Abstract
Communication between pairs of neurones in the central nervous system typically involves classical ‘hard-wired’ synaptic transmission, characterised by high temporal and spatial precision. Over the last two decades, however, knowledge regarding the repertoire of communication modalities used in the brain has notably expanded to include less conventional forms, characterised by a diffuse and less temporally precise transfer of information. These forms are best suited to mediate communication among entire neuronal populations, now recognised to be a fundamental process in the brain for the generation of complex behaviours. In response to an osmotic stressor, the hypothalamic paraventricular nucleus (PVN) generates a multimodal homeostatic response that involves orchestrated neuroendocrine (i.e. systemic release of vasopressin) and autonomic (i.e. sympathetic outflow to the kidneys) components. The precise mechanisms that underlie interpopulation cross-talk between these two distinct neuronal populations, however, remain largely unknown. The present review summarises and discusses a series of recent studies that have identified the dendritic release of neuropeptides as a novel interpopulation signalling modality in the PVN. A current working model is described in which it is proposed that the activity-dependent dendritic release of vasopressin from neurosecretory neurones in the PVN acts in a diffusible manner to increase the activity of distant presympathetic neurones, resulting in an integrated sympathoexcitatory population response, particularly within the context of a hyperosmotic challenge. The cellular mechanism underlying this novel form of intercellular communication, as well as its physiological and pathophysiological implications, is discussed.
Keywords: vasopressin, sympathetic, neuroendocrine, osmotic, hypothalamus
Introduction
Information processing and intercellular communication in the brain are fundamental and highly complex processes that involves a large variety of signalling modalities. This vast array of communication types may be classified, at least in part, according to distinct spatio-temporal features. At one end of the spatio-temporal spectrum is the classical and well-characterised ‘chemical synaptic transmission’. Chemical synapses are structurally organised units with a well-defined physical substrate, and are devised to efficiently transfer information between pairs of neurones in a precise, spatially constrained and temporally fast manner. The most widely used chemical transmitter in central synapses are amino acids, including the excitatory glutamate and inhibitory GABA transmitters. By acting largely (but not uniquely) on postsynaptic ionotrophic receptors, and because they are rapidly removed from the synaptic cleft after their release, amino acid transmitters mediate transient and spatially precise changes in membrane conductance in the postsynaptic neurone. This fast and accurate interneuronal communication modality is critically important, for example, for coherent spike timing between pairs of neurones (1).
The strength and time course of this ‘hard-wired’ interneuronal communication is dependent upon a number of well-characterised factors, including the probability of presynaptic transmitter release, the affinity of the postsynaptic receptors to the transmitter, the density of postsynaptic receptors clustered at highly specialised sites, and the rate of diffusion/uptake of the neurotransmitter at/ from the synaptic cleft (2–5).
As we move along the spatio-temporal spectrum of signalling modalities, we encounter communication modalities that, rather than mediating the precise transfer of information between pairs of neurones, act on multiple targeted neurones. This communication modality, which could be termed ‘intrapopulation’ signalling, is important, for example, for coordinating the activity of multiple or even an entire population of neurones, under particular conditions. An example of a signalling mechanism acting at this level includes gas neurotransmitters such as nitric oxide and carbon monoxide (7). A prototype of a synchronised population activity is the oxytocin (OT) magnocellular neurosecretory system. During parturition and lactation, the firing discharge of OT neurones located in the hypothalamic supraoptic (SON) and paraventricular (PVN) nuclei becomes periodic and highly synchronised among the entire population of OT neurones in the four nuclei, resulting in a massive bolus release of OT into the systemic circulation (8,9). This phenomenon has been studied for many years in different laboratories (10–14), and dendritically-released OT from OT neurones themselves has been shown to be a critical autoregulatory mechanism facilitating synchronised bursting activity and its onset (15–18). Still, how the activity of OT neurones is synchronised among the four nuclei remains to be fully understood.
Finally, at the other end of the spatio-temporal spectrum, we encounter signalling modalities that mediate transfer of information between entire populations of neurones. This type of ‘interpopulation’ communication is now acknowledged to be important for the generation of complex behaviours by the brain, which require the coordinated activity of multiple neuronal populations (19,20).
Compared to fast-acting neurotransmitters at ‘hard-wired’ chemical synapses, an efficient signalling modality transferring information across entire populations of neurones, which in some cases may be located relatively distant one from each other, would need to act in a more diffuse, less spatially-constrained manner (i.e. to reach multiple and far-distant targets) and, consequently, at a slower rate as well. This type of paracrine, slow-acting signalling modality has been described previously in the literature and is known as ‘volume transmission’ (21). Volume transmission implies the diffusion of a signal in the extracellular and/or cerebrospinal fluids, thus lacking temporal and spatial precision. Compared to hard-wired signals, a volume-transmission signal acts within a poorly defined physical substrate, has a slow temporal encoding and long transmission delay, and is also characterised by a high degree of divergence (21,22). In the hard-wired chemical synapse, the specificity and ‘privacy’ of the communication is largely determined by the spatially constrained structure of the synapse. Conversely, in volume transmission, specificity is solely determined by the specificity of the signal/receptor interaction (i.e. the density and location of specific receptors for the signal in question). Finally, another contrasting feature between these two major communication modalities is that, although the concentration of classical transmitters at chemical synapses is high (micromolar range) and they bind to low-affinity (high nanomolar to micromolar) postsynaptic receptors, volume transmission signals are typically found at low concentrations (nanomolar range) and bind to receptors that have a relatively high binding affinity (picomolar to nanomolar range).
Compared to the extensive literature available on conventional, fast synaptic transmission, much less is known about the identity and precise underlying mechanisms of volume-transmitted signals in the brain. Nonetheless, a growing body of evidence suggests that unconventional transmitters, including gaseous molecules, gliotransmitters and neuropeptides, possess unique properties that make them ideal candidates to fulfill this role. The present review provides a summary of recent advances in the neuroendocrine field, highlighting the novel role of neuropeptides as molecules that mediate interpopulation cross-talk in the hypothalamus.
The PVN of the hypothalamus: an ideal model system for studying interpopulation communication signalling
The hypothalamic PVN is a pivotal centre within the central neuronal circuitry involved in the maintenance of bodily homeostasis, playing a major role in the generation of homeostatic neurohumoral responses (23). This complex function is achieved by a rich bidirectional interconnectivity with multiple brain centres, including both visceroceptive afferent inputs, as well as autonomic, endocrine and neuroendocrine motor outputs (24). Based on this multifaceted organisation, the PVN is able to continuously sense and integrate information regarding the status of the body’s internal environment. Moreover, during challenges or conditions that deviate physiological variables from their respective set-points, visceroceptive inputs arising mostly from the brainstem elicit complex patterns of neurosecretory and autonomic motor output patterns from the PVN, which, acting on peripheral tissues, help with the re-establishment of bodily homeostasis.
Numerous distinctive anatomical and physiological features make the PVN an ideal centre model for studying the role of neuropeptides as signalling molecules in mediating interpopulation communication in the brain. First, despite being a relatively small nucleus, the PVN houses many sets of functionally distinct neurones, which have been historically classified into two major groups, namely ‘magnocellular’ and ‘parvocellular’ neurones (25,26). Magnocellular neurosecretory neurones (also found in the neighbouring SON) emit axons that terminate in the posterior pituitary, from where they release the neurohormones OT and vasopressin (VP) into the systemic circulation. These neurones have been shown to play critical roles in reproductive homeostasis, blood pressure regulation and fluid/electrolyte balance. Parvocellular neurosecretory neurones send their axons to the median eminence, from where they release hypophysiotrophic hormones that control the function of the anterior pituitary and the major hypothalamic-pituitary axes. These include corticotrophin-releasing hormone (CRH) neurones, thyrotophin-releasing hormone neurones and somatostatin neurones. Finally, parvocellular preautonomic neurones send long descending projections to sympathetic and parasympathetic centres in the brainstem and spinal cord, including the nucleus of the solitary tract, the rostral ventrolateral medulla (RVLM), the dorsal motor nucleus of the vagus and preganglionic sympathetic neurones in the interme-diolateral cell column of the spinal cord (26). These neurones have been shown to modulate sympathetic and parasympathetic outflows to a variety of target organs, including the heart, the peripheral vasculature and the kidneys, in turn playing major roles in cardiovascular and fluid/electrolyte homeostasis, amongst other functions (27–29). In addition to these neurosecretory and autonomic targets, the PVN also sends projections to hierarchically higher centres in the brain, including the central amygdala, projections recently shown to modulate fear-conditioned responses (30) (Fig. 1).
