Abstract
Background
The FDA is considering reducing the nicotine content in tobacco products as a population-based strategy to reduce tobacco addiction. Research is needed to determine the threshold level of nicotine needed to maintain smoking and the extent of compensatory smoking that could occur during nicotine reduction. Sources of variability in these measures across sub-populations also need to be identified so that policies can take into account the risks and benefits of nicotine reduction in vulnerable populations.
Methods
The present study examined these issues in a rodent nicotine self- administration model of nicotine reduction policy to characterize individual differences in nicotine reinforcement thresholds, degree of compensation, and elasticity of demand during progressive reduction of the unit nicotine dose. The ability of individual differences in baseline nicotine intake and nicotine pharmacokinetics to predict responses to dose reduction was also examined.
Results
Considerable variability in the reinforcement threshold, compensation, and elasticity of demand was evident. High baseline nicotine intake was not correlated with the reinforcement threshold, but predicted less compensation and less elastic demand. Higher nicotine clearance predicted low reinforcement thresholds, greater compensation, and less elastic demand. Less elastic demand also predicted lower reinforcement thresholds.
Conclusions
These findings suggest that baseline nicotine intake, nicotine clearance, and the essential value of nicotine (i.e. elasticity of demand) moderate the effects of progressive nicotine reduction in rats and warrant further study in humans. They also suggest that smokers with fast nicotine metabolism may be more vulnerable to the risks of nicotine reduction.
Keywords: Nicotine self-administration, Tobacco Control Policy, Behavioral economics, Rat, Pharmacokinetics, Reinforcement threshold
1. INTRODUCTION
The Family Smoking Prevention and Tobacco Control Act (FSPTCA) provides the FDA regulatory authority over myriad aspects of tobacco product manufacturing, sales, and marketing. One of the provisions allows the FDA to mandate a reduction of the nicotine content in tobacco products to non-addictive levels (but not to zero) as a population-wide strategy to improve public health by reducing initiation of tobacco use in adolescents and facilitating cessation in current tobacco users (Benowitz and Henningfield, 2013; Hatsukami et al., 2013a). Several recent clinical studies have demonstrated proof of principle and initial feasibility of this approach (Benowitz et al., 2012; Hatsukami et al., 2010a). Despite the potential public health benefits of such a policy, comprehensive regulatory research is still needed to address numerous questions regarding the viability, efficacy, and safety of an industry-wide nicotine reduction policy (see Donny et al., 2012; Hatsukami et al., 2013a).
In order for a nicotine reduction policy to facilitate the greatest net population decrease in smoking, the nicotine content in tobacco products must be reduced to a level that is no longer capable of reinforcing tobacco use (Sofuoglu and LeSage, 2012). Because the FDA cannot simply mandate elimination of nicotine from tobacco products, they must determine the reinforcement threshold for nicotine (i.e., the lowest dose of nicotine in a product that can reinforce (i.e., increase or maintain) self-administration of that product) to guide setting nicotine standards accordingly. This requires extensive nicotine dose-response studies in order to arrive at the most accurate estimates of the reinforcement threshold. Currently, only limited data from relevant human and animal research pertinent to this issue is available, rendering attempts to estimate this threshold tenuous (Benowitz and Henningfield, 2013; Donny et al., 2012; Sofuoglu and LeSage, 2012). Clinical studies examining smoking progressively reduced nicotine content cigarettes have not consistently demonstrated a significant reduction in cigarettes smoked per day (CPD) at nicotine doses as low as 1 mg/cigarette (Benowitz et al., 2012; 2009a; 2007). Moreover, immediately switching to a very low nicotine content (0.05 mg, “denicotinized”) cigarette has been shown to reduce CPD by only 50% over 6–12 weeks (Donny et al., 2007; Hatsukami et al., 2010b). Given that so-called “denicotinized” cigarettes are capable of maintaining substantial rates of smoking that could still produce significant levels of nicotinic receptor binding in brain (Brody et al., 2009, 2008), and that a truly nicotine-free control product does not yet exist, it is unclear whether the lower rates of smoking maintained by a 0.05 mg/cigarette are due to reinforcing effects of nicotine per se or other factors (Shahan et al., 2001, 1999).
Nicotine self-administration (NSA) studies in animals can be useful for examining determinants of the nicotine reinforcement threshold (see Donny et al., 2012 for further discussion). Yet, few NSA studies in animals have explicitly operationally defined and measured the reinforcement threshold. Most studies have typically used a limited number and/or range of doses that did not allow identifying a threshold (Donny et al., 2012), and many have manipulated dose between subjects rather than model the within-subject decrease in nicotine delivery that a nicotine reduction policy would impose on current smokers. Recent studies have begun to address these issues. For example, Smith et al. (2013) reported that, regardless of the form of dose reduction (i.e., gradual versus immediate), extinction of NSA in male rats was observed at unit doses equal to or below 0.00375 mg/kg in most rats, while NSA was maintained above saline levels at doses equal to or higher than 0.0075 mg/kg. A similar threshold was reported by Grebenstein et al. (2013) in male and female rats exposed to gradual dose reduction in an unlimited-access model (0.0032 and 0.0037 mg/kg, respectively). Both studies showed marked variability between subjects, with some rats exhibiting extinction at doses as high as 0.015 mg/kg and others at doses as low as 0.001 mg/kg. More research is needed to examine this range across strains and species, better characterize individual variability, and identify factors that account for that variability (Donny et al., 2012, 2014; Hatsukami et al., 2013a).
An important issue related to nicotine reduction is whether and to what extent a compensatory increase in tobacco use and associated disease risk would occur in individuals attempting to maintain their nicotine intake. For example, smokers switching to cigarettes with reduced nicotine yield (RNY) can show marked compensation (Rose and Behm, 2004; Scherer, 1999). However, the risk of compensation may be less for individuals smoking reduced nicotine content (RNC) cigarettes (Benowitz and Henningfield, 2013), because they actually contain less nicotine and are not highly ventilated. For example, both gradual (i.e., over weeks or months; (Benowitz et al., 2012, 2009a, 2007) and immediate reduction (Donny et al., 2007; Hatsukami et al., 2010b, 2013b) in nicotine content with RNC cigarettes induced small or no compensatory increases in CPD (Hatsukami et al., 2015). However, marked variability in compensation has been apparent in many studies (Benowitz et al., 2009a, 2007, 2006; Hatsukami et al., 2010a). Understanding the factors that contribute to compensation and its individual variability is important to anticipate the differential risk for this potential side effect of nicotine reduction between vulnerable subpopulations, and to identify supportive interventions they may need to minimize it. For instance, a recent study showed that the level of nicotine dependence as indicated by time to smoke the first cigarette of the day (but not the Fagerstrom Test of Cigarette Dependence score) is positively correlated with degree of compensation (Bandiera et al., 2015), suggesting that smokers who are more dependent may be at greater risk of compensation.
Variability in baseline nicotine intake per se may be a factor related to variability in reinforcement thresholds and compensation between smokers. Smoking cessation rates are typically lower in heavy smokers (Abrams et al., 2000; Borland et al., 2010), raising the question of whether they would also be less likely to reduce their smoking when switching to RNC cigarettes and, consequently, exhibit a lower reinforcement threshold for nicotine. Although some nicotine reduction studies have not found a relationship between CPD and degree of compensation (e.g., Bandiera et al., 2015; Hecht et al., 2004; Niaura et al., 2013), CPD is not a precise measure of actual nicotine intake. When higher baseline nicotine intake per se is measured, it has been correlated with lower compensation, despite no correlation between CPD and compensation (Niaura et al., 2013). This is consistent with findings from our analogous dose-reduction studies in rats (Harris et al., 2011, 2009).
Another factor that may moderate the nicotine reinforcement threshold and compensation is nicotine metabolism. Individuals with faster nicotine elimination typically smoke more and experience greater difficulty quitting (Benowitz, 2008; Benowitz et al., 2003; Chenoweth et al., 2014; Schnoll et al., 2014, 2013). A faster rate of decline in plasma nicotine level between cigarettes could increase the rate of smoking needed to achieve a desired blood level and thus reduce the reinforcing effects of cigarettes with very low nicotine content (i.e., increase the reinforcement threshold) and induce greater compensatory increases in smoking. To our knowledge, only one study has examined the relationship between nicotine metabolism and compensation during gradual nicotine reduction in humans, and no correlation was found (Bandiera et al., 2015). Similarly, our previous studies in rats found that nicotine clearance and other pharmacokinetic parameters were not related to compensation during a single 50% reduction in unit dose (Harris et al., 2011, 2009). However, it is possible that clearance may moderate compensation following larger reductions in unit dose. To our knowledge, no studies in humans or animals have examined the relationship between nicotine metabolism and the nicotine reinforcement threshold.
The purpose of the present study was to extend our previous analysis of gradual nicotine dose reduction in rats as a model of nicotine reduction policy (Grebenstein et al., 2013). The primary goal was to exploit the variability in the nicotine reinforcement threshold and compensation in a larger sample of rats to examine whether these measures were associated with baseline nicotine intake and pharmacokinetic variables. Because reducing the unit dose can be conceptualized as increasing the price of nicotine, a behavioral economic demand curve analysis was also conducted to examine individual differences in elasticity of demand for nicotine (Grebenstein et al., 2013; Hursh and Roma, 2013; Hursh and Silberberg, 2008). Behavioral Economics provides a conceptual, methodological, and analytical framework that is commonly applied in human research, but has been infrequently used in preclinical tobacco addiction research (Diergaarde et al., 2011; Grebenstein et al., 2013). By providing a measure of the overall rate of decline in nicotine consumption as unit dose is reduced, demand curve analysis provided a useful way to assess the relative efficacy of dose reduction among rats with different baseline levels of nicotine intake and nicotine pharmacokinetics. Together, findings from the present study provide information relevant to whether the reinforcement threshold, compensation, and overall efficacy of gradual nicotine reduction policy might differ across subgroups of smokers (e.g., heavy versus light smokers; fast versus slow metabolizers).
2. MATERIAL AND METHODS
2.1. Animals
Twenty-seven male Holtzman rats (Harlan, Indianapolis, IN) weighing 300–325 g at arrival had ad lib access to water and were initially given ad lib access to food for one week, followed by a restricted feeding regimen of 18 – 20 g/day for the remainder of the study. Eight rats were fed 22–25 g/day to maintain body weight gain in the range of other rats. Rats were fed daily during the 1-hr interval between experimental sessions (see 2.6). The Holtzman strain was chosen to extend our previous studies that used the same strain to examine individual differences in compensatory NSA following unit dose reduction (Grebenstein et al., 2013; Harris et al., 2009, 2008). Eight rats from the recent study using an identical protocol were included in the present analysis (Grebenstein et al., 2013). Upon arrival, all rats were individually housed in a temperature- and humidity controlled colony room under a reversed 12-h light/dark cycle (lights off at 11:00 hr) for approximately one week. Rats were then moved to operant conditioning chambers and placed on food restriction in a separate room under the same light/dark cycle following recovery from catheter implantation for NSA (see: 2.4). Protocols were approved by the Institutional Animal Care and Use Committee of the Minneapolis Medical Research Foundation in accordance with the 2003 National Research Council Guidelines for the Care and use of Laboratory Animals and the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research.
