Abstract
Background
Neurocardiogenic syncope (NCS) is a common and sometimes debilitating disorder, with no consistently effective treatment. NCS is due to a combination of bradycardia and vasodilation leading to syncope. Although pacemaker devices have been tried in treating the bradycardic aspect of NCS, no device based therapy exists to treat the co-existent vasodilation that occurs. The renal sympathetic innervation has been the target of denervation to treat hypertension. We hypothesized that stimulation of the renal sympathetic nerves can increase blood pressure and counteract vasodilation in NCS.
Methods and Results
High frequency stimulation (800–900 pps, 10V, 30–200s) was performed using a quadripolar catheter in the renal vein of 7 dogs and 1 baboon. A significant increase in blood pressure [mean (SD) systolic BP 117(±28) vs 128(± 33), diastolic BP (75(± 19) vs 87(± 29) mm Hg] was noted during the stimulation which returned to baseline after cessation of stimulation. The mean increase in systolic and diastolic BP was 13.0 (±3.3) (p=0.006) and 10.2 (±4.6) (p=0.08) respectively.
Conclusion
We report the first ever study of feasibility and safety of high frequency electrical stimulation of the renal sympathetic innervation to increase blood pressure in animal models. This has potential applications in the treatment of hypotensive states such as NCS.
Keywords: neurocardiogenic syncope, blood pressure, renal nerve stimulation, sympathetic nervous system, syncope
Introduction
Syncope accounts for 1% to 3% of emergency department visits, 6% of hospital admissions and $2.4 billion in health care expenditure annually in the USA.1, 2 Neurocardiogenic syncope (NCS), the most common cause of syncope, accounts for 20% of new cases of syncope and has a prevalence of 22% in the U.S. population.3 Current therapies including medications and cardiac pacemaker do not reliably prevent syncope in NCS. Two randomized double-blind controlled trials showed recurrence of neurocardiogenic syncope in 30% of patients despite cardiac pacing, similar to that observed in the placebo group.4, 5 Thus, there is a need for effective therapies that will prevent recurrent NCS.
Transient autonomic dysfunction with increased vagal tone and decreased sympathetic output has been implicated in the pathogenesis of neurocardiogenic syncope.3 This results in transient bradycardia and peripheral vasodilation leading to hypotension and syncope. While cardiac pacing can effectively treat bradycardia, peripheral vasodilation persists and likely explains the failure of pacemaker therapy to consistently prevent symptoms. Thus an effective therapy should ameliorate both bradycardia and vasodilation.
The renal arteries are richly innervated by efferent and afferent sympathetic nerves that play an important role in the regulation of renal and cardiovascular function.6 Efferent renal sympathetic activity leads to release of renin, renal vasoconstriction and sodium retention, which in turn contribute to development of chronic hypertension.7–9 Activation of renal afferent fibers acutely results in widespread vascular sympathetic activation and peripheral vasoconstriction.10–12 Renal denervation using splanchnicectomy and more recently percutaneous catheter ablation in the renal artery has shown promise in reducing blood pressure.13, 14 Conversely, we hypothesized that stimulation of renal innervation may increase blood pressure10, 15 and is a potential treatment strategy for neurocardiogenic syncope. Furthermore, due to the close anatomical proximity of the renal artery and vein, stimulation of the renal nerves can potentially be performed from the renal vein. The aim of this investigation was to assess the effect of high frequency electrical stimulation of the renal nerves from the renal vein on blood pressure in the canine and baboon.
