Abstract
3-Hydroxydecanoic acid (3HD) was produced in Escherichia coli by mobilizing (R)-3-hydroxydecanoyl-acyl carrier protein-coenzyme A transacylase (PhaG, encoded by the phaG gene). By employing an isogenic tesB (encoding thioesterase II)-negative knockout E. coli strain, CH01, it was found that the expressions of tesB and phaG can up-regulate each other. In addition, 3HD was synthesized from glucose or fructose by recombinant E. coli harboring phaG and tesB. This study supports the hypothesis that the physiological role of thioesterase II in E. coli is to prevent the abnormal accumulation of intracellular acyl-coenzyme A.
Bacteria are capable of the synthesis of a family of biopolyesters, named polyhydroxyalkanoates (PHAs). As biodegradable (11) and biocompatible (7, 29, 32) thermoplastic polyesters, PHAs are structurally simple macromolecules synthesized by many bacteria grown on various carbon sources and are believed to act as sinks for carbon and reducing equivalents (19). Over 150 different PHAs have been identified (28). All of the monomer units of PHAs are enantiomerically pure, and each was in R configuration (17). 3-Hydroxyalkanoic acids (3HA), as PHA monomers, may serve as intermediates for the synthesis of many valuable chemicals, such as antibiotics, vitamins, aromatics, pheromones, and (S)-β-amino acids (17).
3HA monomers can be prepared either by chemical synthesis or by PHA degradation. Recently, 3HA monomers, including 3-hydroxybutyric acid (8) and 3-hydroxydecanoic acid (3HD) (31), were reported to be produced by recombinant E. coli harboring either phbA, encoding β-ketothiolase, and phbB, encoding acetoacetyl-coenzyme A (CoA) reductase, or phaG, encoding (R)-3-hydroxydecanoyl-acyl carrier protein (3HD-ACP)-CoA transacylase (PhaG), respectively. PhaG was found to link fatty acid de novo biosynthesis to PHA production by converting 3HD-ACP to (R)-3-hydroxydecanoyl-CoA (3HD-CoA) (24). However, how 3HD-CoA is converted to its corresponding free-acid derivative is unclear.
Fatty acyl thioesterase activity in E. coli extracts was first noted by Kass et al. (13). In subsequent experiments, two separable thioesterases were confirmed (1, 2). Thioesterase I, encoded by the tesA gene, was found to be specific for C12 to C18 acyl-CoA esters, but it was inactive for C6 to C10 acyl-CoA esters or 3-hydroxyacyl-CoA esters (20). Klinke et al. reported that cytosolic thioesterase I mediated acyl-ACP intermediates from the fatty acid de novo biosynthesis pathway to fatty acid β-oxidation in E. coli (14), although native thioesterase I was mainly observed as a periplasmic enzyme (6). On the other hand, thioesterase II, an enzyme encoded by the tesB gene that is composed of four identical subunits (3), has broader substrate specificity. Thioesterase II was reported to cleave C6 to C18 acyl-CoA esters, as well as 3-hydroxyacyl-CoA esters (3, 27), while it did not function as a chain-terminating enzyme in fatty acid synthesis (21). Thus, the exact physiological function of thioesterase II in vivo is not known.
In this study, thioesterase II was coexpressed with PhaG to clarify the physiological role of thioesterase II.
MATERIALS AND METHODS
Bacterial strains, plasmids, primers, and genetic techniques.
All bacterial strains and plasmids used in this study are described in Table 1 and Fig. 1, while the primers used (synthesized by BioAsia Co., Shanghai, China) are listed in Table 2. Conjugation was as described by Simon et al. (26), and Eshcrichia coli S17-1 was employed as a donor strain. All other genetic techniques were performed following either the manufacturers' instructions or standard procedures (25).
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant characteristics | Source or reference |
|---|---|---|
| E. coli | ||
| JM105 | supE endA sbcB15 hsdR4 rpsL thi Δ(lac-proAB) | 25 |
| CH01 | Thioesterase II-negative mutant of E. coli JM105; tesB::Cm(r) | This study |
| S17-1 | recA; harbors the tra genes of plasmid RP4 in the chromosome; proA thi-1 | 26 |
| P. putida GPp104 | PHA synthase-negative mutant of P. putida KT2442 | 10 |
| Plasmids | ||
| pBBR1MCS | Cm(r); broad host range; lacPOZ′ | 16 |
| pBBR1MCS-2 | Kn(r); broad host range; lacPOZ′ | 15 |
| pBluescript SK(−) | Ap(r); lacPOZ′ | 25 |
| pGEM-T | Ap(r);T-vector | Promega Co. |
| pTH19ksl | Kn(r); agp5 lacZ′ repAts1 | 9 |
| pLZZGPp | pBluescript SK(−) derivative, containing phaG gene of P. putida under the control of the lac promoter | 31 |
| pLZZH01 | pGEM-T derivative containing tesB gene of E. coli JM105 | This study |
| pLZZH08 | Deletion of the BamHI-BstBI fragment of plasmid pLZZH01 | This study |
| pLZZH09 | pBBR1MCS-2 derivative, containing tesB gene of E. coli JM105 under the control of the lac promoter | This study |
| pLZZH10 | Deletion of the PmlI—SnaBI fragment of plasmid pLZZGPp | This study |
| pLZZH11 | Insertion of the 1.7-kb BamHI-BstBI fragment of pBBR1MCS into plasmid pLZZH01 | This study |
| pLZZH12 | Insertion of the SacII (filled in)-SacI fragment of pLZZH11 into plasmid pTH19ksl digested with HincII and SacI | This study |
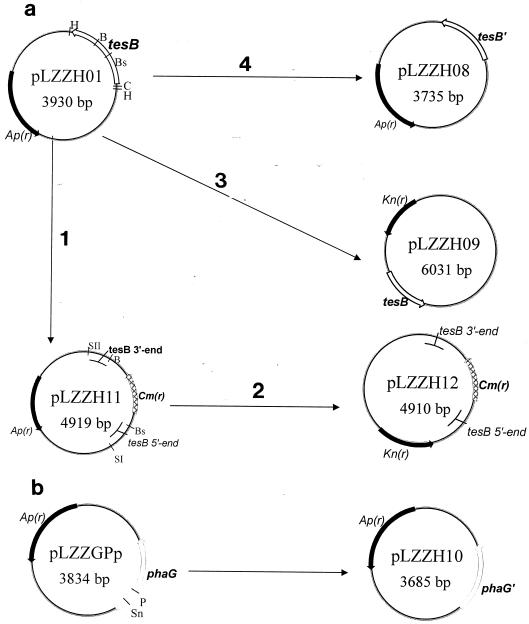
FIG. 1.