2.2. Apparatus
Each operant conditioning chamber (29 cm × 26 cm × 33 cm; Coulbourn Instruments, Allentown, PA) was made of aluminum and Plexiglas walls, an aluminum ceiling, and a stainless steel grid floor. Two response levers were located on the front wall 10 cm above the chamber floor on either side of an aperture for delivery of food pellets (not used in this study) located 2 cm above the floor. LED stimulus lights were located 2 cm above each response lever. Water was continuously available via a spout mounted on the back wall of the chamber. Each chamber was placed inside a sound-attenuating cubicle equipped with an exhaust fan that provided masking noise. Infusion pumps (Model RHSY, Fluid Metering, Syosset, NY) placed outside each cubicle delivered infusions through Tygon tubing connected to a fluid swivel mounted above the chamber, and from the swivel through a spring leash connected to a guide cannula mounted in a harness assembly on the back of the rat. MED-PC IV (Med Associates, St Albans, VT) software was used for operating the apparatus and recording data.
2.3. Drugs
Nicotine bitartrate (Sigma Chemical Co., St. Louis, MO) was dissolved in sterile saline. The pH of the solution was adjusted to 7.4 with dilute NaOH, and heparin (30 units/ml) was added to help maintain catheter patency. Nicotine doses are expressed as the base.
2.4. Surgery
Each rat was implanted with a chronic indwelling catheter into the right jugular vein under i.m. droperidol (2 mg/kg)/fentanyl (0.04 mg/kg) anesthesia, described in detail elsewhere (Harris et al., 2008; LeSage et al., 2002). The catheter was externalized between the scapulae and attached to a vascular-access harness (VAH95AB, Instech Laboratories, Plymouth Meeting, PA) that allowed connection to a fluid swivel via a tether for nicotine administration. Animals were allowed to recover for at least four days after surgery, during which time they received daily i.v. infusions of heparinized saline and ceftriaxone (5.25 mg) and s.c. injections of buprenorphine (0.1 mg/kg; first two days only) for analgesia. Infusions of methohexital (0.1 ml, 10 mg/ml, i.v.) were administered to check patency at the end of the study and on occasions when malfunctions were suspected. If the catheter became occluded prior to the dose-reduction protocol (see 2.6), another catheter was implanted into the contralateral jugular or ipsilateral femoral vein. If catheter failure occurred during the dose-reduction protocol, the rat was excluded from the study.
2.5. NSA training
Rats were trained to self-administer nicotine in daily 23-hr sessions (11:00 am – 10:00 am) according to similar protocols used in our laboratory (LeSage et al., 2002, 2007). Nicotine availability was signaled by illumination of the stimulus light above the active (right) response lever. Following completion of the response requirement, the stimulus light was extinguished and nicotine (0.06 mg/kg/inf) was infused in a volume of 100 μl/kg at a rate of 50 μl/sec. This training dose was chosen because it lies on the descending limb of the NSA dose-response curve (e.g., Corrigall and Coen, 1989; Denoble and Mele, 2006; Grebenstein et al., 2013), allowing measurement of compensatory increases in NSA following unit dose reduction and characterization of both limbs of the “inverted U” dose-response curve. Each infusion was immediately followed by a 7-sec time-out, during which stimulus lights remained off and lever presses were recorded but had no programmed consequence. Following the time-out, the stimulus light was illuminated, indicating availability of the next nicotine infusion. Responses on the other (inactive) lever were recorded but had no programmed consequences. The response requirement was initially a fixed ratio 1 (FR 1), and was gradually increased to FR 3 across several sessions. The criteria for acquisition were a minimum of 10 infusions per day under the FR 3 schedule and a ratio of active to inactive lever presses of at least 2:1 across five consecutive sessions with no trend. No strict time constraint was imposed to acquire NSA or reach stability before beginning the dose-reduction protocol.
2.6. Dose-Reduction Protocol
Following stable NSA (no trend in infusion rates across at least 5 consecutive sessions and a coefficient of variation < 15%), saline was substituted for nicotine. This extinction phase lasted at least seven sessions and until extinction criteria were met (number of infusions per day decreased to below 50% of baseline and no trend was evident for three consecutive sessions). This initial extinction period assured that rats were sensitive to changes in nicotine dose and set a target to define a non-reinforcing dose at the low-end of the dose range. This avoided unnecessary exposure of rats to multiple non-reinforcing nicotine doses. At this point, rats were allowed to reacquire NSA at the training dose. Eight rats required a new catheter during extinction or their baseline infusion rate shifted by more than 10 infusions/session following extinction. These rats were exposed to a second extinction and reacquisition phase. After NSA was reacquired and stable (same criteria as above), the unit dose-reduction phase began. During this phase, the unit dose was reduced weekly until the number of infusions per day during the last three days at a given dose fell within range of the number of infusions during the last three days of the prior extinction phase. Therefore, the number of unit doses to which a rat was exposed differed between rats. Saline was then substituted for nicotine for one week to confirm that the last nicotine unit dose was not reinforcing. For rats exposed to a second extinction phase, the second pre-extinction baseline and extinction phase were used for data analysis (see below). The training dose was then made available to allow reacquisition of NSA. Saline extinction was then arranged for at least three days to ensure complete elimination of nicotine prior to conducting the pharmacokinetic protocol described below. Rats that did not reacquire NSA or exhibit signs of anesthesia upon methohexital infusion were excluded from the study. Twenty-seven rats completed the dose-reduction protocol. Of these, pharmacokinetic data were not collected from six rats that were instead used to collect pilot data for other studies.
2.7. Nicotine Assay and Pharmacokinetic Protocol
Nicotine concentrations in serum samples were measured by gas chromatography with nitrogen phosphorus detection using a standard protocol in our laboratory (Harris et al., 2008; LeSage et al., 2003). Single dose nicotine pharmacokinetic parameters were estimated after completion of the dose-reduction protocol using our previously reported methods (Harris et al., 2008). In brief, rats were anesthetized with droperidol (2 mg/kg)/fentanyl (0.04 mg/kg) i.m. and implanted with a femoral catheter. They received nicotine 0.1 mg/kg i.v. over 10 sec via the catheter used for self-administration (see below), and blood was obtained via the femoral catheter at 15, 30, 60, 120, 180, and 240 min for measurement of the serum nicotine concentration (Harris et al., 2008). Parameter estimates (e.g., volume of distribution, half-life, clearance, and area under the curve (AUC)) were obtained using standard non-compartmental methods using WinNonlin software (version 4.1, Pharsight, Mountain View, CA).
2.8. Data Analysis
Mean lever presses on the active and inactive lever, number of infusions, and nicotine intake across the last three sessions at each unit dose and saline extinction were the primary dependent measures (see Supplementary Materials1 for raw data). Because an unequal number of rats were exposed to lower unit doses, multiple Bonferroni-corrected paired t-tests (significance set at p < 0.0036 for 14 comparisons) were used to examine the changes in dependent measures across unit dose. The proportion of rats maintaining NSA at each dose was examined via multiple Fisher’s exact tests (significance set at p < 0.008 for 6 comparisons).
A compensation index (CI) analogous to a measure of compensation used in smokers (Scherer, 1999) was calculated for each rat using the formula: CI = 1 − (% Change in nicotine intake ÷ % Change in unit dose). The importance of measuring compensation is to assess the potential of an adverse side effect of nicotine reduction, an increase in smoke exposure and its associated disease risk. Percent change, rather than absolute change, in intake is used to calculate the CI in humans and animals because it better reflects the magnitude of impact on each individual (e.g., the same absolute increase in CPD may have a greater adverse health impact on a lighter smoker than a heavier smoker). A CI of 0 indicates no compensation (nicotine intake decreased proportionally with the reduction in unit dose), while a CI of 1.0 indicates complete compensation (nicotine intake was unchanged following dose reduction). Separate CIs for each rat at each unit dose were calculated using the mean intake across the final three days of access to each unit dose, relative to the final three days of access to 0.06 mg/kg at baseline. The statistical significance of each CI was examined via Bonferroni-corrected one-sample t-tests, comparing each CI to a theoretical mean of 0 (significance set to p < 0.0063). To examine predictors of compensation, two CIs were used, the mean of all CIs (CIav) at each dose and the highest CI (CImax). These represented the overall degree of compensation and the greatest degree of compensation at a single dose during the course of reduction, respectively.
Demand curve analysis was conducted according to the exponential model proposed by Hursh and Silberberg (2008) and applied in our prior study (Grebenstein et al., 2013). In the present study, unit price was manipulated by varying the unit dose while the FR size remained constant, rather than the more common approach of varying the FR size while dose remains constant (both manipulations are considered functionally equivalent; Hursh, 1991). The primary parameters of interest, Q0 and α are estimated from the best-fit exponential function and refer to the maximum level of consumption at zero price (i.e., level or “intensity” of demand) and the rate of change in consumption with increases in unit price, respectively. The range of consumption, k, was held constant across all data sets at 2.6 in the present study. The value of α is a measure of reinforcing strength or “essential value” of the commodity being consumed (Hursh and Silberberg, 2008). Higher α values indicate steeper (elastic) demand curves and lower reinforcing strength compared to lower α values associated with slower declining (inelastic) demand curves. Other demand measures of interest included Omax, the maximal response output, and Pmax, the unit price at which maximal response output occurred. Demand functions were generated using a template for GraphPad Prism software (La Jolla, CA) provided by the Institutes for Behavior Resources, Inc. (Baltimore, MD) on their website (http://www.ibrinc.org/index.php?id=181). Using both the exponential demand curve analysis and the CI allowed examining elasticity of demand on two levels. First, the exponential demand analysis measures the rate of change in consumption across all unit prices, providing an aggregate measure of elasticity of demand. Second, the CI measures the change in consumption at individual prices relative to baseline price, which is somewhat analogous to the concept of ”local elasticity” (i.e., elasticity between two adjacent prices; Mackillop et al., 2012). Differential sensitivity to a specific change in price, such as small versus large reductions in nicotine content, could have important implications for specifying the step sizes in a nicotine reduction policy (see: Mackillop et al., 2012).
Linear regression was used to examine the relationship between the predictor variables of baseline nicotine intake, nicotine pharmacokinetic parameters, and indices of demand, and the criterion variables of nicotine intake at each unit dose, overall nicotine intake (area under the nicotine intake dose-response curve (i.e., nicotine AUC), reinforcement threshold, CIav, CImax, and indices of demand. Non-normally distributed data were log transformed (see Table 2), except for the serum nicotine AUC which was inverse transformed because log transformed values were still not normally distributed. Hierarchical multiple regression analysis was used to confirm whether baseline infusion rate and nicotine clearance accounted for unique portions of the variance in each dependent measure, and whether their combination provided greater predictive power than the single predictor with the highest correlation (see Table 3). Assumptions of linearity, independence of errors, lack of co-linearity, homoscedasticity, unusual points, and normality of residuals were met (Howell, 2002). Omax had to be log transformed for this analysis in order meet the assumption of homoscedasticity. Other pharmacokinetic parameters and demand parameters were not included as predictors in the multiple regression analyses because of their significant co-linearity with baseline infusion rate, nicotine clearance, or both.
