3.25; P < 0.05] on discrimination ratio (Figure 3A) confirmed bythe rmANOVA. The post-hoc test did not confirm any differences in lever discrimination between any day of the wheel-access week and the corresponding day of the no-wheel week. However the post-hoc test did confirm that, within the wheel-access week, lever discrimination on T (58%; SEM 10.5%) was significantly lower than W (80.3%; SEM 6.6%) or Th (84.2%; SEM 5.9%).
Figure 2.
Mean (±SEM) infusions obtained four groups self-administering A) methamphetamine (1 hr; 0.05 mg/kg/inf; N=18), B) methamphetamine (2 hr; 0.05 mg/kg/inf; N=13), C) MDMA (2 hr; 0.5 mg/kg/inf; N=11) or D) Methylone (2 hr; 0.5 mg/kg/inf; N=12). Significant differences from the first session within a week are indicated by * and significant differences between Wheel-access and No-Wheel weeks by #.
Figure 3.
Mean (±SEM) lever discrimination ratios from four groups of rats self-administering A) methamphetamine (1 hr; 0.05 mg/kg/inf; N=18), B) methamphetamine (2 hr; 0.05 mg/kg/inf; N=13), C) MDMA (2 hr; 0.5 mg/kg/inf; N=11) or D) Methylone (2 hr; 0.5 mg/kg/inf; N=12). Significant differences from the second session within a week are indicated with &.
There were no confirmed differences in wheel activity between days of the week (2085–2293 quarter rotations, SEM 176–170, was the daily sum range) confirmed in the one-way rmANOVA.
3.2 Experiment 2: Methamphetamine Group 2
The number of infusions of methamphetamine self-administered by the Methamphetamine Group 2 rats was also reduced by the availability of an activity wheel in the ~22 h prior to the session as is illustrated in Figure 2B. The rmANOVA confirmed a significant effect of wheel access condition [F (1, 12) = 5.29; P < 0.05], but not of day of the week or of the interaction of these factors. The post hoc test further confirmed that the number of infusions was significantly lower on T and W of the Wheel-Access week compared with the corresponding days on the No-Wheel week.
There were no significant effects of wheel access week or day of the week on discrimination ratio (Figure 3B) confirmed by the analysis.
3.3 Experiment 3: MDMA
The number of infusions of MDMA that rats self-administered was reduced by the availability of an activity wheel in the ~22 h prior to the session as is illustrated in Figure 2C. The rmANOVA confirmed a significant interaction between the Day of the Week and Wheel-Access condition [F (3, 30) = 3.17; P < 0.05] but not of either factor alone. The posthoc test confirmed that significantly fewer infusions were obtained on T, W and Th compared with M of the Wheel-Access week. Similarly, significantly fewer infusions were obtained on T and Th of the wheel access week compared with the corresponding day of the No-Wheel week. The lever discrimination was poorest on the days following wheel access compared to no-wheel sessions (Figure 3C) but no significant differences in lever discrimination were confirmed in the ANOVA. Finally there were no differences in wheel activity confirmed across the three days (1262–1464 quarter rotations, SEM 195–289, was the daily sum range).
3.4 Experiment 4: Methylone
Self-administration of methylone was decreased by the opportunity for wheel activity in the day prior to the session, Figure 2D. The rmANOVA on drug intake confirmed a significant interaction between the Day of the Week and Wheel-Access condition [F (3, 33) = 3.08; P < 0.05], a significant effect of Day of the Week [F (3, 33) = 3.04; P < 0.05] but not of Wheel-Access alone. The post hoc test confirmed that within the Wheel-access week significantly fewer infusions were obtained on Tu, Wed and Th as compared to M, but there were no differences between days of the week in the No-Wheel week. The posthoc test did not confirm any significant differences in infusions between the wheel access conditions on any of the days of the week. There were no significant differences in lever discrimination confirmed in the analysis (Figure 3D). Finally, the wheel activity was significantly different across the three days [F (1.725, 18.97) = 6.96; P< 0.01] and the posthoc test confirmed that more wheel activity took place prior to the T session (3074 quarter rotations; SEM 686) compared with the W session (1875 quarter rotations; SEM 388).
