Abstract
Background
The grip strength test is widely used; however, little has been investigated about its reliability when used in elderly with subjects thumb carpometacarpal (CMC) osteoarthritis (OA). The purpose of this study was to examine the test–retest reliability of the grip strength test in elderly subjects with thumb CMC OA.
Methods
A total of 78 patients with unilateral thumb CMC OA, 84.6 % female (mean ± SD age 83 ± 5 years), were recruited. Each patient performed three pain free maximal isometric contractions on each hand in two occasions, 1 week apart. Intraclass correlation coefficient (ICC), standard error of measurement (SEM), and 95 % limits of agreement (LOA) were calculated.
Results
Test–retest reliability was excellent for side affected (ICC = 0.947; p = 0.001) and contralateral (ICC = 0.96; p = 0.001) thumb CMC OA.
Conclusions
The present results indicate that maximum handgrip strength can be measured reliably, using the Jamar hand dynamometer, in patients with thumb CMC OA, which enables its use in research and in the clinic to determine the effect of interventions on improving grip.
Keywords: Test–retest, Thumb, Carpometacarpal osteoarthritis, Grip strength
Introduction
The carpometacarpal (CMC) joint of the thumb plays a vital role in optimal hand function. The thumb CMC joint is frequently affected by osteoarthritis (OA), a degenerative condition that can result in deterioration of the joint surfaces [27, 25]. Individuals with CMC joint OA have decreased grip strength that impacts hand function and their ability to perform resistive grip tasks [8, 14]. A strong positive correlation has been found between the variables of grip force control and some parameters of hand function and dexterity in patients with hand OA [17].
To enable the clinician to correctly interpret whether or not a genuine and true change of grip strength has occurred, the tool used to assess grip must be tested and found reliable and valid for use for that patient population [21]. The reliability of measurement can be influenced by several factors such as pain level and loss of normal mobility of the fingers or thumb after injury or disease process [11]. The experience of the examiner also might influence the measurement accuracy. However, studies that examine the impact of pain and disease process on grip strength for patients with hand degenerative conditions are scarce. There are several studies that have examined the reliability of the handgrip strength test in subjects with hand injuries [20, 3] and in people without impairments of the hand [15, 10, 19]. Several studies have evaluated the effect of therapeutic interventions on joint function in patients with CMC OA [9, 28, 29, 24, 26]. Villafañe et al. found high intrarater reliability when assessing grip strength test in the non-involved hand using the Jamar dynamometer [24, 26]. Although the reliability of pinch strength testing of patients with CMC OA has been reported previously [23], no previous study, to our knowledge, has investigated the reliability of handgrip strength testing for use in thumb CMC OA patients.
The reliability of the measurement of grip strength is essential for satisfactory data collection for consistent interpretation of the results. In particular, test–retest reliability is clinically important for correct assessment of follow-up results. Good test–retest reliability enables comparisons to be made over a period of time. Reliable results allow the professional to reach conclusions that are minimally affected by external factors, thereby reducing the chances of error. Therefore, the purpose of this study was to examine the test–retest reliability of the handgrip strength test in elderly subjects with unilateral thumb CMC OA.
Subjects and Methods
A convenience sample of 78 elderly subjects from 70 to 90 years old were recruited for the study from January 2013 to September 2013. Subjects were both male and female and had the medical diagnoses of unilateral thumb CMC OA. All subjects were right-hand dominant. To reach the sample size, the sample calculation was performed based on a priori power calculation on other studies of CMC OA patients, to detect a difference in reliability of 0.98 [24] and 0.72 [29] at 80 % power and a 5 % level of significance. The calculation generated a sample size of 60 individuals. Informed consent was obtained from all participants, and all procedures were conducted according to the Declaration of Helsinki. Patients underwent subjective and physical examination conducted by a therapist with 12 years of experience in treating musculoskeletal disorders. Participants were included if they exhibited a stage III–IV thumb CMC OA in the dominant hand confirmed radiographically according to the Eaton-Littler-Burton classification [12]. The combination of radiological and clinical findings has been recommended for making a more accurate diagnosis of thumb CMC OA [30]. Clinical findings may include reported pain at the CMC joint or a positive grind test. Exclusion criteria were as follows: previous treatment intervention with surgery in the hand or the forearm; corticosteroid injection or any physical therapy intervention within 6 months before the study; multiple pain diagnoses of the upper extremity, e.g., carpal tunnel syndrome, de Quervain’s tenosynovitis, shoulder pathology, and cervical radiculopathy; evidence of systemic illness (rheumatoid arthritis, psoriatic arthritis, systemic lupus erythematosus); fibromyalgia syndrome; complex regional pain syndrome; any degenerative or non-degenerative neurological conditions where pain perception can be altered; presence of any symptom in the non-dominant hand; and evidence of radiographic alterations at the first CMC joint in the non-dominant hand [4, 5].
