Abstract
A single intrathecal dose of adenosine 2A receptor (A2AR) agonist was previously reported to produce a multi-week reversal of allodynia in two different models of neuropathic pain in addition to downregulating glial activation markers in the spinal cord. We aimed to determine whether a single intrathecal administration of an A2AR agonist was able to attenuate motor symptoms induced by experimental autoimmune encephalopathy. Two A2AR agonists (CGS21680 and ATL313) significantly attenuated progression of motor symptoms following a single intrathecal administration at the onset of motor symptoms. OX-42, a marker of microglial activation, was significantly attenuated in the lumbar spinal cord following A2AR administration compared to vehicle. Therefore, A2AR agonists attenuate motor symptoms of EAE by acting on A2AR in the spinal cord.
Keywords: ATL313, CGS21680, myelin oligodendrocyte glycoprotein, multiple sclerosis
Introduction
It has been established that A2AR are located on peripheral immune cells (Hasko and Cronstein, 2004; Hasko et al., 2007) and a variety of cells within the central nervous system (CNS) including glial cells, endothelial cells and neurons (Dare et al., 2007). Adenosine 2A receptor (A2AR) agonists have attracted attention as potential modulators of glial function, based on their anti-inflammatory effects, such as reducing tumor-necrosis factor (TNF)-α production by peripheral immune cells (Dare et al., 2007; Hasko and Cronstein, 2004; Hasko et al., 2007) and by microglial cells (Loram et al., 2013).
It is of importance to note that A2AR agonists have anti-inflammatory effects in vivo when injected directly into the spinal cord in neuropathic pain models, suggesting direct activation of A2AR receptors within the CNS. In support of this, it has been demonstrated that spinal administration of A2AR agonists attenuates glial activation marker expression (for example OX-42) and pro-inflammatory cytokine production in the CNS of neuropathic pain models. Moreover, even a single intrathecal administration of an A2AR agonist reversed neuropathic pain for more than 4 weeks in two animal models, i.e. mechanical allodynia and thermal hyperalgesia (Loram et al., 2009; Loram et al., 2013). Interestingly, mice lacking A2AR showed increased chemokine production with subsequent greater immune cell infiltration into the spinal cord during experimental autoimmune encephalomyelitis (EAE), an inflammatory, demyelinating disease. As a consequence, more motor impairment was observed in these knock-out mice compared to wild type controls (Mills et al., 2012; Yao et al., 2012). In addition, decreases in adenosine A1R expression on microglial cells have been identified in multiple sclerosis patients (Johnston et al., 2001). Considering the importance of A2AR activation as a possible local anti-inflammatory target in the CNS during experimental autoimmune encephalitis (EAE), a model of multiple sclerosis, we questioned whether A2AR agonists improve EAE induced motor impairment via direct effects in the spinal cord?
Materials and Methods
Animals
Pathogen-free male Dark Agouti rats (225–250 g, Harlan Laboratories) were housed in a temperature-controlled environment (23 ± 2°C) with a 12 h light/dark cycle (lights on at 7:00 A.M.). All procedures occurred in the light phase. All rats were allowed one week of acclimation to the colony rooms before experimentation. The Institutional Animal Care and Use Committee of the University of Colorado at Boulder approved all procedures.
Drugs
The A2AR agonist 4-(3-(6-amino-9-(5-cyclopropylcarbamoyl-3,4-dihydroxytetrahydrofuran-2-yl)-9H-purin-2-yl)prop-2-ynyl)piperidine-1- carboxylic acid methyl ester (ATL313) was a gift from Dogwood Pharmaceuticals. The A2AR agonist 2-p-(2-carboxyethyl)phenethylamino-5’-N- ethylcarboxamido adenosine HCl (CGS21680) was purchased from Tocris Bioscience (Bristol, United Kingdom). All of the compounds were dissolved in 100% DMSO to create 10 mM stock concentrations and stored at −20°C. Fresh aliquots were diluted to the appropriate concentration in sterile endotoxin-free isotonic saline (Abbott Laboratories, Abbott Park, IL, USA).
