Abstract
BACKGROUND
Subfertility due to chronic anovulation is common in women with polycystic ovary syndrome (PCOS) and is often treated with IVF. Women with PCOS have an increased ovarian follicle and oocyte count, increased ovarian reserve and/or a slower rate of follicle atresia. If so, one would expect women with PCOS to display a delayed reduction in fertility with advancing age as compared with eumenorrheic women.
METHODS
To test this hypothesis, we compared oocyte count and live birth rates among two groups undergoing IVF, 500 women with PCOS and 500 eumenorrheic women with infertility due to tubal factor only.
RESULTS
Across the age range of 22–41 years, oocyte count and live birth rates remained stable in women with PCOS. In the eumenorrheic comparison group, these parameters decreased significantly with age.
CONCLUSIONS
Women with PCOS display sustained fertility with advancing age as compared with infertile eumenorrheic women.
Keywords: PCOS, ageing, fertility, IVF
Introduction
Polycystic ovary syndrome (PCOS) is a condition characterized by increased ovarian androgen production, insulin resistance and infrequent ovulation (Dunaif, 1997). Despite ovarian dysfunction and attendant subfertility, women with PCOS display increased follicle and oocyte count both unstimulated and during ovarian stimulation and oocyte retrieval for IVF (Homburg et al., 1993). In general, female fertility declines with advancing age (Malizia et al., 2009). Increasing infertility reflects the quantity and quality of the remaining oocyte pool, indeed 50% of women are sterile at the age of 41 (te Velde and Pearson, 2002). The size of the initial follicle stock and the proportion undergoing atresia or commencing growth are mainly genetically determined, and these, together with environmental factors, such as diet, cigarette smoking and cancer treatment, influence the rate of ovarian aging (Alviggi et al., 2009).
Anti-Müllerian hormone (AMH) is secreted by proliferating granulosa cells in growing follicles that have started to mature and Franks and colleagues have demonstrated AMH secretion from the primordial stage onwards. They conclude that ‘even squamous granulosa cells undergo a low rate of cell division’ (Stubbs et al., 2005) and ‘that a proportion of these follicles were growing at a very slow rate’ as also shown in the macaque (Gougeon, 1996) and rat (Hirshfield, 1989).
AMH reaches its maximum secretion in small antral follicles up to 6 mm (Durlinger et al., 2002). Beyond this point, follicular growth becomes FSH dependent and AMH expression diminishes. AMH inhibits the recruitment of primordial follicles (Themmen, 2005) and in the mouse, FSH stimulated pre-antral follicle growth is attenuated in the presence of AMH (Durlinger et al., 2001). AMH expression is less prevalent in primordial and transitional follicles in PCOS women than in controls and it has been speculated whether this may permit increased recruitment of follicles in PCOS women (Stubbs et al., 2005). As they also display prolonged survival of pre-antral follicles, these two factors may contribute to the presence of an increased number of visible follicles in PCOS women (Webber et al., 2007). Both the excessive accumulation of antral follicles and their increased granulosa cell, AMH secretion (Catteau-Jonard et al., 2008) may explain the increased circulating AMH level in PCOS women (Wang et al., 2007). Age-related female infertility is mainly based on changes in ovarian reserve, defined as the number and quality of the remaining follicles in the ovaries at a given age. As AMH correlates well with age and antral follicle count, it may constitute a sensitive marker for ovarian aging. Moreover, the size of the monthly growing follicle pool may reflect the unmeasureable number of primordial follicles in the ovaries and thus indicate the ovarian reserve (Ledger, 2010).
Women with PCOS achieve similar pregnancy and live birth rates per treatment cycle as normo-ovulatory women (Heijnen et al., 2006). Since women with PCOS have both higher follicle counts (Webber et al., 2007) and serum AMH concentrations (Tehrani et al., 2010), we hypothesized that they could also have sustained fertility compared with controls. To test this assumption, we compared oocyte number and live birth rates to chronological age in women with PCOS and eumenorrheic controls undergoing IVF.
Patients and Methods
Selection of patients and study design
Patients in this cohort study were retrospectively identified in our clinical database with detailed records on cycles of assisted reproduction treatments with IVF or ICSI. The complete cohort of women with PCOS (n = 708) was retrieved from the database. The diagnosis of PCOS was based on the 2003 (The Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group, 2004), that is, the presence of at least two of the following conditions: oligo/anovulation, hyperandrogenism and polycystic ovaries. Attenuated 21-hydroxylase activity, Cushing syndrome, androgen-secreting tumors and hyperprolactinemia were excluded by appropriate tests. The retrieved records were manually checked to identify and exclude women with co-existing diseases, such as tubal disease, male factor infertility and endometriosis. The final case cohort contained 500 women with PCOS.