The participants performed a standardized warm-up that consisted of two to three preliminary trials of the test procedure for familiarization with the procedure and instrument used during the procedure. Testing took place between the hours of 9:00 am and 11:30 am. The subjects were given the opportunity to handle the dynamometer before measurement recording. A portable JAMAR Hydraulic Hand Dynamometer (Fabrication Enterprises, Inc.) was used for handgrip strength measurement. The test was performed in the sitting position with the shoulder of tested arm adducted to the side, the elbow flexed at 90°, and the forearm and wrist were set in neutral position [29]. The testing protocol consisted of three pain-free maximal isometric contractions for 3 s, on both hands, with a 1-min pause between measurements. Patients were instructed to squeeze the dynamometer as firmly as possible without eliciting pain. Pain was assessed using the verbal analogue scale. If the subject reported pain during testing, the test was re-administered with instruction to stop squeezing before the onset of pain. Each patient performed three pain-free maximal isometric contractions on each hand on two occasions, one week apart. The mean of these three trials was used for analysis. The trial was designed according to the CONSORT publishing guidelines [13].
Statistical Analysis
Data were analyzed using SPSS version 20.0 (SPSS Inc, Chicago, IL, USA). The results are expressed as means, standard deviations, and/or 95 % confidence intervals. Test–retest data was analyzed using the intraclass correlation coefficient (ICC). ICC values equal or greater than 0.80 are considered high. We calculated ICC for single measures using a two-way random effect model of absolute agreement for the computation of ICC. In order to assess the absolute reliability, the standard error of measurement (SEM) and the 95 % limits of agreement (LOA) were calculated by means of the following equation: SEM = SD × √1-ICC and LOA = inter-trials mean difference ± 1.96 SD of the between trials difference. The SEM expresses measurement error in the same units as the original measurement, and it is not influenced by variability among patients. The inter-trials agreement was also examined graphically by plotting the difference between test and retest against their mean, according to the Bland and Altman approach to calculate LOA [1]. Agreement between two methods of measurement can be inappropriately assessed using the correlation that assesses the relationship of the measurements on a straight line. The LOA calculation plots the differences between test and retest against their mean on both a horizontal and vertical axis. The plot enables a visual inspection of the association between the difference in measurements and the magnitude of grip strength. The statistical analysis was conducted at a 95 % confidence level, and a p < 0.05 was considered statistically significant. ANOVA analysis was performed to determine differences between test and retest handgrip strength values.
