Abstract
Growing evidence suggests that persistent environmental chemicals such as polychlorinated biphenyls may adversely affect human fecundity. The purpose of this study was to evaluate associations between persistent environmental chemicals and semen quality among 501 male partners of couples discontinuing contraception for purposes of becoming pregnant. Men provided a blood specimen and two fresh semen samples collected approximately a month apart that underwent next day analysis for 35 semen quality endpoints. Serum samples were analyzed for 36 polychlorinated biphenyls (congeners #18, 28, 44, 49, 52, 66, 74, 87, 99, 101, 114, 118, 128, 138, 146, 149, 151, 153, 156, 157, 167, 170, 172, 177, 178, 180, 183, 187, 189, 194, 195, 196, 201, 206, 209); 1 polybrominated biphenyl (#153); 9 organochlorine pesticides; and 10 polybrominated diphenyl ethers (congeners #17, 28, 47, 66, 85, 99, 100, 153, 154, 183) using high resolution mass spectrometry. To estimate the effect of chemicals on semen quality, we regressed each semen marker on each chemical while adjusting for research site, age, body mass index, serum lipids, and cotinine levels. Males with chemical concentrations in the fourth quartile, as compared to the first quartile, showed significant associations for several individual chemicals in each chemical class and type of semen quality parameter indicating negative and positive associations with semen quality. Polybrominated diphenyl ethers in particular were associated with several measures of increased abnormal morphology. These exploratory results highlight the role of environmental influences on male fecundity, and are of particular interest given the ubiquitous exposures to these compounds.
Keywords: male fecundity, persistent organic pollutants, polybrominated diphenyl ethers, polychlorinated biphenyls, semen quality, sperm
1. INTRODUCTION
Growing evidence suggests that persistent environmental chemicals such as polychlorinated biphenyls (PCBs) may adversely affect human fecundity, though few prospective couple-based cohort studies have been conducted. Much concern has been raised regarding the reproductive health consequences of exposure to persistent organochlorine pollutants (POPs) as PCBs and dichloro-diphenyltrichloroethane (DDT) in particular have been associated with reduced sperm motility,1–3 and concentration,4 as well as reduced couple fecundity.5 Moreover, these chemicals have been shown to readily penetrate the blood-testis barrier,6 which may alter endocrine homeostasis and impact testicular function. Although these chemicals have also been quantified in seminal fluids, little information has been reported on what these chemical concentrations may mean for reproductive function. Studies relating serum chemical concentrations and semen quality have been limited, however, in that they typically only evaluate a select number of PCBs with a basic semen analysis that focuses only on sperm count, motility, and morphology, and in some cases DNA fragmentation, despite modern technology to evaluate additional functional measures that have been related to fecundity.
Therefore, the objective of this study was to explore potential associations between multiple POPs in serum including polybromated biphenyl (PBB), organochlorine pesticides (OCPs), polybrominated diphenyl ethers (PBDEs), and PCBs, and a comprehensive semen quality assessment in a population based prospective cohort study. These hypotheses are of great interest given the widespread exposure to environmental chemicals and the need for human research at environmentally relevant doses.
2. MATERIALS AND METHODS
2.1 Design and Study Population
The LIFE Study was a prospective cohort study designed to investigate environmental influences on human fecundity and fertility, and its design and methods were described previously in detail.7 In brief, 501 male partners of couples discontinuing contraception for the purposes of becoming pregnant were recruited from 16 counties in Michigan and Texas from 2005–2009 using sampling frameworks tailored for each State allowing for the identification of couples planning pregnancy in the near future. Eligible men were aged 18+ years in a committed relationship; were able to communicate in English or Spanish; and were not surgically or medically sterile. Full human subjects’ approval was granted from all participating institutions prior to obtaining informed consent from all participants.
2.2 Data Collection
Upon enrollment, in-person interviews were conducted with each male partner to ascertain health, demographic, and reproductive histories. All data and biospecimens were collected in the home, and baseline interviews were followed by a standardized anthropometric assessment for determination of body mass index (BMI) conducted by research nurses.8 The research nurse obtained non-fasting blood (~10 mL) for quantification of serum chemicals and lipids using equipment determined to be free of the contaminants under study. Samples were transported on ice to the site laboratories for processing, and remained frozen at −20°C or colder until shipment on ice to the laboratory.
2.3 Serum POP Measurements
All analyses were conducted by the Division of Laboratory Sciences, National Center for Environmental Health, Centers for Disease Control and Prevention, using established protocols for the quantification of POPs in serum. Chemicals included a) 1 PBB (PBB 153); b) 9 OCPs [hexachlorobenzene (HCB), β-hexachlorocyclohexane (β-HCH), γ-hexachlorocyclohexane (γ-HCH), oxychlordane, trans-nonachlor, mirex, p,p′-dichlorodiphenyltrichloroethane (p,p′-DDT) and its metabolites p,p′-dichlorodiphenyldichloroethylene (p,p′-DDE) and o,p′-DDT]; and c) 10 PBDEs (congeners 17, 28, 47, 66, 85, 99, 100, 153, 154, and 183); d) 36 PCBs (congeners 28, 44, 49, 52, 66, 74, 87, 99, 101, 105, 110, 114, 118, 128, 138, 146, 149, 151, 153, 156, 157, 167, 170, 172, 177, 178, 180, 183, 187, 189, 194, 195, 196, 201, 206, and 209). Serum concentrations are reported in nanograms per gram of serum (ppb) and were measured using isotope dilution gas chromatography-high-resolution mass spectrometry using previously published procedures.9,10 We did not substitute by any constant for concentrations below the limit of detection or perform lipid standardization in order to minimize bias associated with these approaches when interested in estimating health effects.11–13 Serum levels of cotinine were quantified using liquid chromatography-isotope dilution tandem mass spectrometry14 for assessment of baseline exposure to smoking with cut-points based on previous literature.15,16 Serum lipids were quantified using commercially available enzymatic methods,17 and reported as total serum lipids (nanograms per gram of serum) using established calculation methods using individual components.18
2.4 Semen Collection and Analysis
A baseline semen sample was obtained followed by a second sample approximately one month apart irrespective of couples’ pregnancy status. Men collected semen samples through masturbation without the use of any lubricant following a recommended two days of abstinence using home collection kits (actual abstinence time: median 3.0 days, mean 4.1 days).19,20 At collection, a glass capillary tube was placed into the semen, and each subject recorded the duration of abstinence, time of semen collection and any information regarding sample collection loss or spillage. Semen samples were shipped via Federal Express overnight to the study’s andrology laboratory at the National Institute for Occupational Health and Safety for analysis representing next-day analysis. Semen delivered to a central andrology laboratory by overnight mail in insulated mailing kits have been successful in maintaining specimens for other studies.19,21,22 Semen analysis after home collection has been reported to be reliable for all semen parameters with the exception of motility parameters.23,24 A percentage of sperm are alive after 24 hours and a next-day motility assessment still can be made and may provide important information on sperm function and survivability.24
We quantified 35 semen parameters including five reflecting general characteristics (volume, straw distance, sperm concentration, total sperm count, hypo-osmotic swollen), eight motility measures, 12 morphometry measures, 8 morphology measures, and two sperm chromatin stability assay measures, using established laboratory protocols inclusive of ongoing quality assurance and control procedures (American Society of Andrology, 1996). Of note is that some parameters are a compilation of other parameters. Specifically, sperm concentration is equal to total sperm count divided by volume, and sperm head area, perimeter, and elongation factor are functions of sperm head length and width. In addition, percent linearity is a function of the straight-line and curvilinear velocity, and percent straightness is a function of straight-line and average path velocity. Both of these composite measures provide overall assessment of sperm motility.
