Abstract
Body growth is rapid in infancy but subsequently slows and eventually ceases due to a progressive decline in cell proliferation that occurs simultaneously in multiple organs. We previously showed that this decline in proliferation is driven in part by postnatal down-regulation of a large set of growth-promoting genes in multiple organs. We hypothesized that this growth-limiting genetic program is orchestrated by microRNAs (miRNAs). Bioinformatic analysis identified target sequences of the miR-29 family of miRNAs to be overrepresented in age–down-regulated genes. Concomitantly, expression microarray analysis in mouse kidney and lung showed that all members of the miR-29 family, miR-29a, -b, and -c, were strongly up-regulated from 1 to 6 weeks of age. Real-time PCR confirmed that miR-29a, -b, and -c were up-regulated with age in liver, kidney, lung, and heart, and their expression levels were higher in hepatocytes isolated from 5-week-old mice than in hepatocytes from embryonic mouse liver at embryonic day 16.5. We next focused on 3 predicted miR-29 target genes (Igf1, Imp1, and Mest), all of which are growth-promoting. A 3′-untranslated region containing the predicted target sequences from each gene was placed individually in a luciferase reporter construct. Transfection of miR-29 mimics suppressed luciferase gene activity for all 3 genes, and this suppression was diminished by mutating the target sequences, suggesting that these genes are indeed regulated by miR-29. Taken together, the findings suggest that up-regulation of miR-29 during juvenile life drives the down-regulation of multiple growth-promoting genes, thus contributing to physiological slowing and eventual cessation of body growth.
Body growth is rapid in early life but gradually slows with age. This decline in growth rate during juvenile life occurs across mammalian species (1), including humans. In each species, growth slows concordantly in major organs to maintain body proportions (2). This growth deceleration is driven primarily by a progressive decline in cell proliferation throughout these different organs (3). We previously showed that this decline in cell proliferation is attributable in part to a growth-limiting genetic program that comprises the postnatal down-regulation of hundreds of growth-promoting genes in multiple organs (4, 5). The molecular mechanisms that orchestrate this genetic program are not well elucidated.
In the current study, we sought to explore the potential role of microRNAs (miRNAs) in the regulation of this growth-limiting genetic program. miRNAs, which represent one class of noncoding RNAs, serve an important role in the regulation of gene expression. Indeed, miRNAs are thought to regulate most genes in the human genome (6), and each miRNA can regulate a larger number of target mRNAs (7). Because of their ability to regulate gene expression, miRNAs play important roles in many physiological, developmental, and disease processes (8). miRNAs are first transcribed as precursor miRNAs from individual genes clustered and located within intergenic regions of the genome or in introns or exons of protein-coding genes (9). These precursor miRNAs are then actively exported to the cytoplasm by the nuclear export receptor exportin 5 and processed by the RNase III endonuclease Dicer along with the double-stranded transactivation-responsive RNA-binding protein, resulting in a small double-stranded RNA structure typically with 22 nucleotides (10, 11). This miRNA duplex is unwound into the mature single-strand form and incorporated into the RNA-induced silencing complex, which guides the complex to bind a complementary sequence within the 3′-untranslated region (UTR) of the target mRNA. In animal cells, this 3′ complementary sequence is typically 6 to 8 nucleotides and is termed the seed region (12). Binding of the miRNA to its target mRNA induces negative regulation of gene expression through mRNA cleavage and/or translational repression, depending on the extent of the miRNA-mRNA pairing, with short and/or imperfect base pairing favoring translational repression (12–14).
In the current study, we sought to identify miRNAs driving the down-regulation of growth-promoting genes with age that contributes to growth deceleration. We first performed microarray analysis to identify changes in miRNA expression during early postnatal life. Because miRNAs negatively regulate gene expression, we focused our attention on miRNAs that were up-regulated with age and thus might contribute to the previously observed down-regulation of multiple mRNAs with age. We particularly focused on miRNAs that were commonly up-regulated with age in multiple organs and thus more likely to contribute to the regulation of a genetic program that is occurring simultaneously in each of these organs. After microarray analysis, quantitative real-time PCR was used for validation. miRNA binding at the 3′-UTR and suppression of gene expression were then confirmed in 3 selected target genes using a luciferase-based assay and site-directed mutagenesis of the seed region.
Materials and Methods
Animal procedures
The animal protocol was approved by the Animal Care and Use Committee, Eunice Kennedy Shiver National Institute of Child Health and Human Development, National Institutes of Health, and the Animal Ethics Committee of Northern Stockholm, Sweden. C57BL/6 male mice (Charles River Laboratories) were killed by carbon dioxide inhalation at the ages of 1, 4, 6, or 8 weeks (n = 6 per group). These time points were selected to analyze the changes that occur as somatic growth decelerates; growth in body mass is rapid at 1 week and gradually slows at 4, 6, and 8 weeks of age. Hepatocytes were isolated from mice at embryonic day (E) 16.5 or at 4 weeks of age and cultured as described previously (15).
miRNA microarray analysis
Total RNA was extracted from kidney and lung of 1- and 6-week-old mice (n = 4 per time point) using a mirVana miRNA Isolation Kit (Applied Biosystems/Ambion). All RNA samples had a 260/280 nm ratio between 1.9 and 2.1. RNA integrity was determined using an Agilent 2100 bioanalyzer (Agilent Technologies) and only high-quality RNA (28S/18S > 1.8; RNA integrity number > 8) was used. Expression of miRNAs were assessed using mouse miRNA microarrays (Agilent Technologies) that interrogate 567 mouse and 10 viral miRNAs (20 probes against each miRNA) from the Sanger database v10.1 (16, 17). Labeling and hybridization of RNA samples were performed according to the manufacturer's protocol. In brief, 100 ng of total RNA was fluorescence labeled with cyanine 3-cytidine bisphosphate and hybridized onto the arrays for 20 hours at 55°C. Slides were scanned with an Agilent microarray scanner G2565BA, and the images obtained were processed with Agilent Feature Extraction Software v10.5.1 to produce raw signal values. The raw signal values were normalized, log transformed, and median centered using Partek Genomics Suite 6.6 (Partek Inc).
Quantitative real-time PCR
Total RNA was extracted using TRIzol (Life Technologies) followed by phenol-chloroform extraction and ethanol precipitation. RNA integrity and quantity were assessed using a bioanalyzer and NanoDrop instrument as described above. To quantify mature miRNAs, we used TaqMan miRNA Reverse Transcription Kits (Life Technologies), which employ stem-loop primers to synthesize a lengthened cDNA product, derived only from the mature miRNA, suitable for quantitative real-time PCR. Total RNA (10 ng) was reverse transcribed using MultiScribe RT enzyme (Life Technologies) in the presence of primers specific for each miRNA. Quantitative real-time PCR was then performed using commercially available TaqMan assays for mmu-miR-29a, mmu-miR-29b, mmu-miR-29c, and snoRNA234. snoRNA234 was used for normalization (18, 19). For studies of Igf1, Mest, and Imp1 expression, reverse transcription was performed using SuperScript III (Life Technologies) and real-time PCR was performed using commercially available TaqMan assays, normalized to 18S ribosomal RNA. Reactions were performed in triplicate on cDNA derived from each animal using the ABI Prism 7900 Sequence Detection System instrument (Life Technologies). The relative quantity of each miRNA was calculated using the formula: relative expression = 2−Ct × 106, where Ct represents the threshold cycle and ΔCt = (Ct of miRNA or gene of interest) − (Ct of snoRNA234 or 18S). Values were multiplied by 106 for convenience of comparison.
