We found that valacyclovir decreases plasma human immunodeficiency virus (HIV)-1 RNA in herpes simplex virus (HSV)-2-negative patients. Acyclovir suppresses plasma HIV-1 levels by blocking HIV-1 reverse transcriptase rather than by decreasing HSV-2-mediated immune activation.
Keywords: HIV-1, HSV-2, acyclovir, herpesvirus, reverse transcriptase inhibitor
Abstract
Background. Acyclovir (ACV), a highly specific anti-herpetic drug, acts as a DNA chain terminator for several human herpesviruses (HHVs), including HHV-2 (HSV-2), a common human immunodeficiency virus (HIV)-1 co-pathogen. Several trials demonstrated that HSV-2 suppressive therapy using ACV or its prodrug valacyclovir (valACV) reduced plasma HIV-1 viral load (VL) in HIV-1/HSV-2 coinfected persons, and this was proposed to be due to a decrease in generalized immune activation. Recently, however, we found that ACV directly suppresses HIV-1 ex vivo in tissues free of HSV-2 but endogenously coinfected with other HHVs. Here, we asked whether valACV suppresses VL in HIV-1 infected HSV-2-seronegative persons.
Methods. Eighteen HIV-1 infected HSV-2-seronegative individuals were randomly assigned in a double blind placebo-controlled, crossover trial. Eligible participants had CD4 cell counts of ≥500 cells/µL and were not taking antiretroviral therapy. Subjects in group A received 12 weeks of valACV 500 mg given twice daily by mouth followed by 2 weeks of a no treatment washout and then 12 weeks of placebo; subjects in group B received 12 weeks of placebo followed by 2 weeks of no treatment washout and then 12 weeks of valACV 500 mg twice daily.
Results. HIV-1 VL in plasma of patients treated with valACV 500 mg twice daily for 12 weeks was reduced on average by 0.37 log10 copies/mL.
Conclusions. These data indicate that the effects of valACV on HIV-1 replication are not related to the suppression of HSV-2-mediated inflammation and are consistent with a direct effect of ACV on HIV-1 replication.
Acyclovir (ACV) was the first safe, potent, and specific antiviral nucleoside analogue to be approved for clinical use [1]. It is a guanosine analog that is efficiently phosphorylated by herpesvirus kinases and acts as specific chain terminator for several human herpesviruses (HHV), including HHV-1, -2 and -3 (respectively, herpes simplex viruses [HSV]-1 and 2 and Varicella zoster virus [VZV]). In the early years of the AIDS epidemic, ACV was tested in vitro for activity against human immunodeficiency virus type 1 (HIV-1) and found to be ineffective [2–4].
HHVs, in particular HSV-2, are among the most common co-pathogens in HIV-1 infected persons, and ACV is commonly used in HIV-1/HSV-2 coinfected patients to treat and prevent symptomatic HSV-2 infection. Several retrospective studies found that ACV treatment of HIV-1 infected individuals was associated with increased survival [5, 6]. These early observations were then corroborated by numerous more recent randomized trials, which demonstrated that HSV-2 suppressive therapy using ACV or its prodrug valacyclovir (valACV) reduced plasma HIV-1 viral load (VL) by 0.25 to 1.23 log10 RNA copies/mL in HIV-1/HSV-2 coinfected persons and delayed HIV-1 disease progression [7–20].
Because ACV has been thought to be inactive against HIV-1, these results were attributed to a decrease of general inflammation due to the suppression of HSV-2 replication (reviewed in [21]).
Surprisingly, we found that in human tissues studied ex vivo, ACV suppresses HIV-1 by directly inhibiting HIV-1 reverse transcriptase (RT) provided that ACV is phosphorylated [22]. These results were confirmed in another ex vivo system [23]. HHV thymidine kinases (TK) are particularly efficient in phosphorylating ACV, and coinfecting herpesviruses present in tissues can supply T cells with sufficient phosphorylated ACV to inhibit HIV [22]. Coinfecting herpesviruses may not be necessary to activate ACV as McMahon et al (2011) recently showed that triphosphorylated ACV could also be detected within cells even in the absence of HHVs [24].
In the present study, we hypothesized that if a similar mechanism occurred in vivo, the suppressive effect of ACV on HIV-1 replication should not be limited to HSV-2 coinfected individuals but should also be demonstrable in HSV-2–seronegative persons. Here, we tested this hypothesis. We undertook a randomized, placebo-controlled, crossover trial to evaluate the impact of valACV on HIV-1 viremia in HSV-2 seronegative persons not receiving antiretroviral therapy (ART).
