Abstract
Flavobacterium psychrophilum is the causative agent of the fish diseases called bacterial cold-water disease and rainbow trout fry syndrome. It has been reported that some isolates of F. psychrophilum are resistant to quinolones; however, the mechanism of this quinolone resistance has been unexplained. In this study, we examined the quinolone susceptibility patterns of 27 F. psychrophilum strains isolated in Japan and the United States. Out of 27 isolates, 14 were resistant to both nalidixic acid (NA) and oxolinic acid (OXA), and the others were susceptible to NA and OXA. When amino acid sequences deduced from gyrA nucleotide sequences of all isolates tested were analyzed, two amino acid substitutions (a threonine residue replaced by an alanine or isoleucine residue in position 83 of GyrA [Escherichia coli numbering] and an aspartic acid residue replaced by a tyrosine residue in position 87) were observed in the 14 quinolone-resistant isolates. These results strongly suggest that, as in other gram-negative bacteria, DNA gyrase is an important target for quinolones in F. psychrophilum.
Flavobacterium psychrophilum (formerly Cytophaga psychrophila and Flexibacter psychrophilus) is a gram-negative bacterium that is the causative agent of bacterial cold-water disease (BCWD) and rainbow trout fry syndrome in salmonid, cyprinid, and other freshwater fishes. This pathogen was originally isolated from juvenile coho salmon (Oncorhynchus kisutch) in the United States in 1948 (2). For the last decade, BCWD and rainbow trout fry syndrome have been reported in the United States, European countries (1), Japan (16), Australia (14), Chile (4), and Korea (8) in many freshwater fish species, e.g., eel (Anguilla anguilla), common carp (Cyprinus carpio), crucian carp (Carassius carassius), tench (Tinca tinca), and oikawa (Zacco platypus) (6, 9). In Japan, since the first isolation of F. psychrophilum on a local ayu (Plecoglossus altivelis) farm in 1987, the bacterium has spread widely in many host species, such as coho salmon, rainbow trout (Oncorhynchus mykiss), and oikawa, in various local areas. Because of serious losses in rivers, as well as in fish farms, BCWD caused by F. psychrophilum is the most economically important fish disease.
In a previous study, some F. psychrophilum isolates from Danish rivers and hatcheries were found to be resistant to oxolinic acid (OXA) (3). In Japan at present, OXA is one of the antimicrobial agents licensed for wide use in aquaculture, and F. psychrophilum isolates from diseased fishes were often reported to be resistant to OXA (6). In gram-negative bacteria, resistance to quinolones most often corresponds to specific amino acid variations in a portion of the A subunit of DNA gyrase (GyrA) referred to as the quinolone resistance-determining region (QRDR) (18). We suspected that a similar mechanism was responsible for natural resistance to quinolones in F. psychrophilum isolates. In this study, to verify this hypothesis, we determined patterns of susceptibility to quinolones, OXA, and nalidixic acid (NA) and sequences of the partial gyrA region, including the QRDR, in 27 F. psychrophilum clinical isolates.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Twenty-seven isolates of F. psychrophilum were examined, including 3 strains from coho salmon, 12 strains from ayu, 6 strains from rainbow trout, 3 strains from amago (Oncorhynchus rhodurus), 2 strains from oikawa, and 1 strain from yamame (Oncorhynchus masou). The strains from coho salmon were isolated in Japan and the United States, and the type strain of F. psychrophilum (NCIMB1947) is included in the group of U.S. isolates. The other strains were isolated in Japan (Table 1). All strains were stored at −80°C and grown in tryptone and yeast extract agar (0.4% tryptone, 0.05% yeast extract, 0.05% CaCl2 · 7H2O, 0.05% MgSO4 · 7H2O, 1.5% agar, distilled water, pH 7.2) at 16°C.
TABLE 1.