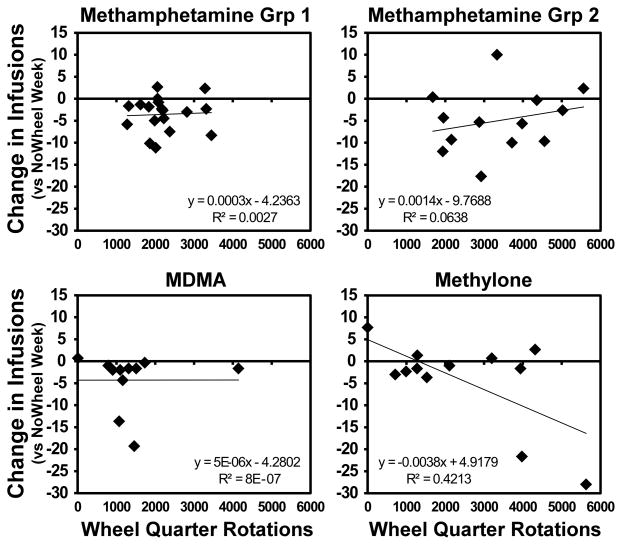
3.5 Correlation of Activity with IVSA Suppression
A correlational analysis between the average wheel activity and intake suppression scores (average intake on T-Th sessions of Wheel-Access weeks – average intake on the T-Th of NoWheel weeks) was performed to address the possibility that exertion on the wheel is the predictor of the suppression of drug intake; Figure 4. There were no significant relationships confirmed by Fisher’s r-to-z test for any of the experimental groups. It was confirmed that the distributions were normal (sphericity test) and there were no significant outliers (> 3 SD from mean). The methylone data was split by sex because the number of wheel rotations and the size of the intake suppression were greater in females (significant interaction of Sex by Wheel Access condition by Day of the Week [F (3, 30) = 3.93; P< 0.01] on intake and significant main effect of Sex [F (1, 10) = 10.49; P< 0.01] and the interaction of Sex with Day of the Week [F (2, 20) = 13.90; P< 0.0005] on wheel rotations); thus, potentially creating a spurious correlation if subjects were treated as a single group. The methylone data was therefore Z-scored to put the females and males on the same scale for analysis, but this correction still did not yield a significant correlation.
Figure 4.
Correlation of wheel quarter-rotations with changes in average drug infusions on the Wheel-Access sessions compared with the corresponding sessions of NoWheel weeks.
4. DISCUSSION
The major finding of this study was that a single day of wheel access immediately prior to the self-administration session was sufficient to reduce the intravenous self-administration (IVSA) of methamphetamine, 3,4-methylenedioxymethamphetamine (MDMA) or methylone in rats. Human epidemiological studies illustrate the potential for physical exercise to prevent or blunt the use of recreational drugs; however, the effects depend on many factors including social context, intensity and type of exercise (Taliaferro et al., 2010; Terry-McElrath and O’Malley, 2011; Terry-McElrath et al., 2011). As one real-world example, it is difficult to dissociate the effects of the social context of sports teams and social/personality factors controlling the intensity of aerobic exercise from the specific effects of the exercise itself in human studies. Therefore, it is possible that the epidemiological link is a consequence of personal or social variables which determine both drug use and sports participation in humans and has little to do, directly, with the exercise itself. The present findings, obtained using a controlled animal model, are therefore critical to confirm a specific link to demonstrate that exercise can attenuate repeated stimulant drug use, and to provide a means to determine potential neurophysiological mechanisms through which exercise effects are mediated.