METHODS
Participants
Twenty-one HIV-1–infected HSV-2 seronegative subjects 18 years and older were enrolled at Asociación Civil Impacta Salud y Educación, in Lima (Peru) and at University Hospitals Case Medical Center in Cleveland Ohio between June 2009 and July 2012. All participants provided written informed consent. The IRBs of the participating institutions approved the study. Inclusion criteria were confirmed HIV-1 infection, absence of serum antibodies for HSV-2, plasma HIV-1 RNA ≥1000 copies/mL, and CD4 cell count ≥500 cells/mm3. We excluded women who were breast-feeding and those of reproductive potential who were not using a reliable contraceptive method, persons with AIDS-defining illnesses, and individuals who had received or were receiving ART, and those who had previous adverse reactions to ACV or valACV.
Study Design
This was a randomized, double-blind, placebo-controlled clinical trial with a crossover design to evaluate the effect of 12 weeks of valACV administration on VL in chronically HIV-1 infected subjects not receiving ART. Twenty-one HIV-infected male and female subjects were divided in 2 groups: Group A received 12 weeks of valACV 500 mg given twice daily by mouth followed by 2 weeks of no treatment washout and then 12 weeks of placebo; Group B received 12 weeks of placebo followed by 2 weeks of no treatment washout and then 12 weeks of valACV 500 mg given twice daily by mouth. ValACV and matched placebo were provided by Glaxo SmithKline Inc. and were stored and distributed by the site pharmacies.
Screening and Follow-up
Baseline laboratory evaluations were obtained and blood was also collected in EDTA containing tubes at weeks 11, 12, 14, 25, and 26. All samples were frozen on site and at the end of the trial were sent for laboratory evaluation. For each patient, a 1 mL sample of coded plasma was shipped to Case Western Reserve University, Cleveland, Ohio for HHV serological tests and a second 1 mL sample was shipped to the Laboratory of Biochemical Pharmacology, Department of Pediatrics at Emory University, Atlanta, Georgia for HIV-1 ultra-deep sequence analyses of RT. Peripheral blood monocular cells (PBMCs) were cryopreserved on site and shipped to the National Institutes of Health (NIH) for HHV DNA VL quantification. Adherence was assessed by participant's self-report.
Laboratory Analyses
Nucleic Acid Extraction and Real-Time Quantitative Polymerase Chain Reaction (PCR)
HHV DNA extraction and VL measurement were performed at NIH as described elsewhere [22] (see Supplementary Data).
HIV-1 Viral Load Measurement
Levels of HIV-1 RNA in plasma were measured in the 2 clinical laboratories, in Lima (Peru) and Cleveland (Ohio) using Food and Drug Administration (FDA)-approved commercial assays (Roche, Abbott).
Ultra-deep Sequencing and Data Analysis
These assays were performed to search for antiretroviral drug resistance mutations (DRMs). Plasma samples from 15 HIV-1 infected subjects were stored in aliquots at −80°C until further processing (see Supplementary Data).
Statistical Analysis
We used medians and interquartile ranges (IQRs) to describe the data. The outcome of interest was the change in plasma HIV-1 RNA from baseline (at study entry) to week 12 and from the end of the washout period (at week 14) to week 26. To assess the effects of the treatment and period, we used graphical exploration as previously described [25]. To analyze the effect of treatment without correction, we used the approach of Senn [26] to compare the change in plasma HIV-1 RNA during treatment with valACV to the change during treatment with placebo. We then fitted an appropriate regression model to account for the possible effect of the treatment period. We tested for the presence of carryover and other treatment-by-period interaction and found no effects on the significance of the viral load difference in the two groups of patients. All tests were 2-sided without correction for multiple comparisons, and P-values <.05 were reported as statistically significant.
RESULTS
Study Participants
The characteristics of the 21 subjects who enrolled in the study are presented in Table 1. The groups were well balanced with regard to demographic characteristics, CD4+ T-cell count, and plasma HIV-1 RNA level at baseline. There were no significant imbalances by study site other than ethnic background.
Table 1.