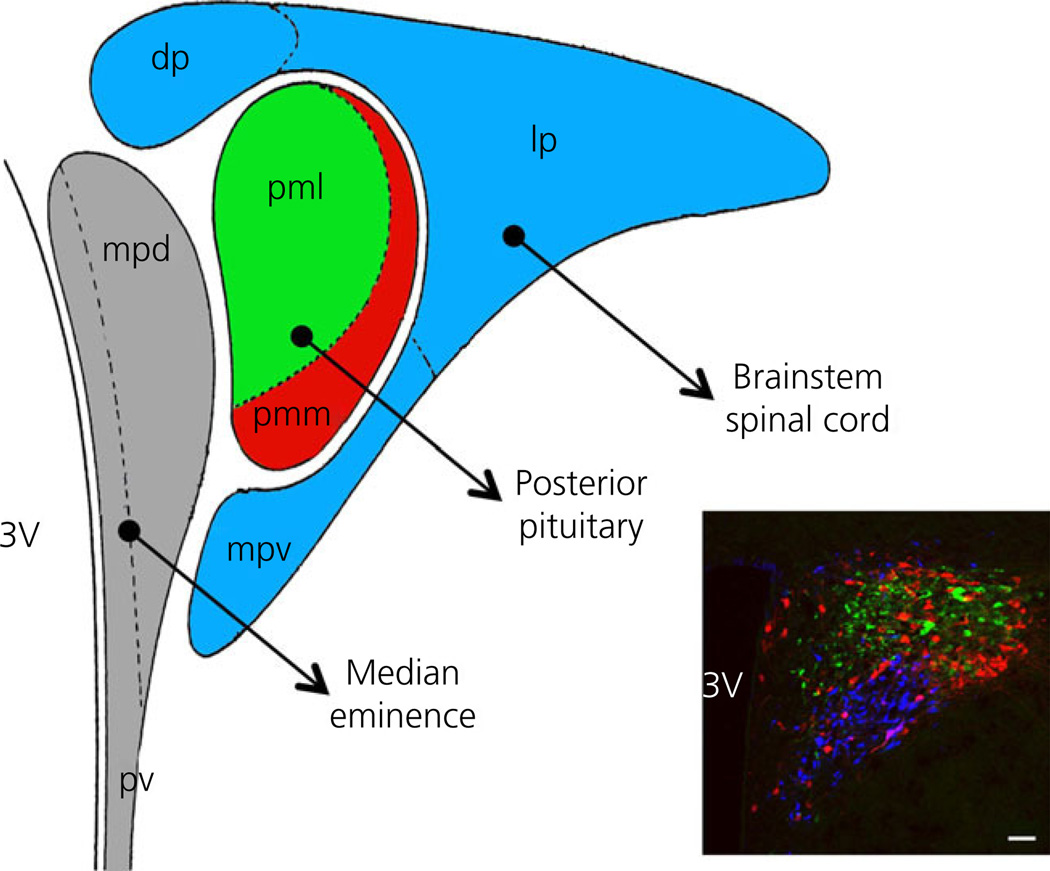
Fig. 1.
Compartmentalised distribution of functionally distinct neurona populations within the hypothalamic paraventricular nucleus (PVN). Graph of the PVN showing its distinct anatomical subdivisions containing neurones that project to the median eminence (grey colour; mpd, medial parvocellular subdivision dorsal; pv, periventricular subnucleus), posterior pituitary [red and green colours; containing primarily oxytocin (OT) and vasopressin (VP) neurones, respectively; pml, posterior magnocellular lateral; pmm, posterior magnocellular medial] and brainstem/spinal cord (light blue colour; dp, dorsal parvocellular subnucleus; lp, lateral parvocellular subnucleus; mpv, media parvocellular ventral subnucleus). Modified from Hatton (70). The inset shows a coronal section of the PVN (bregma = −1.80) showing immunoreactive OT (red), VP (green) and presympathetic retrogradely labelled neurones that innervate the rostral ventrolateral medulla in the brainstem. Scale bar = 100 µm. 3V, third ventricle.
Finally, and perhaps the most significant feature of the PVN in the context of the present review, is that these functionally distinct neuronal populations act in a concerted manner in response to physiological challenges that require the generation of multimodal homeostatic responses. One example that highlights the complex and integrative nature of PVN homeostatic responses occurs during a challenge to the fluid/electrolyte balance. An increase in plasma osmolarity or a loss of blood volume leads to the activation of both magnocellular neurosecretory and presympathetic PVN neurones, resulting in the concurrent systemic release of OT and VP, along with an increase in sympathetic outflow to the kidneys. These two complementary PVN outputs act in concert at the level of the kidneys to properly modulate water and Na+ reabsorption/excretion, restoring fluid and electrolyte homeostasis (31). Moreover, disturbances of fluid homeostasis also result in the activation of CRH neurones with the concomitant engagement of the hypothalamic-pituitary axis (32–34), thus contributing to a multimodal homeostatic response to this type of physiological stressors. Although the general neuroanatomical, neurochemical and physiological features of these neurosecretory and sympathetic responses evoked during an osmotic/fluid challenge are well characterised (31,35,36), knowledge about how the activities of these distinct neuronal populations are coordinated, and whether this coordination results from local integrative processes within the PVN, is largely absent.
Does a compartmentalised anatomical organisation also imply functional compartmentalisation?
Largely based on early neuroanatomical studies performed during the 1980s, the notion that multimodal homeostatic responses coordinated by the PVN involved parallel processing of neurosecretory and autonomic functions has thus far prevailed. These studies have clearly demonstrated a definite compartmentalisation of the distinct PVN neuronal population within discrete anatomical subnuclei within the rat PVN. For example, magnocellular neurosecretory VP neurones are concentrated in the ball-shaped lateral magnocellular subnucleus, whereas magnocellular OT neurones are located predominantly in the medial magnocellular subnucleus. Conversely, presympathetic PVN neurones are predominantly distributed in three distinct subnuclei that include the dorsal cap, the parvocellular posterior and the parvocellular ventromedial subnuclei, whereas CRH neurones are located in the medial parvocellular subnucleus. Precise details about the anatomical distribution of all the distinct PVN neuronal subpopulations are provided elsewhere (25,26,37). This compartmentalised anatomical organisation, together with (i) the fact that PVN neurones innervate distinct targets; (ii) a lack of evidence for axon collaterals within or near the PVN (38,39); and (iii) evidence indicating that afferent inputs into the PVN (e.g. visceroceptive catecholaminergic) are also anatomically segregated (40), supports a minimal ‘hard-wired’ interaction between neurosecretory and preautonomic neuronal populations within the PVN. This idea, however, appears to be counterintuitive given the highly integrative physiological nature of the PVN, along with the close proximity of these distinct neuronal populations within the same nucleus. Noteworthy, in 1982, Pittman et al. (41) suggested that the close anatomical association between the different neuropeptidergic systems raised the possibility of their coordinated activation, which if present, would not be mediated via local ‘hard-wired’ mechanisms. This intriguing idea, however, has never been experimentally tested thus far. As summarised below, recent evidence from our laboratory, along with previous studies conducted in different laboratories, now supports the notion that the dendritic release of neuropeptides serves the role of a ‘wireless’ interpopulation signal participating in the coordination of functionally distinct PVN neuronal populations, as well as in the generation of multi-modal neurohumoral homoestatic responses.
Dendrites are not only receptors, but also sources of signals in the PVN
Dendrites have been classically considered the receptive assemblies in neurones, in which incoming signals from other neurones (inhibitory and excitatory synaptic potentials) are passively integrated and propagated down to the soma and axonal hillock, to evoke and/or modulate the firing output of the receptive neurone. However, the discovery of active conductances throughout the extension of dendritic processes (42–44), as well as the ability of action potentials to propagate in the reverse direction (i.e. from soma to dendrites: back-propagating action potentials) (45), now supports dendrites as major excitable neuronal compartments that actively participate in information processing within the central nervous system. Additionally, dendrites have also been demonstrated not only to be receptive components, but also to act as sources of signalling molecules in the brain. The finding that dopamine is accumulated in, and depleted from, dendrites in substantia nigra dopaminergic neurones (46) constituted the first piece of evidence indicating that dendrites can release neurotransmitters. In 1989, Pow and Morris demonstrated for the first time, using electron microscopy, the presence of omega fusion profiles at dendritic plasma membranes of SON magnocellular neurosecretory neurones (47), representing the sites of exocytosis of large densed-core vesicles. Subsequent to this seminal discovery, work from various groups then conclusively confirmed that both OT and VP neuropeptides could be actively released from the dendrites of magnocellular neurosecretory neurones (19,48,49). The dendritic release of both neuropeptides occurs in an activity-dependent manner and involves a regulated Ca2+-dependent exocytosis (50–53). Moreover, dendritic release can be controlled independently from their release from axonal terminals. For example activation of melanocortin 4 receptor by α-melanocyte-stimulating hormone evokes dendritic but not axonal release of OT from magnocelluar neurones (54). Moreover, the pattern and time course of release from these two sources can be quite distinct, depending on the type of stimuli. Thus, during an osmotic stimulation, OT and VP are released from both dendritic and axonal sources. However, dendritic release is more delayed and longer lasting compared to axonal release (55). Dendritically-released OT and VP act in an autocrine manner to modulate both the efficacy of synaptic inputs, as well as the degree of firing activity of their respective neuronal sources (56–58). These effects, in turn, are critical for optimising their activity during challenging physiological conditions, such as during lactation and in response to osmotic stimulation.
Dendrites as substrates for interpopulation communication within the PVN
By contrast to the anatomical segregation of the somata of the functionally distinct PVN neuronal population, their dendritic processes extend beyond the boundaries of their respective subcompartments. This was demonstrated originally by Rho and Swanson (59), who used a combination of intracellular and retrograde tracing neuroanatomical techniques. More recently, we showed that the dendrites of magnocellular neurosecretory VP neurones extend into PVN subdivisions enriched in presympathetic neurones, becoming in close apposition to the somata and dendrites of these neurones (Fig. 2) (60). Moreover, we showed that, in some instances, dendrites from PVN neurones extend into the contralateral PVN, crossing over the dorsal tip of the third ventricle (61). This close anatomical inter-relationship among the dendrites of neurosecretory VP and presympathetic neurones suggests the presence of dendrodendritic and/or dendrosomatic signalling between these two neuronal populations. In this context, it is noteworthy that dendrites of magnocellular neurosecretory neurones, which account for approximately 80–85% of the total neuronal surface area (14), contain multiple large swellings along their extension, which store the majority of the neuropeptide produced in these neurones (19). Moreover, VP and OT neuropeptides have relatively long half-lives (1–20 min) (62) and are found at high extracellular concentrations in the brain (63). These features together support the notion that dendrites of magnocellular neurones could act as sources of OT and VP neuropeptide signalling within this interpopulation dendrodendritic/somatic microenvironment. We then determined whether the associated presympathetic neurones have the necessary molecular machinery to sense dendritically-released neuropeptides. Using a combination of immunohistochemistry, single-cell reverse transcriptase-polymerase chain reactions and patch-clamp electrophysiology, we showed that presympathetic neurones express the V1a receptor subtype for VP, with clusters of these receptors located in their dendrites. Moreover, we found that their pharmacological activation by focal application of VP evoked membrane depolarisation and a transient increase in firing activity (Fig. 3). These effects persisted in the presence of synaptic block media, and membrane depolarisation was still observed in the presence of the Na+ channel blocker tetrodotoxin, supporting a direct V1a receptor-mediated effect of VP on presympathetic PVN neurones (60).
