Summary
The overall validity of biomarkers in the diagnosis of obstructive sleep apnea (OSA) remains unclear. We conducted a scoping review to provide assessments of biomarkers characteristics in the context of obstructive sleep apnea (OSA) and to identify gaps in the literature. A scoping review of studies in humans without age restriction that evaluated the potential diagnostic value of biological markers (blood, exhaled breath condensate, salivary, and urinary) in the OSA diagnosis was undertaken. Retained articles were those focused on the identification of biomarkers in subjects with OSA, the latter being confirmed with a full overnight or home-based polysomnography (PSG). Search strategies for six different databases were developed. The methodology of selected studies was classified using an adaptation of the evidence quality criteria from the American Academy of Pediatrics. Additionally the biomarkers were classified according to their potential clinical application. We identified 572 relevant studies, of which 117 met the inclusion criteria. Eighty-two studies were conducted in adults, 34 studies involved children, and one study had a sample composed of both adults and children. Most of the studies evaluated blood biomarkers. Potential diagnostic biomarkers were found in 9 pediatric studies and in 58 adults studies. Only 9 studies that reported sensitivity and specificity, which varied substantially from 43% to 100%, and from 45% to 100%, respectively. Thus, studies in adults have focused on the investigation of IL-6, TNF-α and hsCRP. There was not a specific biomarker that was tested by a majority of authors in pediatric studies, and combinatorial urine biomarker approaches have shown preliminary promising results. In adults IL-6 and IL-10 seem to have a favorable potential to become a good biomarker to identify OSA.
Keywords: sleep apnea, biomarker, diagnoses
Introduction
Obstructive sleep apnea (OSA) has now been widely recognized as a major public health concern with numerous and widespread societal consequences that include among others, motor vehicle accidents, increased cardiovascular morbidity, heightened risk for metabolic dysfunction, and mood, behavioral and cognitive deficits leading to impaired work performance and productivity.(1) Although healthcare costs are not normally distributed, i.e., the costliest and the sickest tertile of patients consume 65–82% of all medical-related costs, it has now become apparent that OSA significantly adds to the healthcare cost burden, in addition to its adverse impact on the economy. (2, 3) It is notable that sleep disorders have been assigned as playing a causative role in an estimated 9.1% of work-related injuries. (4)
The prevalence of OSA varies widely, ranging from 14.7% to 36.5%, depending on gender and nationality. (5) It is higher in males (34.2%) than in females (14.7%).(5) Although the prevalence of OSA in Hispanics (36.5%) is similar to American Whites (33.3%), increased risk of OSA occurs in both African American and Asian ethnic groups. (5–8) In contrast, the prevalence of pediatric OSA is reported to be between 1 and 4%, with the caveat that prospective community-based studies using overnight polysomnography (PSG) are lacking.(9, 10)
The standard diagnostic procedure for establishing the presence of OSA is the overnight polysomnography.(11) Except for the a priori reported consensus (11), an original publication or study that provided definitive validation on the use of overnight PSG as the gold standard in OSA diagnosis could not be found even after an extensive literature search. However, notwithstanding the great progress in our understanding of sleep disorders that PSG have afforded over the years, it has also become apparent that overnight PSG are onerous and labor-intensive tests that impose substantial inconvenience to the patients, and are relatively inaccessible. Indeed, waiting times between referral for evaluation to diagnosis commonly take 3–6 months across the United States and around the world. (12)
The relative complexity and high costs associated with overnight PSG as the gold standard approach employed for diagnosing the vast majority of sleep disorders has spurred the quest for alternative diagnostic methods. (12) The development of simple, cheap, and reliable screening tools that permit precise screening of at-risk populations is paramount. If accurate identification of those subjects with or without definitive disease is accomplished using such simplified and less onerous tools, then timely access to clinical care would be possible to a large sector of the population. (12)
During the search for this elusive screening tool, special interest has centered around potential OSA biomarkers. The ideal biomarker should be highly sensitive and specific for OSA, should be dose-responsive and correlate to severity of disease, and should be involved in an important causal pathway, so that changes in the biomarker levels reliably predict improvements in the outcome.(13) Several different OSA biomarkers have been proposed over the last 14 years. However, to the best of our knowledge, no scoping review has been conducted thus far to critically examine what we currently know on the potential viability and use of biomarkers in OSA diagnosis and management. Therefore, the purpose of this study was to map our current understanding regarding biomarkers, and provide assessments of their characteristics in the context of OSA in both adults and children, to identify gaps in the research and help with the dissemination of the findings, and to determine the value of conducting a full systematic review related to this topic.
Methods
This scoping review was done adhering to Arksey and O’Malley’s scoping review proposed reporting framework. (14)
Research question
A scoping review of studies in humans without age restriction that evaluated the potential diagnostic value of biological markers (blood, exhaled breath condensate (EBC), salivary, and urinary) in the diagnostic process of OSA syndrome was undertaken.
Identification of relevant studies
Inclusion criteria
Retained articles were only those studies whose objective was to identify associated biomarkers in subjects with OSA, the latter being confirmed with a full overnight PSG or home-based PSG. Only studies that performed PSG in all subjects were included. The selected studies could include studies in obese and cardiac patients. Studies that assessed the impact of treatment were also included. Studies with and without a control group were selected. Only studies in English, Spanish and Portuguese language were considered.
Exclusion criteria
Studies using day PSG or multichannel polygraphy as the reference diagnostic standard were not included. Studies using biomarkers only to detect the presence of OSA-associated morbidities (cognitive, excessive sleepiness, cardiovascular, metabolic) and/or which the sample included genetic syndromic patients (e.g., Down syndrome, craniofacial anomalies, neuromuscular disorders, etc.), or a cohort of patients with a primary disease for which OSA prevalence is being investigated (e.g., patients with kidney disease, and/or rheumatologic conditions) were omitted. Reviews, letters, conference abstracts and personal opinions were not considered.
Detailed individual search strategies for each of the following bibliographic databases were developed: Cochrane, EMBASE, MEDLINE, PubMed, and LILACS. A partial grey literature search was undertaken using Google Scholar. The end search date for all database searches was March 20, 2014. The references cited in the selected articles were also checked for any citation that could have been missed during the electronic database searches. Additional studies were obtained from a well-published expert in sleep medicine.
Appropriate truncation and word combinations were selected and were adapted for each database search. (Appendix 1) All references were managed by reference manager software (RefWorks -COS is a business unit of ProQuest, LLC. ©7200 Wisconsin Avenue, Suite 601 Bethesda, MD 20866 USA) and duplicate hits were removed.
Study Selection
The selection was completed in two phases. In phase 1, two reviewers independently reviewed the titles and abstracts of all identified electronic database citations (GDL and CPP). A third author was involved when required to make a final decision (SA). Any studies that appeared not to fulfill the inclusion criteria were discarded. In phase 2, the same selection criteria were applied to the full articles to confirm their eligibility. The same two reviewers (GDL and CPP) independently participated in phase 2. The references list of all included articles was reviewed by one examiner (GDL). The articles selected were read by both examiners (GDL and CPP). Any disagreement in either phase was resolved by discussion and mutual agreement between the three reviewers (GDL, CPP, SA). A fourth author with extensive professional experience in sleep medicine (DG) was involved when controversy arose before making a final decision. Final selection was always based on the full-text of the publication.
Charting the data
For all included studies the following information was recorded: year of publication, author, country, sample size, age, name and type of biomarkers, diagnostic PSG-based measure, results, and main conclusion. Authors of potentially eligible full-articles were contacted as necessary to provide further details about their studies.
One author (GDL) collected the required information from the selected articles. A second author (CPP) cross-checked all the collected information. Again, any disagreement in either phase was resolved by discussion and mutual agreement between the three reviewers (GDL, CPP, SA). A fourth author (DG) was involved, when required, to make a final decision.
Level of Evidence
The methodology of selected studies was classified using a non-validated adaptation of the evidence quality criteria from American Academy of Pediatrics (AAP) (11). Two reviewers (GDL and CPP) independently classified the studies into A (well-designed prognostic or diagnostic studies on relevant population), B (prognostic or diagnostic studies with minor limitations, overwhelmingly consistent evidence from observational studies), and C (observational studies (case-control and cohort design). Disagreements were resolved by a third reviewer (DG).
Additionally the biomarker clinical application was classified as (1) potential diagnostic biomarker(s); (2) when the evidence was inconclusive for diagnostic biomarker, and (3) if the evidence was not supportive as potential diagnostic biomarker(s). Two reviewers (GDL and CPP) independently classified the clinical application of biomarkers. A third reviewer (SA) reviewed the classification. Disagreements were resolved by a fourth reviewer (DG).
Collating, summarizing and report the results
Any outcome measurement was considered (risk ratio (RR), odds ratio (OR) or risk difference for dichotomous outcomes; mean difference or standardized mean difference for continuous outcomes; sensitivity and specificity in diagnostic studies).
Results
Study Selection
In phase 1, we found 572 citations across the five electronic databases. After duplicate articles were removed, 279 remaining different citations were retained. A comprehensive evaluation of the abstracts resulting in a final number of 104 articles after phase 1 was performed. We found 40 citations in Google Scholar, but only 4 articles from Google Scholar met our phase 1 inclusion criteria. We identified 29 additional studies from the hand-search of reference lists of these studies, and added six more articles received by an expert (DG). Therefore, we retrieved 143 articles to conduct a full-text review, and subsequently excluded 26 studies (15–40) (Appendix 2). Thus, a total of 117 articles were selected. A flow chart of the process of identification, inclusion, and exclusion of studies is shown in Figure 1.
Fig. 1.
Flow Diagram of literature search and selection criteria.
Study Characteristics
The selected studies were grouped into 2 categories: studies involving children (≤18 years of age) and adults (>18 years of age). Eighty-two studies were conducted in adults and 34 studies involved children (with the exception of one study that included individuals between 12–22 years old(41)). One study had a sample composed of both adults and children (42).
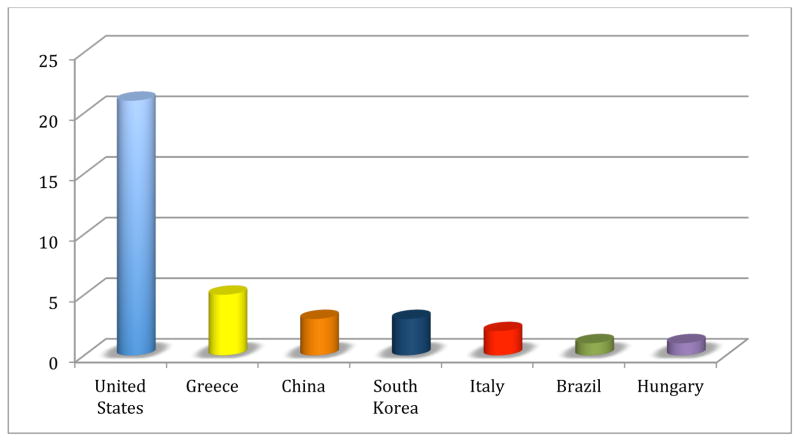
The pediatric studies were published between 2002 and 2014. They were conducted in the USA (41–60), Greece (61–65), China (66–68), South Korea (69–71), Italy (72, 73), Brazil (74), and Hungary (75). (Fig. 2) The diagnostic criterion for OSA was established based on the apnea index (AI), apnea/hypopnea index (AHI), obstructive apnea index (OAI), obstructive apnea-hypopnea index (OAHI), and respiratory disturbance index (RDI). Occasionally, the specific PSG measure used to reach the diagnosis of OSA was not reported (61, 64). When the authors used AHI, the AHI ranged from AHI>1 to AHI >5/hrTST. Most of the studies assessed blood biomarkers (41–44, 46, 49–52, 54–57, 59–62, 66–68, 74), while 7 studied urinary biomarkers (47, 48, 53, 58, 63, 64, 73), 4 explored for potential biomarkers in saliva (69–72), and 3 studies involved EBC (45, 65, 75). A summary of the study descriptive characteristics can be found in Table 1. Complementary information regarding these studies is reported in Appendix 3. The studies in adults were published between 2000 and 2014. The majority was published in China (76–87), USA (42, 88–98), and Japan (99–109) (Fig. 3). The OSA diagnostic criterion was established by AHI, and RDI and occasionally (89, 92, 93, 99, 100, 104, 110–112) it was not specifically reported. When the authors used AHI, the AHI ranged from AHI >5 to AHI ≥30/hrTST. Most of the studies assessed blood-based biomarkers (42, 76, 79–102, 105–111, 113–148), 2 focused on urinary biomarkers (103, 104), and 2 explored for biomarkers in EBC (149, 150), while only 1 study examined saliva (112). Five studies used both blood and urine (77, 151–154) and 4 studies used blood and EBC (78, 155–157). A summary of the study descriptive characteristics can be found in Table 2. Complementary information regarding these studies is reported in Appendix 4.
Fig 2.
Distribution of children studies according to country (n=35). United States (n=21), Greece (n=5), China (n=3), South Korea (n=3), Italy (n=2), Brazil (n=1), Hungary (n=1).
Table 1.