Susceptibilities of 27 F. psychrophilum isolates and mutation of GyrA
| Isolate no.a | Host fish | Isolation yr | Isolation locality | MIC (μg/ml)
|
GyrA mutationb | gyrA accession no. | |
|---|---|---|---|---|---|---|---|
| NA | OXA | ||||||
| NCIMB1947 | Coho salmon | Unknown | United States | 1.56 | 0.2 | — | AB158101 |
| FPC826 | Coho salmon | 1980 | United States | 0.39 | 0.1 | — | AB158102 |
| FPC828 | Coho salmon | 1990 | Miyagi | 3.13 | 0.39 | — | AB158103 |
| FPC840 | Ayu | 1987 | Tokushima | >100 | 12.5 | Thr83-Ala | AB158104 |
| FPC924 | Ayu | 1992 | Wakayama | 50 | 6.25 | Thr83-Ala | AB158105 |
| FPC931 | Ayu | 1993 | Hiroshima | 25 | 1.56 | Thr83-Ala | AB158106 |
| AA9401 | Ayu | 1994 | Aichi | 50 | 6.25 | Thr83-Ala | AB158107 |
| FPC956 | Ayu | 1994 | Shiga | 100 | 12.5 | Thr83-Ala | AB158108 |
| YMA9609 | Ayu | 1996 | Yamanashi | 3.13 | 0.78 | — | AB158109 |
| GFA9604 | Ayu | 1996 | Gifu | 100 | 12.5 | Thr83-Ala | AB158110 |
| KNA9801 | Ayu | 1997 | Kanagawa | 50 | 6.25 | Thr83-Ala | AB158111 |
| OKA9806 | Ayu | 1998 | Okayama | 100 | 12.5 | Thr83-Ile | AB158112 |
| IP980601 | Ayu | 1998 | Iwate | 100 | 3.13 | Thr83-Ala | AB158113 |
| MG980922-1 | Ayu | 1998 | Miyagi | 100 | 6.25 | Thr83-Ala | AB158114 |
| YMA980608 | Ayu | 1998 | Yamagata | 100 | 6.25 | Thr83-Ala | AB158115 |
| FPC814 | Rainbow trout | 1991 | Tokyo | 0.1 | 0.2 | — | AB158116 |
| FPC942 | Rainbow trout | 1994 | Yamagata | 1.56 | 0.78 | — | AB158117 |
| YMR9615 | Rainbow trout | 1996 | Yamanashi | 3.13 | 0.78 | — | AB158118 |
| OKR9801 | Rainbow trout | 1998 | Okayama | 25 | 3.13 | Thr83-Ala | AB158119 |
| OKR9802 | Rainbow trout | 1998 | Okayama | 50 | 6.25 | Thr83-Ala | AB158120 |
| FKR9801 | Rainbow trout | 1998 | Fukui | 100 | 25 | Asp87-Tyr | AB158121 |
| FPC958 | Amago | 1994 | Tottori | 1.56 | 0.39 | — | AB158122 |
| FKM9801 | Amago | 1998 | Fukui | 1.56 | 0.39 | — | AB158123 |
| OKM9801 | Amago | 1998 | Okayama | 1.56 | 0.39 | — | AB158124 |
| FPC945 | Oikawa | 1993 | Hiroshima | 0.78 | 0.2 | — | AB158125 |
| OY99Oik-1 | Oikawa | 1999 | Okayama | 1.56 | 0.39 | — | AB158126 |
| YMY9604 | Yamame | 1996 | Yamanashi | 3.13 | 0.78 | — | AB158127 |
NCIMB, National Collection of Industrial and Marine Bacteria, Aberdeen, United Kingdom. All strains except the type strain, NCIMB1947, were provided by the bacterial collection of the National Research Institute of Aquaculture, Mie, Japan.
Dashes indicate that the amino acid sequences were the same as those of the type strain (NCIMB1947).
Antimicrobial susceptibility testing.
The MICs for F. psychrophilum isolates were determined by following a standardized agar dilution method, which is recommended by the Japanese Society of Chemotherapy. Each isolate was inoculated with a semiautomatic multipoint replicator (Sakuma Seisakusho, Tokyo, Japan) onto freshly prepared Mueller-Hinton agar plates containing twofold serial dilutions (100 to 0.1 μg/ml) of NA (Sigma, St. Louis, Mo.) and OXA (Tanabe Seiyaku, Osaka, Japan). The inoculated plates were incubated at 16°C for 96 h, and MICs were determined. Preliminary reading was done after 48 and 72 h. All determinations were made in duplicate.
DNA extraction.