Subject Characteristics
| Characteristic | Arm A |
Arm B |
|---|---|---|
| ValACV Then Placebo, n = 11 | Placebo Then ValACV, n = 10 | |
| Age, yearsa | 30.4 (22.5–44.9) | 30.9 (24.5–38.5) |
| Male sex, n (%)a | 10 (90.9) | 7 (70) |
| Race, n (%)a | ||
| White | 0 (0) | 2 (20) |
| Black | 2 (22.2) | 1 (10) |
| Mixed | 7 (77.8) | 7 (70) |
| Hispanic ethnicity, n (%)a | 7 (77.8) | 7 (70) |
| Body mass index, kg/m2a | 26.2 (23–28.8) | 26.5 (24.5–27.9) |
| Baseline CD4+ T-cell count, cells/mm3a | 538 (534–650) | 635 (594–792) |
| Baseline plasma HIV-1 RNA, log10 copies/mLa | 4.39 (3.62–4.87) | 4.36 (3.76–4.61) |
Abbreviations: HIV-1, human immunodeficiency virus type 1; IQR, interquartile range; ValACV, valacyclovir.
a Presented are medians and IQR.
Two subjects discontinued the study after randomization and before week 12 and were excluded from the analyses. Another subject was lost to follow-up after randomization and returned to the site only at week 25, when a premature discontinuation visit was completed. There were no study-related toxicities exceeding grade 2, but one subject developed acute hepatitis B infection during the study, which was deemed not to be related to study treatment. Thus, of 21 randomized subjects, 18 HIV-1-positive, HSV-2-negative individuals remained in the trial beyond week 12 and constituted the analysis dataset.
Valacyclovir Decreases Plasma HIV-1 RNA Levels
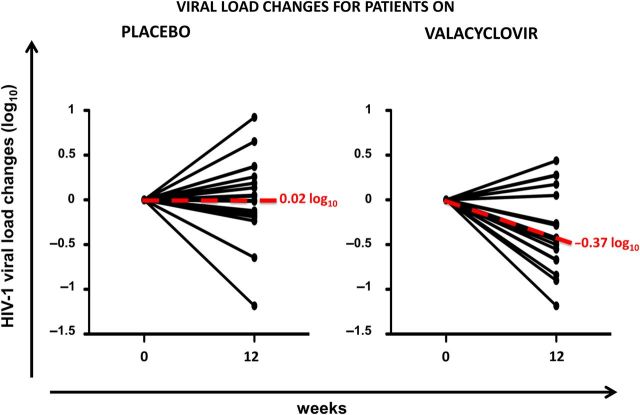
The HIV-1 levels pre- and post-valACV treatment for the above-described 18 individuals are shown in Table 2. The median VL pre- and post-valACV treatment were respectively 4.09 (IQR, 3.42–4.62) and 3.77 (IQR, 3.15–4.33). As shown in Figure 1, administration of valACV was associated with a modest but statistically significant decline in HIV RNA (estimated HIV RNA change during active treatment −0.37 log10 copies/mL, 95% confidence interval, −.62, −.11; P = .009) (Figure 1). We then assessed the model for the presence of a period effect, following the Hills–Armitage approach [27] and found that there was no evidence for a significant period effect (P = .958). Similarly, we fitted a model including an estimate of carryover effect and did not find statistically significant evidence for it (P = .06). Of note, one subject became seropositive for HSV-2 during follow-up. Because the seroconversion was discovered when HSV-2 serologies were repeated at week 26, we could not determine when in the course of trial the seroconversion occurred. Plasma HIV-1 RNA in this patient increased during ACV treatment. We did not exclude this subject's record from the analysis. If we had done so, the overall plasma HIV-1 RNA decrease would have been −0.40 log10.
Table 2.
Changes in Plasma HIV-1 RNA Levels
| Patients | HIV-1 Viral Load at Baseline | HIV-1 Viral Load After valACV Treatment | HIV-1 VL Changes (log10) |
|---|---|---|---|
| 1 | 3.34 | 3.78 | 0.44 |
| 2 | 3.45 | 3.73 | 0.28 |
| 3 | 4.09 | 4.36 | 0.27 |
| 4 | 4.86 | 5.04 | 0.18 |
| 5 | 3.94 | 3.99 | 0.05 |
| 6 | 4.93 | 4.67 | −0.26 |
| 7 | 3.28 | 3.01 | −0.27 |
| 8 | 4.65 | 4.38 | −0.27 |
| 9 | 4.61 | 4.32 | −0.29 |
| 10 | 2.92 | 2.50 | −0.42 |
| 11 | 4.10 | 3.65 | −0.45 |
| 12 | 3.82 | 3.32 | −0.50 |
| 13 | 4.76 | 4.21 | −0.55 |
| 14 | 4.46 | 3.79 | −0.67 |
| 15 | 4.44 | 3.76 | −0.68 |
| 16 | 3.53 | 2.69 | −0.84 |
| 17 | 3.34 | 2.43 | −0.91 |
| 18 | 4.38 | 3.20 | −1.18 |
Abbreviations: HIV-1, human immunodeficiency virus type 1; valACV, valacyclovir; VL, viral load.
