Abstract
Vibrios are ubiquitous marine bacteria that have long served as models for heterotrophic processes and have received renewed attention because of the discovery of increasing numbers of facultatively pathogenic strains. Because the occurrence of specific vibrios has frequently been linked to the temperature, salinity, and nutrient status of water, we hypothesized that seasonal changes in coastal water bodies lead to distinct vibrio communities and sought to characterize their level of differentiation. A novel technique was used to quantify shifts in 16S rRNA gene abundance in samples from Barnegat Bay, N.J., collected over a 15-month period. Quantitative PCR (QPCR) with primers specific for the genus Vibrio was combined with separation and quantification of amplicons by constant denaturant capillary electrophoresis (CDCE). Vibrio populations identified by QPCR-CDCE varied between summer and winter samples, suggesting distinct warm-water and year-round populations. Identification of the CDCE populations by cloning and sequencing of 16S rRNA genes from two summer and two winter samples confirmed this distinction. It further showed that CDCE populations corresponded in most cases to ∼98% rRNA similarity groups and suggested that the abundance of these follows temperature trends. Phylogenetic comparison yielded closely related cultured and often pathogenic representatives for most sequences, and the temperature ranges of these isolates confirmed the trends seen in the environmental samples. Overall, this suggests that temperature is a good predictor of the occurrence of closely related vibrios but that considerable microdiversity of unknown significance coexists within this trend.
The genus Vibrio encompasses a diverse group of heterotrophic marine bacteria including many facultative symbiotic and pathogenic strains. The latter include Vibrio cholerae, the causative agent of cholera, and V. parahaemolyticus and V. vulnificus, which together are responsible for most cases of fatal seafood poisoning (31). Vibrio infections are not limited to humans, as recently highlighted by reports of Vibrio species capable of killing coral tissue (3, 27), and vibrios represent a major source of concern in aquaculture facilities and marine aquaria (11, 40, 47). Because all of these pathogens appear to maintain planktonic populations, considerable interest exists in understanding the prevalence and dynamics of specific Vibrio populations in the environment.
Most studies on Vibrio ecology to date have focused on specific members of the genus, leading to an extensive body of literature on their genetics and ecology. However, the diversity and dynamics of co-occurring Vibrio populations have only rarely been addressed (e.g., see references 1a, 4, 8, 17, 18, and 38) and more rarely still by using quantitative culture-independent methods (8, 17, 18, 38). All quantitative surveys (by molecular techniques) have confirmed the ubiquity of vibrios but have, with the exception of one study (38), also suggested that Vibrio populations are generally <1% of the total bacterioplankton. This is in contrast to culture-based studies, which demonstrate that vibrios can comprise ∼10% of the easily culturable marine bacteria (7, 8).
The distribution of certain coastal Vibrio populations is influenced by environmental factors including salinity e.g., (23, 33), temperature (e.g., see references 20, 21, 22, 33, 35, 39, and 48), and in some cases the abundance of host organisms (e.g., see reference 29). However, many studies of coastal vibrios have been culture dependent and it remains unknown for many of these vibrios whether the observed dynamics reflect shifts in physiology to a viable-but-nonculturable state or represent fluctuations in cell density with temperature. Thus, molecular methods may be better suited to determine shifts in the abundance of Vibrio populations.
Molecular methods have revolutionized the detection and quantification of bacteria in the environment because they circumvent the possible bias and labor intensiveness of cultivation (16). We have recently developed a quantitative PCR (QPCR) protocol that is capable of quantification of diverse, unknown, coexisting 16S rRNA sequences. The approach is similar to denaturant gradient gel electrophoresis (DGGE) (34) but has better resolution and can be made quantitative (30). This method consists of competitive QPCR with group-specific primers targeting a variable region of the 16S rRNA gene (rDNA), followed by separation and quantification of the amplicons by constant denaturant capillary electrophoresis (CDCE) (24). A resolution of a single base pair substitution within 100 bp is routinely achieved, and the detection is quantitative because amplicons are measured by laser-induced fluorescence (31). This method was applied for the first time in the study reported here to explore the co-occurring diversity within a defined bacterial group.
We investigated the dynamics of bacterioplanktonic Vibrio populations in Barnegat Bay, N.J., which represents a temperate coastal water body under the influence of the Gulf Stream. This results in strong seasonal gradients in physiochemical parameters. Because certain Vibrio species have previously been shown to respond strongly to indicators of seasonal change, such as temperature, we hypothesized that distinct Vibrio communities are associated with different seasons in this temperate environment. In this study we address the following questions. (i) What is the diversity of populations coexisting within the Vibrio community? (ii) To what extent does the total Vibrio community vary throughout an annual cycle? (iii) At what level of 16S rRNA divergence between Vibrio populations are different dynamics evident?
MATERIALS AND METHODS
Water samples.
Surface water was collected monthly from Barnegat Bay, N.J. (3°33′48.5"N, 74°1′41.7"W), from July 2001 through September 2002. Water samples were obtained in a bucket lowered off the side of a dock and serially fractionation at the site with 20-, 10-, and 5-μm nylon mesh screens (Spectrum). Between 15 and 80 ml of the <5-μm filtrate was concentrated onto 4.7-cm-diameter 0.22-μm-pore-size polycarbonate filters (Osmotics) by vacuum filtration and immediately stored at −20°C. Surface water temperature, salinity, total chlorophyll, and bacterial abundance in the <5-μm filtrate were measured by standard methods as described previously (36a).
DNA extraction.
DNA was extracted from filters containing the environmental samples by bead beating and chemical lysis. One-half of each 4.7-cm-diameter polycarbonate filter was placed in a 2-ml screw-cap tube with 750 μl of cell lysis buffer (PureGene Cell and Tissue Kit; Gentra Systems) and 0.25 g of 0.1-mm zirconium beads (Biospec Products, Bartlesville, Okla.). Filters were subjected to bead beating at 5,000 rpm for 60 s, followed by incubation at 80°C for 5 min. Subsequently, the samples were incubated with RNase A at 37°C for 30 min with rotation, mixed with 25 μl of protein precipitation solution (PureGene Kit; Gentra Systems), and centrifuged at 15,000 × g for 5 min. The supernatant was transferred to a fresh tube and centrifuged again at 15,000 × g for 5 min to remove residual protein, filter fragments, and beads. An aliquot of the supernatant was transferred to a fresh tube, and DNA was ethanol precipitated, resuspended in Tris-EDTA buffer (pH 8), and stored frozen at −20°C (41).
