Abstract
Aims
Ets1 is an important transcription factor that is expressed in both the cardiac neural crest (NC) and heart mesoderm of vertebrate embryos. Moreover, Ets1 deletion in humans results in congenital heart abnormalities. To clarify the functional contributions of Ets1 in cardiac NC vs. heart mesoderm, we performed tissue-targeted loss-of-function analysis to compare the relative roles of Ets1 in these two tissues during heart formation using Xenopus embryos as a model system.
Methods and results
We confirmed by in situ hybridization analysis that Ets1 is expressed in NC and heart mesoderm during embryogenesis. Using a translation-blocking antisense morpholino to knockdown Ets1 protein selectively in the NC, we observed defects in NC delamination from the neural tube, collective cell migration, as well as segregation of NC streams in the cranial and cardiac regions. Many cardiac NC cells failed to reach their destination in the heart, resulting in defective aortic arch artery formation. A different set of defects was noted when Ets1 knockdown was targeted to heart mesoderm. The formation of the primitive heart tube was dramatically delayed and the endocardial tissue appeared depleted. As a result, the conformation of the heart was severely disrupted. In addition, the outflow tract septum was missing, and trabeculae formation in the ventricle was abolished.
Conclusion
Our study shows that Ets1 is required in both the cardiac NC and heart mesoderm, albeit for different aspects of heart formation. Our results reinforce the suggestion that proper interaction between these tissues is critical for normal heart development.
Keywords: Ets1, Cardiac neural crest, Heart mesoderm, Aortic arch artery, Endocardium
1. Introduction
In all vertebrate embryos, including humans, heart formation involves the integrated morphogenesis of different cell populations with distinct embryonic origins.1 The anterior portion of lateral plate mesoderm contains two presumptive heart anlage that subsequently fuse into a single median heart anlagen, which in turn becomes subdivided into the first heart field that gives rise to the primitive cardiac tube and the second heart field that incorporates into the primitive heart via the arterial and venous poles.2
Vertebrates also have an important cardiac neural crest (NC) contribution to heart development. Arising from the caudal hindbrain, cardiac NC cells in amniotes have been shown to migrate under the ectoderm and settle in the circumpharyngeal area of branchial arches 3, 4, and 6.3 Some NC cells remain in the arches and contribute to the aortic arch arteries,4 whereas others migrate along the aortic arch arteries to the heart and contribute to the outflow tract (OFT) septum that subdivides the OFT into the aorta and pulmonary trunk. This is critical since it effectively separates oxygenated from deoxygenated blood circulation. Cardiac NC cells of amniotes also populate the cardiac cushions, inflow tracts of the heart, the ventricular septum and form the cardiac ganglia.5,6 Both the cardiac NC and heart mesoderm are critical for heart development and many proteins have been identified in each tissue to regulate heart development, including Nkx2-5, Tbx5, Tbx20, Pax6, ADAM19, GATA6,7–16 and Ets1.
Ets1 is a member of a family of winged helix-turn-helix transcription factors that has important roles in a wide variety of biological processes, including regulation of cellular growth, differentiation, migration, and organ development.17 Of particular relevance here, it has been shown to have an important and phylogenetically ancient role in heart development in animals ranging from fly to human. For example, in Drosophila, Ets1 regulates the cardioblast/pericardial cell fate decision.18 In the urochordate, Ciona intestinalis, Ets1/2 directs cardiac lineage specification.19 Zebrafish Ets1 and several Ets1-related proteins (Etsrp/Etv/Fli1) are important for endothelial differentiation and vasculogenesis.20,21 In chick, intravenous injection of antisense Ets1/2 retrovirus affects myocardial morphology and the endocardial to mesenchymal transition, leading to disorganized coronary arteries and ventricular septal defects.22
In addition to its well-characterized presence in the endothelial cell lineage, Ets1 is also expressed in the pre-migratory and migrating cranial, including cardiac, but not trunk NC cells.23,24 In Ets1 knockout mice, cardiac NC failed to enter the proximal portion of the OFT, resulting in failure of membranous interventricular septum formation. Loss of Ets1 also leads to vascular inflammation and remodelling.25 Similarly, deletion of a genomic region containing the Ets-1 gene in humans (Jacobsen syndrome) results in failure of interventricular septum formation.26 However, the relative contributions of Ets1 in cardiac NC vs. heart mesoderm have not been compared in the same animal model. Thus, it remains unclear whether one or both of these tissues contributes to the major heart problems in Ets1 patients.
Here, we take advantage of the power of experimental embryology in the frog, Xenopus laevis, to investigate the role of Ets1 in heart development individually in the NC and heart mesoderm. Xenopus has a three-chambered heart, with one ventricle and a spiral septum that partially separates the OFT, resembling the early phase of conotruncal ridge formation in birds and mice.27 Despite the incomplete physical barrier, it provides a fairly good functional separation of systemic and pulmonary blood at the arterial pole. Xenopus embryos offer several advantages for studying the relative contributions of NC vs. mesoderm to the developing heart. Its accessibility and well-established fate map allows precise targeting of knock-downs to particular tissue. Moreover, development of Xenopus embryos can proceed in the absence of a functional circulation system, allowing defects to be analysed in depth in living embryos.
The results show that Ets1 is expressed in both NC cells and in heart mesodermal cells during Xenopus development. In cardiac NC, Ets1 mainly functions in their delamination and migration, with its loss resulting in defective aortic arch arteries as well as a misshaped OFT, similar to defects observed in DiGeorge patients. In contrast, loss of Ets1 in heart mesoderm leads to severe malformations including loss of the entire endocardium and formation of a single heart chamber comprising a thick layer of cardiomyocytes, a phenotype similar to hypoplastic left heart (HLH) syndrome in humans. These results show that Ets1 in both the cardiac NC cells and heart mesoderm is critical for normal heart formation, with different roles in the two tissues.
2. Methods
Details of experimental methods are provided in Supplementary material online.
2.1. Embryo manipulations, morpholino oligomers, and RNA preparation
Xenopus laevis embryos were microinjected with capped RNAs or morpholino oligomers (MOs) during early cleavage stages. Ets1-MO, RNAs of nuclear beta-galactosidase (nβGal), membrane-tethered EGFP, and mutated Ets1a and Ets1b and their doses used were described in Supplementary material online, Methods. The well-described X. laevis fate map was used to differentially target NC or cardiac mesoderm.
2.2. In situ hybridization
Whole-mount in situ hybridization was performed as previously described28 (also see Supplementary material online, Method). Antisense probes for Ets1a, Ets1b, Twist, Snail2, Sox10, NeuroD, Pax6, Sox3, Nkx2-5, Tbx20, Tbx1, and Mef2 were synthesized with T7 RNA polymerase with linearized plasmid.
2.3. Cranial NC grafting and microscopy
Cranial NC explants were dissected from EGFP-labelled donor embryos at stage 13–1429–31 and inserted isotopically and isochronically into unlabelled host embryos from which cranial NC tissue was removed. The grafted embryos were allowed to heal in MBSH media for 3 h and then transferred to 0.1× MMR before imaged at late tailbud stages. Time-lapse movies were recorded for 12 h using Zeiss LSM 5 EXCITER confocal microscope with a ×10 objective lens to follow the migratory behaviour of grafted NC cells in vivo from stage 16 to stage 30.