The initial sample evaluation consisted of recording the temperature, turbidity, color, liquefaction, and volume of the semen upon arrival at the laboratory. A temperature logging monitor (Maxim Integrated, San Jose, CA, USA) placed on the collection jar determined the temperatures to which the semen had been exposed since collection. Motility assessments, viability estimates, sperm concentrations, the preparation of slides, and preservation of seminal plasma were conducted at this time. Semen volume was measured to the nearest 0.1 ml. An aliquot of semen was heated to 37°C, placed in a 20 micron deep chamber, and sperm motility was assessed using the HTM-IVOS (Hamilton Thorne Biosciences, Beverly, MA) computer assisted semen analysis system (CASA). Sperm concentration was measured using the IVOS system and the IDENT™ stain.25 Sperm viability was conducted by hypo-osmotic swelling (HOS assay).26 The HOS assay determines the structural and functional integrity of the cell membrane. An aliquot of the whole semen was diluted in TNE buffer with glycerol and frozen for SCSA® analysis.
Sperm morphology was determined on a fixed, stained semen smear. Sperm morphology was classified by the two widely accepted classification systems; WHO 3rd Edition (traditional morphology) and WHO 5th Edition (strict morphology).27,28 The main difference between these classification systems is how they classify a “borderline normal” sperm: normal with the traditional scheme and abnormal with the strict scheme.29 Morphometric analyses were conducted by HTM-IVOS CASA (Hamilton Thorne Biosciences, Beverly, MA) and provided objective assessments of individual sperm head size and shape.
Progressive sperm motility was assessed by placing a flat capillary tube filled with hyaluronic acid placed into the fresh ejaculate and the progression of the vanguard sperm was measured when the specimen arrived at the laboratory the next day as a marker of motile sperm at collection (straw distance).20 SCSA® was assayed according to the methods of Evenson, as modified by Breitenstein. 100μl of whole semen were diluted into 500μl TNE buffer and kept frozen at −70°C until analysis.30–32 The SCSA® procedure was conducted on a Coulter Epics Elite Flow Cytometer using the SCSA® program (SCSA diagnostics, Brookings, SD).
The second sample was assessed for a second global fecundity assessment, and was limited to exclusively measurement of volume, concentration, and motility.
2.5 Statistical Analysis
Five men (1 %) were found to be azoospermic on both samples and were excluded from this analysis and were referred to clinical care. Descriptive analysis included the inspection of missing data and influential observations. The study cohort was assessed by select characteristics for male partners by categories of age. Differences in characteristics between age groups were assessed using ANOVA and Fisher’s exact test. The distribution of each chemical was assessed, and the 5th, 25th, 50th, 75th, and 95th percentiles reported.
Linear mixed effects models were used to estimate associations between chemical concentrations and semen quality parameters. Mixed modeling techniques were used to incorporate the inter-sample correlations with random effects for all semen quality endpoints measured in both samples (volume, concentration, next day motility, and sperm head morphology). Quartiles of each chemical were considered with the lowest quartile as the referent group. For chemicals with large numbers of very low levels, tertiles or dichotomous variables were used for comparisons as indicated in the table footnotes. Models were adjusted for age (years), BMI (kg/m2), cotinine (> 40.35 ng/ml), research site, total serum lipids (mg/dl), and fish consumption (more versus less than once per week). Models were also adjusted for abstinence time and sample age, though adjustment for these factors did not appreciably change the results and were not included in the final models for parsimony and because these factors are not confounders of the chemical-semen quality association though these factors are correlated with semen quality.
As a sensitivity analysis, semen quality parameters were also considered with Box-Cox transformation to achieve normality in the linear mixed models. Following Handelsman,33 we found the optimal transformation parameter (λ ranging 0 to 1) using the Shapiro-Wilk W statistic for each semen quality outcome, and reran the analyses to determine whether the obtained results were different from the primary analyses. Given the exploratory nature of this study, and to reduce the overall number of comparisons to preserve our type I error rate, our primary analyses focus on untransformed semen quality with transformed results used as a sensitivity analysis.
3. RESULTS
The LIFE Study cohort comprised 501 male partners of couples attempting to become pregnant, among whom 347 (69%) achieved pregnancy. A total of 468 men had measured chemical concentrations and semen quality and were included in the analysis. The average age of male partners was 31.8 (SD 4.8) years, with an average BMI of 29.9 (SD 5.6). The majority of men were college educated (92%) and self-identified as non-Hispanic white (81%). Men <25 years of age were less likely to be non-Hispanic white, a college graduate, and to ever have fathered a pregnancy prior to study entry (Table 1). Characteristics of the men who did not provide a sample were for the most part similar, though we did observe that they tended to report lower incomes and education and a larger percentage were of Hispanic or other self-identified race/ethnicity. The distribution of exposure for each chemical, and the corresponding sample size, is shown in Table 2.
Table 1.
Sociodemographic characteristics of male partners by age category, LIFE Study, 2005–2009.