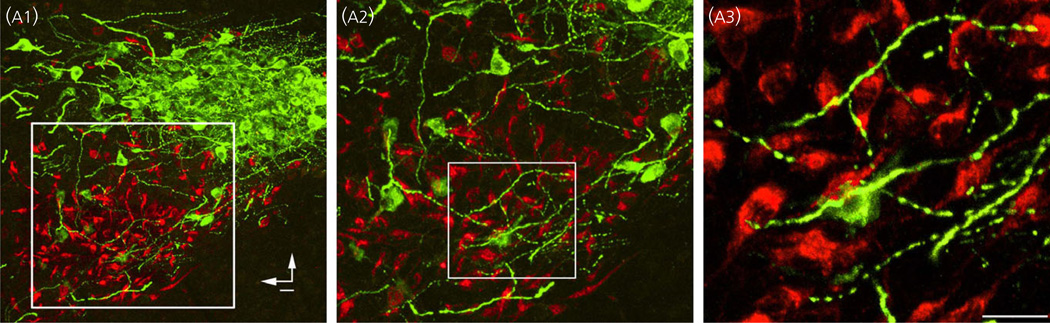
Fig. 2.
Close anatomical inter-relationships among the dendrites of magnocellular neurosecretory and presympatehtic paraventricular nucleus (PVN) neurones. (a1) Photomicrograph showing the topographical segregation between immunoreactive magnocellular vasopressin (VP) neurones (VP, green) and retrogradely labelled presympathetic PVN-rostral ventrolateral medulla neurones. Note the clear anatomical segregation between the somata of these two PVN neurona populations. In (a2) and (a3), the squared regions are shown at progressively higher magnification. Note in (a2) and (a3) that thick and varicose immunoreactive VP dendrites extend beyond the compartment of VP neurones, into the compartment containing presympathetic neurones, becoming in close apposition with somata and dendrites of the latter. Vertical and horizontal bars in (a1) point dorsally and medially, respectively. Modified from Brussaard et al. (56).
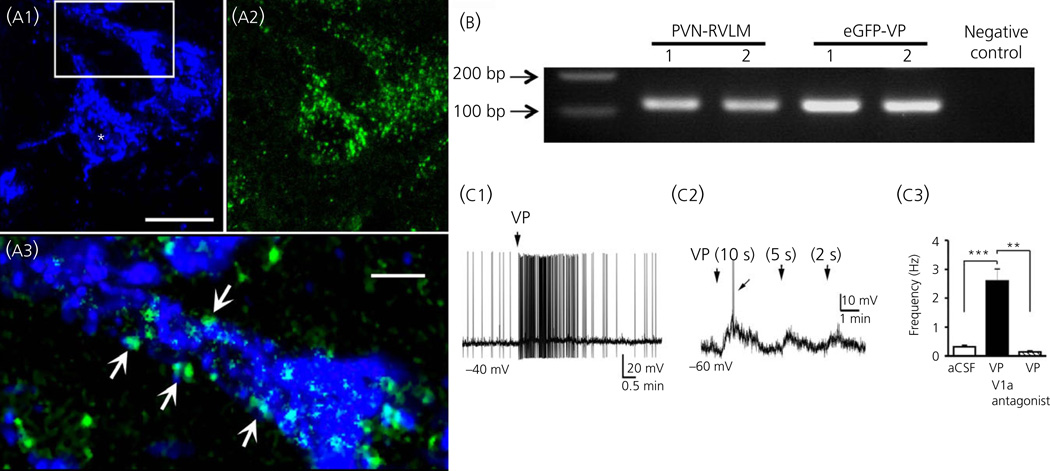
Fig. 3.
Expression of functional vasopressin (VP) V1a receptors in presympathetic paraventricular nucleus (PVN) neurones. (a1) Representative photomicrograph showing a couple of retrogradely-labelled PVN-rostral ventrolateral medulla (RVLM) neurones (blue) that have dense V1a receptor immunoreactivity (green, a2). In (a3), the squared area in (a1) is magnified to better depict dendritic V1a immunoreactive clusters (arrows). (b) Single-cell V1a mRNA expression in identified PVN-RVLM and enhanced green fluorescent protein (eGFP) -VP neurones. A nontemplate negative control is shown on the right, and a small piece of a DNA ladder is shown on the left. Scale bars: (a1, a2) = 20 µm and (a3) = 2.5 µm. (C1) VP (VP, 1 µM) puffed directly onto a presympathetic PVN-RVLM neurone evokes a burst of action potentials. (C2) In the presence of tetrodotoxin (0.5 µM), puffs of VP (1 µM) of decremental durations evoke membrane depolarisation in a proportionally decremental manner. The arrow indicates an evoked Ca2+ spike. (C3) Summary data showing that the increased action potential firing evoked by VP was completely blocked by a V1a receptor antagonist. **P < 0.01 and ***P < 0.001. Modified from Brussaard et al. (56). aCSF, artificial cerobrospinal fluid.
Overall, these observations lead us to test the hypothesis that, in addition to acting in an autocrine fashion, dendritically-released VP from magnocellular neurosecretory neurones diffuses in the extracellular space to modulate the activity of the neighbouring presympathetic neuronal population. In other words, we proposed that dendritic release of peptides within the PVN acts as an interpopulation signal.
Dendritic release of VP mediates intercellular cross-talk between a neuroendocrine VP and a presympathetic PVN neurone
To test whether the endogenous dendritic release of VP was indeed capable of mediating cross-talk between neurosecretory and presympathetic neurones in the PVN, we performed a series of complementary in vitro and in vivo studies. To unequivocally identify both neuronal populations in an in vitro slice preparation, we used transgenic rats that express the enhanced green fluorescent protein (eGFP) driven by the VP promoter (64) (eGFP-VP rat), which received an injection of a fluorescently-labelled retrograde tracer into the RVLM in the brainstem. With this approach, we were able to readily visualise both PVN neuronal populations (Fig. 4). Using slices from these rats, individual VP neurones were activated using laser photolysis of caged-NMDA, at the same time as we monitored the electrical activity of neighbouring presympathetic neurones through a patch pipette. Previous studies demonstrated that the NMDA receptor is a major receptor subtype mediating glutamate actions in magnocellular neurosecretory neurones (65), and also that their activation can efficiently evoke the dendritic release of neuropeptides from these neurones (66). We found that photolysis of caged-NMDA within individual eGFP-VP neurones evoked reproducible excitatory responses in neighbouring presympathetic PVN-RVLM projecting neurones (Fig. 4). These responses occurred with a latency of approximately 5 s and where blocked by a V1a receptor antagonist (60). Taken together, these studies indicate that NMDA receptor-evoked dendritic release of VP from a single neurosecretory VP neurone was sufficient to diffuse in the extracellular space to evoke an excitatory response in neighbouring presympathetic neurones.
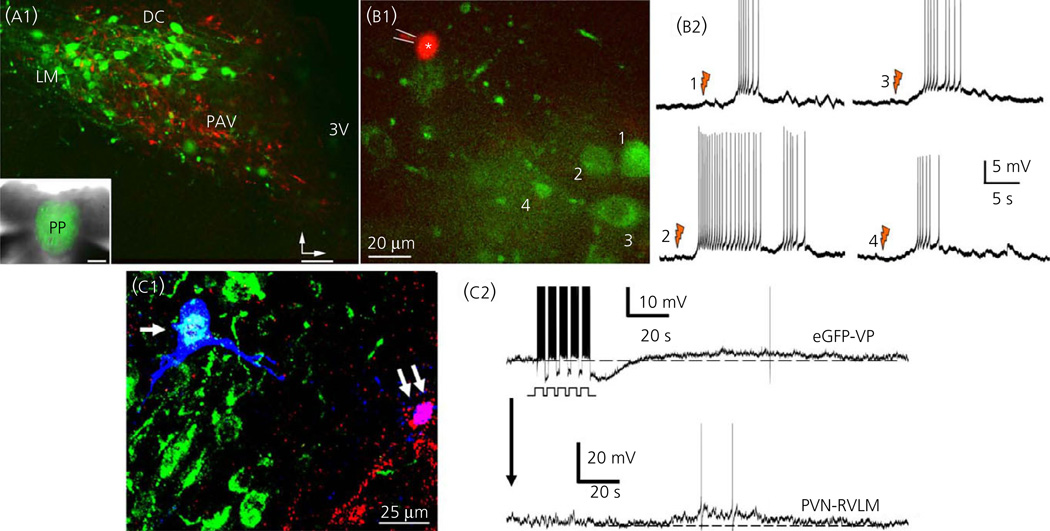
Fig. 4.