Methods
Seven mongrel dogs (weight 30–40 kg) and one baboon (weight 25–35 kg), were anesthetized with ketamine and diazepam for induction and isoflurane (1% to 3% continuous inhalation) for maintenance. Positive pressure ventilation was given through an endotracheal tube. Sheaths were placed percutaneously in the femoral artery and femoral vein. Electrocardiogram and femoral arterial blood pressure were continuously monitored. Angiogram of the renal arteries and veins was performed using a catheter placed in the descending aorta and inferior vena cava, respectively. A commercially available quadripolar catheter (Blazer, Boston Scientific, 7 Fr, 4 mm tip, 2.5 mm electrode spacing) was placed in a unilateral renal vein. High frequency stimulation (HFS) (800–900 pps, 10V) was performed for 30–200s (median 60 s) using a Grass stimulator (Grass Technologies, Warwick, RI) (dose titration study was previously performed for optimization of stimulation). Each animal underwent multiple HFS trials; median 4 (range 1–9). The BP was allowed to return to baseline between each HFS trial. The BP response to HFS was documented. Heart rate immediately before and after each HFS trial was calculated using the mean of 10 consecutive QRS intervals on the surface ECG. The animal was euthanized by induction of ventricular fibrillation at the end of the study. Laparotomy was performed and pathological examination of the kidneys and renal vessels was performed.
Statistical methods
Continuous data is presented as mean (standard deviation) and categorical variables as N (%). The difference in peak SBP and DBP during HFS and that before HFS was computed and included as a response variable in a mixed effects model (PROC MIXED in SAS, version 9.3, Cary, NC) along with a random effect for subject. The mixed effects model was used to account for the within-subject correlation while testing for a significant change in pre vs. peak measures. By fitting subject as a random effect, a common correlation is defined among all data within a subject. The mixed model intercept was estimated and tested to be different from 0, which would indicate a significant difference between pre- and peak-HFS measures. All analyses were performed using the SAS statistical software package (SAS Institute, Cary, NC). A significance level of α=0.05 was used for statistical testing.
Results
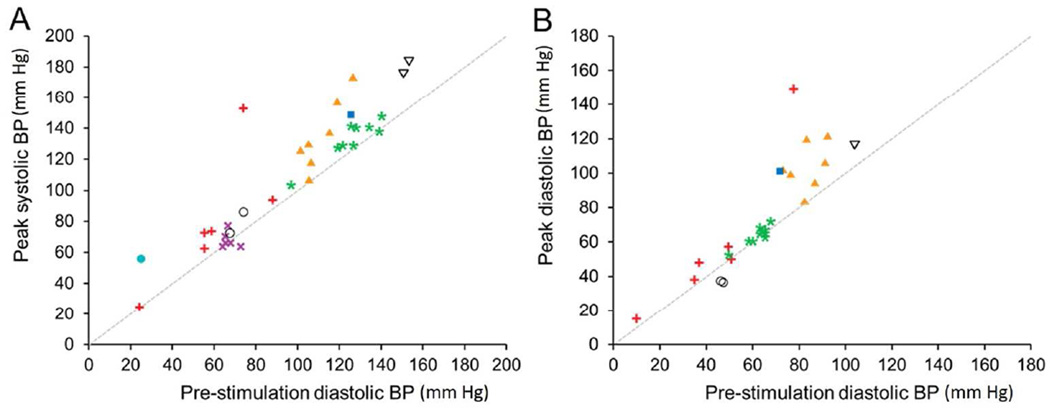
High frequency stimulation was performed in the renal vein on 34 occasions. The mean pre-HFS and peak-HFS systolic blood pressure was 117 (±28) and 128 (±33) mm Hg respectively. The mean pre- and peak- diastolic blood pressure was 75 (±19) and 87 (±29) mm Hg. The systolic and diastolic blood pressure response during each trial of HFS is presented in Figure 1. The mean increase in systolic and diastolic BP was 13.0 (±3.3) (p=0.006) and 10.2 (±4.6) (p=0.08) respectively. The increase in blood pressure was noted to occur within a few seconds of onset of stimulation and was sustained throughout the duration of stimulation (Figure 2). The blood pressure returned to baseline within a few minutes of cessation of HFS. The mean heart rate before and after HFS was 107 (±53) and 111 (±54) bpm respectively. Heart rate did not change significantly immediately after HFS [mean change 0.6 (±1.5), p=0.7].