3. Results
3.1. Ets1 is expressed in both cardiac NC and heart mesoderm during frog development
In amniotes and likely all vertebrates, both the heart mesoderm and cardiac NC make important contributions to the heart and the OFT. As a first step in understanding the possible functions of this gene in heart development, we examined the expression pattern of the two Ets-1 paralogs in Xenopus to determine which may be functionally important for heart development. By in situ hybridization analysis, both Ets1a and Ets1b are expressed in NC cells as well as in heart mesoderm, although there are some distinct differences in the timing and level of expression (Figure 1A). Ets1a is weakly expressed in the neural plate border as well as in non-neural ectoderm during stages of NC specification (stages 12–14). Overlapping the anterior border of neural plate, Ets1a is also expressed in heart mesoderm. At stage 16, Ets1a is strongly expressed in both pre-migratory NC cells (arrow) and heart mesoderm (first heart field and blood islands, arrowhead), and faintly expressed in epidermis. In early tailbud stages (stages 22–24), migrating NC cells express Ets1a as they enter the branchial arches. The future cardiac NC cells reside within the 3rd and 4th migratory streams. At early tailbud stages shown in Figure 1, the 3rd and 4th migratory streams have not yet completely separated. In the heart mesoderm, Ets1a becomes restricted progressively, such that it is only expressed in blood islands by tailbud stages.
Figure 1.
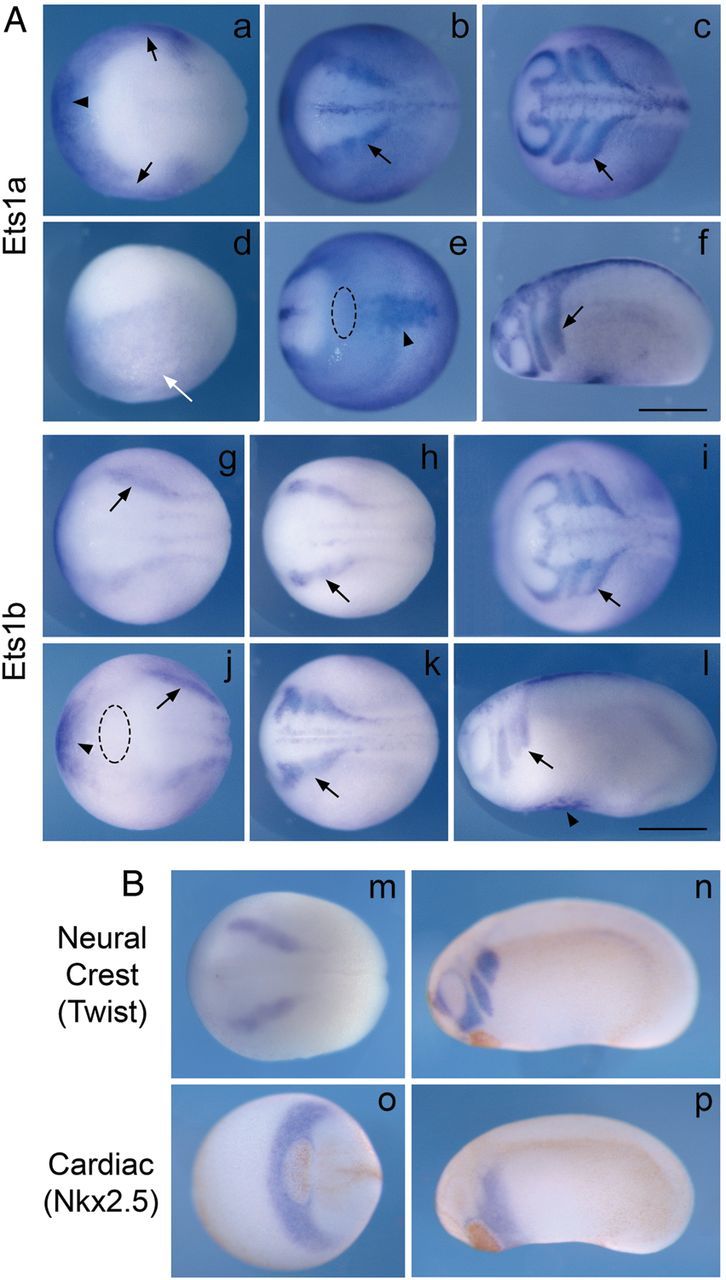
Est1a and Ets1b are expressed in cardiac NC and heart mesoderm during frog development. (A) In situ hybridization analysis shows that both Ets1a (upper panels) and Ets1b (bottom panels) are expressed in pre-migratory and migrating NC cells (black arrows) as well as heart mesodermal cells (arrowheads). Ets1a is also expressed in epidermis (white arrow). (B) The expression patterns of the NC gene Twist and cardiac gene Nkx2-5 at neurula and tailbud stages are shown for comparison. Panels a, d, g, and j are at late gastrula stages (st. 12.5–13); b, e, h, m, and o are at early neurula stages (st. 14–15); k is at late neurula stage (st. 18); c, f, i, l, n, and p are at early tailbud stages (st. 23–24). Panels a, b, c, g, h, k, m are dorsal views; d, f, l, n, and p are lateral views; e is ventral view; i, j and o are anterior views. Dotted ellipse in e and j marks the cement gland (also the brown regions in panels n–p). All embryos have their head to the left. Scale bar = 0.5 mm.
Ets1b is expressed more prominently than Ets1a in presumptive NC cells at the neural plate border starting at stage 12. It is expressed at a similarly robust level in heart mesoderm. Its expression persists in both NC cells (arrows) and heart mesodermal cells (arrowheads) as embryos develop to tailbud stages. Ets1b expression is slightly broader in the heart mesoderm than Ets1a at later stages. Its expression persists in the blood islands and first heart field at tailbud stages. Figure 1B shows the expression of NC marker gene Twist and cardiac marker gene Nkx2-5 at early neurula and tailbud stages for comparison. Nkx2-5 is expressed in the cardiac crescent (mainly second heart field at these stages) adjacent and posterior to the cement gland. The first heart field is immediately posterior to the second heart field. Taken together, these results show that Ets1a and Ets1b have largely overlapping expression patterns suggesting redundancy during heart development, with Ets1b being the more prominent transcript.
3.2. NC formation occurs in the absence of Ets1
Since both Ets1a and Ets1b are expressed in the presumptive cranial NC, we examined whether Ets1 plays an early role in NC development. A single translation-blocking MO was used to inhibit Ets1 protein expression since both Ets1a and Ets1b share a similar translation start site (see Methods). Ets1-MO was injected into one of the dorsal animal cells of Xenopus embryos at 8-cell stage, together with a lineage tracer (nβGal). In this way, it is not only possible to target the presumptive NC on one side of the embryo, but also to compare between the injected and uninjected side as an internal control. Embryos were collected at the neurula stage and in situ hybridization was performed with probes against NC and neural specification genes, such as Snail2, Sox3, and NeuroD. As shown in Figure 2, the expression of Sox10 at early neurula stages was slightly reduced on the Ets1-MO vs. control side of the same embryo (arrowhead, n = 5/13). However, Sox10 expression recovered at later stages, suggesting that Ets1 is not essential for NC formation. Since Ets1 is an important input for Sox10 and FoxD3,32,33 loss of Ets1 most likely delays, but does not inhibit the specification program of NC cells. The neural marker Sox3 is highly expressed in the neural tube and faintly expressed in anterior NC migratory streams, and its neural tube expression was unaffected by Ets1-MO. Pax6, a marker for lens placode, was similarly unaffected by the loss of Ets1. In contrast, NeuroD expression in the trigeminal ganglia was abnormal after Ets1 loss. The lateral tip of NeuroD expression was often reduced or even missing (arrowhead). These results indicate that Ets1 may play a role in the migration or arrangements of NC and/or ectodermal placode cells, which together form the trigeminal ganglion.