| Characteristics: N (%) | Males
|
|||||
|---|---|---|---|---|---|---|
| Overall | 19–24 years |
25–29 years |
30–34 years |
35–51 years |
p-value | |
|
|
||||||
| N (%) | 468 | 15 (3.2) | 151 (32.3) | 173 (37.0) | 129 (27.6) | |
| Age, years; Mean (SD) | 31.8 (4.8) | 22.8 (1.5) | 27.4 (1.3) | 31.8 (1.4) | 37.9 (3.1) | |
| BMI, kg/m2; Mean (SD) | 29.9 (5.6) | 30.6 (2.6) | 30.1 (5.9) | 30.0 (5.7) | 29.4 (5.6) | 0.6957 |
| Abstinence time, days; Mean (SD) | 4.0 (4.5) | 2.3 (0.4) | 3.5 (2.2) | 4.3 (5.7) | 4.5 (4.8) | 0.0972 |
| Self-Identified Race/Ethnicity | 0.0113 | |||||
| Non-Hispanic White | 378 (80.8) | 8 (53.3) | 129 (85.4) | 144 (83.2) | 97 (75.2) | |
| Non-Hispanic Black | 20 (4.3) | 2 (13.3) | 4 (2.7) | 5 (2.9) | 9 (7.0) | |
| Hispanic | 38 (8.1) | 3 (20.0) | 11 (7.3) | 8 (4.6) | 16 (12.4) | |
| Other | 32 (6.8) | 2 (13.3) | 7 (4.6) | 16 (9.3) | 7 (5.4) | |
| College Graduate or Higher | 430 (91.9) | 11 (73.3) | 145 (96.0) | 161 (93.1) | 113 (87.6) | 0.0384 |
| Household Income ($) | 0.0100 | |||||
| <29,999 | 18 (3.9) | 3 (20.0) | 6 (4.0) | 4 (2.3) | 5 (3.9) | |
| 30,000 to 49,999 | 50 (10.7) | 4 (26.7) | 13 (8.6) | 17 (9.8) | 16 (12.4) | |
| 50,000 to 69,999 | 82 (17.5) | 3 (20.0) | 36 (23.8) | 24 (13.9) | 19 (14.7) | |
| ≥70,000 | 311 (66.5) | 5 (33.3) | 93 (61.6) | 125 (72.3) | 88 (68.2) | |
| Health Insurance | 429 (91.7) | 10 (66.7) | 146 (96.7) | 159 (91.9) | 114 (88.4) | 0.0003 |
| Alcohol (per month) | 0.1318 | |||||
| No | 69 (14.7) | 2 (13.3) | 26 (17.2) | 17 (9.8) | 24 (18.6) | |
| Yes | 399 (85.3) | 13 (86.7) | 125 (82.8) | 156 (90.2) | 105 (81.4) | 0.4730 |
| < Once per month | 26 (6.5) | 0 (0.0) | 6 (4.8) | 9 (5.8) | 11 (10.5) | |
| Once per month | 37 (9.3) | 3 (23.1) | 6 (4.8) | 19 (12.2) | 9 (8.6) | |
| 2–3 days per month | 75 (18.8) | 2 (15.4) | 27 (21.6) | 27 (17.3) | 19 (18.1) | |
| Once a week | 104 (26.1) | 4 (30.8) | 39 (31.2) | 36 (23.1) | 25 (23.8) | |
| 2–3 times per week | 120 (30.1) | 3 (23.1) | 35 (28.0) | 49 (31.4) | 33 (31.4) | |
| 4–6 times per week | 25 (6.3) | 0 (0.0) | 7 (5.6) | 12 (7.7) | 6 (5.7) | |
| Every day | 12 (3.0) | 1 (7.7) | 5 (4.0) | 4 (2.6) | 2 (1.9) | |
| Participated in a vigorous exercise program during the last 12 months | 199 (42.5) | 6 (40.0) | 76 (50.3) | 68 (39.3) | 49 (38.0) | 0.1313 |
| Fathered pregnancy prior to study entry | 225 (48.1) | 7 (46.7) | 44 (29.1) | 94 (54.3) | 80 (62.0) | <.0001 |
BMI, body mass index
SD, standard deviation
Table 2.
Distribution of chemical concentrations among males in the LIFE study, 2005–2009.
| Percentile
|
|||||||
|---|---|---|---|---|---|---|---|
| Chemical | N | %<LOD* | 5th | 25th | 50th | 75th | 95th |
| OCPs, ng/g | |||||||
| HCB | 459 | 0.4 | 0.032 | 0.044 | 0.055 | 0.068 | 0.111 |
| β-HCH | 461 | 54.4 | 0.000 | 0.000 | 0.012 | 0.019 | 0.047 |
| γ-HCH (lindane) | 461 | 99.6 | 0.000 | 0.000 | 0.000 | 0.000 | 0.007 |
| oxychlordane | 461 | 10.2 | 0.000 | 0.026 | 0.039 | 0.056 | 0.126 |
| trans-nonachlor | 461 | 2.0 | 0.022 | 0.041 | 0.066 | 0.103 | 0.249 |
| p,p′-DDT | 461 | 43.6 | 0.006 | 0.010 | 0.014 | 0.020 | 0.042 |
| o,p-DDT | 461 | 99.3 | 0.000 | 0.000 | 0.002 | 0.003 | 0.006 |
| p,p′-DDE | 461 | 0.0 | 0.341 | 0.552 | 0.738 | 1.072 | 2.021 |
| mirex | 461 | 55.3 | 0.002 | 0.006 | 0.011 | 0.019 | 0.081 |
| PBB & PBDEs, ng/g | |||||||
| PBB 153 | 461 | 5.9 | 0.002 | 0.006 | 0.010 | 0.018 | 0.119 |
| PBDE 17 | 450 | 79.8 | 0.000 | 0.000 | 0.000 | 0.002 | 0.008 |
| 28 | 461 | 28.9 | 0.000 | 0.002 | 0.007 | 0.015 | 0.043 |
| 47 | 461 | 1.3 | 0.025 | 0.055 | 0.118 | 0.229 | 0.761 |
| 66 | 428 | 84.8 | 0.000 | 0.000 | 0.000 | 0.002 | 0.006 |
| 85 | 461 | 56.2 | 0.000 | 0.001 | 0.002 | 0.004 | 0.016 |
| 99 | 461 | 24.5 | 0.005 | 0.011 | 0.021 | 0.044 | 0.169 |
| 100 | 461 | 1.3 | 0.005 | 0.012 | 0.024 | 0.054 | 0.244 |
| 153 | 461 | 0.0 | 0.013 | 0.027 | 0.056 | 0.154 | 0.653 |
| 154 | 452 | 58.2 | 0.000 | 0.001 | 0.002 | 0.005 | 0.017 |
| 183 | 461 | 66.6 | 0.000 | 0.001 | 0.002 | 0.003 | 0.007 |
| PCBs, ng/g | |||||||
| 28 | 461 | 72.5 | −0.002 | 0.003 | 0.006 | 0.009 | 0.016 |
| 44 | 439 | 85.0 | 0.000 | 0.000 | 0.001 | 0.002 | 0.004 |
| 49 | 439 | 98.6 | 0.000 | 0.000 | 0.000 | 0.001 | 0.002 |
| 52 | 452 | 93.1 | 0.000 | 0.000 | 0.001 | 0.002 | 0.005 |
| 66 | 461 | 49.9 | 0.000 | 0.001 | 0.003 | 0.004 | 0.011 |
| 74 | 461 | 1.3 | 0.006 | 0.010 | 0.014 | 0.020 | 0.038 |
| 87 | 461 | 91.3 | 0.000 | 0.000 | 0.000 | 0.002 | 0.003 |