Total genomic DNA was prepared from individual strains according to the methods used in previous studies (7, 17). Briefly, one loopful of bacterial pellet was mixed with 300 μl of 5% Chelex100 (Sigma) and incubated at 55°C for 30 min. Following mixing by vortexing at high speed for 5 to 10 s, the mixture was boiled for 20 min and then centrifuged for 10 min at 10,000 × g. Without further purification, an aliquot of the supernatant containing DNA was used as a template for PCR amplification.
PCR amplification.
A degenerate universal primer pair, gyrAF and gyrAR (10), and a specific primer pair for the gyrA gene of F. psychrophilum, GYRA-FP1F and GYRA-FP1R, were used. The oligonucleotide sequences of gyrAF, gyrAR, GYRA-FP1F, and GYRA-FP1R were 5′ GAYGGNYTNAARCCNGTNCA 3′, 5′ GCCATNCCNACNGCDATNCC 3′, 5′ GAAACCGGTGCACAGAAGG 3′, and 5′ CCTGTGGCTCCGTTTATTAA 3′, respectively. PCR amplification was performed in a total reaction volume of 10 μl with a Techgene thermal cycler (Techne, Cambridge, United Kingdom). The reaction mixture contained 2 μl of template DNA, 0.1 nmol of each deoxynucleoside triphosphate, 10 pmol of each primer, and 0.5 U of Taq DNA polymerase (Bioneer, Daejeon, Korea). The following temperature profile was used for the amplification: preheating at 94°C for 5 min; 30 cycles of denaturation at 94°C for 30 s, annealing at 48 (gyrAF and gyrAR) or 56°C (GYRA-FP1F and GYRA-FP1R) for 30 s, and extension at 72°C for 90 s; and a final extension at 72°C for 5 min.
Cloning and sequencing of PCR products.
PCR products from three F. psychrophilum isolates (NCIMB1947, FPC814, and FPC840) with the universal primer pair (gyrAF and gyrAR) and PCR products from all F. psychrophilum isolates with the specific primer pair (GYRA-FP1F and GYRA-FP1R) were ligated with the pGEM-T vector (Promega, Madison, Wis.). For the ligation, T4 DNA ligase (Fermentas, Vilnius, Lithuania) was used, and the ligated clones were transformed into the competent E. coli strain EC100 (Epicentre, Madison, Wis.). Sequencing was performed with a CEQ DTCS Quick Start kit in a CEQ 2000 XL DNA analysis system (Beckman Coulter, Fullerton, Calif.).
Nucleotide sequence accession numbers.
The nucleotide sequences of the 396-bp PCR products from 27 F. psychrophilum isolates were determined and deposited in the DDBJ, EMBL, and GenBank nucleotide sequence databases under the accession numbers listed in Table 1.
RESULTS
MICs of NA and OXA.
The MICs of NA and OXA for F. psychrophilum isolates are presented in Table 1. Based on the MICs of NA, two distinct groups were observed. The high-MIC group ranged from 25 to >100 μg/ml, and the low-MIC group ranged from 0.1 to 3.13 μg/ml. The lowest value in the high-MIC group was eightfold higher than the highest value in the low-MIC group. In the case of OXA, the range of MICs was not as wide as for NA. In spite of the narrow range of OXA MICs, the isolates belonging to the NA high-MIC group had higher OXA MICs than those in the low-MIC group. The OXA high- and low-MIC groups ranged from 1.56 to 25 and 0.1 to 0.78 μg/ml, respectively. The isolates belonging to the high-MIC group were regarded as resistant to quinolones.
PCR amplification and sequences of PCR products.
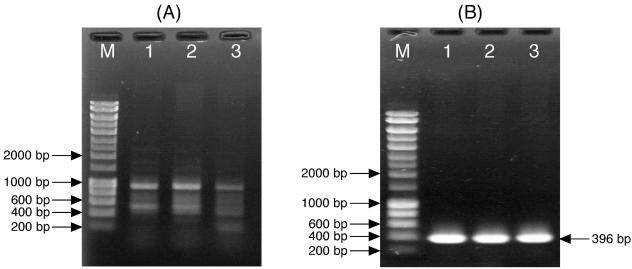
The degenerate universal primers, gyrAF and gyrAR, yielded multiple PCR products from three F. psychrophilum isolates (Fig. 1A). The targeted <450-bp DNA fragment of the gyrA gene was expected to exist in these products. After the cloning and transformation of the products, the length of the inserted DNA in each clone was checked by colony-directed PCR, and then appropriate clones were selected and purified. Following sequence analysis of the products, a specific primer pair, GYRA-FP1F and GYRA-FP1R, was designed. The specific primers successfully yielded an expected single 396-bp PCR product from all isolates tested (Fig. 1B).