Results
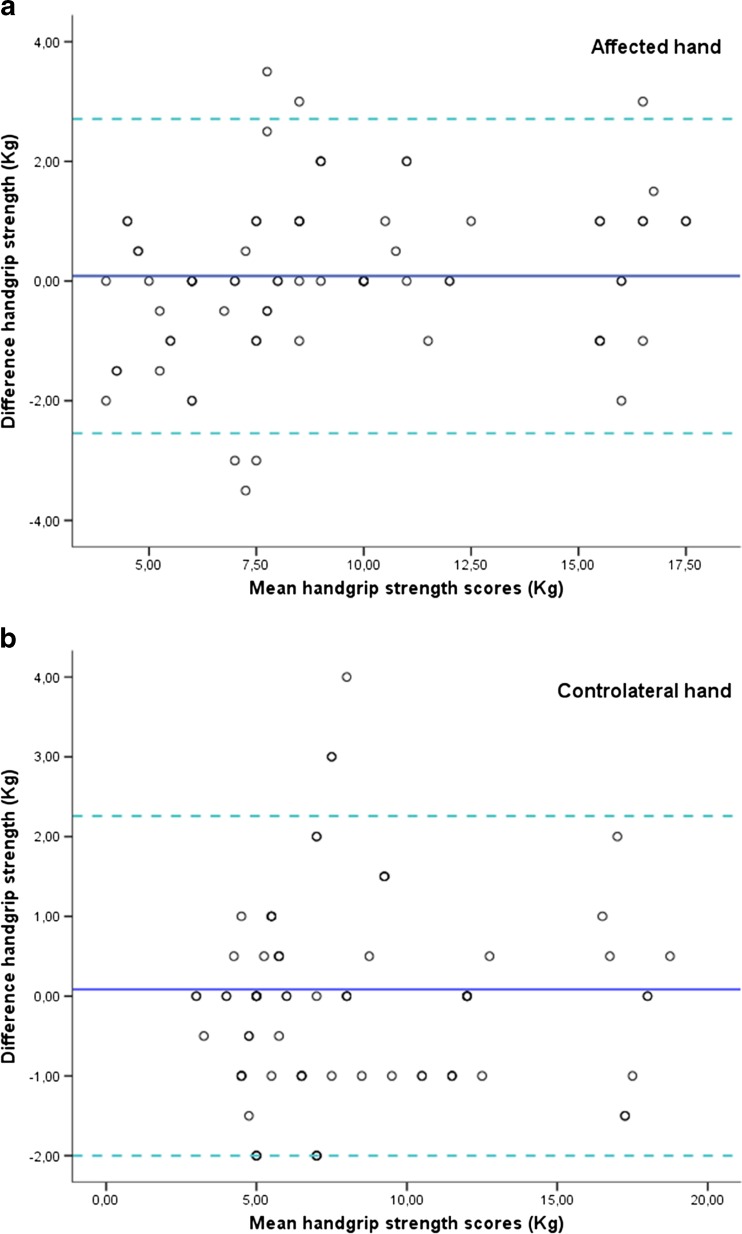
Seventy-eight elderly subjects (66 % females), mean age ± SD 82.9 ± 5.5 years, met all the inclusion criteria and agreed to participate. All subjects exhibited a stage IV thumb CMC OA in the dominant hand (Eaton-Littler-Burton classification). No participants dropped out during the different phases of the study, and no adverse effects were detected after the application of the measurement. None of the participants began drug therapy during the course of the study. The results indicated non-significant differences between test and retest handgrip strength values. The relative reliability between test and retest was very high. Test–retest reliability was excellent for the CMC OA-affected right hand (ICC = 0.947; p = 0.001) and contralateral left hand (ICC = 0.96; p = 0.001). The absolute reliability (SEM and LOA) was good. The mean absolute difference between test and retest was 0.61 and 0.54 kg for the affected right hand and contralateral left hand, respectively. The 95 % limits of agreement ranged from −2.54 to 2.71 kg for the affected hand and from −2.44 to 2.27 kg for the contralateral hand (Fig. 1 and Table 1). The ANOVA analysis found no statistically significant differences between test and retest grip strength values.
Fig. 1.
Bland–Altman plots of the handgrip strength test for a affected right and b contralateral left hand in subjects with thumb CMC OA. The central line characterizes the mean difference between test and retest values; the upper and lower lines characterize the upper and lower 95 % limits of agreement (LOA inter-trials mean difference ± 1.96 SD of the inter-trials difference), respectively
Table 1.