Dendritically-released vasopressin (VP) mediates cross-talk between magnocelluar neurosecretory and the presympathetic paraventricular nucleus (PVN) neurones. (a) Hypothalamic slice obtained from an enhanced green fluorescent protein (eGFP)-VP rat that received an injection of the retrograde tracer rhoda-mine beads in the rostral ventrolateral medulla (RVLM). Magnocellular VP neurones are shown in green and presympathetic PVN neurones are shown in red The inset shows eGFP-stained fibers in the posterior pituitary. Vertical and horizontal bars point dorsally and medially, respectively. (b1) Sample of another hypothalamic slice showing a patched presympathetic PVN-RVLM neurone (red, asterisk) and neighbouring eGFP-VP neurones (green, 1–4). (b2) Laser photolysis of caged NMDA onto eGFP-VP neurones (1,2, orange flashes) resulted in a delayed (approximately 5 s) membrane depolarisation and increased firing activity in the patched presympathetic neurone. (c1) Sample pair of intracellularly labelled (Alexa 633, blue, arrows) PVN neurones during simultaneous dual-patch recordings. The neurone at the left (single arrow) was identified as an eGFP-VP (cyan), whereas the neurone to the right (double arrow) was a retrogradely-labelled PVN-RVLM (purple). (c2) Bursts of action potentials evoked in the eGFP-VP neurone via current injection through the patch pipette resulted in a delayed membrane depolarisation and increased firing discharge in the paired PVN-RVLM neurone. Modified from Brussaard et al. (56). 3V, third ventricle.
This cell-to-cell communication modality was further confirmed using simultaneous dual patch recordings from a pair of identified neurosecretory VP and presympathetic neurones. We found that a series of evoked burst of action potentials in an identified eGFP-VP neurone consistently elicited a delayed excitatory response in the monitored presympathetic neurone (Fig. 4). This intercellular crosstalk was blocked by (i) a V1a receptor antagonist and (ii) when the neurosecretory VP neurones were dialysed with the Ca2+ chelator BAPTA (60). Taken together, these two complementary in vitro approaches show that Ca2+-dependent release of dendritic VP efficiently affects the activity of neighbouring presympathetic neurones.
Astrocytes have been classically considered as non-excitable cells, providing only energy and physical support for surrounding neurones. However, they are now generally recognised as key active players in information processing in the brain (67–69). This is particularly true in the hypothalamic SON and PVN, in which bidirectional neuroglial interactions play critical roles in regulating both neurosecretory and autonomic functions; recent reviews on this topic are provided elsewhere (70–73). In the context of the VP-presympathetic neuronal cross-talk, however, we found that astrocytes were not critical cellular intermediaries: the intercellular cross-talk persisted in the presence of a gliotoxin, and we found the majority of astrocytes did not respond to dendritically-released VP (60).
Dendritic release of VP acts as a diffusible interpopulation signal in the PVN
The results summarised above indicate that the somatodendritic release of VP from an individual neurone can locally diffuse in the extracellular space to affect the activity of a neighbouring presympathetic neurone. To determine whether the average basal activity of the entire VP neuronal population tonically released sufficient amounts of VP to generate a functionally relevant ‘diffusible pool’ of the peptide in the extracellular space, we tested the effects of a V1a antagonist per se on the activity of presympathetic neurones. We found that bath application of a V1a antagonist diminished the ongoing firing activity of presympathetic neurones, supporting the presence of a diffusible pool of VP that tonically stimulates the activity of presympathetic neurones. We found that the strength of the diffusible pool was determined by the degree of activity of the VP neuronal population. Thus, increasing the activity of VP neurones by elevating extracellular K+ levels resulted in a proportionally larger effect of the V1a antagonist on presympathetic firing activity (approximately 75% compared to 52% in normal K+ artificial cerebrospinal fluid). The opposite effect was observed when VP neurones were inhibited by the kappa opioid agonist U50488 (1 µM) (approximately 12% effect), which, as described previously (74), robustly diminished or silenced the firing activity of neurosecretory VP neurones.
The strength of a ‘volume-transmitted’ signal is typically influenced by the half-life of the messenger, as well as the tortuosity of the extracellular space. Thus, to further assess whether VP acted as a diffusible interpopulation signal, we experimentally manipulated these two variables. The half-life of neuropeptides in the brain is determined by their rate of degradation by extracellular peptidases. We found that blockade of tissue aminopeptidase activity with amastatin (10 µm) increased the firing activity of presympathetic neurones, an effect that was blunted in the presence of the V1a receptor blocker (60). These results indicate that extending half-life of VP increased its ability to diffuse more efficiently in the extracelular space to reach its targets. Moreover, we found that reducing the coefficient of diffusion in the extracellular space (by increasing its viscosity using an artificial cerebrospinal fluid containing 5% dextran) (75) significantly diminished the strength of the diffusible pool of VP (60). Thus, our studies are consistent with the notion that tonic somatodendritic release of VP within the PVN mediates an interpopulation cross-talk between neurosecretory and presympathetic neuronal populations.
Role of dendritic release of VP in neurohumoral nomeostatic responses to an osmotic challenge
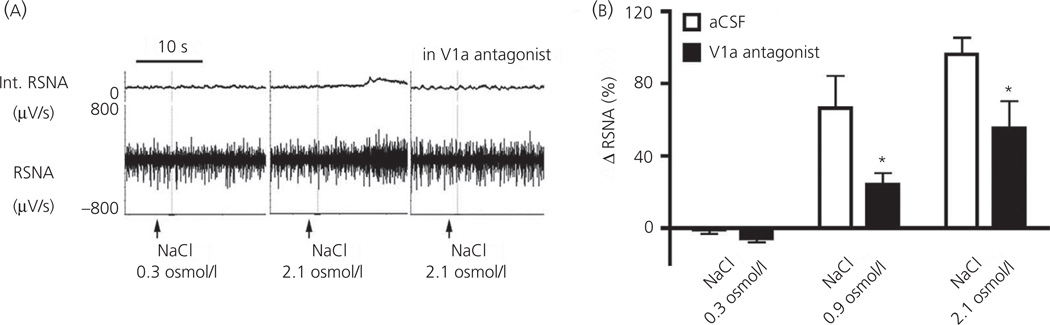
As summarised above, a central osmotic stimulation evokes a complex homeostatic response characterised by an increased systemic release of VP and a concomitant increase in renal sympathetic nerve activity. Importantly, numerous studies support a pivotal role of neurosecretory magnocellular and presympathetic PVN neurones in mediating this neurohumoral response (31,36,76). Still, whether the somatodendritic release of VP within the PVN contributes to the coordinated activation of these two major neuronal population during this multimodal homeostatic response was not known. As previously reported (76), we found that an intracarotid infusion of graded concentrations of NaCl (0.3–2.1 osmol/l NaCl) evoked a dose-dependent increase of renal sympathetic nerve activity and also stimulated intranuclear release of VP, as measured by microdialysis (Fig. 5) (60). Importantly, we found that, when a V1a antagonist was bilaterally microinjected into the PVN before the osmotic challenge, the renal sympathoexcitatory response was inhibited by approximately 50% (Fig. 5). Thus, these in vivo studies indicate that intranuclear somatodendritic release of VP plays a critical role in the recruitment of sympathoexcitatory neurones during a homeostatic challenge that requires an orchestrated neurosecretory and sympathetic response.
Fig. 5.
Dendritically-released vasopressin (VP) contributes to osmotieally-driven renal sympathetic nerve activity (RSNA). (a) Recordings or RSNA after intracar-otid infusions of an isosmotic (NaCl 0.3 osmol/l) or hyperosmotic (NaCl 2.1 osmol/l) solution, in the absence or presence of bilateral microinjections of the V1a receptor antagonist (0.4 mg/ml) into the paraventricular nucleus (PVN). (b) Summary data showing a dose-dependent increase of RSNA after intracarotid infusions of NaCI. Note the blunted sympathetic response after an intra-PVN microinjection of the V1a receptor antagonist [*P < 0.0001 versus respective artificial cerobrospinal fluid (aCSF)]. Modified from Brussaard et al. (56).
Summary and perspectives
The data presented and discussed in the present review support the dendritic release of VP, and its diffusion in the extracellular space, as an efficient signalling mechanism mediating cross-talk between neuroendocrine and autonomic systems in the PVN. This cross-talk operates not only at the cell-to-cell level, influencing individual neuronal firing activity, but also at the whole population level, affecting sympathoexcitatory responses to an osmotic challenge (see the working model in Fig. 6). We consider these findings to be significant within the context of previous anatomical studies demonstrating a highly compartmentalised organisation and segregation among these functionally distinct neuronal populations. Thus, along with lack of evidence for ‘hard-wired’ interconnectivity between the neurosecretory and autonomic subdivisions of the PVN, these anatomical studies have led to the notion that neurosecretory and autonomic information within the PVN is processed in parallel, without much cross-talk or integration occurring within the PVN itself. Our studies indicate, however, that a signalling cross-talk between neurosecretory VP and presympathetic RVLM-projecting neurones does take place in the PVN, and that this inter-population communication is functionally relevant for homeostatic regulation.