To calibrate the detection and quantification of vibrios by QPCR-CDCE, genomic DNA was extracted from the reference bacterial strains V. anguillarum ATCC 19264, V. cholerae ATCC 39315, V. fischeri MJ1, V. parahaemolyticus ATCC 17802. V. splendidus ATCC 33125, V. vulnificus ATCC 27562, and Photobacterium phosphoreum ATCC 11040. Cultures were grown overnight and centrifuged for 5 min at 7,000 × g. Genomic DNA was isolated from bacterial pellets with the QIAGEN DNA Mini Kit (no. 513404) and resuspended in Tris-EDTA buffer (pH 8).
Primer design and synthesis.
Vibrio genus primers were designed against an alignment of published Vibrio and related 16S rRNA sequences and tested in silico with the Ribosomal Database Project (RDP) Probe Match program (version 2.1r3; 4/22/03) (5). The 5′ end of the forward primer was modified with a 54-bp GC-rich clamp and labeled with a fluorescein isothiocyanate molecule for laser-induced fluorescence detection. The primer and GC clamp sequences are as follows: GC clamp, 5′-GCCGCCTGCAGCCCGCGCCCCCCGTGCCCCCGCCCCGCCGCCGGCCCGGGCGCC-3′; 567F, 5′-GGCGTAAAGCGCATGCAGGT-3′; 680R, 5′-GAAATTCTACCCCCCTCTACAG-3′. The GC567F-680R primer pair was highly targeted to vibrios, matching 42 out of 43 sequences of Vibrio type strains, and that of the closely related organism Photobacterium angustum, in the RDP database. Synthesis and labeling of primers was performed by Synthetic Genetics (San Diego, Calif.).
QPCR-CDCE analysis of PCR products.
CDCE was used to identify and quantify different PCR amplicons from mixed- and single-template reactions performed with the primer pair GC567F-680R. For CDCE analysis, PCR products were diluted 50-fold into Milli-Q water and electroinjected into a fused silica capillary (75-μm inner diameter) filled with a replaceable linear polyacrylamide gel matrix (Scientific Polymers, Ontario, N.Y.). Electrophoresis of samples occurred at a current of 10 μA and at a constant optimized separation temperature within a range of 74.9 to 78.0°C. Laser-induced fluorescence of labeled PCR products emerging from the heated zone of the capillary was captured by a photomultiplier (Oriel, Stratford, Conn.) and recorded as a time series of fluorescence signal with the Workbench Data Acquisition Program (Strawberry Tree, Inc., Sunnyvale, Calif.). The relative intensities of fluorescence peaks during CDCE were proportional to the relative abundances of the corresponding amplicons in the PCR product.
QPCR-CDCE quantification of Vibrio populations was accomplished by competitive coamplification with the GC567F-680R primer pair of samples containing Vibrio DNA spiked with known quantities of internal standard DNA. CDCE analysis of the resulting PCR product was used to determine the relative abundances of sample and standard CDCE peaks. The V. cholerae 16S rDNA sequence was identified as a suitable internal standard for competitive QPCR because it was absent from the environmental samples and had migration characteristics sufficiently different from those of the GC567F-680R amplicons of reference and environmental sequences. The V. cholerae internal standard was prepared by PCR amplification of the V. cholerae 16S rDNA with the universal primer pair 27F-1492R, followed by gel purification (QIAGEN gel extraction kit) and quantification by the pico green fluorescence assay (Molecular Probes, Inc., Eugene, Oreg.).
Assay optimization.
To test the specificity of assay conditions for detection of vibrios in the presence of nontarget organisms, PCR amplification of V. cholerae genomic DNA with the GC567F-680R primer pair was challenged with a 100-fold excess of P. phosphoreum genomic DNA. P. phosphoreum has a single base pair mismatch at the 3′-terminal end of each primer (T→C 587, C→T 658 [Escherichia coli numbering]). The dual-template amplifications were performed with SureStart Taq (Stratagene, La Jolla, Calif.) and the following cycling parameters: 8 min at 95°C and then 25 cycles of 1 min at 95°C, 1 min at 50 to 68°C, and 1 min at 72°C. The extent of V. cholerae or P. phosphoreum amplification was determined by analysis of their respective amplicons by CDCE. An annealing temperature of 64°C was selected for environmental sample analysis because it was found to exclusively yield V. cholerae product (Fig. 1A) while less stringent annealing temperatures yielded P. phosphoreum as a dominant amplicon (Fig. 1B to D).
FIG. 1.
CDCE profiles obtained during optimization of the annealing temperature for the Vibrio-specific QPCR-CDCE assay determined by coamplification of DNA from the target species, V. cholerae, with a 100-fold excess of DNA from the nontarget species P. phosphoreum at annealing temperatures of 64°C (A), 61°C (B), 58°C (C), and 55°C (D).
To test whether differences in amplification efficiency would bias QPCR-CDCE quantification of coexisting Vibrio populations, the relative abundances of amplicons within a six-species model Vibrio community were assayed with increasing numbers of PCR cycles. Genomic DNAs from V. fischeri, V. splendidus, V. anguillarum, V. parahaemolyticus, V. vulnificus, and V. cholerae were mixed and coamplified with the GC567F-680R primer pair. The amplicons in the PCR product were analyzed by CDCE, diluted, and then used as the template for a 15-cycle reamplification, after which the process was repeated. The following two sets of Vibrio-specific cycling parameters were tested for extent of coamplification bias: (i) a standard three-stage PCR consisting of 1 min at 95°C, 1 min of annealing at 64°C, and 2 min of elongation at 72°C and dilution twice for a total of 106-fold amplification and (ii) a two-stage PCR eliminating the 72°C elongation step and consisting of only 1 min at 95°C and annealing-elongation for 3 min at 64°C and dilution three times for a total of 6.4 × 1010-fold amplification. Although the bias was relatively small for most templates, the standard three-stage amplification protocol yielded an approximately 2-fold bias for both V. fischeri and V. splendidus after 106-fold amplification. This correlated with both templates having the highest melting domain adjacent to the 680R primer site and led us to hypothesize that they bound the primer with higher efficiency than did the other four Vibrio templates. Thus, to ensure saturation of all templates with primer, the annealing and elongation steps were combined to a single 64°C step (two-stage protocol). This minimized the coamplification bias to an extent that differences in template ratios were within the assay-to-assay variation range (coefficient of variation, ±15%; data not shown).