Figure 1. High frequency stimulation of renal vein causes increases in systolic (A) and diastolic (B) blood pressure.
Effect of separate trials of high frequency stimulation on blood pressure pre- and during stimulation are shown (n = 34). The mean pre-HFS and peak-HFS systolic blood pressure was 117 (±28) and 128 (±33) mm Hg respectively. The mean pre- and peak- diastolic blood pressure was 75 (±19) and 87 (±29) mm Hg.
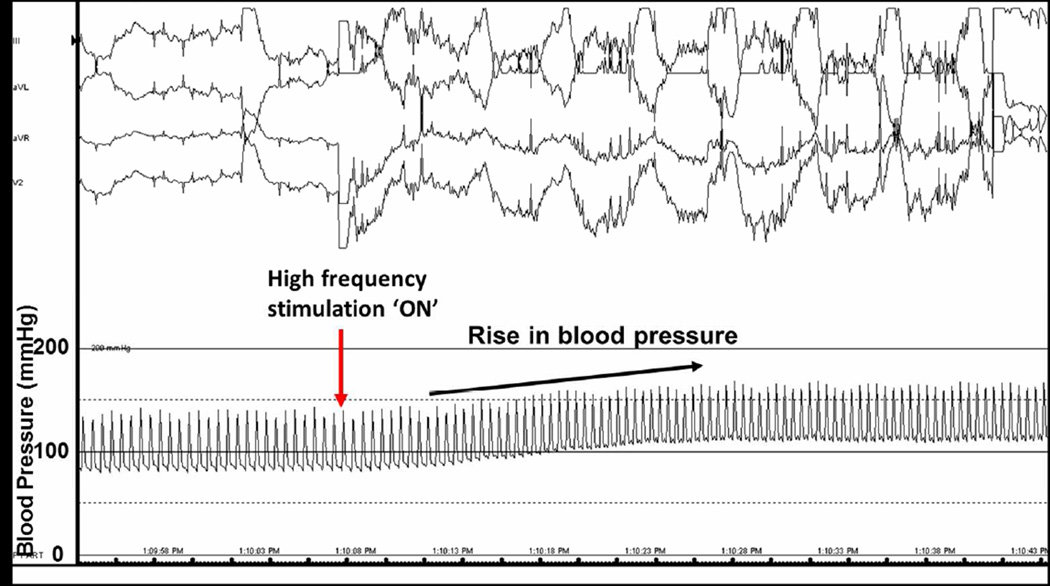
Figure 2. In vivo demonstration of acute increase in mean arterial blood pressure.
During high-frequency stimulation from a quadripolar catheter tip in a unilateral renal vein, a dramatic increase in blood pressure was seen (baseline of 139/80 to 163/104). The mean arterial blood pressure is notably sustainable during stimulation.
Gross pathologic examination showed significant renal hilar, renal capsular, and retroperitoneal hemorrhage in one dog. This likely resulted from perforation of the renal vein during catheterization, although injury as a result of HFS could not be ruled out.
Discussion
We present the first description of the hemodynamic effects of high frequency stimulation in the renal vein of anesthetized canine and baboon. We noted a rapid and modest rise in systolic and diastolic blood pressure that was sustained during stimulation with cessation of effect when stimulation was stopped. We propose that the potential mechanism of this hemodynamic response is stimulation of the renal sympathetic neurons at the renal hilum. This novel observation has potential applications in the treatment of hypotensive states associated with sympathetic withdrawal such as neurocardiogenic syncope.