Scheme of plasmids used in this study. (a) The tesB gene from the E. coli JM105 genome was amplified by PCR with primers P1 and P3 and inserted into the pGEM-T vector to yield the starting plasmid, pLZZH01. The chloramphenicol resistance cassette from pBBR1MCS replaced the BstBI/BamHI fragment of tesB in pLZZH01, leading to the plasmid pLZZH11 (step 1). The SacII (filled in)-SacI fragment from pLZZH11 was inserted into the HincII/SacI sites of the temperature-sensitive vector pTH19ks1, resulting in pLZZH12 (step 2), which was used to construct the tesB knockout mutant E. coli strain. The HincII/ClaI fragment of pLZZH01, containing the tesB gene, was introduced into the corresponding sites of the vector pBBR1MCS-2 to yield pLZZH09 (step 3). BstBI/BamHI double-digested pLZZH01 was sequentially blunt ended by T4 polymerase and ligated to obtain the competitive plasmid pLZZH08 for the tesB transcriptional assay (step 4). (b) pLZZGPp was digested with PmlI and SnaBI, followed by ligation, resulting in pLZZH10, used for the transcriptional assay for phaG. tesB encodes thioesterase II; tesB′, the BstBI/BamHI fragment of tesB, was deleted; phaG encodes 3HD-ACP-CoA transacylase; phaG′, the SnaBI/PmlI fragment of phaG, was deleted. Ap(r), Kn(r), and Cm(r), ampicillin, kanamycin, and chloramphenicol resistance genes, respectively. B, BamHI; Bs, BstBI; C, ClaI; H, HincII; P, PmlI; SI, SacI; SII, SacII; Sn, SnaBI.
TABLE 2.
Primers used in this studya
| No. | Sequence |
|---|---|
| P1 | 5′-TCC ATC GAT GCG GCA GCT TTG TTA CT-3′ |
| P2 | 5′-GGA GCT AAG GAA GCT AAA ATG GAG AA-3′ |
| P3 | 5′-TTA GTC GAC GGT TTT CAC CTC CGG CT-3′ |
| P4 | 5′-TTG TAT GCT GCA AAA GAG ACC GT-3′ |
| P5 | 5′-TAC AGC AGC CAT TCA TTC AAA TT-3′ |
| P6 | 5′-ACG GCC TCG TTC GCC CAG A-3′ |
| P7 | 5′-GTC CAG GAA GTG GCC CGC A-3′ |
All primers were synthesized by BioAsia Co. (Shanghai, China).
Culture media.
Mineral salts medium (23) containing 0.5 g of (NH4)2SO4/liter and Luria-Bertani medium (25) were used in this study. All chemicals were analytical grade.
Flask fermentation conditions.
Recombinant E. coli strains were incubated at 37°C and 200 rpm for 48 h on a rotary shaker (series 25 D; NBS, New Brunswick, N.J.) in 100 ml of Luria-Bertani medium containing 100 mg of ampicillin/liter and 50 mg of kanamycin/liter. IPTG (isopropyl-β-d-thiogalactopyranoside;1 mmol/liter), fructose (20 g/liter), and triclosan (0.1 mg/liter) were added to the culture after 9, 12, and 24 h, respectively. Just before the addition of fructose, 10 ml of broth from each culture was taken and examined for thioesterase II activity and transcriptional intensity. The cellular dry weight (CDW) and extracellular 3HD were analyzed as described below.
PHA synthase (encoded by phaC)-negative mutant P. putida GPp104 strains were inoculated in mineral salts medium containing 1 mmol of IPTG/liter and 20 g of glucose/liter at 30°C and 200 rpm for 48 h. If necessary, 50 mg of kanamycin/liter was added to the broth at the beginning of inoculation. At 12 h, 10 ml of broth from each culture was sampled for transcriptional assay of phaG.
3HD analysis.
Liquid cultures were centrifuged at 10,000 × g for 15 min. The cells were washed twice and dried at 80°C for 8 h to determine the CDW. An aliquot of the supernatant (5 ml) was lyophilized for 48 h. Lyophilized materials were subjected to methanolysis and assayed with a gas chromatograph (18). This analysis was performed by injecting 1 μl of sample into a Hewlett-Packard model 6890 (Plus) series gas chromatograph system equipped with a 0.25-μm-diameter HP-INNOWax capillary column 30 m in length. The standard was a commercial product (H-3648; Sigma). The gas chromatography-mass spectroscopy (GC-MS) (AntoSystem XL GC-TurboMass; Perkin-Elmer, Norwalk, Conn.) analysis was performed using the sample for GC analysis described above. 3HD production ability was presented as the ratio of 3HD to CDW.
Thioesterase assay.
