Abstract
The purpose of this study was to investigate the effect of whole body vibration (WBV) training on maximal strength, squat jump, and flexibility of well-trained combat athletes. Twelve female and 8 male combat athletes (age: 22.8 ± 3.1 years, mass: 65.4 ± 10.7 kg, height: 168.8 ± 8.8 cm, training experience: 11.6 ± 4.7 years, training volume: 9.3 ± 2.8 hours/week) participated in this study. The study consisted of three sessions separated by 48 hours. The first session was conducted for familiarization. In the subsequent two sessions, participants performed WBV or sham intervention in a randomized, balanced order. During WBV intervention, four isometric exercises were performed (26 Hz, 4 mm). During the sham intervention, participants performed the same WBV intervention without vibration treatment (0 Hz, 0 mm). Hand grip, squat jump, trunk flexion, and isometric leg strength tests were performed after each intervention. The results of a two-factor (pre-post[2] × intervention[2]) repeated measures ANOVA revealed a significant interaction (p = 0.018) of pre-post × intervention only for the hand grip test, indicating a significant performance increase of moderate effect (net increase of 2.48%, d = 0.61) after WBV intervention. Squat jump, trunk flexion, and isometric leg strength performances were not affected by WBV. In conclusion, the WBV protocol used in this study potentiated hand grip performance, but did not enhance squat jump, trunk flexion, or isometric leg strength in well-trained combat athletes.
Keywords: trained athlete, ergogenic aid, grip strength, lower body strength, vertical jump
INTRODUCTION
Whole body vibration (WBV) is a neuromuscular training modality to enhance explosive strength, maximal strength, and flexibility [1]. In recent years, WBV has become a popular scientific research field. Numerous studies have been conducted to investigate its effects on biomotor abilities as well as its possible physiological mechanisms. In employing WBV, vibration (mechanical stimulus with an oscillatory motion character) is applied to the whole body via a vibrating platform. The WBV-initiated muscular activation level depends on the vibratory load, which involves four basic variables: frequency, amplitude, acceleration (also defined as magnitude which is the product of angular velocity and amplitude), and duration [2–4]. However, musculoskeletal stiffness and damping of vibrations by tissues and body fluids should also be considered as two other factors affecting the amount of vibration transmitted to body parts [5].
Although there are several recent WBV studies conducted on well-trained/elite athletes [6–9], generally, untrained or recreationally trained individuals constitute the sample for the majority of WBV-related studies, making the generalizability of these study results to well-trained athletes impossible [3]. Well-trained athletes are believed to have no margin for further performance improvement, due to already having highly developed muscular characteristics, such as high levels of reflex sensitivity, fast-twitch fibre recruitment, motor neuron excitability, and stiffer muscle-tendon units [10, 11]. This could be interpreted as well-trained athletes being close to their genetic potentials. Notwithstanding, WBV was shown to successfully enhance performance in elite athletes from Eastern bloc countries long ago. However, most of these results were dependent on observational data from coaches. Therefore, to validate these results, highly controlled scientific studies should be conducted on well-trained athletes, since the sports science literature needs to be enriched with such studies. Without sufficient evidence-based research results, prescribing training programmes with the purpose of enhancing specific biomotor abilities of well-trained athletes is difficult [3].
A limited number of acute WBV studies have been performed on well-trained/elite athletes compared to recreationally trained or untrained individuals. Briefly, some studies conducted on well-trained/elite athletes showed an acute performance-enhancing effect of WBV on vertical jump height and mechanical power [8, 12, 13], flexibility [6, 8, 9, 12], and muscular activity [14]. In contrast, some studies revealed either no change or a significant performance reduction in vertical jump, power performance [7, 9, 11], and flexibility [7].
Although studies on well-trained athletes are available in the literature, further studies are needed to determine optimal WBV protocol(s) and variable combinations for elite/well-trained athletes to enhance performance [3]. All sports have their own specific requirements, such as training intensity, training frequency, training volume, diet, environmental conditions, dominant energy system, psychological factors, body dimensions, and predominantly used muscles. Therefore, due to the differences just mentioned, all sports differ from one another to some extent in terms of responses to an identical training stimulus. No specific study in the literature has investigated whether WBV enhances performance in well-trained combat athletes accustomed to performing high-intensity explosive strength, muscular endurance (two extremes of the strength continuum), flexibility, and technical training in combination over a long time period. Accordingly, the purpose of this study was to investigate the acute effect of commonly used low amplitude-low frequency vertical WBV on basic performance determinants in combat sports — maximal strength, vertical jump, and flexibility [15] — in well-trained combat athletes.
Some studies have shown that different isometric exercise series increased several performance measures of well-trained athletes [8, 9, 12]. Therefore, the hypothesis of the present study was that an acute intervention, including four one-minute isometric exercises performed during vertical WBV at 26 Hz, 4 mm, would enhance the squat jump, trunk flexibility, isometric hand grip strength, and isometric leg strength in well-trained combat athletes.
MATERIALS AND METHODS
Participants
Eight male and 12 female well-trained combat athletes (age: 22.8 ± 3.1 years, mass: 65.4 ± 10.7 kg, height: 168.8 ± 8.8 cm, training experience: 11.6 ± 4.7 years, training volume: 9.3 ± 2.8 hours · week−1) competing in judo, karate, tae kwon do, and Muay Thai, volunteered to participate in this study. None of the participants had previous experience with WBV.