Response to renal vein stimulation: Potential mechanism
Although the mechanism of the hemodynamic response to renal vein stimulation was not investigated in this preliminary study, the most likely mechanism is the stimulation of the renal sympathetic nerves. While elevation of blood pressure as a result of pain during HFS cannot be completely ruled out, it is unlikely since the animals were deeply anesthetized and signs of pain such as piloerection were not noted. Chinushi et al. reported an increase in blood pressure, serum norepinephrine and sympathetic indices of heart rate variability during renal artery stimulation using a quadripolar catheter in dogs.15 This effect was abolished following radiofrequency ablation. Stimulation from the renal vein at high output may also result in stimulation of the renal nerves due to close anatomical association of the vein, artery and neural components at the renal hilum.
Electrical stimulation of renal nerves in experimental animals has been shown to increase blood pressure. This effect may be mediated through renal afferent and/or efferent sympathetic fibers. Efferent sympathetic stimulation leads to decreased renal blood flow, renin release and sodium retention, leading to hypertension.9 Renal denervation has been shown to produce the opposite effects. Electrical stimulation of renal afferents produces a pressor response due to widespread activation of the sympathetic nervous system and norepinephrine mediated increase in vascular tone in several vascular beds including the mesentery and muscles.16–18 Further sustained afferent stimulation leads to a sustained increase in BP mediated by increased levels of vasopressin.17 In addition to these direct cardiovascular effects, afferent nerves also exert inhibitory reflex effects on the efferent input to the kidneys, the renorenal reflex, which lowers blood pressure.9, 19 Stimulation of the renal afferent sympathetic nerves in animals has been shown to predominantly exert a pressor response. In the absence of significant changes in heart rate, peripheral vasoconstriction is the most likely mechanism of the increase in BP seen in this study. Some animals, however, did not show a significant vasopressor response, although none had a decrease in blood pressure. This may be secondary to non-selective activation of afferent nerves that elicit inhibitory and excitatory effects with a variable net response.6, 9
Modulation of renal sympathetic innervation: Potential new applications
Evidence for the critical role of the renal sympathetic nerves in human hypertension comes from past studies of surgical sympathectomy and several recent trials of catheter based renal denervation for the treatment of resistant hypertension.14, 20–22 Renal denervation using radiofrequency ablation in the renal arteries reduced whole body norepinephrine spillover and muscle sympathetic nerve activity confirming the role of renal sympathetic nerves in elevating BP through central adrenergic effects.22 The recently concluded SIMPLICITY-HTN 3 trial however did not show significant decline in BP with catheter ablation compared to a sham-operated group.23 While this has brought into question both the role of catheter based renal denervation in the treatment of hypertension and the methods used to achieve it, the bulk of human and experimental evidence support an important role for renal sympathetic innervation in the regulation of blood pressure.
The findings of the current study show that sympathetic nerve stimulation increases blood pressure and that this can be safely and effectively performed from the renal vein. Potential applications include development of an implantable transvenous device in the renal vein to treat transient hypotensive states such as neurocardiogenic syncope, other reflex syncope, postural orthostatic tachycardia syndrome and postural hypotension related to autonomic neuropathy. It can be hypothesized that stimulation of renal nerves using an implantable electrode catheter in the renal vein in response to sudden bradycardia could be effective in preventing syncope by increasing peripheral sympathetic tone.
Limitations
While elevation of blood pressure as a result of pain during HFS cannot be completely ruled out, it is less likely since the animals were deeply anesthetized and signs of pain such as piloerection were not noted. The mechanism of blood pressure response to stimulation in the renal vein was not investigated and further mechanistic studies including the evaluation of cardiac hemodynamics, peripheral vascular resistance and peripheral autonomic nerve traffic during stimulation are required to confirm the proposed role of the renal sympathetic innervation.
Conclusions
We report for the first time, the feasibility, safety, and efficacy of stimulation of the renal vein as a means to activate the sympathetic nervous system to increase blood pressure in an acute setting. We hypothesize that transvenous stimulation of renal innervation can have potential application in the treatment of reflex syncopal states that have a significant vasodepressor component.
Acknowledgments
This study was funded in part by a grant from the American Heart Association, the NCRP Summer 2012 Clinical Research Program.