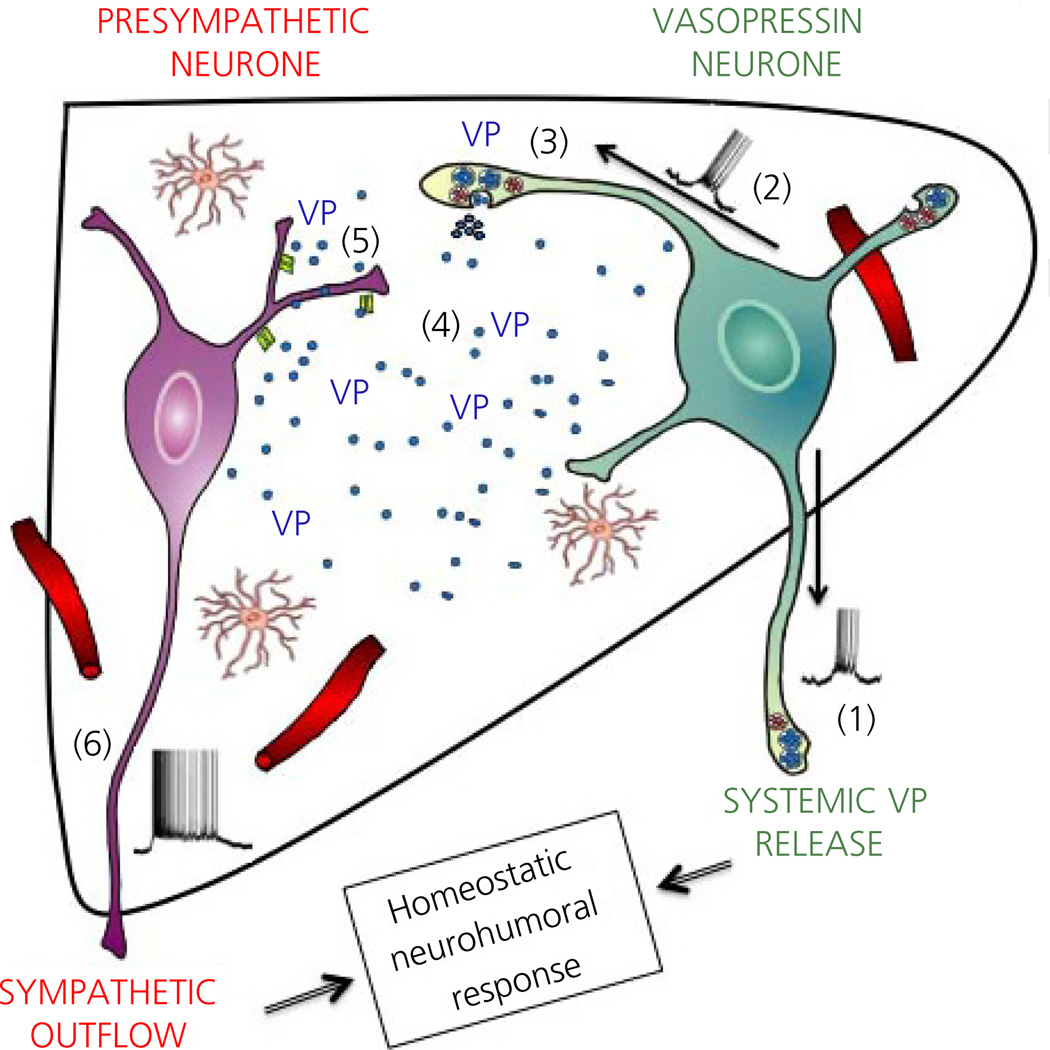
Fig. 6.
Neurosecretory-autonomic cross-talk mediated by dendritically-released vasopressin (VP). (1) Activation of neurosecretory VP neurones (e.g. osmotic stimulus, NMDA receptor activation) leads to a burst of action ootentials that propagates anterogradely to depolarise axonal terminals at the neurohypophysis, resulting in the systemic release of VP. (2) In addition, action potentials back-propagate into dendritic segments resulting (3) in the dendritic, intranuclear release of VP. (4) VP passively diffuses in the extracel-lular space in a volume-transmission manner. (5) Binding of VP to V1a receptor subtypes in presympathetic paraventricular nucleus (PVN) neurones evokes membrane depolarisation and increased firing activity, leading in turn to increased sympathetic outflow to peripheral organs (e.g. kidneys). Taken together, we propose that the concerted activation of neurosecretory VP and presympathetic PVN neurones contributes to a proper multimodal homeostatic neurohumoral response, such as that required during an osmotic challenge.
Two important aspects need to be considered within the context of these results. First, VP is only one of numerous neuropeptides or signalling molecules that can be released from somatodendritic compartments in the PVN. OT and dynorphin (77), as well as endocannabinoids (78), have been shown to be released in an activity-dependent manner from the dendrites of magnocellular neurosecretory neurones. However, whether these other molecules also mediate interpopulation communication in the PVN remains to be determined. Second, our studies focused on two specific neuronal populations of the PVN. Thus, whether other PVN neuronal populations could also be targeted by a similar interpopulation signalling modality remains unknown. Of particular interest is the population of CRH neurosecretory neurones, given that the hypothalamic-pituitary axis is usually activated in tandem with the sympathetic and posterior pituitary systems in response to a variety of physiological challenges.
Finally, although a coordinated activation of the autonomic and neuroendocrine systems is required during physiological homeostatic responses, it is now well established that their imbalanced or exacerbated activation can lead to a disease state. In this sense, a growing body of evidence supports neurohumoral activation as a key pathophysiological mechanism in prevalent diseases, including hypertension, heart failure and diabetes (79,80). For example, numerous studies implicate an exacerbated PVN-driven sympat-hoexcitation (81–85), underlined largely by increased activity of PVN presympathetic neurones (86–89), as a key pathophysiological mechanism in heart failure. Importantly, a clear correlation between this increased sympathoexcitatory drive morbidity and mortality in heart failure has been established (79). Moreover, a growing body of evidence also supports the enhanced activation of VP neurones (88,90–93), as well as elevated circulating levels of VP in experimental models and human patients with heart failure (94–97). Chronically elevated levels of VP have been shown to contribute to altered fluid/electrolyte balance, as well as detrimental myocardial effects in heart failure (98–102). Still, whether the exacerbated activation of VP neurones in heart failure also results in enhanced central levels of VP is unknown at present. If this were the case, we could speculate that, based on our recent studies (60), an enhanced dendritic release of VP and its diffusion in the extracellular space may constitute an underlying mechanism contributing to the exacerbated presympathetic neuronal activity and sympathoexcitatory outflow during heart failure. Moreover, changes in the configuration and/or tortuosity of the extracellular space itself (e.g. as a consequence of astrogliosis), a common finding in numerous diseases, including heart failure, obesity and the metabolic syndrome (103–107), is also likely to affect the diffusion capacity and strength of a volume-transmitted signal, such as dendritically-released VP. Thus, it will be important for future studies to determine whether an altered peptidergic dendritic release process and/or structural remodeling of the extracellular space constitute novel, alternative mechanisms contributing to exacerbated neurohumoral activation in disease conditions. This knowledge, in turn, could lead to the discovery of additional and perhaps more efficient therapeutic targets and approaches for the treatment of these prevalent diseases in the human population.
References
- 1.Galarreta M, Hestrin S. Spike transmission and synchrony detection in networks of GABAergic interneurons. Science. 2001;292:2295–2299. doi: 10.1126/science.1061395. [DOI] [PubMed] [Google Scholar]
- 2.Abbott LF, Regehr WG. Synaptic computation. Nature. 2004;431:796–803. doi: 10.1038/nature03010. [DOI] [PubMed] [Google Scholar]
- 3.Lisman JE, Raghavachari S, Tsien RW. The sequence of events that underlie quantal transmission at central glutamatergic synapses. Nat Rev Neurosci. 2007;8:597–609. doi: 10.1038/nrn2191. [DOI] [PubMed] [Google Scholar]
- 4.Mody I, De Koninck Y, Otis TS, Soltesz I. Bridging the cleft at GABA synapses in the brain. Trends Neurosci. 1994;17:517–525. doi: 10.1016/0166-2236(94)90155-4. [DOI] [PubMed] [Google Scholar]
- 5.Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, Kanai Y, Hediger MA, Wang Y, Schielke JP, Welty DF. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16:675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- 6.Zhang ZW, Kang JI, Vaucher E. Axonal varicosity density as an index of local neuronal interactions. PLoS One. 2011;6:e22543. doi: 10.1371/journal.pone.0022543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dawson TM, Snyder SH. Gases as biological messengers: nitric oxide and carbon monoxide in the brain. J Neurosci. 1994;14:5147–5159. doi: 10.1523/JNEUROSCI.14-09-05147.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belin V, Moos F, Richard P. Synchronization of oxytocin cells in the hypothalamic paraventricular and supraoptic nuclei in suckled rats: direct proof with paired extracellular recordings. Exp Brain Res. 1984;57:201–203. doi: 10.1007/BF00231147. [DOI] [PubMed] [Google Scholar]
- 9.Poulain DA, Wakerley JB. Electrophysiology of hypothalamic magnocelular neurones secreting oxytocin and vasopressin. Neuroscience. 1982;7:773–808. doi: 10.1016/0306-4522(82)90044-6. [DOI] [PubMed] [Google Scholar]
- 10.Boudaba C, Tasker JG. Intranuclear coupling of hypothalamic magnocellular nuclei by glutamate synaptic circuits. Am J Physiol Regul Integr Comp Physiol. 2006;291:R102–R111. doi: 10.1152/ajpregu.00795.2005. [DOI] [PubMed] [Google Scholar]
- 11.Israel JM, Le Masson G, Theodosis DT, Poulain DA. Glutamatergic input governs periodicity and synchronization of bursting activity in oxytocin neurons in hypothalamic organotypic cultures. Eur J Neurosci. 2003;17:2619–2629. doi: 10.1046/j.1460-9568.2003.02705.x. [DOI] [PubMed] [Google Scholar]
- 12.Neumann I, Douglas AJ, Pittman QJ, Russell JA, Landgraf R. Oxytocin released within the supraoptic nucleus of the rat brain by positive feedback action is involved in parturition-related events. J Neuroendocrinol. 1996;8:227–233. doi: 10.1046/j.1365-2826.1996.04557.x. [DOI] [PubMed] [Google Scholar]
- 13.Stern JE, Armstrong WE. Changes in the electrical properties of supraoptic nucleus oxytocin and vasopressin neurons during lactation. J Neurosci. 1996;16:4861–4871. doi: 10.1523/JNEUROSCI.16-16-04861.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stern JE, Armstrong WE. Reorganization of the dendritic trees of oxytocin and vasopressin neurons of the rat supraoptic nucleus during lactation. J Neurosci. 1998;18:841–853. doi: 10.1523/JNEUROSCI.18-03-00841.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moos F, Freund-Mercier MJ, Guerne Y, Guerne JM, Stoeckel ME, Richard P. Release of oxytocin and vasopressin by magnocellular nuclei in vitro: specific facilitatory effect of oxytocin on its own release. J Endocrinol. 1984;102:63–72. doi: 10.1677/joe.0.1020063. [DOI] [PubMed] [Google Scholar]
