Abstract
Objective
Prior studies examining coronary atherosclerosis in the young have been limited by retrospective analyses in small cohorts. We examined the relationship between cardiovascular risk factors (RFs) and prevalence and severity of coronary atherosclerosis in a large, prospective, multinational registry of consecutive young individuals undergoing coronary computerized tomographic angiography (CCTA).
Method and results
Of 27 125 patients undergoing CCTA, 1635 young (<45 years) individuals without known coronary artery disease (CAD) or coronary anomalies were identified. Coronary plaque was assessed for any CAD, obstructive CAD (≥50% stenosis), and presence of calcified plaque (CP) and non-calcified plaque (NCP). Among 1635 subjects (70% men, age 38 ± 6 years), any CAD, obstructive CAD, CP, and NCP were observed in 19, 4, 5, and 8%, respectively. Compared with women, men demonstrated higher rates of any CAD (21 vs. 12%, P < 0.001), CP (6 vs. 3%, P = 0.01), and NCP (9 vs. 5%, P = 0.008), although no difference was observed for rates of obstructive CAD (5 vs. 4%, P = 0.46). Any CAD, obstructive CAD, and NCP were higher for young individuals with diabetes, hypertension, dyslipidaemia, current smoking, or family history of CAD; while only diabetes and dyslipidaemia were associated with CP. Increasing cardiovascular RFs was associated with a greater prevalence and extent and severity of CAD, with individuals with 0, 1, 2, ≥3 RFs manifesting a dose–response increase in any CAD (P < 0.001, for trend), obstructive CAD (P < 0.001, for trend), NCP (P < 0.001, for trend), and CP (P < 0.001, for trend). In multivariable analysis adjusting for sex and cardiovascular RFs, male sex was the strongest predictor for any CAD (odds ratio [OR] = 1.95, 95% confidence interval [CI] = 1.43–2.66, P < 0.001), CP (OR = 1.46, 95% CI = 1.08–1.98, P = 0.01), and NCP (OR = 1.33, 95% CI = 1.06–1.67, P = 0.01); family history of CAD was the strongest predictor for obstructive CAD (OR = 2.71, 95% CI = 1.65–4.45, P < 0.001).
Conclusion
Any and obstructive CAD is present in 1 in 5 and 1 in 20 young individuals, respectively, with family history associated with the greatest risk of obstructive CAD.
Keywords: young adults, coronary artery disease, coronary risk factors, coronary CT angiography
Introduction
As the prevalence of coronary atherosclerosis has generally been demonstrated to be low, the determinants and clinical association of coronary artery disease (CAD) are not well characterized for young patients.1–3 Importantly, prior studies that have investigated the prevalence of coronary atherosclerosis in the young have been limited to small populations,4–11 and the association between CHD and risk factors (RFs) has been inadequately examined.
Coronary computed tomographic angiography (CCTA) is a non-invasive test that permits direct visualization of CAD with high diagnostic performance. In a prospective, multicentre international study of consecutive individuals undergoing CCTA, we evaluated the prevalence of coronary atherosclerosis in young subjects and relationship between coronary atherosclerosis and CAD RFs.
Methods
Patients
CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: an International Multicenter Registry) is an international, multicentre, observational registry of 27 125 consecutive patients who underwent ≥64-detector row CCTA clinically referred for suspected CAD at 12 centres between 2003 and 2009. The study design has been previously described.12 Each centre obtained approval from their respective ethics or institutional review board. Of 27 125 consecutive adult patients at these centres, we excluded 2350 patients with known CAD (previous myocardial infarction [MI] and/or coronary revascularization) and 173 patients with coronary anomaly. Among 1635 remaining patients—defined as 45 years of age or younger—met inclusion criteria for the current study. The age threshold for inclusion in the study criteria was chosen in accordance with prior investigations of young patients, as well as from empirical evidence by CCTA of an exceedingly low prevalence of CAD in this population.
Before the scan, physicians or dedicated nurse professionals prospectively collected information on the presence of categorical CAD RFs in each individual. Systemic arterial hypertension was defined as a documented history of high blood pressure or treatment with antihypertensive medications. Diabetes mellitus was defined by diagnosis of diabetes made previously by a physician and/or use of insulin or oral hypoglycaemic agents. Dyslipidaemia was defined as known but untreated dyslipidaemia or current treatment with lipid-lowering medications. A positive smoking history was defined as current smoking or cessation of smoking within 3 months of testing. Positive family history (FH) of CAD was determined by patient query and was defined as a MI, sudden cardiac death, or need for coronary revascularization in a first-degree relative. Body mass index (BMI) was calculated according to the formula: weight/height in kg/m2. Chest pain symptom was defined as non-cardiac, atypical, or typical angina.
Coronary CTA acquisition
CCTAs were performed on a single-source, 64-slice scanner (Lightspeed VCT, GE Healthcare, Milwaukee, WI, USA; SOMATOM Sensation 64, Siemens Medical Systems, Erlangen, Germany) or dual-source scanner (Definition or Flash, Siemens Medical Systems). Before imaging, in patients without contraindications, oral and/or intravenous metoprolol was administered in an attempt to achieve a target heart rate ≤65 bpm for single-source scanners or ≤75 bpm for dual-source scanners. Whenever possible, 0.4 mg sublingual nitroglycerin was administered 3–5 min before image acquisition. Timing bolus or automated bolus tracking at the proximal ascending aorta was used to determine the time from contrast injection to optimal coronary artery enhancement. Contrast (80–140 mL, depending on site) was injected at 5–6 mL/s (rates >6 mL/s were reserved for very obese patients or patients with very thick chests), and whole-volume image acquisition was completed in a single breath-hold. Acquired image data were initially reconstructed in mid diastole (always) and end systole (when available). When image quality was suboptimal on initial reconstruction, multisector reconstruction algorithm with or without manual electrocardiogram (ECG) editing was used to improve image quality. Reconstructed data were then sent to a workstation, where a minimum of 1 level III equivalent and/or board-certified reader in cardiovascular computed tomography employed all necessary post-processing techniques to determine the presence of CAD in any visible segment ≥2 mm in diameter, with obstructive CAD defined as a luminal diameter stenosis >50%. Any CAD was defined as the presence of any plaque regardless of luminal diameter stenosis.