Footnotes
S. Asirvatham received honoraria/consulting fees from Abiomed, Atricure, Biotronik, Boston Scientific, Medtronic, Spectranetics, St. Jude, Sanofi-Aventis, Wolters Kluwer, and Elsevier, and is a co-patent holder and may receive future royalties from: Aegis (appendage ligation), ATP (atrial fibrillation ablation and coagulum reduction during ablation), Nevro (use of nerve signal modulation to treat central, autonomic, and peripheral nervous system disorders, including pain), Sanovas (lung ablation), Sorin Medical (tricuspid valve project). M. Madhavan and S. Asirvatham are both co-applicants on related patent applications, but have received no royalties at present. C. DeSimone has a pending patent – SJA/MM. S. Johnson, S. Suddendorf, and S. Mikell have an ownership stake in a patent related to this topic but have received no royalties.
Other authors: No disclosures.
References
- 1.Mendu ML, McAvay G, Lampert R, Stoehr J, Tinetti ME. Yield of diagnostic tests in evaluating syncopal episodes in older patients. Arch Intern Med. 2009;169:1299–1305. doi: 10.1001/archinternmed.2009.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun BC, Emond JA, Camargo CA., Jr Direct medical costs of syncope-related hospitalizations in the United States. Am J Cardiol. 2005;95:668–671. doi: 10.1016/j.amjcard.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 3.Chen-Scarabelli C, Scarabelli TM. Neurocardiogenic syncope. BMJ. 2004;329:336–341. doi: 10.1136/bmj.329.7461.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Connolly SJ, Sheldon R, Thorpe KE, Roberts RS, Ellenbogen KA, Wilkoff BL, Morillo C, Gent M. Pacemaker therapy for prevention of syncope in patients with recurrent severe vasovagal syncope: Second Vasovagal Pacemaker Study (VPS II): a randomized trial. JAMA. 2003;289:2224–2229. doi: 10.1001/jama.289.17.2224. [DOI] [PubMed] [Google Scholar]
- 5.Raviele A, Giada F, Menozzi C, Speca G, Orazi S, Gasparini G, Sutton R, Brignole M. A randomized, double-blind, placebo-controlled study of permanent cardiac pacing for the treatment of recurrent tilt-induced vasovagal syncope. The vasovagal syncope and pacing trial (SYNPACE) Eur Heart J. 2004;25:1741–1748. doi: 10.1016/j.ehj.2004.06.031. [DOI] [PubMed] [Google Scholar]
- 6.DiBona GF, Kopp UC. Neural control of renal function. Physiol Rev. 1997;77:75–197. doi: 10.1152/physrev.1997.77.1.75. [DOI] [PubMed] [Google Scholar]
- 7.Kopp U, Aurell M, Nilsson IM, Ablad B. The role of beta-1-adrenoceptors in the renin release response to graded renal sympathetic nerve stimulation. Pflugers Arch. 1980;387:107–113. doi: 10.1007/BF00584260. [DOI] [PubMed] [Google Scholar]
- 8.Kopp U, Bradley T, Hjemdahl P. Renal venous outflow and urinary excretion of norepinephrine, epinephrine, and dopamine during graded renal nerve stimulation. Am J Physiol. 1983;244:E52–E60. doi: 10.1152/ajpendo.1983.244.1.E52. [DOI] [PubMed] [Google Scholar]
- 9.Kopp UC. Neural Control of Renal Function. San Rafael (CA): Morgan & Claypool Life Sciences; 2011. [PubMed] [Google Scholar]