Table 2.
Correlations between baseline infusions and nicotine pharmacokinetic parameters and criterion response measures.
| Predictor | Response Measure
|
|||||||
|---|---|---|---|---|---|---|---|---|
| CIav | 1 CImax | 1 Reinforcement Threshold | 1α | Q0 | Pmax | Omax | Baseline Infusions | |
| Baseline Infusions | − 0.39* | − 0.27 | − 0.12 | − 0.65*** | 0.51** | 0.07 | 0.59** | |
| CL | 0.40^ | 0.46* | − 0.55** | − 0.71*** | − 0.29 | 0.69*** | 0.69*** | 0.22 |
| 1T1/2 | − 0.40^ | − 0.43^ | 0.36 | 0.52* | 0.31 | − 0.46* | − 0.47* | −0.09 |
| Vss | 0.12 | 0.15 | − 0.44* | − 0.47* | − 0.04 | 0.42^ | 0.44* | 0.30 |
| 1AUC | − 0.38^ | −0.43^ | 0.44* | 0.64** | 0.27 | − 0.60** | − 0.59** | −0.20 |
Not normally distributed (log transformed),
p < 0.05,
p < 0.01,
p < 0.001,
p < 0.1
Table 3.
Hierarchical multiple regression coefficients predicting each response measure from baseline nicotine intake (BI) and nicotine clearance (CL). Model 1 includes the single predictor with the highest R2 alone, while Model 2 includes both predictors. The delta symbol indicates the change in the associated statistic between models.
| Model 1 (Single Predictor) | Model 2 (BI + CL) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Response Measure | Predictor | R | R2 | F | R | R2 | F | ΔR2 | ΔF |
| CIav | BI | 0.44 | 0.19 | 4.67* | 0.67 | 0.45 | 7.43** | 0.26 | 8.37* |
| CImax | CL | 0.46 | 0.21 | 5.18* | 0.62 | 0.38 | 5.50* | 0.17 | 4.79* |
| Threshold | CL | 0.55 | 0.30 | 8.23* | 0.55 | 0.30 | 3.91* | 0.00 | 0.004 |
| α | CL | 0.71 | 0.51 | 19.56*** | 0.87 | 0.76 | 28.75*** | 0.25 | 19.12*** |
| Q0 | BI | 0.57 | 0.33 | 9.24** | 0.72 | 0.52 | 9.73** | 0.19 | 7.20* |
| Pmax | CL | 0.69 | 0.48 | 17.44** | 0.70 | 0.49 | 8.70** | 0.01 | 0.46 |
| Omax | CL | 0.71 | 0.51 | 19.56*** | 0.87 | 0.76 | 28.74*** | 0.25 | 19.20*** |
p < 0.05,
p < 0.01,
p < 0.001
3. RESULTS
3.1. Baseline NSA and extinction
The average number of sessions to acquire NSA and achieve stability prior to extinction was 76 ± 7 (SEM, range 23 – 185). Variability in sessions to stability was due to several issues, including the speed of acquisition, time needed for variability to decrease and/or lever discrimination to develop, the need for remedial FR training due to ratio strain, the need to implant a new catheter, or a combination of these and other factors. Despite the varied histories between rats, stable performance was typical (Grebenstein et al., 2013; Harris et al., 2011, 2009) and neither the number of sessions to stability or total cumulative nicotine intake over this period were correlated with any dependent measure or predictor variable. The mean number of infusions at baseline was 31.1 ± 1.6 (SEM, range 16.3 – 46.7). An extinction burst was evident on the first day of extinction, with the mean number of infusions increasing to 130.6% ± 9.0% (SEM, median 119.4, range 51.6% – 229.2%) of baseline. Infusion rates quickly declined below baseline thereafter, with the majority of rats meeting extinction criteria within seven sessions (mean 8.9±1.1 SEM, median 7, range 4 – 31). Neither the extinction burst or days to meet extinction criteria were correlated with any other NSA measure. Mean number of infusions after reacquisition before unit dose reduction began were 30.3 ± 1.5 SEM (range 16.3 – 46.7).
3.2. Individual differences in the reinforcement threshold, compensation, and elasticity of demand
Figure 1 shows the mean number of active and inactive lever presses (left panel) and the percentage or rats maintaining NSA rates above saline (right panel) as a function of dose during progressive unit dose reduction. The curve for active lever responses exhibited an inverted U-shape, with the peak at the 0.01 mg/kg unit dose. The mean number of active lever responses was significantly higher compared to the inactive lever at all unit doses, including saline (all p values < 0.001), and the number of inactive lever presses did not significantly vary with dose. Individual variability in active lever presses increased as dose decreased, with the greatest variability apparent at doses just below the peak of the dose-response curve. The mean reinforcement threshold was 0.0033 ± 0.0027 (SD) mg/kg, with significant variability between subjects (median 0.002 mg/kg, range 0.0005 – 0.01 mg/kg). All rats maintained NSA during reduction down to 0.01 mg/kg. Greater than 50% of rats maintained NSA down to the 0.002 mg/kg dose. Only one rat maintained NSA at the 0.0005 mg/kg dose. All rats had met extinction criteria before reaching the 0.00025 mg/kg dose.
Figure 1.
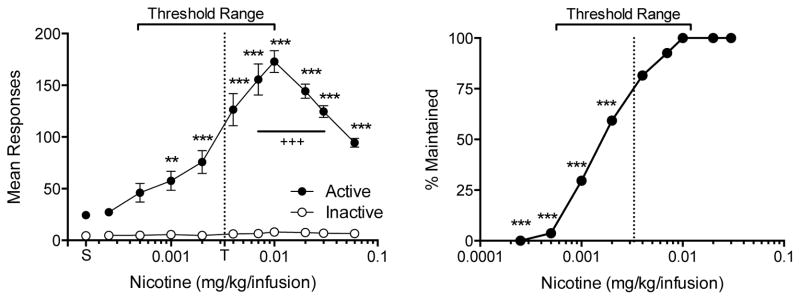
Left panel: Mean (±SEM) number of responses on the active and inactive levers during the course of unit dose reduction. The unit dose for NSA was reduced weekly until responding was within range of responding during saline extinction. Therefore, each point represents the mean of a different number of rats (27 at 0.01 to 0.06 mg/kg; 25, 22, 16, 9, and 1 at each dose from 0.007 to 0.00025 mg/kg, respectively). The vertical dotted line indicates the mean threshold reinforcing unit dose (T). Significant differences from saline, **p < 0.01, ***p < 0.001. Significant differences from 0.06 mg/kg baseline, +++p < 0.001. Right panel: Percentage of rats maintaining NSA infusion rates above the range of infusion rates for saline. Significant difference in proportion from 0.06 mg/kg baseline, ***p < 0.001.
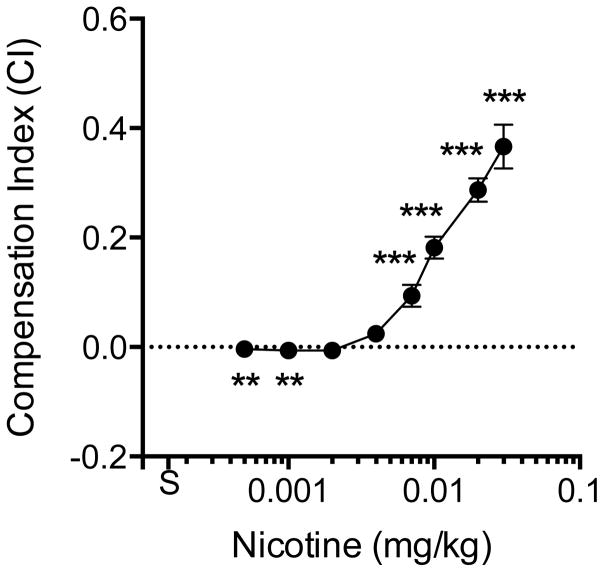
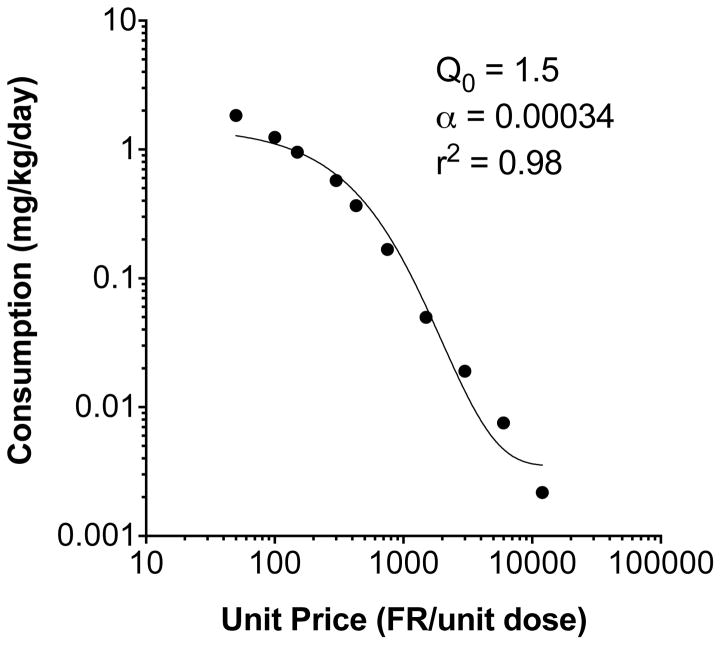
Figure 2 shows the compensation index at each unit dose. Significant compensation was observed during each unit dose reduction down to 0.007 mg/kg, and significant under-compensation (intake proportionally lower than the decrease in dose) occurred at the two lowest unit doses. The highest CI (CImax) was at the first step of dose reduction (0.03 mg/kg) in most rats (22 of 27), and decreased with further reductions in unit dose. The mean CImax, was 0.39 ± 0.19 (SD), with considerable variability between rats (median 0.35, range 0.15 – 1.1). The mean CIav across all doses was 0.142 ± 0.061 (SD), which was also highly variable between subjects (median 0.124, range 0.04 – 0.28). Figure 3 shows the aggregated group demand curve, and Table 1 shows associated demand curve parameters for individual subjects. For rats as a group, the decline in nicotine consumption with increases in unit price was well described by the exponential demand function (r2 = 0.98). Individual consumption was also generally well described (r2 range 0.81 – 1.0). There was considerable individual variability in all demand parameters.
Figure 2.
Mean (±SEM) compensation index (CI) at each unit dose. The horizontal dotted line indicates the CI value if no compensation occurred (i.e., the decrease in intake was proportional to the decrease in unit dose). Points above the dotted line indicate compensation, while points below indicate under-compensation (see text for further discussion). Each point is the mean of up to 27 rats. See Figure 1 for further details. Different from 0, **p<0.01, ***p<0.001.