Environmental sample analysis.
Thirty to 60 ng of DNA from Barnegat Bay samples was used as the template for PCR and was amended with 250, 500, 1,250, 2,500, or 5,000 copies of internal standard DNA. Competitive QPCRs were performed under the following optimized assay conditions: 8 min at 95°C, followed by 35 cycles of 1 min at 95°C and 3 min at 64°C with SureStart Taq (Stratagene) on a Stratagene Robocycler. PCR products were diluted 10-fold, followed by an additional 3 to 15 PCR cycles under the same conditions to obtain a product yield sufficient for CDCE analysis while minimizing formation of heteroduplex DNA (46). Environmental PCR spectra were observed by CDCE, and recurring peaks were identified by aligning spectra from multiple months. Populations observed in multiple months were verified by combining representative samples and observing comigration of CDCE peaks.
The detection limit for quantification of vibrios was determined for each sample as the smallest observable population size by CDCE. This detection limit differs for each month because the lower limit of the assay dynamic range generally traced a level approximately 2 orders of magnitude below the largest population size observed.
CDCE peak areas were measured with AcqKnowledge 2.1 software (Biopac Systems, Santa Barbara, Calif.). The environmental Vibrio populations in each sample were quantified in triplicate and averaged. The abundance of each Vibrio population observed in a sample was determined from the area of its corresponding CDCE peak by the formula Pi/mL = [(APiCStd/AStd) × (VSampleVPCRFDNA/VDNA)]/(9.1 operons/cell), where Pi is the number of organisms in the population, APi is the area of the CDCE peak corresponding to Vibrio population i, CStd is the number of internal standard DNA molecules spiked into the QPCR, AStd is the area of the internal standard CDCE peak, VSample is the volume of water filtered (milliliters), VDNA is the volume of buffer in which filter DNA extracts are suspended (microliters), VPCR is the volume of DNA extract (microliters) added to the PCR mixture as the template, and FDNA is the fraction of DNA recovered by the extraction protocol. Division by a presumptive number of operons per cell converts the number of copies of 16S rDNA templates to the number of organisms. We used an average ribosomal operon copy number for the genus Vibrio of 9.1 as listed by the rRNA Operon Copy Number Database (8/27/03) (published values range from 7 to 13) (25). Vibrio abundances were log transformed to normalize the data, and correlations with temperature, salinity, chlorophyll, and total bacterial counts were determined by the correlation coefficient (R) with Microsoft Excel.
Identification of CDCE Vibrio populations by sequencing.
DNA sequences corresponding to the Vibrio populations observed by CDCE were identified by cloning environmental PCR products from four sampling dates (8/13/01, 12/17/01, 2/8/02, and 8/12/02). PCR products were prepared for (8/13/01, 12/17/01, and 8/12/02) in quadruplicate with the universal primer 27F and the Vibrio-specific primer 680R. Thirty to 60 ng of environmental DNA was amplified under the following parameters optimized for Vibrio specificity: first, 8 min at 94°C, followed by 15 cycles of 45 s at 94°C, and annealing-extension for 2 min at 64°C with SureStart Taq reagents and primer concentrations of 0.4 μM (27F) and 0.1 μM (680R). Next, 5 μl of the PCR product was added to 20 μl of fresh PCR reagents with primers 27F (0.1 μM) and 680R (0.1 μM) and the PCR parameters were 8 min at 94°C, followed by 30 cycles of 45 s at 94°C, 1 min at 58°C, and 2 min at 72°C. A sample from 2/8/02 was amplified under the above parameters and then transferred and amplified for 30 additional cycles. A high numbers of cycles was generally necessary to obtain enough product for cloning since the amplification efficiency was greatly reduced when using the protocol designed to decrease nonspecific amplification of Photobacterium. Finally, PCR products were reconditioned to eliminate heteroduplexes by 10-fold dilution and amplification for three cycles with fresh PCR reagents (46).
PCR products were gel purified (QIAGEN gel extraction kit), and for each month 4 to 10 ng of PCR product was cloned into the PCR 2.1-TOPO vector and transformed with the TOPO cloning kit (Invitrogen). Cultures of positive clones were grown at 37°C for 18 h with shaking. Plasmids were extracted with the RevPrep Orbit workstation (GeneMachines, San Carlos, Calif.).
Sequence analysis and phylogeny.
Direct sequencing of plasmid templates was accomplished with the M13 reverse sequencing primer (Invitrogen), Applied Biosystems Big Dye v. 1.1 reagents, and analysis on an ABI 3700 sequencer. Sequences were edited and sorted into groups containing highly related sequences (>99% similarity) with 100% sequence identity in the region used for CDCE analysis (positions 567 to 680 [E. coli numbering]) (Sequencher software; Molecular Probes). Putative polymerase errors were identified by secondary-structure analysis of the 16S rRNA molecule as noncompensated base changes (excluding G-U pairs) present in a single sequence within a consensus group or as changes at positions conserved in >98% of all bacterial taxa (26).
Sequences with a high probability of being chimeric were identified by two methods. An initial screen was done with the RDP Chimera Check program calibrated to 16S rRNA positions 27 to 680 (E. coli numbering) of 20 unique cultured Vibrio isolates. The average breakpoint value of these sequences was 12 ± 6.3 (standard deviation), where each fragment produced by the break matched an RDP Vibrio sequence with a similarity index of 0.89 or greater. Clones with a breakpoint value 2 standard deviations above the mean (25 or greater, with one fragment having the greatest similarity outside the genus Vibrio or both fragments with a similarity index of ≥0.89 for Vibrio sequences) were scored as highly probable chimeras and excluded from further analysis. Second, the 16S rRNA secondary structures of remaining clones were surveyed for strings of noncompensated base pairing in stem regions (excluding G-U pairs) that would indicate that the sequences were potentially chimeric. The criteria used to detect chimeras among cloned sequences identified intergeneric chimeras formed in silico between database sequences of vibrios and non-vibrios and also between most of the Vibrio sequences examined. However, chimeras formed between closely related vibrios (>99%) could not be identified (e.g., V. parahaemolyticus [accession no. X74721] and V. vulnificus [accession no. X74726]). Thus, the occurrence of chimeras between closely related sequences was likely not detected.
