Abstract
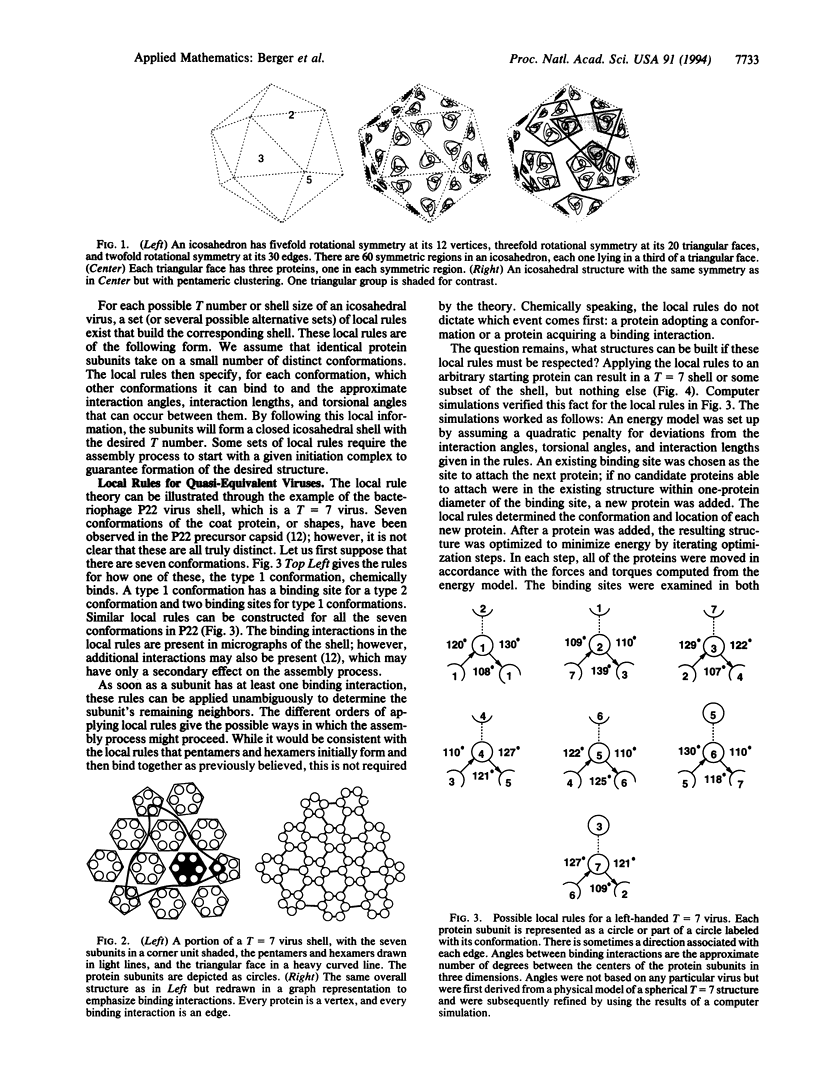
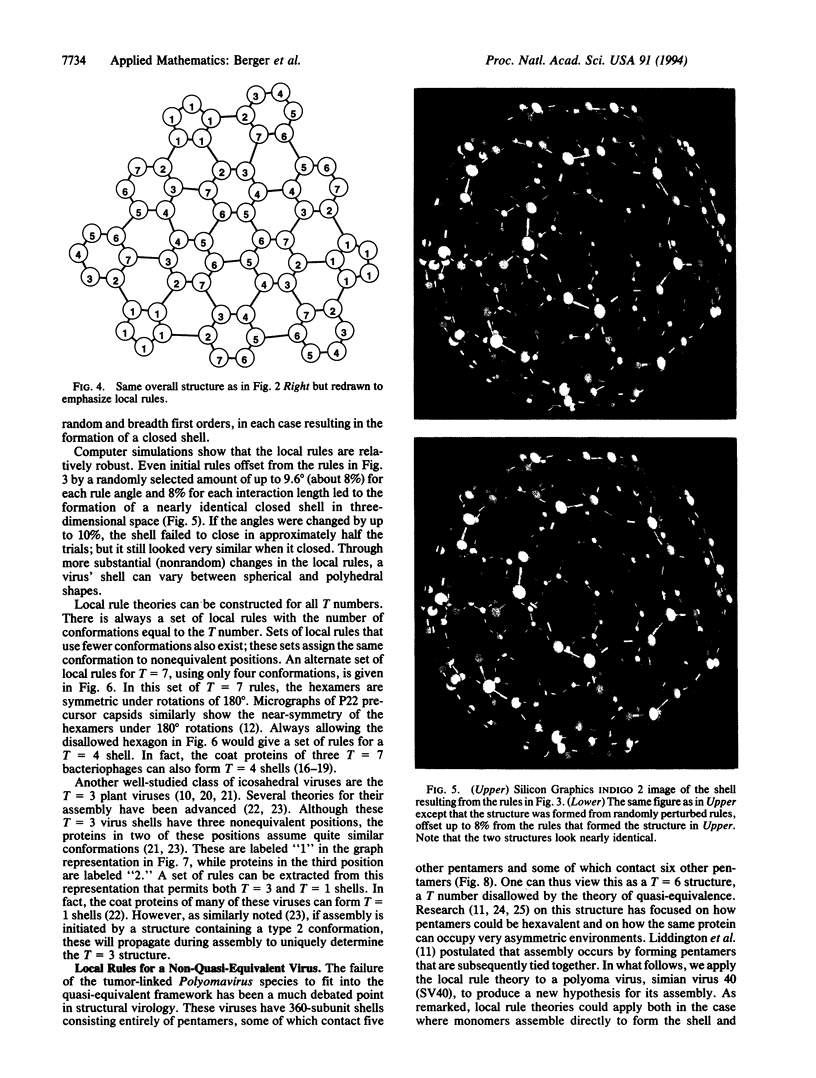
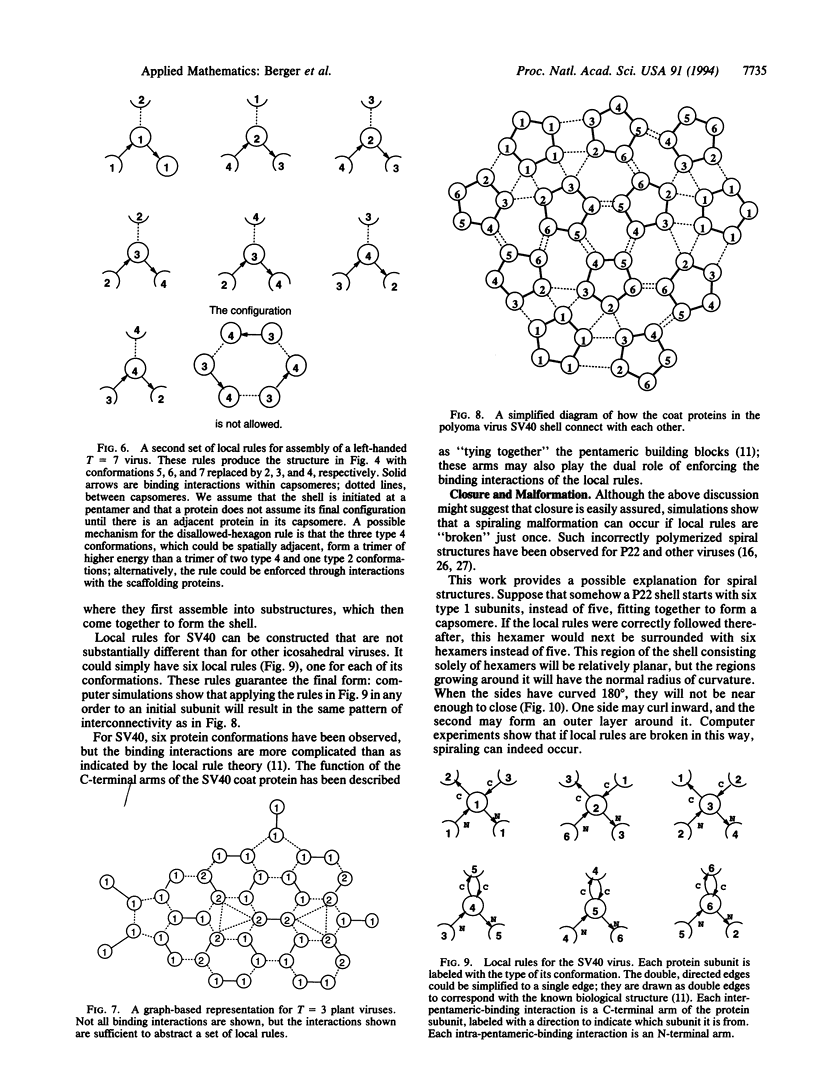
A local rule-based theory is developed which shows that the self-assembly of icosahedral virus shells may depend on only the lower-level interactions of a protein subunit with its neighbors--i.e., on local rules rather than on larger structural building blocks. The local rule theory provides a framework for understanding the assembly of icosahedral viruses. These include both viruses that fall in the quasiequivalence theory of Caspar and Klug and the polyoma virus structure, which violates quasi-equivalence and has puzzled researchers since it was first observed. Local rules are essentially templates for energetically favorable arrangements. The tolerance margins for these rules are investigated through computer simulations. When these tolerance margins are exceeded in a particular way, the result is a "spiraling" malformation that has been observed in nature.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agarwal M., Arthur M., Arbeit R. D., Goldstein R. Regulation of icosahedral virion capsid size by the in vivo activity of a cloned gene product. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2428–2432. doi: 10.1073/pnas.87.7.2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker T. S., Caspar D. L., Murakami W. T. Polyoma virus 'hexamer' tubes consist of paired pentamers. Nature. 1983 Jun 2;303(5916):446–448. doi: 10.1038/303446a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casjens S., King J. Virus assembly. Annu Rev Biochem. 1975;44:555–611. doi: 10.1146/annurev.bi.44.070175.003011. [DOI] [PubMed] [Google Scholar]