Participants had no health problems that would prohibit participation (diabetes, epilepsy, metabolic or neuromuscular diseases, or prostheses). They were required to refrain from vigorous physical activity, consumption of alcohol, any food or drinks containing caffeine or any other types of stimulants at least 24 hours prior to the testing session. Procedures, purpose, and risks of the study were explained in detail to all participants, and they signed an informed written consent form. Approval was granted from the Medical Ethics Committee of the Medical Faculty of Trakya University (protocol number: TÜTF-GOKAEK 2013/39) in accordance with the Declaration of Helsinki.
Procedures
The study consisted of three sessions separated by 48 hours. The first session was conducted to familiarize participants with performance tests, exercises, and the vibration application that would be performed in subsequent sessions. The next two sessions assessed the effects of WBV on selected performance variables. All tests were performed by the same researcher.
Participants were assigned to receive WBV or a sham intervention (Sham) in a randomized, balanced order. Thus, all participants had undertaken each intervention at the end of the study. WBV and Sham were performed at the same time of the day (13:00–15:00) to avoid any effect of circadian rhythms. In WBV, participants received vibration treatment during an isometric exercise protocol including four different exercises (Figure 1) on a vibration platform (Power Plate® Next Generation PRO 5, 2x1A, 2010, USA). In Sham, participants used the vibration platform in the same manner as WBV, except that they received no vibration treatment (0 Hz, 0 mm). Pre-tests were conducted to assess baseline values for hand grip (HG), squat jump (SJ), trunk flexion (TF), and isometric leg strength (ILS) tests. Combat athletes train and compete barefoot; accordingly, the main concern of the present study was assessment of possible performance enhancement using no footwear. Therefore, all performance tests were performed barefoot. Similarly, participants used no footwear during the WBV intervention to avoid any dampening effect from inter-individual differences in footwear. In addition, footwear would have lessened the acceleration of WBV transmitted to the muscles; thus, it would be counterproductive for generating a sufficient training stimulus to enhance test performance in well-trained athletes.
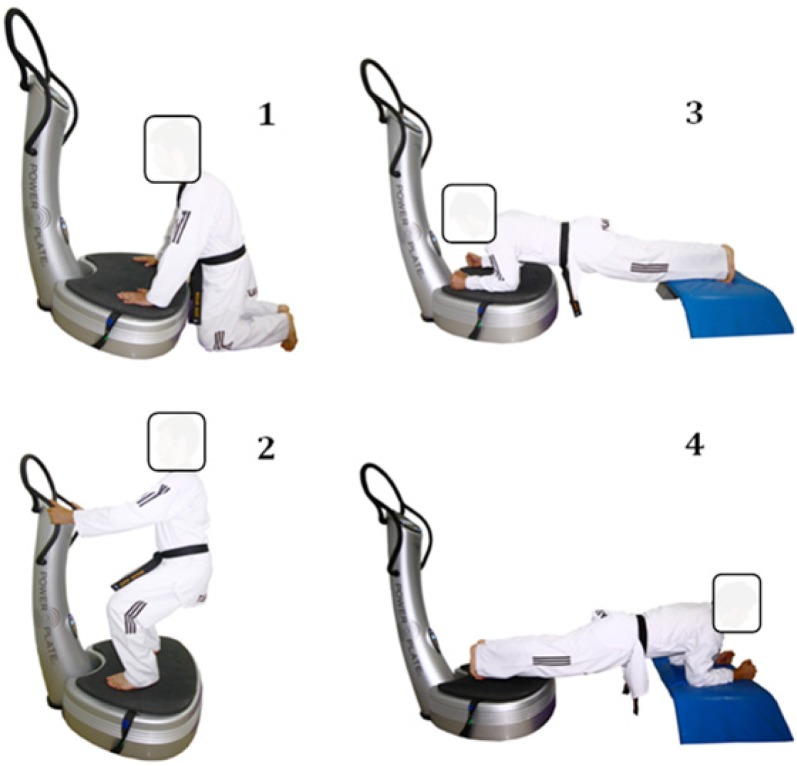
FIG. 1.
Isometric exercises used in the study
Performance assessment procedures for dependent variables
As a warm-up, participants completed six minutes on a cycle ergometer (834 E, Monark, Vansbro, Sweden) at 60–80 rpm with a resistance of 50 W. Performance assessment procedures for dependent variables were started 8.5 minutes after the completion of the stationary cycling period. HG, SJ, TF, and ILS tests were performed in the control test series. Participants performed two maximal trials in each test separated by 30 seconds (one minute for the ILS test), and the highest values were used in the statistical analyses. During isometric strength test trials, when a significant force decrease was detected on the dynamometer, participants were informed to stop exerting force. The highest value obtained in the isometric strength tests were multiplied by 9.81 m · s−2 (gravitational constant) to convert kilograms into Newtons to quantify peak force. Relative isometric strength values were calculated by normalizing absolute values using allometric scaling [16].
Hand grip strength test: Grip strength of the dominant hand was measured using a standard adjustable digital handgrip dynamometer (Takei Physical Fitness Test, TKK 5101, made in PRC) in a standing position with the shoulder adducted and neutrally rotated and the elbow in full extension. The dynamometer was held freely without support, not touching the participant's trunk.
Squat jump test: Participants performed two SJs starting with a knee joint angle of 90° that was measured with a Plastic Goniometer (Lafayette Instrument Europe, Richardson Products, INC., Sammons Preston J00240, 12-inch). During the SJs, participants positioned their hands on their waists. SJ height was assessed with a Takei jump meter (Takei Physical Fitness Test, TKK 5106, made in PRC).