A particular strength of the results is that the four overnight wheel-access experiments differed in methodological detail but produced the same qualitative outcome. Furthermore there was a consistently suppressive effect of wheel access on drug intake when the experiment was conducted in groups of animals that only experienced hours of wheel access following the establishment of stimulant IVSA. This effect was immediate (observed on the following day) and was not present in the same group of animals when wheel access was not provided in the day prior to the session. These data provide further evidence that the effect of wheel activity reported by Smith and colleagues, in a preparation that involved both 6 weeks of access prior to cocaine IVSA as well as ongoing wheel access prior to each IVSA session (Smith and Pitts, 2011; Smith et al., 2011; Smith and Witte, 2012), did not depend on the continual wheel access for weeks prior to the start of IVSA training. An especially important aspect of this finding was that unlimited home cage access to a wheel was capable of greatly reducing established stimulant IVSA. Cosgrove and colleagues (2002) showed that 6 hours of concurrent wheel access could reduce established cocaine IVSA but our prior study (Miller et al., 2012) found that a single hour of concurrent access to an activity wheel was insufficient to alter established methamphetamine self-administration once it had been established.
The present finding extends one recent report which found that wheel activity in the day prior to a cocaine IVSA session decreased the levels of drug intake in a manner that was independent of whether rats were provided wheel access in the 6 weeks prior to the initiation of IVSA training (Smith and Witte, 2012). The present study takes it a critical step farther by showing that wheel-related changes in drug taking occur within-subject, i.e., the effect is reversible and can be with wheel access after stable IVSA is already established. This finding also confirms that such effects are not confined to cocaine IVSA and indeed will likely generalize across many stimulant drugs of abuse. Interestingly, one prior study found, using a small group of rats (N=3), that responding for food under a Progressive Ratio schedule of reinforcement was reduced by 19 h of prior wheel access (Pierce et al., 1986) so this effect may extend to behavior reinforced by many classes of stimuli. Together, these results have important implications for therapeutic translation and support broad recommendations for the use of exercise programs in stimulant cessation attempts. Most encouragingly, these current data imply that a beneficial or therapeutic effect can be obtained in people who have no prior history of exercising. Furthermore, the present results found that there was no individual correlation between the distance traveled on the wheel and subsequent suppression of drug intake. This is similar to a finding of no correlation in a study where wheel access preceded the initiation of cocaine access for six weeks (Smith and Pitts, 2011). Likewise, our prior report found that similar reductions in methamphetamine IVSA were produced by concurrent wheel access during the operant session (from the start of training) in strains of rats that differed about six fold in wheel activity. The most parsimonious explanation is that it is the individually-determined rewarding properties of exercise (Belke, 1997, 2000), rather than the magnitude or duration of physical exertion, which is the critical variable as discussed at length (Miller et al., 2012).
Limitations of the present study include the recognition that only a single wheel-access condition (one day long session of wheel access prior to the IVSA session) was used. Thus, further experimentation will be required to determine if there is an optimal temporal placement and/or duration of acute bouts of exercise relative to drug availability for the reduction of intravenous self-administration; it may be the case that such parameters depend on the drug being used. This study examined a fixed wheel intervention across four different stimulant self-administration models to enhance the ability to generalize but this precludes making precise quantitative inferences. Quantifying the differential impact of wheel access across drugs, as one example, would require a study using more constrained experimental procedures. It must also be considered that exercise may only be effective against a subset of drug use patterns, in terms of amount ingested and frequency of drug use. Future studies may illuminate the necessary exercise conditions and, for example, help to determine if metabolism or distribution of various drugs of abuse is altered sufficiently to explain the outcome. Ultimately, such findings will help to optimize therapeutic approaches using exercise and might also help to explain the heretofore mixed evidence from human smoking cessation trials (Ussher et al., 2008), which may have resulted from suboptimal exercise protocols in some trials.
The effect of wheel activity in the present study and the prior work with cocaine self-administration is proposed to be specific to locomotor activity, but a plausible alternative interpretation is general environmental enrichment. Environmental enrichment (typically via the provision of novel objects in the home cage) has been shown to decrease subsequent self-administration of methylphenidate (Alvers et al., 2012) and cocaine (Puhl et al., 2012) in rats. Environmental enrichment also decreases cocaine place-preference conditioning in mice (Nader et al., 2012) but methamphetamine place conditioning in mice is not so influenced (Thiriet et al., 2011). One unknown factor, however, is the degree to which various kinds of environmental enrichment enhance rats’ spontaneous locomotor behavior which might be the specific factor necessary to affect subsequent drug preference.