Experimental Autoimmune Encephalitis induction
Relapsing-remitting experimental autoimmune encephalomyelitis (rr-EAE) was induced as previously described (Sloane et al., 2009). Recombinant peptide corresponding to the N-terminal sequence of rat myelin oligodendrocyte glycoprotein (MOG; amino acids 1–125) was expressed in Escherichia coli and purified to homogeneity by Ni-Chelate chromatography using a 6-His tag. The purified protein was dissolved in 6 M urea and dialyzed against 10 mM of sodium acetate buffer (NaAc; pH 3.0) then stored at −80 °C. rr-EAE was induced via injection of 8.75 µg MOG in 0.01 M NaAc (pH 3.0) emulsified in incomplete Freund’s adjuvant (IFA; Sigma, St. Louis, MO; in a 1:1 ratio of MOG (8.75 µg in 50 µl): IFA (50 µl)). Rats were briefly anaesthetized with isoflurane and given a single intra-dermal injection (100 µl total volume) into the dorsal skin just rostral to the base of the tail.
Intrathecal drug administration
At the time of onset of motor symptoms CGS21680 (10 pmol), ATL313 (1, 10 and 100 pmol) or vehicle was administered intrathecally (IT) under brief isoflurane anesthesia. The lumbar region was shaved and cleaned. An 18 Gauge guide needle, with the hub removed, was inserted into the L5/6 intervertebral space. A PE-10 catheter was inserted into the guide needle, premarked such that the distal end of the PE-10 tubing rested over the L4–L6 lumbar spinal cord. All drugs were administered over 20 s (1 µl of drug followed by 2 µl of sterile saline flush) with a 30 s delay before removing the catheter and guide needle. Each rat was anesthetized for a maximum of 5 min, and none incurred observable neurological damage from the procedure.
Motor score and body weight
Rats were monitored daily for body mass changes and motor symptoms. During periods of motor symptom impairment with a score greater than 3, the rats’ bladders were manually expressed to help control bladder infections. Motor deficits were scored on a scale from 0 to 7 based on the degree of ascending paralysis. The scores correspond to the following deficits: 0, no symptom expression; 1, partial tail paralysis; 2, full tail paralysis; 3, hindlimb weakness (unsteady gait while walking); 4, partial hindlimb paralysis (no weight bearing but observable movement of the limb); 5, full hindlimb paralysis; 6, partial forelimb paralysis; 7, euthanasia due to disease progression. Motor scores were recorded during the first few hours of the light cycle.
Immunohistochemistry
In the group of rats induced with rr-EAE that received either 10 pmol CGS21680 or vehicle, the animals were injected intraperitoneally with a terminal dose of sodium pentobarbital when their motor score reached 4.5 or higher or at the end of the experiment (24–26 d after onset of motor symptoms). Animals were transcardially perfused with ice-cold heparinized saline followed by 4% paraformaldehyde/0.1M phosphate buffered saline. 6 mm pieces of the L4 –L6 lumbar spinal cord were dissected and post-fixed overnight in 4% paraformaldehyde. The lumbar spinal cord was cryoprotected in 30% sucrose solution until processed for immunohistochemistry.
20 µm sections were mounted onto gelatin-subbed slides, and processed with immunohistochemistry with primary antibodies: monoclonal mouse anti-rat CD68 (1:200, AbD Serotec) or monoclonal mouse anti-rat OX-42 (1:100; BD Biosciences Pharmingen) and incubated with biotinylated secondary antibodies, (1:200; Jackson ImmunoResearch) as described previously (Loram et al., 2009; Sloane et al., 2009) The sections were incubated in avidin-biotin complex (ABC, 1:400; Vector Laboratories), washed, and reacted with 3,3’-diaminobenzadine tetrahydrochloride (DAB) (Sigma-Aldrich). Glucose oxidase and D-glucose were used followed by nickeleous ammonium sulfate to optimize the reaction product. Sections were dried, dehydrated and coverslipped. From each animal’s spinal cord, the left and the right dorsal horn of five to seven sections within the L4 –L6 region were captured at 10× magnification and included in the analysis using NIH ImageJ software. The signal pixels within the dorsal horn were identified above 3.5 SD beyond a control region (lateral column of the cord). The integrated densitometry was calculated as the number of pixels and the average gray scales above the set background. Immunohistochemistry procedures were conducted simultaneously on tissue from all groups for each detection antigen. Specificity of primary antibodies was assessed by omission of primary antibody during staining of selected slides from each treatment group. This omission resulted in a complete lack of signal (Sloane et al., 2009), data not shown.