FIG. 1.
PCR amplification of partial gyrA sequences from F. psychrophilum isolates. Lanes M, molecular size markers (HyperLadder 1; Bioline, London, United Kingdom); lanes 1, 2, and 3, PCR products of the F. psychrophilum type strain (NCIMB1947), FPC814, and FPC840, respectively. (A) Amplification from F. psychrophilum isolates with degenerate universal primers gyrAF and gyrAR. (B) Amplification of the predicted 396-bp fragments (including primers) from F. psychrophilum isolates with specific primers GYRA-FP1F and GYRA-FP1R.
Amino acid sequence analysis.
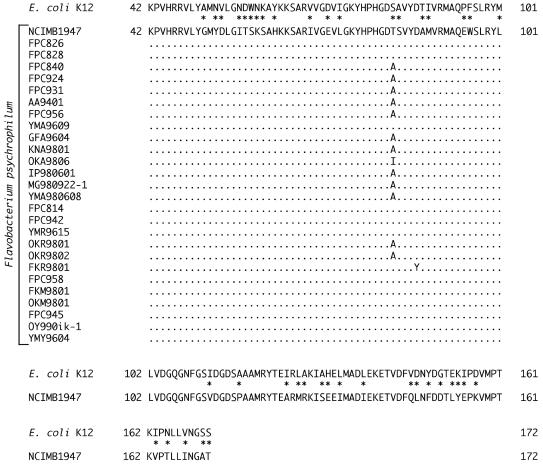
The amino acid sequences of the partial GyrA proteins (131 amino acid residues between positions 42 and 172 in the numbering system used for E. coli) were deduced from the determined nucleotide sequences (Fig. 2). The amino acid sequences of all the F. psychrophilum isolates tested were found to be identical, except for positions 83 and 87. At position 83, a threonine, an alanine, and an isoleucine residue were observed in 14, 12, and 1 isolates, respectively. At position 87, one isolate (FKR9801) possessed a tyrosine residue, and the others possessed an aspartic acid residue.
FIG. 2.
Alignment of amino acid sequences of GyrA from F. psychrophilum isolates and E. coli K-12 (accession no. X57174). For F. psychrophilum, the dots represent amino acids identical to those of the type strain of F. psychrophilum (NCIMB1947). All of the amino acids from 102 to 172 were identical in F. psychrophilum isolates. For E. coli, the asterisks indicate amino acids different from those of NCIMB1947.
DISCUSSION
DNA gyrase and topoisomerase IV are type II DNA topoisomerases catalyzing DNA topological changes necessary for DNA replication and transcription. DNA gyrase is usually the natural target of the quinolone antibiotics in gram-negative bacteria. This enzyme is composed of two subunits, A and B, encoded by gyrA and gyrB, respectively. Acquired resistance to quinolones in gram-negative bacteria most often corresponds to mutations in the gyrA DNA sequence, especially in a specific 40-amino-acid region between positions 67 and 106 within the N-terminal portion of the GyrA protein. This region, the QRDR, is near the putative active site supposed to be the interaction site between the A subunit of DNA gyrase and quinolones (18).
In the present study, we attempted to verify our hypothesis that variations in the QRDR contribute intrinsic quinolone resistance in F. psychrophilum isolates. We first determined the MICs of NA and OXA for 27 clinical isolates of F. psychrophilum. A PCR approach using the previously reported universal primers gyrAF and gyrAR was employed to amplify a portion of the F. psychrophilum gyrA QRDR. These universal primers have been determined by alignment of several gyrA sequences from various gram-negative and gram-positive bacterial species and correspond to positions 39 to 45 and 173 to 179 of the amino acid sequence of the E. coli GyrA protein (10). Although the gyrA sequences of members of the genus Flavobacterium have not yet been reported and were not used for designing the primers gyrAF and gyrAR, these universal primers were able to amplify the gyrA region of F. psychrophilum. This indicates that positions 39 to 45 and 173 to 179 of GyrA are regions conserved among many bacterial species, including F. psychrophilum. On the basis of the determined DNA sequences of gyrA amplified with the universal primers, we designed successful primers specific for gyrA of F. psychrophilum (PSY-G1F and PSY-G1R) and determined the nucleotide sequences of all the isolates tested. With a preliminary experiment, the specificity of these primers for F. psychrophilum was confirmed using other bacteria belonging to the genus Flavobacterium (data not shown). This suggests the feasibility of using these primers for PCR detection and identification in F. psychrophilum.