Trunk flexion test: The Standing Trunk Flexion Meter (Takei Physical Fitness Test, TKK 5103, made in PRC) was used to assess trunk flexion. During the test, participants held one hand exactly on the other one and flexed their trunk in a controlled manner (no rapid movement). Maximum distance reached was held for two seconds.
Isometric leg strength test: ILS was measured with an electronic dynamometer (Takei Physical Fitness Test, TKK 5102, made in PRC). During the ILS, participants stood on the platform of the dynamometer with an erect body, and their knee joint angle was set at ∼130°–140° using a plastic goniometer. The dynamometer was adjusted (sensitivity of 0.02 m) by attaching the suitable chain ring to the dynamometer for each participant separately, keeping the knee joint angle within the proper range. The individualized length of the chain used in the testing process was recorded (by determining the sequence number of the used chain ring) to ensure that each participant was tested using their pre-determined chain length in repeated measures (intra-day and inter-day). Self-selected foot position was marked on the platform to avoid any possible intra-individual differences in body posture during trials. Participants were instructed to exert maximal effort, starting with a progressively increasing manner to extend their knees. Participants were strictly warned not to use any upper body muscle during their effort.
WBV intervention
Participants rested passively for 20 minutes after the completion of the control tests. Afterwards, they performed the same cycling protocol used in the control tests. Two minutes after the cycling protocol, a WBV exercise series consisting of the following four one-minute isometric exercises targeting forearm, leg, and trunk muscles was performed: (1) kneeling on the ground with arms straight and hands placed on the platform, (2) squatting at a 90° knee joint angle, keeping the torso perpendicular to the floor, (3) prone bridge forward, and (4) prone bridge backward (Figure 1); the exercises were performed at a frequency of 26 Hz and amplitude of 4 mm and were separated by 30-second passive rest intervals. This frequency and amplitude combination is considered a safe and effective exercise intervention [12]. Post-tests were performed in the same order one minute after completing the last isometric exercise.
Sham intervention
Participants performed the same WBV intervention (mentioned above), except that they received no vibration treatment (0 Hz, 0 mm) during the isometric exercise protocol.
The order between performed interventions and control tests was allocated in a randomized, balanced design to avoid any possible biased study results due to an order effect.
Statistical analysis
Data were analysed using the IBM SPSS Statistics for Windows version 20 software (IBM Corp., 2011, Armonk, NY). A Shapiro-Wilk test was performed, and histograms with a normal curve were checked to test for normality. If the data were not normally distributed, skewness and kurtosis were taken into account to determine the level of non-normality. Since a violation of normality assumption was detected, natural logarithmic (log) transformation was performed on performance measures to normalize the data. Afterwards, an analysis of the normality assumption for log-transformed data was repeated using the same methods. As all skewness and kurtosis values of related variables fell between the range of –1 and 1, and no statistical significance was found in Shapiro-Wilk tests, parametric statistics were performed using log-transformed data. Data of male and female participants were pooled prior to statistical analyses since the homogeneity of variance assumption was met, and no statistically significant difference was found in investigated variables between genders.
Four separate 2 × 2 (pre-post × intervention) two-factor within-subject design analyses of variance (ANOVA) were used to investigate the main effects of pre-post and intervention (Sham and WBV), as well as interaction effect of pre-post and intervention. Afterwards, four separate one-factor within-subject design ANOVAs with post-hoc LSD tests were conducted to identify the effects of WBV and Sham on performance measures, regardless of whether there was a significant interaction between pre-post and intervention. This was done to determine the effect sizes of differences in the dependent variables. The statistical significance level was set at p ≤ 0.05 for all analyses. Intra-class correlation coefficients (ICC), 95% confidence intervals (95% CIs; computed using two-factor mixed-effects single-measure reliability, absolute agreement), coefficient of variation (CV), and standard error of the measurement (SEM) were investigated to estimate test-retest reliability of control test measures.
RESULTS
There was high test-retest relative reliability for control test measures of HG, SJ, TF, and ILS (0.99 [0.97-1.00], 0.90 [0.75-0.96], 0.96 [0.90-0.98], and 0.91 [0.78-0.96], respectively, p < 0.001). These high intra-class ICC were also supported by low SEM values (12.2 for HG, 4.63 for SJ, 1.20 for TF, and 95.6 for ILS) and low CV values (3.1% for HG, 8.6% for SJ, 7.4% for TF, and 8.7% for ILS).