Figure 1.
Trajectories of plasma HIV RNA by study arm. The left panel shows trajectories of VL in participants who received placebo during 12 weeks. The right panel shows trajectories of VL in participants who received valacyclovir (valACV) during 12 weeks. Abbreviations: HIV, human immunodeficiency virus; VL, viral load.
HHV DNA in PBMC of the Enrolled Subjects
HHV DNAs were measured in PBMCs at enrollment. The limits of detection of the PCR assays for HHV DNAs were between 5 and 10 copies depending on the virus [22]. HSV-2 positive individuals were excluded from the trial; as expected, we did not find HSV-2 DNA in PBMC of any enrolled individuals. Also, HSV-1, VZV, or cytomegalovirus (CMV) DNA was not found. Other HHV DNAs were identified however. Epstein–Barr virus (EBV) DNA was present in PBMC of all 18 subjects. HHV-7 and HHV-6 DNAs were found in PBMCs samples of 15 and 6 individuals, respectively. HHV-8 DNA was found in one patient sample. Levels of viral DNA varied significantly for each HHV. EBV DNA content ranged from 127 to 8692 copies per 106 erv-3 cellular genes; HHV-7 ranged from 447 to 50 767 copies per 106 erv-3 cellular genes; HHV-6 ranged from 44 to 146 copies per 106 erv-3 cellular genes. No correlations were observed between the HHV DNA copy number and the change in HIV-1 VL during the treatment.
Seroprevalence for HHVs was determined at enrollment and at completion of the study. We found a prevalence of anti-EBV, anti-CMV, anti-HHV-6, and anti-HSV-1 antibodies, respectively, of 94% (17/18), 89% (16/18), 83% (15/18), and 78% (14/18) at enrollment. One patient seroconverted for HSV-2 during the study.
The concordance between the HHV DNA in PBMC and seropositivity varied for different HHVs: EBV DNA and serology were concordant in all tested patients except one in which serology was negative while PCR was positive for EBV DNA. A similar pattern was observed for HHV-6 in which a positive serology was found in 5 out of the 6 patients who had a positive viral DNA PCR.
PCR and serology were discordant for CMV and HSV-1 as no viral DNA was detected by PCR in any of the tested samples, whereas serology was positive in 89% and 78% of the study subjects.
Emergence of Drug Resistance Mutations in HIV-1 RT
Prolonged exposure to high dose ACV in vitro has been associated with the development of resistance mutations in HIV-1 RT [28, 29]. Here, we sequenced the HIV-1 RT in plasma samples from enrolled subjects to investigate whether ACV selective pressure is associated with the emergence of resistance mutations in vivo. Conventional sequencing determined that all subjects were infected with clade B HIV-1 (data not shown). Ultradeep pyrosequencing was successfully performed in a total of 34 samples. Nine samples (from 6 different subjects) of the 34 samples analyzed (26%) exhibited DRM with a score of ≥1% based on Stanford v6.3.
DRM were found in 9 samples from 6 subjects. In group A, one subject had V118I (2.5%) at week-26 post-placebo; a second subject had D67N (19.9%) and K70R (99.7%) at week-0 pre-valACV treatment, and a third subject had 5 DRM (T69D (1.1%), T69N (98.4%), K101E (13.7%), V108I (5.1%), V118I (15.4%)) at week-0 pre-valACV and 3 DRM at week-12 post-valACV (T69D (29.2%), T69N (70.2%), V118I (13.7%)), and the same 3 DRM at week-26 post-placebo (T69D (14.8%), T69N (84.8%), V118I (36.7%)).
In group B, in 3 subjects with DRM, there was V75I (19.9%) at week-0 pre-placebo, and K70R (98.3%) at week-26 post-valACV in 1 subject. The second subject also had V75I (5.9%) at week-26 post-valACV. The third subject had DRM K70R (5.4%) at week-26 post-valACV.