In terms of plausible neurological mechanisms, this study suggests that there are transient, short-term effects of exercise on mechanisms that influence voluntary stimulant drug intake. This effect in a rat model is similar to epidemiological data that show transient and short-term protective effects after exercise, for example decreased nicotine craving or smoking cue reactivity immediately after workout sessions (Bock et al., 1999; Elibero et al., 2011; Taylor and Katomeri, 2007). Consequently, the mechanism cannot involve persisting effects on, e.g., neurodegeneration or neurogenesis, as has been suggested in studies in which rats (Engelmann et al., 2014; Sobieraj et al., 2014) or mice (Mustroph et al., 2015) exercise for several weeks prior to experiencing drug access. One potential mechanism of the effect of exercise may lie with endogenous opioid signaling. For example Chen and colleagues found that 25 min of treadmill running (35 m/min) increased leucine-enkephalin (L-ENK) levels in caudate-putamen neurons in rat brains up to 180 min post-exercise (Chen et al., 2007); dynorphin expression in the paraventricular nucleus was also elevated by acute exercise 30 min after, but there was evidence that chronic activity might have upregulated dynorphin.
In summary, these data provide clear evidence from an animal model that access to wheels in the home cage for the entire day prior to the self-administration session greatly reduced the intake of methylone, MDMA or methamphetamine. Therefore the epidemiological evidence of reduced substance use in adolescents who engage in sports (Terry-McElrath and O’Malley, 2011; Terry-McElrath et al., 2011) is at least partially attributable to the exercise itself and not exclusively due to the social context of teams or the self-selection into athletics. These data from a rodent model further support the use of exercise in human stimulant drug user cessation attempts.
Abuse of psychomotor stimulants is a public health challenge
One day of wheel access inhibits established METH self-administration
Methylone and MDMA self-administration is also reduced by wheel access
Exercise can reduce drug use even absent a prior exercise history
Acknowledgments
Role of Funding Source. The study was conducted under the support of USPHS grants DA024105, DA024705 and DA035281 (MAT). The NIH/NIDA had no role in study design, collection, analysis and interpretation of data, in the writing of the report, or in the decision to submit the paper for publication.
The authors are grateful for the work of Brittani D. Vaillancourt (support T34 GM087193) who participated in related wheel-access studies critical for advancing our designs but not directly included here. This is publication #22050 from The Scripps Research Institute.
Footnotes
Contributors MAT designed the study with significant input from MLM and SMA. KMC, SMA, SAV and MLM collected and organized the data, and completed initial data analyses. MLM and SMA conducted literature searches and provided summaries of previous related work. MAT and SMA undertook the statistical analysis and MAT, SMA and MLM created figures and drafted the manuscript. All authors contributed to and have approved the final manuscript.