Construction and mutagenesis of luciferase construct
The 3′-UTR regions of Igf1, Mest, and Imp1 containing predicted miR-29 target sequences were amplified by PCR using the following primers: Igf1, seed region 1 forward 5′-GGCCTCGAGCTCGAGAGGAAGTGCAGGAA-3′ and reverse 5′-CTAGCGGCCGCGCGGCC GCATTAAAAAGAAA-3′; Igf1, seed region 2 forward 5′-GGCCTCGAGTTGTAGACTTTGCTATGGAGGTAAATTG-3′ and reverse 5′-CTAGCGGCCGCCCATTTGCCTGAAGTTATTTTTGC-3′; Mest forward 5′-GGCCTCGAGGCTGGAAAGAGTAGCCTCCCT-3′ and reverse 5′-CTAGCGGCCGCTAATCAATAATGCTGCTTTAACTAAGTTAAAA-3′; and Imp1 forward 5′-GGCCTCGAGGCCGGCCATCTTTCTACATC-3′ and reverse 5′-GTAGCGGCCGCGATTATGAAAAACACACGCTCCAG. Amplicons were restriction digested with XhoI and NotI (New England Biolabs) and cloned into psiCHECK-2 plasmid (Promega). Site-directed mutagenesis was performed on the psiCHECK-2 plasmids containing 3′-UTRs to introduce 3-nucleotide deletions in the sequence complementary to the miR29 seed region, using the GeneArt Site-Directed Mutagenesis System (Life Technologies), following the manufacturer's instructions and with the following primers: Igf1 target region 1, 5′-TGTTGTTTTTTAGTACAATGCTATTTTGTAGTTTGTTATA-3′ and 5′-TATAACAAACTACAAAATAGCATTGTACTAAAAAACAACA-3′; Igf1 target region 2, 5′-TCCTGGGGGATTGGGGTGTGCTTGCCCAGGGCTAGGGAGC-3-′ and 5′-GCTCCCTAGCCCTGGGCAAGCACACCCCAATCCCCCAGGA-3′; Mest, 5′-GAAAGACCTAAGAGCAAATGCTGAATACTTTTTTAAGCC-3′ and 5′-GGCTTAAAAAAGTATTCAGCATTTGCTCTTAGGTCTTTC-3′; and Imp1, 5′-TCCTGGGGGATTGGGGTGTGCTTGCCCAGGGCTAGGGAGC-3′ and 5′-GCTCCCTAGCCCTGGGCAAGCACACCCCAATCCCCCAGGA-3′.
Luciferase assay in HEK293 cells
HEK293 cells were maintained in DMEM supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin. The day before transfection, HEK293 cells were seeded on 96-well plates at 50% confluence. Cells were transfected using Lipofectamine 2000 (Life Technologies) according to the manufacturer's protocol. For example, in a final volume of 100 μL, cells were transfected with 1 μL of Lipofectamine 2000, 25 ng of psiCHECK-2, and 10 nM miR-29a mimic (Dharmacon). Cells were lysed 48 hours after transfection, and luciferase expression was assessed using the Dual-Luciferase Reporter Assay System (Promega) and a spectrophotometer. The psiCHECK-2 plasmid contains Renilla luciferase reporter gene upstream of the 3′-UTR to be examined, as well as a Firefly reporter gene used for normalization of transfection efficiency. Interference of luciferase expression by the binding of the miRNA mimic on 3′-UTR is therefore represented by a reduction in the ratio of Renilla to Firefly luciferase activity.
Bioinformatics and statistical analysis
miRNA targets were predicted in Partek using the Microcosm algorithm and the TargetScan algorithm. To obtain the short list of miR-29 target “growth” genes in Table 3, we first filtered the full list of miR-29 targets from TargetScan using our previous microarray data (5) to only include genes down-regulated from 1 to 4 weeks of age in mouse heart, kidney, and lung (false discovery rate [FDR] < 0.05). A second filter was then used to include only genes identified in a search for “growth/size” in the Mouse Genome Informatics (MGI) website “Genes and Markers Query Form,” search field “Mouse phenotypes & mouse models of human disease.” ANOVA, χ2 test, and Mann-Whitney rank sum test were performed using SigmaPlot 10. P values were corrected for multiple comparisons, whenever applicable, using the Holm-Šidák method.
Table 3.