Nucleotide BLAST and phylogenetic analyses were used to determine the relatedness of environmental Vibrio populations to previously described Vibrio isolates. A clone matching the consensus sequence of each >99% consensus group was selected to represent that group for phylogenetic analysis. Clones without a >99% consensus group were included in the phylogenetic analysis if they met both chimera check criteria. Sequences were aligned with ClustalX and visually corrected with secondary-structure information. Phylogenetic analysis was done with PAUP*, version 4.0b10 (43). Relationships were determined by the neighbor-joining method with Jukes-Cantor correction and checked for consistency with Parsimony. For each analysis, the robustness was tested by bootstrap resampling with the minimum evolution method with 100 replicates.
To determine which cloned sequences corresponded to Vibrio populations observed in environmental spectra, plasmids containing the cloned 16S rDNA sequences were amplified with the GC567F-680R primer pair and analyzed by CDCE. Clones with a CDCE peak matching a peak in the environmental spectra were confirmed by comigration of cloned and environmental DNA.
Nucleotide sequence accession numbers.
All partial 16S rRNA sequences from the four sampling dates included in the phylogenetic analysis were deposited in the GenBank database under accession numbers AY374379 to AY374413.
RESULTS
Vibrio community dynamics.
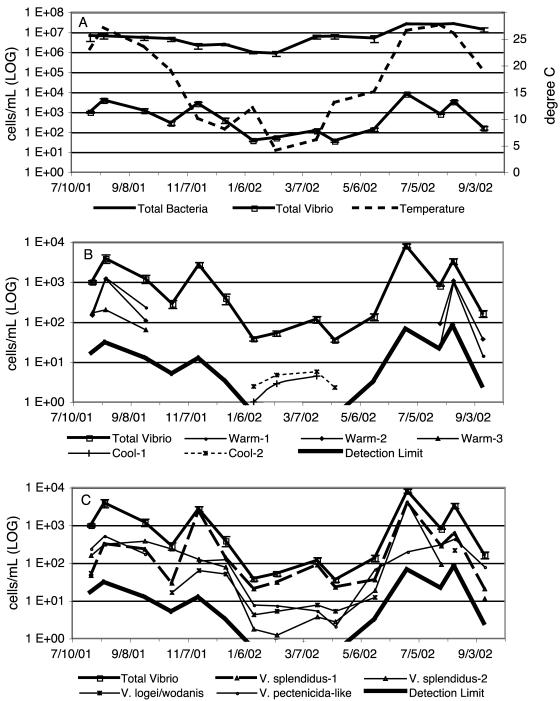
CDCE analysis of samples collected from Barnegat Bay, N.J., over a 15-month period showed the coexistence of multiple Vibrio populations that followed distinct, recurring seasonal patterns. Four or more dominant populations were observed in most samples, but both proportion and occurrence varied considerably. Total vibrios, defined as the sum of individual populations detected by CDCE, comprised 5 × 10−4 to 0.1% of the total bacterioplankton population, reaching a maximum abundance of 8.0 × 103± 9.2 × 102 cells/ml (June 2002) and a minimum of 37 ± 5 cells/ml (April 2002) while total bacterial direct counts ranged from 8.4 × 105 (February 2002) to 2.5 × 107 (August 2002) cells/ml (Fig. 2A).
FIG. 2.
Comparison of Vibrio dynamics within Barnegat Bay bacterioplankton populations over a 15-month period. Numbers of cells per milliliter were obtained from QPCR-CDCE detection of gene copies by division with an average value of 9.1 rRNA operons per cell (rrndb) as explained in Materials and Methods. The values shown are temperature, total bacterial cell counts determined by epifluorescence microscopy, and total Vibrio abundance as the sum of individual CDCE populations (A); seasonal Vibrio CDCE populations (B); and year-round Vibrio CDCE populations (C). Data points represent averages of triplicate runs. Coefficients of variation for quantification ranged from 10 to 24% for total vibrios and from 5 to 50% for individual populations, excluding four data points measured near the detection limit (1/15/02, Cool-1; 2/8/02, Cool-2 and V. splendidus-2; and 4/10/02, V. pectenicida-like), where coefficients of variation ranged between 56 and 77% (for clarity, error bars representing standard deviations are shown only for total vibrios).
Two seasonally differentiated groups of populations were suggested by the distribution of CDCE peaks (Fig. 2B and C). First, three populations only occurred during the summer, when water temperatures were between 19 and 27.5°C (Warm-1, Warm-2, and Warm-3, Fig. 2B and 3). During the warm months, typically four to six populations were seen with abundances between 14 ± 7 and 3.9 × 103± 4.3 × 102 cells/ml and changing patterns of dominance (Fig. 2B and C). Second, four populations were observed consistently during the winter and spring, when water temperatures ranged from 4 to 15°C, and intermittently during warmer months (Fig. 2C). Finally, two populations were detected only from January to April 2002 (Fig. 2C). The number of populations observed in the winter was highest, but their size was generally small, with only a single population reaching a level of >102 cells/ml.
FIG. 3.
Phylogenetic relationships of partial 16S rRNA sequences of vibrios. Relationships were determined by distance analysis with one representative sequence from each 99% consensus group of the clones from four sampling dates and sequences from reference strains. Terminal nodes are labeled according to the sampling date (13 August 2001 = 2, 17 December 2001 = 6, 8 February 2002 = 8, 12 August 2001 = 14), a clone identifier, and (in brackets) the number of clones recovered for each 99% consensus group. Nodes with bootstrap support of >50% are indicated on the tree. GenBank accession numbers for reference species are listed as they appear on the tree from top to bottom (AY217770, Vibrio sp. strain R10; AJ316181, Vibrio sp. strain R-15052; AJ440005, V. coralliilyticus; AF007115, V. shilonii; X74710, V. mediterranei; X74692, V. campbellii; X74721, V. parahaemolyticus; X74722, V. pelagius; AF319770, Vibrio sp. strain Ex97; AF388387, V. parahaemolyticus operon Vp16; Y13830, V. pectenicida; AY136105, V. splendidus MED22; AB038026, Vibrio sp. strain OC25; AY046955, V. splendidus B17; AY069971, Vibrio sp. strain QY101; X74718, V. anguillarum; AY136129, Vibrio sp. strain RED47; AB013297, V. rumoiensis; AJ132227, V. wodanis; AF022410, Vibrio sp. strain ANG.HOH; AJ437616, V. logei; X74687, P. phosphoreum).