Cells sampled as described above were washed twice with 0.1 mol of Tris-hydrochloride buffer (pH 8.0)/liter and suspended in the same buffer, followed by homogenization on ice. Crude extracts were centrifuged at 1,000 × g and 4°C for 5 min. The resulting supernatants were monitored for thioesterase activity. The total-protein concentration was determined by the method of Bradford (5) using Coomassie Plus Protein Assay Reagent (Pierce) with a UV-visible-light spectrophotometer (Ultraspec 3300; Biochrom) and adjusted to 10 μg/ml. The assay for thioesterase II contained (per milliliter) 0.1 mmol Tris-hydrochloride buffer (pH 8.0), 100 nmol 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB; Sigma), 20 nmol decanoyl-CoA (Sigma), and 100 μl of crude cell extract. Reduction of DTNB by CoA liberated in the thioesterase reaction was measured at 412 nm (22). The initial rates were measured using a recording spectrophotometer (Specord 200; Analytik Jena AG, Jena, Germany) (22). A unit of enzyme activity was defined as the amount of the enzyme catalyzing cleavage of 1 μmol of decanoyl-CoA per min under the above-mentioned conditions. The molar extinction coefficient of reduced DTNB was taken as 13,600 (22).
RNA analysis.
Total RNA was isolated and purified using commercial kits (Shenergy Biocolor Biological Science and Technology Co., Shanghai, China). Purified total RNA was quantified using an Ultraspec 3300 UV-visible-light spectrophotometer. Reverse transcriptase (RT) PCR was performed with a cDNA Cycle kit (Invitrogen). The transcriptional analysis procedure was as described by Zhang and Cronan (30) employing Ex Taq DNA polymerase (TakaRa; Dalian, China). Briefly, 2 μg of isolated total RNA was reverse transcribed into cDNA in a 10-μl reaction mixture. Plasmid pLZZH08 or pLZZH10 was used as the competitive DNA and was carefully diluted in a series of concentrations that were determined using an Ultraspec 3300 UV-visible-light spectrophotometer. The reverse-transcriptase reaction product (1 μl) and different concentrations of competitive DNA (as specified in the figure legends [see Fig. 5 and 6]) were added to a 20-μl PCR mixture. The products were separated on a 2.5% agarose gel and stained with ethidium bromide, and the amounts of the products were estimated by video densitometry analysis using LabWorks Analysis software (UVP). The ratios of the fluorescence intensities of the PCR products of the competitive DNA to those of the RT-PCR products were plotted as a function of the concentration of the competitive DNA (30).
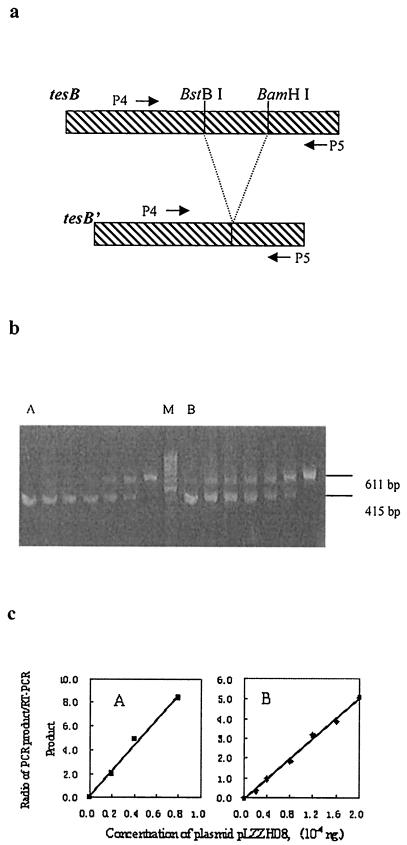
FIG. 5.
Semiquantitative RT-PCR assay of tesB transcription in both the phaG-negative strain E. coli(pBluescript SK(−), pBBR1MCS-2) (A) and the phaG-containing strain E. coli JM105(pLZZGPp, pBBR1MCS-2) (B). The strains were sampled from the flask cultures (see the text for details). (a) RT-PCR and PCR were performed by employing primers P4 and P5. Plasmid pLZZH08, harboring a DNA fragment, tesB′ (tesB without a central BstBI/BamHI fragment), was used as competitive DNA. (b and c) Competitive DNA (2.0 × 10−4, 1.6 × 10−4, 1.2 × 10−4, 0.8 × 10−4, 0.4 × 10−4, 0.2 × 10−4, and 0 ng) was added to lanes from left to right, respectively, in gels A and B. Lane M is a GeneRuler 50-bp DNA ladder (MBI). The fluorescence intensities of the bands on the agarose gel in each lane were quantified by densitometry following ethidium bromide staining. The ratio of the intensity of the PCR product (415 bp) of competitive DNA to that of the RT-PCR product (611 bp) was calculated for each reaction, and these ratios were plotted as a function of the competitive DNA concentration (c). The data are shown as means of six experiments. The error bars for SEM are not visible because they are smaller than the symbol size.
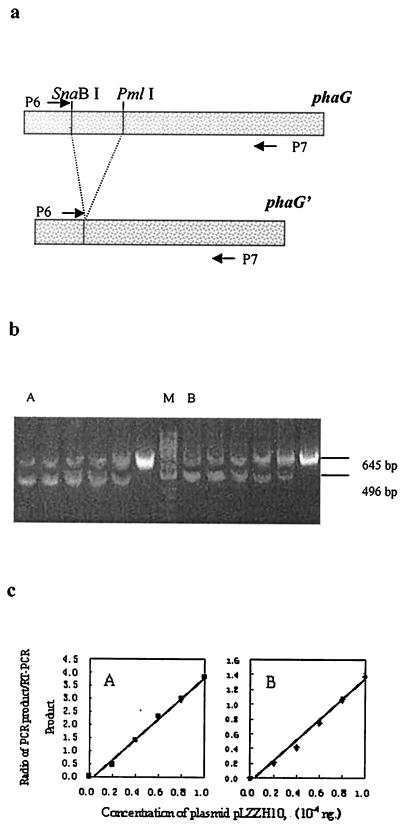
FIG. 6.