DISCUSSION
Accumulating evidence suggests that a number of copathogens including viruses can affect HIV transmission and pathogenesis. In particular, HSV-2 infection increases the efficiency of both acquisition and transmission of HIV-1 and is associated with increased plasma and genital HIV-1 levels (reviewed in [21]). The effect on acquisition and transmission was attributed to the local disruption of epithelial genital barriers and to the enrichment of submucosal layers with activated immune cells, whereas the effect on HIV-1 replication was ascribed to recruitment of target cells and increased systemic inflammation [21]. Therefore, suppression of HSV-2 with ACV seemed to be a meaningful strategy for both reducing HIV-1 transmission and delaying HIV-1 disease progression by reducing HIV-1 levels.
Over the past 7 years, at least 14 clinical trials have demonstrated that ACV treatment, though ineffective in preventing HIV-1 transmission and acquisition [13, 14, 30], resulted in reduced genital and plasma HIV-1 levels [7–20] and in some cases, delayed HIV-1 disease progression in coinfected individuals [5, 6, 14, 19, 31].
Until recently, the decrease of HIV-1 VL by ACV has been attributed to the suppression of HSV-2-triggered systemic inflammation as no direct effect of ACV on HIV-1 replication could be documented in in vitro studies.
We recently reported in studies utilizing human tissues ex vivo that ACV suppresses HIV-1 replication directly and acts as an HIV-1 RT inhibitor [22]. In those ex vivo systems, HIV-1 suppression did not require HSV-2 but was conditioned to the presence of other HHVs.
Here we evaluated the effect of valACV, a prodrug of ACV, on plasma HIV-1 RNA levels in HIV-1 infected individuals not coinfected with HSV-2. ValACV was used because of its enhanced bioavailability relative to ACV.
On average, valACV reduced HIV-1 VL in plasma by 0.37 log10 copies/mL. Plasma HIV-1 concentrations rebounded to pre-enrollment levels within 2 weeks of termination of valACV treatment, consistent with short drug half-life and lack of intracellular accumulation. Although the effect of valACV on plasma HIV-1 RNA is modest, it is comparable to monotherapy with other antiretrovirals. For example, an approximately 0.5 log10 decrease in HIV-1 viremia was reported for either zidovudine or stavudine monotherapy [32, 33]. Moreover, the reduction of plasma HIV-1 VL mediated by ACV could have a measurable beneficial biological effect, as mathematical modeling suggests that progression to an AIDS-defining illness or death decreases by 25% with every 0.3 log10 decrement in plasma HIV-1 RNA [34] and that a decrease in plasma HIV-1 RNA of 0.74 log10 copies/mL reduces heterosexual transmission risk by 50% [35].
The reduction of HIV-1 VL observed in our study provides evidence that the effect of ACV on HIV-1 VL is not restricted to the presence of HSV-2 and suggests that ACV has a direct anti-HIV-1 activity in vivo. A direct effect of ACV on HIV-1 RT rather than an indirect effect on systemic inflammation is also supported by the results of other more recent clinical trials: (i) In a controlled trial assessing the impact of valACV on systemic immune activation and inflammation in ART-treated patients, valACV had no impact on the fraction of activated CD8+ T cells, hsCRP, interleukin 6 (IL-6), or sICAM-1, or on other immune and inflammatory markers [36]; (ii) Although treatment with valACV of HIV-1 coinfected pregnant women was associated with lower plasma HIV-1 RNA levels, the frequencies of both CD4+ and CD8+ CD38+HLA-DR+ T cells in these subjects was not different from those in placebo recipients [37]; (iii) Although both ACV and valACV completely suppressed genital HSV-2 shedding, valACV treatment was associated with a greater reduction in HIV-1 VL than ACV [20].
Our trial supports the evidence that ACV directly inhibits HIV-1 in vivo and that HSV-2 is not required for this effect. As expected, however, we found that almost every subject enrolled in this study carried one or several other HHVs, as evaluated by the presence of HHV DNAs. Moreover, serology showed the presence of anti-HHV antibodies, an evidence of HHV infection history. Although we previously demonstrated that HHVs mediate phosphorylation of ACV and are sufficient and necessary to confer anti-HIV-1 activity to ACV ex vivo, we did not find any correlation between the pattern of infection with different HHVs and the extent of HIV-1 VL reduction in our trial. It remains unknown whether the TK of these HHVs is sufficient to mediate ACV phosphorylation, or if an unknown cellular kinase [24] is critical for ACV phosphorylation and the subsequent anti-HIV-1 effect observed in vivo.