Summary of study descriptive characteristics of included pediatric articles (n=35). The biomarker clinical application was classified as (1) potential diagnostic biomarker(s); (2) inconclusive for diagnostic biomarker, and (3) evidence not supportive as potential diagnostic biomarker(s). The level of evidence was classified in A (well designed prognostic or diagnostic studies on relevant population), B (prognostic or diagnostic studies with minor limitations, overwhelmingly consistent evidence from observational studies), C (observational studies (case-control and cohort design).
| Year | Author | Country | Cases | Control | Mean Age or range if provided |
Type of Biomarker |
OSA diagnostic criteria (PSG) |
Biomarker | Classification as a Biomarker |
Level of Evidence |
|---|---|---|---|---|---|---|---|---|---|---|
| 2002 | Gozal et al(42) | United States | OSA (n=41/23 male) | - | 7.6 | Blood | AI>5 | VEGF | 2 | B |
| 2004 | Tauman et al(43) | United States | Consecutive snoring OSA (n=66/37 male) | Consecutive snoring non-OSA (n=15/10 male) | 3–18 (9.3) | Blood | AHI≥5 | CRP | 2 | B |
| 2005 | Kaditis et al(61) | Greece | AHI ≥5 (n=30/17 male) | AHI<5 (n=80/47 male) | 2–13 | Blood | Not reported | HOMA | 3 | B |
| 2005 | Larkin et al(44) | United States | Mild OSA (n=143) Moderate OSA (AHI 5–15) Severe OSA (AHI ≥15) |
- | 13–18 | Blood | AHI>1 | CRP | 2 | C |
| 2006 | Goldbart et al(45) | United States | Snoring (n=50): Mild OSA (n=29/58% male) OSA (n=21/57% male) |
Non-snoring (n=12/58% male) | 6–16 | EBC | AHI>5 | PGE2, LTB4, Cys-LTs, LTC4, LTD4, LTE4 | 2 (LTB4, Cys-LTs, LTC4, LTD4, LTE4) 3 (PGE2) |
B |
| 2006 | Kheirandish-Gozal et al(46) | United States | Non-obese with OSA before and after adenotonsillectomy (n=20/55% boys) | - | 7.3 | Blood | AHI ≥5 | CRP | 2 | B |
| 2006 | Krishna et al(47) | United States | Snoring or suspected OSA (n=11/9 male) | Healthy non-snoring (n=11/8 male) | 3–14 (6.8) | Urine | AHI>5 | Proteomic analysis | 2 | B |
| 2006 | Li et al(66) | China | Obese with Mild OSA: AHI: 1–10 (n=47) Moderate to Severe OSA: AHI>10 (n=13) |
Non-OSA snoring: AHI<1 (n=34) |
7–18 | Blood | AHI>1 | Lipid profile, Insulin | 1 (insulin) | B |
| 2006 | Montgomery-Downs et al(48) | United States | OSA (n=47) | - | 6.6 | Urine | OAHI ≥1 | IsoP-m | 3 | B |
| 2006 | O’Brien et al(49) | United States | Mild OSA (n=47/64% male) OSA (n=39/44% male) |
Non-OSA (n=42/43% male) | 4–10 (6.9) | Blood | AHI>5 | ICAM-1, P-selectin | 1 (P-selectin) 3(ICAM-1) |
B |
| 2006 | Shah et al(50) | United States | OSA (n=20) | Non-OSA with HS (n=20) | 3–12 | Blood | AHI >5 | Proteomic patterns | 1 | B |
| 2007 | Kaditis et al(62) | Greece | PS (n=19) Mild OSA (n=30) OSA (n=25) |
- | 3–13 | Blood | AHI> 1 | cICAM-1 | 3 | B |
| 2007 | Tauman et al(51) | United States | Mild OSA (n=42) OSA (n=43) |
Non-OSA (n=45) | 1–17 (8.2) | Blood | AHI> 1 | Leptin, Adiponectin, Glucose, Insulin, CRP | 3 | B |
| 2008 | Gozal et al(52) | United States | Non-obese OSA (n=20/12 male) | Non-snoring (n=20/12 male) | 4–9 | Blood | AHI>1 | IL-6 IL-10 |
2 | B |
| 2008 | Li et al(68) | China | Mild OSA (n=23) Moderate OSA (n=22) |
Non-OSA (n=96) | 8.5–12.8 | Blood | OAI>1 | CRP | 2 | B |
| 2009 | Gozal et al(53) | United States | PS (n=30/16 male) OSA (n=60/32 male) |
Non-OSA (n=30/16 male) | 2–9 | Urine | OAI > and/or OAHI > 2 | Kallikrein-1, Uromodulin, Urocortin-3, Orosomucoid-1 | 1 | B |
| 2009 | Kaditis et al(63) | Greece | PS (n=26) Mild OSA (n=29) Moderate to severe OSA (n=19) |
History of recurrent tonsillitis and without snoring (n=18) | 6.0 | Urine | OAHI ≥2 | Cys-LTs | 2 | B |
| 2009 | Kaditis et al(64) | Greece | Mild hypoxemia (n=22/9 male) Moderate hypoxemia (n=20/11 male) Severe hypoxemia (n=12/9 male) |
Non-snoring (n=10/7 male) | 5.2 | Urine | Not reported | Norepinephrine, Epinephrine, Normetanephrine, Metanephrine | 2 | B |
| 2010 | Kim et al(54) | United States | Mild OSA (n=106/60.4% male) Moderate-to-severe OSA (n=34/61.8% male) |
Non-OSA (n=115/55.7% male) | 5–10 (7.6) | Blood | AHI≥ 1 | MRP 8/14 | 2 | B |
| 2010 | Li et al(67) | China | HS and OSA symptoms (n=141/96 male). | - | 8.5–12.8 | Blood | OAI>1 | Adipokines | 3 | B |
| 2011 | Bhushan et al(55) | United States | OSA Non-Obese (n=92) OSA Obese (n=94) |
Non-OSA Non-Obese (n=90) Obese (n=33) |
5–8 | Blood | AHI≥1 | FABP4 | 2 | B |
| 2012 | DeBoer(41) | United States | OSA (n=9/4 male) | Non-OSA (n=15/10 male) | 12–22 | Blood | AHI>2.5 | hsCRP | 3 | B |
| 2012 | Khalyfa et al(56) | United States | OSA (n=131) | Non-OSA (n=323) | 5–8 | Blood | OAI≥ 1 and AHI≥ 5 | MIF, hsCRP, insulin, glucose | 1 | B |
| 2012 | Malakasioti et al(65) | Greece | Mild OSA (n=22/11 male) Moderate-to severe OSA (n=12/6 male) |
Non-OSA (n=16/8 male) | 4–14 | EBC | AHI >1 | H2O2 Sum of nitrate NOx | 2 | B |
| 2012 | Stefanini et al(74) | Brazil | OSA (n=28) | Non-OSA with HS (n=22) | 3–13 | Blood | AHI≥1 | Hemoglobin, Hematocrit, Glucose, Insulin, Triglycerides, Total cholesterol, HDL, LDL, VLDL, TSH, T4 | 3 | B |
| 2013 | Benedek et al(75) | Hungary | OSA (n=18) | Non-OSA with HS (n=10) | 8.5 | EBC | AHI ≥ 1 | VOCs mixtures | 1 | B |
| 2013 | Gozal et al(57) | United States | OSA with endothelial dysfunction (n=35) OSA without endothelial dysfunction (n=47) |
Healthy Non-OSA (n=35) | 5–10 (7.2) | Blood | AHI ≥2 | Plasma adropin | 3 | B |
| 2013 | Kheirandish-Gozal et al(58) | United States | OSA (n=50) | Non-OSA (n=20) | 3–12 (6.3) | Urine | AHI≥2 | Urinary Neurotransmitters | 1 | B |
| 2013 | Kim et al(59) | United States | Mild OSA (n=53) Moderate to severe OSA (n=9) |
Non-OSA (n=44) | 5–10 | Blood | AHI ≥ 1 | TREM-1, Pentraxin-3, hsCRP, MRP 8/14 | 2 | B |
| 2013 | Park et al(69) | Korea | OSA (n=48/32 male) | Non-OSA, (n=32/13 male) | 3–13 (7.1) | Saliva | AHI > 1 | Salivary cortisol (r-sCor, n-sCor, m-sCor) | 2(m-sCor, r-sCor) 3 (n-sCor) |
B |
| 2014 | Jeong et al(70) | Korea | OSA with enlarged tonsils/adenoids (n=13/11 male) | - | 3–11 | Saliva | AHI>1 | Salivary cortisol (n-sCor, m-sCor, sub-sCor, r-sCor) | 1 | B |
| 2014 | Kheirandish-Gozal(60) | United States | OSA Non-obese (n=57/54.3% male) OSA obese (n=53/58.5% male) |
Non-OSA Non-obese (n=59/54.2% male) Non-OSA/Obese (n=50/54% male) |
6.8 | Blood | AHI≥ 2 | LBP | 1 | B |
| 2014 | Park et al(71) | Korea | OSA with enlarged tonsils/adenoids (n=41/30 male) | OSA with enlarged tonsils/adenoids (n=26/9 male) | 3–16 | Saliva | AHI ≥1 | Alpha-amylase | 1 | B |
| 2014 | Patacchioli et al(72) | Italy | Mild OSA (n=13) Moderate-to-severe OSA (n=14) |
Non-OSA (n=7) | 4.9 | Saliva | AHI >1 | Salivary cortisol a-amylase diurnal trajectory and production | 2 (salivary cortisol) 3(a-amylase diurnal trajectory and production) |
B |
| 2014 | Villa et al(73) | Italy | AHI<5 (n=28/21 male) AHI≥5 (n=37/20 male) |
- | 5.9 | Urine | AHI ≥5 | 8-isoprostane | 1 | B |
All terms that mean obstructive sleep apnea (SDB, SRDB, OSAS) were standardized as OSA.
Abbreviations:
AHI=apnea/hypopnea index, cICAM-1=circulating intercellular adhesion molecule 1, CRP=C reactive protein, Cys-LTs=cysteinyl leukotrienes, EBC=exhaled breath condensate, FABP4=fatty acid binding protein 4, H2O2 =hydrogen peroxide, HDL=high density lipoprotein, HOMA=homeostasis model assessment, HS=habitual snoring, hsCRP=high-sensitivity C-reactive protein, ICAM-1 intercellular adhesion molecule 1, IL-10=interleukin-10, IsoP-m=isoprostane metabolites, LBP=lipopolysaccharide-binding protein, LTB4=leukotriene B4, LTC4=leukotriene C4, LTD4= leukotriene D4, LTE4= leukotriene E4, m-sCor=salivary cortisol after, PSG morning salivary cortisol, MIF=macrophage migration inhibitory factor, MRP=myeloid-related protein, n-sCor =salivary cortisol before PSG night salivary cortisol, NOx=nitrate mono-nitrogen oxides, OAHI= obstructive apnea-hypopnea index, OAI= obstructive apnea index, OSA=obstructive sleep apnea, Ptx3=Pentraxin-3, PGE2= Prostaglandin E2, PS=primary snoring, PSG=polysomnography, r-sCor= salivary cortisol ratio, sub-sCor = subtract salivary cortisol, T4= thyroxine, TREM-1=triggering receptor expressed on myeloid cells-1, TSH=thyroid stimulating hormone, VEGF= vascular endothelial growth factor, VLDL=very low density lipoprotein, VOCs=complex volatile organic compounds.
Fig 3.
Distribution of adults’ studies according to country (n=83). China (n=12), United States (n=12), Japan (n=11), Greece (n=8), Turkey (n=8), Spain (n=6), Brazil (n=3), UK (n=3), Germany (n=2), South Korea (n=2), Taiwan (n=2). In the following countries only one study was done: Arabia, Australia, Canada, France, India, Ireland, Israel, Israel/Sweden, Italy, Poland, Portugal, Sweden, Switzerland, Thailand. These countries are not represented in the graph.
Table 2.
Summary of study descriptive characteristics of included articles (adults, n=83) The biomarker clinical application was classified as (1) potential diagnostic biomarker(s); (2) inconclusive for diagnostic biomarker, and (3) evidence not supportive as potential diagnostic biomarker(s). The level of evidence was classified in A (well designed prognostic or diagnostic studies on relevant population), B (prognostic or diagnostic studies with minor limitations, overwhelmingly consistent evidence from observational studies), C (observational studies (case-control and cohort design).