CCTA interpretation
CCTA interpretation was performed in an intent-to-diagnose fashion for all CCTAs, in accordance with prior reported investigations. All uninterpretable segments were scored with the same stenosis severity as the most adjacent proximal evaluable segment, in accordance with standard protocols from prior multicentre studies.13,14 A 16-segment AHA coronary artery tree model was used.15 Coronary atherosclerotic lesions were quantified for lumen diameter stenosis by visual estimation and graded as none (0% luminal stenosis), mild (1–49%), moderate (50–69%), or severe (≥70%). Plaque composition in each coronary segment was classified as calcified, non-calcified, or partially calcified as we have previously described,16 and the presence of any plaque, calcified, non-calcified, and partially calcified plaque was also evaluated.
Plaque severity was scored on a per-patient, per-vessel, and per-segment level. Per-patient maximal plaque severity was defined by maximal intraluminal stenosis in any of the coronary segments at the 50% stenosis threshold. Any CAD was defined as patients who had any plaque, irrespective of grade of stenosis.
Statistical analysis
All statistical calculations were performed using STATA (Version 11, StataCorp LP, College Station, TX, USA) and SAS version 9.2 (SAS Institute, Cary, NC, USA) for Windows. Categorical variables are presented as frequencies and continuous variables as mean ± SD. Variables were compared with Pearson's χ2 test for categorical variables or Fisher's exact test where there were cell counts <6, and by Student's unpaired t-test for continuous variables. Comparison of BMIs between genders was performed with the Kruskal–Wallis non-parametric test. Stepwise multivariable logistic regression analysis including age, sex, and coronary RFs was performed to determine the association between these variables and the presence of obstructive CAD, any CAD, and calcified plaque (CP) and non-calcified plaque (NCP); these relationships were expressed as odds ratio (OR) and 95% confidence intervals (CIS). A value of P < 0.05 was considered significant. The χ2 test for trend was used to compare patients with and without obstructive CAD, any CAD and the presence of CP and NCP across ordered categorical variables.
Results
Clinical characteristics of the study cohort
Characteristics of study population are detailed in Table 1. Among 1635 subjects, 70% was men, 6% had diabetes, 37% had dyslipidaemia, 31% had hypertension, 21% had current smoking, and 33% had FH of CAD. Thirty-three per cent were asymptomatic. The mean age of the overall study group was 38 ± 6 years. Men exhibited higher BMI [Median: 27 kg/m2, inter-quartile range (IQR) [24–30] for males vs. Median: 25 kg/m2, IQR [22–31] for females (P = 0.0001)] and higher frequency of dyslipidaemia (male 40% vs. female 30%, P < 0.001). There were no significant differences in rates of diabetes, hypertension, current smoking, FH of CAD, and typical chest pain between males and females. The frequency of asymptomatic status was higher in males than in females (P < 0.001).
Table 1.
Baseline patient characteristics
| Variables | All patients, n = 1635 | Male, n = 1143 | Female, n = 492 | P-value |
|---|---|---|---|---|
| Age, years (mean ± SD) | 38 ± 6 | 38 ± 6 | 38 ± 6 | 0.34 |
| BMI, kg/m2a (median, IQR) | (27, 24–30) | (27, 24–30) | (25, 22–31) | 0.0001 |
| Diabetes, % | 6 | 5 | 7 | 0.31 |
| Dyslipidaemia, % | 37 | 40 | 30 | <0.001 |
| Hypertension, % | 31 | 31 | 30 | 0.65 |
| Current smoking, % | 21 | 23 | 18 | 0.05 |
| Family history of CAD, % | 33 | 32 | 35 | 0.18 |
| Typical chest pain, % | 10 | 9 | 11 | 0.29 |
| Atypical chest pain, % | 50 | 48 | 56 | 0.002 |
| Non-cardiac chest pain, % | 7 | 6 | 9 | 0.07 |
| Asymptomatic, % | 33 | 37 | 25 | <0.001 |
| Asymptomatic patients | All patients, n = 545 | Male, n = 424 | Female, n = 121 | P-value |
| Age, years (mean ± SD) | 38 ± 6 | 39 ± 6 | 38 ± 6 | 0.14 |
| BMI, kg/m2a (median, IQR) | (26, 24–30) | (27, 24–30) | (25, 22–29) | 0.0003 |
| Diabetes, % | 6 | 6 | 5 | 0.63 |
| Dyslipidaemia, % | 37 | 40 | 27 | 0.009 |
| Hypertension, % | 31 | 32 | 28 | 0.46 |
| Current smoking, % | 17 | 18 | 16 | 0.57 |
| Family history of CAD, % | 31 | 31 | 32 | 0.78 |
| Symptomatic patients | All patients, n = 1090 | Male, n = 719 | Female, n = 371 | P-value |
| Age, years (mean ± SD) | 37 ± 6 | 37 ± 7 | 38 ± 6 | 0.02 |
| BMI, kg/m2a (median, IQR) | (27, 24–30) | (27, 24–30) | (26, 23–31) | 0.01 |
| Diabetes, % | 6 | 5 | 7 | 0.12 |
| Dyslipidaemia, % | 37 | 40 | 31 | 0.002 |
| Hypertension, % | 31 | 31 | 31 | 0.92 |
| Current smoking, % | 23 | 25 | 19 | 0.02 |
| Family history of CAD, % | 34 | 32 | 36 | 0.20 |
| Typical chest pain, % | 14 | 14 | 14 | 0.94 |
| Atypical chest pain, % | 75 | 76 | 74 | 0.58 |
| Non-cardiac chest pain, % | 10 | 10 | 12 | 0.38 |
SD, standard deviation; BMI, body mass index; IQR, inter-quartile range; CAD, coronary artery disease.