Conflict of Interest. The authors have no conflicts of interest to report for this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aarde SM, Angrish D, Barlow DJ, Wright MJ, Jr, Vandewater SA, Creehan KM, Houseknecht KL, Dickerson TJ, Taffe MA. Mephedrone (4-methylmethcathinone) supports intravenous self-administration in Sprague-Dawley and Wistar rats. Addict Biol. 2013;18:786–799. doi: 10.1111/adb.12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aarde SM, Huang PK, Dickerson TJ, Taffe MA. Binge-like acquisition of 3,4-methylenedioxypyrovalerone (MDPV) self-administration and wheel activity in rats. Psychopharmacology (Berl) 2014 doi: 10.1007/s00213-014-3819-4. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvers KM, Marusich JA, Gipson CD, Beckmann JS, Bardo MT. Environmental enrichment during development decreases intravenous self-administration of methylphenidate at low unit doses in rats. Behav Pharmacol. 2012;23:650–657. doi: 10.1097/FBP.0b013e3283584765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belke TW. Running and responding reinforced by the opportunity to run: effect of reinforcer duration. J Exp Anal Behav. 1997;67:337–351. doi: 10.1901/jeab.1997.67-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belke TW. Studies of wheel-running reinforcement: parameters of Herrnstein’s (1970) response-strength equation vary with schedule order. J Exp Anal Behav. 2000;73:319–331. doi: 10.1901/jeab.2000.73-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock BC, Marcus BH, King TK, Borrelli B, Roberts MR. Exercise effects on withdrawal and mood among women attempting smoking cessation. Addict Behav. 1999;24:399–410. doi: 10.1016/s0306-4603(98)00088-4. [DOI] [PubMed] [Google Scholar]
- Chen JX, Zhao X, Yue GX, Wang ZF. Influence of acute and chronic treadmill exercise on rat plasma lactate and brain NPY, L-ENK, DYN A1-13. Cell Mol Neurobiol. 2007;27:1–10. doi: 10.1007/s10571-006-9110-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove KP, Hunter RG, Carroll ME. Wheel-running attenuates intravenous cocaine self-administration in rats: sex differences. Pharmacol Biochem Behav. 2002;73:663–671. doi: 10.1016/s0091-3057(02)00853-5. [DOI] [PubMed] [Google Scholar]
- Creehan KM, Vandewater SA, Taffe MA. Intravenous self-administration of mephedrone, methylone and MDMA in female rats. Neuropharmacology. 2015;92:90–7. doi: 10.1016/j.neuropharm.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De La Garza R, 2nd, Fabrizio KR, Gupta A. Relevance of rodent models of intravenous MDMA self-administration to human MDMA consumption patterns. Psychopharmacology (Berl) 2006;189:425–434. doi: 10.1007/s00213-005-0255-5. [DOI] [PubMed] [Google Scholar]
- Elibero A, Janse Van Rensburg K, Drobes DJ. Acute effects of aerobic exercise and Hatha yoga on craving to smoke. Nicotine Tob Res. 2011;13:1140–1148. doi: 10.1093/ntr/ntr163. [DOI] [PubMed] [Google Scholar]
- Engelmann AJ, Aparicio MB, Kim A, Sobieraj JC, Yuan CJ, Grant Y, Mandyam CD. Chronic wheel running reduces maladaptive patterns of methamphetamine intake: regulation by attenuation of methamphetamine-induced neuronal nitric oxide synthase. Brain Struct Funct. 2014;219:657–672. doi: 10.1007/s00429-013-0525-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber JC, Barbee RW, Bielitzki JT, Clayton LA, Donovan JC, Hendriksen CFM, Kohn DF, Lipman NS, Locke PA, Melcher J, Quimby FW, Turner PV, Wood GA, Wurbel H. Guide for the Care and Use of Laboratory Animals. 8. National Academies Press; Washington D.C: 2011. [Google Scholar]
- Miller ML, Vaillancourt BD, Wright MJ, Jr, Aarde SM, Vandewater SA, Creehan KM, Taffe MA. Reciprocal inhibitory effects of intravenous d-methamphetamine self-administration and wheel activity in rats. Drug Alcohol Depend. 2012;121:90–96. doi: 10.1016/j.drugalcdep.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustroph ML, Merritt JR, Holloway AL, Pinardo H, Miller DS, Kilby CN, Bucko P, Wyer A, Rhodes JS. Increased adult hippocampal neurogenesis is not necessary for wheel running to abolish conditioned place preference for cocaine in mice. Eur J Neurosci. 