Figure 3.
Exponential demand curve describing mean consumption as a function of unit price. Demand curve parameters are derived from the curve fit to the aggregate mean values, not from averaging the parameters from curve fits to individual rat data, as in Table 1.
Table 1.
Exponential demand curve parameters for individual subjects (k set to 2.6 log units globally).
| Subject ID | α | Q0 | r2 | Pmax | Omax |
|---|---|---|---|---|---|
| 5305 | 0.00042 | 2.2 | 1.00 | 221.9 | 160.9 |
| 5310 | 0.00032 | 2.7 | 1.00 | 237.3 | 211.2 |
| 5311 | 0.00057 | 2.3 | 0.98 | 156.4 | 118.6 |
| 5306 | 0.00052 | 3.5 | 0.96 | 112.7 | 130 |
| 5667 | 0.00059 | 2.2 | 0.99 | 158.0 | 114.5 |
| 7488 | 0.00036 | 1.9 | 0.99 | 299.8 | 187.7 |
| 5940 | 0.00033 | 2.4 | 0.99 | 258.9 | 204.8 |
| 6658 | 0.00033 | 1.1 | 0.99 | 564.9 | 204.8 |
| 7487 | 0.00046 | 2.7 | 0.87 | 165.1 | 146.9 |
| 7037 | 0.00054 | 1.7 | 0.99 | 223.4 | 125.1 |
| 7121 | 0.00031 | 1.9 | 0.99 | 348.1 | 218 |
| 7612 | 0.00029 | 2.5 | 0.99 | 282.8 | 233 |
| 7489 | 0.0004 | 1.9 | 0.94 | 269.8 | 168.9 |
| 7670 | 0.00059 | 1.8 | 1.00 | 193.1 | 114.5 |
| 7816 | 0.00033 | 3.8 | 1.00 | 163.5 | 204.8 |
| 7802 | 0.00041 | 2.0 | 1.00 | 250.1 | 164.8 |
| 7817 | 0.00019 | 2.0 | 0.99 | 539.6 | 355.7 |
| 7920 | 0.00038 | 1.9 | 0.99 | 284.0 | 177.8 |
| 8050 | 0.00035 | 1.6 | 0.93 | 366.2 | 193.1 |
| 9020 | 0.00021 | 2.3 | 1.00 | 424.5 | 321.8 |
| 9004 | 0.00045 | 1.3 | 1.00 | 350.5 | 150.2 |
| 6798 | 0.00094 | 2.6 | 0.81 | 83.9 | 71.9 |
| 7227 | 0.00035 | 2.0 | 0.99 | 292.9 | 193.1 |
| 6799 | 0.00037 | 1.1 | 0.98 | 503.8 | 182.6 |
| 7069 | 0.00023 | 1.6 | 0.96 | 557.2 | 293.8 |
| 7557 | 0.00051 | 3.2 | 0.98 | 125.6 | 132.5 |
| 7776 | 0.00061 | 1.8 | 0.99 | 186.8 | 110.8 |
| Mean | 0.00042 | 2.1 | 0.97 | 282.3 | 181.2 |
| SD | 0.00016 | 0.7 | 0.04 | 136.9 | 65.2 |
3.3. Predictors of individual differences: Baseline nicotine intake
Table 2 shows the coefficients for correlations between predictor variables and criterion response measures. Figure 4 shows the scatterplot and curve fit for correlations between baseline infusions and the three primary dependent variables. The mean number of baseline infusions was not significantly correlated with reinforcement threshold (Figure 4, left panel) or the maximum level of compensation (Table 2), but was significantly, negatively correlated with the overall CI (Figure 4, center panel; r = −0.39, p < 0.05) and elasticity of demand (α, Figure 4, right panel; r = −0.68, p < 0.0001). Thus, rats with higher baseline intake exhibited a lower overall level of compensation and less elastic demand (i.e., less sensitivity to dose reduction/price increase). Baseline infusion rate was also significantly, positively correlated with other demand indices, such as intensity of demand (Q0, r = 0.51, p < 0.01) and maximum effort expenditure (Omax, r = 0.59, p < 0.01), but not Pmax.
Figure 4.
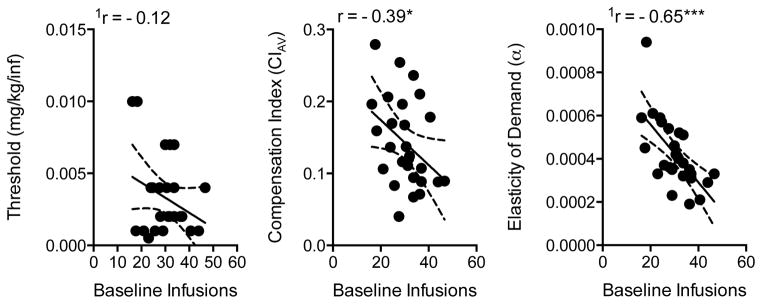
Scatterplots, regression lines, and correlation coefficients describing the relationship between baseline infusions and the reinforcement threshold (left panel), mean compensation index (middle panel), and elasticity of demand (right panel). Dotted lines represent the 95% confidence band of the regressions line. Scatterplots show non-transformed data. 1Correlation based on transformed data. Significantly different from zero, *p < 0.05, ***p < 0.001.
3.4. Predictors of individual differences: Nicotine pharmacokinetics
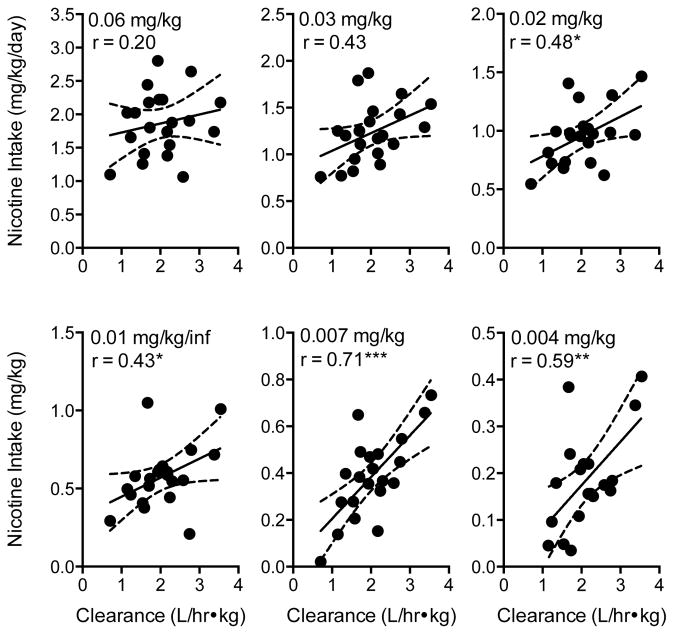
Figure 5 shows the correlation between nicotine intake and nicotine clearance at each unit dose. Although the tendency for higher nicotine intake to be associated with faster clearance was not significant at the two highest unit doses, this relationship was statistically significant at lower doses, especially those just above the mean reinforcement threshold (see Figure 5 for statistic and p values). In addition, overall nicotine intake during the entire dose-reduction protocol (calculated as area under the nicotine intake dose-response curve) was significantly, positively correlated with nicotine clearance, such that rats with faster clearance had higher overall nicotine intake (r = 0.58, r2 = 0.34, p < 0.05). Figure 6 shows that nicotine clearance was negatively correlated with the nicotine reinforcement threshold (left panel, r = 0.55, p = 0.01), such that faster clearance was associated with lower reinforcement thresholds. Faster nicotine clearance tended to be associated with greater overall compensation, but this relationship only approached significance (Figure 6, center panel; r = 0.39, p < 0.1). However, faster clearance, shorter half-life, and smaller AUC were associated with greater maximal compensation (Table 2). All pharmacokinetic parameters, except half-life, were also associated with indices of demand (Table 2). Faster clearance, higher volume of distribution, and lower AUC were associated with less elastic demand (i.e., lower α, Table 2). Faster clearance and lower AUC were also associated with higher Pmax and Omax values, indicating that demand remains inelastic at lower nicotine doses and maximal effort expenditure for nicotine is greater in rats with faster nicotine clearance. Nicotine clearance was negatively correlated with elasticity of demand (Figure 7, right panel; −0.61 p < 0.01). None of the pharmacokinetic parameters were associated with intensity of demand (Q0).
Figure 5.
Scatterplots, regression lines, and correlation coefficients describing the relationship between nicotine clearance and nicotine intake during exposure to the indicated unit nicotine dose. Significantly different from zero, *p < 0.05, **p < 0.01, ***p < 0.001. See Figure 4 for further details.
Figure 6.
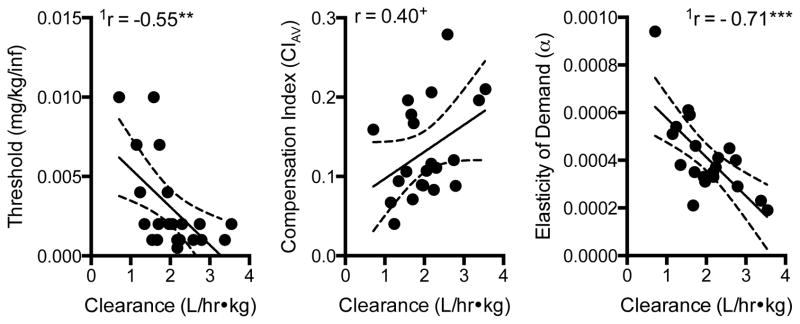
Scatterplots, regression lines, and correlation coefficients describing the relationship between nicotine clearance and the nicotine reinforcement threshold (left panel), average compensation index (middle panel), and elasticity of demand (right panel). Significantly different from zero, **p < 0.01. See Figure 4 for further details.
Figure 7.
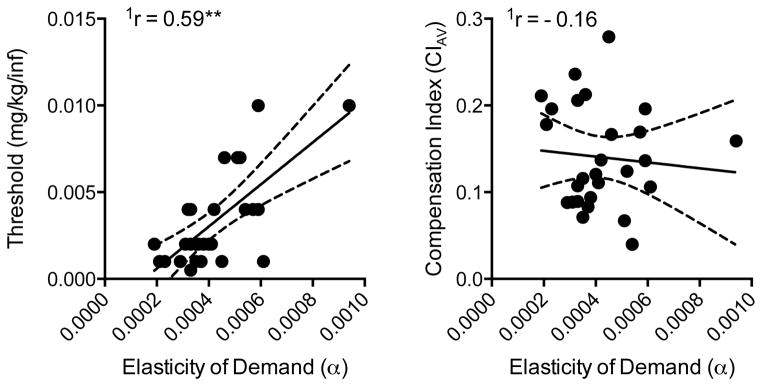
Scatterplots, regression lines, and correlation coefficients describing the relationship between elasticity of demand and the nicotine reinforcement threshold (left panel) and average compensation index (right panel). Significantly different from zero, **p < 0.01. See Figure 4 for further details.