The control group consisted of eumenorrheic women who underwent IVF or ICSI for tubal factor infertility only (n = 856). We excluded women with other co-existing conditions. These initial control cohorts differed with respect to mean age (PCOS, 31.3 years; tubal factor, 33.4 years; P < 0.001) and the shape of age distributions. To reduce bias in subsequent regression analyses and to obtain a similar age distribution in the case and control groups, one woman from the tubal factor cohort was matched with each woman in the PCOS cohort according to age across the age range of 20–44 years. Matching was performed with the nearest month of birth. Finally, each group consisted of 500 patients after matching.
Ovarian stimulation and IVF
Ovarian stimulation and IVF were performed as previously described (Bjercke et al., 2005). Briefly, pituitary down-regulation was achieved with GnRH agonist (Suprecur, Sanofi-Aventis, Germany or Synarela, Pfizer, Belgium) administered intranasally from the mid-luteal phase of the preceding cycle in patients with regular menses. Patients with PCOS who were oligomenorrheic were given a progestine to induce endometrial sloughing. Recombinant FSH (Gonal-F, Merck Serono, Geneva, Switzerland or Puregon, Organon, Oss, The Netherlands) or human menopausal gonadotrophin (Menopur, Ferring, Denmark) was given for ovarian stimulation. The standard starting dose was 75 IU daily in the PCOS group and 150 IU daily in the control group. The dose was adjusted after 5–9 days according to the ovarian response. In some cases (n = 7 in each group), down-regulation with GnRH agonist was not performed and spontaneous ovulation was prevented by administration of GnRH antagonist (Cetrotide, Serono, Germany or Orgalutran, Organon, The Netherlands 0.25 mg daily), beginning on the sixth day of stimulation with FSH or the day when the largest follicle reached the diameter of 14 mm. Transvaginal oocyte retrieval was performed 34–36 h after administration of 6500 or 10 000 IU human chorionic gonadotrophin (hCG, Ovitrelle, Serono or Pregnyl, Organon). One or two embryos were transferred on Day 2 or Day 3 after follicle aspiration. Luteal phase support was provided as intravaginal progesterone capsules (Progestan, Organon, 600 mg daily) or gel (Crinone, Serono, 180 mg daily).
To avoid selection bias, only the first treatment was included in the analysis. Pregnancy was defined by serum hCG concentration >20 IU/l on Day 12 after embryo transfer. Ovarian hyperstimulation syndrome was diagnosed by a combination of clinical symptoms (abdominal distension, nausea, excessive follicle development of >25 follicles per ovary, or ascites) independent of hospitalization or outpatient management. Body mass index (BMI, kg/m2) was assessed at the first visit.
Data analysis
Data are shown as mean and standard deviation or median and range. Between-group comparisons were performed with chi-square test, t-test or Mann–Whitney test as appropriate. P < 0.05 was considered statistically significant. To assess age-related ovarian capacity to mature follicles in response to FSH stimulation, multiple regression analyses were performed in the case and control group using age, mean daily FSH dose and BMI as independent variables (Fig. 1). In the PCOS group, a history of ovarian surgery was also considered as a covariate. The analysis was performed for the groups separately, and regression coefficients (slopes) for age were compared between the groups with t-test.
Figure 1.
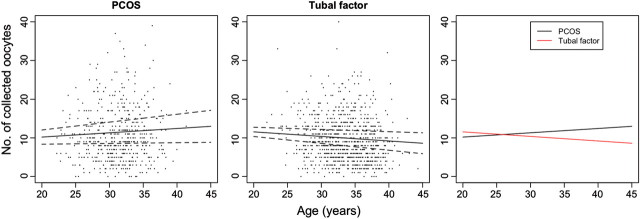
Number of collected oocytes during first treatment with IVF in women with PCOS or tubal factor infertility. Dotted lines indicate SE.
Age-related changes in live birth rate were compared between the groups by calculating age-specific regression coefficients with logistic regression analysis. The age of the woman and the number of replaced embryos were used as covariates in these analyses (Fig. 2).
Figure 2.
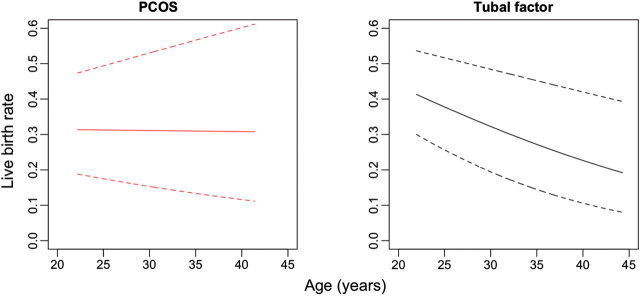
Predicted age-specific live birth rates for women with PCOS or tubal factor infertility undergoing first treatment with IVF. Dotted lines indicate SE.