Statistical analysis
Motor deficit scores were analyzed using a non-parametric Wilcoxon-Rank sum test. Body mass was evaluated using 2-way ANOVA. The mean immunohistochemical densitometry values for the left and right dorsal horn were averaged and analyzed by two-tailed t-test. The Kaplan-Meier survival curve was analyzed with log-rank test. Bonferroni post-hoc tests were used where appropriate and P < 0.05 was considered statistically significant.
Results
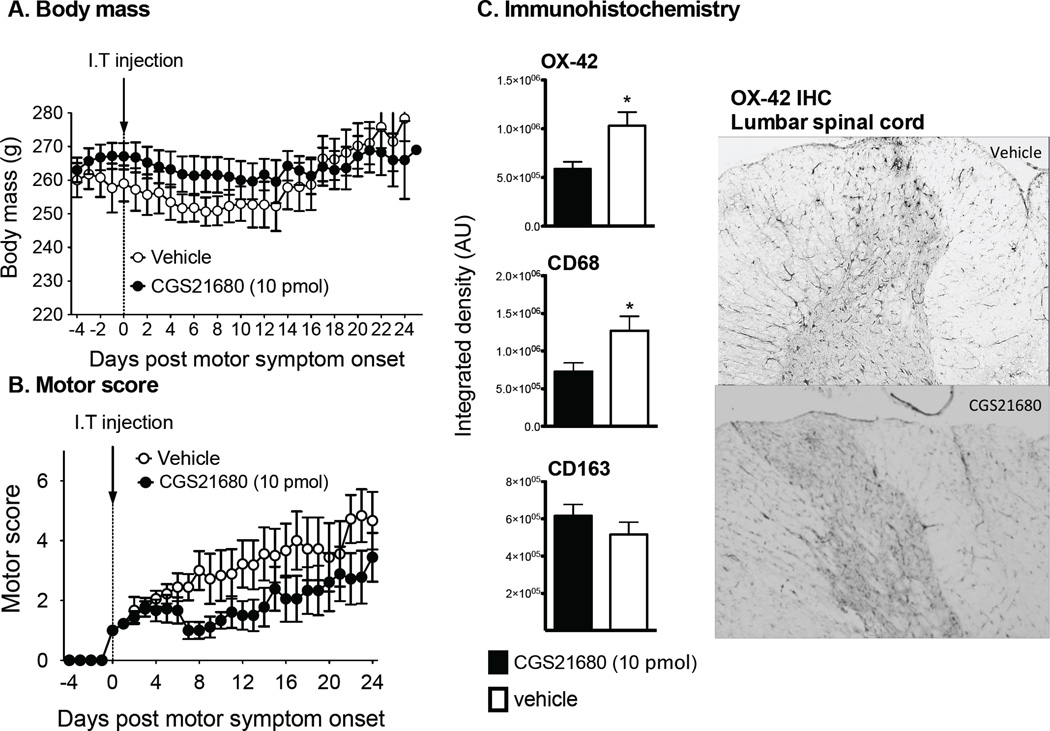
MOG peptide induced motor impairment within 7–12 d following inoculation. The rats receiving the single intrathecal (IT) vehicle injection demonstrated the relapsing remitting motor impairment (Fig. 1 and Fig. 2) as described previously (Ledeboer et al., 2003; Sloane et al., 2009). In the rats given a single IT injection of the A2AR agonist CGS21680, the body mass throughout the experiment was not significantly different over time (2-way-ANOVA, P=0.16), regardless of drug administered (Fig. 1A). There was a significant drug effect (2-way-ANOVA, P=0.007), but there was no significant interaction (2-way-ANOVA, P=0.99). Therefore, body mass was well maintained throughout the experiment with rats in the CGS21680 group having a higher body mass for the duration of the experiment. At the onset of motor symptoms, a single intrathecal dose of 10 pmol CGS21680 significantly improved rr- EAE-induced motor symptoms (Wilcoxon, P<0.0001) for the duration of the experiment (last test day was 24–26 d after intrathecal injection), see Fig 1B.
Figure 1.