| Year | Author | Country | Cases* | Controls | Age (mean in years) |
Type of Biomarker |
OSA diagnostic criteria at PSG |
Biomarker | Classification as a biomarker |
Level of Evidence |
|---|---|---|---|---|---|---|---|---|---|---|
| 2000 | Chin et al(99) | Japan | OSA patients treated with CPAP (n=23/23 male) | - | 51.0 | Blood | Not reported | Soluble intercellular adhesion molecules | 2 | B |
| 2002 | Carpagnano et al(149) | UK | OSA (n=18/13 male) Obese (n=10/4 male) |
Healthy adults (n=15/8 men) | 41.7# | EBC | AHI>20 | IL-6, 8-isoprostane | 1 | B |
| 2002 | Gozal et al(42) | United states | OSA (n=68/47 male) | - | 54.4 | Blood | AHI ≥15 | VEGF | 1 | B |
| 2002 | Lavie et al Study 1(110) | Israel and Sweden | OSA (n=85) | - | 52.8 | Blood | Not reported | VEGF | 2 | B |
| 2002 | Lavie et al Study 2(110) | Israel and Sweden | Severe OSA (n=5) | Control group 1: healthy adults (n=6) Control group 2 (n=6) Snoring OSA suspected but AHI <10 (n=6) |
40.0# | Blood | AHI≥10 | VEGF | 2 | B |
| 2002 | Lavie et al Study 3(110) | Israel and Sweden | CPAP patients (n=22) | - | 54.3 | Blood | Not reported | VEGF | 2 | B |
| 2002 | Schulz et al(114) | Germany | OSA with severe hypoxia (n=10) OSA with moderate hypoxia (n=10) |
Non-OSA (n=10) | Not reported | Blood | AHI>10 | VEGF | 1 | B |
| 2002 | Shamsuzzaman et al(88) | United states | OSA (n=22/18 male) | Non-OSA (n=20/15 male) | 45.5# | Blood | AHI≥5 | CRP | 1 | B |
| 2003 | Carpagnano et al(155) | UK | OSA (n=18/13 male) | Non-OSA (n=12/8 male) | 47.0# | Blood EBC | AHI>20 | 8-Isoprotane | 1 | B |
| 2003 | Christou et al(113) | Greece | OSA (n=17/16 male) | Non-OSA (n=8/4 male) | 51.4# | Blood | AHI>10 | Antioxidant capacity | 2 | B |
| 2003 | Ohga et al(100) | Japan | OSA (n=20/20 male) | Non-OSA (n=10/10 male) | 48.35# | Blood | Not reported | ICAM-1, IL-8, MCP-1 | 1 | C |
| 2003 | Yokoe et al(101) | Japan | Mild OSA (n=13) Moderate to severe OSA (N=17) |
Obese subjects (n=14/14 male) | 51.2# | Blood | AHI>5 | CRP, IL-6 | 1 | B |
| 2004 | Guilleminault et al(89) | United states | OSA (n=146) and UARS (n=39) | Control (n=54) | 43.8# | Blood | Not reported | CRP | 2 | B |
| 2004 | Imagawa et al(102) | Japan | Severe OSA (n=110) | Non-OSA (n=45) | Not reported | Blood | AHI ≥30 | IL-6, TNF-α | 2 | C |
| 2005 | Alzoghaibi et al(115) | Saudi Arabia | Nonsmoker patients with severe OSA (n=25) | Healthy nonsmokers (n=17) | 40.1# | Blood | AHI ≥ 5 | SOD, Lipid peroxidation, Cytokines, IL-8, GCP-2 | 3 (SOD Lipid peroxidation Cytokines) 1 (IL-8 GCP-2) |
B |
| 2005 | Sukegawa et al(103) | Japan | OSA (n=17/17 male) | - | 53.7 | Urine | AHI ≥5 | Urinary catecholamines | 2 | B |
| 2005 | Yamauchi et al(104) | Japan | Non-severe OSA (n=70) Severe OSA (n=58) |
- | 49.1# | Urine | Not reported | 8-OHdG | 1 | B |
| 2006 | Braga et al(116) | Brazil | OSA (n=29/29male) | Non-OSA (n=17/17 male) | 36.5# | Blood | AHI ≥ 5 | S100B, NSE | 1 | B |
| 2006 | Htoo et al(90) | United states | Mild to Moderate OSA (n=6/4 male) Severe OSA (n=7/6 male) |
Non-OSA (n=9/6 male) | 39.2# | Blood | AHI>10 | NF-kB | 1 | B |
| 2006 | Lentini et al(117) | Germany | Mild to Moderate OSA (n=93/17 male) Severe OSA (n=89/71 male) |
Non-OSA (n=19/8 male) | 54.9 | Blood | AHI>5 | CK levels | 1 | B |
| 2006 | Mehra et al(91) | United states | RDI 0–4.9 (n=177/58 male) RDI 5.0–9.9 (n=50/22 male) RDI 10–14.9 (n=39/17 male) RDI 15.0–29.9 (n=62/29 male) |
- | 46.9# | Blood | RDI≥5 | IL-6, slL-R | 1 (slL-R) 3(IL-6) |
A |
| 2007 | Peled et al(118) | Israel | OSA (n=100/61 male) | - | 58.1 | Blood | AHI ≥10 | VEGF | 3 | B |
| 2007 | Phillips et al(151) | Australia | Subjects after one and seven nights of withdrawal from CPAP (n=20/19 male) | - | 54.0 | Blood Urine | RDI ≥15 | hsCRP, hsIL-6 and hsTNF-α, VEGF, urinary catecholamines | 1 Noradrenaline 3(hsCRP, hsIL-6 and hsTNF-α, VEGF, adrenaline | B |
| 2007 | Punjabi et al(92) | United states | OSA (n=69/69 male) | - | 40.2 | Blood | Not reported | CRP | 2 | B |
| 2007 | Ryan et al(119) | Ireland | Mild/Moderate OSA (n=35) Severe OSA (n=31) Severe Obese OSA (n=14) |
Non-OSA (n=30) | 41.3# | Blood | AHI >5 | CRP, Homocysteine | 3 | C |
| 2007 | Ursavas et al(120) | Turkey | Moderate-to-severe OSA (n=39/30 male) | Non-OSA (n=34/23 male) | 50.5# | Blood | AHI ≥5 | ICAM-1, VCAM-1 | 1 | B |
| 2007 | Ye et al(76) | China | Mild OSA (n=23/23 male) Moderate to severe OSA (n=28/28 male) |
Obese men (n=25) | 49.8# | Blood | AHI ≥ 5 | CRP, MMP-9 | 1 | B |
| 2008 | Antonopoulou et al(156) | Greece | OSA (n=45/37 male, 28 smokers) | Healthy subjects non-ramdomly selected (n=25/18 male, 15 smokers) | 51.5# | Blood EBC | AHI ≥ 5 | EBD: pH, 8-isoprostane, TNF-α, IL-6 Plasma: leptin |
3 | B |
| 2008 | Arias et al(152) | Spain | OSA (n=30/30 male) | Obese subjects (n=15/15 male) | 50.0# | Blood Urine | AHI ≥10 | sTNFR-1, IL-6, LTB4, TNF-α, Norepinephrine, Epinephrine | 1 sTNF, Norepinephrine Epinephrine) 3(R-1 IL-6 LTB4 TNF-α) |
B |
| 2008 | Burioka et al(105) | Japan | Severe OSA (n=9/9 male) | - | 48.2 | Blood | AHI>5 | IL-6 | 1 | B |
| 2008 | Constantinidis et al(121) | Greece | Obese OSA (n=13/13 male) Overweighted OSA (n=11/11 male) |
Overweighted (n=12/12 male) and obese (n=15/15 male) | 26–54 (45.1) | Blood | AHI>5 | IL-1β,, IL-6, TNF-a | 1(IL-6 TNF-a) 3 (IL-1β,) |
B |
| 2008 | Kanbay et al(122) | Turkey | OSA (n=106) | Non-OSA (n=32) | 48.9# | Blood | AHI ≥5 | Adiponectin, TNF-α | 1 | B |
| 2008 | Li et al(157) | Thailand | Mild OSA: 22 Moderate OSA: 22 Severe OSA: 24 |
Non-OSA (n=22) | 48.5 | Blood EBC | AHI ≥5 | 8-isoprostane, IL-6, TNF-α, IL-10 | 1 | B |
| 2008 | Norman et al(93) | United states | OSA (n=109) | - | 48.5 | Blood | Not reported | Serum Aminotransferase | 2 | B |
| 2008 | Petrosyan et al(150) | Greece | Obese non-OSA (n=9) OSA (n=26) |
Non-obese and non-OSA (n=10) | 48.4# | EBC | AHI>20 | nNO, eNO, eCO, LTB4, nitrates, H2O2 | 1 | B |
| 2008 | Takahashi et al(106) | Japan | OSA candidates to CPAP (n=41/38 male) | Non-OSA (n=12/11 male) | 48.3# | Blood | AHI>5 | TRX, Adiponectin | 1 | B |
| 2008 | Zamarron et al(123) | Spain | OSA suspected (n=96/96 male) divided in two groups: OSA OSA with hypertension |
- | 53.3 | Blood | AHI>10 | PAI-1 | 1 | C |
| 2009 | Kim et al(124) | South Korea | Severe OSA (n=40/40 male) | Non-OSA (n=34/34 male) | 44.8# | Blood | AHI ≥ 5 | Nine proteins: Haptoglobin alpha 2 chain Haptoglobin beta chain Chain B, Alpha-Ferrous-Carbon monoxy (T-state) Apolipoprotein M Complement component 3 precursor Serum paraoxonase Complement factor B Complement C4 precursor Complement component C4a |
2 | B |
| 2009 | Kuramoto et al(107) | Japan | Without or mild OSA (n=35/27 male) Moderate OSA (n=35/26 male) Severe OSA (n=46/43 male) |
- | 49.5# | Blood | Without or mild OSA: AHI<20 | SAA, Brachial-ankle PWV | 1 | B |
| 2009 | Lam et al(77) | China | AHI <5 (n=25/25 male) AHI ≥5 (n=69/69 male) |
43.7 | Blood Urine | AHI ≥5 | Urinary catecholamines, Cortisol, Insulin, Glucose, Lipids | 1(Urinary catecholamines) 2(Cortisol Insulin Glucose lipids) |
B | |
| 2009 | Lederer et al(94) | United states | OSA (n=11/6 male) | Non-smoking healthy (n=10/5 male) | 40.0 | Blood | AHI ≥5 | KL-6 | 2 | C |
| 2009 | Li et al(78) | China | Mild OSA (n=22) Moderate OSA (n=22) Severe OSA (n=24) |
Smoker control group (n=10) Non OSA (n=22) |
44# | Blood EBC | AHI ≥5 | 8-isoprostane, TNF-α, IL-6, IL-10 | 1 (IL-6, IL-10) 2(8-isoprostane, TNF-α) | |
| 2009 | Lui et al(79) | China | AHI 0–15 (n=35/35 male) AHI 15to<30 (n=32/32 male) AHI ≥30 (n=44/44 male) |
- | 44.3# | Blood | AHI ≥5 | CRP | 1 | B |
| 2009 | Ting et al(111) | Taiwan | OSA (n=263/56.6% male) divided in four groups: Constant Low (n=138) Morning Drop (n=34) Constant High (n=63) Morning Surge (n=28) |
- | 44.7 | Blood | Not reported | Biochemical markers, SSBP | 2 | B |
| 2009 | Ucar et al(125) | Turkey | Snoring OSA (n=62/48 male) | Snoring non-OSA (n=18/10 male) | 48.9# | Blood | AHI ≥5 | Arterial lactate levels | 1 | B |
| 2010 | Lee et al(126) | Taiwan | OSA (n=65/65 male) | - | 38.2 | Blood | AHI ≥5 | hs-CRP | 1 | B |
| 2010 | Pallayova et al(95) | United states | Severely obese adults from bariatric surgery (n=45/9 male) | - | 36.0 | Blood | AHI ≥5 | Glucose, Insulin, Selected cytokines, HOMA-IS, HOMA-B | 2 | B |
| 2010 | Steiropoulo s et al(127) | Greece | OSA: (n=38/33 male) | Consecutive non-OSA (n=23/17 male) | 44.6# | Blood | AHI≥ 15 | TNF-α, Il-6, CRP, fibrinogen levels | 1 (TNF-α, Il-6) 3(CRP, fibrinogen levels) |
B |
| 2010 | Ye et al(80) | China | Mild (n=43/32 male) Moderate (n=39/31 male) Severe (n=45/39 male) |
Non-OSA (n=52/37 male) | 45.3# | Blood | AIH ≥5 | DNA, IL-6, MDA | 1 | B |
| 2011 | Akinnusi et al(96) | United states | Non-smokers OSA (n=38/38 male) | Healthy subjects (n=12/12 male) | 50.0# | Blood | AHI>5 | pLOX-1 circulating apoptotic endothelial cells (CD146+, CD45−, CD31+) | 1 | B |
| 2011 | Cintra et al(128) | Brazil | OSA (n=75/75 male) | Non-OSA (n=75/75 male) | 53.2# | Blood | AHI>5 | Cysteine, Homocysteine | 1 | B |
| 2011 | Jurado-Gamez et al (130) | Spain | OSA (n=46/34 male) | non-OSA: (n=23/15 male) | 35–65 (47.5#) | Blood | AHI ≥5 | IRH, Oxidative Stress | 2 | B |
| 2011 | Kohler et al(153) | Switzerland | Therapeutic CPAP (n=20/19 male) Sub-therapeutic CPAP (n=21/21 male |
- | 20.5# | Blood Urine | Not reported | Urinary catecholamine, Lipids, insulin resistance | 1 (Urinary catecholamines) 2 (Lipids, insulin resistance) |
B |
| 2011 | Ladesich et al(97) | United states | None/Mild OSA (n=228/122 male) Moderate OSA (n=70/50 male) Severe OSA (n=52/36 male) |
- | 54.0# | Blood | None/Mild OSA: AHI 0–14 | RBC omega-3 fatty acids | 2 | B |
| 2011 | Pallayova et al(98) | United states | Morbidly obese (n=23) | - | Older than 21 years old | Blood | AHI ≥5 | TNF-α receptor 2 | 1 | B |
| 2011 | Zamarron et al(132) | Spain | OSA (n=20/20 male) | - | 33–64 (49.9) | Blood | AHI≥10 | ICAM-1, PAI-1, E-selectin, Endothelin-1, vWF | 1 (ICAM-1, PAI-1) 3(E-selectin, Endoth elin-1, vWF) |
B |
| 2012 | Duru et al(133) | Turkey | OSA (n=43/25 male) | Non-OSA (n=25/17 male) | 45.5# | Blood | AHI ≥ 5 | S100B | 1 | B |
| 2012 | Feng et al(81) | China | OSA (n=132/132 male) | Non-OSA (n=108/108 male) | 47.4# | Blood | AHI ≥5 | Chemerin | 1 | C |
| 2012 | Guven et al(134) | Turkey | OSA (n= 47/9 male) | Non-OSA (n=29/5 male) | 52.8# | Blood | AHI ≥ 5 | hs-CRP | 1 | B |
| 2012 | Hira et al(129) | India | OSA (n=40/36 male) | Non-OSA (n=40/36 male) | Control group: <40=12 40–50=16 >50=12 Study group: <40=10 40–50=20 >50=10 |
Blood | AHI ≥ 5 | Uric acid | 1 | C |
| 2012 | Jurado-Gamez et al(135) | Spain | OSA (n=68/49 male) divided in: Mild-moderate desaturation group (n=31/22 male) Severe group (n=37/27 male) |
48.0 | Blood | AHI ≥ 5 | MDA, ICAM-1, IRH, P-selectin | 2 | B | |
| 2012 | Lee et al(136) | South Korea | Mild to moderate OSA (n=31) Severe OSA (n=22) |
Non-OSA (n=20) | 45.7# | Blood | AHI ≥5 | OxLDL, GPX, LDL, TAS, SOD, 8-isoprostano, PO-56 | 3 | B |
| 2012 | Mancuso et al(137) | Italy | Mild OSA (n=7) Moderate OSA (n=15) Severe OSA (n=19) |
Healthy (n=32/18 male) | 54.0# | Blood | AHI <15 | AOPP, FRAP, GSH | 2 | C |
| 2012 | Papaioannou et al(112) | UK | Community adults (n=22/20male) | Community adults (n=22/17 male) | 46.5# | Saliva | Not reported | Melatonin | 3 | C |
| 2012 | Simiakakis et al(138) | Greece | Consecutive subjects referred to Sleep Laboratory: (n=42/27 male) | Consecutive subjects referred to Sleep Laboratory: (n=24/12 male) | 46.3# | Blood | AHI ≥15 | d-ROMs, BAP | 2 | B |
| 2012 | Sokucu et al(139) | Turkey | Adults referred to Sleep Laboratory with OSA symptoms (n=108/72 male) | - | 49.2 | Blood | AHI ≥5 | RDW | 1 | C |
| 2012 | Svensson et al(131) | Sweden | AHI <15 (n=262) AHI ≥15 (n=136) |
50.0 | Blood | AHI ≥15 | CRP, TNF-α, IL-6 MPO, lysozyme | 3 | A | |
| 2013 | Aihara et al(108) | Japan | Consecutive OSA (n=38/21 male), none had been previously diagnosed with or treated for OSA divided in: Group sputum + (n=28/15 male) Group sputum – (n=10/6 male) |
- | 55.4# | Blood Sputum | AHI ≥ 5 | Leptin, Cytokine, Albumin | 2 | B |
| 2013 | Chung et al(140) | Canada | Preoperative patients (n=384/289 male) | - | 60.0 | Blood | AHI>5 | HCO3 | 2 | B |
| 2013 | Cofta et al(141) | Poland | Mild-OSA-1 (n=21) Moderate-OSA-2 (n=18) Severe-OSA-3 (n=21) |
Non-smoking: ol-OSA-0 (n=20) | 54.5# | Blood | AHI ≥ 5 | E-selectin, L-selectin, P-selectin | 1 | B |
| 2013 | Ferrarini et al(142) | Spain | Non-severe OSA (n=15/11 male) and severe OSA (n=18/12 male) | - | Non-severe: OSA 32–81 years Severe OSA: 34–85 years |
Blood | AHI ≥ 5 | Glycerophosh oplipids, Pophyrins, Fatty Acyls, Amino acid, metabolities and derivates, Peptides, PE | 2 | B |
| 2013 | Guo et al(82) | China | OSA suspected: Mild OSA (n=14) Moderate OSA (n=11) Severe OSA (n=29) |
OSA suspected: (n=9) | 47.8# | Blood | AHI ≥ 5 | TRX | 1 | B |
| 2013 | Hirotsu et al(143) | Brazil | OSA (n=339/18.8% male) | non-OSA (n=682/25.9% male) | 44.6# | Blood | AHI ≥ 15 | Uric acid | 1 | A |
| 2013 | Kurt et al(144) | Turkey | Group A (n=20): AHI< 5/h Group B (n=15): AHI 5–14 Group C (n=26): AHI 15–29.9 Group D (n=37): AHI ≥30/h |
- | 52.5# | Blood | AHI ≥5 | PDW, CRP, MPV, RDW | 1 (PDW) 3(CRP, MPV, RDW) |
B |
| 2013 | Murase et al(109) | Japan | Mild OSA (n=37/24 male) Moderate OSA (n=24/18 male) Severe OSA (n=26/18 male) |
Non OSA (n=15/8 male) | 54.8# | Blood | AHI≥ 5 | Plasma Ngal | 2 | B |
| 2013 | Ntalapasch a et al(145) | Greece | OSA (n=18) | Non-OSA (n=13) | 49.5# | Blood | AHI >30 | GSH, GSSG, 8-isoprostane, TBARS, catalase activity, SOD, TAC | 1 (GSH, GSSG) 3(8-isoprost ane, TBARS, catalas e activity, SOD, TAC) |
B |
| 2013 | Ozben et al(146) | Turkey | OSA (n=60/33 male) | Healthy (n=23/19 male) | 49.8# | Blood | AHI ≥5 | Copeptin | 3 | B |
| 2013 | Pinto et al(154) | Portugal | Mild/Moderate OSA (n=36) Severe OSA (n=31) |
- | 49.5# | Blood Urine | RDI ≥5 | NOx U-NE levels | 1 | B |
| 2013 | Shi et al(83) | China | OSA (n=126/126 male) | Non-OSA (n=74) | 49.0# | Blood | AHI>5 | S100A12 | 1 | B |
| 2013 | Wang et al(85) | China | Moderate to severe OSA (n=20/18 male | Non-OSA (n=15/14 male) | 38.5# | Blood | AHI ≥15 | Fractalkine | 1 | C |
| 2013 | Wang et al(84) | China | OSA (n=192/192 male) | Non-OSA (n=144/144 male) | 49.0# | Blood | AHI≥5 | Ometin-1 | 1 | B |
| 2013 | Zhang et al(86) | China | Mild OSA (n=15/15 male) Moderate OSA (n=24/24 male) Severe OSA (n=36/36 male) |
Non-OSA (n=23/23 male) | 32.5# | Blood | AHI≥5 | Cystatin C, hsCRP | 1 | B |
| 2014 | Tual-Chalot et al(147) | France | OSA (n=20/20 male) | Non-OSA (n=15/15 male) | 43.3# | Blood | AHI≥5 | Circulating microparticles | 2 | B |
| 2014 | Vavougios et al(148) | Greece | OSA (n=120/100 male) | - | 48 | Blood | AHI>5 | DJ-1 protein | 1 | B |
| 2014 | Wang et al(87) | China | OSA (n=159/98 male) | Healthy (n=104/64 male) | 53.0# | Blood | AHI>5 | YKL-40 | 1 | B |
All terms that mean obstructive sleep apnea (SDB, SRDB, OSAS) were standardized as OSA.