Figure 2.
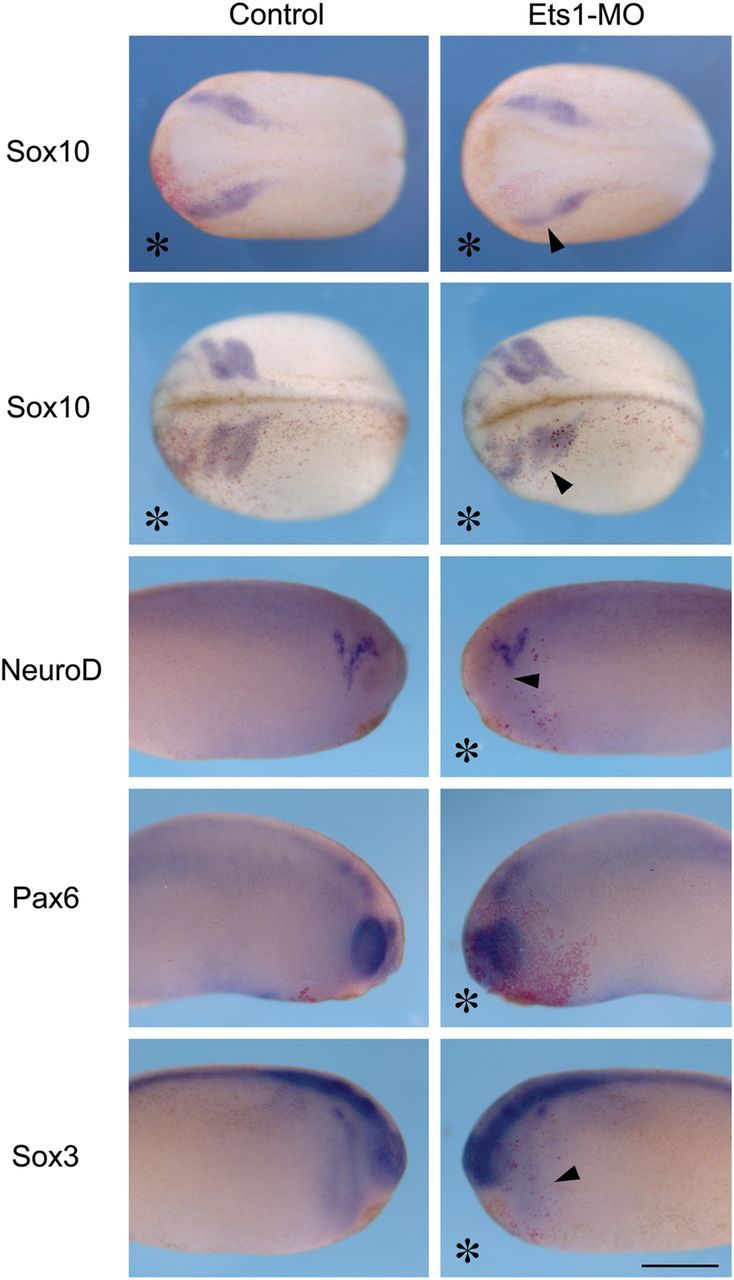
Loss of Ets1 primarily affects NC cell migration. Embryos receiving 10 ng of Ets1-MO on one side (marked by *) were analysed for NC, neural, or placodal gene expression by in situ hybridization. While the expression of Sox10 is slightly reduced on the injected side during NC specification, its expression pattern is strongly altered on the Ets1-MO-injected side at stages of NC migration. Specification of the trigeminal ganglia (NeuroD), lens placode (Pax6), and neural tube (Sox3) is unaffected, although the patterning of trigeminal ganglia is disrupted. These results suggest that Ets1 is mainly required for NC migration. Upper panels are dorsal views with heads to the left, lower panels are lateral views of the same embryo with their heads towards the middle. Scale bar = 0.5 mm. n = 25–40 for each sample.
3.3. Ets1 is required for NC migration
Because the abnormalities in trigeminal ganglion formation may be a secondary effect due to aberrant cell migration, we next examined the migratory behaviour of NC cells by both static and dynamic analysis. First we analysed the expression pattern of the NC gene Sox10, which is prominently expressed by all migrating NC cells at tailbud stages. Our results show that Ets1-MO disrupts NC cell migration when cranial crest cells are migrating towards the branchial arches (Figure 2). NC cells on the Ets1-MO injected side (arrowhead) migrated a much shorter distance than those on the control side of the same embryo.
To confirm this migration defect is autonomous to the NC, cranial NC explants from EGFP-labelled donor embryos, treated with either Ets1-MO or control-MO, were transplanted to wild-type host embryos of the same stage from which the endogenous NC tissue was removed (Figure 3). When control EGFP-labelled cranial NC cells were grafted, they migrated efficiently to the branchial arches and segregated into four migratory streams. In contrast, grafts of Ets1-MO treated NC only migrated short distances and failed to segregate into many migratory streams. Consistent with Ets1’s expression throughout cranial NC, all four cranial (including cardiac) crest streams were affected. To control for specificity of the morpholino knockdown, we conducted a rescue experiment in which embryos were co-injected with Ets1-MO plus 50 pg of mRNA constructs encoding Ets1a and Ets1b, but lacking the MO-recognition site. Under these conditions, NC migration was largely restored, demonstrating that the effect is specifically due to loss of Ets1 rather than off-target effects.
Figure 3.