A single intrathecal injection of CGS21680 at the time of motor symptom onset reduces EAE induced motor paralysis. Here, body mass (A) and motor scores (B) are displayed relative to the first day that motor deficits were observed (score of 1 or greater). Black circles represent the group receiving CGS21680 (n=9) with standard error bars. Open circles represent the group receiving intrathecal vehicle (n = 9). A significant reduction in the severity of motor paralysis was observed following CGS21680 therapy without a significant change in body mass over time. CGS21680 (10 pmol) administered in animals with EAE attenuates expression of the microglial activation marker (OX-42) and CD68 in lumbar L4 –L6 spinal cord sections of the dorsal horns (panel C). n = 11–12/group) for both OX-42, CD68 and CD163 immunohistochemistry. Representative images of OX-42 staining for the vehicle control and CGS21680 group are presented. Data are presented as mean ± SEM. *p < 0.05, between groups indicated.
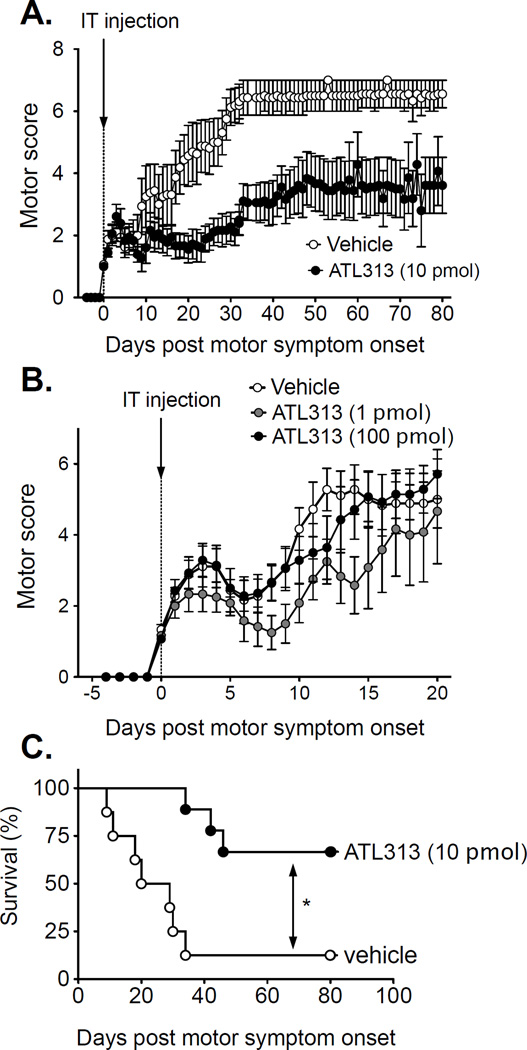
Figure 2.
A single intrathecal injection of ATL313 (1, 10 and 100 pmol), at the time of motor symptom onset, reduces EAE induced motor paralysis. Here, motor scores for 0 and 10 pmol ATL313 (A) and 0, 1 and 100 pmol ATL313 (B) are displayed relative to the first day that motor deficits were observed (score of 1 or greater). All data are presented as the mean and SEM. Open circles represent the group receiving intrathecal vehicle (n = 8/9). Black circles represent the group receiving ATL313 100 pmol (n=7) in panel A and C and ATL313 10 pmol (n=9) in panel B and D. Grey circles represent ATL313 1 pmol in panel B and D (n=6). A significant reduction in the severity of motor paralysis was observed following ATl313 at 1 and 10 pmol without a significant change in body mass over time. Kaplan-Meier survival curve of ATL313 10 pmol (black circles) compared to vehicle (open circles) in rats induced with EAE (C). A single intrathecal injection of ATL313 significantly extended the survival of the rats with EAE compared to vehicle (P<0.01) to beyond 80 days when the experiment was terminated.
ATL313 (1, 10 and 100 pmol), a more specific A2AR agonist, was injected IT at the onset of motor symptoms in the same way as detailed for CGS21680. Two groups were run such that 1 and 100 pmol was compared to a vehicle group and a second experiment was done comparing 10 pmol with vehicle. The body mass was significantly affected over time (2-way-ANOVA, P<0.0001), regardless of drug administered (data not shown). There was no significant difference in body mass with 10 or 100 pmol ATl313 compared to vehicle (P>0.05), but was significantly higher in the 1 pmol ATL313 group compared to vehicle (P<0.0001, data not shown).