Semiquantitative RT-PCR assay of phaG transcription in the tesB-negative strain P. putida GPp104(pBBR1MCS-2) (A) and the tesB-containing strain P. putida GPp104(pLZZH09) (B). The strains were sampled from the flask cultures (described in Materials and Methods). (a) RT-PCR and PCR were performed by employing primers P6 and P7. Plasmid pLZZH10, harboring a DNA fragment, phaG′ (phaG without a central SnaBI/PmlI fragment), was used as competitive DNA. (b and c) Competitive DNA (1.0 × 10−4, 0.8 × 10−4, 0.6 × 10−4, 0.4 × 10−4, 0.2 × 10−4, and 0 ng) was added to lanes from left to right, respectively, in gels A and B. Lane M is a GeneRuler 50-bp DNA ladder (MBI). The fluorescence intensities of the bands on the agarose gel in each lane were quantified by densitometry following ethidium bromide staining. The ratio of the intensity of the PCR product (496 bp) of competitive DNA to that of the RT-PCR product (645 bp) was calculated for each reaction, and these ratios were plotted as a function of the competitive DNA concentration (c). The data are shown as means of five experiments. The error bars for SEM are not visible because they are smaller than the symbol size.
Statistical analysis.
The data were presented as means ± standard errors of the mean (SEM). Statistical comparisons were performed using the Student t test with SPSS software. P values of <0.05 were considered statistically significant.
RESULTS
Construction and characterization of an isogenic tesB knockout mutant of E. coli.
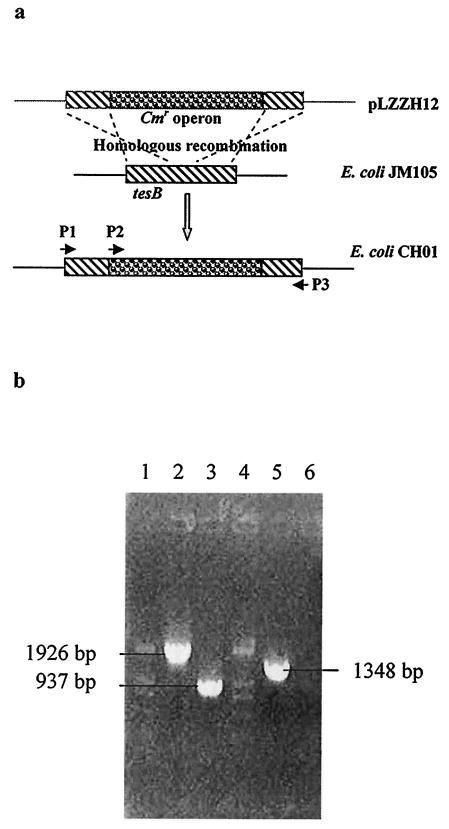
The coding region of tesB (including the native ribosome binding site) from E. coli JM105 was amplified by PCR employing primers P1 and P3 (Table 2). The ClaI and HincII restriction sites were introduced into the PCR product. The 929-bp product was inserted into the commercial vector pGEM-T (Promega), resulting in plasmid pLZZH01 (Fig. 1a). The DNA sequence of tesB obtained from the inserted DNA fragment (sequenced by BioAsia Co.) exactly matched that found in the GenBank database (accession number AE000151). The isogenic tesB knockout mutant E. coli CH01 was obtained by replacing a central 200-bp region of tesB with a chloramphenicol resistance cassette from pBBR1MCS and employing a temperature-sensitive vector, pTH19ks1 (Fig. 1a and 2). The isogenic mutant was verified by PCR using primers P1 and P3 and primers P2 and P3, respectively (Fig. 2).
FIG. 2.
Scheme of construction (a) and identification (b) of E. coli CH01. (a) The derivative of the temperature-sensitive vector pTh19ks1, pLZZH12, harboring a DNA fragment in which the chloramphenicol resistance cassette from pBBR1MCS replaced the BstBI/BamHI fragment of tesB, was introduced into E. coli JM105. Due to homologous recombination, a tesB knockout mutant strain, E. coli CH01, was obtained. P1 anneals with the 5′ end of tesB, P2 anneals with the chloramphenicol resistance gene (Cmr), and P3 anneals with the 3′ end of tesB. (b) Lanes 1 and 4, DL2000 DNA marker (TaKaRa); lanes 2 and 3, PCR analysis of E. coli CH01 and E. coli JM105 with primers P1 and P3 (the expected product sizes determined from sequence data were 1,926 and 937 bp, respectively); lanes 5 and 6, PCR analysis of E. coli CH01 with primers P2 and P3 (lane5; the expected product size was 1,348 bp) and of the control strain, E. coli JM105, with the same primers (lane 6; no product was expected).
3HD is synthesized by various recombinant E. coli strains.