aAnalysis of body mass index was performed in 1588 (97%) patients (1107 males and 481 females).
CCTA findings associated with gender and CAD risk
Among all study subjects, any CAD, obstructive CAD, CP, and NCP were observed in 19, 4, 5, and 8%, respectively. Compared with women, men demonstrated higher rates of any CAD (21 vs. 12%, P < 0.001), NCP (9 vs. 5%, P = 0.008), and CP (6 vs. 3%, P = 0.01), although no difference was observed for rates of obstructive CAD (5 vs. 4%, P = 0.46). Any CAD, obstructive CAD, and NCP were significantly higher for individuals with diabetes, current smoking, dyslipidaemia, FH of CAD or hypertension; only diabetes and dyslipidaemia were significantly associated with CP (Table 2).
Table 2.
Association of sex and cardiovascular risk factors to coronary atherosclerosis in young patients in CCTA
| Overall | Malea | Femalea | P | DMa | No DMa | P |
|---|---|---|---|---|---|---|
| Any CAD | 21 | 12 | <0.001 | 30 | 18 | 0.003 |
| Obstructive CAD | 4 | 4 | 0.46 | 9 | 4 | 0.03 |
| Presence of NCP | 9 | 5 | 0.008 | 14 | 7 | 0.02 |
| Presence of CP | 6 | 3 | 0.01 | 10 | 4 | 0.02 |
| SM | No SM | P | CHOL | No CHOL | P | |
| Any CAD | 23 | 17 | 0.01 | 23 | 16 | 0.001 |
| Obstructive CAD | 6 | 4 | 0.03 | 7 | 3 | <0.001 |
| Presence of NCP | 10 | 7 | 0.04 | 11 | 6 | 0.001 |
| Presence of CP | 4 | 5 | 0.69 | 6 | 4 | 0.01 |
| FH | No FH | P | HTN | No HTN | P | |
| Any CAD | 23 | 17 | 0.002 | 24 | 16 | <0.001 |
| Obstructive CAD | 7 | 3 | <0.001 | 7 | 3 | 0.001 |
| Presence of NCP | 10 | 7 | 0.009 | 10 | 7 | 0.01 |
| Presence of CP | 5 | 4 | 0.49 | 5 | 4 | 0.44 |
| Asymptomatic patients | Malea | Femalea | P | DMa | No DMa | P |
| Any CAD | 22 | 15 | 0.09 | 34 | 19 | 0.04 |
| Obstructive CAD | 3 | 3 | 1.00 | 6 | 3 | 0.26 |
| Presence of NCP | 9 | 6 | 0.26 | 19 | 8 | 0.03 |
| Presence of CP | 8 | 3 | 0.10 | 19 | 6 | 0.007 |
| SM | No SM | P | CHOL | No CHOL | P | |
| Any CAD | 18 | 21 | 0.51 | 24 | 18 | 0.10 |
| Obstructive CAD | 4 | 3 | 0.52 | 6 | 1 | 0.009 |
| Presence of NCP | 7 | 8 | 0.73 | 10 | 7 | 0.31 |
| Presence of CP | 7 | 7 | 0.87 | 9 | 6 | 0.19 |
| FH | No FH | P | HTN | No HTN | P | |
| Any CAD | 27 | 17 | 0.009 | 24 | 19 | 0.18 |
| Obstructive CAD | 6 | 2 | 0.002 | 3 | 3 | 1.00 |
| Presence of NCP | 12 | 6 | 0.02 | 8 | 8 | 0.97 |
| Presence of CP | 9 | 6 | 0.25 | 10 | 6 | 0.12 |
| Symptomatic patients | Malea | Femalea | P | DMa | No DMa | P |
| Any CAD | 21 | 11 | <0.001 | 28 | 17 | 0.03 |
| Obstructive CAD | 5 | 4 | 0.27 | 10 | 4 | 0.05 |
| Presence of NCP | 9 | 5 | 0.02 | 12 | 7 | 0.21 |
| Presence of CP | 4 | 2 | 0.14 | 5 | 4 | 0.47 |
| SM | No SM | P | CHOL | No CHOL | P | |
| Any CAD | 25 | 16 | <0.001 | 22 | 15 | 0.004 |
| Obstructive CAD | 7 | 4 | 0.046 | 7 | 3 | 0.009 |
| Presence of NCP | 11 | 6 | 0.007 | 11 | 6 | 0.001 |
| Presence of CP | 3 | 4 | 0.68 | 5 | 3 | 0.03 |
| FH | No FH | P | HTN | No HTN | P | |
| Any CAD | 21 | 16 | 0.047 | 24 | 15 | <0.001 |
| Obstructive CAD | 8 | 3 | 0.002 | 9 | 3 | <0.001 |
| Presence of NCP | 9 | 7 | 0.12 | 11 | 6 | 0.002 |
| Presence of CP | 4 | 4 | 0.97 | 3 | 4 | 0.68 |
CCTA, coronary CT angiography; DM, diabetes; CAD, coronary artery disease; CP, calcified plaque; NCP, non-calcified plaque; SM, current smoking; CHOL, dyslipidaemia; FH, family history of coronary artery disease; HTN, hypertension.
aReported as percentages.