There was a significant change in motor score over time with all groups in the classic relapsing remitting profile characteristic of the model. At the onset of motor symptoms, a single IT dose of 1 pmol ATL313 significantly improved rr-EAE-induced motor symptoms (Wilcoxon, P<0.0001, Fig. 2B), but 100 pmol ATL313 did not improve motor symptoms compared to vehicle (Wilcoxon, P=0.83, Fig. 2B). ATL313 at 10 pmol dose significantly reduced motor paralysis (Wilcoxon, P<0.0001, Fig. 2A) and dramatically extended survival to at least 80 days when the experiment was terminated (Log-rank, P<0.01, Fig. 2C).
Immunohistochemistry
The lumbar spinal cord was collected and processed for OX-42, CD68 and CD163 immunoreactivity, all markers of the monocyte lineage (Fig. 1C). The integrated pixel density of the mean of the combined left and right dorsal horns was evaluated and levels of OX-42 and CD68 markers were significantly lower in A2AR agonist-treated rats than those receiving vehicle (t-test, P=<0.05). CD163 in the combined left and right dorsal horns was not significantly different between groups (t-test, P=0.27).
Discussion
This series of experiments demonstrate that a single IT administration of an A2AR agonist at the time of motor symptom onset significantly attenuated the motor symptoms induced by rr-EAE. In addition, the A2AR agonist treatment decreased markers of activation in microglia and monocyte-lineage cells (CD68 and OX-42) in a region of the lumbar spinal cord affected by rr-EAE. Unlike CD68 and OX-42, CD163 was not different between those rats receiving A2AR agonist and the vehicle control. CD163 is a scavenger receptor used to identify alternatively activated macrophages and is often associated with increased expression of IL-10 known to be increased with A2AR agonism (Buechler et al., 2013; Loram et al., 2009). These experiments evaluated the efficacy of intrathecal A2AR agonism in a model of robust neuroinflammation, rr- EAE, and identified a long duration of effect in arresting progression of motor impairment from a single administration and significantly extended survival. Interestingly, there appears to present a U-shaped dose response function of ATL313 where higher doses are less effective than lower doses.
The role of adenosine in the severity and disease progression of EAE is complex. A bidirectional effect of adenosine receptor activation is identified in neuroinflammation and brain injury (Dai and Zhou, 2011). In some instances the lack of adenosine is neuroprotective, as seen in CD73 deficient mice. CD73 is a receptor that converts extracellular ADP or AMP to adenosine. CD73 −/− mice were protected from EAE (Mills et al., 2008) suggesting that the lack of extracellular adenosine synthesis protects the mice against EAE. In contrast, when CD4+ cells from CD73 −/− mice were adoptively transferred into mice lacking CD4+ cells, the EAE was exacerbated compared to adoptively transferring WT CD4+ cells (Mills et al., 2008). In addition, studies have demonstrated that both A1R and A2AR null mice had more severe EAE disease and motor impairment than wild type controls demonstrating a protective effect in neuroinflammation (Mills et al., 2012; Tsutsui et al., 2004; Yao et al., 2012). This bidirectional effect may result in the microenvironment of the macrophages, and e.g. microglia in EAE. Recent data demonstrated that pro-inflammatory macrophages may phenotypically switch to a regulatory macrophage, which is anti-inflammatory, in the presence of IL-10, converting them to regulatory macrophages. This phenotypic switch has been demonstrated following G-protein-coupled receptor activation, such as A2AR (Cohen et al., 2013). This switch can only occur with already classically activated macrophages potentially explaining why in non-inflamed environments, such as glutamate mediated excitatory tissue damage (Dai and Zhou, 2011), A2AR agonism is not protective, but in an already inflamed EAE model, as demonstrated here, the A2AR agonism is protective. In addition, most studies have evaluated the effect of adenosine and adenosine receptors at the time EAE induction and not once motor symptoms have presented. It is possible that adenosine receptors have different roles depending on the phase of the disease. For example, an adenosine antagonist, caffeine, given the day before MOG induction and every three days after induction, reduced EAE clinical scores, shown to be from reduced recruitment of CD4+ cells into the CNS (Mills et al., 2008). The data presented here are the first to demonstrate that in the EAE model, intrathecal administration of an A2AR agonist given at the time of motor symptom onset decreases the severity of these motor symptoms.