Test and retest values, and index of relative and absolute reliability of handgrip strength in patients with thumb CMC OA
| Test (kg) | Retest (kg) | Bias (kg) | ICC (95 % CI) | 95 % LOA (kg) | SEM (kg) | ||
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Affected right hand | 9.38 ± 4.14 | 9.30 ± 3.84 | 0.083 | 0.94 (0.91; 0.96) | −2.543 | 2.709 | 0.61 |
| Contralateral left hand | 8.17 ± 4.26 | 8.26 ± 4.20 | −0.083 | 0.96 (0.94; 0.97) | −2.439 | 2.272 | 0.54 |
Data are expressed as means ± standard deviation
Bias difference between test and retest, ICC intraclass correlation coefficient; 95 % CI 95 % confidence interval, 95 % LOA 95 % limits of agreement, SEM standard error of measurement
Discussion
In our study, high ICC was found for the reliability of grip strength measurements for both affected and contralateral hands in subjects with CMC OA. However, there were no significant differences, in both absolute and relative handgrip strength between affected and unaffected hands. The results suggest that hand-held dynamometry can be a reliable assessment technique when practiced by a single experienced tester. This supports findings of a prior study that reported that a single examiner could reliably use a dynamometer when assessing grip on neurologically impaired individuals [2]. Not all studies use a single tester for all measurements; future studies should address the inter-rater reliability of grip testing. Future work should concentrate on measuring the dynamic strength of individuals with CMC OA because dynamic strength is used by individuals’ everyday when performing activities of daily living such as opening jars and food packaging. Pain can also affect grip strength and testing reliability. Perhaps this study’s subjects grip strength would have been greater if they were allowed to squeeze the dynamometer into a painful range. The patients performed grip strength testing on two occasions, 1 week apart in this study; perhaps, 1 week was not a long-enough period and strength should have been assessed at 1 month and 6 months as well.
These findings agree with the work of Coldham et al. [6] who demonstrated excellent test–retest reliability for the evaluation grip strength in symptomatic subjects and with Stratford et al. [22] and Nitschke et al. [16] for the reliability of pain-free grip strength in populations with painful conditions. The SEMs in this study were small, ranging from 0.54 kg for grip with the right hand to 0.64 kg for grip with the left hand. Clinically, this implies that a change as small as ±1.2 kg (2 SEM) is indicative of a true change in grip strength for patients with CMC OA [18]. A change smaller than the standard error of measurement (SEM) is likely to be the result of measurement error rather than a true observed change. Patients achieving a difference in grip of at least one standard error of measurement (0.54 kg right, 0.64 kg left) may help the clinician determine that a patient has achieved a minimal clinically important difference [7].
In an attempt to control known risk of errors, we followed the American Society for Hand Therapists’ standardized position [23]. Comparison of the reliability results between this and other studies can only be made where the sample size and subject demographics are similar. Results may vary in a population of patients with different stages of CMC OA or with subjects that have differing degree of pain. This study used a short retest interval of 1 min for recovery from possible fatigue. The high degree of reliability and low SEMs found in this study may be affected if testing with an alternative rest interval.
While clinical tests are important physical measures, it is also important to incorporate patients’ perceptions of their OA symptoms as well as changes in those symptoms [22]. We recommend that clinicians incorporate a patient-centered outcome measure in their clinical practice to assess change from the patient’s perspective.
There are some limitations to this study. First, participants were elderly subjects with stage IV thumb CMC OA and, undoubtedly, they were not representative of the entire population that would be measured in clinical practice. Additional studies are needed to examine grip strength among other age groups and other stages of CMC OA. Additional studies are needed to examine these relationships among other age groups and other stages of CMC OA. We also did not recruit participants for the study, but rather used a sample of convenience. Another limitation is that the purpose of this study was to measure the test–retest reliability of the grip strength test in elderly subjects with unilateral thumb CMC OA; however, this is not a comprehensive measure of hand function.
The results of our study indicate that reliable grip strength assessment can be obtained with the Jamar dynamometer if the Jamar is calibrated properly and standardized testing procedures are followed. Maximum handgrip strength can be measured reliably, using the Jamar hand dynamometer, in patients with thumb CMC OA, which enables its use in research and in the clinic to determine the effect on interventions on improving grip.
Acknowledgments
This work was supported by personal funds of Jorge Hugo Villafañe (JHV). The authors thank C.C, for their assistance.
Conflict of Interest
Jorge Hugo Villafañe declares that he has no conflict of interest.
Kristin Valdes declares that he has no conflict of interest.
Statement of Informed Consent
Informed consent was obtained from all participants and all procedures were conducted according to the Declaration of Helsinki.
Contributor Information
Jorge Hugo Villafañe, Phone: +39.011. 9065495, Email: mail@villafane.it.
Kristin Valdes, Email: hotglassgal@comcast.net.
Carla Vanti, Email: carla.vanti@unibo.it.
Paolo Pillastrini, Email: paolo.pillastrini@unibo.it.