Another important question is whether ACV administration may lead to the selection of resistance mutations. In in vitro studies, a V75I mutation in RT renders HIV-1 less sensitive to ACV; this mutation was selected under the pressure of supratherapeutic dose of ACV or its prodrug derivative in both single cell cultures of CD4+ lymphoblasts and in the MT-4 cell line [28, 29]. In contrast, in vivo HSV-2 suppressive therapy using therapeutic concentrations of ACV or valACV did not select for specific HIV-1 resistance in HIV-1/HSV-2 coinfected persons [38–40]. To assess whether or not HIV-1 DRM were selected by valACV therapy in this study, we performed ultradeep sequencing of the HIV-1 RT. Although our findings of decreased HIV-1 levels in plasma imply that there was some selection pressure on HIV-1 and presumably on its RT, our findings were consistent with the 3 previously published studies, which showed that valACV did not select for specific HIV-1 resistance mutations [38–40]. Indeed, in the present in vivo study, the V75I mutation was only found in 2 individuals and at low frequency (<20%); one appeared only at baseline of week-0 pre placebo, and the other only at week-26 post-ACV. Despite these findings, we cannot discount the possibility that a more pronounced evolution of specific HIV-1 ACV resistance mutations might have been observed following a longer period (>12 weeks) of valACV monotherapy. The discrepancy between in vitro and in vivo results may be explained by the weaker selective pressure exerted by low-dose ACV and a significant fitness cost that may outweigh the advantage of ACV resistance. Indeed, a comparison of the fitness of wild-type vs V75I virus in the presence of ACV in the single round infectivity assay showed that replication of wild-type virus is favored over the mutant [29].
Currently, the primary indication for use of ACV or valACV in HIV-1 infected individuals remains treatment of HSV-2 clinical infection. Our data indicate that valACV may decrease HIV-1 VL in HIV-1-infected HSV-2-negative patients, and thus the drug's benefits for HIV-infected patients may go beyond the suppression of HSV-2. Larger randomized trials and cost effectiveness analyses could be warranted to further explore the potential of this antiviral agent in the context of HIV-1 infection, in particular in combination with other antivirals.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We gratefully acknowledge the contributions of the participants for this study. We also thank Payel Chatterjee for ultradeep sequencing sample preparation and Dr Ting Nie for Sanger sequencing assistance (Emory University).
Financial support. This research was supported, in part, by the Intramural Research Program of the National Institute of Child Health and Human Development, National Institute of Health (NIH) and by the NIH Bench-to-Bedside Program. M. M. L. and B. R. are supported in part by NIH grants AI 68636, AI 76174 and by the CWRU Center for AIDS Research AI36219. R. F. S. is partly supported by the NIH sponsored CFAR grant 2P30-AI-050409 and the Department of Veterans Affairs.
Potential conflicts of interest. All authors: No potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Elion GB. The biochemistry and mechanism of action of acyclovir. J Antimicrob Chemother 1983; 12(suppl B):9–17. [DOI] [PubMed] [Google Scholar]
- 2.Patil SD, Koga M, Schneller SW, Snoeck R, De Clercq E. (+-)-carbocyclic 5′-nor-2′-deoxyguanosine and related purine derivatives: synthesis and antiviral properties. J Med Chem 1992; 35:2191–5. [DOI] [PubMed] [Google Scholar]
- 3.Jeong LS, Yoo SJ. Synthesis and antiviral activity of novel isodideoxy nucleosides with exocyclic methylene. Bioorg Med Chem Lett 1998; 8:847–52. [DOI] [PubMed] [Google Scholar]
- 4.Balzarini J, Haller-Meier F, De Clercq E, Meier C. Antiviral activity of cyclosaligenyl prodrugs of acyclovir, carbovir and abacavir. Antivir Chem Chemother 2001; 12:301–6. [DOI] [PubMed] [Google Scholar]
- 5.Mitsuya H, Broder S. Strategies for antiviral therapy in AIDS. Nature 1987; 325:773–8. [DOI] [PubMed] [Google Scholar]
- 6.Ioannidis JP, Collier AC, Cooper DA, et al. Clinical efficacy of high-dose acyclovir in patients with human immunodeficiency virus infection: a meta-analysis of randomized individual patient data. J Infect Dis 1998; 178:349–59. [DOI] [PubMed] [Google Scholar]
- 7.Ouedraogo A, Nagot N, Vergne L, et al. Impact of suppressive herpes therapy on genital HIV-1 RNA among women taking antiretroviral therapy: a randomized controlled trial. AIDS 2006; 20:2305–13. [DOI] [PubMed] [Google Scholar]