Vibrio diversity.
Of 152 sequences obtained from amplification with the 27F-680R primer pair, 23 were excluded as probable chimeras. The remaining sequences were closely related to the genus Vibrio and contained the 567F primer site. The distribution of sequences obtained from the August 2001 and 2002, December 2001, and February 2002 sampling dates confirmed the existence of distinct warm-water and year-round populations suggested by CDCE. Sequences from the two summer months were more similar to each other than to sequences from the two winter months and vice versa. Distance analysis showed that in most cases, the sequences identified by a CDCE peak conformed roughly to 98% similarity groups. However, several exceptions existed. Most notably, resolution for the CDCE population identified as V. pectenicida-like in Fig. 3 was lower, with a maximum distance of 4.5% between the sequences detected in winter and summer samples. In two cases (Warm-1 and V. splendidus-2), CDCE populations were polyphyletic owing to apparent conversion or conservation of the CDCE target region in two resolved clades (Fig. 3). Thus, although overall good agreement between sequence clades and CDCE populations was observed, these examples highlight the necessity to corroborate rapid diversity screening methods with sequence identification even when using high-resolution methods such as CDCE.
Phylogenetic analysis showed that almost all of the sequences recovered had close cultured relatives (Fig. 3). The majority of the clones (34 of 62) from the warm-water sampling dates were closely related to the V. campbellii-V. parahaemolyticus-V. carchariae cluster. Additional clones from both summer libraries were related to coral-associated vibrios, including the marine pathogen V. coralliilyticus, and sequences related to V. rumoiensis and V. splendidus and distantly related to V. pectenicida (94 to 95%). In the winter libraries, V. splendidus sequences were well represented, with 7 of 40 and 27 of 27 sequences obtained for December 2001 and February 2002, respectively. V. splendidus populations had a single nucleotide polymorphism in the region amplified by the GC567F-680R primer pair, leading to the identification of two CDCE populations, V. splendidus-1 and V. splendidus-2. The dynamics of the V. splendidus-1 CDCE population are ambiguous during summer months owing to the superposition of CDCE peaks under assay conditions with the August 2001 V. rumoiensis clone (dashed line, Fig. 2C). Additional winter clones were related to V. pectenicida (99.5%) (8 of 40) and to the V. logei-V. wodanis group (9 of 40), having sequence identity in the 567-to-680 region with V. logei. In several cases correspondence between CDCE populations and cloned sequences was not determined. This was the case for V. anguillarum sequences (2 of 40) from the December 2001 library and CDCE populations Cool-1 and Cool-2 (Fig. 2C).
Correlation of dynamics with environmental parameters.
Vibrio community size was strongly correlated with temperature (R = 0.69), with distinct temperature responses evident in individual populations. The strongest correlations were evident in the year-round populations V. pectenicida-like and V. splendidus-2 (R = 0.75 and R = 0.74, respectively). Summer populations Warm-1, Warm-2, and Warm-3 (V. parahaemolyticus-V. campbellii group and V. coralliilyticus group) were positively correlated with temperature (R = 0.41 to 0.66) and were only detected between 19 and 27.5°C. Salinity, total chlorophyll, and bacterial counts were also considered in the correlation analysis; however, the temperature dependence of these parameters was stronger than the correlation with any Vibrio population, suggesting that temperature was the most significant factor determining population occurrence.
DISCUSSION
Quantification of Vibrio populations by QPCR-CDCE combined with sequence identification by clone library analysis showed that Vibrio populations are present year round in Barnegat Bay, with elevated population sizes during summer. A cold-ocean community with a seasonal shift in structure toward warm-ocean populations during the summer and fall is suggested by comparison of the sequences recovered with growth characteristics observed in closely related cultured vibrios. These warm-ocean populations contain sequences closely related to noted pathogens of humans and marine fauna. The Barnegat Bay Vibrio community displayed a population size and dynamics comparable to those observed in other studies by fluorescence in situ hybridization (FISH) (8, 18). Assuming that the ribosomal operon copy numbers for environmental vibrios do not differ significantly from the rrndb database average (25), the maximum abundance detected in Barnegat Bay corresponds well to the maximum Vibrio abundance observed in North Sea bacterioplankton (8) (Table 1). However, the vibrios in the North Sea study appeared to be primarily particle attached while the measurement of the Barnegat Bay vibrios was skewed toward free-living cells since the water was prefiltered with a 5-μm cutoff. Furthermore, a study of the abundance and dynamics of Vibrio and Photobacterium populations in the Choptank River estuary measured with a FISH probe targeting both genera revealed a similar seasonality (18). Thus, higher relative abundance during warm-water periods appears to be a general trait of vibrioplankton in temperate waters.
TABLE 1.
Comparison of estimates of Vibrio community and population sizes obtained by culture-independent methods in this and other studies
| Environment | Sampling dates | Pretreatment | Assay type | Target genera | Abundance (cells/ml)
|
Reference | |
|---|---|---|---|---|---|---|---|
| Total genus | Specific populations | ||||||
| Barnegat Bay, N.J. | July 2001-Sept. 2002 | <5-μm prefiltration | QPCR-CDCE | Vibrio | 37-8.0 × 103 | 2-3.9 × 103 | This study |
| Choptank River, Md. | April-Dec. 1996 | <64-μm prefiltration | FODCb | Vibrio, Photo- bacterium | 5 × 103-1 × 105 | 50-6 × 103 | 18 |
| North Sea | Sept., Nov. 1997; Feb., Aug. 1998 | None | FISHa | Vibrio | <8 × 103c (except Sept. 1997 [1 × 104]) | NDd | 8 |
FISH by microscopy.
FODC, fluorescent-oligonucleotide direct counts by flow cytometry.
Below limit of detection.
ND, not determined.
Diversity of sequences corresponding to a single CDCE population suggested coherent dynamics when sequences diverged less than 2%. For example, sequences within the V. parahaemolyticus and coral-associated similarity clusters, respectively, were only detected among clones obtained during summer months (Fig. 3). This suggests that adaptation to warm environments is a defining feature of these groups of related organisms. In contrast, when sequence divergence exceeded 2%, no seasonal correlation within single CDCE populations was evident. This was the case for the V. pectenicida-like and V. splendidus-2 populations, for which sequences with the maximum divergence (i.e., 2 to 4.5%) were distributed across summer and winter samples.