Alberto Borboni, Email: alberto.borboni@ing.unibs.it.
References
- 1.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307–310. doi: 10.1016/S0140-6736(86)90837-8. [DOI] [PubMed] [Google Scholar]
- 2.Bohannon RW. Test-retest reliability of hand-held dynamometry during a single session of strength assessment. Phys Ther. 1986;66(2):206–209. doi: 10.1093/ptj/66.2.206. [DOI] [PubMed] [Google Scholar]
- 3.Brown A, Cramer LD, Eckhaus D, Schmidt J, Ware L, MacKenzie E. Validity and reliability of the dexter hand evaluation and therapy system in hand-injured patients. J Hand Ther. 2000;13(1):37–45. doi: 10.1016/S0894-1130(00)80051-4. [DOI] [PubMed] [Google Scholar]
- 4.Chiarotto A, Fernandez-de-Las-Penas C, Castaldo C, Negrini S, Villafañe JH. Widespread pressure pain hypersensitivity in elderly subjects with unilateral thumb carpometacarpal osteoarthritis. Hand (N Y) 2013;8(4):422–429. doi: 10.1007/s11552-013-9537-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiarotto A, Fernandez-de-Las-Penas C, Castaldo M, Villafañe JH. Bilateral pressure pain hypersensitivity over the hand as potential sign of sensitization mechanisms in individuals with thumb carpometacarpal osteoarthritis. Pain Med. 2013;14(10):1585–1592. doi: 10.1111/pme.12179. [DOI] [PubMed] [Google Scholar]
- 6.Coldham F, Lewis J, Lee H. The reliability of one vs. three grip trials in symptomatic and asymptomatic subjects. J Hand Ther. 2006;19(3):318–326. doi: 10.1197/j.jht.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 7.Copay AG, Subach BR, Glassman SD, Polly DW, Schuler TC. Understanding the minimum clinically important difference: a review of concepts and methods. Spine J. 2007;7:541–546. doi: 10.1016/j.spinee.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 8.Dominick KL, Jordan JM, Renner JB, Kraus VB. Relationship of radiographic and clinical variables to pinch and grip strength among individuals with osteoarthritis. Arthritis Rheum. 2005;52(5):1424–1430. doi: 10.1002/art.21035. [DOI] [PubMed] [Google Scholar]
- 9.Fess E, Moran C. Clinical assessment recommendations. Garner: American Society of Hand Therapists; 1981. [Google Scholar]
- 10.Hamilton A, Balnave R, Adams R. Grip strength testing reliability. J Hand Ther. 1994;7(3):163–170. doi: 10.1016/S0894-1130(12)80058-5. [DOI] [PubMed] [Google Scholar]
- 11.Hopkins WG, Schabort EJ, Hawley JS. Reliability of power in physical performance tests. Sports Med. 2001;31(3):211–234. doi: 10.2165/00007256-200131030-00005. [DOI] [PubMed] [Google Scholar]
- 12.Jaggi R, Morris S. Practice tips. Rule of thumb: update on first carpometacarpal joint osteoarthritis. Can Fam Physician. 2007;53(8):1309–1310. [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson C, Green B. Submitting manuscripts to biomedical journals: common errors and helpful solutions. J Manip Physiol Ther. 2009;32(1):1–12. doi: 10.1016/j.jmpt.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 14.Lee HJ, Paik NJ, Lim JY, Kim KW, Gong HS. The impact of digit-related radiographic osteoarthritis of the hand on grip-strength and upper extremity disability. Clin Orthop Relat Res. 2012;470(8):2202–2208. doi: 10.1007/s11999-012-2253-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mathiowetz V, Weber K, Volland G, Kashman M. Reliability and validity of grip and pinch strength evaluations. J Hand Surg [Am] 1984;9(2):222–226. doi: 10.1016/S0363-5023(84)80146-X. [DOI] [PubMed] [Google Scholar]
- 16.Nitschke JE, McMeeken JM, Burry HC, Matyas TA. When is a change a genuine change? A clinically meaningful interpretation of grip strength measurements in healthy and disabled women. J Hand Ther. 1999;12(1):25–30. doi: 10.1016/S0894-1130(99)80030-1. [DOI] [PubMed] [Google Scholar]