List of 38 miR-29 Target “Growth” Genes (Predicted by TargetScan) That Were Down-Regulated From 1 to 4 Weeks in Mouse Kidney, Lung, and Heart, and Have a Growth/Size Phenotype in Knockout Mouse Model (MGI)
| Target Gene | Gene Name | Conserved Sites |
Aggregate PCTa | |||
|---|---|---|---|---|---|---|
| Total | 8mer | 7mer-m8b | 7mer-1Ac | |||
| ADAMTS2 | ADAM metallopeptidase with thrombospondin type 1 motif, 2 | 3 | 1 | 2 | 0 | 0.98 |
| AGTR2 | Angiotensin II receptor, type 2 | 1 | 0 | 1 | 0 | 0.82 |
| BLMH | Bleomycin hydrolase | 1 | 0 | 1 | 0 | 0.82 |
| BMP1 | Bone morphogenetic protein 1 | 1 | 0 | 1 | 0 | 0.72 |
| COL11A1 | Collagen, type XI, α 1 | 2 | 0 | 2 | 0 | 0.93 |
| COL1A1 | Collagen, type I, α 1 | 3 | 1 | 0 | 2 | >0.99 |
| COL1A2 | Collagen, type I, α 2 | 2 | 1 | 0 | 1 | 0.96 |
| COL2A1 | Collagen, type II, α 1 | 1 | 1 | 0 | 0 | 0.9 |
| COL3A1 | Collagen, type III, α 1 | 2 | 2 | 0 | 0 | 0.98 |
| COL4A1 | Collagen, type IV, α 1 | 2 | 1 | 0 | 1 | 0.99 |
| COL5A1 | Collagen, type V, α 1 | 4 | 2 | 2 | 0 | > 0.99 |
| COL5A2 | Collagen, type V, α 2 | 2 | 1 | 0 | 1 | > 0.99 |
| COL6A3 | Collagen, type VI, α 3 | 1 | 1 | 0 | 0 | 0.92 |
| CTNNBIP1 | Catenin, β interacting protein 1 | 1 | 0 | 1 | 0 | 0.83 |
| DNMT3A | DNA (cytosine-5-)-methyltransferase 3 α | 1 | 0 | 1 | 0 | 0.8 |
| DNMT3B | DNA (cytosine-5-)-methyltransferase 3 β | 1 | 1 | 0 | 0 | 0.94 |
| FBN1 | Fibrillin 1 | 2 | 1 | 1 | 0 | > 0.99 |
| FBN2 | Fibrillin 2 | 1 | 0 | 1 | 0 | 0.51 |
| FRAS1 | Fraser syndrome 1 | 1 | 0 | 1 | 0 | 0.83 |
| FREM1 | FRAS1 related extracellular matrix 1 | 1 | 0 | 1 | 0 | 0.77 |
| IGF1 | Insulin-like growth factor 1 (somatomedin C) | 2 | 2 | 0 | 0 | > 0.99 |
| IMP1 | Insulin-like growth factor 2 mRNA binding protein 1 | 1 | 0 | 1 | 0 | 0.73 |
| KIRREL | Kin of IRRE like (Drosophila) | 1 | 0 | 1 | 0 | 0.83 |
| LAMC1 | Laminin, γ1 (formerly LAMB2) | 1 | 1 | 0 | 0 | 0.83 |
| LASP1 | LIM and SH3 protein 1 | 2 | 0 | 1 | 1 | 0.99 |
| MAP4K4 | Mitogen-activated protein kinase kinase kinase kinase 4 | 2 | 0 | 1 | 1 | > 0.99 |
| MEST | Mesoderm specific transcript homolog (mouse) | 1 | 0 | 1 | 0 | 0.83 |
| MMP2 | Matrix metallopeptidase2 (gelatinase A, 72 kDa gelatinase, 72 kDa type IV collagenase) | 1 | 0 | 1 | 0 | 0.83 |
| MYCN | V-myc myelocytomatosis viral related oncogene, neuroblastoma derived (avian) | 1 | 0 | 1 | 0 | 0.72 |
| NPAS3 | Neuronal PAS domain protein 3 | 1 | 1 | 0 | 0 | 0.85 |
| PDGFRB | Platelet-derived growth factor receptor, β polypeptide | 1 | 0 | 1 | 0 | 0.81 |
| REST | RE1-silencing transcription factor | 1 | 0 | 1 | 0 | 0.63 |
| SERPINH1 | Serpin peptidase inhibitor, cladeH (heat shock protein 47), member 1, (collagen binding protein 1) | 1 | 0 | 0 | 1 | 0.94 |
| TET1 | Tet oncogene 1 | 4 | 0 | 3 | 1 | > 0.99 |
| TGFB2 | Transforming growth factor, β 2 | 1 | 0 | 1 | 0 | 0.83 |
| TPM1 | Tropomyosin1 (α) | 1 | 1 | 0 | 0 | 0.29 |
| TRAM2 | Translocation associated membrane protein 2 | 1 | 0 | 1 | 0 | 0.67 |
| TUBB2B | Tubulin, β 2B | 1 | 0 | 1 | 0 | 0.83 |
| ZDHHC15 | Zinc finger, DHHC-type containing 15 | 1 | 0 | 0 | 1 | 0.73 |
PCT (preferentially conserved targeting) = (S/B − 1)/(S/B), where S/B = signal-to-background ratio for each site at each site's branch length. Aggregate PCT = 1 − [(1 − PCT) site 1 × (1 − PCT) site 2 × (1 − PCT) site 3… ] when multiple sites are present.
7mer-m8: an exact match to positions 2 to 8 of the mature miRNA (the seed + position 8).
7mer-1A: an exact match to positions 2 to 7 of the mature miRNA (the seed) followed by an A.
Results
Changes in miRNA expression during juvenile growth
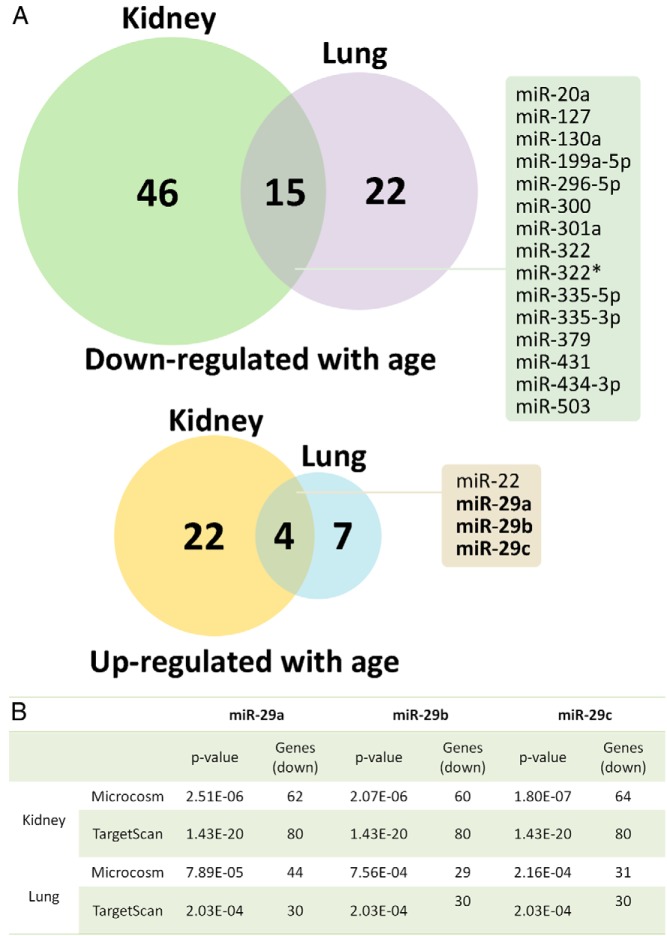
Microarray analysis was used to identify changes in miRNA expression from 1 to 6 weeks of age in mouse kidney and lung. Of the 567 miRNAs included in the microarray, 87 miRNAs changed significantly (defined by ≥2-fold, FDR < 0.05, ANOVA) in the kidney and 48 miRNAs changed significantly in the lung. A larger number of miRNAs were down-regulated than were up-regulated with age (Figure 1A). Of these, 15 miRNAs were commonly down-regulated and 4 miRNAs were commonly up-regulated with age in both kidney and lung (Figure 1A and Table 1). The overlap between the 2 organs was significantly greater than would be expected by chance (P < .001 for both up- and down-regulation, Pearson χ2 test), suggesting a tendency of miRNA to be commonly regulated with age in the 2 organs.
Figure 1.
A, Venn diagram showing the number of miRNAs commonly down- or up-regulated significantly (defined by ≥2-fold, FDR < 0.05, ANOVA) from 1 to 6 weeks of age in mouse kidney and lung. miRNA expression was measured by microarray. Names of commonly down- or up-regulated miRNAs are shown in boxes. B, Predicted binding sites for miR-29a, -b, and -c were overrepresented among genes that were up- or down-regulated with age in both mouse kidney and lung. Gene expression was based on our prior expression microarray data in 1-, 4-, and 8-week-old mice. Both Microcosm and TargetScan algorithms were used, and only results for the miR-29 family are shown (overall data are presented in Table 2).