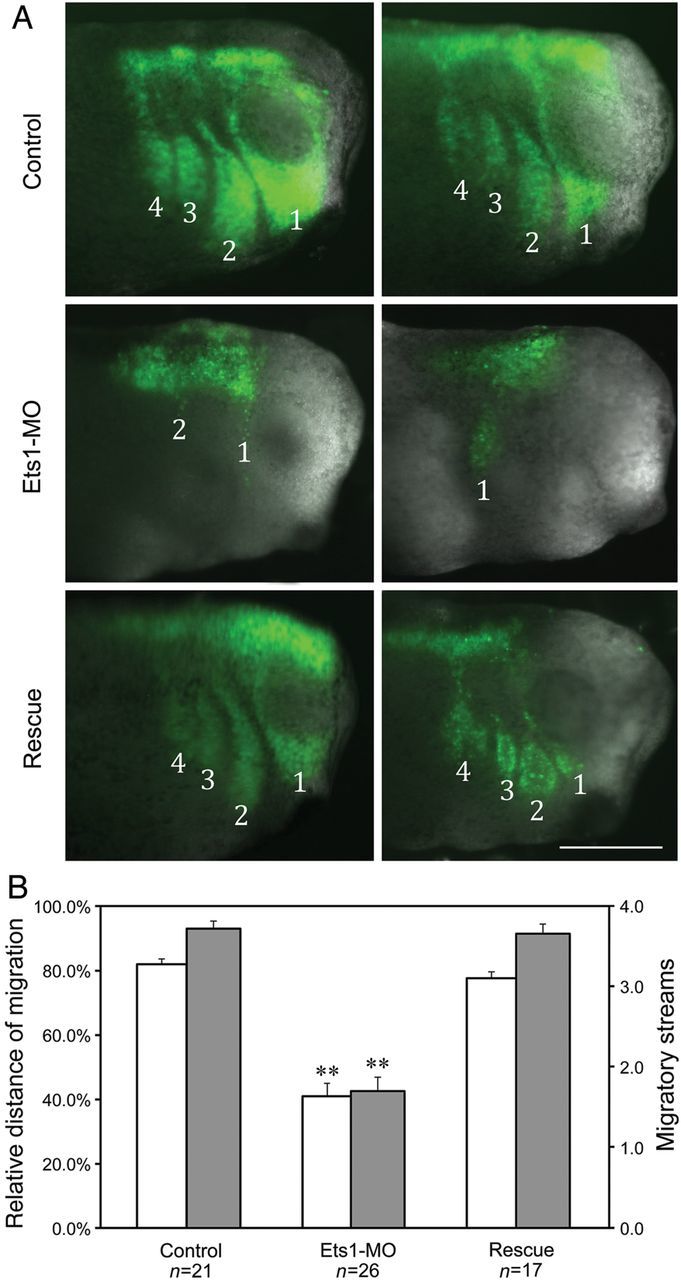
Ets1 is required cell-autonomously for NC migration. Cranial NC explants were dissected from injected embryos and grafted into stage-matched uninjected host embryos. (A) The migration of NC grafts in host embryos was visualized by coexpressed EGFP. While control NC grafts segregated into four migratory steams and migrated into the branchial arches, Ets1-MO expressing grafts failed to migrate very far. There were also fewer migratory streams. Coexpressing Ets1-MO plus Ets1a and Ets1b rescued the effect. Stage 30 embryo heads are shown with anterior to the right. Scale bar = 0.5 mm. (B) The farthest distances of NC migration along the D-V axis were measured in every embryo and the relative migration distance was calculated by comparing that to the entire D-V length of the embryo. In addition, the numbers of migratory streams were counted and summarized in the bar graph. Control grafts on average migrated 82 ± 2% along the D-V axis and segregated into 3.7 ± 0.1 streams. Ets1-MO decreased those into 40 ± 4% of distance and 1.7 ± 0.2 streams. Adding back Ets1a/b rescued the effect significantly, restoring the migration distance to 78 ± 2% of the axis and migratory streams to 3.6 ± 0.1. Asterisks indicates that Ets1-MO inhibited NC migration significantly when comparing to control or rescue conditions (with P < 0.01).
To quantitate these results, we measured the relative distance of migration of the leading NC cells along the dorsal-ventral axis as well as the number of distinct migratory streams. While control grafts travelled over 82% of the entire D-V axis and on average segregated into three to four (avg. 3.7) streams, Ets1-MO grafts only migrated 40% along the axis and segregated into one to two (avg. 1.7) streams. Coinjection of Ets1a/b mRNA efficiently restored migration and stream number to control levels (78% of D-V axis and 3.6 streams; Figure 3B). Both the migration distance and streams of segregation were significantly reduced by Ets1-MO when compared with control or rescue conditions (P << 0.01). In contrast, there was no significant difference between control and rescued conditions (P = 0.1/0/7), suggesting the rescue is efficient.
To perform a dynamic analysis of the effects of loss of Ets1, time-lapse movies were recorded from 3 h post grafting to early tadpole stages to visualize the process of NC migration (Supplementary material online, Movies S1–S3). As expected, control NC cells left the neural tube and rapidly migrated laterally as a cohesive group, undergoing collective cell migration.34 In contrast, many Ets1-MO-treated crest cells failed to leave the neural tube. Those that did migrate away from the neural tube tended to do so as individual cells or small cell clusters, suggesting a loss of collective cell migration. As a result, they migrated shorter distances and in a disorganized manner.
3.4. Ets1 is required in cardiac NC for the formation of aortic arches
We next asked whether these defects in early NC migration might translate to later defects in the NC contribution to the cardiovascular system. To examine these longer-term effects, Ets1-MO was introduced into both sides of the NC forming region and embryos were raised to stages 28 and 45 for analysis.
At stage 28, NC cells have not yet entered the primitive heart tube, which solely comprises heart mesoderm. In haematoxylin and eosin sections through control embryos, we observed a single endothelial heart tube and an almost closed myocardial tube (Figure 4A). After bilateral Ets1-MO knockdown targeted to the NC, the formation of the myocardial tube appeared normal (arrow), but the endocardial cells were delayed such that they were still condensing without a central dilatation (arrowheads). This may due to a minor developmental delay when embryos receive MO.
Figure 4.
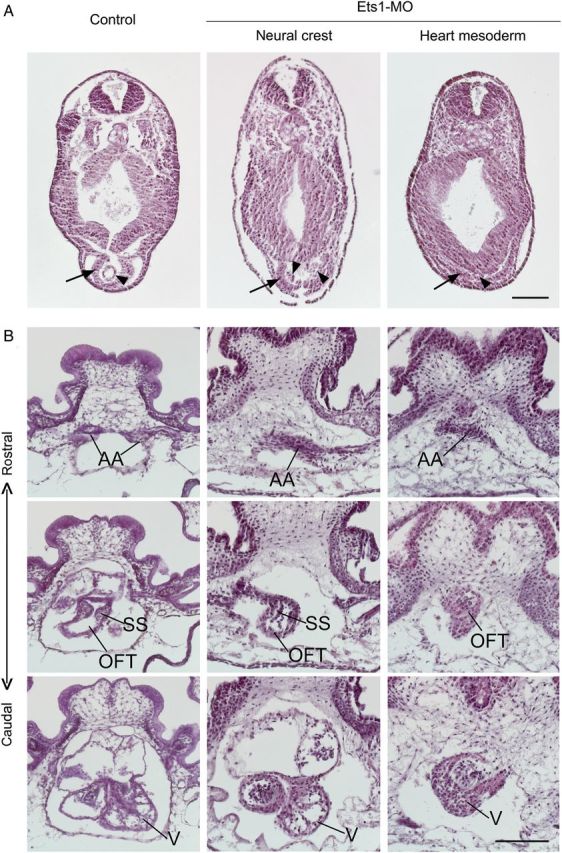
Ets1 is required in both cardiac NC and heart mesoderm for heart formation. Embryos receiving Ets1-MO in NC cells or heart mesoderm on both sides were collected at stage 28 (A) for examining primitive heart tube formation or stage 45 (B) for chambered heart formation. The embryos were transversely sectioned and stained with haematoxylin–eosin for better visualization of their histology. (A) In control embryo, a single endothelial heart tube forms inside a partially closed myocardial heart tube (n = 3). Ets1-MO in NC does not affect such heart tube formation despite a slight delay in the fusion of the endocardial tube (n = 2). In contrast, Ets1-MO expression in heart mesoderm abolishes the formation of the primitive heart tube (n = 4). The layer of cardiac myocytes is present but fails to form a tube. Arrows indicate myocardium and arrowheads indicate endocardium. (B) At stage 45, three-chambered heart is formed. From rostral to caudal sections, paired aortic arch arteries (AA) join at the OFT, which then connects with a single ventricle (V). Spiral septum (SS) shows as partial ridges in the section. Two atria are also visible dorsal to the ventricle. When Ets1 is knocked down in NC, the formation of aortic arch arteries is disrupted (n = 4). The vessels of the artery are not distinct, and often missing on one side. The OFT is also somewhat misshapen, although the spiral septum is still present. Most caudally, the ventricle and atria are largely unaffected. When Ets1-MO is expressed in heart mesoderm, the formation of the heart is essentially destroyed (n = 3). The heart is basically a single chamber with thick myocardium, continues from OFT to ventricle, with blood trapping inside. All sections are arranged with dorsal to the top. Scale bar = 0.2 mm.