It is possible that the bidirectional effect of A2AR agonism also lies in the route of administration or dose administered. Selective and non-selective adenosine receptor antagonists are given systemically, the symptoms of EAE in wild type mice or rats are less severe (Chen et al., 2010; Mills et al., 2012; Mills et al., 2008; Wang et al., 2014; Wei et al., 2013). Moreover, ATL313 administered systemically into a murine sepsis model significantly improved survival and reduced plasma pro-inflammatory cytokines (Moore et al., 2008). Therefore, it appears that in inflammatory models, the antiinflammatory response is independent of route of administration of an A2AR agonist. In the same experiment a dose response function demonstrated that the lowest dose produced the most robust effect. Of interest to note, in the same experiment a dose response function was done in the murine sepsis model identified that the lowest dose tested (5 ug/kg) produced the most robust response (Moore et al., 2008). Therefore, it is possible that the higher doses are toxic or counterproductive in this tightly regulated system between adenosine and ATP, as demonstrated in the data presented here.
A2AR are found on most immune cells in both peripheral immune cells and immunocompetent cells within the CNS (Dare et al., 2007; Deaglio et al., 2007; Hasko and Cronstein, 2004; Zarek et al., 2008). EAE is induced through CD4+ cell activation and infiltration into the CNS. A2AR −/− mice induced with EAE had an increased CD4+ cell count in the spinal cord than wild type controls (Mills et al., 2012; Yao et al., 2012). This increased CD4+ cell infiltration may in part be from peripheral mechanisms of increased T-cell expansion in the spleen (Yao et al., 2012). Therefore, there is a role of A2AR agonism in immune cells in the periphery. However, intrathecal administration of an A2AR agonist, as done here, focuses on the role of A2AR within the spinal cord, demonstrating that A2AR agonists exert an anti-inflammatory effect within the spinal cord resulting in improved clinical scores. It is unlikely that such low doses and such small volumes administered that the effects noted in these experiments are from leakage of the drug out of the CNS and into the periphery. In addition, the reduction in markers of microglia and macrophages within the spinal cord suggests local effects of the A2AR administered IT. The reduced OX-42 immunoreactivity has been previously shown in neuropathic models 7 d after a single A2AR spinal administration (Loram et al., 2009). Therefore, our immunohistochemical observations support direct actions of the A2AR agonism on microglia and macrophages within the spinal cord.
The immunohistochemistry presented here support previous studies where macrophages in the spinal cord, subsequent to A2AR agonist administration, were less pro-inflammatory than vehicle controls. A2AR agonism in macrophages and microglia has been identified to induce a more anti-inflammatory phenotype with reduced TNFα and increased IL-10 production (Ferrante et al., 2013; Loram et al., 2013). Monocytes recruited to the site of inflammation differentiate when entering the tissues. The macrophage differentiation is dependent on the microenvironment to determine the phenotype of the macrophages. In A2AR −/− mice, there was an increased number of IBA-1+ and CD11b/F480+ cells in the spinal cord and brain compared to wild type controls when induced with EAE (Mills et al., 2012; Yao et al., 2012). The IBA-1 positive cells were more amoeboid than ramified morphology suggestive of activated microglia. The lack of effect on CD163 is not surprising given new evidence suggesting a regulatory phenotype where IL-10 and arginase are increased, but the alternatively activated markers, such as CD163 are not affected (Mosser and Edwards, 2008). Studies investigating the effect of A2AR agonism on arginase in spinal microglia are worthy of further investigation.
The extended survival in the EAE rats receiving a single administration of the A2AR agonist may result from a reduction in infiltrating immune cells into spinal cord because of reduced inflammation and lesions (Ajami et al., 2011; Loram et al., 2009). In the A2AR −/− mice, the acute presentation of EAE was less severe than WT controls. However, after 10 d the disease presentation was comparable (Mills et al., 2012). The same reduction in macrophage activation markers is seen in the spinal cord of EAE rats subsequent to IL-10 gene therapy administered intrathecally in EAE rats (Sloane et al., 2009). It is not known whether the microglial markers would look comparable to that of the earlier time points, as these tissues were not evaluated. But, it is not unreasonable to assume that given the unusually long survival of these animals that the immunohistochemistry of the microglia would not demonstrate as much activation than that of the vehicle control animals.