- 16.Jourdain P, Israel JM, Dupouy B, Oliet SH, Allard M, Vitiello S, Theodosis DT, Poulain DA. Evidence for a hypothalamic oxytocin-sensitive pattern-generating network governing oxytocin neurons in vitro. J Neurosci. 1998;18:6641–6649. doi: 10.1523/JNEUROSCI.18-17-06641.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Israel JM, Poulain DA, Oliet SH. Oxytocin-induced postinhibitory rebound firing facilitates bursting activity in oxytocin neurons. J Neurosci. 2008;28:385–394. doi: 10.1523/JNEUROSCI.5198-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freund-Mercier MJ, Richard P. Electrophysiological evidence for facilitatory control of oxytocin neurones by oxytocin during suckling in the rat. J Physiol. 1984;352:447–466. doi: 10.1113/jphysiol.1984.sp015302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ludwig M, Leng G. Dendritic peptide release and peptide-dependent behaviours. Nat Rev Neurosci. 2006;7:126–136. doi: 10.1038/nrn1845. [DOI] [PubMed] [Google Scholar]
- 20.Vizi ES, Kiss JP, Lendvai B. Nonsynaptic communication in the central nervous system. Neurochem Int. 2004;45:443–451. doi: 10.1016/j.neuint.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 21.Fuxe K, Dahlstrom A, Hoistad M, Marcellino D, Jansson A, Rivera A, Diaz-Cabiale Z, Jacobsen K, Tinner-Staines B, Hagman B, Leo G, Staines W, Guidolin D, Kehr J, Genedani S, Belluardo N, Agnati LF. From the Golgi-Cajal mapping to the transmitter-based characterization of the neuronal networks leading to two modes of brain communication: wiring and volume transmission. Brain Res Rev. 2007;55:17–54. doi: 10.1016/j.brainresrev.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 22.Zoli M, Agnati LF. Wiring and volume transmission in the central nervous system: the concept of closed and open synapses. Prog Neurobiol. 1996;49:363–380. doi: 10.1016/0301-0082(96)00020-2. [DOI] [PubMed] [Google Scholar]
- 23.Swanson LW, Sawchenko PE. Paraventricular nucleus: a site for the integration of neuroendocrine and autonomic mechanisms. Neuroendocrinology. 1980;31:410–417. doi: 10.1159/000123111. [DOI] [PubMed] [Google Scholar]
- 24.Palkovits M. Interconnections between the neuroendocrine hypothalamus and the central autonomic system. Geoffrey Harris Memorial Lecture, Kitakyushu, Japan, October 1998. Front Neuroendocrinol. 1999;20:270–295. doi: 10.1006/frne.1999.0186. [DOI] [PubMed] [Google Scholar]
- 25.Armstrong WE, Warach S, Hatton GI, McNeill TH. Subnuclei in the rat hypothalamic paraventricular nucleus: a cytoarchitectural, horseradish peroxidase and immunocytochemical analysis. Neuroscience. 1980;5:1931–1958. doi: 10.1016/0306-4522(80)90040-8. [DOI] [PubMed] [Google Scholar]
- 26.Swanson LW, Kuypers HG. The paraventricular nucleus of the hypothalamus: cytoarchitectonic subdivisions and organization of projections to the pituitary, dorsal vagal complex, and spinal cord as demonstrated by retrograde fluorescence double-labeling methods. J Comp Neurol. 1980;194:555–570. doi: 10.1002/cne.901940306. [DOI] [PubMed] [Google Scholar]
- 27.Chen QH, Toney GM. Identification and characterization of two functionally distinct groups of spinal cord-projecting paraventricular nucleus neurons with sympathetic-related activity. Neuroscience. 2003;118:797–807. doi: 10.1016/s0306-4522(03)00033-2. [DOI] [PubMed] [Google Scholar]
- 28.Stocker SD, Keith KJ, Toney GM. Acute inhibition of the hypothalamic paraventricular nucleus decreases renal sympathetic nerve activity and arterial blood pressure in water-deprived rats. Am J Physiol Regul Integr Comp Physiol. 2004;286:R719–R725. doi: 10.1152/ajpregu.00494.2003. [DOI] [PubMed] [Google Scholar]
- 29.Zhang K, Patel KP. Effect of nitric oxide within the paraventricular nucleus on renal sympathetic nerve discharge: role of GABA. Am J Physiol. 1998;275:R728–R734. doi: 10.1152/ajpregu.1998.275.3.R728. [DOI] [PubMed] [Google Scholar]
- 30.Knobloch HS, Charlet A, Hoffmann LC, Eliava M, Khrulev S, Cetin AH, Osten P, Schwarz MK, Seeburg PH, Stoop R, Grinevich V. Evoked axona oxytocin release in the central amygdala attenuates fear response. Neuron. 2012;73:553–566. doi: 10.1016/j.neuron.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 31.Bourque CW. Central mechanisms of osmosensation and systemic osmoregulation. Nat Rev Neurosci. 2008;9:519–531. doi: 10.1038/nrn2400. [DOI] [PubMed] [Google Scholar]
- 32.Aguilera G, Lightman SL, Kiss A. Regulation of the hypothalamic-pitui-tary-adrenal axis during water deprivation. Endocrinology. 1993;132:241–248. doi: 10.1210/endo.132.1.8380375. [DOI] [PubMed] [Google Scholar]
- 33.Arnhold MM, Wotus C, Engeland WC. Differential regulation of parvocellular neuronal activity in the paraventricular nucleus of the hypothalamus following single vs. repeated episodes of water restriction-induced drinking. Exp Neurol. 2007;206:126–136. doi: 10.1016/j.expneurol.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wotus C, Arnhold MM, Engeland WC. Dehydration-induced drinking decreases Fos expression in hypothalamic paraventricular neurons expressing vasopressin but not corticotropin-releasing hormone. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1349–R1358. doi: 10.1152/ajpregu.00304.2006. [DOI] [PubMed] [Google Scholar]
- 35.Toney GM, Chen QH, Cato MJ, Stocker SD. Central osmotic regulation of sympathetic nerve activity. Acta Physiol Scand. 2003;177:43–55. doi: 10.1046/j.1365-201X.2003.01046.x. [DOI] [PubMed] [Google Scholar]
- 36.Toney GM, Stocker SD. Hyperosmotic activation of CNS sympathetic drive: implications for cardiovascular disease. J Physiol. 2010;588:3375–3384. doi: 10.1113/jphysiol.2010.191940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hatton GI, Hutton UE, Hoblitzell ER, Armstrong WE. Morphological evidence for two populations of magnocellular elements in the rat paraventricular nucleus. Brain Res. 1976;108:187–193. doi: 10.1016/0006-8993(76)90176-1. [DOI] [PubMed] [Google Scholar]
- 38.Hatton GI, Cobbett P, Salm AK. Extranuclear axon collaterals of paraventricular neurons in the rat hypothalamus: intracellular staining, immunocytochemistry and electrophysiology. Brain Res Bull. 1985;14:123–132. doi: 10.1016/0361-9230(85)90072-3. [DOI] [PubMed] [Google Scholar]
- 39.Ludwig M. Dendritic release of vasopressin and oxytocin. J Neuroendocrinol. 1998;10:881–895. doi: 10.1046/j.1365-2826.1998.00279.x. [DOI] [PubMed] [Google Scholar]
- 40.Sawchenko PE, Swanson LW. The organization of noradrenergic pathways from the brainstem to the paraventricular and supraoptic nuclei in the rat. Brain Res. 1982;257:275–325. doi: 10.1016/0165-0173(82)90010-8. [DOI] [PubMed] [Google Scholar]
- 41.Pittman QJ, Veale WL, Lederis K. Central neurohypophyseal peptide pathways: interactions with endocrine and other autonomic functions. Peptides. 1982;3:515–520. doi: 10.1016/0196-9781(82)90118-8. [DOI] [PubMed] [Google Scholar]
- 42.Frick A, Johnston D. Plasticity of dendritic excitability. J Neurobiol. 2005;64:100–115. doi: 10.1002/neu.20148. [DOI] [PubMed] [Google Scholar]
- 43.Kim S, Guzman SJ, Hu H, Jonas P. Active dendrites support efficient initiation of dendritic spikes in hippocampal CA3 pyramidal neurons. Nat Neurosci. 2012;15:600–606. doi: 10.1038/nn.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Magee J, Hoffman D, Colbert C, Johnston D. Electrical and calcium signaling in dendrites of hippocampal pyramidal neurons. Annu Rev Physiol. 1998;60:327–346. doi: 10.1146/annurev.physiol.60.1.327. [DOI] [PubMed] [Google Scholar]
- 45.Spruston N, Schiller Y, Stuart G, Sakmann B. Activity-dependent action potential invasion and calcium influx into hippocampal CA1 dendrites. Science. 1995;268:297–300. doi: 10.1126/science.7716524. [DOI] [PubMed] [Google Scholar]
- 46.Bjorklund A, Lindvall O. Dopamine in dendrites of substantia nigra neurons: suggestions for a role in dendritic terminals. Brain Res. 1975;83:531–537. doi: 10.1016/0006-8993(75)90849-5. [DOI] [PubMed] [Google Scholar]
- 47.Pow DV, Morris JF. Dendrites of hypothalamic magnocellular neurons release neurohypophysial peptides by exocytosis. Neuroscience. 1989;32:435–439. doi: 10.1016/0306-4522(89)90091-2. [DOI] [PubMed] [Google Scholar]