By stage 45, the formation of the three-chambered heart is completed in wild-type embryos. In contrast, embryos injected with Ets1-MO into the NC forming region displayed severe craniofacial abnormalities, a slight cardiac dysplasia, and cranial and pericardial edema (Supplementary material online, Figure S1). Rostral to caudal sections through the hearts of control embryos reveal two branches of aortic arch arteries that merge into aortic sac and extend into OFT with a spiral septum, which then goes into the ventricle (Figure 4B). In Ets1-MO expressing embryos, however, the aortic arch arteries were often malformed or missing on one side, consistent with the NC origin of the aortic arch arteries.35 Despite the finding that the OFT was slightly misshapen and the spiral septum was less prominent, the OFT and the ventricle appeared relatively intact. Although there is no evidence that cardiac NC cells contribute to the spiral septum in the OFT, their interaction with heart mesenchyme cells may be required during the formation of the OFT and the spiral septum. The function of the heart appeared affected as well, since blood was trapped in the heart of experimental but not control embryos, suggesting that the circulation was impaired.
3.5. Ets1 is required in heart mesoderm for endocardium development
Ets1 is expressed in both the cranial NC and heart mesoderm of the frog. To test the functional significance of Ets1 in heart mesoderm, Ets1-MO was targeted to both sides of the presumptive cardiac region at 8-cell stage. Embryos were then examined at stage 28 and stage 45 as described above (Supplementary material online, Figure S1). At stage 28, the myocardial layer appeared flat in Ets1-MO embryos compared with the trough shaped myocardium in controls (Figure 4A, right panel). The pericardial cavity was missing and the endocardium consisted of very few, non-descript cells. Such a severe defect suggests problems in the formation of the primitive heart tube from the first heart field.
At stage 45, the formation of the heart was incomplete in Ets1-MO-treated embryos (Figure 4B, right panels). At rostral levels, the aortic arches appeared malformed. More caudally, the OFT was misshapen and the spiral septum was unrecognizable. Finally, the ventricle was underdeveloped, containing trapped blood inside. Interestingly, this phenotype is reminiscent of HLH syndrome in humans, a disorder for which there is no currently existing animal model.36 The heart chamber appeared to be a simple expansion of the OFT that lacked the characteristic trabeculae normally present in its inner wall. Since trabeculae formation requires signals from the endocardium, the failure of trabeculae formation may be a secondary effect to the missing endocardium. These results suggest that Ets1 is critical for endocardium formation during heart development.
Since the heart malformation may be secondary to the loss of endocardium tissue, we next examined the requirement for Ets1 in endocardium formation by directly analysing the expression of cardiac genes. Embryos receiving Ets1-MO on one side, targeted to either NC or heart mesoderm regions, were collected at stage 22 and subjected to in situ hybridization against Nkx2-5, Tbx20, and Tbx1 (Figure 5 and Supplementary material online, Figure S2). When Ets1-MO was targeted to the NC, all three genes were expressed symmetrically in the bilateral heart primodia (n = 13–15). In contrast, when Ets1-MO was targeted to cardiogenic region, the expression of cardiac genes was affected. While the expression area of Nkx2-5 was mildly reduced (n = 10/17), the expression level and area of Tbx20 were decreased significantly (arrow, n = 15/19). Tbx20 is normally expressed near the ventral midline, where the two heart primodium meet to form the primitive heart tube. However, this expression was largely abolished on the Ets1-MO injected side. Similarly, Tbx1 expression in heart primodium was also reduced (closed arrowheads). The reduced expression of cardiac genes was rescued by adding back Ets1 in cardiac mesoderm. Since Nkx2-5 is mainly expressed in the myocardium, while Tbx20 is expressed in both myocardium and endocardium, including high levels in endocardial cushions,37,38 their differential responses to Ets1 knockdown suggest that Ets1 is specifically required for endocardium development.
Figure 5.
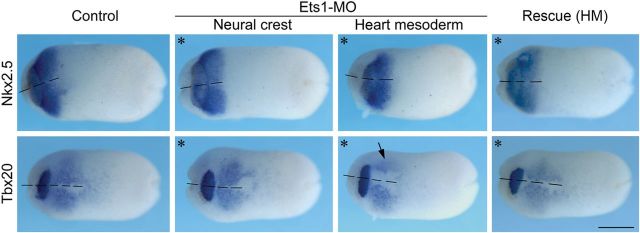
Ets1-MO is required for endocardial gene expression. Embryos receiving 10 ng of Ets1-MO on one side (marked by asterisk) were analysed at stage 22 for cardiac gene expression by in situ hybridization. When Ets1-MO was targeted to NC cells, the expression of cardiac genes was not affected (n = 13–15). In contrast, Ets1 knockdown in heart mesoderm reduced the expression of Nkx2-5 and Tbx20. While the expression of myocardial gene Nkx2-5 was only mildly reduced (n = 10/17), the expression of Tbx20 that is required for both myocardium and endocardium was markedly decreased (arrow, n = 15/19). The reduction in cardiac gene expression was rescued by coexpressing Ets1. All panels are ventral views with anterior to the left. The dashed lines mark the ventral midline. Scale bar = 0.5 mm.
3.6. Ets1 is also required for cranial cartilage formation
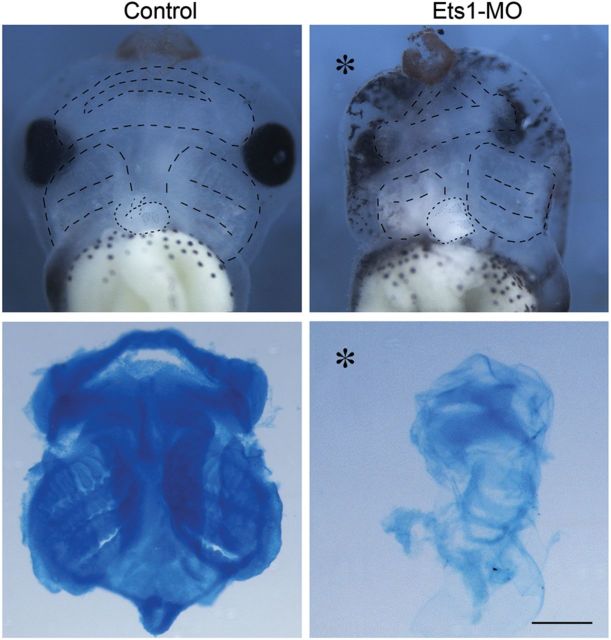
Since cranial NC cells contribute to many other important derivatives in addition to the cardiovascular system, such as the facial cartilage and bones, we asked whether the migration defects caused by Ets1-MO had later consequences on cartilage formation. Alcian blue staining was performed to examine craniofacial cartilage in stage 45 embryos. The results show that NC-derived mandibular, hyoid, and branchial arch cartilages were completely abolished on the injected side (Figure 6). Thus, the defect in NC migration later results in a general failure in facial cartilage formation. This is particularly interesting since patients with Jacobsen's syndrome and DiGeorge syndrome have craniofacial defects in additional to heart defects.
Figure 6.