We have previously demonstrated that a single intrathecal administration of an A2AR agonist significantly decreased neuropathic allodynia in multiple models of neuropathic pain on rats (Loram et al., 2009). Neuroinflammation contributing to neuropathic pain is mild compared to aggressive neuroinflammation in autoimmune models of MS. Still, a single intrathecal delivery of A2AR agonists is effective in reducing motor symptoms associated with reduced neuroinflammation i.e. microglial activation via direct spinal effects.
Research highlight.
A single intrathecal A2AR agonist attenuated motor symptoms induced by experimental autoimmune encephalopathy.
Acknowledgments
Financial support for these studies was provided by Dogwood Pharmaceuticals, LLC, and by NIH grants DA02422 and RC1-NS067807 and Department of Defense grant DM102108.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ajami B, Bennett JL, Krieger C, McNagny KM, Rossi FM. Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nat Neurosci. 2011;14:1142–1149. doi: 10.1038/nn.2887. [DOI] [PubMed] [Google Scholar]
- Buechler C, Eisinger K, Krautbauer S. Diagnostic and prognostic potential of the macrophage specific receptor CD163 in inflammatory diseases. Inflammation & allergy drug targets. 2013;12:391–402. doi: 10.2174/18715281113126660060. [DOI] [PubMed] [Google Scholar]
- Chen GQ, Chen YY, Wang XS, Wu SZ, Yang HM, Xu HQ, He JC, Wang XT, Chen JF, Zheng RY. Chronic caffeine treatment attenuates experimental autoimmune encephalomyelitis induced by guinea pig spinal cord homogenates in Wistar rats. Brain Res. 2010;1309:116–125. doi: 10.1016/j.brainres.2009.10.054. [DOI] [PubMed] [Google Scholar]
- Cohen HB, Briggs KT, Marino JP, Ravid K, Robson SC, Mosser DM. TLR stimulation initiates a CD39-based autoregulatory mechanism that limits macrophage inflammatory responses. Blood. 2013;122:1935–1945. doi: 10.1182/blood-2013-04-496216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai SS, Zhou YG. Adenosine 2A receptor: a crucial neuromodulator with bidirectional effect in neuroinflammation and brain injury. Reviews in the neurosciences. 2011;22:231–239. doi: 10.1515/RNS.2011.020. [DOI] [PubMed] [Google Scholar]
- Dare E, Schulte G, Karovic O, Hammarberg C, Fredholm BB. Modulation of glial cell functions by adenosine receptors. Physiology & behavior. 2007;92:15–20. doi: 10.1016/j.physbeh.2007.05.031. [DOI] [PubMed] [Google Scholar]
- Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen JF, Enjyoji K, Linden J, Oukka M, Kuchroo VK, Strom TB, Robson SC. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. The Journal of experimental medicine. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrante CJ, Pinhal-Enfield G, Elson G, Cronstein BN, Hasko G, Outram S, Leibovich SJ. The adenosine-dependent angiogenic switch of macrophages to an M2-like phenotype is independent of interleukin-4 receptor alpha (IL-4Ralpha) signaling. Inflammation. 2013;36:921–931. doi: 10.1007/s10753-013-9621-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasko G, Cronstein BN. Adenosine: an endogenous regulator of innate immunity. Trends Immunol. 2004;25:33–39. doi: 10.1016/j.it.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Hasko G, Pacher P, Deitch EA, Vizi ES. Shaping of monocyte and macrophage function by adenosine receptors. Pharmacol Ther. 2007;113:264–275. doi: 10.1016/j.pharmthera.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston JB, Silva C, Gonzalez G, Holden J, Warren KG, Metz LM, Power C. Diminished adenosine A1 receptor expression on macrophages in brain and blood of patients with multiple sclerosis. Annals of neurology. 2001;49:650–658. [PubMed] [Google Scholar]
- Ledeboer A, Wierinckx A, Bol JG, Floris S, Renardel de Lavalette C, De Vries HE, van den Berg TK, Dijkstra CD, Tilders FJ, van dam AM. Regional and temporal expression patterns of interleukin-10, interleukin-10 receptor and adhesion molecules in the rat spinal cord during chronic relapsing EAE. J Neuroimmunol. 2003;136:94–103. doi: 10.1016/s0165-5728(03)00031-6. [DOI] [PubMed] [Google Scholar]