Impact of gender or CAD risk factors on coronary atherosclerosis
In multivariable logistic regression analyses considering age and CAD risk factors, male gender was the strongest predictor for any CAD (OR = 1.95, 95% CI = 1.43–2.66, P < 0.001), CP (OR = 1.46, 95% CI = 1.08–1.98, P = 0.01), and NCP (OR = 1.33, 95% CI = 1.06–1.67, P = 0.01; Tables 3 and 4), while FH of CAD was the strongest predictor for obstructive CAD (OR = 2.71, 95% CI = 1.65–4.45, P < 0.001; Table 3).
Table 3.
Multivariate predictors of any and obstructive CAD on CCTA among young patients
| Variables | Any CAD |
Obstructive CAD |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value | |
| Male | 2.06 | 1.50–2.82 | <0.001 | 1.21 | 0.69–2.13 | 0.51 |
| Age | 1.11 | 1.08–1.14 | <0.001 | 1.07 | 1.01–1.14 | 0.02 |
| Diabetes | 1.67 | 1.02–2.73 | 0.04 | 1.58 | 0.70–3.56 | 0.27 |
| Dyslipidaemia | 1.14 | 0.87–1.49 | 0.34 | 1.84 | 1.10–3.07 | 0.02 |
| Current smoking | 1.37 | 1.01–1.84 | 0.04 | 1.56 | 0.91–2.67 | 0.10 |
| Family history of CAD | 1.40 | 1.07–1.83 | 0.01 | 2.56 | 1.56–4.21 | <0.001 |
| Hypertension | 1.40 | 1.07–1.84 | 0.02 | 1.82 | 1.10–3.03 | 0.02 |
| Asymptomatic patients | ||||||
| Male | 1.50 | 0.85–2.66 | 0.16 | 0.75 | 0.23–2.47 | 0.64 |
| Age | 1.14 | 1.08–1.21 | <0.001 | 1.15 | 0.97–1.36 | 0.10 |
| Diabetes | 2.13 | 0.93–4.85 | 0.07 | 2.66 | 0.51–13.86 | 0.25 |
| Dyslipidaemia | 0.98 | 0.62–1.54 | 0.92 | 3.16 | 1.05–9.56 | 0.04 |
| Current smoking | 0.81 | 0.45–1.47 | 0.50 | 1.60 | 0.48–5.29 | 0.44 |
| Family history of CAD | 1.76 | 1.12–2.77 | 0.01 | 3.70 | 1.30–10.53 | 0.01 |
| Hypertension | 1.31 | 0.82–2.11 | 0.26 | 0.71 | 0.23–2.20 | 0.55 |
| Symptomatic patients | ||||||
| Male | 2.26 | 1.54–3.31 | <0.001 | 1.50 | 0.79–2.87 | 0.22 |
| Age | 1.10 | 1.06–1.14 | <0.001 | 1.06 | 1.00–1.13 | 0.06 |
| Diabetes | 1.47 | 0.79–2.76 | 0.23 | 1.44 | 0.56–3.70 | 0.45 |
| Dyslipidaemia | 1.19 | 0.86–1.67 | 0.30 | 1.54 | 0.85–2.76 | 0.15 |
| Current smoking | 1.69 | 1.18–2.41 | 0.004 | 1.50 | 0.82–2.76 | 0.19 |
| Family history of CAD | 1.27 | 0.91–1.78 | 0.16 | 2.31 | 1.30–4.10 | 0.004 |
| Hypertension | 1.48 | 1.06–2.08 | 0.02 | 2.46 | 1.37–4.43 | 0.003 |
CAD, coronary artery disease; CCTA, coronary CT angiography; OR, odds ratio; CI, confidence interval.
Table 4.