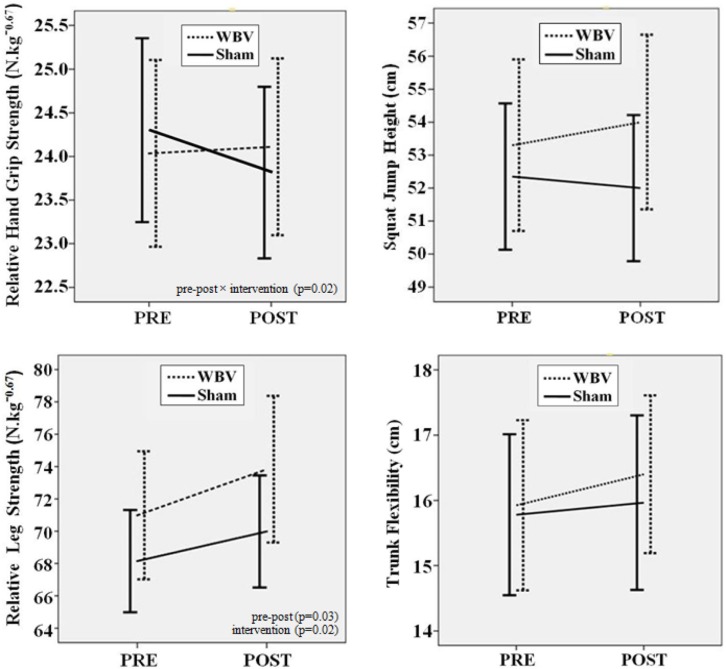
No significant main effect for “pre-post” was detected in HG, SJ, or TF tests (p = 0.871, p = 0.232, and p = 0.308, respectively). In contrast, a significant main effect was found for ILS (p = 0.029). No significant main effect for “intervention” was found in HG, SJ, or TF tests (p = 0.281, p = 0.773, and p = 0.771, respectively). In contrast, a significant main effect was found for ILS (p = 0.018). A significant interaction effect was found between “pre-post” and “intervention” for the HG test, indicating that while Sham decreased performance by 2.06% (absolute decrease of 0.496 N · kg−0.67), WBV increased HG performance by 0.42% (absolute increase of 0.083 N · kg−0.67), resulting in a net increase of 2.48% (p = 0.018). In contrast, no significant interaction effect was detected for SJ, TF, or ILS tests (p = 0.492, p = 0.464, and p = 0.661, respectively). In addition, one-factor ANOVA also revealed no significant difference for these tests (p = 0.483, p = 0.159, and p = 0.743, respectively). Simple, main, and interaction effects are provided in more detail in Figure 2 and Table 1.
FIG. 2.
Effects of whole body vibration (WBV) and sham intervention on performance measures
TABLE 1.
Comparison of changes in performance measures between interventions
| ∆ (Post-Pre) | ∆WBV—∆Sham | †p | †ES [95% CIs] | †1–β | |||
|---|---|---|---|---|---|---|---|
| HG (N · kg −0.67 ) | Post-WBV Pre-WBV |
24.1 ± 4.6 24.0 ± 4.8 |
0.083 ± 1.057 | 0.579 ± 0.984 | 0.018* | 0.61 [0.35–0.86] | 0.735 |
| Post-Sham Pre-Sham |
23.8 ± 4.4 24.3 ± 4.7 |
-0.496 ± 0.891 | |||||
| SJ (cm) | Post-WBV Pre-WBV |
54.0 ± 11.8 53.3 ± 11.6 |
0.700 ± 3.181 | 1.05 ± 6.60 | 0.483* | 0.26 [– 0.11–0.63] | 0.197 |
| Post-Sham Pre-Sham |
52.0 ± 9.9 52.4 ± 9.9 |
-0.350 ± 5.071 | |||||
| TF (cm) | Post-WBV Pre-WBV |
16.4 ± 5.4 15.9 ± 5.8 |
0.475 ± 1.155 | 0.290 ± 1.728 | 0.159* | 0.55 [– 0.16–1.27] | 0.646 |
| Post-Sham Pre-Sham |
16.0 ± 6.0 15.8 ± 5.5 |
0.185 ± 1.091 | |||||
| ILS (N · kg −0.67 ) | Post-WBV Pre-WBV |
73.8 ± 20.3 71.0 ± 17.7 |
2.85 ± 7.20 | 1.02 ± 9.29 | 0.743* | 0.11 [– 0.22–0.44] | 0.075 |
| Post-Sham Pre-Sham |
70.1 ± 15.5 68.2 ± 14.2 |
1.84 ± 7.20 |
Note: Descriptive statistics are shown as mean ± standard deviation of raw data; †Calculated based on natural log-transformed data
p < 0.05
∆ = absolute change; CIs = confidence intervals; ES = effect size (Cohen's d,<0.2 = trivial, 0.2 ≤ d ≤ 0.5 = small, 0.5 ≤d ≤0.8 = medium, > 0.8 = large effect; 1–β = statistical power; HG = hand grip; ILS = isometric leg strength; SJ = squat jump; TF = trunk flexion; WBV = whole body vibration
DISCUSSION
This study enriches the literature concerning the effects of acute WBV on physical performance variables of well-trained athletes; moreover, to our knowledge, this is the first study investigating the acute effects of WBV on maximal isometric leg strength. The main findings showed that acute WBV had a significant performance-enhancing effect on HG strength in well-trained combat athletes, while having no statistically significant effect on SJ, TF, or ILS.
There is no consensus in the literature on WBV variable combinations (frequency, amplitude, rest intervals, exposure time, repetition number, contraction type, body posture, using additional loads, or only body weight) that optimize performance improvements [3, 17, 18]. Some investigators have proposed that frequency and amplitude should be defined as separate biomotor abilities [19]. In addition, the timing of post-vibration testing is a crucial factor in assessing acute WBV effects as they are time-sensitive [20, 21]. As no standardization of WBV variables was present in the acute study protocols, variations in any of the above variables in different studies make comparing and interpreting results challenging and impractical [4].