Table 1.
List of miRNAs Commonly Up- or Down-Regulated From 1- to 6-Week-Old Mouse Kidney and Lung by Microarray Analysis
| Probe Set Identification No. | Gene Name | Kidney 1- to 6-Wk |
Lung 1- to 6-Wk |
||
|---|---|---|---|---|---|
| Fold Change | P Value | Fold Change | P Value | ||
| A_54_P1309 | mmu-miR-22 | 2.15 | 4.49E–04 | 2.22 | 7.49E–06 |
| A_54_P2489 | mmu-miR-29a | 24.67 | 1.03E–08 | 9.00 | 8.26E–08 |
| A_54_P1012 | mmu-miR-29b | 29.47 | 2.53E–08 | 8.49 | 2.67E–07 |
| A_54_P2493 | mmu-miR-29c | 12.93 | 9.05E–08 | 9.98 | 1.88E–08 |
| A_54_P1306 | mmu-miR-20a | −4.81 | 2.14E–04 | −2.05 | 5.19E–04 |
| A_54_P2287 | mmu-miR-127 | −2.58 | 8.86E–04 | −5.03 | 7.54E–05 |
| A_54_P1044 | mmu-miR-130a | −3.84 | 3.57E–05 | −2.60 | 3.42E–06 |
| A_54_P2377 | mmu-miR-199a-5p | −7.22 | 4.51E–05 | −2.97 | 3.73E–05 |
| A_54_P4376 | mmu-miR-296–5p | −5.72 | 4.07E–08 | −2.49 | 1.01E–03 |
| A_54_P4378 | mmu-miR-300 | −2.26 | 1.40E–03 | −4.21 | 9.89E–07 |
| A_54_P2440 | mmu-miR-301a | −4.93 | 1.66E–04 | −2.87 | 3.26E–03 |
| A_54_P2503 | mmu-miR-322 | −6.62 | 4.90E–05 | −2.81 | 2.42E–07 |
| A_54_P3487 | mmu-miR-322* | −4.05 | 5.86E–07 | −2.41 | 2.73E–03 |
| A_54_P2692 | mmu-miR-335–3p | −3.33 | 5.90E–04 | −2.25 | 1.07E–03 |
| A_54_P2687 | mmu-miR-335–5p | −8.06 | 5.38E–08 | −4.69 | 9.19E–05 |
| A_54_P1528 | mmu-miR-379 | −3.96 | 3.56E–04 | −6.55 | 9.53E–06 |
| A_54_P2730 | mmu-miR-431 | −2.17 | 9.14E–04 | −2.65 | 1.46E–04 |
| A_54_P2741 | mmu-miR-434–3p | −3.05 | 6.03E–04 | −4.24 | 3.36E–03 |
| A_54_P2843 | mmu-miR-503 | −5.43 | 4.00E–08 | −2.65 | 1.23E–03 |
All 3 members of the miR-29 family are up-regulated with age in multiple organs
Based on our initial hypothesis that specific miRNAs contribute to the down-regulation of growth-promoting genes with age, we focused our attention on miRNAs that were commonly up-regulated with age, even though miRNAs that were commonly down-regulated with age could also be potentially important and may warrant further studies in the future. Interestingly, 3 of the 4 miRNAs up-regulated with age in both organs were miR-29a, -b, and -c, the 3 members of the miR-29 miRNA family (Figure 1A and Table 1). Interestingly, the magnitude of up-regulation for miR-29 was substantially greater than that of any of the other up- or down-regulated miRNAs; miR-29a, -b, and -c were all up-regulated by 10- to 30-fold from 1- to 6-week-old kidney and lung.
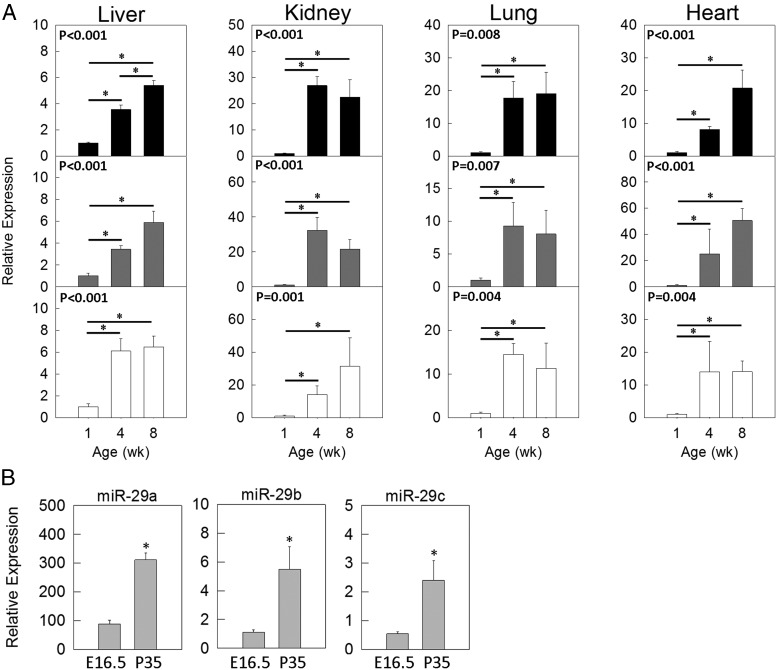
Real-time PCR was first used to validate our findings in the microarray (Figure 2A) using 1-, 4- and 8-week-old kidney and lung. To test whether the increase in miR-29 expression with age is a more general phenomenon also occurring in other tissues, we performed real-time PCR in liver and heart of the same 3 age groups and found similar increases in miR-29 expression with age in these organs (Figure 2A). We also compared expression of miR-29 in hepatocytes isolated from 5-week-old liver with that in hepatocytes isolated from embryonic liver at E16.5, both after 3 days in primary culture (Figure 2B). Expression levels of miR-29b and -c in 5-week hepatocytes were similar to those in 4-week-old liver, and miR-29a showed even higher expression levels in the hepatocytes (Figure 2B), suggesting that the miR-29 expression, at least in the liver, is attributed to the parenchymal cells in the organ. Interestingly, hepatocytes from 5-week-old animals showed higher expression of miR-29 than fetal hepatocytes from E16.5 animals (P < .01 for miR-29a, -b, and -c, ANOVA) (Figure 2B), suggesting that the increase in miR-29 expression with age is at least partly cell autonomous rather than solely dependent on the microenvironment of the organs.
Figure 2.
miR-29 expression increases with age. A, Age-related changes in miR-29 expression (means ± SEM) in mouse liver, kidney, lung, and heart. The relative expression levels of miR-29a (black bars), -b (gray bars), or -c (white bars) were analyzed by real-time PCR and normalized to snoRNA234 expression. P values in the upper left corners of each panel refer to overall changes in expression with age (ANOVA). *, (P < .05 for each pairwise comparison, corrected with the Holm-Šidák method. B, Comparison of miR-29 expression in primary hepatocytes isolated from embryos at gestational age 16.5 days (E16.5) and postnatal mice at 5 weeks of age (P35). *, P < .01 (ANOVA).