Ets1-MO disrupts cranial cartilage formation. Ventral views of the stage 45 heads illustrate formation of cranial cartilage outlined in upper panels. Alcian blue stained cartilage were dissected and shown in the lower panels. While mandibular, hyoid, and posterior branchial arch cartilages were distinct in the control head (n = 12), cartilage formation was completely abolished by Ets1-MO (asterisk marks the injected half; n = 14). Cartilage formation in the contralateral side of Ets1-MO injected embryo was also impaired, likely due to a delay in cartilage maturation. Scale bar = 0.5 mm.
4. Discussion
In this study, we have examined the role of Ets1 in heart development using X. laevis as an experimental system in which we can individually target either the NC or the cardiogenic mesoderm. The results show that Ets1 is required in both cardiac NC and cardiogenic mesoderm for proper heart formation, albeit with distinct roles for each tissue of origin. After loss of Ets1 in the cardiac NC, the formation of the aortic arch arteries is disrupted, consistent with the findings that aortic arch arteries are largely derived from cardiac NC in the frog cardiovascular system.35 In contrast, Ets1 loss-of-function in heart mesoderm abolishes endocardium formation and dramatically impairs heart morphogenesis, suggesting an essential role for Ets1 in the development of the endothelium.
4.1. Ets1 in NC cell adhesion and cell migration
Our grafting experiments demonstrate that Ets1-MO affects the delamination and collective migration of NC cells, as well as their segregation into discrete streams. In chick embryos, Ets1 is expressed in the cranial but not trunk NC. Whereas cranial NC cells migrate collectively, trunk NC cells migrate largely as individuals. However, over-expression of Ets1 in the trunk converts trunk NC to a more collective type of migration and results in local degradation of basal lamina.39 Thus it is possible that Ets1 directly or indirectly regulates cell adhesion amongst NC cells and/or between NC cells and neighbouring tissue to control their migratory behaviour.
During the cranial NC epithelial to mesenchymal transition, the levels of N-cadherin and E-cadherin appear to be fine-tuned to allow NC cells to first detach from the neural tube and next dissociate with each other, thus initiating migration.40 During NC migration, cell adhesion is also dynamically modulated. NC cells not only interact with each other to generate cell polarity and directionality through contact inhibition and co-attraction to efficiently migrate, but also sort into different migratory streams based on the different receptors they express.41–47 The role of Ets1 in these processes is not yet known, but given that Ets1 is an important regulator of NC specifier genes like FoxD3 and Sox10,32,33 it may be upstream of many migratory and adhesive events. The present results are consistent with this possibility, since we observed pronounced defects in NC migration and segregation into streams after loss of Ets1.
4.2. Ets1 in endothelium differentiation and heart morphogenesis
Loss of Ets1 in heart mesoderm results in highly abnormal heart development. Formation of the primitive heart tube is severely delayed and the three-chambered heart conformation is lost. Although myocardium formation appears to occur relatively normally, there are serious defects during endocardium development. Through an endocardial–mesenchymal transition, the endocardium serves as the source of mesenchymal cells in the endocardial cushions that give rise to valves and septum, including the membranous portion of interventricular septum.48 The lack of these structures and the partial loss of Tbx20 expression suggest that endothelium development is abolished by Ets1-MO. This is consistent with observations from other studies that Ets family members are involved in endothelial differentiation.17,49
The defective heart conformation may be a secondary effect to the loss of endocardium tissue. For example, endocardium plays an important role in signalling to cardiomyocytes for the formation of trabecular myocardium.50,51 Specifically, Neuregulin1 secreted by endocardium can activate ErbB2/ErbB4 in myocardium to promote trabeculae formation,52 while Notch from the endocardium activates BMP10 in myocardium to promote trabeculae proliferation.53
How Ets1 regulates endothelium development is not yet clear. In mouse, Ets1 has been shown to potentially bind to a distal GATA4 enhancer that is specifically expressed in endocardium,54 suggesting that Ets1 may be involved in endocardium specification. In vitro, Ets transcription factors have also been implicated in regulating endothelium cell adhesion in a mouse endothelial cell line.55 Ets1 has been shown to directly activate myocyte enhancer factor 2 (MEF2) during endothelium development,56 suggesting that Ets1 may also play a role in its differentiation. Consistent with this possibility, our results show that MEF2 expression in cardiac region is slightly reduced by the loss of Ets1 (Supplementary material online, Figure S2). However, we cannot rule out the possibility that there may be an early defect in artery formation and/or a defect in artery maintenance.
4.3. Interactions between cardiac NC and heart mesoderm during heart formation
The present results show that Ets1 is required in both NC and heart mesoderm for proper heart formation, with Ets1 likely playing different roles in the two tissues. In the case of aortic arch artery formation, cardiac NC cells migrated into pharyngeal arch arteries and interact with the endothelial cells lining the great vessels during the formation and remodelling of the arch arteries. Although the requirement for the cardiac NC and the endothelial cells are different during the development of aortic arch arteries, the loss of Ets1 in either tissue leads to similar defective aortic arch formation. The observation that loss of Ets1 in cardiac mesoderm does not affect the migration of cardiac NC cells into pharyngeal arches (data not shown) suggests that the interactions between the two populations happen after they meet in circumpharyngeal area. For example, the cardiac NC cells colonize the aortic arch arteries and differentiate into smooth muscle cells may receive signals from the endothelial lining to make remodelling decisions. On the other hand, cardiac NC may also influence the development of cardiac mesoderm. Cardiac crest cells migrate through the pharyngeal arches and associate with the second heart field before entering the heart. The time and position of the formation of second heart field overlaps with the position where cardiac NC cells pause and rest.5 Thus, they may influence each other during the formation of the aortic arch arteries and OFT. Consistent with this possibility, cardiac NC ablation disrupts the development of arterial pole by interfering with the second heart field cell's integration into the heart, suggesting that the interaction with cardiac NC is required for the development of second heart field cells.57 Our results also support this notion that, although cardiac NC does not contribute to OFT in Xenopus,35 disrupted cardiac crest by Ets1-MO also led to misshapen and smaller OFT formation, suggesting that fewer second heart field cells migrated into the OFT to lengthen the heart tube.
Our NC loss-of-Ets1 embryos phenocopies several cardiac and craniofacial defects associated with DiGeorge syndrome or 22q11 deletion syndrome.58,59 One of the genes in the locus and thought to be responsible for much of the phenotype is Tbx1.60,61 Interestingly, Tbx1 is expressed in the pharyngeal ectoderm, endoderm, and second heart field, but not in NC cells, suggesting that Tbx1 acts in a non-cell-autonomous manner on NC cells. Consistently, we observed a decrease in Tbx1 expression in heart primodium after loss of Ets1 in the Xenopus cardiac mesoderm, confirming a positive influence of cardiac mesoderm on cardiac NC cells.
In summary, the present results have revealed new functions for an important developmental regulator, Ets1, in heart development. Although the role of Ets1 in the NC has been well established using mouse models, the defects observed in patients with mutations in Ets1 are more complicated than those observed in animals with conditional knock-outs of Ets1 in the NC. By using a complementary animal model system in which it is possible to separately target knock-outs to NC vs. presumptive cardiac mesoderm, the present experiments highlight the importance of Ets1 in the mesoderm and provide an animal model for further study of Ets1 downstream targets. Some of the direct targets of Ets1 in the NC have recently been elucidated. For example, Ets1 is a direct transcriptional regulator of Sox10 and FoxD3, both critical genes in the NC lineage.33,34 In contrast, direct targets of Ets1 in the cardiac mesoderm are not yet known. Our data suggest that in Xenopus Tbx1 may be such a target, perhaps partially accounting for the phenotype that may be involved in disorders like DiGeorge syndrome. Thus, further analysis of direct, tissue-specific targets of Ets1 is likely to provide new insights into the ontogeny of cardiovascular defects.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Conflict of interest: none declared.