Mean calculated by author.
Abbreviations
8-OHdG=8-hydroxy-2′-deoxyguanosine, AHI=apnea/hypopnea index, AIC=akaike information criterion, ALT=alanine aminotransferase, AOPP=advanced oxidation protein products, AST=aspartate aminotransferase, BAP=biological anti-oxidant capacity, BMI=body mass index, BP=blood pressure, CK=creatine phosphokinase, CPAP=continuous positive airway pressure, CRP=C-reactive protein, d-ROMs=derivates of reactive oxygen metabolites, DPB= diastolic blood pressure, EBC= exhaled breath condensate, eCO=exhaled carbon monoxide, eNO=exhaled nitric oxide, FFA= free fatty acids, FPG=fasting plasma glucose, FRAP=ferric reducing antioxidant power, GPX=glutathione peroxidase, GSH= reduced glutathione, GSH=total glutathione, GSSG = oxidized glutathione, H2O2=hydrogen peroxide, HCO3=bicarbonate, HOMA-B=pancreatic beta-cell function, HOMA-IR=homeostasis model assessment of insulin resistance, HOMA-IS=homeostasis model assessment estimates of insulin sensitivity, hs-CRP=high sensitivity C-reactive protein, ICAM-1=intercellular adhesion molecule-1, IL-6=interleukin-6, IL-8=interleukin-8, IRH=ischemic reactive hyperemia, LDA=linear discriminant analysis, LDL=serum low-density lipoprotein, LH=luteinizing hormone, LTB4=leukotriene B4, MAP=mean arterial pressure, MCP-1=monocyte chemoattractant protein-1, MDA=malondialdehyde, MMP-9=metalloproteinase-9, MPO=myeloperoxidase, MPV=mean platelet volume, MVDA=multivariate data analysis, NE=norepinephrine, NF-kB=proinflamatory transcription nuclear factor, Ngal=neutrophil gelatinase, NME=normetanephrine, nNO=nasal nitric oxide, NOx=reduced plasma nitrate, NSE=neuron-specific enolase, ODI=oxygen desaturation index, OSA=obstructive sleep apnea, oxLDL =oxidized low-density lipoprotein cholesterol, PAI-1=plasminogen activator inhibitor-1, PDW=platelet distribution width, PE=phosphoethanolamie, pLOX-1=plasma LOX-1, PRL=prolactin, PSG=polysomnography, PWV=pulse wave velocity, RDI=respiratory disturbance index, RDW=red cell distribution width, SAA=serum amyloid, SBP=systolic blood pressure, slL-R=soluble IL-6 receptor, SOD=superoxide dysmutase, SSBP=post- to pre-overnight sleep systolic blood pressure, sTNFR-1=soluble tumor necrosis factor-α receptor, TAC=total antioxidant capacity, TAS=total antioxidant status, TBARS =thiobarbituric acid-reactive substances, TNF-α=tumor necrose factor alpha, TRH=thyroid releasing hormone, TRX=thioredoxin, TSH=thyroid stimulating hormone, U-NE=urinary norepinephrine, UVDA=univariate data analysis, VCAM-1=vascular cell adhesion molecule-1, VEGF=vascular endothelial growth factor, vWF=von Willebrand factor, WHR=waist-to-hip ratio, WL=weight loss.
Level of Evidence
In studies involving children, nearly all studies were classified as B (prognostic or diagnostic studies with minor limitations, overwhelmingly consistent evidence from observational studies). Only one study (44) was classified as C (case-control and cohort design). No study was classified as A (well designed prognostic or diagnostic studies on relevant population).
In adult-based studies 70 were classified as B, with 11 studies being classified as C. Only 3 studies were classified as fulfilling A criteria (91, 131, 143).
Synthesis of Results
When biomarkers were classified according to their clinical application, the biomarkers studied in 9 pediatric studies were designated as potential diagnostic biomarkers; in 15 studies, findings were inconclusive for a diagnostic biomarker, and 8 studies presented evidence that was not supportive as potential diagnostic biomarkers. Three studies had different classifications for two biomarkers studied. The classification for each study is presented in Table 1. All potential biomarkers for pediatric OSA are presented in Table 3.
Table 3.
Potential biomarkers identified in children.
| Potential Biomarkers | Amount of studies that investigated this biomarker |
|---|---|
| Insulin | 2 |
| 8-isoprostane | 1 |
| Alpha-amylase | 1 |
| Glucose | 1 |
| hsCRP | 1 |
| Kallikrein-1 | 1 |
| Lipopolysaccharide-binding Protein | 1 |
| Macrophage migration inhibitory factor | 1 |
| Orosomucoid-1 | 1 |
| P-selectin | 1 |
| Proteomic patterns | 1 |
| Salivary cortisol (n-sCor, m-sCor, sub-sCor, r-sCor) | 1 |
| Urinary Neurotransmitters | 1 |
| Urocortin-3 | 1 |
| Uromodulin | 1 |
| VOCs mixtures | 1 |
hsCRP=high sensitivity C-reactive protein, VOCs=complex volatile organic compounds.
In studies involving adults, classification of the biomarkers according to their clinical application yielded 58 studies where the biomarkers were considered as potential diagnostic biomarkers, while 19 studies were inconclusive for diagnostic biomarkers, and 3 studies presented evidence not supportive as potential diagnostic biomarkers. One study had different classifications for two biomarkers concomitantly studied. Regarding the potential diagnostic biomarkers, interleukin-6 (IL-6), tumor necrosis factor α (TNF-α) and high sensitivity C-reactive protein (hsCRP) were the most frequently assessed biomarkers. The classification for each study is presented in Table 1. The potential biomarkers for adults identified are shown in Table 4.
Table 4.
Potential biomarkers identified in adults.
| Potential Biomarkers | Amount of studies that investigated this biomarker |
|---|---|
| IL-6 | 7 |
| TNF-α | 5 |
| CRP | 4 |
| 8-isoprostane, hs-CRP, ICAM-1. | 3 |
| Adiponectin, IL-10, IL-8, PAI-1, S100B, TRX, Urinary catecholamines, VEGF, uric acid | 2 |
| 8-OHdG, Arterial lactate levels, Brachial-ankle PWV, Chemerin, CK, Cystatin C, Cysteine, DJ-1, E-selectin, eCO, eNO, Epinephrine, Fractalkine, GSH, GSSG, H2O2, Homocysteine, L-selectin, LTB4, MCP-1, MMP-9, NF-kB, Nitrates, nNO, Noradrenaline, Norepinephrine, NOx, NSE, Ometin-1, P-selectin, PDW, pLOX-1RDW, S100A12, SAA, slL-R, sTNFR-1, TNF-α receptor 2, VCAM-1, YKL-40. | 1 |
8-OHdG=*-hydroxy-2′-deoxyguanosine, CK=creatine phosphokinase, DJ-1= gene that is involved in tumorigenesis and in maintaining mitochondrial homeostasis, eCO=exhaled carbon monoxide, eNO=exhaled nitric oxide, GSH=reduced glutathione, GSSG=oxidized glutathione, H2O2=hydrogen peroxide, hs-CRP=high sensitivity C-reactive protein, ICAM-1=intercellular adhesion molecule-1, IL-10=interleukin-10, IL-8=interleukin-8, LTB4=leukotriene B4, MCP-1=monocyte chemoattractant protein-1, MMP-9=matrix metallopeptidade-9, NF-kB=proinflamatory transcription nuclear factor kappa B, nNO=nasal nitric oxide, NOx=nitrate mono-notrogen oxides, NSE=neuron-specific enolase, PAI-1=plasminogen activator inhibitor-1, PDW=platelet distribution width, pLOX-1=plasma lectin-like oxidized low-density lipoprotein receptor-1, PWV=pulse wave velocity, RDW=red cell distribution width, SAA=serum amyloid, S100B=S100 calcium binding protein B,, slL-6R=soluble interleukin-6 receptor, sTNFR-1=soluble tumor necrosis factor receptor-1, TNF-α rR2= tumor necrose factor alpha receptor 2, TRX=thioredoxin, VCAM-1=vascular cell adhesion molecule-1, VEGF=vascular endothelial growth factor, YKL-40=human cartilage glycoprotein-40.
Discussion
The present scoping review investigated the available evidence regarding biomarkers for the diagnosis of OSA. The gold standard for OSA -PSG- imposes several important limitations, such as cost and reduced widespread availability. Moreover, this technique is potentially inconvenient since it requires that the patient will sleep outside the home environment. (158) Therefore we need to develop methods that would allow for the large-scale screening of at-risk populations, and enable the accurate identification of the subjects with or without the disease, could potentially revolutionize the field.(12) This pressing need to find an ideal biomarker for OSA as an alternative to the PSG may account for the large number of studies that have addressed this topic since 2000. In addition, the realization that OSA is associated with elevated levels of biochemical or inflammatory markers that may contribute to an increased risk of cardiovascular disease further propelled the field forward in the quest for diagnostic biomarkers. (128)
The ideal biomarker should have some critical characteristics, such as disease specificity, mandatory presence in all affected patients (i.e., high sensitivity and specificity), reversibility following proper treatment, and detectability before patients develop obvious clinical manifestations. Furthermore, ideal biomarkers should reflect not only the severity of the disease, but also provide indicative information over the cumulative history of the disease, as well as enable a cut-off value with minimal overlap between normal and disease. (159) In addition, an optimal diagnostic policy employing biomarkers would be expected to minimize the total cost and burden of diagnosing a patient, in which the economic value would consist of the sum of two financially-driven components, namely measurement costs and the costs associated with misdiagnosis. (160)
Before we discuss the actual findings of this scoping review, some technical and methodological considerations regarding the studies included merit specific commentary. Different groups of researchers have attempted to identify OSA biomarkers in children and adults throughout the world. Interestingly, most of the pediatric studies have been performed in United States and Greece, primarily by 2 major research groups led by Gozal and Kaditis, respectively, while most studies involving adults have been conducted in China, the United States and Japan, with no particular predominance of any specific group of investigators. Studies in adults have primarily focused on the investigation of IL-6, TNF-α, and hsCRP. On the other hand, we did not find any particular trend towards a specific biomarker among the pediatric studies. Also noteworthy was the wide variation in the OSA diagnostic criteria employed by the pediatric studies. The AHI was the most frequently used diagnostic PSG measure of OSA severity. However, the use of AHI was associated with two major limitations. Firstly, the clinically accepted consensus for the cut-off AHI value for either the presence or absence of OSA remains unresolved. Secondly, no widely accepted agreement has been reached regarding whether children with PSG-based AHI values between the “normal cutoff” and 5/hrTST should undergo surgical adenotonsillectomy. (159) Based on these considerations, it becomes apparent that the definitive diagnosis of OSA solely based on the low-end spectrum of the PSG-based measures (i.e., AHI, RDI, OAHI, etc..) is difficult if not impossible. It is also apparent that the lack of consensus on the PSG-based diagnostic criteria is the result of the relative dichotomy that exists between PSG-derived measures and clinical symptoms. For example, children who are very symptomatic may present with a “normal PSG” in the presence of habitual snoring. Conversely, asymptomatic snoring children may exhibit severe respiratory disturbance in their PSG. (160) Similar, albeit less vague overlap exists among adult patients, even if the PSG criteria for the presence of OSA have been more firmly established and accepted around the world (161–166).