Predicted miR-29 binding sequences are overrepresented in age–down-regulated genes
In parallel with the miRNA microarray analysis, we used a bioinformatic approach to identify miRNAs that may contribute to changes in gene expression that occur during early postnatal life. Previously, we had performed expression microarrays in 1-, 4-, and 8-week-old mouse kidney and lung and identified hundreds of genes that change with age in these organs (5). In the current study, we used a bioinformatic algorithm to predict which miRNAs are likely to cause these changes by identifying overrepresentation of miRNA binding sequences in these genes. This approach is independent of our miRNA microarray analysis because it did not take into account the changes in miRNA expression with age. To increase the validity of our analysis we used both TargetScan and Microcosm as miRNA target prediction algorithms. Both algorithms indicated that miR-29a, -b, and -c target sequences are overrepresented in genes down-regulated with age in the kidney and genes down-regulated with age in the lung (Figure 1B and Table 2), which supports our hypothesis that up-regulation of miR-29 contributes to the down-regulation of genes with age. In contrast, target sequences of miR-22, the only other miRNA up-regulated in both kidney and lung, were not overrepresented in age–down-regulated genes. Interestingly, when using genes that are up-regulated with age in the kidney or in the lung for prediction, we did not find any miRNAs commonly predicted by both TargetScan and Microcosm in either organ. Using Microcosm, four miRNAs (miR-218–2, miR-23b, miR-542–3p, and miR-653) were commonly implicated in genes up-regulated in kidney and lung. However, none of these 4 miRNAs were found to be down-regulated with age in our miRNA microarray.
Table 2.
List of miRNAs for Which the Target Sequence Is Most Overrepresented in Genes Down-Regulated from 1 to 4 Weeks in Mouse Kidney and Lung
| miRNAa | Kidney Down-Regulated mRNA (1–4 Wk)b |
Lung Down-Regulated mRNA (1–4 Wk) |
||
|---|---|---|---|---|
| Microcosm | TargetScan | Microcosm | TargetScan | |
| mmu-mir-369–3p | 2.43E–10 | 5.69E–11 | 2.26E–04 | 5.96E–06 |
| mmu-mir-29a | 2.51E–06 | 1.43E–20 | 7.89E–05 | 2.03E–04 |
| mmu-mir-29c | 1.80E–07 | 1.43E–20 | 2.16E–04 | 2.03E–04 |
| mmu-mir-29b | 2.07E–06 | 1.43E–20 | 7.56E–04 | 2.03E–04 |
| mmu-mir-101a | 3.37E–03 | 1.26E–09 | 5.16E–04 | 1.57E–04 |
| mmu-mir-200c | 3.02E–05 | 7.01E–11 | 2.25E–04 | 7.15E–03 |
| mmu-mir-9 | 9.01E–03 | 8.16E–11 | 1.37E–03 | 1.13E–03 |
| mmu-mir-200b | 2.03E–03 | 7.01E–11 | 6.04E–03 | 7.15E–03 |
Columns 2 to 5 contain P values representing the likelihood that the observed number of miR-29 binding sites occurred by chance.
Only the 8 miRNAs with P < .01 in all 4 cases were listed. There were a total of 36 miRNAs with P < .05 in all 4 cases.
When up-regulated mRNAs were used for prediction instead, no miRNA reached significance (P < .05) for all 4 cases.
Predicted miR-29 target genes significantly overlap with age–down-regulated genes
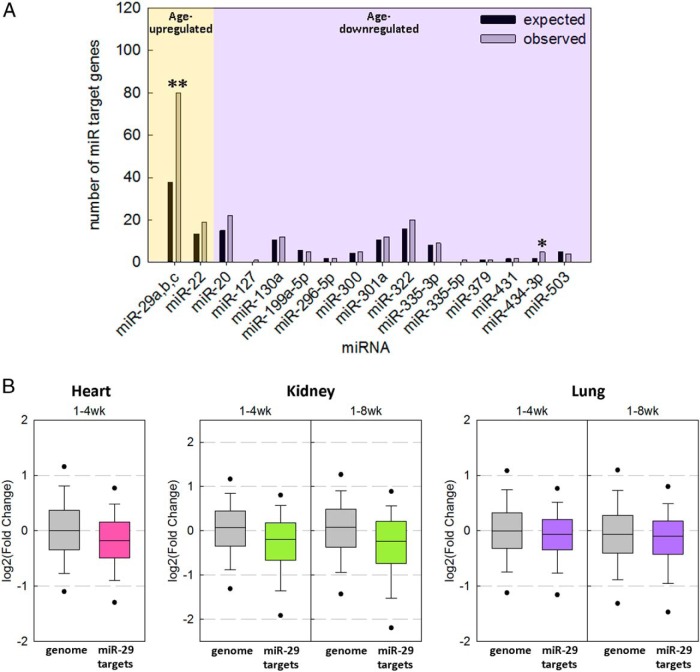
We next analyzed our previous mRNA microarray expression data of 1-, 4-, and 8-week-old mouse kidney and lung (5), asking whether predicted miR-29 targets in the genome tend to be down-regulated with age. TargetScan predicted 1080 genes in the genome as miR-29 target genes, 80 of which were significantly down-regulated from 1 to 4 weeks of age in all 3 mouse organs we studied (kidney, lung, and heart, FDR of < 0.05), much greater than would be expected by chance (P < .001, χ2 test) (Figure 3A). In contrast, when we performed the same analysis for the only other miRNA up-regulated with age (miR-22) or the 15 miRNAs down-regulated with age shown in Figure 1A, only miR-434-3p approached significance (P = .036, not significant after correction for multiple comparisons) (Figure 3A). This contrast suggests that miR-29 represents a uniquely important miR regulator of gene expression during this juvenile period.
Figure 3.
Down-regulation of predicted miR-29 target genes with age. mRNA expression was previously assessed by microarrays in lung and kidney of 1-, 4-, and 8-week-old mice and in heart of 1- and 4-week-old mice (Gene Expression Omnibus [GEO] data set GSE38754). Target genes of each miRNA were based on the prediction by TargetScan. A, Bar graph comparing the expected (black bar) and observed (white bar) numbers of target genes significantly regulated from 1 to 4 weeks (all 3 organs FDR < 0.05) in the direction predicted by the up- or down-regulation of the miRNA (eg, for age–up-regulated miRNAs, analysis was performed on age–down-regulated genes; **, P < .001; *, P < .05 (χ2 test). B, Box-and whiskers plots showing changes in expression of predicted miR-29 target genes with age in mouse kidney, lung, and heart. The fold change in expression of predicted miR-29 target genes from 1 to 4 or from 1 to 8 weeks of age was compared with changes in expression of all genes represented on the microarray (genome). The line within each box represents the median fold change of the genes in the designated gene set. The upper and lower boundaries of the box indicate the 75th and 25th percentiles, respectively. Whiskers (error bars) above and below the box indicate the 90th and 10th percentiles and outlying dots indicate the 95th and 5th percentiles. All gene sets showed significant down-regulation with age (P < .001, Mann-Whitney rank sum test).