The counter movement jump (CMJ) has been used in many studies investigating the effects of WBV on explosive strength and jumping performance [7–9, 12, 22]. Adams et al. [23] reported that the CMJ potentially reflects the integrative responses of contractile, neural, and elastic elements that could be affected acutely by WBV. Similarly, Cochrane and Stannard [12] used the CMJ with arm swing to assess the acute effects of WBV on jump height. They stated that the CMJ is commonly used in various sports. Furthermore, they concluded that detecting possible effects of WBV on jump height using the CMJ is more reasonable; this is because WBV is more likely to affect this type of jump, which involves a stretch-shortening cycle and activation of stretch receptors (spinal reflexes) in the eccentric phase. Various lower body movements in combat sports include stretch-shortening cycles as in the CMJ. In this context, assessing explosive strength using the CMJ seems to better match combat athletes than the SJ. However, the CMJ also includes various parameters that could cause possible intra-individual differences in jump height, such as arm swing (if used), squat depth at the end of the eccentric phase, muscle lengths at this depth, elongation speed of muscles during the eccentric phase, joint angles (hip, knee, and ankle joint), contributions of the elastic components, and reflex mechanisms, all of which are interrelated. Possible intra-individual differences in these parameters could lead to biased results in comparing pre- and post-test data unless these parameters are controlled. Investigating effects of WBV on vertical jump performance using the CMJ makes it extremely difficult to assess whether obtained study results are due to WBV or any possible intra-individual differences in the above-mentioned parameters. Therefore, the present study used only the SJ, which was also used by Despina et al. [8], Dallas and Kirialanis [9], and Bullock et al. [11], and which has been used to assess explosive lower-body strength and the ability to recruit motor units [24], to keep all other confounding factors constant.
Despina et al. [8] reported a statistically significant 1.9 cm increase in the SJ after WBV (side-alternating vibration at 30 Hz, 2 mm, 5 × 15 s, 15 s inter-exercise rest interval) including five different isometric exercises. A similar WBV protocol was used by Dallas and Kirialanis [9] except for the vibration type (vertical vibration). Results obtained by Dallas and Kirialanis [9] (1.96% insignificant increase in SJ) contradicted those obtained by Despina et al. [8]. Since no control measurement (measurements after the same study protocol performed on WBV device performed at 0 Hz, 0 mm) was performed, it is impossible to identify to what extent WBV contributed to this performance change. It is possible that the effects of WBV might be overestimated if the isometric exercises used in this study had a potentiating effect on muscles. In contrast, if the exercises had a fatiguing effect, then the effects of WBV may have been underestimated. Therefore, interpreting these results is challenging. Bullock et al. [11] found an insignificant decrease (5.5 ± 4.4%) with a medium effect size (d = 0.57) in SJ performance after a vertical WBV protocol performed at 30 Hz, 4 mm, including 3 × 60 s isometric squats on toes with a 110° knee joint angle (inter-set rest intervals of three minutes).
Since the WBV protocol in the present study was highly different from the above mentioned studies, it is difficult to determine the exact reasons why these studies revealed different results. Well-trained athletes, especially those who are accustomed to high-intensity stretch-shortening type activities, have fast-twitch muscle fibres that have no margin for further performance improvement; this is due to already existing high levels of reflex sensitivity, fast-twitch fibre recruitment, and motor neuron excitability. In addition, the stiffer muscle-tendon units of athletes compared to untrained individuals could be another reason for no improvement, since stiffer muscle-tendon units minimize muscle length changes during WBV, reducing transmission of the vibratory stimulus to related body parts and resulting in a lower WBV load. This implies that performance enhancement, especially that related to speed-strength activities, in well-trained athletes via WBV is more subtle than in untrained individuals [10, 11]. Similarly, the insignificant changes in SJ obtained in the present study might also have resulted from the physiological and morphological characteristics of well-trained athletes who constituted the present study sample. Although the frequency-amplitude combination in the present study has been shown to be optimal for enhancing vertical jump performance [23], no enhancement was detected in SJ height. As this optimal combination was determined on untrained subjects by Adams et al. [23], this combination might not have fit the well-trained sample of the present study. Moreover, it is also possible that other variables, such as body posture, WBV duration, and work-to-rest ratio, together with the amplitude and frequency used in the present study, may have led to a vibratory stimulus that was insufficient to enhance SJ performance.
Each individual's unique muscular activation response to the same WBV exercise should also be taken into consideration. Di Giminiani et al. [25] found that eight weeks of WBV conducted with individualized vibration frequencies that maximized vastus lateralis muscle activation of each participant significantly increased SJ height (11% vs 3%) and mechanical power during continuous rebound jumping performance (22% vs 3%) compared to WBV with a fixed-vibration frequency of 30 Hz. Therefore, the fixed-vibration parameters in the present study might not have elicited optimal SJ performance.
There are contradictory results related to the effects of acute WBV on force generation capacity [26, 27]. One possible force-enhancing mechanism suggested is a reduction in the recruitment threshold of fast-twitch motor units due to vibration. Although this phenomenon was demonstrated with local vibration exposure, it has been reported that preferential activation of fast-twitch muscle fibres may also occur with WBV via the same mechanism [2, 28]. However, one report indicates that acute vibration has inhibitory rather than facilitatory effects on muscle strength [5]. Although the WBV duration used for each exercise in the present study was suitable for enhancing subsequent isometric strength performance, the inter-exercise rest intervals were quite short [29]. This WBV work-to-rest ratio may be a cause of unchanged ILS performance.
De Ruiter et al. [30] found no significant increase in isometric strength of knee extensors after WBV, including body weight isometric squats (30 Hz, 8 mm, 5 × 1 min WBV, 2 min inter-set intervals, 110° knee joint angle). They stated that during WBV, both antagonist and agonist muscles were exposed to vibration, and this could lead to increased inhibition of both muscles. In addition, they suggested that additional motor unit recruitment could be limited during WBV. These physiological mechanisms might provide an explanation for the unchanged isometric leg strength measures. Similarly, Garcia-Lopez et al. [31] found no significant difference in isometric squat strength after an acute WBV intervention that included intermittent body weight isometric squats (30 Hz, 2.5 mm, 5 × 1 min WBV, 1 min inter-set intervals, 90° knee joint angle). Although these studies were not performed on well-trained athletes, the results are consistent with the present study.