Regarding the type of biomarkers explored in our assessment, the majority of studies evaluated blood biomarkers, with only few studies having evaluated either urine, saliva and/or EBC, although such approaches are noninvasive and easily collected, and particularly suitable for children. Analyzing the level of evidence, only three studies were classified as A(91, 131, 143). Mehra et al (91) evaluated biomarkers in participants of the Cleveland Family Study, a longitudinal genetic epidemiological study in United States. This study was designed to investigate the causal factors and natural history of OSA. Svensson et al (131) selected women from general population in Sweden. Hirotsu et al (143) used subjects from an epidemiologic sleep study namely EPISONO, in Brazil. Considering these three studies, (91, 131, 143) we are able to identify only two potential biomarkers: sIL-6R (91) and uric acid (143). Most studies were classified as level of evidence B, because they used samples from sleep laboratories or patients with suspected OSA rather than community-based approaches.
In the context of the properties of potential diagnostic biomarkers, the importance of reporting receiver operator curves and other measures of diagnostic performance can not be overemphasized (158). However, even though it is impossible to properly assess the real diagnostic capability of any alternative test without such measures, we found only nine studies that reported sensitivity and specificity. The sensitivity and specificity for these 9 studies (50, 53, 58, 75)(78, 82, 117, 120, 143) varied substantially from 43% to 100%, and from 45% to 100%, respectively. Only five studies reported excellent sensitivity: Li et al (78)(100%), Gozal et al (53)(95%), Shah et al (50)(93%), Guo et al (82)(91%) and Kheirandish-Gozal et al (58) (82%). From these five studies (50, 53, 58, 75, 82), only Gozal et al (53) and Li et al (78) also reported excellent specificity (both 97%). Is it important to emphasize that the results reported when the biomarkers were combined in Gozal et al (53) and Kheirandish-Gozal et al (58) showed better accuracy measurements than when the biomarkers tested in these studies were analyzed individually.
In summary, this review provides up-to-date insights of the current state of knowledge about biological markers and their potential applicability in OSA diagnosis. Over the last 14 years, a substantial number of studies have aimed to identify an ideal biomarker or set of biomarkers for OSA. Although, no simple and useful disease marker panel for OSA is currently available and routinely used in clinical practice, considerable progress has been made, thereby justifying efforts to provide a critical appraisal of this field, and further indicate future research directions that rely on the cumulative evidence presented heretofore. (159) We should emphasize that despite our comprehensive search strategy, 29 studies were found by hand-searching in the reference list, and that the absence of universally agreed upon PSG criteria for the diagnosis of OSA along with the systematic inclusion of patient referral populations may further alter any conclusions pertaining to the validity of a proposed set of promising biomarkers. Notwithstanding such concerns, the cumulative data support the concept that biological markers should provide valid tools to identify OSA in both children and adults, even if a specific set of biomarkers cannot be firmly recommended at this preliminary stage of discovery and validation.
Conclusions
The majority of pediatric studies have been performed in the USA and Greece, while adult studies were primarily conducted in China, USA and Japan. Most of studies used blood biomarkers. Studies in adults primarly explored the investigation of IL-6, TNF-α, and hsCRP as potentially promising biomarkers. There was not a specific biomarker that was tested by a majority of authors in pediatric studies, i.e., each paper evaluated different non-overlapping types of biomarkers.
Only the combination of kallikrein-1, uromodulin, urocortin-3 and orosomucoid-1 appears to provide sufficient accuracy to be considered a potential OSA diagnostic test in children. In adults, IL-6 and IL-10 appear to exhibit a favorable profile as biomarkers aiming to discriminate patients with and without OSA.
Practice points
The present scoping review shows that:
Although there are a substantial number of studies published in the literature, most of the explored approaches do not identify definitive biomarkers.
The combination of kallikrein-1, uromodulin, urocortin-3 and orosomucoid-1 appears to have sufficient accuracy to be considered an OSA diagnostic test in children.
IL-6 and IL-10 exhibit favorable potential to become a good biomarker to identify OSA and non-OSA adults.
Research agenda
In the future we need to:
Improve the reporting methodology by calculating and reporting sensitivity and specificity, using samples from community, and employing a definitive AHI cut-off value for PSG-based diagnosis of OSA.
Prepare systematic review and meta-analysis to critically evaluate the diagnostic value of biomarkers in OSA diagnosis.
Estimate cost-effectiveness of biomarkers tests.
To formulate potential future exploratory research directions and unbiased discovery approaches aiming at advancing this promising area
Supplementary Material
Table S1. Search
Table S2. Excluded articles and reasons for exclusion (n=26).
Table S3. Complementary information regarding included pediatric articles (n=35).
Table S4. Complementary information regarding adults selected studies.*
Abbreviation box
- AAP
American Academy of Pediatrics
- AHI
apnea/hypopnea index
- AI
apnea index
- CRP
C reactive protein
- EBC
exhaled breath condensate
- EPISONO
Epidemiologic sleep study
- hr/TST
hour total sleep time
- hs-CRP
high sensitivity C-reactive protein
- IL-6
interleukin-6
- OAHI
obstructive apnea-hypopnea index
- OAI
obstructive apnea index
- OSA
obstructive sleep apnea
- PSG
polysomnography
- sIL-6R
soluble interleukin-6 receptor
- TNF-α
tumor necrosis factor α
Footnotes
Conflict of interest
The authors have no conflict of interest to declare.
Supplementary data related to this article can be found on-line.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Dr. Graziela De Luca Canto, Adjunct professor, Department of Dentistry, Federal University of Santa Catarina, Florianopolis, SC, Brazil. Post Doctoral Fellow, School of Dentistry, Faculty of Medicine and Dentistry, University of Alberta, Canada
Dr. Camila Pachêco-Pereira, Master student, School of Dentistry, Faculty of Medicine and Dentistry, School of Dentistry, University of Alberta, Canada
Dr. Secil Aydinoz, Associate Professor of Pediatrics at GATA Haydarpasa Teaching Hospital, Istanbul, Turkey, and Visiting Scholar in the Section of Pediatric Sleep Medicine, Department of Pediatrics, The University of Chicago, USA
Dr. Paul W. Major, Professor and Chair, School of Dentistry, Senior Associate Dean, Dental Affairs, School of Dentistry, Faculty of Medicine and Dentistry, University of Alberta, Canada
Dr. Carlos Flores-Mir, Associate Professor, Division Head of Orthodontics, School of Dentistry, Faculty of Medicine and Dentistry, University of Alberta, Canada
Dr. David Gozal, Professor and Chair, Department of Pediatrics, Pritzker School of Medicine, Biological Sciences Division, The University of Chicago, and Physician-in-Chief, Comer Children’s Hospital, USA
References
- 1.Leger D, Bayon V, Laaban JP, Philip P. Impact of sleep apnea on economics. Sleep medicine reviews. 2012;16(5):455–62. doi: 10.1016/j.smrv.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Tarasiuk A, Reuveni H. The economic impact of obstructive sleep apnea. Current opinion in pulmonary medicine. 2013;19(6):639–44. doi: 10.1097/MCP.0b013e3283659e1e. [DOI] [PubMed] [Google Scholar]
- 3.Skaer TL, Sclar DA. Economic implications of sleep disorders. PharmacoEconomics. 2010;28(11):1015–23. doi: 10.2165/11537390-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 4.Hillman DRMA, Pezzullo L. The economic cost of sleep disorders. Sleep. 2006;29:299–305. doi: 10.1093/sleep/29.3.299. [DOI] [PubMed] [Google Scholar]
- 5.Yamagishi K, Ohira T, Nakano H, Bielinski SJ, Sakurai S, Imano H, et al. Cross-cultural comparison of the sleep-disordered breathing prevalence among Americans and Japanese. The European respiratory journal. 2010;36(2):379–84. doi: 10.1183/09031936.00118609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ralls FM, Grigg-Damberger M. Roles of gender, age, race/ethnicity, and residential socioeconomics in obstructive sleep apnea syndromes. Current opinion in pulmonary medicine. 2012;18(6):568–73. doi: 10.1097/MCP.0b013e328358be05. [DOI] [PubMed] [Google Scholar]
- 7.Mirrakhimov AEST, Erkin M, Mirrakhimov EM. Prevalence of obstructive sleep apnea in Asian adults: a systematic review of the literature. BMC pulmonary medicine. 2013;13:1–10. doi: 10.1186/1471-2466-13-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buxbaum SG, Elston RC, Tishler PV, Redline S. Genetics of the apnea hypopnea index in Caucasians and African Americans: I. Segregation analysis. Genetic epidemiology. 2002;22(3):243–53. doi: 10.1002/gepi.0170. [DOI] [PubMed] [Google Scholar]
- 9.Lumeng JC, Chervin RD. Epidemiology of pediatric obstructive sleep apnea. Proceedings of the American Thoracic Society. 2008;5(2):242–52. doi: 10.1513/pats.200708-135MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.EOB Sleep disordered breathing in children in a general population sample: prevalence and risk factors. Sleep. 2009;32(6):731–6. doi: 10.1093/sleep/32.6.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11*.Marcus CL, Brooks LJ, Draper KA, Gozal D, Halbower AC, Jones J, et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130(3):576–84. doi: 10.1542/peds.2012-1671. [DOI] [PubMed] [Google Scholar]
- 12.Gozal D. Serum, urine, and breath-related biomarkers in the diagnosis of obstructive sleep apnea in children: is it for real? Current opinion in pulmonary medicine. 2012;18(6):561–7. doi: 10.1097/MCP.0b013e328358be2d. [DOI] [PubMed] [Google Scholar]
- 13.Shih JL, Malhotra A. Could vitamins be helpful to patients with sleep apnea? Chest. 2011;139(2):237–8. doi: 10.1378/chest.10-2017. [DOI] [PubMed] [Google Scholar]
- 14.Arksey HOML. Scoping studies: towards a methodological framework. International Journal of Social Research Methodology. 2005;8(1):19–32. [Google Scholar]
- 15.Bratel T, Wennlund A, Carlstrom K. Pituitary reactivity, androgens and catecholamines in obstructive sleep apnoea. Effects of continuous positive airway pressure treatment (CPAP) Respiratory medicine. 1999;93(1):1–7. doi: 10.1016/s0954-6111(99)90068-9. [DOI] [PubMed] [Google Scholar]
- 16.Calvin AD, Somers VK, Steensma DP, Rio Perez JA, van der Walt C, Fitz-Gibbon JM, et al. Advanced heart failure and nocturnal hypoxaemia due to central sleep apnoea are associated with increased serum erythropoietin. European journal of heart failure. 2010;12(4):354–9. doi: 10.1093/eurjhf/hfq005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cholidou KG, Kostakis ID, Manali ED, Perrea D, Margeli A, Vougas K, et al. Calprotectin: a protein related to cardiovascular risk in adult patients with obstructive sleep apnea. Cytokine. 2013;61(3):917–23. doi: 10.1016/j.cyto.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 18.Culla B, Guida G, Brussino L, Tribolo A, Cicolin A, Sciascia S, et al. Increased oral nitric oxide in obstructive sleep apnoea. Respiratory medicine. 2010;104(2):316–20. doi: 10.1016/j.rmed.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 19.El-Solh AA, Mador MJ, Sikka P, Dhillon RS, Amsterdam D, Grant BJ. Adhesion molecules in patients with coronary artery disease and moderate-to-severe obstructive sleep apnea. Chest. 2002;121(5):1541–7. doi: 10.1378/chest.121.5.1541. [DOI] [PubMed] [Google Scholar]
- 20.Gozal D, Crabtree VM, Sans Capdevila O, Witcher LA, Kheirandish-Gozal L. C-reactive protein, obstructive sleep apnea, and cognitive dysfunction in school-aged children. American journal of respiratory and critical care medicine. 2007;176(2):188–93. doi: 10.1164/rccm.200610-1519OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gozal D, Serpero LD, Kheirandish-Gozal L, Capdevila OS, Khalyfa A, Tauman R. Sleep measures and morning plasma TNF-alpha levels in children with sleep-disordered breathing. Sleep. 2010;33(3):319–25. doi: 10.1093/sleep/33.3.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khalyfa A, Gharib SA, Kim J, Capdevila OS, Kheirandish-Gozal L, Bhattacharjee R, et al. Peripheral blood leukocyte gene expression patterns and metabolic parameters in habitually snoring and non-snoring children with normal polysomnographic findings. Sleep. 2011;34(2):153–60. doi: 10.1093/sleep/34.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim J, Bhattacharjee R, Khalyfa A, Kheirandish-Gozal L, Capdevila OS, Wang Y, et al. DNA methylation in inflammatory genes among children with obstructive sleep apnea. American journal of respiratory and critical care medicine. 2012;185(3):330–8. doi: 10.1164/rccm.201106-1026OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kishida K, Funahashi T, Shimomura I. Adiponectin as a routine clinical biomarker. Best practice & research Clinical endocrinology & metabolism. 2014;28(1):119–30. doi: 10.1016/j.beem.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 25.Lin QC, Xie HS, Liu XJ, Zhou JL, Zhao JM. Relationship between obstructive sleep apnea-hypopnea syndrome and high sensitivity C-reactive protein in non-obese subjects. Zhonghua yi xue za zhi. 2013;93(30):2355–8. [PubMed] [Google Scholar]