Expression of predicted miR-29 target genes decreases with age in kidney, lung, and heart
We next performed a similar analysis, asking whether there is a tendency for the expression of predicted miR-29 targets in the genome to either increase or decrease with age. Among genes predicted to be miR-29 target genes by TargetScan, there was an overall decline in expression from 1 to 4 weeks and from 1 to 8 weeks in both kidney and lung (P < .001 by Mann-Whitney rank sum test) (Figure 3B). When we restricted our analysis to changes of miR-29 target gene expression from 4 to 8 weeks, there was still a modest and yet significant decline (P < .05) (Supplemental Figure 1). A similar general decline in miR-29 target gene expression was also observed in the 1- and 4-week-old mouse heart (P < .001, data unavailable at 8 weeks) (Figure 3B), further supporting our hypothesis.
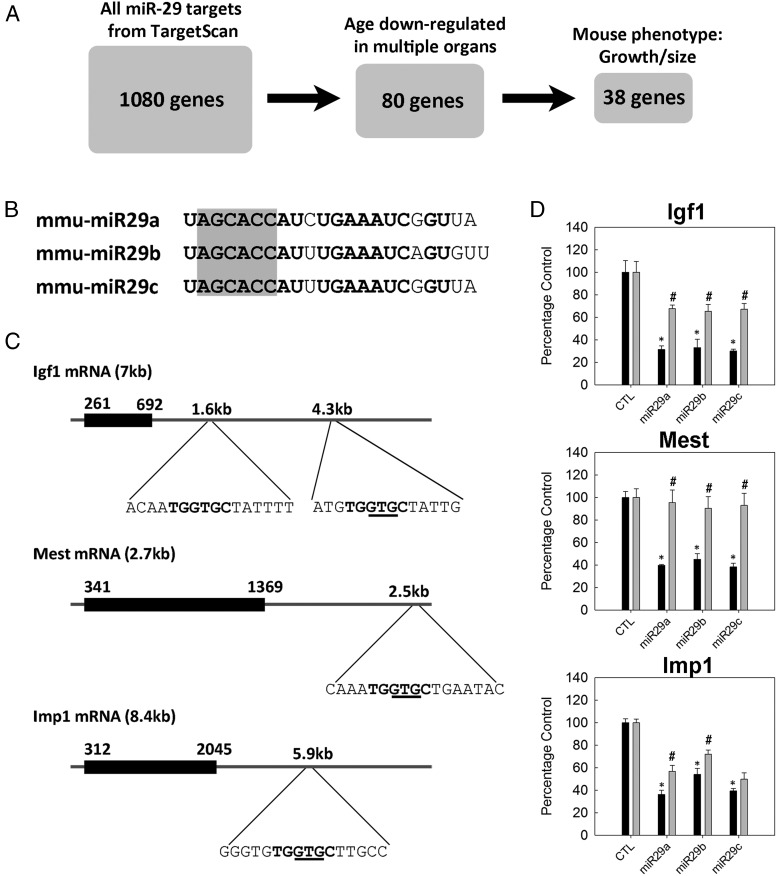
miR-29s act on 3′-UTRs of Igf1, Mest, and Imp1 mRNA
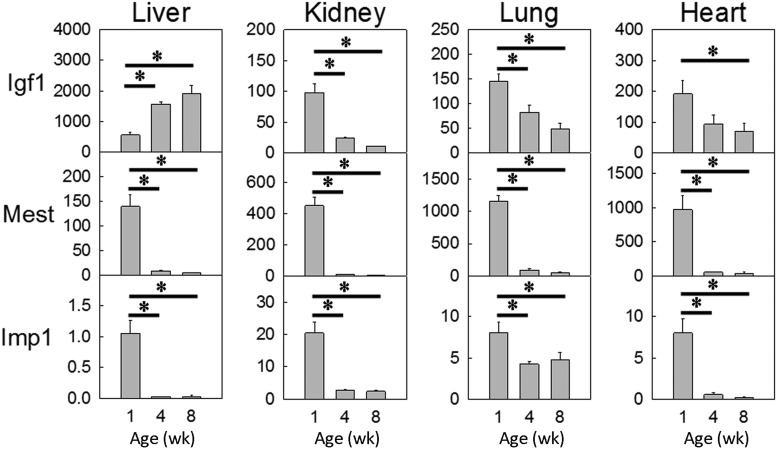
We next aimed to assess whether specific genes important for juvenile growth are indeed regulated by miR-29. To this end, we generated a list of miR-29 predicted target genes that are relevant to our study (Figure 4A). To generate this list, we first extracted the 1080 genes predicted by TargetScan to be miR-29 targets in the genome. Of these 1080 genes, we next identified the 80 genes that were down-regulated by expression microarray from 1 to 4 weeks of age in all 3 mouse organs studied (kidney, lung, and heart, FDR < 0.05). Finally, we narrowed our focus to the 38 genes that were known to affect body growth, based on the presence of a growth/size phenotype in knockout mouse models (from MGI) (Figure 4A, Table 3, and Supplemental Table 1). Of these 38 genes, we selected Igf1, Mest, and Imp1 to experimentally assess regulation by miR-29. All 3 genes are growth promoting based on previous in vivo studies. Igf1 is a well-known stimulator of growth that acts in both endocrine (secreted by liver) and paracrine/autocrine fashion, whereas both Mest and Imp1 knockout mice showed growth retardation (20, 21). We first confirmed down-regulation of these 3 genes with age using real-time PCR. Mest and Imp1 were down-regulated in all 4 tissues studied, whereas Igf1 was down-regulated in kidney, lung, and heart, but not in the liver (Figure 5).
Figure 4.
In vitro validation of selected miR-29 target genes. A, A list of predicted miR-29 target genes that are relevant to our study was generated by filtering for down-regulation of expression with age in multiple organs and also for a phenotype involving body growth and/or body size in mice. B, Sequence of mouse miR-29a, -b, and -c. Seed regions are highlighted in gray. C, mRNA structures of mouse Igf1, Mest, and Imp1. Black boxes indicate coding regions; horizontal lines indicate UTRs. 3′-UTR regions containing predicted miR-29 target sites are expanded, with the sequence complementary to the seed regions shown in bold and the 3-nucleotides deleted by site-directed mutagenesis underlined. D, Igf1, Mest, or Imp1 3′-UTR regions were placed downstream from a luciferase reporter gene and then transfected, along with miR mimics, into HEK293 cells. miR-29a, -b, or -c mimics significantly reduced luciferase activity (black bars, means ± SEM) compared with that for a random mimic (CTL). *, P < .05 (ANOVA) compared with CTL. This reduction in luciferase activity was significantly diminished (gray bars) when 3 nucleotides were deleted from the miR-29 target region using site-directed mutagenesis. #, P < .05 (ANOVA) compared to the corresponding wild-type construct (adjacent black bar).