The ecological differentiation of co-occurring bacterial populations with small-scale differences in 16S rRNA sequences remains an open question (37). On the one hand, the genomics of closely related strains have revealed unexpectedly large differences in gene content and genome architecture (14) and closely related strains can differ in potentially relevant physiological and metabolic properties (10, 42). On the other hand, we have recently suggested that the phylogenetic architecture of marine microbial communities does not provide evidence of strong ecological differentiation among organisms with closely related 16S rRNA sequences (1). By large-scale sequencing of clone libraries constructed from both salt marsh sediment and coastal bacterioplankton communities (1, 26), we found that at least half of the retrieved 16S rRNA sequences fell into large, discrete clusters containing <1% sequence divergence. This pattern is consistent with theoretical considerations that sequence clusters are evidence of past selective sweeps and persist because competitive mechanisms are too weak to purge diversity from within the clusters (1). A critical test of these theories is whether microdiverse bacterial taxa respond to ecological factors in a cohesive manner. Although this will require detailed analysis of environmental dynamics of specific genomic variants, evidence provided here suggests that microdiverse taxa respond cohesively to the temperature of their environment. At a minimum, this suggests that adaptation to temperature may be a relatively conservative trait among these Vibrio populations.
The distinction between warm-water and year-round vibrios in Barnegat Bay is consistent with the origin and growth properties of related Vibrio isolates. Most of the V. splendidus sequences in winter-dominant CDCE population V. splendidus-1 were closely related to marine pathogens isolated from temperate waters of the Pacific and Atlantic Oceans (e.g., see references 19 and 44). In contrast, a more distantly related warm-water clone in the V. splendidus-2 population had the highest similarity with MED22, a tropical isolate from the Mediterranean Sea (36). The populations dominating the summer Vibrio community of Barnegat Bay are highly related to tropical strains, including V. campbellii and the noted food-borne human pathogen V. parahaemolyticus, which are both routinely isolated from waters above 20°C (12, 13), and to V. carchariae, an agent of disease in sharks and human wound infections (9). Sequences clustering with tropical coral epiflora and temperature-dependent pathogens (V. coralliilyticus, V. mediterranei, and V. shilonii) were detected in Barnegat Bay during both summers, and the 30°C growth optimum of V. rumoiensis (49) is consistent with detection of its sequence in warm waters.
Population dynamics observed by CDCE combined with sequence-based identification lends insight into the ecology of several Vibrio populations. Sequences from three clone libraries have the highest degree of similarity with V. pectenicida, a strain isolated from temperate environments and proposed to be restricted to animal hosts (e.g., the scallop Pecten maximus) (28, 45). Our observations of the CDCE population year-round and highly related sequences (99.5%) in December 2001 suggest a group of organisms related to V. pectenicida that are more planktonic and diverse than originally described. Year-round detection of the V. logei-V. wodanis group by CDCE supports the characterization of V. logei as a cosmopolitan psychrophilic population (9, 45); however, the closely related group V. wodanis has only been described as a pathogen of North Atlantic salmon. Our detection of V. wodanis-like ribotypes (greater than 99% similarity) in Barnegat Bay indicates that the ecology of this group may also be more cosmopolitan than originally described (2, 32, 45). The high degree of correspondence between the population dynamics observed by CDCE, the distribution of sequence types detected in our four clone libraries, and the growth properties of closely related Vibrio isolates corroborate our observation that the prevalence of year-round and warm-water Vibrio communities seasonally alternates within the bacterioplankton of Barnegat Bay, N.J.
Blooms of tropical and subtropical vibrios in temperate regions during warm seasons may be initiated by a variety of mechanisms and may have implications for interpretation of recurrence, or possibly spread, of potential human and marine pathogens owing to increased sea surface temperatures (6, 15). It has been proposed that such populations may overwinter within sediments or in association with marine fauna (e.g., see references 3 and 22), and association of Vibrio species with sediments and zooplankton during winter months has been observed in the Chesapeake Bay (17, 21, 22). Alternatively, Barnegat Bay may be inoculated with subtropical strains transported into temperate waters by the Gulf Stream. Elucidation of whether one of these mechanisms dominates requires quantification of population sizes in the various compartments of seawater and sediments during winter months and during the onset of blooms. However, it may provide important information on what mechanisms determine the population size of warmth-adapted potential human pathogens in temperate waters.
Acknowledgments
We thank William Thilly of MIT, who generously provided the CDCE machine, and Tony Baca, who helped the project with great dedication. Reference Vibrio strains were the generous gift of Kathy Boetcher, University of Maine, and John Mekalanos, Harvard Medical School. Plasmid preps were carried out at the W. M. Keck Ecological and Evolutionary Genetic Facility in the Josephine Bay Paul Center for Comparative Molecular Biology and Evolution at the Marine Biological Laboratory, Woods Hole, Mass. Sequencing reactions were run at the Harvard Biopolymers Facility, Boston, Mass.
Funding was for this study was provided by grants from Seagrant and NOAA to M.F.P. and graduate fellowships from the National Science Foundation, the Switzer Environmental Science Foundation, and the Whittaker Health Sciences Foundation to J.R.T.