Funding
This work was supported by American Heart Association postdoctoral fellowship (12POST8610001) and National Institutes of Health career grant (1K99DE022796) to S.N., and National Institutes of Health (R01HD037105) to M.E.B.
References
- 1.Vincent SD, Buckingham ME. How to make a heart: the origin and regulation of cardiac progenitor cells. Curr Top Dev Biol 2010;90:1–41. [DOI] [PubMed] [Google Scholar]
- 2.Evans SM, Yelon D, Conlon FL, Kirby ML. Myocardial lineage development. Circ Res 2010;107:1428–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Phillips MT, Kirby ML, Forbes G. Analysis of cranial neural crest distribution in the developing heart using quail-chick chimeras. Circ Res 1987;60:27–30. [DOI] [PubMed] [Google Scholar]
- 4.Waldo K, Miyagawa-Tomita S, Kumiski D, Kirby ML. Cardiac neural crest cells provide new insight into septation of the cardiac outflow tract: aortic sac to ventricular septal closure. Dev Biol 1998;196:129–144. [DOI] [PubMed] [Google Scholar]
- 5.Le Lievre CS, Le Douarin NM. Mesenchymal derivatives of the neural crest: analysis of chimaeric quail and chick embryos. J Embryol Exp Morphol 1975;34:125–154. [PubMed] [Google Scholar]
- 6.Kirby ML, Gale TF, Stewart DE. Neural crest cells contribute to normal aorticopulmonary septation. Science 1983;220:1059–1061. [DOI] [PubMed] [Google Scholar]
- 7.Lyons I, Parsons LM, Hartley L, Li R, Andrews JE, Robb L, Harvey RP. Myogenic and morphogenetic defects in the heart tubes of murine embryos lacking the homeo box gene Nkx2-5. Genes Dev 1995;9:1654–1666. [DOI] [PubMed] [Google Scholar]
- 8.Bartlett HL, Sutherland L, Kolker SJ, Welp C, Tajchman U, Desmarais V, Weeks DL. Transient early embryonic expression of Nkx2-5 mutations linked to congenital heart defects in human causes heart defects in Xenopus laevis. Dev Dyn 2007;236:2475–2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruneau BG, Nemer G, Schmitt JP, Charron F, Robitaille L, Caron S, Conner DA, Gessler M, Nemer M, Seidman CE, Seidman JG. A murine model of Holt-Oram syndrome defines roles of the T-Box transcription factor Tbx5 in cardiogenesis and disease. Cell 2001;106:709–721. [DOI] [PubMed] [Google Scholar]
- 10.Brown DD, Martz SN, Binder O, Goetz SC, Price BM, Smith JC, Conlon FL. Tbx5 and Tbx20 act synergistically to control vertebrate heart morphogenesis. Development 2005;132:553–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horb ME, Thomsen GH. Tbx5 is essential for heart development. Development 1999;126:1739–1751. [DOI] [PubMed] [Google Scholar]
- 12.Szeto DP, Griffin KJ, Kimelman D. HrT is required for cardiovascular development in zebrafish. Development 2002;129:5093–5101. [DOI] [PubMed] [Google Scholar]
- 13.Mommersteeg MT, Andrews WD, Ypsilanti AR, Zelina P, Yeh ML, Norden J, Kispert A, Chedotal A, Christoffels VM, Parnavelas JG. Slit-roundabout signaling regulates the development of the cardiac systemic venous return and pericardium. Circ Res 2013;112:465–475. [DOI] [PubMed] [Google Scholar]
- 14.Epstein JA, Li J, Lang D, Chen F, Brown CB, Jin F, Lu MM, Thomas M, Liu E, Wessels A, Lo CW. Migration of cardiac neural crest cells in splotch embryos. Development 2000;127:1869–1878. [DOI] [PubMed] [Google Scholar]
- 15.Komatsu K, Wakatsuki S, Yamada S, Yamamura K, Miyazaki J, Sehara-Fujisawa A. Meltrin beta expressed in cardiac neural crest cells is required for ventricular septum formation of the heart. Dev Biol 2007;303:82–92. [DOI] [PubMed] [Google Scholar]
- 16.Lepore JJ, Mericko PA, Cheng L, Lu MM, Morrisey EE, Parmacek MS. Gata-6 regulates semaphorin 3C and is required in cardiac neural crest for cardiovascular morphogenesis. J Clin Invest 2006;116:929–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sato Y. Role of ETS family transcription factors in vascular development and angiogenesis. Cell Struct Funct 2001;26:19–24. [DOI] [PubMed] [Google Scholar]
- 18.Alvarez AD, Shi W, Wilson BA, Skeath JB. Pannier and pointedP2 act sequentially to regulate Drosophila heart development. Development 2003;130:3015–3026. [DOI] [PubMed] [Google Scholar]
- 19.Davidson B, Shi W, Beh J, Christiaen L, Levine M. FGF signaling delineates the cardiac progenitor field in the simple chordate, Ciona intestinalis. Genes Dev 2006;20:2728–2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sumanas S, Lin S. Ets1-related protein is a key regulator of vasculogenesis in zebrafish. PLoS Biol 2006;4:e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pham VN, Lawson ND, Mugford JW, Dye L, Castranova D, Lo B, Weinstein BM. Combinatorial function of ETS transcription factors in the developing vasculature. Dev Biol 2007;303:772–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lie-Venema H, Gittenberger-de Groot AC, van Empel LJ, Boot MJ, Kerkdijk H, de Kant E, DeRuiter MC. ETS-1 and ETS-2 transcription factors are essential for normal coronary and myocardial development in chicken embryos. Circ Res 2003;92:749–756. [DOI] [PubMed] [Google Scholar]
- 23.Tahtakran SA, Selleck MA. Ets-1 expression is associated with cranial neural crest migration and vasculogenesis in the chick embryo. Gene Expr Patterns 2003;3:455–458. [DOI] [PubMed] [Google Scholar]
- 24.Oikawa T, Yamada T. Molecular biology of the Ets family of transcription factors. Gene 2003;303:11–34. [DOI] [PubMed] [Google Scholar]
- 25.Gao Z, Kim GH, Mackinnon AC, Flagg AE, Bassett B, Earley JU, Svensson EC. Ets1 is required for proper migration and differentiation of the cardiac neural crest. Development 2010;137:1543–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grossfeld PD, Mattina T, Lai Z, Favier R, Jones KL, Cotter F, Jones C. The 11q terminal deletion disorder: a prospective study of 110 cases. Am J Med Genet A 2004;129A:51–61. [DOI] [PubMed] [Google Scholar]
- 27.Kolker SJ, Tajchman U, Weeks DL. Confocal imaging of early heart development in Xenopus laevis. Dev Biol 2000;218:64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nie S, Kee Y, Bronner-Fraser M. Caldesmon regulates actin dynamics to influence cranial neural crest migration in Xenopus. Mol Biol Cell 2011;22:3355–3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Borchers A, Epperlein HH, Wedlich D. An assay system to study migratory behavior of cranial neural crest cells in Xenopus. Dev Genes Evol 2000;210:217–222. [DOI] [PubMed] [Google Scholar]
- 30.Alfandari D, Cousin H, Gaultier A, Hoffstrom BG, DeSimone DW. Integrin alpha5beta1 supports the migration of Xenopus cranial neural crest on fibronectin. Dev Biol 2003;260:449–464. [DOI] [PubMed] [Google Scholar]