- 26.Loubaki L, Jacques E, Semlali A, Biardel S, Chakir J, Series F. Tumor necrosis factor-alpha expression in uvular tissues differs between snorers and apneic patients. Chest. 2008;134(5):911–8. doi: 10.1378/chest.08-0886. [DOI] [PubMed] [Google Scholar]
- 27.Makino S, Handa H, Suzukawa K, Fujiwara M, Nakamura M, Muraoka S, et al. Obstructive sleep apnoea syndrome, plasma adiponectin levels, and insulin resistance. Clinical endocrinology. 2006;64(1):12–9. doi: 10.1111/j.1365-2265.2005.02407.x. [DOI] [PubMed] [Google Scholar]
- 28.Osorio RS, Ayappa I, Mantua J, Gumb T, Varga A, Mooney AM, et al. The interaction between sleep-disordered breathing and apolipoprotein E genotype on cerebrospinal fluid biomarkers for Alzheimer’s disease in cognitively normal elderly individuals. Neurobiology of aging. 2014;35(6):1318–24. doi: 10.1016/j.neurobiolaging.2013.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oyama J, Yamamoto H, Maeda T, Ito A, Node K, Makino N. Continuous positive airway pressure therapy improves vascular dysfunction and decreases oxidative stress in patients with the metabolic syndrome and obstructive sleep apnea syndrome. Clinical cardiology. 2012;35(4):231–6. doi: 10.1002/clc.21010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel SR, Zhu X, Storfer-Isser A, Mehra R, Jenny NS, Tracy R, et al. Sleep duration and biomarkers of inflammation. Sleep. 2009;32(2):200–4. doi: 10.1093/sleep/32.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Przybylowski T, Bielicki P, Kumor M, Hildebrand K, Maskey-Warzechowska M, Fangrat A, et al. Exhaled nitric oxide in patients with obstructive sleep apnea syndrome. Pneumonologia i alergologia polska. 2006;74(1):21–5. [PubMed] [Google Scholar]
- 32.Roche F, Gaspoz JM, Pichot V, Picard-Kossovsky M, Maudoux D, Garcin A, et al. Association between C-reactive protein and unrecognised sleep-disordered breathing in the elderly. The European respiratory journal. 2009;33(4):797–803. doi: 10.1183/09031936.00023208. [DOI] [PubMed] [Google Scholar]
- 33.Rubinsztajn R, Kumor M, Byskiniewicz K, Chazan R. The influence of 3 weeks therapy with continuous positive airway pressure on serum leptin and homocysteine concentration in patients with obstructive sleep apnea syndrome. Pneumonologia i alergologia polska. 2006;74(1):63–7. [PubMed] [Google Scholar]
- 34.Salord N, Gasa M, Mayos M, Fortuna-Gutierrez AM, Montserrat JM, Sanchez-de-la-Torre M, et al. Impact of OSA on biological markers in morbid obesity and metabolic syndrome. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2014;10(3):263–70. doi: 10.5664/jcsm.3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Staats R, Stoll P, Zingler D, Virchow JC, Lommatzsch M. Regulation of brain-derived neurotrophic factor (BDNF) during sleep apnoea treatment. Thorax. 2005;60(8):688–92. doi: 10.1136/thx.2004.038208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uysal A, Liendo C, McCarty DE, Kim PY, Paxson C, Chesson AL, et al. Nocturnal hypoxemia biomarker predicts sleepiness in patients with severe obstructive sleep apnea. Sleep & breathing = Schlaf & Atmung. 2014;18(1):77–84. doi: 10.1007/s11325-013-0851-2. [DOI] [PubMed] [Google Scholar]
- 37.Van Hoorenbeeck K, Franckx H, Debode P, Aerts P, Wouters K, Ramet J, et al. Weight loss and sleep-disordered breathing in childhood obesity: effects on inflammation and uric acid. Obesity. 2012;20(1):172–7. doi: 10.1038/oby.2011.282. [DOI] [PubMed] [Google Scholar]
- 38.Wang YN, Yang Y, Luo YQ, Chen LL. Effects of nasal continuous positive airway pressure short-term treatment on C-reactive protein and intercellular adhesion molecule-1 in patients with obstructive sleep apnea-hypopnea syndrome. Zhong nan da xue xue bao Yi xue ban = Journal of Central South University Medical sciences. 2005;30(6):666–9. [PubMed] [Google Scholar]
- 39.Wang Y, Wang JJ, Zhao MQ, Liu SM, Li YZ. Changes of Serum Brain-Derived Neurotrophic Factor in Children with Obstructive Sleep Apnoea-Hypopnoea Syndrome following Adenotonsillectomy. Journal of International Medical Research. 2010;38(6):1942–51. doi: 10.1177/147323001003800607. [DOI] [PubMed] [Google Scholar]
- 40.Wang W, Redline S, Khoo MC. Autonomic markers of impaired glucose metabolism: effects of sleep-disordered breathing. Journal of diabetes science and technology. 2012;6(5):1159–71. doi: 10.1177/193229681200600521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deboer MD, Mendoza JP, Liu L, Ford G, Yu PL, Gaston BM. Increased systemic inflammation overnight correlates with insulin resistance among children evaluated for obstructive sleep apnea. Sleep & breathing = Schlaf & Atmung. 2012;16(2):349–54. doi: 10.1007/s11325-011-0499-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gozal D, Lipton AJ, Jones KL. Circulating vascular endothelial growth factor levels in patients with obstructive sleep apnea. Sleep. 2002;25(1):59–65. doi: 10.1093/sleep/25.1.59. [DOI] [PubMed] [Google Scholar]
- 43.Tauman R, Ivanenko A, O’Brien LM, Gozal D. Plasma C-reactive protein levels among children with sleep-disordered breathing. Pediatrics. 2004;113(6):e564–9. doi: 10.1542/peds.113.6.e564. [DOI] [PubMed] [Google Scholar]
- 44.Larkin EK, Rosen CL, Kirchner HL, Storfer-Isser A, Emancipator JL, Johnson NL, et al. Variation of C-reactive protein levels in adolescents: association with sleep-disordered breathing and sleep duration. Circulation. 2005;111(15):1978–84. doi: 10.1161/01.CIR.0000161819.76138.5E. [DOI] [PubMed] [Google Scholar]
- 45.Goldbart AD, Krishna J, Li RC, Serpero LD, Gozal D. Inflammatory mediators in exhaled breath condensate of children with obstructive sleep apnea syndrome. Chest. 2006;130(1):143–8. doi: 10.1378/chest.130.1.143. [DOI] [PubMed] [Google Scholar]
- 46.Kheirandish-Gozal L, Capdevila OS, Tauman R, Gozal D. Plasma C-reactive protein in nonobese children with obstructive sleep apnea before and after adenotonsillectomy. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2006;2(3):301–4. [PMC free article] [PubMed] [Google Scholar]
- 47.Krishna J, Shah ZA, Merchant M, Klein JB, Gozal D. Urinary protein expression patterns in children with sleep-disordered breathing: preliminary findings. Sleep medicine. 2006;7(3):221–7. doi: 10.1016/j.sleep.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 48.Montgomery-Downs HE, Krishna J, Roberts LJ, 2nd, Gozal D. Urinary F2-isoprostane metabolite levels in children with sleep-disordered breathing. Sleep & breathing = Schlaf & Atmung. 2006;10(4):211–5. doi: 10.1007/s11325-006-0079-5. [DOI] [PubMed] [Google Scholar]
- 49.O’Brien LM, Serpero LD, Tauman R, Gozal D. Plasma adhesion molecules in children with sleep-disordered breathing. Chest. 2006;129(4):947–53. doi: 10.1378/chest.129.4.947. [DOI] [PubMed] [Google Scholar]
- 50*.Shah ZA, Jortani SA, Tauman R, Valdes R, Jr, Gozal D. Serum proteomic patterns associated with sleep-disordered breathing in children. Pediatric research. 2006;59(3):466–70. doi: 10.1203/01.pdr.0000198817.35627.fc. [DOI] [PubMed] [Google Scholar]
- 51.Tauman R, Serpero LD, Capdevila OS, O’Brien LM, Goldbart AD, Kheirandish-Gozal L, et al. Adipokines in children with sleep disordered breathing. Sleep. 2007;30(4):443–9. doi: 10.1093/sleep/30.4.443. [DOI] [PubMed] [Google Scholar]
- 52.Gozal D, Serpero LD, Sans Capdevila O, Kheirandish-Gozal L. Systemic inflammation in non-obese children with obstructive sleep apnea. Sleep medicine. 2008;9(3):254–9. doi: 10.1016/j.sleep.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53*.Gozal D, Jortani S, Snow AB, Kheirandish-Gozal L, Bhattacharjee R, Kim J, et al. Two-dimensional differential in-gel electrophoresis proteomic approaches reveal urine candidate biomarkers in pediatric obstructive sleep apnea. American journal of respiratory and critical care medicine. 2009;180(12):1253–61. doi: 10.1164/rccm.200905-0765OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim J, Bhattacharjee R, Snow AB, Capdevila OS, Kheirandish-Gozal L, Gozal D. Myeloid-related protein 8/14 levels in children with obstructive sleep apnoea. The European respiratory journal. 2010;35(4):843–50. doi: 10.1183/09031936.00075409. [DOI] [PubMed] [Google Scholar]
- 55.Bhushan B, Khalyfa A, Spruyt K, Kheirandish-Gozal L, Capdevila OS, Bhattacharjee R, et al. Fatty-acid binding protein 4 gene polymorphisms and plasma levels in children with obstructive sleep apnea. Sleep medicine. 2011;12(7):666–71. doi: 10.1016/j.sleep.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Khalyfa A, Kheirandish-Gozal L, Capdevila OS, Bhattacharjee R, Gozal D. Macrophage migration inhibitory factor gene polymorphisms and plasma levels in children with obstructive sleep apnea. Pediatric pulmonology. 2012;47(10):1001–11. doi: 10.1002/ppul.22560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gozal D, Kheirandish-Gozal L, Bhattacharjee R, Molero-Ramirez H, Tan HL, Bandla HP. Circulating adropin concentrations in pediatric obstructive sleep apnea: potential relevance to endothelial function. The Journal of pediatrics. 2013;163(4):1122–6. doi: 10.1016/j.jpeds.2013.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58*.Kheirandish-Gozal L, McManus CJ, Kellermann GH, Samiei A, Gozal D. Urinary neurotransmitters are selectively altered in children with obstructive sleep apnea and predict cognitive morbidity. Chest. 2013;143(6):1576–83. doi: 10.1378/chest.12-2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim J, Gozal D, Bhattacharjee R, Kheirandish-Gozal L. TREM-1 and pentraxin-3 plasma levels and their association with obstructive sleep apnea, obesity, and endothelial function in children. Sleep. 2013;36(6):923–31. doi: 10.5665/sleep.2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kheirandish-Gozal L, Peris E, Wang Y, Tamae Kakazu M, Khalyfa A, Carreras A, et al. Lipopolysaccharide-binding protein plasma levels in children: effects of obstructive sleep apnea and obesity. The Journal of clinical endocrinology and metabolism. 2014;99(2):656–63. doi: 10.1210/jc.2013-3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kaditis AG, Alexopoulos EI, Damani E, Karadonta I, Kostadima E, Tsolakidou A, et al. Obstructive sleep-disordered breathing and fasting insulin levels in nonobese children. Pediatric pulmonology. 2005;40(6):515–23. doi: 10.1002/ppul.20306. [DOI] [PubMed] [Google Scholar]
- 62.Kaditis AG, Alexopoulos EI, Kalampouka E, Hatzi F, Karadonta I, Kyropoulos T, et al. Nocturnal change of circulating intercellular adhesion molecule 1 levels in children with snoring. Sleep & breathing = Schlaf & Atmung. 2007;11(4):267–74. doi: 10.1007/s11325-007-0117-y. [DOI] [PubMed] [Google Scholar]
- 63.Kaditis AG, Alexopoulos E, Chaidas K, Ntamagka G, Karathanasi A, Tsilioni I, et al. Urine concentrations of cysteinyl leukotrienes in children with obstructive sleep-disordered breathing. Chest. 2009;135(6):1496–501. doi: 10.1378/chest.08-2295. [DOI] [PubMed] [Google Scholar]
- 64.Kaditis AG, Alexopoulos EI, Damani E, Hatzi F, Chaidas K, Kostopoulou T, et al. Urine levels of catecholamines in Greek children with obstructive sleep-disordered breathing. Pediatric pulmonology. 2009;44(1):38–45. doi: 10.1002/ppul.20916. [DOI] [PubMed] [Google Scholar]
- 65.Malakasioti G, Alexopoulos E, Befani C, Tanou K, Varlami V, Ziogas D, et al. Oxidative stress and inflammatory markers in the exhaled breath condensate of children with OSA. Sleep & breathing = Schlaf & Atmung. 2012;16(3):703–8. doi: 10.1007/s11325-011-0560-7. [DOI] [PubMed] [Google Scholar]
- 66.Li AM, Chan MH, Chan DF, Lam HS, Wong EM, So HK, et al. Insulin and obstructive sleep apnea in obese Chinese children. Pediatric pulmonology. 2006;41(12):1175–81. doi: 10.1002/ppul.20508. [DOI] [PubMed] [Google Scholar]
- 67.Li AM, Ng C, Ng SK, Chan MM, So HK, Chan I, et al. Adipokines in children with obstructive sleep apnea and the effects of treatment. Chest. 2010;137(3):529–35. doi: 10.1378/chest.09-2153. [DOI] [PubMed] [Google Scholar]
- 68.Li AM, Chan MH, Yin J, So HK, Ng SK, Chan IH, et al. C-reactive protein in children with obstructive sleep apnea and the effects of treatment. Pediatric pulmonology. 2008;43(1):34–40. doi: 10.1002/ppul.20732. [DOI] [PubMed] [Google Scholar]
- 69.Park CS, Guilleminault C, Hwang SH, Jeong JH, Park DS, Maeng JH. Correlation of salivary cortisol level with obstructive sleep apnea syndrome in pediatric subjects. Sleep medicine. 2013;14(10):978–84. doi: 10.1016/j.sleep.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 70.Jeong JH, Guilleminault C, Park CS, Son HL, Lee HK, Hwang SH, et al. Changes in salivary cortisol levels in pediatric patients with obstructive sleep apnea syndrome after adenotonsillectomy. Sleep medicine. 2014 doi: 10.1016/j.sleep.2013.12.019. [DOI] [PubMed] [Google Scholar]
- 71.Park CS, Guilleminault C, Park HJ, Cho JH, Lee HK, Son HL, et al. Correlation of salivary alpha amylase level and adenotonsillar hypertrophy with sleep disordered breathing in pediatric subjects. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2014;10(5):559–66. doi: 10.5664/jcsm.3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Patacchioli FR, Tabarrini A, Ghiciuc CM, Dima-Cozma LC, Prete A, Bianchini C, et al. Salivary biomarkers of obstructive sleep apnea syndrome in children. Pediatric pulmonology. 2014 doi: 10.1002/ppul.22972. [DOI] [PubMed] [Google Scholar]