- 10.Calaresu FR, Stella A, Zanchetti A. Haemodynamic responses and renin release during stimulation of afferent renal nerves in the cat. J Physiol. 1976;255:687–700. doi: 10.1113/jphysiol.1976.sp011303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katholi RE, Whitlow PL, Hageman GR, Woods WT. Intrarenal adenosine produces hypertension by activating the sympathetic nervous system via the renal nerves in the dog. J Hypertens. 1984;2:349–359. [PubMed] [Google Scholar]
- 12.Katholi RE, Whitlow PL, Winternitz SR, Oparil S. Importance of the renal nerves in established two-kidney, one clip Goldblatt hypertension. Hypertension. 1982;4:166–174. [PubMed] [Google Scholar]
- 13.Smithwick RH, Thompson JE. Splanchnicectomy for essential hypertension; results in 1,266 cases. J Am Med Assoc. 1953;152:1501–1504. doi: 10.1001/jama.1953.03690160001001. [DOI] [PubMed] [Google Scholar]
- 14.Esler MD, Krum H, Schlaich M, Schmieder RE, Bohm M, Sobotka PA. Renal sympathetic denervation for treatment of drug-resistant hypertension: one-year results from the Symplicity HTN-2 randomized, controlled trial. Circulation. 2012;126:2976–2982. doi: 10.1161/CIRCULATIONAHA.112.130880. [DOI] [PubMed] [Google Scholar]
- 15.Chinushi M, Izumi D, Iijima K, Suzuki K, Furushima H, Saitoh O, Furuta Y, Aizawa Y, Iwafuchi M. Blood pressure and autonomic responses to electrical stimulation of the renal arterial nerves before and after ablation of the renal artery. Hypertension. 2013;61:450–456. doi: 10.1161/HYPERTENSIONAHA.111.00095. [DOI] [PubMed] [Google Scholar]
- 16.Calaresu FR, Stella A, Zanchetti A. Haemodynamic responses and renin release during stimulation of afferent renal nerves in the cat. The Journal of physiology. 1976;255:687–700. doi: 10.1113/jphysiol.1976.sp011303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caverson MM, Ciriello J. Effect of stimulation of afferent renal nerves on plasma levels of vasopressin. Am J Physiol. 1987;252:R801–R807. doi: 10.1152/ajpregu.1987.252.4.R801. [DOI] [PubMed] [Google Scholar]
- 18.Caverson MM, Ciriello J. Contribution of paraventricular nucleus to afferent renal nerve pressor response. Am J Physiol. 1988;254:R531–R543. doi: 10.1152/ajpregu.1988.254.3.R531. [DOI] [PubMed] [Google Scholar]
- 19.Saeki Y, Terui N, Kumada M. Physiological characterization of the renal-sympathetic reflex in rabbits. Jpn J Physiol. 1988;38:251–266. doi: 10.2170/jjphysiol.38.251. [DOI] [PubMed] [Google Scholar]
- 20.Catheter-based renal sympathetic denervation for resistant hypertension: durability of blood pressure reduction out to 24 months. Hypertension. 2011;57:911–917. doi: 10.1161/HYPERTENSIONAHA.110.163014. [DOI] [PubMed] [Google Scholar]
- 21.Krum H, Schlaich M, Whitbourn R, Sobotka PA, Sadowski J, Bartus K, Kapelak B, Walton A, Sievert H, Thambar S, Abraham WT, Esler M. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet. 2009;373:1275–1281. doi: 10.1016/S0140-6736(09)60566-3. [DOI] [PubMed] [Google Scholar]
- 22.Schlaich MP, Sobotka PA, Krum H, Lambert E, Esler MD. Renal sympathetic-nerve ablation for uncontrolled hypertension. N Engl J Med. 2009;361:932–934. doi: 10.1056/NEJMc0904179. [DOI] [PubMed] [Google Scholar]
- 23.Bhatt DL, Kandzari DE, O'Neill WW, D'Agostino R, Flack JM, Katzen BT, Leon MB, Liu M, Mauri L, Negoita M, Cohen SA, Oparil S, Rocha-Singh K, Townsend RR, Bakris GL. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370:1393–1401. doi: 10.1056/NEJMoa1402670. [DOI] [PubMed] [Google Scholar]