REFERENCES
- 1.Acinas, S. G., V. Klepac-Ceraj, D. E. Hunt, C. Pharino, I. Ceraj, D. L. Distel, and M. F. Polz. Fine-scale phylogenetic architecture of a complex bacterial community. Nature, in press. [DOI] [PubMed]
- 1a.Barbieri, E., L. Falzano, and C. Fiorentini. 1999. Occurrence, diversity, and pathogenicity of halophilic Vibrio spp. and non-O1 Vibrio cholerae from estuarine waters along the Italian Adriatic Coast. Appl. Environ. Microbiol. 65:2748-2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benediktsdottir, E., L. Verdonck, C. Sproer, S. Helgason, and J. Swings. 2000. Characterization of Vibrio viscosus and Vibrio wodanis isolated at different geographical locations: a proposal for reclassification of Vibrio viscosus as Moritella viscosa comb. nov. Int. J. Syst. Evol. Microbiol. 50:479-488. [DOI] [PubMed] [Google Scholar]
- 3.Ben-Haim, Y., F. L. Thompson, C. C. Thompson, M. C. Cnockaert, B. Hoste, J. Swings, and E. Rosenberg. 2003. Vibrio coralliilyticus sp nov., a temperature-dependent pathogen of the coral Pocillopora damicornis. Int. J. Syst. Evol. Microbiol. 53:309-315. [DOI] [PubMed] [Google Scholar]
- 4.Caldini, G., A. Neri, and S. Cresti. 1997. High prevalence of Vibrio cholerae non-O1 carrying heat-stable-enterotoxin-encoding genes among Vibrio isolates from a temperate-climate river basin of central Italy. Appl. Environ. Microbiol. 63:2934-2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cole, J. R., B. Chai, T. L. Marsh, R. J. Farris, Q. Wang, S. A. Kulam, S. Chandra, D. M. McGarrell, T. M. Schmidt, G. M. Garrity, and J. M. Tiedje. 2003. The Ribosomal Database Project (RDP-II): previewing a new autoaligner that allows regular updates and the new prokaryotic taxonomy. Nucleic Acids Res. 31:442-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colwell, R. R. 1996. Global climate and infectious disease: the cholera paradigm. Science 274:2025-2031. [DOI] [PubMed] [Google Scholar]
- 7.Eilers, H., J. Pernthaler, and R. Amann. 2000. Succession of pelagic marine bacteria during enrichment: a close look at cultivation-induced shifts. Appl. Environ. Microbiol. 66:4634-4640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eilers, H., J. Pernthaler, F. O. Glockner, and R. Amann. 2000. Culturability and in situ abundance of pelagic bacteria from the North Sea. Appl. Environ. Microbiol. 66:3044-3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farmer, J. J., and F. W. Hickman-Brenner. 1999. The genera Vibrio and Photobacterium. In M. E. A. Dworkin (ed.), The prokaryotes: an evolving electronic resource for the microbiological community, 2nd ed., release 3.0. Springer Verlag, New York, N.Y. [Online.] http://link.springer-ny.com/link/service/books/10125/.
- 10.Fox, G. E., J. D. Wisotzkey, and J. P. Jurtshuk. 1992. How close is close: 16S rRNA sequence identity may not be sufficient to guarantee species identity. Int. J. Syst. Bacteriol. 42:166-170. [DOI] [PubMed] [Google Scholar]
- 11.Gorant, C., F. Merien, R. Berthe, E. Mermoud, and P. Perolat. 1999. Arbitrarily primed PCR to type Vibrio spp. pathogenic for shrimp. Appl. Environ. Microbiol. 65:1145-1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grimes, D. J. 1991. Ecology of estuarine bacteria capable of causing human disease: a review. Estuaries 14:345-360. [Google Scholar]
- 13.Grimes, D. J., P. R. Brayton, P. A. West, F. L. Singleton, and R. R. Colwell. 1986. The probabilistic identification of Vibrio spp. isolated from surface seawater with special reference to Vibrio campbellii. Lett. Appl. Microbiol. 2:93-95. [Google Scholar]
- 14.Hacker, J., and E. Carniel. 2001. Ecological fitness, genomic islands and bacterial pathogenicity—a Darwinian view of the evolution of microbes. EMBO Rep. 2:376-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harvell, C. D., C. E. Mitchell, J. R. Ward, S. Altizer, A. P. Dobson, R. S. Ostfeld, and M. D. Samuel. 2002. Climate warming and disease risks for terrestrial and marine biota. Science 296:2158-2162. [DOI] [PubMed] [Google Scholar]
- 16.Head, I. M., J. R. Saunders, and R. W. Pickup. 1998. Microbial evolution, diversity, and ecology: a decade of ribosomal RNA analysis of uncultivated microorganisms. Microb. Ecol. 35:1-21. [DOI] [PubMed] [Google Scholar]
- 17.Heidelberg, J. F., K. B. Heidelberg, and R. R. Colwell. 2002. Bacteria of the γ-subclass Proteobacteria associated with zooplankton in Chesapeake Bay. Appl. Environ. Microbiol. 68:5498-5507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heidelberg, J. F., K. B. Heidelberg, and R. R. Colwell. 2002. Seasonality of Chesapeake Bay bacterioplankton species. Appl. Environ. Microbiol. 68:5488-5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jensen, S., O. B. Samuelsen, K. Andersen, L. Torkildsen, C. Lambert, G. Choquet, C. Paillard, and O. Bergh. 2003. Characterization of strains of Vibrio splendidus and V. tapetis isolated from corkwing wrasse Symphodus melops suffering vibriosis. Dis. Aquat. Organisms 53:25-31. [DOI] [PubMed] [Google Scholar]
- 20.Jiang, S. C., and W. Fu. 2001. Seasonal abundance and distribution of Vibrio cholerae in coastal waters quantified by a 16S-23S intergenic spacer probe. Microb. Ecol. 42:540-548. [DOI] [PubMed] [Google Scholar]
- 21.Kaneko, T., and R. R. Colwell. 1978. Annual cycle of Vibrio parahaemolyticus in Chesapeake Bay. Microb. Ecol. 4:135-155. [DOI] [PubMed] [Google Scholar]
- 22.Kaneko, T., and R. R. Colwell. 1973. Ecology of Vibrio parahaemolyticus in Chesapeake Bay. J. Bacteriol. 113:24-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaspar, C. W., and M. L. Tamplin. 1993. Effects of temperature and salinity on the survival of Vibrio vulnificus in seawater and shellfish. Appl. Environ. Microbiol. 59:2425-2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khrapko, K. H., J. S. Hanekamp, and W. G. Thilly. 1994. Constant denaturant capillary electrophoresis (CDCE)—a high resolution approach to mutational analysis. Nucleic Acids Res. 22:364-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klappenbach, J. A., P. R. Saxman, J. R. Cole, and T. M. Schmidt. 2001. rrndb: the Ribosomal RNA Operon Copy Number Database. Nucleic Acids Res. 29:181-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klepac-Ceraj, V., M. Bahr, B. C. Crump, A. P. Teske, J. E. Hobbie, and M. F. Polz. 2004. High overall diversity and dominance of microdiverse relationships in salt marsh sulfate-reducing bacteria. Environ. Microbiol. 6:585-598. [DOI] [PubMed] [Google Scholar]