- Caspar D. L. Movement and self-control in protein assemblies. Quasi-equivalence revisited. Biophys J. 1980 Oct;32(1):103–138. doi: 10.1016/S0006-3495(80)84929-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dokland T., Lindqvist B. H., Fuller S. D. Image reconstruction from cryo-electron micrographs reveals the morphopoietic mechanism in the P2-P4 bacteriophage system. EMBO J. 1992 Mar;11(3):839–846. doi: 10.1002/j.1460-2075.1992.tb05121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnshaw W., King J. Structure of phage P22 coat protein aggregates formed in the absence of the scaffolding protein. J Mol Biol. 1978 Dec 25;126(4):721–747. doi: 10.1016/0022-2836(78)90017-7. [DOI] [PubMed] [Google Scholar]
- Eppler K., Wyckoff E., Goates J., Parr R., Casjens S. Nucleotide sequence of the bacteriophage P22 genes required for DNA packaging. Virology. 1991 Aug;183(2):519–538. doi: 10.1016/0042-6822(91)90981-g. [DOI] [PubMed] [Google Scholar]
- Fraenkel-Conrat H., Williams R. C. RECONSTITUTION OF ACTIVE TOBACCO MOSAIC VIRUS FROM ITS INACTIVE PROTEIN AND NUCLEIC ACID COMPONENTS. Proc Natl Acad Sci U S A. 1955 Oct 15;41(10):690–698. doi: 10.1073/pnas.41.10.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller M. T., King J. Assembly in vitro of bacteriophage P22 procapsids from purified coat and scaffolding subunits. J Mol Biol. 1982 Apr 15;156(3):633–665. doi: 10.1016/0022-2836(82)90270-4. [DOI] [PubMed] [Google Scholar]