The MICs of quinolones and GyrA substitutions were clearly associated with each other. F. psychrophilum isolates whose GyrA amino acid sequences were equivalent to that of the type strain of F. psychrophilum were susceptible to NA and OXA in the ranges of 0.1 to 3.13 and 0.1 to 0.78 μg/ml, respectively, while isolates possessing GyrA mutations different from the type strain of F. psychrophilum (Thr83-Ala or Ile and Asp87-Tyr) were resistant to both NA and OXA (Table 1). It is known that, in other bacteria, the amino acid substitution(s) at position(s) 83 and/or 87 in GyrA is responsible for quinolone resistance (12, 15). These data strongly indicate that in F. psychrophilum as well, the QRDR in GyrA is an important target for quinolones. In vitro mutagenesis experiments at positions 83 and 87 in GyrA are needed to verify the reasonable hypothesis that QRDR is a target of quinolones in F. psychrophilum.
Bruun et al. reported that F. psychrophilum isolates for which the MICs were <1 μg/ml should be susceptible to OXA, depending on the maximum serum drug concentration obtained in rainbow trout after OXA treatment (3). This assumption for susceptible MICs of OXA in F. psychrophilum is closely matched by our results. In this study, F. psychrophilum isolates for which the MICs ranged from 0.1 to 0.78 μg/ml were susceptible to OXA and possessed GyrA amino acid substitutions compared to the type strain of F. psychrophilum, while isolates for which the MICs ranged from 1.56 to 12.5 μg/ml were resistant. Among the F. psychrophilum isolates from ayu, 11 out of 12 were resistant to NA and OXA. This significantly higher rate of isolates resistant to quinolones may correlate with the previous situation for use of antibiotics in ayu farming: in previous epidemic vibriosis in ayu caused by Listonella anguillara (formerly Vibrio anguillarum), OXA was widely used as a “wonder drug ” for the disease in Japan (11, 13). Further investigations are needed to clarify the relationship between antibiotic treatments in fish farming and the appearance of antibiotic-resistant bacteria.
Studies of bacterial mechanisms of quinolone resistance involving DNA gyrase and/or topoisomerase IV mutations have been done since the 1970s, and it has been revealed that many bacteria pathogenic to humans or domestic animals behave the same way in resisting quinolones (12, 15). In the case of fish disease treatments, quinolone antibiotics, such as OXA, have been commonly used in most fish farms or hatcheries, and a study of the quinolone resistance mechanism of Yersinia ruckeri, which causes enteric redmouth disease in salmonid fishs, was recently reported (5). However, this kind of study of bacteria pathogenic to fish is not sufficient. In this study, we explained the quinolone resistance mechanism of F. psychrophilum, and the present study is the first investigation of Flavobacterium species. When antibiotic treatments of cultured fish are done, immersion treatment is more convenient than feeding treatment with drugs intermixed with food, especially in freshwater fish species, such as ayu. Immersion treatment of cultured fish with antibiotics might pose a risk that the targeted pathogenic bacteria, as well as other bacteria naturally inhabiting the water, will acquire resistance to the antibiotics. Because selective pressure caused by the excessive dosage of antibiotics is a common factor in creating antibiotic-resistant strains of bacteria, studies of the antibiotic resistance of bacteria pathogenic to aquatic animals are needed for aquaculture management and the ecological study of aquatic microorganisms.