3.5 Predictors of individual differences: Multiple predictor model
Results of multiple regression analysis are reported in Table 3. For all response measures other than the reinforcement threshold and Pmax, baseline infusion rate and nicotine clearance accounted for significant independent portions of the variance in each dependent measure, such that the regression model including both variables was better at predicting a given response measure than the best single-predictor model (i.e., the single predictor with the highest r2).
3.6. Elasticity of demand as a predictor of the reinforcement threshold and compensation
Figure 7 shows correlations between elasticity of demand and the reinforcement threshold (left panel) and compensation (right panel). Elasticity of demand was positively correlated with the nicotine reinforcement threshold, such that rats exhibiting less elastic demand (i.e., less sensitivity to decreases in unit dose) had a lower reinforcement threshold (r = 0.55, p < 0.01). Elasticity of demand was not correlated with either measure of compensation.
4. DISCUSSION
The present study found that NSA during progressive unit dose reduction resulted in marked individual variability in the nicotine reinforcement threshold, degree of compensation, and elasticity of demand. Significant sources of this variability included individual differences in baseline nicotine intake and nicotine clearance. Specifically, higher baseline nicotine intake predicted lower overall compensation and elasticity of demand (i.e., reduced sensitivity to increases in unit price), but was not associated with the reinforcement threshold. Faster nicotine clearance predicted a lower reinforcement threshold, greater degree of compensation, and less elastic demand for nicotine. Lower elasticity of demand predicted a lower reinforcement threshold. Together, these results provide new information on the determinants of NSA in rats, extend the validity of animal NSA models with respect to nicotine pharmacokinetics, and have important implications for future regulatory research in humans and development of nicotine reduction policy.
4.1. Variability in the reinforcement threshold, compensation, and elasticity of demand
4.1.1. Reinforcement threshold
The mean reinforcement threshold of 0.0033 mg/kg/infusion observed in the present study is consistent with other studies that have examined the unit dose-response curve for maintenance of NSA. Several studies that have used unit doses below 0.004 mg/kg/infusion have shown that 0.003 to 0.004 mg/kg/infusion can maintain NSA above saline levels, whereas lower doses do not (Corrigall and Coen, 1989; Cox et al., 1984; Donny et al., 1995; Grebenstein et al., 2013; Shoaib et al., 1997; Sorge and Clarke, 2009; Valentine et al., 1997; Watkins et al., 1999). However, other studies have reported that higher doses (7.5 to 30 mg/kg/infusion) did not maintain NSA (see: Donny et al., 2012 for review). This marked variability between studies could be due to several subject and methodological factors, including stage of NSA [i.e., acquisition versus maintenance; (Donny et al., 2012; Smith et al., 2014), strain (Brower et al., 2002), infusion speed (Sorge and Clarke, 2009), and type of environmental cues paired with nicotine delivery (Caggiula et al., 2002, 2001; Chaudhri et al., 2006; Donny et al., 2003)]. Together, these findings underscore the point that the primary goal of animal research on nicotine reduction is not to specify an absolute value for the nicotine reinforcement threshold. Rather, it is to specify factors that influence the threshold that may be worth examining in human studies.
4.1.2. Compensation
The general inverted-U shape of the dose-response curve and the degree of compensation in the present study were similar to other studies that have examined the effects of single or multiple unit dose reductions on NSA in rats (Denoble and Mele, 2006; Grebenstein et al., 2013; Harris et al., 2009; Shoaib et al., 1997). The finding that compensation was only partial, highly variable, and decreases with nicotine dose is consistent with studies in humans smoking cigarettes with either progressively-reduced nicotine yield or content (Benowitz et al., 2012, 2009a, 2007). However, the overall magnitude of compensation may be somewhat higher in humans exposed to progressive nicotine reduction (e.g., 60% following a 50–60% reduction in nicotine content (Benowitz et al., 2009a), compared to the level of compensation observed in the present study (e.g., 29–37% compensation following a 50–60% reduction in dose). One potential reason for this difference is that the nicotine self-administration assay in rats does not model the constant level of tar and associated peripheral sensory stimuli and CNS effects derived from smoking RNC or RNY cigarettes. Some non-nicotine components of tar may have reinforcing and discriminative stimulus effects of their own or modulate the reinforcing effects of nicotine [e.g., acetaldehyde (Hoffman and Evans, 2013), minor alkaloids (Bardo et al., 1999; Clemens et al., 2009; Green et al., 2000), beta carbolines (Arnold et al., 2014)]. The discriminative stimulus, conditioned reinforcing, and primary reinforcing effects of these stimuli may be more effective at maintaining smoking of RNC cigarettes compared to the visual and auditory cues that may be maintaining NSA at very low unit doses in animal models. This may limit the translational relevance and validity of self-administration models that use nicotine alone. Continued work incorporating other sensory cues (e.g., olfactory and gustatory stimuli) and combining non-nicotine constituents with nicotine delivery in self-administration models is vital to addressing this issue (Chen et al., 2011; Costello et al., 2014).
4.1.3. Elasticity of demand
The present study confirms our previous observations of the precision of exponential demand curve analysis for describing the overall relationship between nicotine consumption and nicotine dose (i.e., unit price) in a unit dose reduction model. Goodness-of-fit of the exponential curves and associated demand parameter estimates were similar to our prior study of sex differences in elasticity of demand for nicotine using the same nicotine reduction model (Grebenstein et al., 2013). The correlations between elasticity of demand and other measures of NSA in the present study indicate that the precision of exponential demand curve analysis is sufficient to distinguish between subpopulations of rats, as has been reported in other NSA studies (Diergaarde et al., 2011). Together, these findings are similar to those with other drugs of abuse and further support the utility of behavioral economic approaches to model drug abuse policies in animals (Hursh, 1991; Hursh and Roma, 2013).
4.2. Predictors of the reinforcement threshold, compensation, and elasticity of demand
4.2.1. Baseline nicotine intake
Because heavier smokers typically have lower quit rates (Borland et al., 2010), they might also be more likely to continue smoking RNC cigarettes and have a lower reinforcement threshold compared to lighter smokers. Consistent with this notion, some behavioral economic studies have shown that higher baseline CPD predicts greater persistence of hypothetical cigarette consumption despite increases in the price of cigarettes (Bidwell et al., 2012; Mackillop et al., 2008; Murphy et al., 2011). Although there was some tendency in the present study for rats with higher baseline nicotine intake to have lower reinforcement thresholds, this relationship was not statistically significant. Nonetheless, the association between baseline nicotine intake per se and the nicotine content required for extinction of smoking (i.e., reinforcement threshold) needs to be directly examined in humans.
The negative correlation between baseline nicotine intake and compensation observed in the present study is consistent with three previous studies examining this relationship in dose-reduction and access-reduction models in rats (Harris et al., 2011, 2009, 2008). In all of these studies, rats with higher baseline infusion rates exhibited lower levels of compensation. Similarly, a clinical study by Niaura et al. (2013) showed that participants with higher baseline mid-day plasma nicotine levels showed less compensation after switching to low nicotine yield cigarettes, even though this and other studies (Bandiera et al., 2015) showed no correlation between CPD and compensation. These findings suggest that baseline plasma nicotine concentrations may be a better predictor of compensation than CPD, and that smokers with higher baseline nicotine intake may be at lower risk for compensatory smoking during nicotine reduction.
Harris et al. (2009) suggested that the inverse relationship between baseline intake and compensation may be accounted for by individual differences in nicotine potency. That is, given similar peaks in the dose-response curve for individual rats, those in which nicotine potency is lower would have higher baseline infusion rates at the training dose. This dose would therefore be closer to the peak of the curve, leaving less “potential” for compensation in those rats. Yet, lower potency would also be expected to raise the reinforcement threshold in rats with high baseline intake in the present study, but this was not the case. Rather, the opposite trend was apparent, suggesting that other factors likely contribute to the negative correlation between baseline intake and compensation (e.g., pharmacodynamic processes, stimulus conditioning factors; see Sections 4.2.2. and 4.3.2.). Regardless of the underlying mechanism, these studies suggest that baseline nicotine intake could be useful for identifying smokers at risk of compensation in response to nicotine reduction policies and tailoring supportive therapies to minimize that risk.
The negative correlation between baseline nicotine intake and elasticity of demand in the present study suggests that the reinforcing efficacy of nicotine is greater in rats with high baseline NSA rates. This is consistent with human studies showing that heavier smokers exhibit less elastic demand for cigarettes using the Cigarette Purchase Task (Bidwell et al., 2012; Mackillop et al., 2008; Murphy et al., 2011). The present finding is also in accord with studies showing that smokers with higher baseline plasma nicotine levels (Niaura et al., 2013) or CPD (Borland et al., 2010) have a harder time quitting, even though they may also exhibit less compensation when cigarette nicotine yield is reduced (Niaura et al., 2013). Together, these studies suggest that baseline nicotine intake may be useful for identifying those smokers most likely to continue smoking despite gradual reductions in cigarette nicotine content.
4.2.2. Nicotine pharmacokinetics
The four-fold range of individual variability in nicotine clearance observed in the present study is similar to our prior studies in rats (Harris et al., 2008, 2009), as well as that reported in humans (Benowitz et al., 1982; Harris et al., 2009). The lack of correlation between nicotine clearance and baseline infusion rate is also in accord with our previous studies using the same nicotine training dose (Harris et al., 2009). The present study extends examination of this relationship across a wide range of unit nicotine doses. Nicotine clearance was positively correlated with overall nicotine intake during the dose-reduction protocol, and this correlation was strongest at unit doses just above the mean nicotine reinforcement threshold (e.g., 0.004 and 0.007 mg/kg). These findings are similar to those with oral NSA in mice (Klein et al., 2004; Siu et al., 2006) and clinical studies showing that smokers with slow nicotine metabolism smoke fewer cigarettes per day and consume less nicotine per cigarette (Benowitz et al., 2003; Chenoweth et al., 2014; Malaiyandi et al., 2005; Pianezza et al., 1998; Tyndale et al., 1999). Given that the rate of nicotine clearance is independent of dose (Benowitz et al., 2009b), it is unclear why the correlation between clearance and intake at the two highest unit doses was not significant (although a trend was apparent). It is possible that the magnitude and time course of pharmacodynamic effects (e.g., nicotinic receptor activation, desensitization, and reactivation; recruitment of non-nicotinic receptor systems) of high unit nicotine doses overshadowed the influence of individual differences in clearance. The significant correlations between clearance and intake at the lower unit doses (0.004 – 0.02 mg/kg), which are more similar to the nicotine obtained from one puff up to one cigarette, provide new evidence supporting the validity of NSA in animals as a model of tobacco use in humans and the notion that unit doses in this range may be more relevant to smoking in humans than higher doses commonly used for NSA (Matta et al., 2007).