Figure 5.
Igf1, Mest, and Imp1 expression (means ± SEM) decreases with age. Relative expression was analyzed by real-time PCR and normalized to 18S expression. In all 4 tissues and for all 3 genes studied, the overall changes in expression with age were statistically significant (P < .05, ANOVA). *, P < .05 for each pairwise comparison, corrected with the Holm-Šidák method.
For each of these 3 genes, a 1-kb region in the 3′-UTR of each mRNA containing the miR-29 predicted binding sequence (Figure 4, B and C) was placed downstream of a plasmid with a luciferase gene (psiCHECK-2). The plasmid was then cotransfected into HEK293 cells with mimics of miR-29a, -b, or -c or a negative control miRNA. The presence of miR-29a, -b, or -c mimics caused an approximately 60% reduction in luciferase activity when the luciferase gene was placed upstream of the Igf1, Mest, or Imp1 3′-UTR (Figure 4D, black bars). For Igf1, the effect was only observed when we used the 3′-UTR containing the distal seed region (approximately 4.3-kb downstream from the transcription start site) (Figure 4C) but not the proximal seed region (approximately 1.6 kb from the transcription start site) (data not shown). Importantly, when the luciferase assay was repeated using a mutant 3′-UTR with a 3-nucleotide deletion in the center of the sequence complementary to the miR-29 seed region (Figure 4C), the reduction in luciferase activity was almost completely abolished for Mest and partially blocked for Igf1 and Imp1 (Figure 4D, gray bars). The reasons for the incomplete effect on Igf1 and Imp1 3′-UTRs are unclear but could involve retained mRNA-miRNA binding, which depends both on the sequence of the seed region and on the mRNA structure. However, the overall effect of miR-29 on luciferase activity and the diminution in that effect with mutation of the binding site support the bioinformatics prediction that miR-29a, -b, and -c negatively regulate the expression of Igf1, Mest, and Imp1 mRNA.
Discussion
In humans and other mammals, body growth is rapid in early life but slows with age due to declining cell proliferation rates in multiple organs. We previously found evidence that this decline in cell proliferation is driven by a growth-limiting genetic program that involves the down-regulation of many growth-promoting genes in multiple organs. In the current study, we found several lines of evidence suggesting that this genetic program is orchestrated in part by increasing levels of miR-29a, -b, and -c. First, using miRNA arrays, we identified 4 miRNAs that were up-regulated with age during juvenile life in both mouse kidney and lung. Of these 4 miRNAs, 3 were the members of one miRNA family, miR-29a, -b, and -c. Real-time PCR showed that this up-regulation of miR-29a, -b, and -c occurred not only in kidney and lung but also in liver and heart. In an independent analysis, we used a bioinformatic approach to identify predicted miRNA binding sites in mRNAs that are down-regulated with age in kidney and lung, and we found that the predicted binding sites for miR-29a, -b, and -c were among the most overrepresented binding sites in these age–down-regulated genes. Conversely, miR-29 predicted target genes were overrepresented among age–down-regulated genes and, as a group, were significantly down-regulated with age in kidney, lung, and heart. Finally, for 3 growth-promoting genes that are down-regulated with age, Igf1, Mest, and Imp1, we found evidence that miR-29a, -b, and -c act on the 3′-UTR regions to inhibit gene expression.
Taken together, our findings support the hypothesis that the miR-29 family of miRNAs is up-regulated with age in multiple tissues, which causes down-regulation of multiple growth-promoting genes. In previous studies, we found evidence that this program of gene expression contributes to the slowing of proliferation and body growth that occurs in multiple organs of juvenile mammals (1, 4, 5). Therefore, in combination, current evidence suggests that up-regulation of miR29s may help orchestrates the physiological slowing of body growth.
This study has several important limitations. It relies in part on the identification of miRNA target genes using bioinformatic algorithms. These predictions are imprecise with both substantial false positives and false negatives occurring (22). We therefore did not rely on these algorithms to predict individual interactions between one specific miRNA and a specific mRNA, but instead used the algorithms for broad analyses of large sets of genes to find general patterns. For specific miRNA-mRNA interactions, we confirmed the bioinformatics predictions empirically. However, this specific confirmation was performed for only 3 genes. A second limitation is that the bioinformatics analysis used a set of genes down-regulated with age at the mRNA level. Because miRNAs regulate gene expression by altering both mRNA stability and translation, it would also be of great interest to identify miRNAs that target genes down-regulated with age at the protein level. Ultimately, the hypothesis that miR-29s help orchestrate a juvenile growth-regulating genetic program might be tested by in vivo genetic manipulation of miR-29 expression in a mouse model.
Our findings that Igf1 was down-regulated with age in kidney, lung, and heart but up-regulated with age in the liver suggest an important difference between paracrine and endocrine IGF-1 regulation. In kidney, lung, and heart, tissues in which IGF-1 is thought to act primarily in a paracrine fashion, miR-29 was up-regulated with age and Igf1 mRNA levels declined. Thus, our findings suggest that miR-29 helps drive the down-regulation of paracrine-acting IGF-1, contributing to body growth deceleration. In contrast, in liver, which is the primary source of endocrine-acting IGF-1 (23), miR-29 levels increased with age, yet Igf1 mRNA levels also increased. This unique increase in hepatic Igf1 mRNA probably contributes to the observed increase in circulating IGF-1 that occurs with age and suggests that, in liver, other regulatory mechanisms override miR-29 regulation. These differences in the liver could involve the liver-specific mechanisms that drive the high Igf1 expression observed in that organ, for example, chromatin accessibility at Igf1 Stat5-binding domains (24).
The up-regulation of miR-29 expression with age in multiple organs reported here and its proposed role in suppressing postnatal growth is consistent with findings by other groups. Using microarrays, Cao et al (25) recently reported that miR-29a is up-regulated in rat cardiomyocytes from postnatal day 2 to 4 weeks of age and further demonstrated its role in suppressing cardiomyocyte proliferation by blocking the G1/S and G2/M cell cycle transition. Similarly, Wei et al (26) recently reported the up-regulation of miR-29 family members with age in postnatal mouse skeletal muscle and during muscle differentiation and showed that miR-29 negatively regulates muscle cell proliferation by targeting the Akt signaling pathway. These findings, combined with numerous reports on miR-29 as a tumor suppressor (27–29), collectively suggest that miR-29 plays an important role in regulating proliferation of normal cells during postnatal life as well as malignant cells.