In contrast, McBride et al. [21] found significant improvements in maximal isometric strength of the plantar flexors immediately and eight minutes after WBV intervention that included intermittent body weight isometric squats (30 Hz, 3.5 mm, 6 × 30 s WBV, 1 min inter-set intervals, 100° knee joint angle). However, the mechanism for this increase was not clarified, since muscle activation and motor neuron excitability remained unchanged. These different results between quadriceps and plantar flexor muscles could be explained by the suggestions of Cochrane and Stannard [12]. They emphasized that muscles distal to the vibration source show less pronounced performance enhancement compared to muscles that are more proximal. This may have limited improvements in lower body strength in the present study. It has been stated that vibration magnitude should be sufficient to elicit strength improvement [26, 27]. In the present study, only two of the four exercises had direct foot contact with the WBV platform, and only one of these two exercises was similar to the body posture used during lower body strength testing. Body postures during WBV in the present study, together with other WBV variables, may have resulted in insufficient transmission of the vibratory stimulus to the muscles recruited during the ILS test. In addition, oscillating platforms are more effective than vertical vibration platforms; thus the type of WBV platform employed could be another factor affecting the lack of performance improvement [31].
In the meta-analysis of Marin and Rhea [32], the acute effects of vibration on force generation were insignificant, and changes in performance variables had very low effect sizes, consistent with the present findings. In addition, Cardinale and Erskine [3] suggested that it is hard for athletes to benefit from vibration training unless used in combination with traditional resistance training exercises; this is because frequency and amplitude ranges in available platforms are insufficient to elicit a sufficient stimulus for already highly trained muscles. In contrast, Bosco et al. [13] observed acute improvement in force generation capacities in well-trained athletes. Explaining the possible causes of such conflicting results is very difficult, since WBV studies conducted on well-trained athletes are very limited in the literature, and no standardized training protocols were used in these studies. However, as mentioned previously, one possible explanation for the ineffectiveness of WBV for vertical jump and isometric leg strength in the present study may be the distance between vibration source and target muscles, reducing transmission of the vibratory stimulus to the target muscles due to absorption by the soft tissues, leading to an insufficient vibration load [4]. This interpretation is supported by the results of several local vibration studies. Local vibration intervention significantly improved mechanical power [1, 33] and neuromuscular efficiency [33] in elite athletes during explosive bilateral elbow flexion performance. In these studies, the vibration stimulus was transmitted through the handles of the vibration device, which might have generated an optimal vibratory load for performance enhancement since the investigated (target) muscles were more proximal to the vibration source [12, 26].
It is generally accepted that acute or chronic WBV has no effect on HG strength performance [12, 26, 27]. Cochrane and Stannard [12] reported no significant effect of WBV on HG performance, and they stated that muscles not directly exposed to vibration would show less pronounced performance enhancement compared to directly vibrated muscles. Based on the purpose of their study, forearm muscles were not directly exposed to vibration. Hence, the lack of improvement in HG strength performance was an expected result validating their hypothesis. This may also explain the lack of significant improvement in handgrip performance in the studies of Torvinen et al. [26] and Torvinen et al. [27], in which only standing types of exercises, such as squatting and slight jumps, were performed on the WBV platform. The contradictory finding in the present study may have resulted from the different exercises used. Since forearm muscles of participants in the present study were very close to the vibration source during the first and third exercise (Figure 1), the direct exposure to vibration might have resulted in the net 2.4% HG strength performance improvement with a medium to large effect size (ES = 0.61). However, whether this improvement has practical significance is debatable, as the 95% confidence intervals of this effect size are quite large (0.35–0.86). It should also be taken into consideration that the isometric exercise protocol performed with no vibration (0 Hz, 0 mm) in the present study led to a 2.06% reduction in HG performance, possibly due to a fatiguing effect. However, when the net performance change is considered, WBV compensated for this reduction and potentiated a statistically significant performance enhancement.
Some studies have investigated the effects of WBV on flexibility in well-trained athletes [12], especially in gymnasts [6, 8, 9]. The present study design was similar to that of Cochrane and Stannard [12]. However, they found a significant increase in flexibility performance that is inconsistent with the present results. On the other hand, although the relative flexibility increase (5.3%) in our study was statistically insignificant (p = 0.159), the effect size of this increase was medium to large (ES = 0.55); this could be interpreted as a lower degree of flexibility enhancement than the 8.2% increase observed by Cochrane and Stannard [12]. Nevertheless, when the large 95% CI of this effect size was considered (–0.16–1.27), it is unlikely that the 5.4% improvement in flexibility performance in the present study is of practical significance. However, it should also be considered that the increases in flexibility performance relative to the control group in the study of Cochrane and Stannard [12] and in the present study were 2.9% and 5.4%, respectively. Although a higher relative performance increase was observed in the current study, the lack of statistical significance might have been due to the high variability in flexibility measures of participants. On the other hand, Cochrane and Stannard [12] reported no effect size and no CIs for the flexibility improvement, making it difficult to interpret whether their statistically significant results were of practical importance.
Despina et al. [8] and Dallas and Kirialanis [9] also found significant improvements in flexibility performance. However, interpreting the results of WBV on flexibility in the study by Dallas and Kirialanis [9] is challenging, as no control measurement was performed.