The finding that faster clearance, shorter half-life, and lower AUC were all correlated with greater overall or maximal compensation, or both, supports the notion that faster metabolizers may be at greater risk for compensation when smoking RNC cigarettes. However, a recent study showed no correlation between nicotine metabolism (i.e. nicotine metabolite ratio (NMR)) and compensation in smokers exposed to gradual nicotine reduction (Bandiera et al., 2015). This discrepancy may be due, in part, to compensation being measured in terms of absolute change in CPD, rather than the use of a formal compensation index based on actual nicotine intake as in the present study. In addition, NMR was not associated with CPD at any level of nicotine content, suggesting that individual variability in the NMR may not have been sufficient to detect a correlation with compensation. Myriad differences between smoking and nicotine metabolism in humans and NSA in rats could also be involved (Matta et al., 2007).
The present findings also contrast with our prior report that compensatory increases in NSA during a single 50% reduction in unit dose were not correlated with nicotine pharmacokinetics (Harris et al., 2009). This may be attributable to differences in the experimental design between studies. The present study measured the average compensation over repeated reductions in dose and the maximal degree of compensation at any single dose. Although compensation in the present study tended to be greatest at the same dose used in the previous study (0.03 mg/kg), some rats showed greater compensation at lower doses. Therefore, compensation may have been underestimated in some rats in the previous study. The wider range of CI and pharmacokinetic parameter values in the present study may have also facilitated detection of correlations with pharmacokinetic parameters in the present study.
Faster nicotine clearance and lower AUC was also predictive of less elastic demand and a lower nicotine reinforcement threshold. Individual differences in the elasticity of demand for a drug could be considered a reflection of the relative reinforcing efficacy of nicotine between individuals, and therefore individual differences in the abuse potential/addictiveness of nicotine (Hursh and Silberberg, 2008; Mackillop et al., 2008). As such, the present findings suggest nicotine may have greater abuse liability in people with fast nicotine metabolism, which is in accordance with studies in adult smokers showing fast metabolizers of nicotine are more nicotine dependent, smoke more CPD, and have greater difficulty quitting than slow metabolizers (Chenoweth et al., 2014; Schnoll et al., 2014; 2013).
4.2.3. Baseline nicotine intake and nicotine clearance as co-predictors
Baseline nicotine intake and nicotine clearance were not correlated with each other, and each accounted for unique portions of the variance in several measures of NSA. As such, the combination of these variables in a multivariate regression model lead to a significant improvement in the prediction of compensation and elasticity of demand. Although the portion of the variance uniquely associated with nicotine clearance clearly suggests the role of a specific pharmacokinetic mechanism in these NSA measures, it is unclear what mechanism underlies the portion of the variance that is uniquely associated with baseline intake. Perhaps it reflects a role of conditioning and pharmacodynamic processes independent of nicotine pharmacokinetics (or at least nicotine clearance). Regardless of underlying mechanisms, the present findings suggest that multivariate regression models that include baseline nicotine intake and nicotine clearance may be especially useful for predicting smokers’ responses to RNCs in clinical studies.
4.2.4. Behavioral economic indices of demand
Together with the findings discussed above, the observation that elasticity of demand was correlated with the reinforcement threshold but not compensation, suggests a complex interaction of mechanisms that control NSA during dose reduction. The significant positive correlation between elasticity of demand and the reinforcement threshold indicates that rats in which nicotine has greater reinforcing efficacy are also those that are more sensitive to the reinforcing effects of low nicotine doses (i.e., nicotine is more potent, in that a lower dose is needed to produce a reinforcing effect). However, those rats also tended to have faster nicotine clearance, which would reduce the potency of nicotine (i.e., higher doses would be needed to produce a reinforcing effect). In addition, the lack of correlation between elasticity of demand and compensation is counterintuitive, as both provide a measure of the degree to which a rat defends its baseline level of intake. This finding is reminiscent of our prior study showing sex differences in compensation, but not elasticity of demand or the nicotine reinforcement threshold (Grebenstein et al., 2013). As Grebenstein et al. (2013) discussed, the complex pattern of relationships in the present study could result, in part, from the ways the NSA dose-response curve can be influenced by the relative degree of control exerted by nicotine per se versus the discriminative and conditioned-reinforcing effects of the stimuli with which nicotine is associated. Greater control by nicotine would serve to enhance compensation, reduce elasticity of demand, and lower the reinforcement threshold. In contrast, greater control by discriminative stimulus and conditioned reinforcing effects of nicotine-paired stimuli would not necessarily influence compensation (because nicotine has relatively less control in this case), but still reduce elasticity of demand and the reinforcement threshold by maintaining lever pressing in their own right. Therefore, it is possible that a rat with less elastic demand and a lower reinforcement threshold would exhibit less compensation. Individual differences in nicotine pharmacokinetics and/or pharmacodynamics could also moderate the relative roles of nicotine and nicotine-paired stimuli. Ultimately, the NSA dose-response function is the net result of the interactions between all of these interrelated variables. It is also important to note that the CI and α are to some extent mathematically distinct. The former is the magnitude of change in consumption as a proportion of baseline intake at a given unit dose. The later is an aggregate measure of the rate of change in consumption over all unit doses, independent of baseline intake. Regardless of the mechanisms mediating the observed relationships, the present findings suggest that elasticity of demand for nicotine/cigarettes could be a useful predictor of the nicotine reinforcement threshold in smokers exposed to progressive nicotine reduction.
4.3. Conclusions and future directions
The present study identifies individual differences in baseline nicotine intake and nicotine pharmacokinetics as potential determinants of the nicotine reinforcement threshold, elasticity of demand, and compensation. It also identifies elasticity of demand for nicotine as a potential determinant of the reinforcement threshold. Future studies that directly manipulate these variables are needed to determine the causal nature of these relationships. Nonetheless, these findings suggest factors that could be studied in humans exposed to RNC cigarettes, and may be helpful in predicting how subpopulations of smokers (e.g., heavy vs. light smokers, slow vs. fast nicotine metabolizers) might respond to nicotine reduction interventions and policies. Specifically, measures of nicotine intake, nicotine pharmacokinetics, and demand for nicotine may be helpful for anticipating potential risks (e.g., compensatory smoking) and benefits of nicotine regulation. Importantly, such measures may be relatively easy to obtain in human studies to determine their predictive utility. For instance, measurement of baseline nicotine intake or nicotine metabolism (i.e., the nicotine metabolite ratio) could be accomplished with a single mid-day blood sample (Niaura et al. 2013). Moreover, demand for nicotine/cigarettes can be easily measured via the Cigarette Purchase Task, a validated self-report measure that has been useful for assessing demand for cigarettes (Bidwell et al., 2012; Mackillop et al., 2008; Murphy et al., 2011). These measures may also be useful for individualizing supportive treatments to minimize compensation and facilitate reduction or cessation (e.g., high-dose NRT for heavy smokers and/or fast metabolizers; (Lerman et al., 2006; Schnoll et al., 2013; Selby et al., 2013)).
The present study focused on adult rats because the intent was to model the effects of a gradual nicotine reduction policy in established smokers, similar to studies in humans (Benowitz et al., 2012; 2009a). However, a primary question raised by the prospect of nicotine regulation is whether reducing nicotine delivery below the reinforcement threshold in current adult smokers would actually be sufficient to prevent the development of nicotine addiction in adolescents. The present study identifies a range of nicotine doses to address this question in future studies. Although several studies suggest that adolescent rats might be more sensitive than adults to the reinforcing effects of nicotine (Belluzzi et al., 2005; Chen et al., 2007; Levin et al., 2007, 2003), to our knowledge, none have examined NSA at doses below the reinforcement threshold observed in adults in the present study. Moreover, studies suggest that the reinforcement threshold for acquisition may be higher than that for maintenance of NSA (Smith et al., 2014), and factors that moderated maintenance of NSA in the present study may moderate acquisition in different ways (e.g. nicotine pharmacokinetics (Garcia et al., 2015; Rubinstein et al., 2013)). Direct comparison of the reinforcement threshold in adolescents and adults will be necessary to determine whether the clinical and policy implications of the present study are limited to the effects of RNC cigarettes in current adult smokers.
Supplementary Material
Highlights.
A gradual nicotine reduction policy was modeled in rats.
High baseline nicotine intake predicted less compensation and less elastic demand.
Higher nicotine clearance predicted lower reinforcement thresholds.
Higher nicotine clearance predicted greater compensation, and less elastic demand.
Less elastic demand predicted lower reinforcement thresholds.
Acknowledgments
Role of funding source
Funding for this study was provided by NIH/NIDA grant R01-026444 (MGL) and a career development award (MGL) and postdoctoral fellowship (PEG) from the Minneapolis Medical Research Foundation. These funding institutions had no role in the study design, data collection, data analysis, interpretation of the data, manuscript preparation, or decisions to submit the manuscript for publication.
The authors thank Christine Hernandez and Luke Kane for their excellent technical assistance in conducting the experiment, and Dr. Andrew Harris for his comments on earlier versions of the manuscript. The authors also thank Drs. Steven Hursh and Pete Roma from the Institutes for Behavior Resources (Baltimore, MD) and Johns Hopkins University School of Medicine for providing the software for demand curve analysis and their assistance with conducting the analysis.
Footnotes
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Contributors
MGL designed and supervised conduct of the study. DB conducted the study. PEG, MGL, PRP, and SAR analyzed the data. PEG, MGL, and PRP wrote drafts of the manuscript. All authors contributed to and approved the final manuscript.
Conflict of interest
The authors have no conflicts to disclose.