- 48.Landgraf R, Neumann ID. Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Front Neuroendocrinol. 2004;25:150–176. doi: 10.1016/j.yfrne.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 49.Ludwig M, Pittman QJ. Talking back: dendritic neurotransmitter release. Trends Neurosci. 2003;26:255–261. doi: 10.1016/S0166-2236(03)00072-9. [DOI] [PubMed] [Google Scholar]
- 50.Ludwig M, Bull PM, Tobin VA, Sabatier N, Landgraf R, Dayanithi G, Leng G. Regulation of activity-dependent dendritic vasopressin release from rat supraoptic neurones. J Physiol. 2005;564:515–522. doi: 10.1113/jphysiol.2005.083931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ludwig M, Sabatier N, Bull PM, Landgraf R, Dayanithi G, Leng G. Intracellular calcium stores regulate activity-dependent neuropeptide release from dendrites. Nature. 2002;418:85–89. doi: 10.1038/nature00822. [DOI] [PubMed] [Google Scholar]
- 52.Tobin V, Leng G, Ludwig M. The involvement of actin, calcium channels and exocytosis proteins in somato-dendritic oxytocin and vasopressin release. Front Physiol. 2012;3:261. doi: 10.3389/fphys.2012.00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tobin V, Schwab Y, Lelos N, Onaka T, Pittman QJ, Ludwig M. Expression of exocytosis proteins in rat supraoptic nucleus neurones. J Neuroendocrinol. 2012;24:629–641. doi: 10.1111/j.1365-2826.2011.02237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sabatier N, Caquineau C, Dayanithi G, Bull P, Douglas AJ, Guan XM, Jiang M, Van der Ploeg L, Leng G. Alpha-melanocyte-stimulating hormone stimulates oxytocin release from the dendrites of hypothalamic neurons while inhibiting oxytocin release from their terminals in the neurohypophysis. J Neurosci. 2003;23:10351–10358. doi: 10.1523/JNEUROSCI.23-32-10351.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ludwig M, Callahan MF, Neumann I, Landgraf R, Morris M. Systemic osmotic stimulation increases vasopressin and oxytocin release within the supraoptic nucleus. J Neuroendocrinol. 1994;6:369–373. doi: 10.1111/j.1365-2826.1994.tb00595.x. [DOI] [PubMed] [Google Scholar]
- 56.Brussaard AB, Kits KS, de Vlieger TA. Postsynaptic mechanism of depression of GABAergic synapses by oxytocin in the supraoptic nucleus of immature rat. J Physiol. 1996;497:495–507. doi: 10.1113/jphysiol.1996.sp021783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gouzenes L, Desarmenien MG, Hussy N, Richard P, Moos FC. Vasopressin regularizes the phasic firing pattern of rat hypothalamic magnocelular vasopressin neurons. J Neurosci. 1998;18:1879–1885. doi: 10.1523/JNEUROSCI.18-05-01879.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ludwig M, Leng G. Autoinhibition of supraoptic nucleus vasopressin neurons in vivo: a combined retrodialysis/electrophysiological study in rats. Eur J Neurosci. 1997;9:2532–2540. doi: 10.1111/j.1460-9568.1997.tb01682.x. [DOI] [PubMed] [Google Scholar]
- 59.Rho JH, Swanson LW. A morphometric analysis of functionally defined subpopulations of neurons in the paraventricular nucleus of the rat with observations on the effects of colchicine. J Neurosci. 1989;9:1375–1388. doi: 10.1523/JNEUROSCI.09-04-01375.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Son SJ, Filosa JA, Potapenko ES, Biancardi VC, Zheng H, Patel KP, Tobin VA, Ludwig M, Stern JE. Dendritic peptide release mediates interpopulation crosstalk between neurosecretory and preautonomic networks. Neuron. 2013;78:1036–1049. doi: 10.1016/j.neuron.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stern JE. Electrophysiological and morphological properties of preautonomic neurones in the rat hypothalamic paraventricular nucleus. J Physiol. 2001;537:161–177. doi: 10.1111/j.1469-7793.2001.0161k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stark H, Burbach JP, Van der Kleij AA, De Wied D. In vivo conversion of vasopressin after microinjection into limbic brain areas of rats. Peptides. 1989;10:717–720. doi: 10.1016/0196-9781(89)90102-2. [DOI] [PubMed] [Google Scholar]
- 63.Mens WB, Witter A, van Wimersma Greidanus TB. Penetration of neu-rohypophyseal hormones from plasma into cerebrospinal fluid (CSF): half-times of disappearance of these neuropeptides from CSF. Brain Res. 1983;262:143–149. doi: 10.1016/0006-8993(83)90478-x. [DOI] [PubMed] [Google Scholar]
- 64.Ueta Y, Fujihara H, Serino R, Dayanithi G, Ozawa H, Matsuda K-I, Kawata M, Yamada J, Ueno S, Fukuda A, Murphy D. Transgenic expression of enhanced green fluorescent protein enables direct visualization for fhysiological studies of vasopressin neurons and isolated nerve terminals of the fat. Endocrinology. 2005;146:406–413. doi: 10.1210/en.2004-0830. [DOI] [PubMed] [Google Scholar]
- 65.Hu B, Bourque CW. NMDA receptor-mediated rhythmic bursting activity in rat supraoptic nucleus neurones in vitro. J Physiol. 1992;458:667–687. doi: 10.1113/jphysiol.1992.sp019440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.de Kock CP, Burnashev N, Lodder JC, Mansvelder HD, Brussaard AB. NMDA receptors induce somatodendritic secretion in hypothalamic neurones of lactating female rats. J Physiol. 2004;561:53–64. doi: 10.1113/jphysiol.2004.069005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Araque A, Navarrete M. Glial cells in neuronal network function. Philos Trans R Soc Lond B Biol Sci. 2010;365:2375–2381. doi: 10.1098/rstb.2009.0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Volterra A, Meldolesi J. Astrocytes, from brain glue to communication elements: the revolution continues. Nat Rev Neurosci. 2005;6:626–640. doi: 10.1038/nrn1722. [DOI] [PubMed] [Google Scholar]
- 69.Araque A, Navarrete M. Glial cells in neuronal network function. Philos Trans R Soc Lond B Biol Sci. 2010;365:2375–2381. doi: 10.1098/rstb.2009.0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hatton GI. Dynamic neuronal-glial interactions: an overview 20 years later. Peptides. 2004;25:403–411. doi: 10.1016/j.peptides.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 71.Oliet SH, Panatier A, Piet R, Mothet JP, Poulain DA, Theodosis DT. Neuron-glia interactions in the rat supraoptic nucleus. Prog Brain Res. 2008;170:109–117. doi: 10.1016/S0079-6123(08)00410-X. [DOI] [PubMed] [Google Scholar]
- 72.Stern JE, Filosa JA. Bidirectional neuroglial signaling modalities in the hypothalamus: role in neurohumoral regulation. Auton Neurosci. 2013;175:51–60. doi: 10.1016/j.autneu.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tasker JG, Oliet SH, Bains JS, Brown CH, Stern JE. Glial regulation of neuronal function: from synapse to systems physiology. J Neuroendocrinol. 2012;24:566–576. doi: 10.1111/j.1365-2826.2011.02259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brown CH, Ludwig M, Leng G. kappa-opioid regulation of neuronal activity in the rat supraoptic nucleus in vivo. J Neurosci. 1998;18:9480–9488. doi: 10.1523/JNEUROSCI.18-22-09480.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Piet R, Vargova L, Sykova E, Poulain DA, Oliet SH. Physiological contribution of the astrocytic environment of neurons to intersynaptic crosstalk. Proc Natl Acad Sci USA. 2004;101:2151–2155. doi: 10.1073/pnas.0308408100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen QH, Toney GM. AT(1)-receptor blockade in the hypothalamic PVN reduces central hyperosmolality-induced renal sympathoexcitation. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1844–R1853. doi: 10.1152/ajpregu.2001.281.6.R1844. [DOI] [PubMed] [Google Scholar]
- 77.Brown CH, Bourque CW. Autocrine feedback inhibition of plateau potentials terminates phasic bursts in magnocellular neurosecretory cells of the rat supraoptic nucleus. J Physiol. 2004;557:949–960. doi: 10.1113/jphysiol.2004.063818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Di S, Boudaba C, Popescu IR, Weng FJ, Harris C, Marcheselli VL, Bazan NG, Tasker JG. Activity-dependent release and actions of endocannabinoids in the rat hypothalamic supraoptic nucleus. J Physiol. 2005;569:751–760. doi: 10.1113/jphysiol.2005.097477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cohn JN, Levine TB, Olivari MT, Garberg V, Lura D, Francis GS, Simon AB, Rector T. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N Engl J Med. 1984;311:819–823. doi: 10.1056/NEJM198409273111303. [DOI] [PubMed] [Google Scholar]