Although no physiological assessment was performed in the present study, the main reason for the lack of change in flexibility performance might be insufficient neural potentiation of the stretch reflex loop, due to insufficient activation of Ia inhibitory inter-neurons of the antagonist muscles. Lack of antagonistic inhibition might not have adjusted muscular coordination to levels appropriate for reducing braking forces around the hip and lower back joints; thus, trunk flexion performance remained unchanged [29].
Variable combinations — frequency, amplitude, rest intervals, exposure time, repetition number, contraction type, body posture, using no additional loads — used in the present WBV intervention may not have generated a sufficient vibratory stimulus to elicit acute training responses in participants whose highly trained muscles were already close to their genetic potentials. Different combinations of WBV variables generating higher vibratory loads on muscles of well-trained athletes may be more effective in performance enhancement. Prescribing WBV programmes with the purpose of enhancing specific biomotor abilities of well-trained athletes may be possible, but further study is needed to verify this.
CONCLUSIONS
In conclusion, the isometric exercise protocol used in the present study led to a reduction in HG performance; however, WBV created a potentiating effect and compensated for this reduction, resulting in a statistically significant performance enhancement. However, this protocol was ineffective for enhancement of SJ, TF, and ILS performance in well-trained combat athletes. Further research is needed to assess optimal WBV variable combinations to maximize performance gains in well-trained athletes. In particular, studies designed to investigate the effects of WBV protocols and their residual effects on a single performance variable, rather than several performance variables, will provide more beneficial and practical knowledge for the WBV literature.
Conflict of interests
The authors declared no conflict of interests regarding the publication of this manuscript.
REFERENCES
- 1.Issurin VB, Tenenbaum G. Acute and residual effects of vibratory stimulation on explosive strength in elite and amateur athletes. J Sport Sci. 1999;17:177–182. doi: 10.1080/026404199366073. [DOI] [PubMed] [Google Scholar]
- 2.Rittweger J. Vibration as an exercise modality: How it may work, and what its potential might be. Eur J Appl Physiol. 2010;108:877–904. doi: 10.1007/s00421-009-1303-3. [DOI] [PubMed] [Google Scholar]
- 3.Cardinale M, Erskine JA. Vibration training in elite sport: Effective training solution or just another fad? Int J Sport Physiol. 2008;3:232–239. doi: 10.1123/ijspp.3.2.232. [DOI] [PubMed] [Google Scholar]
- 4.Cochrane DJ. The potential neural mechanisms of acute indirect vibration. J Sport Sci Med. 2011;10:19–30. [PMC free article] [PubMed] [Google Scholar]
- 5.Cochrane DJ. Vibration exercise: The potential benefits. Int J Sports Med. 2011;32:75–99. doi: 10.1055/s-0030-1268010. [DOI] [PubMed] [Google Scholar]
- 6.George D, Vasilis K, Vasilis M, Giorgos P. Acute effect of whole-body vibration combined with stretching on bridge performance in artistic gymnasts. J Biol Exer. 2012;8:47–57. [Google Scholar]
- 7.Margarita EG, Pavlos EE, Stavros T, Giorgos PP. Acute effects of dynamic whole body vibration in well trained track & field sprinters. J Physical Edu Sport. 2013;13:270–277. [Google Scholar]
- 8.Despina T, George D, George T, Sotiris P, Alessandra DC, George K, Maria R, Stavros K. Short-term effect of whole-body vibration training on balance, flexibility and lower limb explosive strength in elite rhythmic gymnasts. Hum Movement Sci. 2014;33:149–158. doi: 10.1016/j.humov.2013.07.023. [DOI] [PubMed] [Google Scholar]
- 9.Dallas G, Kirialanis P. The effect of two different conditions of whole-body vibration on flexibility and jumping performance on artistic gymnasts. Sci Gymnastics J. 2013;5:67–77. [Google Scholar]
- 10.Delecluse C, Roelants M, Diels R, Koninckx E, Verschueren S. Effects of whole body vibration training on muscle strength and sprint performance in sprint-trained athletes. Int J Sports Med. 2005;26:662–668. doi: 10.1055/s-2004-830381. [DOI] [PubMed] [Google Scholar]
- 11.Bullock N, Martin D.T, Ross A, Rosemond CD, Jordan MJ, Marino FE. Acute effect of whole-body vibration on sprint and jumping performance in elite skeleton athletes. J Strength Cond Res. 2008;22:1371–1374. doi: 10.1519/JSC.0b013e31816a44b5. [DOI] [PubMed] [Google Scholar]
- 12.Cochrane DJ, Stannard SR. Acute whole body vibration training increases vertical jump and flexibility performance in elite female field hockey players. Brit J Sport Med. 2005;39:860–865. doi: 10.1136/bjsm.2005.019950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bosco C, Colli R, Introini E, Cardinale M, Tsarpela O, Madella A, Tihanyi J, Viru A. Adaptive responses of human skeletal muscle to vibration exposure. Clin Physiol. 1999;19:183–187. doi: 10.1046/j.1365-2281.1999.00155.x. [DOI] [PubMed] [Google Scholar]