Orchestration of the multiorgan juvenile genetic program appears also to involve mechanisms other than miRNA regulation. A recent study shows evidence that E2f3 and E2f1 transcription factors may bind to the promoter region of multiple age–down-regulated growth-promoting genes in this program and thus contribute to its orchestration (15). Similarly, genes that are down-regulated during juvenile life in multiple organs show shifts in methylation of histone 3 at lysine resides 4 and 27 that might also contribute to the observed temporal regulation of expression (30). Additional transcription factors, miRNAs, epigenetic changes, and other yet undiscovered mechanisms may also play a role.
In summary, we found evidence that miR-29a, -b, and -c are up-regulated with age in multiple organs in mice and that this up-regulation helps orchestrate a juvenile multiorgan genetic program which involves the down-regulation of many growth-promoting genes with age. These findings suggest that up-regulation of the miR-29 family of miRNAs serves as one of the regulatory mechanisms that allows rapid proliferation and body growth in early life but then suppresses proliferation, causing the rate of body growth to slow and eventually approach zero in adulthood.
Acknowledgments
This work was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health. O.N. and A.C.A. were supported by the Swedish Research Council (Project 2012-99X-221998-01-3 and K2015-54X-22736-01-4), Karolinska Institutet, the Swedish Society of Medicine, Stiftelsen Frimurare Barnhuset Stockholm, Sällskapet Barnavård, and HKH Kronprinsessan Lovisas Förening för Barnasjukvård.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- E
- embryonic day
- FDR
- false discovery rate
- MGI
- Mouse Genome Informatics
- miRNA
- microRNA
- UTR
- untranslated region.
References
- 1. Delaney A, Padmanabhan V, Rezvani G, et al. Evolutionary conservation and modulation of a juvenile growth-regulating genetic program. J Mol Endocrinol. 2014;52:269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lui JC, Baron J. Mechanisms limiting body growth in mammals. Endocr Rev. 2011;32:422–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chang M, Parker EA, Muller TJ, et al. Changes in cell-cycle kinetics responsible for limiting somatic growth in mice. Pediatr Res. 2008;64:240–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lui JC, Forcinito P, Chang M, Chen W, Barnes KM, Baron J. Coordinated postnatal down-regulation of multiple growth-promoting genes: evidence for a genetic program limiting organ growth. FASEB J. 2010;24:3083–3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Finkielstain GP, Forcinito P, Lui JC, et al. An extensive genetic program occurring during postnatal growth in multiple tissues. Endocrinology. 2009;150:1791–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. [DOI] [PubMed] [Google Scholar]
- 7. Fabian MR, Sonenberg N, Filipowicz W. Regulation of mRNA translation and stability by microRNAs. Annu Rev Biochem. 2010;79:351–379. [DOI] [PubMed] [Google Scholar]
- 8. Sayed D, Abdellatif M. MicroRNAs in development and disease. Physiol Rev. 2011;91:827–887. [DOI] [PubMed] [Google Scholar]
- 9. Lee Y, Kim M, Han J, et al. MicroRNA genes are transcribed by RNA polymerase II. EMBO J. 2004;23:4051–4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yi R, Qin Y, Macara IG, Cullen BR. Exportin-5 mediates the nuclear export of pre-microRNAs and short hairpin RNAs. Genes Dev. 2003;17:3011–3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lund E, Güttinger S, Calado A, Dahlberg JE, Kutay U. Nuclear export of microRNA precursors. Science. 2004;303:95–98. [DOI] [PubMed] [Google Scholar]
- 12. Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mathonnet G, Fabian MR, Svitkin YV, et al. MicroRNA inhibition of translation initiation in vitro by targeting the cap-binding complex eIF4F. Science. 2007;317:1764–1767. [DOI] [PubMed] [Google Scholar]
- 14. Petersen CP, Bordeleau ME, Pelletier J, Sharp PA. Short RNAs repress translation after initiation in mammalian cells. Mol Cell. 2006;21:533–542. [DOI] [PubMed] [Google Scholar]
- 15. Lui JC, Baron J. Evidence that Igf2 down-regulation in postnatal tissues and up-regulation in malignancies is driven by transcription factor E2f3. Proc Natl Acad Sci USA. 2013;110:6181–6186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kozomara A, Griffiths-Jones S. miRBase: annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res. 2014;42:D68–D73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Griffiths-Jones S, Saini HK, van Dongen S, Enright AJ. miRBase: tools for microRNA genomics. Nucleic Acids Res. 2008;36:D154–D158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Matouskova P, Bartikova H, Bousova I, Hanusova V, Szotakova B, Skalova L. Reference genes for real-time PCR quantification of messenger RNAs and microRNAs in mouse model of obesity. PLoS One. 2014;9:e86033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bouhaddioui W, Provost PR, Tremblay Y. Identification of most stable endogenous control genes for microRNA quantification in the developing mouse lung. PLoS One. 2014;9:e111855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lefebvre L, Viville S, Barton SC, Ishino F, Keverne EB, Surani MA. Abnormal maternal behaviour and growth retardation associated with loss of the imprinted gene Mest. Nat Genet. 1998;20:163–169. [DOI] [PubMed] [Google Scholar]
- 21. Hansen TV, Hammer NA, Nielsen J, et al. Dwarfism and impaired gut development in insulin-like growth factor II mRNA-binding protein 1-deficient mice. Mol Cell Biol. 2004;24:4448–4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Witkos TM, Koscianska E, Krzyzosiak WJ. Practical aspects of microRNA target prediction. Curr Mol Med. 2011;11:93–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yakar S, Liu JL, Stannard B, et al. Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc Natl Acad Sci USA. 1999;96:7324–7329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Santhanam M, Chia DJ. Hepatic-specific accessibility of Igf1 gene enhancers is independent of growth hormone signaling. Mol Endocrinol. 2013;27:2080–2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cao X, Wang J, Wang Z, et al. MicroRNA profiling during rat ventricular maturation: a role for miR-29a in regulating cardiomyocyte cell cycle re-entry. FEBS Lett. 2013;587:1548–1555. [DOI] [PubMed] [Google Scholar]
- 26. Wei W, He HB, Zhang WY, et al. miR-29 targets Akt3 to reduce proliferation and facilitate differentiation of myoblasts in skeletal muscle development. Cell Death Dis. 2013;4:e668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bae HJ, Noh JH, Kim JK, et al. MicroRNA-29c functions as a tumor suppressor by direct targeting oncogenic SIRT1 in hepatocellular carcinoma. Oncogene. 2014;33:2557–2567. [DOI] [PubMed] [Google Scholar]
- 28. Jiang H, Zhang G, Wu JH, Jiang CP. Diverse roles of miR-29 in cancer (review). Oncol Rep. 2014;31:1509–1516. [DOI] [PubMed] [Google Scholar]
- 29. Wu Z, Huang X, Huang X, Zou Q, Guo Y. The inhibitory role of Mir-29 in growth of breast cancer cells. J Exp Clin Cancer Res. 2013;32:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lui JC, Chen W, Cheung CS, Baron J. Broad shifts in gene expression during early postnatal life are associated with shifts in histone methylation patterns. PLoS One. 2014;9:e86957. [DOI] [PMC free article] [PubMed] [Google Scholar]