- 73.Villa MP, Supino MC, Fedeli S, Rabasco J, Vitelli O, Del Pozzo M, et al. Urinary concentration of 8-isoprostane as marker of severity of pediatric OSAS. Sleep & breathing = Schlaf & Atmung. 2014 doi: 10.1007/s11325-013-0934-0. [DOI] [PubMed] [Google Scholar]
- 74.de Stefanini DO, Barros EL, Stefanini R, Pradella-Hallinan ML, Pignatari SS, Fujita RR. Comparing the clinical profile of non obese children with sleep apnea and snoring. Brazilian journal of otorhinolaryngology. 2012;78(5):22–6. doi: 10.5935/1808-8694.20120004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75*.Benedek P, Lazar Z, Bikov A, Kunos L, Katona G, Horvath I. Exhaled biomarker pattern is altered in children with obstructive sleep apnoea syndrome. International journal of pediatric otorhinolaryngology. 2013;77(8):1244–7. doi: 10.1016/j.ijporl.2013.04.025. [DOI] [PubMed] [Google Scholar]
- 76.Ye J, Liu H, Li Y, Liu X, Zhu JM. Increased serum levels of C-reactive protein and matrix metalloproteinase-9 in obstructive sleep apnea syndrome. Chinese medical journal. 2007;120(17):1482–6. [PubMed] [Google Scholar]
- 77.Lam JC, Yan CS, Lai AY, Tam S, Fong DY, Lam B, et al. Determinants of daytime blood pressure in relation to obstructive sleep apnea in men. Lung. 2009;187(5):291–8. doi: 10.1007/s00408-009-9161-7. [DOI] [PubMed] [Google Scholar]
- 78*.Li Y, Chongsuvivatwong V, Geater A, Liu A. Exhaled breath condensate cytokine level as a diagnostic tool for obstructive sleep apnea syndrome. Sleep medicine. 2009;10(1):95–103. doi: 10.1016/j.sleep.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 79.Lui MM, Lam JC, Mak HK, Xu A, Ooi C, Lam DC, et al. C-reactive protein is associated with obstructive sleep apnea independent of visceral obesity. Chest. 2009;135(4):950–6. doi: 10.1378/chest.08-1798. [DOI] [PubMed] [Google Scholar]
- 80.Ye L, Ma GH, Chen L, Li M, Liu JL, Yang K, et al. Quantification of circulating cell-free DNA in the serum of patients with obstructive sleep apnea-hypopnea syndrome. Lung. 2010;188(6):469–74. doi: 10.1007/s00408-010-9253-4. [DOI] [PubMed] [Google Scholar]
- 81.Feng X, Li P, Zhou C, Jia X, Kang J. Elevated levels of serum chemerin in patients with obstructive sleep apnea syndrome. Biomarkers : biochemical indicators of exposure, response, and susceptibility to chemicals. 2012;17(3):248–53. doi: 10.3109/1354750X.2012.658864. [DOI] [PubMed] [Google Scholar]
- 82*.Guo Q, Wang Y, Li QY, Li M, Wan HY. Levels of thioredoxin are related to the severity of obstructive sleep apnea: based on oxidative stress concept. Sleep & breathing = Schlaf & Atmung. 2013;17(1):311–6. doi: 10.1007/s11325-012-0692-4. [DOI] [PubMed] [Google Scholar]
- 83.Shi YK, Chen JX, Huang Y, Li AY. Serum S100A12 levels are associated with the presence and severity of obstructive sleep apnea syndrome in male patients. Sleep & breathing = Schlaf & Atmung. 2013 doi: 10.1007/s11325-013-0876-6. [DOI] [PubMed] [Google Scholar]
- 84.Wang Q, Feng X, Zhou C, Li P, Kang J. Decreased levels of serum omentin-1 in patients with obstructive sleep apnoea syndrome. Annals of clinical biochemistry. 2013;50(Pt 3):230–5. doi: 10.1177/0004563212473275. [DOI] [PubMed] [Google Scholar]
- 85.Wang Y, Li Y, Chen P, Luo Y, Yang Y, Yang Y. Elevated fractalkine in patients with obstructive sleep apnea hypopnea syndrome. Sleep & breathing = Schlaf & Atmung. 2013;17(1):203–8. doi: 10.1007/s11325-012-0674-6. [DOI] [PubMed] [Google Scholar]
- 86.Zhang XB, Lin QC, Deng CS, Chen GP, Cai ZM, Chen H. Elevated serum cystatin C in severe OSA younger men without complications. Sleep & breathing = Schlaf & Atmung. 2013;17(1):235–41. doi: 10.1007/s11325-012-0678-2. [DOI] [PubMed] [Google Scholar]
- 87.Wang X, Xing GH. Serum YKL-40 concentrations are elevated and correlated with disease severity in patients with obstructive sleep apnea syndrome. Scandinavian journal of clinical and laboratory investigation. 2014;74(1):74–8. doi: 10.3109/00365513.2013.859726. [DOI] [PubMed] [Google Scholar]
- 88.Shamsuzzaman ASM. Elevated C-Reactive Protein in Patients With Obstructive Sleep Apnea. Circulation. 2002;105(21):2462–4. doi: 10.1161/01.cir.0000018948.95175.03. [DOI] [PubMed] [Google Scholar]
- 89.Guilleminault C, Kirisoglu C, Ohayon MM. C-reactive protein and sleep-disordered breathing. Sleep. 2004;27(8):1507–11. doi: 10.1093/sleep/27.8.1507. [DOI] [PubMed] [Google Scholar]
- 90.Htoo AK, Greenberg H, Tongia S, Chen G, Henderson T, Wilson D, et al. Activation of nuclear factor kappaB in obstructive sleep apnea: a pathway leading to systemic inflammation. Sleep & breathing = Schlaf & Atmung. 2006;10(1):43–50. doi: 10.1007/s11325-005-0046-6. [DOI] [PubMed] [Google Scholar]
- 91.Mehra R, Storfer-Isser A, Kirchner HL, Johnson N, Jenny N, Tracy RP, et al. Soluble interleukin 6 receptor: A novel marker of moderate to severe sleep-related breathing disorder. Archives of internal medicine. 2006;166(16):1725–31. doi: 10.1001/archinte.166.16.1725. [DOI] [PubMed] [Google Scholar]
- 92.Punjabi NM, Beamer BA. C-reactive protein is associated with sleep disordered breathing independent of adiposity. Sleep. 2007;30(1):29–34. doi: 10.1093/sleep/30.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Norman D, Bardwell WA, Arosemena F, Nelesen R, Mills PJ, Loredo JS, et al. Serum aminotransferase levels are associated with markers of hypoxia in patients with obstructive sleep apnea. Sleep. 2008;31(1):121–6. doi: 10.1093/sleep/31.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lederer DJ, Jelic S, Basner RC, Ishizaka A, Bhattacharya J. Circulating KL-6, a biomarker of lung injury, in obstructive sleep apnoea. The European respiratory journal. 2009;33(4):793–6. doi: 10.1183/09031936.00150708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pallayova M, Steele KE, Magnuson TH, Schweitzer MA, Hill NR, Bevans-Fonti S, et al. Sleep apnea predicts distinct alterations in glucose homeostasis and biomarkers in obese adults with normal and impaired glucose metabolism. Cardiovascular diabetology. 2010;9:83. doi: 10.1186/1475-2840-9-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Akinnusi ME, Laporta R, El-Solh AA. Lectin-like oxidized low-density lipoprotein receptor-1 modulates endothelial apoptosis in obstructive sleep apnea. Chest. 2011;140(6):1503–10. doi: 10.1378/chest.11-0302. [DOI] [PubMed] [Google Scholar]
- 97.Ladesich JB, Pottala JV, Romaker A, Harris WS. Membrane level of omega-3 docosahexaenoic acid is associated with severity of obstructive sleep apnea. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2011;7(4):391–6. doi: 10.5664/JCSM.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pallayova M, Steele KE, Magnuson TH, Schweitzer MA, Smith PL, Patil SP, et al. Sleep apnea determines soluble TNF-alpha receptor 2 response to massive weight loss. Obesity surgery. 2011;21(9):1413–23. doi: 10.1007/s11695-011-0359-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chin K, Nakamura T, Shimizu K, Mishima M, Nakamura T, Miyasaka M, et al. Effects of nasal continuous positive airway pressure on soluble cell adhesion molecules in patients with obstructive sleep apnea syndrome. The American journal of medicine. 2000;109(7):562–7. doi: 10.1016/s0002-9343(00)00580-5. [DOI] [PubMed] [Google Scholar]
- 100.Ohga E, Tomita T, Wada H, Yamamoto H, Nagase T, Ouchi Y. Effects of obstructive sleep apnea on circulating ICAM-1, IL-8, and MCP-1. Journal of applied physiology. 2003;94(1):179–84. doi: 10.1152/japplphysiol.00177.2002. [DOI] [PubMed] [Google Scholar]
- 101.Yokoe T. Elevated Levels of C-Reactive Protein and Interleukin-6 in Patients With Obstructive Sleep Apnea Syndrome Are Decreased by Nasal Continuous Positive Airway Pressure. Circulation. 2003;107(8):1129–34. doi: 10.1161/01.cir.0000052627.99976.18. [DOI] [PubMed] [Google Scholar]
- 102.Imagawa S, Yamaguchi Y, Ogawa K, Obara N, Suzuki N, Yamamoto M, et al. Interleukin-6 and tumor necrosis factor-alpha in patients with obstructive sleep apnea-hypopnea syndrome. Respiration; international review of thoracic diseases. 2004;71(1):24–9. doi: 10.1159/000075645. [DOI] [PubMed] [Google Scholar]
- 103.Sukegawa M, Noda A, Sugiura T, Nakata S, Yoshizaki S, Soga T, et al. Assessment of continuous positive airway pressure treatment in obstructive sleep apnea syndrome using 24-hour urinary catecholamines. Clinical cardiology. 2005;28(11):519–22. doi: 10.1002/clc.4960281106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yamauchi M, Nakano H, Maekawa J, Okamoto Y, Ohnishi Y, Suzuki T, et al. Oxidative stress in obstructive sleep apnea. Chest. 2005;127(5):1674–9. doi: 10.1378/chest.127.5.1674. [DOI] [PubMed] [Google Scholar]
- 105.Burioka N, Miyata M, Fukuoka Y, Endo M, Shimizu E. Day-night variations of serum interleukin-6 in patients with severe obstructive sleep apnea syndrome before and after continuous positive airway pressure (CPAP) Chronobiology international. 2008;25(5):827–34. doi: 10.1080/07420520802384101. [DOI] [PubMed] [Google Scholar]
- 106.Takahashi K, Chin K, Nakamura H, Morita S, Sumi K, Oga T, et al. Plasma thioredoxin, a novel oxidative stress marker, in patients with obstructive sleep apnea before and after nasal continuous positive airway pressure. Antioxidants & redox signaling. 2008;10(4):715–26. doi: 10.1089/ars.2007.1949. [DOI] [PubMed] [Google Scholar]
- 107.Kuramoto E, Kinami S, Ishida Y, Shiotani H, Nishimura Y. Continuous positive nasal airway pressure decreases levels of serum amyloid A and improves autonomic function in obstructive sleep apnea syndrome. International journal of cardiology. 2009;135(3):338–45. doi: 10.1016/j.ijcard.2008.03.078. [DOI] [PubMed] [Google Scholar]
- 108.Aihara K, Oga T, Chihara Y, Harada Y, Tanizawa K, Handa T, et al. Analysis of systemic and airway inflammation in obstructive sleep apnea. Sleep & breathing = Schlaf & Atmung. 2013;17(2):597–604. doi: 10.1007/s11325-012-0726-y. [DOI] [PubMed] [Google Scholar]
- 109.Murase K, Mori K, Yoshimura C, Aihara K, Chihara Y, Azuma M, et al. Association between plasma neutrophil gelatinase associated lipocalin level and obstructive sleep apnea or nocturnal intermittent hypoxia. PloS one. 2013;8(1):e54184. doi: 10.1371/journal.pone.0054184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lavie L, Kraiczi H, Hefetz A, Ghandour H, Perelman A, Hedner J, et al. Plasma vascular endothelial growth factor in sleep apnea syndrome: effects of nasal continuous positive air pressure treatment. American journal of respiratory and critical care medicine. 2002;165(12):1624–8. doi: 10.1164/rccm.20110-040OC. [DOI] [PubMed] [Google Scholar]
- 111.Ting H, Lo HS, Chang SY, Chung AH, Kuan PC, Yuan SC, et al. Post- to pre-overnight sleep systolic blood pressures are associated with sleep respiratory disturbance, pro-inflammatory state and metabolic situation in patients with sleep-disordered breathing. Sleep medicine. 2009;10(7):720–5. doi: 10.1016/j.sleep.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 112.Papaioannou I, Twigg GL, Kemp M, Roughton M, Hooper J, Morrell MJ, et al. Melatonin concentration as a marker of the circadian phase in patients with obstructive sleep apnoea. Sleep medicine. 2012;13(2):167–71. doi: 10.1016/j.sleep.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 113.Christou K, Moulas AN, Pastaka C, Gourgoulianis KI. Antioxidant capacity in obstructive sleep apnea patients. Sleep medicine. 2003;4(3):225–8. doi: 10.1016/s1389-9457(02)00253-8. [DOI] [PubMed] [Google Scholar]
- 114.Schulz R, Hummel C, Heinemann S, Seeger W, Grimminger F. Serum levels of vascular endothelial growth factor are elevated in patients with obstructive sleep apnea and severe nighttime hypoxia. American journal of respiratory and critical care medicine. 2002;165(1):67–70. doi: 10.1164/ajrccm.165.1.2101062. [DOI] [PubMed] [Google Scholar]
- 115.Alzoghaibi MA, Bahammam AS. Lipid peroxides, superoxide dismutase and circulating IL-8 and GCP-2 in patients with severe obstructive sleep apnea: a pilot study. Sleep & breathing = Schlaf & Atmung. 2005;9(3):119–26. doi: 10.1007/s11325-005-0022-1. [DOI] [PubMed] [Google Scholar]
- 116.Braga CW, Martinez D, Wofchuk S, Portela LV, Souza DO. S100B and NSE serum levels in obstructive sleep apnea syndrome. Sleep medicine. 2006;7(5):431–5. doi: 10.1016/j.sleep.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 117*.Lentini S, Manka R, Scholtyssek S, Stoffel-Wagner B, Luderitz B, Tasci S. Creatine phosphokinase elevation in obstructive sleep apnea syndrome: an unknown association? Chest. 2006;129(1):88–94. doi: 10.1378/chest.129.1.88. [DOI] [PubMed] [Google Scholar]
- 118.Peled N, Shitrit D, Bendayan D, Peled E, Kramer MR. Association of elevated levels of vascular endothelial growth factor in obstructive sleep apnea syndrome with patient age rather than with obstructive sleep apnea syndrome severity. Respiration; international review of thoracic diseases. 2007;74(1):50–5. doi: 10.1159/000095675. [DOI] [PubMed] [Google Scholar]
- 119.Ryan S, Nolan GM, Hannigan E, Cunningham S, Taylor C, McNicholas WT. Cardiovascular risk markers in obstructive sleep apnoea syndrome and correlation with obesity. Thorax. 2007;62(6):509–14. doi: 10.1136/thx.2006.066720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120*.Ursavas A, Karadag M, Rodoplu E, Yilmaztepe A, Oral HB, Gozu RO. Circulating ICAM-1 and VCAM-1 levels in patients with obstructive sleep apnea syndrome. Respiration; international review of thoracic diseases. 2007;74(5):525–32. doi: 10.1159/000097770. [DOI] [PubMed] [Google Scholar]