| 99 | 452 | 1.8 | 0.005 | 0.008 | 0.012 | 0.017 | 0.036 |
| 101 | 461 | 61.4 | 0.000 | 0.001 | 0.002 | 0.003 | 0.007 |
| 105 | 461 | 28.6 | 0.000 | 0.002 | 0.004 | 0.005 | 0.012 |
| 110 | 452 | 88.1 | 0.000 | 0.000 | 0.001 | 0.002 | 0.004 |
| 114 | 461 | 87.4 | 0.000 | 0.000 | 0.001 | 0.002 | 0.004 |
| 118 | 461 | 1.1 | 0.007 | 0.012 | 0.017 | 0.025 | 0.054 |
| 128 | 461 | 95.9 | 0.000 | 0.000 | 0.000 | 0.000 | 0.002 |
| 138 | 461 | 0.4 | 0.015 | 0.026 | 0.037 | 0.057 | 0.135 |
| 146 | 448 | 9.8 | 0.000 | 0.005 | 0.007 | 0.010 | 0.024 |
| 149 | 450 | 96.4 | 0.000 | 0.000 | 0.000 | 0.001 | 0.002 |
| 151 | 461 | 96.1 | 0.000 | 0.000 | 0.000 | 0.000 | 0.002 |
| 153 | 461 | 0.2 | 0.022 | 0.040 | 0.057 | 0.084 | 0.187 |
| 156 | 461 | 6.9 | 0.002 | 0.005 | 0.007 | 0.012 | 0.027 |
| 157 | 461 | 70.3 | 0.000 | 0.000 | 0.002 | 0.003 | 0.007 |
| 167 | 461 | 71.2 | 0.000 | 0.000 | 0.000 | 0.003 | 0.006 |
| 170 | 461 | 1.1 | 0.006 | 0.011 | 0.017 | 0.026 | 0.057 |
| 172 | 461 | 62.5 | 0.000 | 0.000 | 0.002 | 0.004 | 0.008 |
| 177 | 461 | 39.9 | 0.000 | 0.002 | 0.003 | 0.005 | 0.011 |
| 178 | 461 | 40.6 | 0.000 | 0.002 | 0.003 | 0.005 | 0.011 |
| 180 | 461 | 0.0 | 0.016 | 0.030 | 0.045 | 0.067 | 0.145 |
| 183 | 461 | 17.1 | 0.000 | 0.003 | 0.005 | 0.008 | 0.018 |
| 187 | 449 | 3.8 | 0.004 | 0.009 | 0.014 | 0.022 | 0.049 |
| 189 | 461 | 93.7 | 0.000 | 0.000 | 0.000 | 0.001 | 0.003 |
| 194 | 460 | 9.6 | 0.000 | 0.006 | 0.010 | 0.016 | 0.038 |
| 195 | 460 | 61.1 | 0.000 | 0.000 | 0.002 | 0.004 | 0.008 |
| 196 | 461 | 3.7 | 0.003 | 0.007 | 0.010 | 0.016 | 0.034 |
| 201 | 461 | 6.7 | 0.002 | 0.006 | 0.010 | 0.016 | 0.038 |
| 206 | 461 | 10.4 | 0.002 | 0.004 | 0.005 | 0.008 | 0.019 |
| 209 | 461 | 51.8 | 0.000 | 0.002 | 0.002 | 0.004 | 0.009 |
| Creatinine, mg/dl | 421 | 0.0 | 28.020 | 70.390 | 139.970 | 201.330 | 294.850 |
| Total Lipids, mg/dl | 460 | 0.0 | 478.112 | 593.048 | 691.252 | 811.149 | 1103.751 |
| Cotinine, ng/ml | 462 | 24.5 | 0.004 | 0.015 | 0.039 | 0.896 | 395.893 |
Machine read values were used in all analyses for values below the limit of detection (we did not substitute by any constant or perform lipid standardization).
β-hexachlorocyclohexane (β-HCH), hexachlorobenzene (HCB), γ-hexachlorocyclohexane (γ-HCH), limit of detection (LOD), p,p′-dichlorodiphenyltrichloroethane (p,p′-DDT) and its metabolites p,p′-dichlorodiphenyldichloroethylene (p,p′-DDE) and o,p′-DDT, organochlorine pesticides (OCPs), polybromated biphenyl (PBB), polybrominated diphenyl ethers (PBDEs), polychlorinated biphenyls (PCBs), SE, standard error
Males with chemical concentrations in the fourth quartile, as compared to the first quartile, showed significant associations at the 0.05 level for several individual POPs and semen quality parameters (Figures 1–2, with details regarding significant associations at the 0.01 level in Table 3). Though the majority of comparisons were null, we did observe associations between each chemical class and each type of semen quality parameter, with results indicating both positive and negative associations with semen quality. Specifically, OCPs were associated with multiple semen quality parameters (Figure 1, Table 3). Of note, β-HCH, was associated with increased values of all motility parameters, increased percent sperm head with acrosome, decreased percent round, and high DNA stainability, as well as increased percent cytoplasmic droplet. In addition, p,p′-DDT, o,p′-DDT, p,p′-DDE, were all associated with increased percent motility. o,p′-DDT, in particular, was also associated with multiple motility, morphometry, and morphology parameters. PBB 153 was associated with increased sperm concentration, and select morphometry (increased elongation factor, decreased sperm head length, decreased percent round) and morphology (decreased percent amorphous, increased % strict criteria) parameters (Figure 1, Table 3). Select PBDEs were also associated with semen quality, with most associations indicating adverse effects on semen quality, specifically increased percentages of abnormal morphology (increased percent bicephalic, number of immature sperm, percent tapered). However, PBDE 153 was associated with increased sperm concentration. Multiple PCBs were associated with overall increases in semen volume, decreased percent with high DNA stainability, and select motility parameters, most commonly increased percent linearity (Figure 2, Table 3).
Figure 1.