- Galisteo M. L., King J. Conformational transformations in the protein lattice of phage P22 procapsids. Biophys J. 1993 Jul;65(1):227–235. doi: 10.1016/S0006-3495(93)81073-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogle J. M., Maeda A., Harrison S. C. Structure and assembly of turnip crinkle virus. I. X-ray crystallographic structure analysis at 3.2 A resolution. J Mol Biol. 1986 Oct 20;191(4):625–638. doi: 10.1016/0022-2836(86)90450-x. [DOI] [PubMed] [Google Scholar]
- Katsura I. Structure and inherent properties of the bacteriophage lambda head shell. IV. Small-head mutants. J Mol Biol. 1983 Dec 15;171(3):297–317. doi: 10.1016/0022-2836(83)90095-5. [DOI] [PubMed] [Google Scholar]
- King J., Casjens S. Catalytic head assembling protein in virus morphogenesis. Nature. 1974 Sep 13;251(5471):112–119. doi: 10.1038/251112a0. [DOI] [PubMed] [Google Scholar]
- King J., Griffin-Shea R., Fuller M. T. Scaffolding proteins and the genetic control of virus shell assembly. Q Rev Biol. 1980 Dec;55(4):369–393. doi: 10.1086/411981. [DOI] [PubMed] [Google Scholar]
- Liddington R. C., Yan Y., Moulai J., Sahli R., Benjamin T. L., Harrison S. C. Structure of simian virus 40 at 3.8-A resolution. Nature. 1991 Nov 28;354(6351):278–284. doi: 10.1038/354278a0. [DOI] [PubMed] [Google Scholar]
- Prasad B. V., Prevelige P. E., Marietta E., Chen R. O., Thomas D., King J., Chiu W. Three-dimensional transformation of capsids associated with genome packaging in a bacterial virus. J Mol Biol. 1993 May 5;231(1):65–74. doi: 10.1006/jmbi.1993.1257. [DOI] [PubMed] [Google Scholar]