- 17.Nunes PM, de Oliveira DG, Aruin AS, dos Santos MJ. Relationship between hand function and grip force control in women with hand osteoarthritis. J Rehabil Res Dev. 2012;49(6):855–865. [PubMed] [Google Scholar]
- 18.Portney L, Watkins M. Foundations of clinical research. New Jersey: Prentice Hall Health; 2000. [Google Scholar]
- 19.Schreuders TA, Roebroeck ME, Goumans J, van Nieuwenhuijzen JF, Stijnen TH, Stam HJ. Measurement error in grip and pinch force measurements in patients with hand injuries. Phys Ther. 2003;83(9):806–815. [PubMed] [Google Scholar]
- 20.Spijkerma NDC, Snijders CJ, Stijnen T, Lankhorst GJ. Standardization of grip strength measurements. Effects on repeatability and peak force. Scand J Rehabil Med. 1991;23(4):203–206. [PubMed] [Google Scholar]
- 21.Stone CA, Nolan B, Lawlor PG, Kenny RA. Hand-held dynamometry: tester strength is paramount, even in frail populations. J Rehabil Med. 2011;43(9):808–811. doi: 10.2340/16501977-0860. [DOI] [PubMed] [Google Scholar]
- 22.Stratford P, Levy D, Gauldie S, Levy K, Miseferi D. Extensor carpi radialis tendonitis: a validation of selected outcome measures. Physiother Can. 1987;39:250–255. [Google Scholar]
- 23.Villafañe JH, Valdes K. Reliability of pinch grip strength test in elderly subjects with unilateral thumb carpometacarpal osteoarthritis. J Phys Ther Sci. 2014;26(7):993–995. doi: 10.1589/jpts.26.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Villafañe JH, Silva GB, Diaz-Parreno SA, Fernandez-Carnero J. Hypoalgesic and motor effects of kaltenborn mobilization on elderly patients with secondary thumb carpometacarpal osteoarthritis: a randomized controlled trial. J Manipu Physiol Ther. 2011;34(8):547–556. doi: 10.1016/j.jmpt.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 25.Villafañe JH, Silva GB, Bishop MD, Fernandez-Carnero J. Radial nerve mobilization decreases pain sensitivity and improves motor performance in patients with thumb carpometacarpal osteoarthritis: a randomized controlled trial. Arch Phys Med Rehabil. 2012;93(3):396–403. doi: 10.1016/j.apmr.2011.08.045. [DOI] [PubMed] [Google Scholar]
- 26.Villafañe JH, Silva GB, Fernandez-Carnero J. Effect of thumb joint mobilization on pressure pain threshold in elderly patients with thumb carpometacarpal osteoarthritis. J Manip Physiol Ther. 2012;35(2):110–120. doi: 10.1016/j.jmpt.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 27.Villafañe JH, Bishop MD, Fernandez-de-Las-Penas C, Langford D. Radial nerve mobilisation had bilateral sensory effects in people with thumb carpometacarpal osteoarthritis: a randomised trial. J Physiother. 2013;59(1):25–30. doi: 10.1016/S1836-9553(13)70143-7. [DOI] [PubMed] [Google Scholar]
- 28.Villafañe JH, Cleland JA, Fernandez-de-Las-Penas C. Bilateral sensory effects of unilateral passive accessory mobilization in patients with thumb carpometacarpal osteoarthritis. J Manip Physiol Ther. 2013;36(4):232–237. doi: 10.1016/j.jmpt.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 29.Villafañe JH, Cleland JA, Fernandez-de-Las-Penas C. The effectiveness of a manual therapy and exercise protocol in patients with thumb carpometacarpal osteoarthritis: a randomized controlled trial. J Orthop Sports Phys Ther. 2013;43(4):204–213. doi: 10.2519/jospt.2013.4524. [DOI] [PubMed] [Google Scholar]
- 30.Zhang W, Doherty BF, Leeb L, Alekseeva NK, Arden JW, Bijlsma F, et al. EULAR evidence-based recommendations for the diagnosis of hand osteoarthritis: report of a task force of ESCISIT. Ann Rheum Dis. 2009;68(1):8–17. doi: 10.1136/ard.2007.084772. [DOI] [PubMed] [Google Scholar]