- 80.Esler MD, Lambert GW, Ferrier C, Kaye DM, Wallin BG, Kalff V, Kelly MJ, Jennings GL. Central nervous system noradrenergic control of sympathetic outflow in normotensive and hypertensive humans. Clin Exp Hypertens. 1995;17:409–423. doi: 10.3109/10641969509087081. [DOI] [PubMed] [Google Scholar]
- 81.Guggilam A, Patel KP, Haque M, Ebenezer PJ, Kapusta DR, Francis J. Cytokine blockade attenuates sympathoexcitation in heart failure: cross-talk between nNOS, AT-1R and cytokines in the hypothalamic paraventricular nucleus. Eur J Heart Fail. 2008;10:625–634. doi: 10.1016/j.ejheart.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Leenen FH. Brain mechanisms contributing to sympathetic hyperactivity and heart failure. Circ Res. 2007;101:221–223. doi: 10.1161/CIRCRESAHA.107.158261. [DOI] [PubMed] [Google Scholar]
- 83.Yu Y, Zhang ZH, Wei SG, Serrats J, Weiss RM, Felder RB. Brain perivascular macrophages and the sympathetic response to inflammation in rats after myocardial infarction. Hypertension. 2010;55:652–659. doi: 10.1161/HYPERTENSIONAHA.109.142836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang K, Li YF, Patel KP. Blunted nitric oxide-mediated inhibition of renal nerve discharge within PVN of rats with heart failure. Am J Physiol Heart Circ Physiol. 2001;281:H995–H1004. doi: 10.1152/ajpheart.2001.281.3.H995. [DOI] [PubMed] [Google Scholar]
- 85.Zucker IH, Schultz HD, Li YF, Wang Y, Wang W, Patel KP. The origin of sympathetic outflow in heart failure: the roles of angiotensin II and nitric oxide. Prog Biophys Mol Biol. 2004;84:217–232. doi: 10.1016/j.pbiomolbio.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 86.Biancardi VC, Son SJ, Sonner PM, Zheng H, Patel KP, Stern JE. Contribution of central nervous system endothelial nitric oxide synthase to neurohumoral activation in heart failure rats. Hypertension. 2011;58:454–463. doi: 10.1161/HYPERTENSIONAHA.111.175810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Han TH, Lee K, Park JB, Ahn D, Park JH, Kim DY, Stern JE, Lee SY, Ryu PD. Reduction in synaptic GABA release contributes to target-selective elevation of PVN neuronal activity in rats with myocardial infarction. Am J Physiol Regul Integr Comp Physiol. 2010;299:R129–R139. doi: 10.1152/ajpregu.00391.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Potapenko ES, Biancardi VC, Zhou Y, Stern JE. Astrocytes modulate a postsynaptic NMDA-GABAA-receptor crosstalk in hypothalamic neurosecretory neurons. J Neurosci. 2013;33:631–640. doi: 10.1523/JNEUROSCI.3936-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang ZH, Francis J, Weiss RM, Felder RB. The renin-angiotensin-aldosterone system excites hypothalamic paraventricular nucleus neurons in heart failure. Am J Physiol Heart Circ Physiol. 2002;283:H423–H433. doi: 10.1152/ajpheart.00685.2001. [DOI] [PubMed] [Google Scholar]
- 90.Patel KP, Zhang K, Kenney MJ, Weiss M, Mayhan WG. Neuronal expression of Fos protein in the hypothalamus of rats with heart failure. Brain Res. 2000;865:27–34. doi: 10.1016/s0006-8993(00)02186-7. [DOI] [PubMed] [Google Scholar]
- 91.Vahid-Ansari F, Leenen FH. Pattern of neuronal activation in rats with CHF after myocardial infarction. Am J Physiol. 1998;275:H2140–H2146. doi: 10.1152/ajpheart.1998.275.6.H2140. [DOI] [PubMed] [Google Scholar]
- 92.Stern JE, Potapenko ES. Enhanced NMDA receptor-mediated intracellular calcium signaling in magnocellular neurosecretory neurons in heart failure rats. Am J Physiol Regul Integr Comp Physiol. 2013;305:R414–R422. doi: 10.1152/ajpregu.00160.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Potapenko ES, Biancardi VC, Zhou Y, Stern JE. Altered astrocyte glutamate transporter regulation of hypothalamic neurosecretory neurons in heart failure rats. Am J Physiol Regul Integr Comp Physiol. 2012;303:R291–R300. doi: 10.1152/ajpregu.00056.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Francis GS, Benedict C, Johnstone DE, Kirlin PC, Nicklas J, Liang CS, Kubo SH, Rudin-Toretsky E, Yusuf S. Comparison of neuroendocrine activation in patients with left ventricular dysfunction with and without congestive heart failure. A substudy of the Studies of Left Ventricular Dysfunction (SOLVD) Circulation. 1990;82:1724–1729. doi: 10.1161/01.cir.82.5.1724. [DOI] [PubMed] [Google Scholar]
- 95.Goldsmith SR, Francis GS, Cowley AW, Jr, Levine TB, Cohn JN. Increased plasma arginine vasopressin levels in patients with congestive heart failure. J Am Coll Cardiol. 1983;1:1385–1390. doi: 10.1016/s0735-1097(83)80040-0. [DOI] [PubMed] [Google Scholar]
- 96.Riegger GA, Liebau G, Bauer E, Kochsiek K. Vasopressin and renin in high output heart failure of rats: hemodynamic effects of elevated plasma hormone levels. J Cardiovasc Pharmacol. 1985;7:1–5. doi: 10.1097/00005344-198501000-00001. [DOI] [PubMed] [Google Scholar]
- 97.Szatalowicz VL, Arnold PE, Chaimovitz C, Bichet D, Berl T, Schrier RW. Radioimmunoassay of plasma arginine vasopressin in hyponatremic patients with congestive heart failure. N Engl J Med. 1981;305:263–266. doi: 10.1056/NEJM198107303050506. [DOI] [PubMed] [Google Scholar]
- 98.Goldsmith SR, Francis GS, Cowley AW., Jr Arginine vasopressin and the renal response to water loading in congestive heart failure. Am J Cardiol. 1986;58:295–299. doi: 10.1016/0002-9149(86)90065-2. [DOI] [PubMed] [Google Scholar]
- 99.Goldsmith SR, Francis GS, Cowley AW, Jr, Goldenberg IF, Cohn JN. Hemodynamic effects of infused arginine vasopressin in congestive heart failure. J Am Coll Cardiol. 1986;8:779–783. doi: 10.1016/s0735-1097(86)80417-x. [DOI] [PubMed] [Google Scholar]
- 100.Nakamura Y, Haneda T, Osaki J, Miyata S, Kikuchi K. Hypertrophic growth of cultured neonatal rat heart cells mediated by vasopressin V (1A) receptor. Eur J Pharmacol. 2000;391:39–48. doi: 10.1016/s0014-2999(99)00775-x. [DOI] [PubMed] [Google Scholar]
- 101.Packer M, Lee WH, Kessler PD, Gottlieb SS, Bernstein JL, Kukin ML. Role of neurohormonal mechanisms in determining survival in patients with severe chronic heart failure. Circulation. 1987;75:IV80–IV92. [PubMed] [Google Scholar]
- 102.Rouleau JL, Packer M, Moye L, de Champlain J, Bichet D, Klein M, Rouleau JR, Sussex B, Arnold JM, Sestier F, et al. Prognostic value of neurohumoral activation in patients with an acute myocardial infarction: effect of captopril. J Am Coll Cardiol. 1994;24:583–591. doi: 10.1016/0735-1097(94)90001-9. 10. [DOI] [PubMed] [Google Scholar]
- 103.Berkseth KE, Guyenet SJ, Melhorn SJ, Lee D, Thaler JP, Schur EA, Schwartz MW. Hypothalamic gliosis associated with high-fat diet feeding is reversible in mice: a combined immunohistochemical and magnetic resonance imaging study. Endocrinology. 2014;155:2858–2867. doi: 10.1210/en.2014-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Horvath TL, Sarman B, Garcia-Caceres C, Enriori PJ, Sotonyi P, Shanabrough M, Borok E, Argente J, Chowen JA, Perez-Tilve D, Pfluger PT, Bronneke HS, Levin BE, Diano S, Cowley MA, Tschop MH. Synaptic input organization of the melanocortin system predicts diet-induced hypothalamic reactive gliosis and obesity. Proc Natl Acad Sci USA. 2010;107:14875–14880. doi: 10.1073/pnas.1004282107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Salazar AP, Quagliotto E, Alves J, Oliveira FA, Saur L, Xavier LL, Pagnussat AS, Rasia-Filho A. Effect of prior exercise training and myocardia infarction-induced heart failure on the neuronal and glial densities and the GFAP-immunoreactivity in the posterodorsal medial amygdala of rats. Histol Histopathol. 2014;29:1423–1435. doi: 10.14670/HH-29.1423. [DOI] [PubMed] [Google Scholar]
- 106.Tomassoni D, Nwankwo IE, Gabrielli MG, Bhatt S, Muhammad AB, Lok-handwala MF, Tayebati SK, Amenta F. Astrogliosis in the brain of obese Zucker rat: a model of metabolic syndrome. Neurosci Lett. 2013;543:136–141. doi: 10.1016/j.neulet.2013.03.025. [DOI] [PubMed] [Google Scholar]
- 107.Wu Q, Howell MP, Palmiter RD. Ablation of neurons expressing agouti-related protein activates fos and gliosis in postsynaptic target regions. J Neurosci. 2008;28:9218–9226. doi: 10.1523/JNEUROSCI.2449-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]