In order to evaluate the functionality of thioesterase II in E. coli, we produced various E. coli recombinants and assessed the 3HA production in these cells. Extracellular 3HD was confirmed by GC-MS (Fig. 3). Plasmid pLZZH01 was digested with ClaI and HincII. The 918-bp tesB gene fragment was inserted into the ClaI and SmaI restriction sites in vector pBBR1MCS-2, leading to plasmid pLZZH09, which contained the tesB gene under the control of the lac promoter (Fig. 1a). Overexpression of tesB in recombinant E. coli JM105 did not result in 3HD production, and tesB-containing knockout mutants grew normally (Table 3). However, compared with E. coli JM105 harboring only phaG, the yield of extracellular 3HD produced by the same strain harboring both tesB and phaG was significantly increased (Table 3). Extracellular 3HD production by the tesB-negative recombinant E. coli CH01(pLZZGPp, pBBR1MCS-2) was ∼3% of the CDW, significantly lower than that of the tesB-positive strain E. coli JM105(pLZZGPp, pBBR1MCS-2), which was ∼30% of the CDW. Introduction of the tesB gene in E. coli CH01 [as E. coli CH01(pLZZGPp, pLZZH09)] returned 3HD production to the same level as that of the tesB-positive control strain [as E. coli JM105(pLZZGPp, pLZZH09)] (Table 3). The results revealed that thioesterase II plays an important role in converting 3HD-CoA to free 3HD in E. coli.
FIG. 3.
Identification of extracellular 3-hydroxydecanoic acid. Supernantant (5 ml) was lyophilized for 48 h. The lyophilized material was subjected to methanolysis, and a GC-MS assay was performed (AntoSystem XL GC-TurboMass).
TABLE 3.
Extracellular accumulation of 3HD by various recombinant E. coli strainsa
| E. coli strain | Plasmid | CDW (g/liter) | 3HD (mg/liter) | %3HD/CDW (wt/wt) |
|---|---|---|---|---|
| JM105 | pBluescript SK(−), pBBR1MCS-2 | 1.84 ± 0.05 | NDb | |
| pBluescript SK (−), pLZZH09 | 1.75 ± 0.03 | ND | ||
| pLZZGPp, pBBR1MCS-2 | 2.11 ± 0.04 | 642 ± 12 | 30.4 ± 0.5 | |
| pLZZGPp, pLZZH09 | 2.20 ± 0.04 | 1,021 ± 22 | 45.9 ± 0.8 | |
| CH01 | pBluescript SK(−), pBBR1MCS-2 | 1.81 ± 0.03 | ND | |
| pLZZGPp, pBBR1MCS-2 | 2.10 ± 0.05 | 72 ± 6 | 3.4 ± 0.1 | |
| pLZZGPp, pLZZH09 | 2.21 ± 0.03 | 956 ± 18 | 43.3 ± 0.6 |
Recombinant E. coli strains were cultivated in 500-ml conical flasks containing 100 ml of Luria-Bertain medium supplemented with 100 mg of ampicillin/liter and 50 mg of kanamycin/liter according to the resistance properties of the plasmids (Table 1 and Fig. 1). E. coli CH01 is the isogenic tesB-negative knockout mutant of E. coli JM105. Plasmid pLZZGPp is a derivative of the vector pBluescript SK(−) harboring phaG under the control of the lac promoter, while pLZZH09 is a derivative of the vector pBBR1MCS-2 harboring tesB under the control of the lac promoter. Cells were grown at 37°C and 200 rpm for 48 h; 1 mmol of IPTG/liter, 20 g of fructose/liter, and 0.1 mg of triclosan/liter were added to the culture after 9, 12, and 24 h, respectively. The data are shown as means ± SEM (n = 6).
ND, not detectable.
Thioesterase II assay of recombinant E. coli strains.
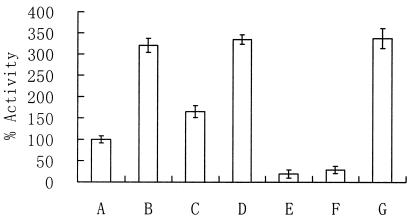
Sole heterogenous expression of phaG in E. coli JM105 was accompanied by a 1.5-fold increase in thioesterase II activity compared with the control strain, E. coli JM105(pBluescript SK(−), pBBR1MSC-2), which was 15.8 mU/mg of total protein and was defined as 100% (Fig. 4). No effect was observed when the host was a tesB-negative strain, E. coli CH01.
FIG. 4.
Enzyme activity assay of thioesterase II enzymes from recombinant E. coli strains. The mean of three independent samples of E. coli JM105(pBluescript SK(−), pBBR1MCS-2) was 15.8 mU/mg of protein, which was defined as 100%. Bars: A, E. coli JM105(pBluescript SK(−), pBBR1MCS-2); B, E. coli JM105(pBluescript SK(−), pLZZH09); C, E. coli JM105(pLZZGPp, pBBR1MCS-2); D, E. coli JM105(pLZZGPp, pLZZH09); E, E. coli CH01(pBluescript SK(−), pBBR1MCS-2); F, E. coli CH01(pLZZGPp, pBBR1MCS-2); G, E. coli CH01(pLZZGPp, pLZZH09). E. coli CH01 is the isogenic tesB-negative mutant of E. coli JM105. Plasmid pLZZGPp is a derivative of the vector pBluescript SK(−) harboring phaG under the control of the lac promoter, while pLZZH09 is a derivative of vector pBBR1MCS-2 harboring tesB under the control of the lac promoter. The data are shown as means ± SEM (n = 3).
Transcriptional assay of tesB.