- Loram LC, Harrison JA, Sloane EM, Hutchinson MR, Sholar P, Taylor FR, Berkelhammer D, Coats BD, Poole S, Milligan ED, Maier SF, Rieger J, Watkins LR. Enduring reversal of neuropathic pain by a single intrathecal injection of adenosine 2A receptor agonists: a novel therapy for neuropathic pain. J Neurosci. 2009;29:14015–14025. doi: 10.1523/JNEUROSCI.3447-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loram LC, Taylor FR, Strand KA, Harrison JA, Rzasalynn R, Sholar P, Rieger J, Maier SF, Watkins LR. Intrathecal injection of adenosine 2A receptor agonists reversed neuropathic allodynia through protein kinase (PK)A/PKC signaling. Brain Behav Immun. 2013;33:112–122. doi: 10.1016/j.bbi.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills JH, Kim DG, Krenz A, Chen JF, Bynoe MS. A2A adenosine receptor signaling in lymphocytes and the central nervous system regulates inflammation during experimental autoimmune encephalomyelitis. J Immunol. 2012;188:5713–5722. doi: 10.4049/jimmunol.1200545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills JH, Thompson LF, Mueller C, Waickman AT, Jalkanen S, Niemela J, Airas L, Bynoe MS. CD73 is required for efficient entry of lymphocytes into the central nervous system during experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2008;105:9325–9330. doi: 10.1073/pnas.0711175105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CC, Martin EN, Lee GH, Obrig T, Linden J, Scheld WM. An A2A adenosine receptor agonist, ATL313, reduces inflammation and improves survival in murine sepsis models. BMC infectious diseases. 2008;8:141. doi: 10.1186/1471-2334-8-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nature reviews. Immunology. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloane E, Ledeboer A, Seibert W, Coats B, van Strien M, Maier SF, Johnson KW, Chavez R, Watkins LR, Leinwand L, Milligan ED, Van Dam AM. Anti-inflammatory cytokine gene therapy decreases sensory and motor dysfunction in experimental Multiple Sclerosis: MOG-EAE behavioral and anatomical symptom treatment with cytokine gene therapy. Brain Behav Immun. 2009;23:92–100. doi: 10.1016/j.bbi.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsui S, Schnermann J, Noorbakhsh F, Henry S, Yong VW, Winston BW, Warren K, Power C. A1 adenosine receptor upregulation and activation attenuates neuroinflammation and demyelination in a model of multiple sclerosis. J Neurosci. 2004;24:1521–1529. doi: 10.1523/JNEUROSCI.4271-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Xi NN, Chen Y, Shang XF, Hu Q, Chen JF, Zheng RY. Chronic caffeine treatment protects against experimental autoimmune encephalomyelitis in mice: Therapeutic window and receptor subtype mechanism. Neuropharmacology. 2014;86C:203–211. doi: 10.1016/j.neuropharm.2014.06.029. [DOI] [PubMed] [Google Scholar]
- Wei W, Du C, Lv J, Zhao G, Li Z, Wu Z, Hasko G, Xie X. Blocking A2B adenosine receptor alleviates pathogenesis of experimental autoimmune encephalomyelitis via inhibition of IL-6 production and Th17 differentiation. J Immunol. 2013;190:138–146. doi: 10.4049/jimmunol.1103721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao SQ, Li ZZ, Huang QY, Li F, Wang ZW, Augusto E, He JC, Wang XT, Chen JF, Zheng RY. Genetic inactivation of the adenosine A(2A) receptor exacerbates brain damage in mice with experimental autoimmune encephalomyelitis. J Neurochem. 2012;123:100–112. doi: 10.1111/j.1471-4159.2012.07807.x. [DOI] [PubMed] [Google Scholar]
- Zarek PE, Huang CT, Lutz ER, Kowalski J, Horton MR, Linden J, Drake CG, Powell JD. A2A receptor signaling promotes peripheral tolerance by inducing T-cell anergy and the generation of adaptive regulatory T cells. Blood. 2008;111:251–259. doi: 10.1182/blood-2007-03-081646. [DOI] [PMC free article] [PubMed] [Google Scholar]