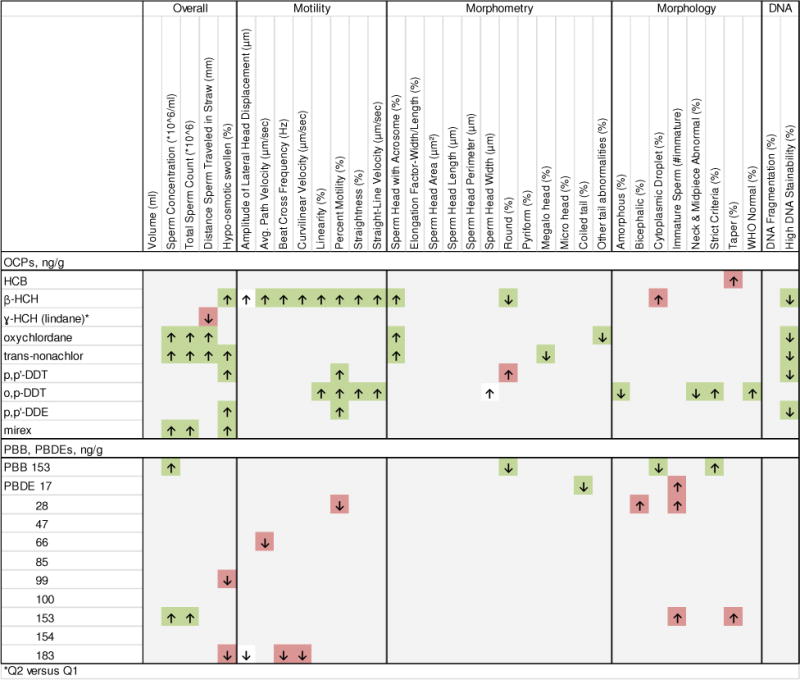
Significant associations (p<0.05) between organochlorine pesticides (OCPs), polybromated biphenyl (PBB) and polybrominated diphenyl ethers (PBDEs), and semen quality parameters, with ↓ and ↑ indicating significant negative and positive associations between the fourth quartile for each chemical concentration compared to the first quartile unless otherwise indicated. Associations shaded in red are thought to be generally associated with diminished semen quality, whereas associations shaded in green are thought to be associated with improvements in semen quality parameters.
β-hexachlorocyclohexane (β-HCH), hexachlorobenzene (HCB), γ-hexachlorocyclohexane (γ-HCH), p,p′-dichlorodiphenyltrichloroethane (p,p′-DDT) and its metabolites p,p′-dichlorodiphenyldichloroethylene (p,p′-DDE) and o,p′-DDT; organochlorine pesticides (OCPs); PBB, polybromated biphenyl; PBDEs, polybrominated diphenyl ethers
Figure 2.

Significant associations (p<0.05) between polychlorinated biphenyls (PCBs) and semen quality parameters with ↓ and ↑ indicating significant negative and positive associations between the fourth quartile for each chemical concentration compared to the first quartile unless otherwise indicated. Associations shaded in red are thought to be generally associated with diminished semen quality, whereas associations shaded in green are thought to be associated with improvements in semen quality parameters.
*Q2 versus Q1; **Q3 versus Q1; PCBs, polychlorinated biphenyls
Table 3.
Significant associations at the α=0.01 level between organochlorine pesticides (OCPs), polybromated biphenyl (PBB), polybrominated diphenyl ethers (PBDEs), polychlorinated biphenyls (PCBs) and semen quality parameters, the LIFE Study, 2005–2009.
| Chemical | Semen Quality Parameter | Beta | SE | |
|---|---|---|---|---|
| β-HCH | Motility | Amplitude of Lateral Head Displacement (μm) | 0.52 | 0.19 |
| Avg. Path Velocity (μm/sec) | 6.19 | 1.81 | ||
| Curvilinear Velocity (μm/sec) | 9.93 | 3.10 | ||
| Linearity (%) | 6.16 | 1.82 | ||
| Straightness (%) | 9.05 | 2.82 | ||
| Straight-Line Velocity (μm/sec) | 4.98 | 1.48 | ||
| Morphology | Cytoplasmic Droplet (%) | 2.22 | 0.85 | |
| Sperm Chromatin Stability | High DNA Stainability (%) | −2.20 | 0.77 | |
|
| ||||
| oxychlordane | Overall | Sperm Concentration (*10^6/ml) | 26.21 | 9.22 |
| Sperm Chromatin Stability | High DNA Stainability (%) | −2.58 | 0.86 | |
|
| ||||
| trans-nonachlor | Overall | Distance Sperm Traveled in Straw (mm) | 3.05 | 1.12 |
| Sperm Chromatin Stability | High DNA Stainability (%) | −3.17 | 0.84 | |
|
| ||||
| p,p′-DDT | Motility | Percent Motility (%) | 5.30 | 1.87 |
|
| ||||
| o,p-DDT | Morphology | Strict Criteria (%) | 4.18 | 1.33 |
| WHO Normal (%) | 5.05 | 1.67 | ||
|
| ||||
| p,p′-DDE | Motility | Percent Motility (%) | 5.57 | 1.92 |
|
| ||||
| mirex | Overall | Sperm Concentration (*10^6/ml) | 25.97 | 9.93 |
| Hypo-osmotic swollen (%) | 4.51 | 1.66 | ||
|
| ||||
| PBDE 17 | Morphometry | Coiled tail (%) | −4.05 | 1.53 |
| Morphology | Immature Sperm (#immature) | 6.47 | 2.42 | |
|
| ||||
| PBDE 28 | Morphology | Bicephalic (%) | 0.69 | 0.26 |
|
| ||||
| PCB 74 | Morphometry | Sperm Head with Acrosome (%) | 2.08 | 0.75 |
|
| ||||
| PCB 99 | Sperm Chromatin Stability | High DNA Stainability (%) | −3.48 | 0.82 |
|
| ||||
| PCB 118 | Morphometry | Sperm Head with Acrosome (%) | 2.33 | 0.75 |
| Sperm Chromatin Stability | High DNA Stainability (%) | −2.58 | 0.83 | |
|
| ||||
| PCB 128* | Morphology | Strict Criteria (%) | 5.56 | 1.89 |
| WHO Normal (%) | 6.99 | 2.36 | ||
|
| ||||
| PCB 138 | Sperm Chromatin Stability | High DNA Stainability (%) | −2.80 | 0.86 |
|
| ||||
| PCB 146 | Motility | Avg. Path Velocity (μm/sec) | 6.78 | 2.09 |
| Linearity (%) | 8.28 | 2.10 | ||
| Percent Motility (%) | 5.25 | 1.95 | ||
| Straightness (%) | 11.00 | 3.26 | ||
| Straight-Line Velocity (μm/sec) | 5.62 | 1.71 | ||
| Morphometry | Sperm Head with Acrosome (%) | 2.37 | 0.80 | |
| Sperm Chromatin Stability | High DNA Stainability (%) | −2.39 | 0.88 | |
|
| ||||
| PCB 153 | Overall | Distance Sperm Traveled in Straw (mm) | 3.65 | 1.22 |
| Sperm Chromatin Stability | High DNA Stainability (%) | −2.41 | 0.90 | |
|
| ||||
| PCB 157 | Motility | Linearity (%) | 6.41 | 1.91 |
| Straightness (%) | 9.34 | 2.96 | ||
| Straight-Line Velocity (μm/sec) | 4.09 | 1.56 | ||
|
| ||||
| PCB 172 | Motility | Avg. Path Velocity (μm/sec) | 4.97 | 1.88 |
| Linearity (%) | 5.02 | 1.89 | ||
| Straight-Line Velocity (μm/sec) | 4.69 | 1.53 | ||
|
| ||||
| PCB 177 | Motility | Linearity (%) | 5.25 | 2.01 |
|
| ||||
| PCB 178 | Motility | Linearity (%) | 6.37 | 2.07 |
|
| ||||
| PCB 183 | Motility | Linearity (%) | 5.14 | 1.97 |
| Percent Motility (%) | 5.49 | 1.86 | ||
| Straight-Line Velocity (μm/sec) | 4.61 | 1.60 | ||
| Sperm Chromatin Stability | High DNA Stainability (%) | −2.40 | 0.82 | |
|
| ||||
| PCB 189** | Overall | Hypo-osmotic swollen (%) | 3.10 | 1.19 |
| Motility | Percent Motility (%) | 4.82 | 1.48 | |
|
| ||||
| PCB 196 | Motility | Linearity (%) | 6.00 | 2.26 |
Q2 versus Q1
Q3 versus Q1
β-hexachlorocyclohexane (β-HCH), hexachlorobenzene (HCB), γ-hexachlorocyclohexane (γ-HCH), p,p′-dichlorodiphenyltrichloroethane (p,p′-DDT) and its metabolites p,p′-dichlorodiphenyldichloroethylene (p,p′-DDE) and o,p′-DDT; organochlorine pesticides (OCPs); PBB, polybromated biphenyl; PBDEs, polybrominated diphenyl ethers; PCBs, polychlorinated biphenyls; SE, standard error;
Semen outcomes are not transformed. Mixed effects model (for volume, concentration, next day motility, and sperm head morphology) and linear regression model (for the others) were used, and adjusted for age (years), BMI (kg/ m2), study site (Texas/Michigan), cotinine (> 40.35 ng/ml), total lipids (ng/g), and fish consumption (more or less than once a week). Results presented are for the fourth quartile compared to the first quartile unless otherwise indicated.