Semiquantitative RT-PCR was employed to study the level of tesB transcription in phaG-containing E. coli JM105(pLZZGPp, pBBR1MCS-2) sampled from the flask cultures with E. coli JM105(pBluescript SK(−), pBBR1MCS-2) as a control. The principle of this method is the application of a known concentration of plasmid pLZZH08 to compete with the RT-PCR product. To obtain pLZZH08, pLZZH01 was digested with BamHI and BstBI, followed by blunt-ending and ligation processes (Fig. 1a). RT-PCR was carried out by adding known concentrations of competitive DNA to the reaction mixtures as described in Materials and Methods. Total-RNA preparations from E. coli JM105(pBluescript SK(−), pBBR1MCS-2) and E. coli JM105(pLZZGPp, pBBR1MCS-2) were used as RT reaction templates, and the concentration of cDNA formed was taken to be proportional to the mRNA concentration. P4 and P5 were annealed to central sequences of the tesB gene (Fig. 5a); thus, the products of tesB transcripts were 611 and 415 bp for pLZZH08 (Fig. 5b). The ratios of the fluorescence intensities of the PCR products to those of the RT-PCR products were plotted as a function of the concentration of the competitive DNA (Fig. 5c). When the ratio was 1, the molar concentration of the competitive DNA added to the reaction mixture was identical to the molar concentration of the tesB cDNA, which was proportional to the tesB mRNA concentration (30). This result revealed a remarkable increase in transcription of tesB when phaG was expressed in E. coli JM105.
Heterologous expression of tesB in Pseudomonas putida GPp104.
Heterologous expression of tesB in P. putida GPp104 led to the extracellular accumulation of 3HD, while no 3HD was detected in the growth media inoculated with P. putida GPp104 and recombinant P. putida GPp104 harboring only the vector pBBR1MCS-2 (Table 4). On the other hand, pLZZH10, the derivative plasmid of pLZZGPp (Fig. 1b), was used as the competitive template for semiquantitative RT-PCR assay of phaG with the primer pair P6 and P7 (Fig. 6a). The PCR product of pLZZH10 was 496 bp, while the RT-PCR product was 645 bp (Fig. 6b). Interestingly, heterologous expression of tesB in P. putida GPp104 led to a significant increase in phaG transcription (Fig. 6c).
TABLE 4.
Extracellular accumulation of 3HD by recombinant P. putida GPp104 strains from glucosea
| Plasmid | CDW (g/liter) | 3HD (mg/liter) |
|---|---|---|
| 1.13 ± 0.06 | NDb | |
| pBBR1MCS-2 | 0.77 ± 0.05 | ND |
| pLZZH09 | 0.97 ± 0.03 | 208 ± 10 |
Recombinant P. putida GPp104 strains were cultivated in 500-ml conical flasks containing 100 ml of mineral salts medium containing 1 mmol of IPTG/liter and 20 g of glucose/liter. If necessary, 50 mg of kanamycin/liter was added to the broth at the beginning of fermentation. Plasmid pLZZH09 was the derivative of the vector pBBR1MCS-2 harboring tesB under the control of the lac promoter. The cells were grown at 30°C and 200 rpm for 48 h. The data are shown as means ± SEM (n = 5).
ND, not detectable.
DISCUSSION
As monomers of PHA, 3HA have great potential for many applications as intermediates for the synthesis of valuable compounds (17). The introduction of β-ketothiolase (phbA) and acetoacetyl-CoA reductase (phbB) genes from Ralstonia eutropha and phosphotransbutyrylase (ptb) and butyrate kinase (buk) genes from Clostridium acetobutylicum into E. coli established a pathway for extracellular 3-hydroxybutyric acid production starting from acetyl-CoA (8). Expression of PhaG could lead to extracellular accumulation of valuable medium-chain-length-3HA, such as 3HD, by recombinant E. coli from glucose or fructose (31). This study demonstrated that PhaG directs the fatty acid de novo biosynthesis pathway to 3HD production, as well as to PHA synthesis (24). However, medium-chain-length-3HA synthesis utilizing PhaG involves a complex process.
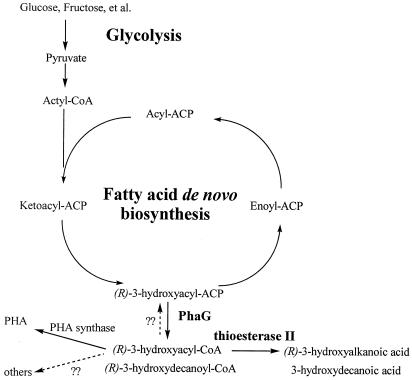
In this study, for the first time, tesB of E. coli was expressed in vivo together with phaG in order to understand how the two genes affect 3HD biosynthesis. The engineered pathway used carbon sources from glycolysis, followed by fatty acid de novo biosynthesis. Next, PhaG converted 3HD-ACP to 3HD-CoA (24). Finally, the thioester of 3HD-CoA was cleaved to yield 3HD (Fig. 7). As a cytosolic tetrameric protein (3), thioesterase II plays an important role in this pathway, as demonstrated by three facts: (i) in the presence of PhaG, overexpression of tesB in E. coli led to increased 3HD synthesis; (ii) in contrast to a tesB-positive strain, 3HD production was lowered significantly in a tesB-negative E. coli strain harboring phaG; and (iii) the reintroduction of tesB restored 3HD production ability (Table 3).
FIG. 7.
Schematic illustration of biosynthesis pathway for 3HD and PHA from unrelated carbon sources, such as glucose and fructose. 3HD-ACP-CoA transacylase (PhaG) is encoded by the gene phaG from P. putida, while thioesterase II is encoded by the gene tesB from E. coli. This engineered pathway began with glycolysis, followed by fatty acid de novo biosynthesis. Under the catalysis of the transacylase, 3HD-ACP is converted to 3HD-CoA. 3HD-CoA is either cleaved by thioesterase II to yield 3HD or polymerized by PHA synthase to yield PHA. In native PHA synthase-negative P. putida GPp104, 3HD-CoA may be converted to other intermediates or back to 3HD-ACP, due to the absence of thioesterase II.