Multivariate predictors of presence of non-calcified plaque and calcified plaque on CCTA
| Variables | Non-calcified plaque |
Calcified plaque |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value | |
| Male | 1.83 | 1.16–2.90 | 0.01 | 2.23 | 1.20–4.13 | 0.01 |
| Age | 1.08 | 1.04–1.13 | <0.001 | 1.14 | 1.07–1.22 | <0.001 |
| Diabetes | 1.66 | 0.87–3.17 | 0.13 | 2.15 | 1.00–4.63 | 0.05 |
| Dyslipidaemia | 1.40 | 0.96–2.05 | 0.08 | 1.36 | 0.85–2.20 | 0.20 |
| Current smoking | 1.41 | 0.93–2.13 | 0.10 | 0.84 | 0.47–1.51 | 0.56 |
| Family history of CAD | 1.51 | 1.04–2.20 | 0.03 | 1.10 | 0.67–1.78 | 0.71 |
| Hypertension | 1.33 | 0.90–1.97 | 0.15 | 0.96 | 0.58–1.60 | 0.89 |
| Asymptomatic patients | ||||||
| Male | 1.55 | 0.66–3.62 | 0.31 | 2.35 | 0.80–6.91 | 0.12 |
| Age | 1.05 | 0.98–1.13 | 0.16 | 1.20 | 1.07–1.35 | 0.002 |
| Diabetes | 3.31 | 1.19–9.21 | 0.02 | 3.31 | 1.16–9.48 | 0.03 |
| Dyslipidaemia | 1.02 | 0.53–1.97 | 0.95 | 0.96 | 0.47–1.95 | 0.90 |
| Current smoking | 0.86 | 0.36–2.01 | 0.72 | 1.12 | 0.46–2.70 | 0.80 |
| Family history of CAD | 2.17 | 1.15–4.11 | 0.02 | 1.53 | 0.75–3.11 | 0.24 |
| Hypertension | 0.87 | 0.43–1.75 | 0.69 | 1.54 | 0.75–3.17 | 0.24 |
| Symptomatic patients | ||||||
| Male | 1.93 | 1.11–3.36 | 0.02 | 1.79 | 0.83–3.86 | 0.14 |
| Age | 1.10 | 1.04–1.16 | 0.001 | 1.11 | 1.03–1.20 | 0.007 |
| Diabetes | 1.13 | 0.47–2.70 | 0.78 | 1.45 | 0.41–5.06 | 0.56 |
| Dyslipidaemia | 1.61 | 1.00–2.58 | 0.049 | 1.79 | 0.92–3.47 | 0.09 |
| Current smoking | 1.70 | 1.04–2.78 | 0.03 | 0.79 | 0.35–1.76 | 0.56 |
| Family history of CAD | 1.33 | 0.83–2.12 | 0.24 | 0.88 | 0.45–1.76 | 0.73 |
| Hypertension | 1.61 | 1.00–2.60 | 0.049 | 0.66 | 0.32–1.38 | 0.27 |
CAD, coronary artery disease; CCTA, coronary CT angiography; OR, odds ratio; CI, confidence interval.
Impact of number of CAD RFs on coronary atherosclerosis
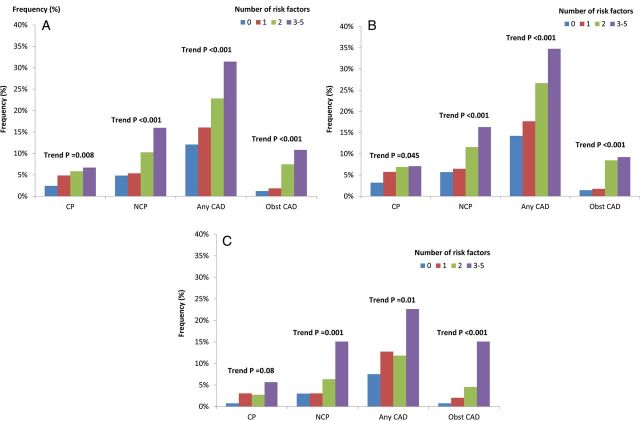
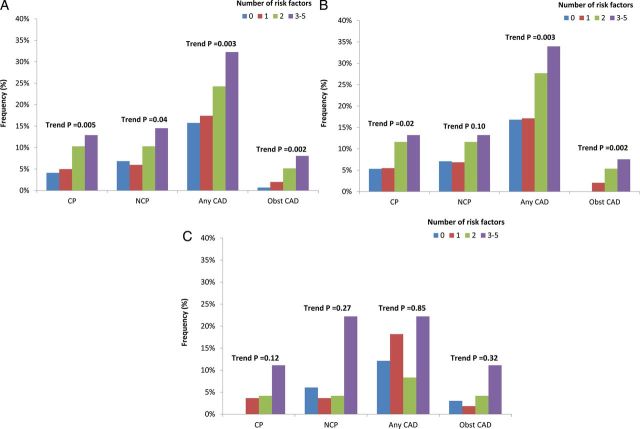
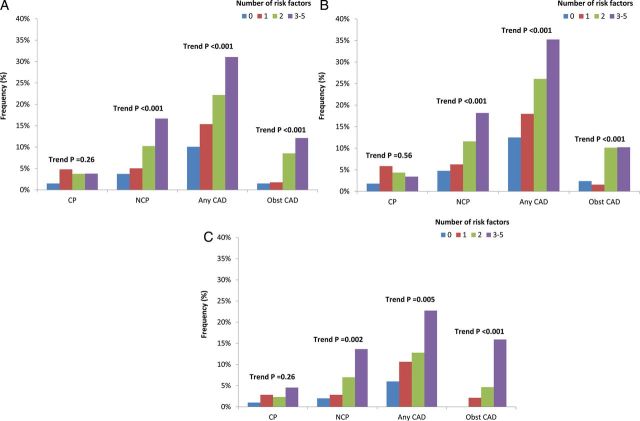
Increasing CAD RFs was associated with greater prevalence, extent, and severity of CAD, with individuals with 0, 1, 2, ≥3 RFs manifesting a dose–response increase in CP (P = 0.008, for trend), NCP (P < 0.001, for trend), any CAD (P < 0.001, for trend), and obstructive CAD (P < 0.001, for trend). For male subjects, increasing number of RFs was also associated with a greater frequency of CP (P = 0.045, for trend), NCP (P < 0.001, for trend), any plaque (P < 0.001, for trend), and obstructive CAD (P < 0.001, for trend). However, for female subjects, increasing number of RFs was not significantly associated with CP (P = 0.085, for trend), while that was significantly correlated with NCP (P = 0.001, for trend), any CAD (P = 0.014, for trend), and obstructive CAD (P < 0.001, for trend) (Figure 1). When classified by symptom state, asymptomatic patients with increasing numbers of RFs possessed increased CP, NCP, any CAD, and obstructive CAD, a finding that was evident more for males than for females (Figure 2). For symptomatic patients, these trends were observed for NCP, any CAD, and obstructive CAD but not CP (Figure 3). These relationships were similar for men and women.