- 121.Constantinidis J, Ereliadis S, Angouridakis N, Konstantinidis I, Vital V, Angouridaki C. Cytokine changes after surgical treatment of obstructive sleep apnoea syndrome. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies. 2008;265(10):1275–9. doi: 10.1007/s00405-008-0627-7. [DOI] [PubMed] [Google Scholar]
- 122.Kanbay A, Kokturk O, Ciftci TU, Tavil Y, Bukan N. Comparison of serum adiponectin and tumor necrosis factor-alpha levels between patients with and without obstructive sleep apnea syndrome. Respiration; international review of thoracic diseases. 2008;76(3):324–30. doi: 10.1159/000134010. [DOI] [PubMed] [Google Scholar]
- 123.Zamarron C, Ricoy J, Riveiro A, Gude F. Plasminogen activator inhibitor-1 in obstructive sleep apnea patients with and without hypertension. Lung. 2008;186(3):151–6. doi: 10.1007/s00408-008-9076-8. [DOI] [PubMed] [Google Scholar]
- 124.Kim J, Lee S, In K, Kim J, You S, Kang K, et al. Increase in serum haptoglobin and apolipoprotein M in patients with obstructive sleep apnoea. Journal of sleep research. 2009;18(3):313–20. doi: 10.1111/j.1365-2869.2008.00725.x. [DOI] [PubMed] [Google Scholar]
- 125.Ucar ZZ, Taymaz Z, Erbaycu AE, Kirakli C, Tuksavul F, Guclu SZ. Nocturnal hypoxia and arterial lactate levels in sleep-related breathing disorders. Southern medical journal. 2009;102(7):693–700. doi: 10.1097/SMJ.0b013e3181a93897. [DOI] [PubMed] [Google Scholar]
- 126.Lee LA, Chen NH, Huang CG, Lin SW, Fang TJ, Li HY. Patients with severe obstructive sleep apnea syndrome and elevated high-sensitivity C-reactive protein need priority treatment. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2010;143(1):72–7. doi: 10.1016/j.otohns.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 127.Steiropoulos P, Papanas N, Nena E, Antoniadou M, Serasli E, Papoti S, et al. Inflammatory markers in middle-aged obese subjects: does obstructive sleep apnea syndrome play a role? Mediators of inflammation. 2010;2010:675320. doi: 10.1155/2010/675320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cintra F, Tufik S, D’Almeida V, Calegare BF, de Paola A, Oliveira W, et al. Cysteine: a potential biomarker for obstructive sleep apnea. Chest. 2011;139(2):246–52. doi: 10.1378/chest.10-0667. [DOI] [PubMed] [Google Scholar]
- 129.Hira HS, Shukla A, Kaur A, Kapoor S. Serum uric acid and lactate levels among patients with obstructive sleep apnea syndrome: which is a better marker of hypoxemia? Annals of Saudi medicine. 2012;32(1):37–42. doi: 10.5144/0256-4947.2012.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jurado-Gamez B, Fernandez-Marin MC, Gomez-Chaparro JL, Munoz-Cabrera L, Lopez-Barea J, Perez-Jimenez F, et al. Relationship of oxidative stress and endothelial dysfunction in sleep apnoea. The European respiratory journal. 2011;37(4):873–9. doi: 10.1183/09031936.00027910. [DOI] [PubMed] [Google Scholar]
- 131.Svensson M, Venge P, Janson C, Lindberg E. Relationship between sleep-disordered breathing and markers of systemic inflammation in women from the general population. Journal of sleep research. 2012;21(2):147–54. doi: 10.1111/j.1365-2869.2011.00946.x. [DOI] [PubMed] [Google Scholar]
- 132.Zamarron C, Riveiro A, Gude F. Circulating levels of vascular endothelial markers in obstructive sleep apnoea syndrome. Effects of nasal continuous positive airway pressure. Archives of medical science : AMS. 2011;7(6):1023–8. doi: 10.5114/aoms.2011.26615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Duru S, Hikmet Firat I, Colak N, Ginis Z, Delibasi T, Ardic S. Serum S100B protein: a useful marker in obstructive sleep apnea syndrome. Neurologia i neurochirurgia polska. 2012;46(5):450–5. doi: 10.5114/ninp.2012.31355. [DOI] [PubMed] [Google Scholar]
- 134.Guven SF, Turkkani MH, Ciftci B, Ciftci TU, Erdogan Y. The relationship between high-sensitivity C-reactive protein levels and the severity of obstructive sleep apnea. Sleep & breathing = Schlaf & Atmung. 2012;16(1):217–21. doi: 10.1007/s11325-011-0492-2. [DOI] [PubMed] [Google Scholar]
- 135.Jurado-Gamez B, Cabrera CB, Ballesteros LC, Hinojosa CM, Cabrera LM, Perez-Jimenez F, et al. Association of Cellular Adhesion Molecules and Oxidative Stress with Endothelial Function in Obstructive Sleep Apnea. Internal Medicine. 2012;51(4):363–8. doi: 10.2169/internalmedicine.51.6571. [DOI] [PubMed] [Google Scholar]
- 136.Lee SD, Ju G, Choi JA, Kim JW, Yoon IY. The association of oxidative stress with central obesity in obstructive sleep apnea. Sleep & breathing = Schlaf & Atmung. 2012;16(2):511–7. doi: 10.1007/s11325-011-0536-7. [DOI] [PubMed] [Google Scholar]
- 137.Mancuso M, Bonanni E, LoGerfo A, Orsucci D, Maestri M, Chico L, et al. Oxidative stress biomarkers in patients with untreated obstructive sleep apnea syndrome. Sleep medicine. 2012;13(6):632–6. doi: 10.1016/j.sleep.2011.10.030. [DOI] [PubMed] [Google Scholar]
- 138.Simiakakis M, Kapsimalis F, Chaligiannis E, Loukides S, Sitaras N, Alchanatis M. Lack of effect of sleep apnea on oxidative stress in obstructive sleep apnea syndrome (OSAS) patients. PloS one. 2012;7(6):e39172. doi: 10.1371/journal.pone.0039172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sokucu SN, Karasulu L, Dalar L, Seyhan EC, Altin S. Can red blood cell distribution width predict severity of obstructive sleep apnea syndrome? Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2012;8(5):521–5. doi: 10.5664/jcsm.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chung F, Chau E, Yang Y, Liao P, Hall R, Mokhlesi B. Serum bicarbonate level improves specificity of STOP-Bang screening for obstructive sleep apnea. Chest. 2013;143(5):1284–93. doi: 10.1378/chest.12-1132. [DOI] [PubMed] [Google Scholar]
- 141.Cofta S, Wysocka E, Dziegielewska-Gesiak S, Michalak S, Piorunek T, Batura-Gabryel H, et al. Plasma selectins in patients with obstructive sleep apnea. Advances in experimental medicine and biology. 2013;756:113–9. doi: 10.1007/978-94-007-4549-0_15. [DOI] [PubMed] [Google Scholar]
- 142.Ferrarini A, Ruperez FJ, Erazo M, Martinez MP, Villar-Alvarez F, Peces-Barba G, et al. Fingerprinting-based metabolomic approach with LC-MS to sleep apnea and hypopnea syndrome: a pilot study. Electrophoresis. 2013;34(19):2873–81. doi: 10.1002/elps.201300081. [DOI] [PubMed] [Google Scholar]
- 143*.Hirotsu C, Tufik S, Guindalini C, Mazzotti DR, Bittencourt LR, Andersen ML. Association between uric acid levels and obstructive sleep apnea syndrome in a large epidemiological sample. PloS one. 2013;8(6):e66891. doi: 10.1371/journal.pone.0066891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kurt OK, Yildiz N. The importance of laboratory parameters in patients with obstructive sleep apnea syndrome. Blood coagulation & fibrinolysis : an international journal in haemostasis and thrombosis. 2013;24(4):371–4. doi: 10.1097/MBC.0b013e32835d53d4. [DOI] [PubMed] [Google Scholar]
- 145.Ntalapascha M, Makris D, Kyparos A, Tsilioni I, Kostikas K, Gourgoulianis K, et al. Oxidative stress in patients with obstructive sleep apnea syndrome. Sleep & breathing = Schlaf & Atmung. 2013;17(2):549–55. doi: 10.1007/s11325-012-0718-y. [DOI] [PubMed] [Google Scholar]
- 146.Ozben S, Guvenc TS, Huseyinoglu N, Sanivar HS, Hanikoglu F, Cort A, et al. Low serum copeptin levels in patients with obstructive sleep apnea. Sleep & breathing = Schlaf & Atmung. 2013;17(4):1187–92. doi: 10.1007/s11325-013-0822-7. [DOI] [PubMed] [Google Scholar]
- 147.Tual-Chalot S, Gagnadoux F, Trzepizur W, Priou P, Andriantsitohaina R, Martinez MC. Circulating microparticles from obstructive sleep apnea syndrome patients induce endothelin-mediated angiogenesis. Biochimica et biophysica acta. 2014;1842(2):202–7. doi: 10.1016/j.bbadis.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 148.Vavougios G, Pastaka C, Tsilioni I, Natsios G, Seitanidis G, Florou E, et al. The DJ-1 protein as a candidate biomarker in obstructive sleep apnea syndrome. Sleep & breathing = Schlaf & Atmung. 2014 doi: 10.1007/s11325-014-0952-6. [DOI] [PubMed] [Google Scholar]
- 149.Carpagnano GE, Kharitonov SA, Resta O, Foschino-Barbaro MP, Gramiccioni E, Barnes PJ. Increased 8-isoprostane and interleukin-6 in breath condensate of obstructive sleep apnea patients. Chest. 2002;122(4):1162–7. doi: 10.1378/chest.122.4.1162. [DOI] [PubMed] [Google Scholar]
- 150.Petrosyan M, Perraki E, Simoes D, Koutsourelakis I, Vagiakis E, Roussos C, et al. Exhaled breath markers in patients with obstructive sleep apnoea. Sleep & breathing = Schlaf & Atmung. 2008;12(3):207–15. doi: 10.1007/s11325-007-0160-8. [DOI] [PubMed] [Google Scholar]
- 151.Phillips CL, Yang Q, Williams A, Roth M, Yee BJ, Hedner JA, et al. The effect of short-term withdrawal from continuous positive airway pressure therapy on sympathetic activity and markers of vascular inflammation in subjects with obstructive sleep apnoea. Journal of sleep research. 2007;16(2):217–25. doi: 10.1111/j.1365-2869.2007.00589.x. [DOI] [PubMed] [Google Scholar]
- 152.Arias MA, Garcia-Rio F, Alonso-Fernandez A, Hernanz A, Hidalgo R, Martinez-Mateo V, et al. CPAP decreases plasma levels of soluble tumour necrosis factor-alpha receptor 1 in obstructive sleep apnoea. The European respiratory journal. 2008;32(4):1009–15. doi: 10.1183/09031936.00007008. [DOI] [PubMed] [Google Scholar]
- 153.Kohler M, Stoewhas AC, Ayers L, Senn O, Bloch KE, Russi EW, et al. Effects of continuous positive airway pressure therapy withdrawal in patients with obstructive sleep apnea: a randomized controlled trial. American journal of respiratory and critical care medicine. 2011;184(10):1192–9. doi: 10.1164/rccm.201106-0964OC. [DOI] [PubMed] [Google Scholar]
- 154.Pinto P, Barbara C, Montserrat JM, Patarrao RS, Guarino MP, Carmo MM, et al. Effects of CPAP on nitrate and norepinephrine levels in severe and mild-moderate sleep apnea. BMC pulmonary medicine. 2013;13:13. doi: 10.1186/1471-2466-13-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Carpagnano GE, Kharitonov SA, Resta O, Foschino-Barbaro MP, Gramiccioni E, Barnes PJ. 8-Isoprostane, a marker of oxidative stress, is increased in exhaled breath condensate of patients with obstructive sleep apnea after night and is reduced by continuous positive airway pressure therapy. Chest. 2003;124(4):1386–92. doi: 10.1378/chest.124.4.1386. [DOI] [PubMed] [Google Scholar]
- 156.Antonopoulou S, Loukides S, Papatheodorou G, Roussos C, Alchanatis M. Airway inflammation in obstructive sleep apnea: is leptin the missing link? Respiratory medicine. 2008;102(10):1399–405. doi: 10.1016/j.rmed.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 157.Li Y, Chongsuvivatwong V, Geater A, Liu A. Are biomarker levels a good follow-up tool for evaluating obstructive sleep apnea syndrome treatments? Respiration; international review of thoracic diseases. 2008;76(3):317–23. doi: 10.1159/000119542. [DOI] [PubMed] [Google Scholar]
- 158.Brockmann PE, Schaefer C, Poets A, Poets CF, Urschitz MS. Diagnosis of obstructive sleep apnea in children: a systematic review. Sleep medicine reviews. 2013;17(5):331–40. doi: 10.1016/j.smrv.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 159.Wong TK. The search on an ideal disease marker for childhood obstructive sleep apnea syndrome. Sleep. 2011;34(2):133–4. doi: 10.1093/sleep/34.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Gozal D, Kheirandish-Gozal L. New approaches to the diagnosis of sleep-disordered breathing in children. Sleep medicine. 2010;11(7):708–13. doi: 10.1016/j.sleep.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 161.Nerfeldt P, Aoki F, Friberg D. Polygraphy vs. polysomnography: missing osas in symptomatic snorers--a reminder for clinicians. Sleep & breathing = Schlaf & Atmung. 2014;18(2):297–303. doi: 10.1007/s11325-013-0884-6. [DOI] [PubMed] [Google Scholar]
- 162.Parati G, Lombardi C, Hedner J, Bonsignore MR, Grote L, Tkacova R, et al. Recommendations for the management of patients with obstructive sleep apnoea and hypertension. The European respiratory journal. 2013;41(3):523–38. doi: 10.1183/09031936.00226711. [DOI] [PubMed] [Google Scholar]
- 163.Grigg-Damberger MM. The AASM Scoring Manual four years later. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2012;8(3):323–32. doi: 10.5664/jcsm.1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Balk EM, Moorthy D, Obadan NO, Patel K, Ip S, Chung M, et al. AHRQ Comparative Effectiveness Reviews. Rockville (MD): 2011. Diagnosis and Treatment of Obstructive Sleep Apnea in Adults. [PubMed] [Google Scholar]
- 165.Collop NA, Anderson WM, Boehlecke B, Claman D, Goldberg R, Gottlieb DJ, et al. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep Medicine. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2007;3(7):737–47. [PMC free article] [PubMed] [Google Scholar]
- 166.Health Quality O. Polysomnography in patients with obstructive sleep apnea: an evidence-based analysis. Ontario health technology assessment series. 2006;6(13):1–38. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Search
Table S2. Excluded articles and reasons for exclusion (n=26).
Table S3. Complementary information regarding included pediatric articles (n=35).
Table S4. Complementary information regarding adults selected studies.*