2015;41:216–226. doi: 10.1111/ejn.12782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader J, Claudia C, Rawas RE, Favot L, Jaber M, Thiriet N, Solinas M. Loss of environmental enrichment increases vulnerability to cocaine addiction. Neuropsychopharmacology. 2012;37:1579–1587. doi: 10.1038/npp.2012.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce WD, Epling WF, Boer DP. Deprivation and satiation: the interrelations between food and wheel running. J Exp Anal Behav. 1986;46:199–210. doi: 10.1901/jeab.1986.46-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puhl MD, Blum JS, Acosta-Torres S, Grigson PS. Environmental enrichment protects against the acquisition of cocaine self-administration in adult male rats, but does not eliminate avoidance of a drug-associated saccharin cue. Behav Pharmacol. 2012;23:43–53. doi: 10.1097/FBP.0b013e32834eb060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk S, Colussi-Mas J, Do J, Bird J. Profile of MDMA self-administration from a large cohort of rats: MDMA develops a profile of dependence with extended testing. J Drug Alcohol Res. 2012;1:1–6. [Google Scholar]
- Schenk S, Hely L, Lake B, Daniela E, Gittings D, Mash DC. MDMA self-administration in rats: acquisition, progressive ratio responding and serotonin transporter binding. Eur J Neurosci. 2007;26:3229–3236. doi: 10.1111/j.1460-9568.2007.05932.x. [DOI] [PubMed] [Google Scholar]
- Smith MA, Pitts EG. Access to a running wheel inhibits the acquisition of cocaine self-administration. Pharmacol Biochem Behav. 2011;100:237–243. doi: 10.1016/j.pbb.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Walker KL, Cole KT, Lang KC. The effects of aerobic exercise on cocaine self-administration in male and female rats. Psychopharmacology. 2011;218:357–369. doi: 10.1007/s00213-011-2321-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Witte MA. The effects of exercise on cocaine self-administration, food-maintained responding, and locomotor activity in female rats: importance of the temporal relationship between physical activity and initial drug exposure. Exp Clin Psychopharmacol. 2012;20:437–446. doi: 10.1037/a0029724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobieraj JC, Kim A, Fannon MJ, Mandyam CD. Chronic wheel running-induced reduction of extinction and reinstatement of methamphetamine seeking in methamphetamine dependent rats is associated with reduced number of periaqueductal gray dopamine neurons. Brain struct Funct. 2014 doi: 10.1007/s00429-014-0905-7. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taliaferro LA, Rienzo BA, Donovan KA. Relationships between youth sport participation and selected health risk behaviors from 1999 to 2007. J School Health. 2010;80:399–410. doi: 10.1111/j.1746-1561.2010.00520.x. [DOI] [PubMed] [Google Scholar]
- Taylor A, Katomeri M. Walking reduces cue-elicited cigarette cravings and withdrawal symptoms, and delays ad libitum smoking. Nicotine Tob Res. 2007;9:1183–1190. doi: 10.1080/14622200701648896. [DOI] [PubMed] [Google Scholar]
- Terry-McElrath YM, O’Malley PM. Substance use and exercise participation among young adults: parallel trajectories in a national cohort-sequential study. Addiction. 2011;106:1855–1865. doi: 10.1111/j.1360-0443.2011.03489.x. discussion 1866-1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry-McElrath YM, O’Malley PM, Johnston LD. Exercise and substance use among American youth, 1991–2009. Am J Prev Med. 2011;40:530–540. doi: 10.1016/j.amepre.2010.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiriet N, Gennequin B, Lardeux V, Chauvet C, Decressac M, Janet T, Jaber M, Solinas M. Environmental enrichment does not reduce the rewarding and neurotoxic effects of methamphetamine. Neurotox Res. 2011;19:172–182. doi: 10.1007/s12640-010-9158-2. [DOI] [PubMed] [Google Scholar]
- Ussher MH, Taylor A, Faulkner G. Exercise interventions for smoking cessation. Cochrane Database Syst Rev. 2008:CD002295. doi: 10.1002/14651858.CD002295.pub4. [DOI] [PubMed] [Google Scholar]
- Watterson LR, Hood L, Sewalia K, Tomek SE, Yahn S, Johnson CT, Wegner S, Blough BE, Marusich JA, Olive MF. The reinforcing and rewarding effects of methylone, a synthetic cathinone commonly found in “bath salts”. J Addict Res Ther. 2012;S9:1–8. doi: 10.4172/2155-6105.S9-002. [DOI] [PMC free article] [PubMed] [Google Scholar]