Figure 1.
Association between coronary atherosclerosis and number of cardiovascular RFs for (A) the entire study cohort, (B) for male subjects, and (C) for female subjects. CAD, coronary artery disease; obst CAD, obstructive coronary artery disease, pre CP, presence of calcified plaque; pre NCP, presence of non-calcified plaque.
Figure 2.
Association between coronary atherosclerosis and number of cardiovascular RFs among asymptomatic patients according to (A) the entire study cohort, (B) for male subjects, and (C) for female subjects.
Figure 3.
Association between coronary atherosclerosis and number of cardiovascular RFs among symptomatic patients according to (A) the entire study cohort, (B) for male subjects, and (C) for female subjects. CAD, coronary artery disease; obst CAD, obstructive coronary artery disease; pre CP, presence of calcified plaque; pre NCP, presence of non-calcified plaque.
Discussion
In this prospective, multicentre international study of young patients <45 years of age, we examined prevalence and severity of CAD and their association to gender and CAD RFs. Further, we examined the relationship of demographic and CAD RFs to coronary plaque composition in a fashion that has not been previously studied. We identified a common occurrence of CAD by CCTA in young individuals, with approximately one in five individuals exhibiting any CAD. Further, despite their young age, there existed a 1 in 20 prevalence of obstructive CAD in these individuals referred for clinically indicated CCTA. These prevalences were disproportionate when comparing gender and CAD RFs, with increasing rates of any obstructive CAD in male individuals compared with their female counterpart, as well as for individuals with increasing numbers of traditional RFs. These observational findings indicate an early pathogenesis of manifest atherosclerosis that may help inform future interventions aimed at halting progression of the insidious CAD process.
Prior autopsy-based studies investigating young soldiers who died from non-cardiac causes have demonstrated that coronary atherosclerosis begins early in life. These pathologic studies reported a frequency of coronary atherosclerosis to be 77.3% among Korean war-era soldiers,5,6,11 while Vietnam war soldiers exhibited identifiable coronary atherosclerosis in only 45%, with more severe coronary atherosclerosis observed in 5%.9 Subsequent autopsy studies reported that the incidence of coronary stenosis increases with age in studies with subjects ranging from 21 to 39 or 30 to 34 years,4,7 and a higher atherosclerosis presence in men than in women.8 More contemporary analyses employing intravascular ultrasound (IVUS) on 262 donor heart of a mean age of 33.4 ± 13.2 years within 1 month of cardiac transplantation observed a prevalence of coronary atherosclerosis that varied from 17% in donor hearts from individuals <20 years of age to 85% in donor hearts from individuals ≥50 years of age.10
The explanation of the variability of CAD prevalence in these studies is likely multi-fold in nature, but it may certainly be linked to different study subjects, different methods of CAD examination, dissimilar definitions of coronary atherosclerosis, and small study populations. Our present study findings directly extend these prior studies by prospective evaluation of a significantly larger population that is international in scope, employing a more systematic and comprehensive evaluation of coronary atherosclerosis by a non-invasive method with high diagnostic performance. Importantly, among the study cohort, 70% were asymptomatic and suggest a hypothesis-generating notion that these findings may be similar in a population-based study. The results of this present study should be viewed with caution. Given the lack of prognostic data associated with the study, no concrete conclusion can be drawn regarding the need or timing of early treatment in young patients for reduction of future adverse cardiovascular events. Future larger studies evaluating the prognostic utility of any CAD, CP, and NCP will be needed to address this question.
Previous epidemiologic studies have demonstrated a significant association between subclinical coronary atherosclerosis and RFs even in children, adolescents, and young adults.4,8,17,18 Given its magnitude, the current study was able to further expand these prior findings by assessing the relationship of sex and CAD risk factors to the prevalence and severity of coronary atherosclerosis. In this regard, we identified male gender as the strongest predictor of any coronary atherosclerosis, as well as for the presence of both CP and NCP in young patients. We were able not only to identify which demographic and CAD RFs are associated with higher CAD prevalence, but also the degree to which these factors predict CAD presence, severity, and plaque composition. One prior prospective cohort deserved to be mentioned, having analysed 14 786 middle-aged (age: 25–64 years) men and women in Finland, and investigators found that relative difference in CHD risk between sexes was largest among 25–49 year old,19 a finding that is in direct accordance with the present study.2 These observations may be explained, in part, by several studies that have demonstrated a cardioprotective effect of oestrogen through glucose metabolism and haemostatic system;20,21 although an array of other factors deserve equal consideration. Interestingly, in this Finnish study, differences in RFs were more favourable in women, but sex differences in RF levels diminished with increasing age.19 These findings suggest a cumulative differential progression of atherosclerosis due to higher rates of RFs and longer exposure in men than in women. In the present study, FH of CAD was the strongest predictor for obstructive CAD. This result is concordant with the prior studies that have shown an association between coronary atherosclerosis and FH of CAD in young patient,22,23 and the quantification of the direct association of this factor is likely explained by both genetic and environmental factors.24 Future studies will be invaluable to further assess this novel observation.