- 27.Kushmaro, A., E. Banin, Y. Loya, E. Stackebrandt, and E. Rosenberg. 2001. Vibrio shiloi sp. nov., the causative agent of bleaching of the coral Oculina patagonica. Int. J. Syst. Evol. Microbiol. 51:1383-1388. [DOI] [PubMed] [Google Scholar]
- 28.Lambert, C., J. L. Nicolas, V. Cilia, and S. Corre. 1998. Vibrio pectenicida sp. nov., a pathogen of scallop (Pecten maximus) larvae. Int. J. Syst. Bacteriol. 48:481-487. [DOI] [PubMed] [Google Scholar]
- 29.Lee, K.-H., and E. G. Ruby. 1994. Effect of the squid host on the abundance and distribution of symbiotic Vibrio fischeri in nature. Appl. Environ. Microbiol. 60:1565-1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lim, E. L., A. V. Tomita, W. G. Thilly, and M. F. Polz. 2001. Combination of competitive quantitative PCR and constant denaturant capillary electrophoresis for high-resolution detection and enumeration of microbial cells. Appl. Environ. Microbiol. 67:3897-3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lipp, E. K., and J. B. Rose. 1997. The role of seafood in foodborne diseases in the United States of America. Rev. Sci. Tech. 16:620-640. [DOI] [PubMed] [Google Scholar]
- 32.Lunder, T., H. Sorum, G. Holstad, A. G. Steigerwalt, P. Mowinckel, and D. J. Brenner. 2000. Phenotypic and genotypic characterization of Vibrio viscosus sp. nov. and Vibrio wodanis sp. nov. isolated from Atlantic salmon (Salmo salar) with ‘winter ulcer'. Int. J. Syst. Evol. Microbiol. 50:427-450. [DOI] [PubMed] [Google Scholar]
- 33.Motes, M. L., A. DePaola, D. W. Cook, J. E. Veazey, J. C. Hunsucker, W. E. Garthright, R. J. Blodgett, and S. J. Chirtel. 1998. Influence of water temperature and salinity on Vibrio vulnificus in northern Gulf and Atlantic Coast oysters (Crassostrea virginica). Appl. Environ. Microbiol. 64:1459-1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muyzer, G., and K. Smalla. 1998. Application of denaturant gradient gel electrophoresis (DGGE) and temperature gradient gel electrophoresis (TGGE) in microbial ecology. Antonie van Leeuwenhoek 73:127-141. [DOI] [PubMed] [Google Scholar]
- 35.Nishiguchi, M., E. Ruby, and M. McFall-Ngai. 1998. Competitive dominance among strains of luminous bacteria provides an unusual form of evidence for parallel evolution in sepiolid squid-vibrio symbioses. Appl. Environ. Microbiol. 64:3209-3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pinhassi, J., and T. Berman. 2003. Differential growth response of colony-forming alpha- and gamma-proteobacteria in dilution culture and nutrient addition experiments from Lake Kinneret (Israel), the eastern Mediterranean Sea, and the Gulf of Eilat. Appl. Environ. Microbiol. 69:199-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36a.Randa, M. A., M. F. Polz, and E. L. Lim. Population dynamics of Vibrio vulnificus in a North Atlantic estuary. Appl. Environ. Microbiol., in press. [DOI] [PMC free article] [PubMed]
- 37.Rappé, M. S., and S. J. Giovannoni. 2003. The uncultured microbial majority. Annu. Rev. Microbiol. 57:369-394. [DOI] [PubMed] [Google Scholar]
- 38.Rehnstam, A.-S., S. Bäckman, D. C. Smith, F. Azam, and Å. Hagström. 1993. Blooms of sequence-specific culturable bacteria in the sea. FEMS Microbiol. Ecol. 102:161-166. [Google Scholar]
- 39.Ruby, E. G., and K. H. Nealson. 1978. Seasonal changes in species composition of luminous bacteria in nearshore seawater. Limnol. Oceanogr. 23:530-533. [Google Scholar]
- 40.Ruple, A. D., and D. W. Cook. 1992. Vibrio vulnificus and indicator bacteria in shellfish stock and commercially produced oysters from the Gulf Coast. J. Food Prot. 55:667-671. [DOI] [PubMed] [Google Scholar]
- 41.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor Laboratory, N.Y.
- 42.Sass, H., E. Wieringa, H. Cypionka, H.-D. Babenzien, and J. Overmann. 1998. High genetic and physiological diversity of sulfate-reducing bacteria isolated from an oligotrophic lake sediment. Arch. Microbiol. 170:243-251. [DOI] [PubMed] [Google Scholar]
- 43.Swofford, D. L. 1991. PAUP: phylogenetic analysis using parsimony, 3.0s ed. Illinois Natural History Survey, Champaign.
- 44.Takeuchi, K., K. Tajima, M. M. Iqbal, T. Sawabe, and Y. Ezura. 1999. Taxonomical and serological studies on the causative bacteria of the disease of sea urchin Strongylocentrotus intermedius occurring at low water temperatures. Fish. Sci. 65:264-268. [Google Scholar]
- 45.Thompson, F. L., B. Hoste, K. Vandemeulebroecke, and J. Swings. 2001. Genomic diversity amongst Vibrio isolates from different sources determined by fluorescent amplified fragment length polymorphism. Syst. Appl. Microbiol. 24:520-538. [DOI] [PubMed] [Google Scholar]
- 46.Thompson, J. R., L. A. Marcelino, and M. F. Polz. 2002. Heteroduplexes in mixed-template amplifications: formation, consequences and elimination by 'reconditioning PCR'. Nucleic Acids Res. 30:2083-2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tison, D. L., M. Nishibuchi, J. D. Greenwood, and K. J. Seidler. 1982. Vibrio vulnificus biogroup 2: new biogroup pathogenic for eels. Appl. Environ. Microbiol. 44:640-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wright, A. C., R. T. Hill, J. A. Johnson, M. C. Roghman, R. R. Colwell, and J. G. Morris. 1996. Distribution of Vibrio vulnificus in the Chesapeake Bay. Appl. Environ. Microbiol. 62:717-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yumoto, I., H. Iwata, T. Sawabe, K. Ueno, N. Ichise, H. Matsuyama, H. Okuyama, and K. Kawasaki. 1999. Characterization of a facultatively psychrophilic bacterium, Vibrio rumoiensis sp. nov., that exhibits high catalase activity. Appl. Environ. Microbiol. 65:67-72. [DOI] [PMC free article] [PubMed] [Google Scholar]