- 8.Nagot N, Ouedraogo A, Foulongne V, et al. Reduction of HIV-1 RNA levels with therapy to suppress herpes simplex virus. [see comment]. N Engl J Med 2007; 356:790–9. [DOI] [PubMed] [Google Scholar]
- 9.Zuckerman RA, Lucchetti A, Whittington WL, et al. Herpes simplex virus (HSV) suppression with valacyclovir reduces rectal and blood plasma HIV-1 levels in HIV-1/HSV-2-seropositive men: a randomized, double-blind, placebo-controlled crossover trial. J Infect Dis 2007; 196:1500–8. [DOI] [PubMed] [Google Scholar]
- 10.Baeten JM, Strick LB, Lucchetti A, et al. Herpes simplex virus (HSV)-suppressive therapy decreases plasma and genital HIV-1 levels in HSV-2/HIV-1 coinfected women: a randomized, placebo-controlled, cross-over trial. J Infect Dis 2008; 198:1804–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunne EF, Whitehead S, Sternberg M, et al. Suppressive acyclovir therapy reduces HIV cervicovaginal shedding in HIV- and HSV-2-infected women, Chiang Rai, Thailand. J Acquir Immune Defic Syndr 2008; 49:77–83. [DOI] [PubMed] [Google Scholar]
- 12.Delany S, Mlaba N, Clayton T, et al. Impact of aciclovir on genital and plasma HIV-1 RNA in HSV-2/HIV-1 co-infected women: a randomized placebo-controlled trial in South Africa. AIDS 2009; 23:461–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Celum C, Wald A, Lingappa JR, et al. Acyclovir and transmission of HIV-1 from persons infected with HIV-1 and HSV-2. N Engl J Med 2010; 362:427–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lingappa JR, Baeten JM, Wald A, et al. Daily acyclovir for HIV-1 disease progression in people dually infected with HIV-1 and herpes simplex virus type 2: a randomised placebo-controlled trial. Lancet 2010; 375:824–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanton C, Weiss HA, Rusizoka M, et al. Long-term impact of acyclovir suppressive therapy on genital and plasma HIV RNA in Tanzanian women: a randomized controlled trial. J Infect Dis 2010; 201:1285–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mugwanya K, Baeten JM, Mugo NR, Irungu E, Ngure K, Celum C. High-dose valacyclovir HSV-2 suppression results in greater reduction in plasma HIV-1 levels compared with standard dose acyclovir among HIV-1/HSV-2 coinfected persons: a randomized, crossover trial. J Infect Dis 2011; 204:1912–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drake AL, Roxby AC, Ongecha-Owuor F, et al. Valacyclovir suppressive therapy reduces plasma and breast milk HIV-1 RNA levels during pregnancy and postpartum: a randomized trial. J Infect Dis 2012; 205:366–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roxby AC, Drake AL, Ongecha-Owuor F, et al. Effects of valacyclovir on markers of disease progression in postpartum women co-infected with HIV-1 and herpes simplex virus-2. PloS One 2012; 7:e38622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reynolds SJ, Makumbi F, Newell K, et al. Effect of daily aciclovir on HIV disease progression in individuals in Rakai, Uganda, co-infected with HIV-1 and herpes simplex virus type 2: a randomised, double-blind placebo-controlled trial. Lancet Infect Dis 2012; 12:441–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perti T, Saracino M, Baeten JM, et al. High-dose valacyclovir decreases plasma HIV-1 RNA more than standard-dose acyclovir in persons coinfected with HIV-1 and HSV-2: a randomized crossover trial. J Acquir Immune Defic Syndr 2013; 63:201–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van de Perre P, Segondy M, Foulongne V, et al. Herpes simplex virus and HIV-1: deciphering viral synergy. Lancet Infect Dis 2008; 8:490–7. [DOI] [PubMed] [Google Scholar]
- 22.Lisco A, Vanpouille C, Tchesnokov EP, et al. Acyclovir is activated into a HIV-1 reverse transcriptase inhibitor in herpesvirus-infected human tissues.[see comment]. Cell Host & Microbe 2008; 4:260–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McMahon MA, Siliciano JD, Lai J, et al. The anti-herpetic drug acyclovir inhibits HIV replication and selects the V75i reverse transcriptase multi-drug resistance mutation. J Biol Chem 2008; 283:31289–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McMahon MA, Parsons TL, Shen L, Siliciano JD, Siliciano RF. Consistent inhibition of HIV-1 replication in CD4+ T cells by acyclovir without detection of human herpesviruses. J Virol 2011; 85:4618–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones B, Kenward MG. Design and analysis of cross-over trials. 2nd ed. Chapman & Hall/CRC monographs on statistics & applied probability, 2003.