Finally, we have also determined that increasing prevalence of CAD RFs were significantly associated with the presence of CP, NCP, any CAD, and obstructive CAD. These results may suggest that CAD treatment measures in young patients at the time of atherosclerosis identification may be salutary in nature, and future studies would be useful to evaluate the therapeutic benefit of treatment of early coronary atherosclerosis.
Study limitations
This study is not without limitations. First, our study findings were derived from a cohort of individuals who were referred for clinically indicated CCTA by their physician for suspected CAD, and thus, extrapolation of our study findings to population-based cohorts should be done with caution. Secondly, our study findings indicate the significant associations between the presence and extent of coronary atherosclerosis self-report which may be subject to ascertainment bias; however, this method is that which is commonly applied in clinical practice, and thus, the results of our multinational study may be considered widely generalizable. Third, given the current limited spatial and temporal resolution of CCTA, it remains possible that CAD was underestimated compared with IVUS or autopsy studies. Fourth, the radiation dose conferred by CCTA is non-negligible, and care must be taken to minimize exposure in this young population, given the hypothetical risk of future cancers. Fifth, current generation CCTA is dependent on single-energy image acquisition and calcium artefacts—including ‘blooming’ from partial volume artefacts are still known to occur. While the present manuscript employed standard definitions of CP that have been used consistently in prior investigations, the likelihood of small degrees of NCP obscured by CPs cannot be discounted. These findings could also be influenced by the ‘real-world’ design of the study which did not employ a dedicated core laboratory for image interpretation. Notably, all CCTA readers were Level III-equivalent physicians with extensive experience interpreting CCTAs. Further, the pharmacologic therapy of the study cohort was not known nor were changes in treatment after the CCTA. Future studies evaluating these findings as it relates to CAD progression and prognosis are necessary and are currently being pursued by our laboratory. Sixth, the cross-sectional nature of the current study limited the long-term assessment of the conventional RFs and how they might influence the risk of CAD progression over time. Forthcoming longitudinal studies utilizing time-updated measures are needed to further our understanding of how the conventional RFs might impact the progression of CAD. Finally, the prognostic utility of the findings related to any CAD and the presence of CP or NCP was not examined in the present study. Larger observational cohort studies are needed to determine the ability of these CAD findings by CCTA to further risk stratify patients beyond traditional CAD risk factors.
Conclusion
Any CAD and obstructive CAD are present in 1 in 5 and 1 in 20 young individuals, respectively, with FH associated with the greatest risk of obstructive CAD.
Funding
J.K.M. receives research support from the National Institutes of Health-National Heart Lung and Blood Institute (NIH-NHLBI) (R01HL111141, R01HL115150, R01HL118019) and the Dalio Institute of Cardiovascular Imaging. This research was also supported by Leading Foreign Research Institute Recruitment Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (MSIP) (2012027176). This study was also funded, in part, by a generous gift from the Dalio Institute of Cardiovascular Imaging (New York, NY) and the Michael Wolk Foundation (New York, NY). J.K.M. also receives modest medical advisory board compensation from GE Healthcare and serves as a consultant to HeartFlow. S.A. received grant support from Siemens and Bayer Schering Pharma and has served as a consultant for Servier. M.Al-M. received support from the American Heart Association, BCBS Foundation of Michigan, and Astellas. F.C. received grant support from GE Healthcare and has served on the Speakers' Bureau of Bracco and as a consultant for Servier; E.M. received grant support from GE Healthcare. K.C. received grant support from Bayer Pharma and Blue Cross Blue Shield Blue Care MI. B.J.W.C. received research and fellowship support from GE Healthcare, research support from Pfizer and AstraZeneca, and educational support from TeraRecon. J.H. received a research grant from Siemens Medical Systems. P.K. received institutional research support from GE Healthcare and grant support from Swiss National Science Foundation. G.R. received grant support from Siemens, Blue Cross Blue Shield Blue Care MI, and Bayer Pharma. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
Conflict of interest: None declared.