- 31.DeSimone DW, Davidson L, Marsden M, Alfandari D. The xenopus embryo as a model system for studies of cell migration. Methods Mol Biol 2005;294:235–245. [DOI] [PubMed] [Google Scholar]
- 32.Betancur P, Bronner-Fraser M, Sauka-Spengler T. Genomic code for Sox10 activation reveals a key regulatory enhancer for cranial neural crest. Proc Natl Acad Sci USA 2010;107:3570–3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simoes-Costa MS, McKeown SJ, Tan-Cabugao J, Sauka-Spengler T, Bronner ME. Dynamic and differential regulation of stem cell factor FoxD3 in the neural crest is encrypted in the genome. PLoS Genet 2012;8:e1003142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Theveneau E, Mayor R. Can mesenchymal cells undergo collective cell migration? The case of the neural crest. Cell Adh Migr 2011;5:490–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee YH, Saint-Jeannet JP. Cardiac neural crest is dispensable for outflow tract septation in Xenopus. Development 2011;138:2025–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grossfeld PD. The genetics of hypoplastic left heart syndrome. Cardiol Young 1999;9:627–632. [DOI] [PubMed] [Google Scholar]
- 37.Brown DD, Binder O, Pagratis M, Parr BA, Conlon FL. Developmental expression of the Xenopus laevis Tbx20 orthologue. Dev Genes Evol 2003;212:604–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gessert S, Kuhl M. Comparative gene expression analysis and fate mapping studies suggest an early segregation of cardiogenic lineages in Xenopus laevis. Dev Biol 2009;334:395–408. [DOI] [PubMed] [Google Scholar]
- 39.Theveneau E, Duband JL, Altabef M. Ets-1 confers cranial features on neural crest delamination. PLoS One 2007;2:e1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rogers CD, Saxena A, Bronner ME. Sip1 mediates an E-cadherin-to-N-cadherin switch during cranial neural crest EMT. J Cell Biol 2013;203:835–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carmona-Fontaine C, Matthews HK, Kuriyama S, Moreno M, Dunn GA, Parsons M, Stern CD, Mayor R. Contact inhibition of locomotion in vivo controls neural crest directional migration. Nature 2008;456:957–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Teddy JM, Kulesa PM. In vivo evidence for short- and long-range cell communication in cranial neural crest cells. Development 2004;131:6141–6151. [DOI] [PubMed] [Google Scholar]
- 43.Theveneau E, Marchant L, Kuriyama S, Gull M, Moepps B, Parsons M, Mayor R. Collective chemotaxis requires contact-dependent cell polarity. Dev Cell 2010;19:39–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu X, Li WE, Huang GY, Meyer R, Chen T, Luo Y, Thomas MP, Radice GL, Lo CW. Modulation of mouse neural crest cell motility by N-cadherin and connexin 43 gap junctions. J Cell Biol 2001;154:217–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith A, Robinson V, Patel K, Wilkinson DG. The EphA4 and EphB1 receptor tyrosine kinases and ephrin-b2 ligand regulate targeted migration of branchial neural crest cells. Curr Biol 1997;7:561–570. [DOI] [PubMed] [Google Scholar]
- 46.Mellott DO, Burke RD. Divergent roles for Eph and ephrin in avian cranial neural crest. BMC Dev Biol 2008;8:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Helbling PM, Tran CT, Brandli AW. Requirement for epha receptor signaling in the segregation of Xenopus third and fourth arch neural crest cells. Mech Dev 1998;78:63–79. [DOI] [PubMed] [Google Scholar]
- 48.Snarr BS, Kern CB, Wessels A. Origin and fate of cardiac mesenchyme. Dev Dyn 2008;237:2804–2819. [DOI] [PubMed] [Google Scholar]
- 49.De Val S, Black BL. Transcriptional control of endothelial cell development. Dev Cell 2009;16:180–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stankunas K, Hang CT, Tsun ZY, Chen H, Lee NV, Wu JI, Shang C, Bayle JH, Shou W, Iruela-Arispe ML, Chang CP. Endocardial Brg1 represses ADAMTS1 to maintain the microenvironment for myocardial morphogenesis. Dev Cell 2008;14:298–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wagner M, Siddiqui MA. Signal transduction in early heart development (ii): ventricular chamber specification, trabeculation, and heart valve formation. Exp Biol Med (Maywood) 2007;232:866–880. [PubMed] [Google Scholar]
- 52.Lee KF, Simon H, Chen H, Bates B, Hung MC, Hauser C. Requirement for neuregulin receptor erbB2 in neural and cardiac development. Nature 1995;378:394–398. [DOI] [PubMed] [Google Scholar]
- 53.Grego-Bessa J, Luna-Zurita L, del Monte G, Bolos V, Melgar P, Arandilla A, Garratt AN, Zang H, Mukouyama YS, Chen H, Shou W, Ballestar E, Esteller M, Rojas A, Perez-Pomares JM, de la Pompa JL. Notch signaling is essential for ventricular chamber development. Dev Cell 2007;12:415–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schachterle W, Rojas A, Xu SM, Black BL. Ets-dependent regulation of a distal Gata4 cardiac enhancer. Dev Biol 2012;361:439–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mattot V, Vercamer C, Soncin F, Calmels T, Huguet C, Fafeur V, Vandenbunder B. Constitutive expression of the DNA-binding domain of Ets1 increases endothelial cell adhesion and stimulates their organization into capillary-like structures. Oncogene 2000;19:762–772. [DOI] [PubMed] [Google Scholar]
- 56.De Val S, Anderson JP, Heidt AB, Khiem D, Xu SM, Black BL. Mef2c is activated directly by Ets transcription factors through an evolutionarily conserved endothelial cell-specific enhancer. Dev Biol 2004;275:424–434. [DOI] [PubMed] [Google Scholar]
- 57.Waldo KL, Hutson MR, Stadt HA, Zdanowicz M, Zdanowicz J, Kirby ML. Cardiac neural crest is necessary for normal addition of the myocardium to the arterial pole from the secondary heart field. Dev Biol 2005;281:66–77. [DOI] [PubMed] [Google Scholar]
- 58.Shprintzen RJ. Velo-cardio-facial syndrome: 30 years of study. Dev Disabil Res Rev 2008;14:3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Van Mierop LH, Kutsche LM. Cardiovascular anomalies in digeorge syndrome and importance of neural crest as a possible pathogenetic factor. Am J Cardiol 1986;58:133–137. [DOI] [PubMed] [Google Scholar]
- 60.Jerome LA, Papaioannou VE. DiGeorge syndrome phenotype in mice mutant for the T-box gene, Tbx1. Nat Genet 2001;27:286–291. [DOI] [PubMed] [Google Scholar]
- 61.Merscher S, Funke B, Epstein JA, Heyer J, Puech A, Lu MM, Xavier RJ, Demay MB, Russell RG, Factor S, Tokooya K, Jore BS, Lopez M, Pandita RK, Lia M, Carrion D, Xu H, Schorle H, Kobler JB, Scambler P, Wynshaw-Boris A, Skoultchi AI, Morrow BE, Kucherlapati R. TBX1 is responsible for cardiovascular defects in velo-cardio-facial/DiGeorge syndrome. Cell 2001;104:619–629. [DOI] [PubMed] [Google Scholar]