Sensitivity analysis was used to evaluate the effects under the Box-Cox family of transformations and results were similar, though more significant associations were observed with sperm concentration in the transformed analyses (data not shown).
4. DISCUSSION
Overall, in this exploratory study we observed associations between each class of POPs and semen quality parameters among men from the general population in a prospective cohort of male partners of couples enrolled prior to conception and seeking pregnancy. Associations were suggestive of both positive and negative beneficial associations with semen quality, and were observed at environmentally relevant exposure levels. These results highlight the role of environmental influences on male fecundity, and should be of concern given the ubiquitous exposures to these compounds. This is the first study to evaluate a comprehensive panel of chemical exposures with a detailed semen analysis, and additional research is needed in this area to confirm these findings.
Our results are in line with other studies that have noted associations with POPs, specifically PCBs, and semen quality parameters, particularly reduced sperm motility.1–4,34–37 Other studies have looked at PCB 153 and p,p′-DDE in particular and observed associations with reduced sperm motility,1–3 concentration,4 and total count.3 In our study we observed that PCB153 was associated with increased semen volume, increased straw distance, and decreased percent high DNA stainability, but not associated with motility, and that p,p′-DDE was associated with increased percent motility. These results may suggest improvements in semen quality, though these differences may also be a result of our next-day motility assessment which would increase the variability in measurement, or that the levels of concentrations tended to be slightly lower in the present study, although we observed associations with several other congeners and reductions in measures of motility. Previous studies have been limited in their assessment of select PCB congeners and OCPs, and general semen quality parameters. Our study expands on previous work to evaluate a broad spectrum of individual chemicals. Interestingly, we observed that several OCPs and PCBs were associated with increases in sperm concentration and volume, though were also associated with both increases and decreases in percent hypoosmotic swollen, a marker of a functional and intact plasma membrane.38 It is possible that these findings may be indicative of healthier men with better semen quality characteristics and higher fish consumption and chemical exposure, though our results were adjusted for fish consumption. It is important to note that what generally might be interpreted as improvements in individual semen quality parameters (e.g., increases in volume, concentration, or motility) are difficult to interpret overall, as these changes may still collectively indicate disturbances in normal function and may or may not have beneficial effects on fecundity.
There are fewer prior studies evaluating the role of PBDEs and semen quality, with one study in particular among 52 adult men recruited from an infertility clinic.34 These authors observed that semen mobility was associated with PBDEs 47 and 100. Although we did not observe associations with these specific congeners, our results highlight that there are many potential chemical signals that may influence male fecundity and semen quality that require further study. In particular, we observed that PBDE 17, 28, and 153, were all associated with increased immature sperm, and PBDE28 was associated with reduced percent motility. These results are suggestive of declines in semen quality and increased abnormal morphology.
Several potential mechanisms have been hypothesized to explain associations between POPs and semen quality, though exact mechanisms are unknown. These chemicals are known for their endocrine-disrupting qualities, and PCBs in particular have potential estrogenic, antiestrogenic or anti-androgenic effects depending on the congener, with DDT and its metabolites also acting as estrogen receptor agonists and androgen receptor antagonists.39–41 In addition, these PCBs have been shown to readily penetrate the blood-testis barrier, and may thus have direct effects on spermatogenesis.6 Others have hypothesized that effects may be due to increasing gonadotropin-releasing hormone, which would have downstream effects on luteinizing hormone production and release.42 A range of effects, as was observed in our study, is plausible given the varying modes of action and varying biologic activity of the chemicals of interest.
This study has several strengths, including a large number of participants recruited irrespective of exposure or pregnancy outcome and for whom serum chemicals were individually quantified. The presented results are adjusted for measured BMI and lipid concentrations given the strong associations between BMI and semen quality and that these are lipophilic chemicals.43 This exploratory study offers a comprehensive and hypothesis generating picture of potential associations between multiple POPs and a comprehensive semen quality assessment, though we cannot rule out that some of the associations may be a result of multiple comparisons. We were limited in our assessment of next-day motility. However, we were able to globally assess the presence of motile sperm at collection through the glass straw methods described above. Though the variability in measurement is increased (reducing efficiency), there is no evidence to support that the use of the next day analysis introduces bias. We recognize that the next day analysis is not suitable for clinical purposes, but is utilized here for large population-based studies in designing work responsive to studying environmental chemicals in populations not seeking clinical care for either infertility or pregnancy. In addition, no differences were observed between various semen endpoints (excluding motility) between samples collected at home the night before compared to samples analyzed within 1.5 hours.21,22 Moreover, men in the LIFE study had chemical exposures that were comparable to adult men in the National Health and Nutrition Examination Survey (NHANES) representing the US population, though geometric mean levels were lower for most chemicals measured (PBB 153: LIFE 1.74 (95% confidence interval 1.58, 1.91), NHANES 2.76 (95% confidence interval 2.21, 3.45); p,p′DDE: LIFE 111 (95% confidence interval 106, 116), NHANES 235 (95% confidence interval 193, 288)) possibly given the younger age distribution in our study cohort than NHANES.44
In conclusion, our findings demonstrate that select persistent environmental chemicals in each of the four classes evaluated were observed to be associated with markers of semen quality and male fecundity, indicating positive and negative effects on semen quality. The exact mechanisms remain elusive, but effects on sperm quality warrant additional study given widespread exposure and possible male mediated effects on couple fecundity.