Preliminary data from this study were presented at the 19th Annual Meeting of the Society for Research on Nicotine and Tobacco, Boston, MA; March 2013.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrams DB, Herzog TA, Emmons KM, Linnan L. Stages of change versus addiction: a replication and extension. Nicotine Tob Res. 2000;2:223–229. doi: 10.1080/14622200050147484. [DOI] [PubMed] [Google Scholar]
- Arnold MM, Loughlin SE, Belluzzi JD, Leslie FM. Reinforcing and neural activating effects of norharmane, a non-nicotine tobacco constituent, alone and in combination with nicotine. Neuropharmacology. 2014;85:293–304. doi: 10.1016/j.neuropharm.2014.05.035. [DOI] [PubMed] [Google Scholar]
- Bandiera FC, Ross KC, Taghavi S, Delucchi K, Tyndale RF, Benowitz NL. Nicotine dependence, nicotine metabolism, and the extent of compensation in response to reduced nicotine content cigarettes. Nicotine Tob Res. 2015 doi: 10.1093/ntr/ntu337. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardo MT, Green TA, Crooks PA, Dwoskin LP. Nornicotine is self-administered intravenously by rats. Psychopharmacology. 1999;146:290–296. doi: 10.1007/s002130051119. [DOI] [PubMed] [Google Scholar]
- Belluzzi JD, Wang R, Leslie FM. Acetaldehyde enhances acquisition of nicotine self-administration in adolescent rats. Neuropsychopharmacology. 2005;30:705–712. doi: 10.1038/sj.npp.1300586. [DOI] [PubMed] [Google Scholar]
- Benowitz NL. Clinical pharmacology of nicotine: implications for understanding, preventing, and treating tobacco addiction. Clin Pharmacol Ther. 2008;83:531–541. doi: 10.1038/clpt.2008.3. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Dains KM, Hall SM, Stewart S, Wilson M, Dempsey D, Jacob P. Progressive commercial cigarette yield reduction: biochemical exposure and behavioral assessment. Cancer Epidemiol Biomarkers Prev. 2009a;18:876–883. doi: 10.1158/1055-9965.EPI-08-0731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, Dains KM, Hall SM, Stewart S, Wilson M, Dempsey D, Jacob P. Smoking behavior and exposure to tobacco toxicants during 6 months of smoking progressively reduced nicotine content cigarettes. Cancer Epidemiol Biomarkers Prev. 2012;21:761–769. doi: 10.1158/1055-9965.EPI-11-0644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, Hall SM, Stewart S, Wilson M, Dempsey D, Jacob P. Nicotine and carcinogen exposure with smoking of progressively reduced nicotine content cigarette. Cancer Epidemiol Biomarkers Prev. 2007;16:2479–2485. doi: 10.1158/1055-9965.EPI-07-0393. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Henningfield JE. Reducing the nicotine content to make cigarettes less addictive. Tob Control. 2013;22(Suppl 1):i14–i17. doi: 10.1136/tobaccocontrol-2012-050860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, Hukkanen J, Jacob P., III . Handbook of Experimental Pharmacology. Springer; Berlin Heidelberg, Berlin, Heidelberg: 2009b. Nicotine Chemistry, Metabolism, Kinetics and Biomarkers. In: Nicotine Psychopharmacology; pp. 29–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, Jacob P, Herrera B. Nicotine intake and dose response when smoking reduced-nicotine content cigarettes. Clin Pharmacol Ther. 2006;80:703–714. doi: 10.1016/j.clpt.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Jacob P, Jones RT, Rosenberg J. Interindividual variability in the metabolism and cardiovascular effects of nicotine in man. J Pharmacol Exp Ther. 1982;221:368–372. [PubMed] [Google Scholar]
- Benowitz NL, Pomerleau OF, Pomerleau CS, Jacob P. Nicotine metabolite ratio as a predictor of cigarette consumption. Nicotine Tob Res. 2003;5:621–624. doi: 10.1080/1462220031000158717. [DOI] [PubMed] [Google Scholar]
- Bidwell LC, Mackillop J, Murphy JG, Tidey JW, Colby SM. Latent factor structure of a behavioral economic cigarette demand curve in adolescent smokers. Addict Behav. 2012;37:1257–1263. doi: 10.1016/j.addbeh.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borland R, Yong HH, O’Connor RJ, Hyland A, Thompson ME. The reliability and predictive validity of the Heaviness of Smoking Index and its two components: findings from the International Tobacco Control Four Country study. Nicotine Tob Res. 2010;12(Suppl):S45–50. doi: 10.1093/ntr/ntq038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Costello MR, Abrams AL, Scheibal D, Farahi J, London ED, Olmstead RE, Rose JE, Mukhin AG. Brain nicotinic acetylcholine receptor occupancy: effect of smoking a denicotinized cigarette. Int J Neuropsychopharmacol. 2009;12:305–316. doi: 10.1017/S146114570800922X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Olmstead RE, Allen-Martinez Z, Scheibal D, Abrams AL, Costello MR, Farahi J, Saxena S, Monterosso J, London ED. Ventral striatal dopamine release in response to smoking a regular vs a denicotinized cigarette. Neuropsychopharmacology. 2008;34:282–289. doi: 10.1038/npp.2008.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brower VG, Fu Y, Matta SG, Sharp BM. Rat strain differences in nicotine self-administration using an unlimited access paradigm. Brain Res. 2002;930:12–20. doi: 10.1016/s0006-8993(01)03375-3. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, Chaudhri N, Perkins KA, Evans-Martin FF, Sved AF. Importance of nonpharmacological factors in nicotine self-administration. Physiol Behav. 2002;77:683–687. doi: 10.1016/s0031-9384(02)00918-6. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, Hoffman A, Perkins KA, Sved AF. Cue dependency of nicotine self-administration and smoking. Pharmacol Biochem Behav. 2001;70:515–530. doi: 10.1016/s0091-3057(01)00676-1. [DOI] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib M, Craven L, Palmatier MI, Liu X, Sved AF. Operant responding for conditioned and unconditioned reinforcers in rats is differentially enhanced by the primary reinforcing and reinforcement-enhancing effects of nicotine. Psychopharmacology. 2006;189:27–36. doi: 10.1007/s00213-006-0522-0. [DOI] [PubMed] [Google Scholar]
- Chen H, Matta SG, Sharp BM. Acquisition of nicotine self-administration in adolescent rats given prolonged access to the drug. Neuropsychopharmacology. 2007;32:700–709. doi: 10.1038/sj.npp.1301135. [DOI] [PubMed] [Google Scholar]
- Chen H, Sharp BM, Matta SG, Wu Q. Social interaction promotes nicotine self-administration with olfactogustatory cues in adolescent rats. Neuropsychopharmacology. 2011;36:2629–2638. doi: 10.1038/npp.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenoweth MJ, Novalen M, Hawk LW, Schnoll RA, George TP, Cinciripini PM, Lerman C, Tyndale RF. Known and novel sources of variability in the nicotine metabolite ratio in a large sample of treatment-seeking smokers. Cancer Epidemiol Biomarkers Prev. 2014;23:1773–1782. doi: 10.1158/1055-9965.EPI-14-0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens K, Caillé S, Stinus L, Cador M. The addition of five minor tobacco alkaloids increases nicotine-induced hyperactivity, sensitization and intravenous self-administration in rats. Int J Neuropsychopharmacol. 2009;12:1–12. doi: 10.1017/S1461145709000273. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM. Nicotine maintains robust self-administration in rats on a limited-access schedule. Psychopharmacology. 1989;99:473–478. doi: 10.1007/BF00589894. [DOI] [PubMed] [Google Scholar]
- Costello MR, Reynaga DD, Mojica CY, Zaveri NT, Belluzzi JD, Leslie FM. Comparison of the reinforcing properties of nicotine and cigarette smoke extract in rats. Neuropsychopharmacology. 2014;39:1843–1851. doi: 10.1038/npp.2014.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox BM, Goldstein A, Nelson WT. Nicotine self-administration in rats. Br J Pharmacol. 1984;83:49–55. doi: 10.1111/j.1476-5381.1984.tb10118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denoble VJ, Mele PC. Intravenous nicotine self-administration in rats: effects of mecamylamine, hexamethonium and naloxone. Psychopharmacology. 2006;184:266–272. doi: 10.1007/s00213-005-0054-z. [DOI] [PubMed] [Google Scholar]
- Diergaarde L, van Mourik Y, Pattij T, Schoffelmeer ANM, De Vries TJ. Poor impulse control predicts inelastic demand for nicotine but not alcohol in rats. Addict Biol. 2011;17:576–587. doi: 10.1111/j.1369-1600.2011.00376.x. [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Knopf S, Brown C. Nicotine self-administration in rats. Psychopharmacology. 1995;122:390–394. doi: 10.1007/BF02246272. [DOI] [PubMed] [Google Scholar]
- Donny EC, Chaudhri N, Caggiula AR, Evans-Martin FF, Booth S, Gharib MA, Clements LA, Sved AF. Operant responding for a visual reinforcer in rats is enhanced by noncontingent nicotine: implications for nicotine self-administration and reinforcement. Psychopharmacology. 2003;169:68–76. doi: 10.1007/s00213-003-1473-3. [DOI] [PubMed] [Google Scholar]
- Donny EC, Hatsukami DK, Benowitz NL, Sved AF, Tidey JW, Cassidy RN. Reduced nicotine product standards for combustible tobacco: building an empirical basis for effective regulation. Prev Med. 2014;68:17–22. doi: 10.1016/j.ypmed.2014.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donny EC, Houtsmuller E, Stitzer ML. Smoking in the absence of nicotine: behavioral, subjective and physiological effects over 11 days. Addiction. 2007;102:324–334. doi: 10.1111/j.1360-0443.2006.01670.x. [DOI] [PubMed] [Google Scholar]
- Donny EC, Taylor TG, LeSage MG, Levin M, Buffalari DM, Joel D, Sved AF. Impact of tobacco regulation on animal research: new perspectives and opportunities. Nicotine Tob Res. 2012;14:1319–1338. doi: 10.1093/ntr/nts162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia KLP, Coen K, Miksys S, Lê AD, Tyndale RF. Effect of brain CYP2B inhibition on brain nicotine levels and nicotine self-administration. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.40. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grebenstein P, Burroughs D, Zhang Y, LeSage MG. Sex differences in nicotine self-administration in rats during progressive unit dose reduction: Implications for nicotine regulation policy. Pharmacol Biochem Behav. 2013:114–115. 70–81. doi: 10.1016/j.pbb.2013.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green TA, Phillips SB, Crooks PA, Dwoskin LP, Bardo MT. Nornicotine pretreatment decreases intravenous nicotine self-administration in rats. Psychopharmacology. 2000;152:289–294. doi: 10.1007/s002130000524. [DOI] [PubMed] [Google Scholar]
- Harris AC, Burroughs D, Pentel PR, LeSage MG. Compensatory nicotine self-administration in rats during reduced access to nicotine: an animal model of smoking reduction. Exp Clin Psychopharm. 2008;16:86–97. doi: 10.1037/1064-1297.16.1.86. [DOI] [PubMed] [Google Scholar]