Interestingly, when phaG was expressed in E. coli, both the activity and the transcription level of thioesterase II increased (Fig. 4 and 5). It appeared that the abnormal accumulation of 3HD-CoA induced by PhaG up-regulated tesB gene transcription. On the other hand, when tesB was expressed in the phaC-negative strain P. putida GPp104, 3HD produced by the recombinant strain was detected in the culture, and the transcription level of phaG was remarkably increased (Table 4 and Fig. 6). These results indicate a mechanism for 3HD synthesis like that described in Fig. 7. As is known, in a phaC-positive P. putida strain, 3HD-CoA was removed by PHA synthase, resulting in the accumulation of PHA polymers as a sink for reducing power (19). When PhaG was introduced in recombinant E. coli, 3HD-CoA accumulated to an abnormally high level. Due to the absence of PHA synthase in E. coli, thioesterase II seems to play a role in reducing the accumulation of 3HD-CoA by removing CoA ester to release its free-acid derivative (Fig. 7). In phaC-negative P. putida GPp104, there should be another mechanism to reduce CoA ester accumulation, while the heterologous expression of tesB led to increased transcription of phaG by removing the product of the transacylation reaction catalyzed by PhaG (Fig. 6 and 7). Thus, both PhaG and thioesterase II are essential for extracellular production of 3HD.
Surprisingly, two thioesterases whose activities are similar to those of E. coli were found in Rhodopseudomonas sphaeroides (4). Similar thioesterase complementation occurring in dissimilar bacteria suggests that these enzymes play important roles (22). However, the physiological function of thioesterase II was not fully understood, as no obvious physiological or biochemical defect was observed in E. coli with tesB overexpression or deletion (21, 22). However, the high specificity for acyl-CoA substrates suggests that one role of this enzyme could be to prevent the accumulation of intracellular acyl-CoA (27), although the normal cellular enzyme level is generally sufficient to preclude acyl-CoA accumulation (12). In the present study, by employing a direct 3HD biosynthesis pathway for high-level accumulation of intracellular 3HD-CoA, thioesterase II was indeed found to play a role in preventing the accumulation of intracellular acyl-CoA and thus in maintaining an appropriate acyl-CoA pool in vivo.
Acknowledgments
This research was supported by the National Nature Science Foundation for Distinguished Young Scholars (grant no. 30225001). The State 863 Funds (no. 2002AA213051) also contributed financially to this study.
The phaG gene of P. putida and the phaC-negative strain P. putida GPp104 were kindly provided by A. Steinbüchel of the Westfälische Wilhelms-Universität Münster, Münster, Germany, and B. Witholt of the University of Groningen, Groningen, The Netherlands, respectively. The temperature-sensitive vector pTH19ks1 was provided by the Cloning Vector Collection, Department of Microbial Genetics, National Institute of Genetics, Mishima, Japan. We thank Peiyu Liao and Powen Yan for their assistance in the early stages of this project. We also gratefully acknowledge the constructive comments and warmhearted help of Cheng-Dui Yang in GC-MS analysis and Zheng Wang in the thioesterase II activity assay.
REFERENCES
- 1.Barnes, E. M., Jr., A. C. Swindell, and S. J. Wakil. 1970. Purification and properties of a palmityl thioesterase II from Escherichia coli. J. Biol. Chem. 245:3122-3128. [PubMed] [Google Scholar]
- 2.Barnes, E. M., Jr., and S. J. Wakil. 1968. Studies on the mechanism of fatty acid synthesis. Purification and general properties of palmityl thioesterase. J. Biol. Chem. 243:2955-2962. [PubMed] [Google Scholar]
- 3.Bonner, W. M., and K. Bloch. 1972. Purification and properties of fatty acyl thioesterase I from Escherichia coli. J. Biol. Chem. 247:3123-3133. [PubMed] [Google Scholar]
- 4.Boyce, S. G., and D. R. Leuking. 1984. Purification and characterization of a long-chain acyl coenzyme A thioesterase from Rhodopseudomonas sphaeroides. Biochemistry 23:141-147. [Google Scholar]
- 5.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 6.Cho, H., and J. E. Cronan, Jr. 1993. Escherichia coli thioesterase I, molecular cloning and sequencing of the structural gene and identification as a periplasmic enzyme. J. Biol. Chem. 268:9238-9245. [PubMed] [Google Scholar]
- 7.Deng, Y., X. S. Lin, Z. Zheng, J. G. Deng, J. C. Chen, H. Ma, and G. Q. Chen. 2003. Poly(hydroxybutyrate-co-hydroxyhexanoate) promoted production of extracellular matrix of articular cartilage chondrocytes in vitro. Biomaterials 24:4273-4281. [DOI] [PubMed] [Google Scholar]
- 8.Gao, H. J., Q. Wu, and G. Q. Chen. 2002. Enhanced production of d-(−)-3-hydroxybutyric acid by recombinant Escherichia coli. FEMS Microbiol. Lett. 213:59-65. [DOI] [PubMed] [Google Scholar]
- 9.Hashimoto-Gotoh, T., M. Yamaguchi, K. Yasojima, A. Tsuijimura, Y. Wakabayashi, and Y. Watanabe. 2000. A set of temperature sensitive-replication/-segregation and temperature resistant plasmid vectors with different copy numbers and in an isogenic background (chloramphenicol, kanamycin, lacZ, repA, par, polA). Gene 241:185-191. [DOI] [PubMed] [Google Scholar]