Highlights.
Chemicals in each of four classes of POPs associated with semen quality
Associations indicate both positive and negative effects on semen quality
POPs at environmentally relevant levels associated with semen quality
PBDEs 17, 28 and 153 associated with higher percentage of abnormal sperm morphology
OCPs and PCBs associated with lower DNA stainability, morphometry and morphology
Acknowledgments
This work was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) (contracts #N01-HD-3-3355, N01-HD-3-3356 and N01-HD-3-3358). We acknowledge the Reproductive Health Assessment Team, Biomonitoring and Health Assessment Branch, National Institute for Occupational Health and Safety (NIOSH) for the analysis of semen samples under a Memo of Understanding with the NICHD. We also acknowledge Andreas Sjodin, John T. Bernert and Pam Olive for analysis of chemical and lipid concentrations in serum.
Abbreviations
- β-HCH
β-hexachlorocyclohexane
- DDE
1,1-dichloro-2,2-bis(p-chlorophenyl)ethylene
- DDT
1,1,1-trichloro-2,2-bis(p-chlorophenyl)ethane
- γ-HCH
γ-hexachlorocyclohexane
- HCB
hexachlorobenzene
- LIFE Study
Longitudinal Investigation of Fertility and the Environment Study
- LOD
Limit of Detection
- OCPs
organochlorine pesticides
- PBB
polybrominated biphenyl
- PBDEs
polybrominated diphenyl ethers
- PCBs
polychlorinated biphenyls
- POPs
persistent organic pollutants
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Sunni L. Mumford, Email: mumfords@mail.nih.gov.
Sungduk Kim, Email: kims2@mail.nih.gov.
Zhen Chen, Email: chenzhe@mail.nih.gov.
Robert E. Gore-Langton, Email: rlangton@emmes.com.
Dana Boyd Barr, Email: dbbarr@emory.edu.
Germaine M. Buck Louis, Email: louisg@mail.nih.gov.
References
- 1.Toft G, Rignell-Hydbom A, Tyrkiel E, et al. Semen quality and exposure to persistent organochlorine pollutants. Epidemiology. 2006;17(4):450–458. doi: 10.1097/01.ede.0000221769.41028.d2. [DOI] [PubMed] [Google Scholar]
- 2.Rignell-Hydbom A, Rylander L, Giwercman A, et al. Exposure to CB-153 and p,p′-DDE and male reproductive function. Hum Reprod. 2004;19(9):2066–2075. doi: 10.1093/humrep/deh362. [DOI] [PubMed] [Google Scholar]
- 3.Bonde JP, Toft G, Rylander L, et al. Fertility and markers of male reproductive function in Inuit and European populations spanning large contrasts in blood levels of persistent organochlorines. Environ Health Perspect. 2008;116(3):269–277. doi: 10.1289/ehp.10700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haugen TB, Tefre T, Malm G, et al. Differences in serum levels of CB-153 and p,p′-DDE, and reproductive parameters between men living south and north in Norway. Reprod Toxicol. 2011;32(3):261–267. doi: 10.1016/j.reprotox.2011.06.072. [DOI] [PubMed] [Google Scholar]
- 5.Buck Louis GM, Sundaram R, Schisterman EF, et al. Persistent environmental pollutants and couple fecundity: the LIFE study. Environ Health Perspect. 2013;121(2):231–236. doi: 10.1289/ehp.1205301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bush B, Bennett AH, Snow JT. Polychlorobiphenyl congeners, p,p′-DDE, and sperm function in humans. Arch Environ Contam Toxicol. 1986;15(4):333–341. doi: 10.1007/BF01066399. [DOI] [PubMed] [Google Scholar]
- 7.Buck Louis GM, Schisterman EF, Sweeney AM, et al. Designing prospective cohort studies for assessing reproductive and developmental toxicity during sensitive windows of human reproduction and development–the LIFE Study. Paediatr Perinat Epidemiol. 2011;25(5):413–424. doi: 10.1111/j.1365-3016.2011.01205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anthropometric Standardization Reference Manual. Champaign, IL: Human Kinetics Books; 1988. [Google Scholar]
- 9.Kuklenyik Z, Needham LL, Calafat AM. Measurement of 18 perfluorinated organic acids and amides in human serum using on-line solid-phase extraction. Anal Chem. 2005;77(18):6085–6091. doi: 10.1021/ac050671l. [DOI] [PubMed] [Google Scholar]
- 10.Sjodin A, Jones RS, Lapeza CR, et al. Semiautomated high-throughput extraction and cleanup method for the measurement of polybrominated diphenyl ethers, polybrominated biphenyls, and polychlorinated biphenyls in human serum. Anal Chem. 2004;76(7):1921–1927. doi: 10.1021/ac030381+. [DOI] [PubMed] [Google Scholar]
- 11.Richardson DB, Ciampi A. Effects of exposure measurement error when an exposure variable is constrained by a lower limit. Am J Epidemiol. 2003;157(4):355–363. doi: 10.1093/aje/kwf217. [DOI] [PubMed] [Google Scholar]
- 12.Schisterman EF, Whitcomb BW, Louis GM, Louis TA. Lipid adjustment in the analysis of environmental contaminants and human health risks. Environ Health Perspect. 2005;113(7):853–857. doi: 10.1289/ehp.7640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schisterman EF, Vexler A, Whitcomb BW, Liu A. The limitations due to exposure detection limits for regression models. Am J Epidemiol. 2006;163(4):374–383. doi: 10.1093/aje/kwj039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernert JT, Jr, Turner WE, Pirkle JL, et al. Development and validation of sensitive method for determination of serum cotinine in smokers and nonsmokers by liquid chromatography/atmospheric pressure ionization tandem mass spectrometry. Clin Chem. 1997;43(12):2281–2291. [PubMed] [Google Scholar]
- 15.Pirkle JL, Bernert JT, Caudill SP, et al. Trends in the exposure of nonsmokers in the U.S. population to secondhand smoke: 1988–2002. Environ Health Perspect. 2006;114(6):853–858. doi: 10.1289/ehp.8850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wall MA, Johnson J, Jacob P, Benowitz NL. Cotinine in the serum, saliva, and urine of nonsmokers, passive smokers, and active smokers. Am J Public Health. 1988;78(6):699–701. doi: 10.2105/ajph.78.6.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akins JR, Waldrep K, Bernert JT., Jr The estimation of total serum lipids by a completely enzymatic ‘summation’ method. Clin Chim Acta. 1989;184(3):219–226. doi: 10.1016/0009-8981(89)90054-5. [DOI] [PubMed] [Google Scholar]