REFERENCES
- 1.Bernardet, J. F., F. Baudin-Laurencin, and G. Tixerant,. 1988. First identification of Cytophaga psychrophila in France. Bull. Eur. Assoc. Fish Pathol. 8:104-105. [Google Scholar]
- 2.Borg, A. F. 1960. Studies on myxobacteria associated with diseases in salmonid fishes. Wildl. Dis. 8:85. [Google Scholar]
- 3.Bruun, M. S., A. S. Schmidt, L. Madsen, and I. Dalsgaard. 2000. Antimicrobial resistance patterns in Danish isolates of Flavobacterium psychrophilum. Aquaculture 187:201-212. [Google Scholar]
- 4.Bustos, P. A., J. Calbuyahue, J. Montana, B. Opazo, P. Entrala, and R. Solervicens. 1995. First isolation of Flexibacter psychrophilus, as causative agent of rainbow trout fry syndrome (RTFS), producing rainbow trout mortality in Chile. Bull. Eur. Assoc. Fish Pathol. 15:162-164. [Google Scholar]
- 5.Gibello, A., M. C. Porrero, M. M. Blanco, A. I. Vela, P. Liebana, M. A. Moreno, J. F. Fernandez-Garayzabal, and L. Dominguez. 2004. Analysis of the gyrA gene of clinical Yersinia ruckeri isolates with reduced susceptibility to quinolones. Appl. Environ. Microbiol. 70:599-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iida, Y., and A. Mizokami. 1996. Outbreaks of coldwater disease in wild ayu and pale chub. Fish Pathol. 31:157-164. [Google Scholar]
- 7.Izumi, S., and H. Wakabayashi. 1997. Use of PCR to detect Cytophaga psychrophila from apparently healthy juvenile ayu and coho salmon eggs. Fish Pathol. 32:169-173. [Google Scholar]
- 8.Lee, K. B., and G. J. Heo. 1998. First isolation and identification of Cytophaga psychrophila from cultured ayu in Korea. Fish Pathol. 33:37-38. [Google Scholar]
- 9.Lehmann, J., D. Mock, F. J. Sturenberg, and J. F. Bernardet. 1991. First isolation of Cytophaga psychrophila from a systemic disease in eel and cyprinids. Dis. Aquat. Org. 10:217-220. [Google Scholar]
- 10.Maurin, M., C. Abergel, and D. Raoult. 2001. DNA gyrase-mediated natural resistance to fluoroquinolones in Ehrlichia spp. Antimicrob. Agents Chemother. 45:2098-2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muroga, K., N. Yoneyama, and Y. Jo. 1978. Vibriostatic agent and non-sensitive Vibrio anguillarum isolated from ayu. Fish Pathol. 13:159-162. [Google Scholar]
- 12.Piddock, L. J. V., V. Ricci, I. Mclaren, and D. J. Griggs. 1998. Role of mutation in the gyrA and parC genes of nalidixic-acid-reisitant salmonella serotypes isolated from animals in the United Kingdom. J. Antimicrob. Chemother. 41:635-641. [DOI] [PubMed] [Google Scholar]
- 13.Sako, H., and R. Kusuda. 1978. Chemotherapeutic studies on trimethoprim against vibriosis of pond-cultured ayu. I. Microbiological evaluation of trimethoprim and sulfonamides on the causative agent Vibrio anguillarum. Fish Pathol. 13:91-96. [Google Scholar]
- 14.Schmidtke, L. M., and J. Carson. 1995. Characteristics of Flexibacter psychrophilus isolated from Atlantic salmon in Australia. Dis. Aquat. Org. 21:157-161. [Google Scholar]
- 15.Sugino, A., C. L. Peebles, K. N. Kreuzer, and N. R. Cozzarelli. 1977. Mech-anism of action of nalidixic acid: purification of Escherichia coli nalA gene product and its relationship to DNA gyrase and a novel nicking-closing enzyme. Proc. Natl. Acad. Sci. USA 74:4767-4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wakabayashi, H., M. Horinouchi, T. Bunya, and G. Hoshiai. 1991. Outbreaks of cold-water disease in coho salmon in Japan. Fish Pathol. 26:211-212. [Google Scholar]
- 17.Walsh, P. S., D. A. Metzger, and R. Higuchi. 1991. Chelex100 as a medium for simple extraction of DNA for PCR based typing from forensic material. BioTechniques 10:507-513. [PubMed] [Google Scholar]
- 18.Yoshida, H., M. Bogaki, M. Nakamura, and S. Nakamura. 1990. Quinolone resistance-determining region in the DNA gyrase gyrA gene of Escherichia coli. Antimicrob. Agents Chemother. 34:1271-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]