- Harris AC, Pentel PR, Burroughs D, Staley MD, LeSage MG. A lack of association between severity of nicotine withdrawal and individual differences in compensatory nicotine self-administration in rats. Psychopharmacology. 2011;217:153–166. doi: 10.1007/s00213-011-2273-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AC, Pentel PR, LeSage MG. Correlates of individual differences in compensatory nicotine self-administration in rats following a decrease in nicotine unit dose. Psychopharmacology. 2009;205:599–611. doi: 10.1007/s00213-009-1567-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami DK, Benowitz NL, Donny E, Henningfield J, Zeller M. Nicotine reduction: strategic research plan. Nicotine Tob Res. 2013a;15:1003–1013. doi: 10.1093/ntr/nts214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami DK, Donny EC, Koopmeiners JS, Benowitz NL. Compensatory smoking from gradual and immediate reduction in cigarette nicotine content. Cancer Epidemiol Biomarkers Prev. 2015;24:472–476. doi: 10.1158/1055-9965.EPI-14-0739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami DK, Heishman SJ, Vogel RI, Denlinger RL, Roper-Batker AN, Mackowick KM, Jensen J, Murphy SE, Thomas BF, Donny E. Dose-response effects of spectrum research cigarettes. Nicotine Tob Res. 2013b;15:1113–1121. doi: 10.1093/ntr/nts247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami DK, Kotlyar M, Hertsgaard LA, Zhang Y, Carmella SG, Jensen JA, Allen SS, Shields PG, Murphy SE, Stepanov I, Hecht SS. Reduced nicotine content cigarettes: effects on toxicant exposure, dependence and cessation. Addiction. 2010a;105:343–355. doi: 10.1111/j.1360-0443.2009.02780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami DK, Perkins KA, LeSage MG, Ashley DL, Henningfield JE, Benowitz NL, Backinger CL, Zeller M. Nicotine reduction revisited: science and future directions. Tob Control. 2010b;19:e1–10. doi: 10.1136/tc.2009.035584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht SS, Murphy SE, Carmella SG, Zimmerman CL, Losey L, Kramarczuk I, Roe MR, Puumala SS, Li YS, Le C, Jensen J, Hatsukami DK. Effects of reduced cigarette smoking on the uptake of a tobacco-specific lung carcinogen. J Natl Cancer Inst. 2004;96:107–115. doi: 10.1093/jnci/djh016. [DOI] [PubMed] [Google Scholar]
- Hoffman AC, Evans SE. Abuse potential of non-nicotine tobacco smoke components: acetaldehyde, nornicotine, cotinine, and anabasine. Nicotine Tob Res. 2013;15:622–632. doi: 10.1093/ntr/nts192. [DOI] [PubMed] [Google Scholar]
- Howell DC. Statistical Methods for Psychology. 5. Wadsworth Group; Pacific Grove, CA: 2002. [Google Scholar]
- Hursh SR. Behavioral economics of drug self-administration and drug abuse policy. J Exp Anal Behav. 1991;56:377–393. doi: 10.1901/jeab.1991.56-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursh SR, Roma PG. Behavioral economics and empirical public policy. J Exp Anal Behav. 2013;99:98–124. doi: 10.1002/jeab.7. [DOI] [PubMed] [Google Scholar]
- Hursh SR, Silberberg A. Economic demand and essential value. Psychol Rev. 2008;115:186–198. doi: 10.1037/0033-295X.115.1.186. [DOI] [PubMed] [Google Scholar]
- Klein LC, Stine MM, Vandenbergh DJ, Whetzel CA, Kamens HM. Sex differences in voluntary oral nicotine consumption by adolescent mice: a dose-response experiment. Pharmacol Biochem Behav. 2004;78:13–25. doi: 10.1016/j.pbb.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Lerman C, Tyndale R, Patterson F, Wileyto E, Shields P, Pinto A, Benowitz N. Nicotine metabolite ratio predicts efficacy of transdermal nicotine for smoking cessation. Clin Pharm Therpeutics. 2006;79:600–608. doi: 10.1016/j.clpt.2006.02.006. [DOI] [PubMed] [Google Scholar]
- LeSage MG, Keyler DE, Burroughs D, Pentel PR. Effects of pregnancy on nicotine self-administration and nicotine pharmacokinetics in rats. Psychopharmacology. 2007;194:413–421. doi: 10.1007/s00213-007-0830-z. [DOI] [PubMed] [Google Scholar]
- LeSage MG, Keyler DE, Collins G, Pentel PR. Effects of continuous nicotine infusion on nicotine self-administration in rats: relationship between continuously infused and self-administered nicotine doses and serum concentrations. Psychopharmacology. 2003;170:278–286. doi: 10.1007/s00213-003-1539-2. [DOI] [PubMed] [Google Scholar]
- LeSage MG, Keyler DE, Shoeman D, Raphael D, Collins G, Pentel PR. Continuous nicotine infusion reduces nicotine self-administration in rats with 23-h/day access to nicotine. Pharmacol Biochem Behav. 2002;72:279–289. doi: 10.1016/s0091-3057(01)00775-4. [DOI] [PubMed] [Google Scholar]
- Levin ED, Lawrence SS, Petro A, Horton K, Rezvani AH, Seidler FJ, Slotkin TA. Adolescent vs. adult-onset nicotine self-administration in male rats: duration of effect and differential nicotinic receptor correlates. Neurotoxicol Teratol. 2007;29:458–465. doi: 10.1016/j.ntt.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Rezvani AH, Montoya D, Rose JE, Swartzwelder HS. Adolescent-onset nicotine self-administration modeled in female rats. Psychopharmacology. 2003;169:141–149. doi: 10.1007/s00213-003-1486-y. [DOI] [PubMed] [Google Scholar]
- Mackillop J, Few LR, Murphy JG, Wier LM, Acker J, Murphy C, Stojek M, Carrigan M, Chaloupka F. High-resolution behavioral economic analysis of cigarette demand to inform tax policy. Addiction. 2012;107:2191–2200. doi: 10.1111/j.1360-0443.2012.03991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackillop J, Murphy JG, Ray LA, Eisenberg DTA, Lisman SA, Lum JK, Wilson DS. Further validation of a cigarette purchase task for assessing the relative reinforcing efficacy of nicotine in college smokers. Exp Clin Psychopharmacol. 2008;16:57–65. doi: 10.1037/1064-1297.16.1.57. [DOI] [PubMed] [Google Scholar]
- Malaiyandi V, Sellers EM, Tyndale RF. Implications of CYP2A6 genetic variation for smoking behaviors and nicotine dependence. Clin Pharmacol Therpeutics. 2005;77:145–158. doi: 10.1016/j.clpt.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Matta SG, Balfour DJ, Benowitz NL, Boyd RT, Buccafusco JJ, Caggiula AR, Craig CR, Collins AC, Damaj MI, Donny EC, Gardiner PS, Grady SR, Heberlein U, Leonard SS, Levin ED, Lukas RJ, Markou A, Marks MJ, Mccallum SE, Parameswaran N, Perkins KA, Picciotto MR, Quik M, Rose JE, Rothenfluh A, Schafer WR, Stolerman IP, Tyndale RF, Wehner JM, Zirger JM. Guidelines on nicotine dose selection for in vivo research. Psychopharmacology. 2007;190:269–319. doi: 10.1007/s00213-006-0441-0. [DOI] [PubMed] [Google Scholar]
- Murphy JG, Mackillop J, Tidey JW, Brazil LA, Colby SM. Validity of a demand curve measure of nicotine reinforcement with adolescent smokers. Drug Alcohol Depend. 2011;113:207–214. doi: 10.1016/j.drugalcdep.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niaura RS, Pearson JL, Abrams DB. Compensation predicts smoking cessation failure. Psychopharmacology. 2013;230:261–266. doi: 10.1007/s00213-013-3150-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pianezza ML, Sellers EM, Tyndale RF. Nicotine metabolism defect reduces smoking. Nature. 1998;393:750–750. doi: 10.1038/31623. [DOI] [PubMed] [Google Scholar]
- Rose J, Behm F. Effects of low nicotine content cigarettes on smoke intake. Nicotine Tob Res. 2004;6:309–319. doi: 10.1080/14622200410001676378. [DOI] [PubMed] [Google Scholar]
- Rubinstein ML, Shiffman S, Moscicki AB, Rait MA, Sen S, Benowitz NL. Nicotine metabolism and addiction among adolescent smokers. Addiction. 2013;108:406–412. doi: 10.1111/j.1360-0443.2012.04026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer G. Smoking behaviour and compensation: a review of the literature. Psychopharmacology. 1999;145:1–20. doi: 10.1007/s002130051027. [DOI] [PubMed] [Google Scholar]
- Schnoll RA, George TP, Hawk L, Cinciripini P, Wileyto P, Tyndale RF. The relationship between the nicotine metabolite ratio and three self-report measures of nicotine dependence across sex and race. Psychopharmacology. 2014;231:2515–2523. doi: 10.1007/s00213-013-3421-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnoll RA, Wileyto EP, Leone FT, Tyndale RF, Benowitz NL. High dose transdermal nicotine for fast metabolizers of nicotine: a proof of concept placebo-controlled trial. Nicotine Tob Res. 2013;15:348–354. doi: 10.1093/ntr/nts129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selby P, Andriash K, Zawertailo L, Persad D, Zack M, Busto UE. Escalating doses of transdermal nicotine in heavy smokers. J Clin Psychopharmacol. 2013;33:667–674. doi: 10.1097/JCP.0b013e31829a829d. [DOI] [PubMed] [Google Scholar]
- Shahan TA, Bickel WK, Badger GJ, Giordano LA. Sensitivity of nicotine-containing and de-nicotinized cigarette consumption to alternative non-drug reinforcement: a behavioral economic analysis. Behav Pharmacol. 2001;12:277–284. doi: 10.1097/00008877-200107000-00006. [DOI] [PubMed] [Google Scholar]
- Shahan TA, Bickel WK, Madden GJ, Badger GJ. Comparing the reinforcing efficacy of nicotine containing and de-nicotinized cigarettes: a behavioral economic analysis. Psychopharmacology. 1999;147:210–216. doi: 10.1007/s002130051162. [DOI] [PubMed] [Google Scholar]
- Shoaib M, Schindler CW, Goldberg SR. Nicotine self-administration in rats: strain and nicotine pre-exposure effects on acquisition. Psychopharmacology. 1997;129:35–43. doi: 10.1007/s002130050159. [DOI] [PubMed] [Google Scholar]
- Siu ECK, Wildenauer DB, Tyndale RF. Nicotine self-administration in mice is associated with rates of nicotine inactivation by CYP2A5. Psychopharmacology. 2006;184:401–408. doi: 10.1007/s00213-006-0306-6. [DOI] [PubMed] [Google Scholar]
- Smith TT, Levin ME, Schassburger RL, Buffalari DM, Sved AF, Donny EC. Gradual and immediate nicotine reduction result in similar low-dose nicotine self-administration. Nicotine Tob Res. 2013;15:1918–1925. doi: 10.1093/ntr/ntt082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TT, Schassburger RL, Buffalari DM, Sved AF, Donny EC. Low-dose nicotine self-administration is reduced in adult male rats naïve to high doses of nicotine: implications for nicotine product standards. Exp Clin Psychopharmacol. 2014;22:453–459. doi: 10.1037/a0037396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, LeSage MG. The reinforcement threshold for nicotine as a target for tobacco control. Drug Alcohol Depend. 2012;125:1–7. doi: 10.1016/j.drugalcdep.2012.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorge R, Clarke P. Rats self-administer intravenous nicotine delivered in a novel smoking-relevant procedure: effects of dopamine antagonists. J Pharmacol Exp Ther. 2009;330:633–640. doi: 10.1124/jpet.109.154641. [DOI] [PubMed] [Google Scholar]
- Tyndale RF, Pianezza ML, Sellers EM. A common genetic defect in nicotine metabolism decreases risk for dependence and lowers cigarette consumption. Nicotine Tob Res. 1999;1:S63–S67. doi: 10.1080/14622299050011831. [DOI] [PubMed] [Google Scholar]
- Valentine JD, Hokanson JS, Matta SG, Sharp BM. Self-administration in rats allowed unlimited access to nicotine. Psychopharmacology. 1997;133:300–304. doi: 10.1007/s002130050405. [DOI] [PubMed] [Google Scholar]
- Watkins SS, Epping-Jordan MP, Koob GF, Markou A. Blockade of nicotine self-administration with nicotinic antagonists in rats. Pharmacol Biochem Behav. 1999;62:743–751. doi: 10.1016/s0091-3057(98)00226-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.