References
- 1.Arzamendi D, Benito B, Tizon-Marcos H, Flores J, Tanguay JF, Ly H, et al. Increase in sudden death from coronary artery disease in young adults. Am Heart J 2011;161:574–80. [DOI] [PubMed] [Google Scholar]
- 2.Drory Y, Turetz Y, Hiss Y, Lev B, Fisman EZ, Pines A, et al. Sudden unexpected death in persons less than 40 years of age. Am J Cardiol 1991;68:1388–92. [DOI] [PubMed] [Google Scholar]
- 3.Rubin JB, Borden WB. Coronary heart disease in young adults. Curr Atheroscler Rep 2012;14:140–9. [DOI] [PubMed] [Google Scholar]
- 4.Berenson GS, Srinivasan SR, Bao W, Newman WP, 3rd, Tracy RE, Wattigney WA. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study. N Engl J Med 1998;338:1650–6. [DOI] [PubMed] [Google Scholar]
- 5.Enos WF, Jr, Beyer JC, Holmes RH. Pathogenesis of coronary disease in American soldiers killed in Korea. J Am Med Assoc 1955;158:912–4. [DOI] [PubMed] [Google Scholar]
- 6.Enos WF, Holmes RH, Beyer J. Coronary disease among United States soldiers killed in action in Korea; preliminary report. J Am Med Assoc 1953;152:1090–3. [DOI] [PubMed] [Google Scholar]
- 7.McGill HC, Jr, McMahan CA, Zieske AW, Sloop GD, Walcott JV, Troxclair DA, et al. Associations of coronary heart disease risk factors with the intermediate lesion of atherosclerosis in youth. The Pathobiological Determinants of Atherosclerosis in Youth (PDAY) Research Group. Arterioscler Thromb Vasc Biol 2000;20:1998–2004. [DOI] [PubMed] [Google Scholar]
- 8.McGill HC, Jr, McMahan CA, Zieske AW, Tracy RE, Malcom GT, Herderick EE, et al. Association of coronary heart disease risk factors with microscopic qualities of coronary atherosclerosis in youth. Circulation 2000;102:374–9. [DOI] [PubMed] [Google Scholar]
- 9.McNamara JJ, Molot MA, Stremple JF, Cutting RT. Coronary artery disease in combat casualties in Vietnam. JAMA 1971;216:1185–7. [PubMed] [Google Scholar]
- 10.Tuzcu EM, Kapadia SR, Tutar E, Ziada KM, Hobbs RE, McCarthy PM, et al. High prevalence of coronary atherosclerosis in asymptomatic teenagers and young adults: evidence from intravascular ultrasound. Circulation 2001;103:2705–10. [DOI] [PubMed] [Google Scholar]
- 11.Virmani R, Robinowitz M, Geer JC, Breslin PP, Beyer JC, McAllister HA. Coronary artery atherosclerosis revisited in Korean war combat casualties. Arch Pathol Lab Med 1987;111:972–6. [PubMed] [Google Scholar]
- 12.Min JK, Dunning A, Lin FY, Achenbach S, Al-Mallah MH, Berman DS, et al. Rationale and design of the CONFIRM (COronary CT Angiography EvaluatioN For Clinical Outcomes: An InteRnational Multicenter) Registry. J Cardiovasc Comput Tomogr 2011;5:84–92. [DOI] [PubMed] [Google Scholar]
- 13.Budoff MJ, Dowe D, Jollis JG, Gitter M, Sutherland J, Halamert E, et al. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J Am Coll Cardiol 2008;52:1724–32. [DOI] [PubMed] [Google Scholar]
- 14.Meijboom WB, Meijs MF, Schuijf JD, Gitter M, Sutherland J, Halamert E, et al. Diagnostic accuracy of 64-slice computed tomography coronary angiography: a prospective, multicenter, multivendor study. J Am Coll Cardiol 2008;52:2135–44. [DOI] [PubMed] [Google Scholar]
- 15.Austen WG, Edwards JE, Frye RL, Gensini GG, Gott VL, Griffith LS, et al. A reporting system on patients evaluated for coronary artery disease. Report of the Ad Hoc Committee for Grading of Coronary Artery Disease, Council on Cardiovascular Surgery, American Heart Association. Circulation 1975;51:5–40. [DOI] [PubMed] [Google Scholar]
- 16.Lin F, Shaw LJ, Berman DS, Callister TQ, Weinsaft JW, Wong FJ, et al. Multidetector computed tomography coronary artery plaque predictors of stress-induced myocardial ischemia by SPECT. Atherosclerosis 2008;197:700–9. [DOI] [PubMed] [Google Scholar]
- 17.Berenson GS, Wattigney WA, Tracy RE, Newman WP, 3rd, Srinivasan SR, Webber LS, et al. Atherosclerosis of the aorta and coronary arteries and cardiovascular risk factors in persons aged 6 to 30 years and studied at necropsy (The Bogalusa Heart Study). Am J Cardiol 1992;70:851–8. [DOI] [PubMed] [Google Scholar]
- 18.McGill HC, Jr, McMahan CA. Determinants of atherosclerosis in the young. Pathobiological Determinants of Atherosclerosis in Youth (PDAY) Research Group. Am J Cardiol 1998;82:30T–6T. [DOI] [PubMed] [Google Scholar]
- 19.Jousilahti P, Vartiainen E, Tuomilehto J, Puska P. Sex, age, cardiovascular risk factors, and coronary heart disease: a prospective follow-up study of 14 786 middle-aged men and women in Finland. Circulation 1999;99:1165–72. [DOI] [PubMed] [Google Scholar]
- 20.Grady D, Rubin SM, Petitti DB, Fox CS, Black D, Ettinger B, et al. Hormone therapy to prevent disease and prolong life in postmenopausal women. Ann Intern Med 1992;117:1016–37. [DOI] [PubMed] [Google Scholar]
- 21.Shahar E, Folsom AR, Salomaa VV, Stinson VL, McGovern PG, Shimakawa T, et al. Relation of hormone-replacement therapy to measures of plasma fibrinolytic activity. Atherosclerosis Risk in Communities (ARIC) Study Investigators. Circulation 1996;93:1970–5. [DOI] [PubMed] [Google Scholar]
- 22.Hoit BD, Gilpin EA, Henning H, Maisel AA, Dittrich H, Carlisle J, et al. Myocardial infarction in young patients: an analysis by age subsets. Circulation 1986;74:712–21. [DOI] [PubMed] [Google Scholar]
- 23.Zimmerman FH, Cameron A, Fisher LD, Ng G. Myocardial infarction in young adults: angiographic characterization, risk factors and prognosis (Coronary Artery Surgery Study Registry). J Am Coll Cardiol 1995;26:654–61. [DOI] [PubMed] [Google Scholar]
- 24.Topol EJ, McCarthy J, Gabriel S, Moliterno DJ, Rogers WJ, Newby LK, et al. Single nucleotide polymorphisms in multiple novel thrombospondin genes may be associated with familial premature myocardial infarction. Circulation 2001;104:2641–4. [DOI] [PubMed] [Google Scholar]