- 26.Senn S. Cross-Over Trials in Clinical Research. In: Chichester JWaS, 2002:35–88. [Google Scholar]
- 27.Hills M, Armitage P. The two-period cross-over clinical trial. Br J Clin Pharmacol 1979; 8:7–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tchesnokov EP, Obikhod A, Massud I, et al. Mechanisms associated with HIV-1 resistance to acyclovir by the V75I mutation in reverse transcriptase. J Biol Chem 2009; 284:21496–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McMahon MA, Siliciano JD, Kohli RM, Siliciano RF. Sensitivity of V75I HIV-1 reverse transcriptase mutant selected in vitro by acyclovir to anti-HIV drugs. AIDS 2010; 24:319–23. [DOI] [PubMed] [Google Scholar]
- 30.Watson-Jones D, Weiss HA, Rusizoka M, et al. Effect of herpes simplex suppression on incidence of HIV among women in Tanzania.[see comment]. N Engl J Med 2008; 358:1560–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ludema C, Cole SR, Poole C, Chu H, Eron JJ. Meta-analysis of randomized trials on the association of prophylactic acyclovir and HIV-1 viral load in individuals coinfected with herpes simplex virus-2. AIDS 2011; 25:1265–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Katzenstein DA, Hughes M, Albrecht M, et al. Virologic and CD4+ cell responses to new nucleoside regimens: switching to stavudine or adding lamivudine after prolonged zidovudine treatment of human immunodeficiency virus infection. ACTG 302 Study Team. AIDS Clinical Trials Group. AIDS Res Hum Retroviruses 2000; 16:1031–7. [DOI] [PubMed] [Google Scholar]
- 33.Rey D, Hughes M, Pi JT, Winters M, Merigan TC, Katzenstein DA. HIV-1 reverse transcriptase codon 215 mutation in plasma RNA: immunologic and virologic responses to zidovudine. The AIDS Clinical Trials Group Study 175 Virology Team. J Acquir Immune Defic Syndr Hum Retrovirol 1998; 17:203–8. [DOI] [PubMed] [Google Scholar]
- 34.Modjarrad K, Chamot E, Vermund SH. Impact of small reductions in plasma HIV RNA levels on the risk of heterosexual transmission and disease progression. AIDS 2008; 22:2179–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lingappa JR, Hughes JP, Wang RS, et al. Estimating the impact of plasma HIV-1 RNA reductions on heterosexual HIV-1 transmission risk. PloS One 2010; 5:e12598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yi TJ, Walmsley S, Szadkowski L, et al. A randomized controlled pilot trial of valacyclovir for attenuating inflammation and immune activation in HIV/herpes simplex virus 2-coinfected adults on suppressive antiretroviral therapy. Clin Infect Dis 2013; 57:1331–8. [DOI] [PubMed] [Google Scholar]
- 37.Roxby AC, Liu AY, Drake AL, et al. Short communication: T cell activation in HIV-1/herpes simplex virus-2-coinfected Kenyan women receiving valacyclovir. AIDS Res Hum Retroviruses 2013; 29:94–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim HN, Wang J, Hughes J, et al. Effect of acyclovir on HIV-1 set point among herpes simplex virus type 2-seropositive persons during early HIV-1 infection. J Infect Dis 2010; 202:734–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.LeGoff J, Tanton C, Delaugerre C, et al. No selection of nucleoside reverse transcriptase inhibitor resistance associated mutations by acyclovir suppressive therapy in herpes simplex virus-2/HIV-1 dually infected persons. AIDS 2010; 24:2595–6. [DOI] [PubMed] [Google Scholar]
- 40.Baeten JM, Lingappa J, Beck I, et al. Herpes simplex virus type 2 suppressive therapy with acyclovir or valacyclovir does not select for specific HIV-1 resistance in HIV-1/HSV-2 dually infected persons. J Infect Dis 2011; 203:117–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.