- Prevelige P. E., Jr, Thomas D., King J. Nucleation and growth phases in the polymerization of coat and scaffolding subunits into icosahedral procapsid shells. Biophys J. 1993 Mar;64(3):824–835. doi: 10.1016/S0006-3495(93)81443-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevelige P. E., Jr, Thomas D., King J. Scaffolding protein regulates the polymerization of P22 coat subunits into icosahedral shells in vitro. J Mol Biol. 1988 Aug 20;202(4):743–757. doi: 10.1016/0022-2836(88)90555-4. [DOI] [PubMed] [Google Scholar]
- Rayment I., Baker T. S., Caspar D. L., Murakami W. T. Polyoma virus capsid structure at 22.5 A resolution. Nature. 1982 Jan 14;295(5845):110–115. doi: 10.1038/295110a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossmann M. G. Constraints on the assembly of spherical virus particles. Virology. 1984 Apr 15;134(1):1–11. doi: 10.1016/0042-6822(84)90267-8. [DOI] [PubMed] [Google Scholar]
- Silva A. M., Rossmann M. G. Refined structure of southern bean mosaic virus at 2.9 A resolution. J Mol Biol. 1987 Sep 5;197(1):69–87. doi: 10.1016/0022-2836(87)90610-3. [DOI] [PubMed] [Google Scholar]
- Sorger P. K., Stockley P. G., Harrison S. C. Structure and assembly of turnip crinkle virus. II. Mechanism of reassembly in vitro. J Mol Biol. 1986 Oct 20;191(4):639–658. doi: 10.1016/0022-2836(86)90451-1. [DOI] [PubMed] [Google Scholar]
- Teschke C. M., King J., Prevelige P. E., Jr Inhibition of viral capsid assembly by 1,1'-bi(4-anilinonaphthalene-5-sulfonic acid). Biochemistry. 1993 Oct 12;32(40):10658–10665. doi: 10.1021/bi00091a016. [DOI] [PubMed] [Google Scholar]