- 10.Huisman, G. W., E. Wonink, R. Meima, B. Kazemier, O. Terostra, and B. Witholt. 1991. Metabolism of poly(3-hydroxyalkanoates) by Pseudomonas oleovorans: identification and sequences of genes and function of the encoded proteins in the synthesis and degradation of PHA. J. Biol. Chem. 266:2191-2198. [PubMed] [Google Scholar]
- 11.Jendrossek, D., A. Schirmer, and H. G. Schlegel. 1996. Biodegradation of polyhydroxyalkanoic acids. Appl. Microbiol. Biotechnol. 46:451-463. [DOI] [PubMed] [Google Scholar]
- 12.Jiang, P., and J. E. Cronan, Jr. 1994. Inhibition of fatty acid synthesis in Escherichia coli in the absence of phospholipid synthesis and release of inhibition by thioesterase action. J. Bacteriol. 176:2814-2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kass, L. R., D. J. H. Brock, and K. Bloch. 1967. β-Hydroxydecanoyl thioester dehydrase. I. Purification and properties. J. Biol. Chem. 242:4418-4431. [PubMed] [Google Scholar]
- 14.Klinke, S., Q. Ren, B. Witholt, and B. Kessler. 1999. Production of medium-chain-length poly(3-hydroxyalkanoates) from gluconate by recombinant Escherichia coli. Appl. Environ. Microbiol. 65:540-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kovach, M. E., P. H. Elzer, D. S. Hill, G. T. Robertson, M. A. Farris, R. M. Roop II, and K. M. Peterson. 1995. Four new derivatives of the broad-host-range cloning vector pBBRMCS, carrying different antibiotic-resistance cassettes. Gene 166:175-176. [DOI] [PubMed] [Google Scholar]
- 16.Kovach, M. E., R. W. Phillips, P. H. Elzer, R. M. Roop II, and K. M. Peterson. 1994. pBBR1MCS: a broad-host-range cloning vector. BioTechniques 16:800-802. [PubMed] [Google Scholar]
- 17.Lee, S. Y., S. H. Park, Y. Lee, and S. H. Lee. 2003. Production of Chiral and other valuable compounds from microbial polyesters, p. 375-387. In A. Steinbüchel (ed.), Biopolymers, vol. 4. Wiley-Vch. Verkag GmbH, Weinheim, Germany. [Google Scholar]
- 18.Lee, Y., S. H. Park, I. T. Lim, K. Han, and S. Y. Lee. 2000. Preparation of alkyl (R)-(−)-3-hydroxybutyrate by acidic alcoholysis of poly-(R)-(−)-3-hydroxybutyrate. Enzyme Microb. Technol. 27:33-36. [DOI] [PubMed] [Google Scholar]
- 19.Madison, L. L., and G. M. Huisman. 1999. Metabolic engineering of poly(3-hydroxyakanoates): from DNA to plastic. Microbiol. Mol. Biol. Rev. 63:21-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Magnuson, K., S. Jackowski, C. O. Rock, and J. E. Cronan, Jr. 1993. Regulation of fatty acid biosynthesis in Escherichia coli. Microbiol. Rev. 57:522-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naggert, J., M. L. Narasimhan, L. DeVeaux, H. Cho, Z. I. Randhawa, J. E. Cronan, Jr., B. N. Green, and S. Smith. 1991. Cloning, sequencing, and characterization of Escherichia coli thioesterase II. J. Biol. Chem. 266:11044-11050. [PubMed] [Google Scholar]
- 22.Narasimhan, M. L., J. L. Lampi, and J. E. Cronan, Jr. 1986. Genetic and biochemical characterization of an Escherichia coli K-12 mutant deficient in acyl-coenzyme A thioesterase II. J. Bacteriol. 165:911-917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramsay, B. A., K. Lomaliza, C. Chavarie, B. Dubé, P. Bataille, and J. A. Ramsay. 1990. Production of poly-(β-hydroxybutyric-co-β-hydroxyvaleric) acids. Appl. Environ. Microbiol. 56:2093-2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rehm, B. H. A., N. Kruger, and A. Steinbüchel. 1998. A new metabolic link between fatty acid de novo synthesis and polyhydroxyalkanoic acid synthesis. J. Biol. Chem. 273:24044-24051. [DOI] [PubMed] [Google Scholar]
- 25.Sambrook, J., and D. W. Russel. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 26.Simon, R., U. Priefer, and A. Pühler. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram-negative bacteria. Bio/Technology 1:784-791. [Google Scholar]
- 27.Spencer, A. K., A. D. Greenspan, and J. E. Cronan, Jr. 1978. Thioesterase I and II of Escherichia coli. J. Biol. Chem. 253:5922-5926. [PubMed] [Google Scholar]
- 28.Steinbüchel, A., and T. Lutke-Eversloh. 2003. Metabolic engineering and pathway construction for biotechnological production of relevant polyhydroxyalkanoates in microorganisms. Biochem. Eng. J. 16:81-96. [Google Scholar]
- 29.Williams, S. F., D. P. Martin, D. H. Horowitz, and O. P. Peoples. 1999. PHA applications: addressing the price performance issue. I. Tissue engineering. Int. J. Biol. Macromol. 25:111-121. [DOI] [PubMed] [Google Scholar]
- 30.Zhang, Y., and J. E. Cronan, Jr. 1998. Transcriptional analysis of essential genes of the Escherichia coli fatty acid biosynthesis gene cluster by functional replacement with the analogous Salmonella typhimurium gene cluster. J. Bacteriol. 180:3295-3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zheng, Z., M. J. Zhang, G. Zhang, and G. Q. Chen. 2004. Production of 3-hydroxydecanoic acid by recombinant Escherichia coli HB101 harboring phaG gene. Antonie Leeuwenhoek 85:93-101. [DOI] [PubMed] [Google Scholar]
- 32.Zheng, Z., Y. Deng, X. S. Lin, L. X. Zhang, and G. Q. Chen. 2003. Induced production of rabbit articular cartilage-derived chondrocytes collagen II on polyhydroxyalkanoate blends. J. Biomater. Sci. Polym. Ed. 14:615-624. [DOI] [PubMed] [Google Scholar]