- 14.Cardinale M, Lim J. Electromyography activity of vastus lateralis muscle during whole-body vibrations of different frequencies. J Strength Cond Res. 2003;17:621–624. doi: 10.1519/1533-4287(2003)017<0621:eaovlm>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 15.Heller J, Peric T, Dlouha R, Kohlikova E, Melichna J, Novakova H. Physiological profiles of male and female taekwon-do (ITF) black belts. J Sport Sci. 1998;16:243–249. doi: 10.1080/026404198366768. [DOI] [PubMed] [Google Scholar]
- 16.Markovic G, Jaric S. Is vertical jump height a body size-independent measure of muscle power? J Sport Sci. 2007;25:1355–1363. doi: 10.1080/02640410601021713. [DOI] [PubMed] [Google Scholar]
- 17.Cardinale M, Lim J. The acute effects of two different whole body vibration frequencies on vertical jump performance. Med Sport. 2003;56:287–292. [Google Scholar]
- 18.Delecluse C, Roelants M, Verschueren S. Strength increase after whole-body vibration compared with resistance training. Med Sci Sport Exer. 2003;35:1033–1041. doi: 10.1249/01.MSS.0000069752.96438.B0. [DOI] [PubMed] [Google Scholar]
- 19.Gerodimos V, Zafeiridis A, Karatrantou K, Vasilopoulou T, Chanou K, Pispirikou E. The acute effects of different whole-body vibration amplitudes and frequencies on flexibility and vertical jumping performance. J Sci Med Sport. 2010;13:438–443. doi: 10.1016/j.jsams.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Bedient AM, Skidmore EC, Signorile JF. Displacement and frequency for maximizing power output resulting from a bout of whole body vibration. Med Sci Sport Exer. 2008;40:161–162. doi: 10.1519/JSC.0b013e3181b45bdc. [DOI] [PubMed] [Google Scholar]
- 21.McBride JM, Nuzzo JL, Dayne AM, Israetel MA, Nieman DC, Triplett NT. Effect of an acute bout of whole body vibration exercise on muscle force output and motor neuron excitability. J Strength Cond Res. 2010;24:184–189. doi: 10.1519/JSC.0b013e31819b79cf. [DOI] [PubMed] [Google Scholar]
- 22.Cronin J, Nash M, Whatman C. The acute effects of hamstring stretching and vibration on dynamic knee joint range of motion and jump performance. Phys Ther Sport. 2008;9:89–96. doi: 10.1016/j.ptsp.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 23.Adams JB, Edwards D, Serviette D, Bedient AM, Huntsman E, Jacobs KA, Del Rossi G, Roos BA, Signorile JF. Optimal frequency, displacement, duration, and recovery patterns to maximize power output following acute whole-body vibration. J Strength Cond Res. 2009;23:237–245. doi: 10.1519/JSC.0b013e3181876830. [DOI] [PubMed] [Google Scholar]
- 24.Da Silva-Grigoletto ME, De Hoyo M, Sanudo B, Carrasco L, Garcia-Manso JM. Determining the optimal whole-body vibration dose-response relationship for muscle performance. J Strength Cond Res. 2011;25:3326–3333. doi: 10.1519/JSC.0b013e3182163047. [DOI] [PubMed] [Google Scholar]
- 25.Di Giminiani R, Tihanyi J, Safar S, Scrimaglio R. The effects of vibration on explosive and reactive strength when applying individualized vibration frequencies. J Sport Sci. 2009;27:169–177. doi: 10.1080/02640410802495344. [DOI] [PubMed] [Google Scholar]
- 26.Torvinen S, Kannus P, Sievanen H, Jarvinen TAH, Pasanen M, Kontulainen S, Jarvinen TLN, Jarvinen M, Oja P, Vuori I. Effect of a vibration exposure on muscular performance and body balance. Randomized cross-over study. Clin Physiol Funct Imaging. 2002;22:145–152. doi: 10.1046/j.1365-2281.2002.00410.x. [DOI] [PubMed] [Google Scholar]
- 27.Torvinen S, Sievanen H, Jarvinen TAH, Pasanen M, Kontulainen S, Kannus P. Effect of 4-min vertical whole body vibration on muscle performance and body balance: A randomized cross-over study. Int J Sports Med. 2002;23:374–379. doi: 10.1055/s-2002-33148. [DOI] [PubMed] [Google Scholar]
- 28.Pollock RD, Woledge RC, Martin FC, Newham DJ. Effects of whole body vibration on motor unit recruitment and threshold. J Appl Physiol. 2012;112:388–395. doi: 10.1152/japplphysiol.01223.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cardinale M, Bosco C. The use of vibration as an exercise intervention. Exerc Sport Sci Rev. 2003;31:3–7. doi: 10.1097/00003677-200301000-00002. [DOI] [PubMed] [Google Scholar]
- 30.de Ruiter CJ, van der Linden RM, van der Zijden MJ, Hollander AP, de Haan A. Short-term effects of whole-body vibration on maximal voluntary isometric knee extensor force and rate of force rise. Eur J Appl Physiol. 2003;88:472–475. doi: 10.1007/s00421-002-0723-0. [DOI] [PubMed] [Google Scholar]
- 31.Garcia-Lopez D, Garatachea N, Marin PJ, Martin T, Herrero AJ. Acute effects of whole-body vibrations on balance, maximal force and perceived exertion: Vertical platform versus oscillating platform. Eur J Sport Sci. 2012;12:425–430. [Google Scholar]
- 32.Marin PJ, Rhea MR. Effects of vibration training on muscle strength: A meta-analysis. J Strength Cond Res. 2010;24:548–556. doi: 10.1519/JSC.0b013e3181c09d22. [DOI] [PubMed] [Google Scholar]
- 33.Bosco C, Cardinale M, Tsarpela O. Influence of vibration on mechanical power and electromyogram activity in human arm flexor muscles. Eur J Appl Physiol. 1999;79:306–311. doi: 10.1007/s004210050512. [DOI] [PubMed] [Google Scholar]