- 18.Phillips DL, Pirkle JL, Burse VW, et al. Chlorinated hydrocarbon levels in human serum: effects of fasting and feeding. Arch Environ Contam Toxicol. 1989;18(4):495–500. doi: 10.1007/BF01055015. [DOI] [PubMed] [Google Scholar]
- 19.Royster MO, Lobdell DT, Mendola P, et al. Evaluation of a container for collection and shipment of semen with potential uses in population-based, clinical, and occupational settings. J Androl. 2000;21(3):478–484. [PubMed] [Google Scholar]
- 20.Turner TW, Schrader SM. Sperm migration assay as a measure of recently ejaculated sperm motility in specimens shipped overnight. J Androl. 2006 [Google Scholar]
- 21.Luben TJ, Olshan AF, Herring AH, et al. The healthy men study: an evaluation of exposure to disinfection by-products in tap water and sperm quality. Environ Health Perspect. 2007;115(8):1169–1176. doi: 10.1289/ehp.10120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olshan AF, Perreault SD, Bradley L, et al. The healthy men study: design and recruitment considerations for environmental epidemiologic studies in male reproductive health. Fertil Steril. 2007;87(3):554–564. doi: 10.1016/j.fertnstert.2006.07.1517. [DOI] [PubMed] [Google Scholar]
- 23.Morris RA, Jeffay SC, Strader LF, et al. Evaluation of sperm chromatin structure assay (SCSA) in human sperm after simulated overnight shipment. J Androl. 2003 [Google Scholar]
- 24.Stovall DW, Guzick DS, Berga SL, et al. Sperm recovery and survival: two tests that predict in vitro fertilization outcome. Fertil Steril. 1994;62(6):1244–1249. doi: 10.1016/s0015-0282(16)57193-3. [DOI] [PubMed] [Google Scholar]
- 25.Zinaman MJ, Uhler ML, Vertuno E, et al. Evaluation of computer-assisted semen analysis (CASA) with IDENT stain to determine sperm concentration. J Androl. 1996;17(3):288–292. [PubMed] [Google Scholar]
- 26.Jeyendran RS, Van der Ven HH, Perez-Pelaez M, et al. Development of an assay to assess the functional integrity of the human sperm membrane and its relationship to other semen characteristics. J Reprod Fertil. 1984;70(1):219–228. doi: 10.1530/jrf.0.0700219. [DOI] [PubMed] [Google Scholar]
- 27.World Health Organization. Laboratory manual for the examination of human semen and semen-cervical mucus interaction. 3. Cambridge: Cambridge University Press; 1992. [Google Scholar]
- 28.World Health Organization. Laboratory manual for the examination and processing of human semen. 5. Geneva: World Health Organization Press; 2010. [Google Scholar]
- 29.Rothmann SA, Bort AM, Quigley J, Pillow R. Sperm morphology classification: a rational method for schemes adopted by the world health organization. Methods Mol Biol. 2013;927:27–37. doi: 10.1007/978-1-62703-038-0_4. [DOI] [PubMed] [Google Scholar]
- 30.Breitenstein MJ, Clark JC, Schrader SM, Simon SD. The Use of a Half-Mirror for the Measurement of át in the Sperm Chromatin Structure Assay. J Androl. 1994;14:P45. [Google Scholar]
- 31.Evenson DP, Jost LK, Baer RK, et al. Individuality of DNA denaturation patterns in human sperm as measured by the sperm chromatin structure assay. Reprod Toxicol. 1991;5(2):115–125. doi: 10.1016/0890-6238(91)90039-i. [DOI] [PubMed] [Google Scholar]
- 32.Evenson DP, Larson KL, Jost LK. Sperm chromatin structure assay: its clinical use for detecting sperm DNA fragmentation in male infertility and comparisons with other techniques. J Androl. 2002;23(1):25–43. doi: 10.1002/j.1939-4640.2002.tb02599.x. [DOI] [PubMed] [Google Scholar]
- 33.Handelsman DJ. Optimal power transformations for analysis of sperm concentration and other semen variables. J Androl. 2002;23(5):629–634. [PubMed] [Google Scholar]
- 34.Abdelouahab N, Ainmelk Y, Takser L. Polybrominated diphenyl ethers and sperm quality. Reprod Toxicol. 2011;31(4):546–550. doi: 10.1016/j.reprotox.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 35.Hauser R, Chen Z, Pothier L, et al. The relationship between human semen parameters and environmental exposure to polychlorinated biphenyls and p,p′-DDE. Environ Health Perspect. 2003;111(12):1505–1511. doi: 10.1289/ehp.6175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McAuliffe ME, Williams PL, Korrick SA, et al. The association between sperm sex chromosome disomy and semen concentration, motility and morphology. Hum Reprod. 2012;27(10):2918–2926. doi: 10.1093/humrep/des302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meeker JD, Hauser R. Exposure to polychlorinated biphenyls (PCBs) and male reproduction. Syst Biol Reprod Med. 2010;56(2):122–131. doi: 10.3109/19396360903443658. [DOI] [PubMed] [Google Scholar]
- 38.Ramu S, Jeyendran RS. The hypo-osmotic swelling test for evaluation of sperm membrane integrity. Methods Mol Biol. 2013;927:21–25. doi: 10.1007/978-1-62703-038-0_3. [DOI] [PubMed] [Google Scholar]
- 39.Bonefeld-Jorgensen EC, Andersen HR, Rasmussen TH, Vinggaard AM. Effect of highly bioaccumulated polychlorinated biphenyl congeners on estrogen and androgen receptor activity. Toxicology. 2001;158(3):141–153. doi: 10.1016/s0300-483x(00)00368-1. [DOI] [PubMed] [Google Scholar]
- 40.Korach KS, Sarver P, Chae K, et al. Estrogen receptor-binding activity of polychlorinated hydroxybiphenyls: conformationally restricted structural probes. Mol Pharmacol. 1988;33(1):120–126. [PubMed] [Google Scholar]
- 41.Kelce WR, Gray LE, Wilson EM. Antiandrogens as environmental endocrine disruptors. Reprod Fertil Dev. 1998;10(1):105–111. doi: 10.1071/r98051. [DOI] [PubMed] [Google Scholar]
- 42.Jansen HT, Cooke PS, Porcelli J, et al. Estrogenic and antiestrogenic actions of PCBs in the female rat: in vitro and in vivo studies. Reprod Toxicol. 1993;7(3):237–248. doi: 10.1016/0890-6238(93)90230-5. [DOI] [PubMed] [Google Scholar]
- 43.Eisenberg ML, Kim S, Chen Z, et al. The relationship between male BMI and waist circumference on semen quality: data from the LIFE study. Hum Reprod. 2014;29(2):193–200. doi: 10.1093/humrep/det428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Centers for Disease Control and Prevention. Fourth National Report on Human Exposure to Environmental Chemicals. 2009 [Google Scholar]