The R program package (available at www.r-project.org) version 2.5.0 was used for statistical analysis and plotting the data.
Results
The age was similar in the two groups but women with PCOS had higher BMI than women with tubal factor infertility and received lower FSH dosage than controls, with a lower daily dose but a more prolonged stimulation (Table I). The number of collected oocytes was statistically comparable between the groups, whereas the number of diploid fertilized oocytes was lower in the PCOS compared with the tubal factor infertility group. The number of cycles in which no embryos were transferred was higher in the PCOS than in the control group. No statistical differences were revealed among the groups in terms of total pregnancy rate and pregnancy outcome (Table I).
Table I.
Clinical characteristics, ovarian stimulation and treatment outcome among age-matched women with PCOS and tubal factor infertility undergoing IVF.
| PCOS (n = 500) | Tubal factor infertility (n = 500) | P-value | |
|---|---|---|---|
| Age (years) | 31.3 (3.6) | 31.6 (3.2) | |
| BMI (kg/m2) | 26.5 (5.2) | 23.5 (3.9) | <0.001 |
| Total FSH dose (IU) | 1650 (525–9825) | 1725 (525–2500) | 0.04 |
| FSH treatment (days) | 12 (6–39) | 11 (6–28) | <0.001 |
| Number of collected oocytes | 9 (0–39) | 9 (0–33) | 0.20 |
| Number of diploid fertilized oocytes | 5 (0–25) | 6 (0–23) | <0.001 |
| Number of cycles with transfer of embryos | <0.001 | ||
| 0 embryo | 65 (13%) | 35 (7%) | |
| 1 embryo | 102 (20%) | 79 (16%) | |
| 2 embryos | 333 (67%) | 386 (77%) | |
| No. of pregnancies | 169 (33.8%) | 169 (33.8%) | 0.99 |
| No. of spontaneous abortions | 47 (9.4%) | 36 (7.2%) | 0.25 |
| No. of ectopic pregnancies | 0 | 6 | |
| No. of stillbirths | 1 | 0 | |
| No. of live births | 121 (24.2%) | 124 (24.8%) | 0.82 |
| Ovarian hyperstimulation syndrome | 11 (2.2%) | 3 (0.6%) | 0.03 |
In the PCOS group, no significant association between age and number of collected oocytes was observed [regression coefficient (slope) +0.11, SE: 0.09, P= 0.23]. In the tubal factor infertility group, advanced age was associated with a reduced number of retrieved oocytes (slope −0.12, SE: 0.06, P = 0.05). The slopes for regression lines (Fig. 1) differed significantly between the PCOS group and tubal factor infertility (P = 0.01).
In the PCOS group, a significant association between age and live birth rate was not observed (coefficient: −0.001, P= 0.96). In the tubal factor infertility group, advanced age was associated with a significantly reduced probability of live birth (coefficient: −0.049, P = 0.03) (Fig. 2).
Discussion
The results indicate that women with PCOS maintain a stable oocyte count and live birth rate across the age range of 22–41 years during IVF treatment, whereas normo-ovulatory women with tubal factor infertility experience a significant decline with increasing age during this period. Although the finding support the hypothesis that women with PCOS have a sustained fertility compared with controls, conclusions about the pace of ovarian aging cannot be drawn, since the age range of 22–41 years does not cover subsequent events during reproductive aging, such as the age at cessation of fertility or menopause.
Sustained fertility in PCOS women is supported by many observations. First, women who had been infertile due to anovulation experienced spontaneous pregnancies late in reproductive age (Lunde et al., 2001; Vulpoi et al., 2007) and entered menopause later than ovulatory women (Dahlgren et al., 1992). Second, increased ovarian volume and follicle number was observed in PCOS compared with controls across the age range of 20 years to menopause (Asamarai et al., 2009), which is in accordance with the observation that the serum AMH level is increased and shows a slower rate of decline with aging in PCOS women (Mulders et al., 2004; Tehrani et al., 2010). Finally, when treated with in vitro maturation, which does not entail FSH stimulation, women with PCOS have an increased number of oocytes collected and higher live birth rate compared with controls (Child et al., 2001). A sustained number of growing follicles, however, fails to gain support by some other studies. Inhibin B is a marker of ovarian antral follicle cohort in PCOS (Bili et al., 2001) and eumenorrheic women (Danforth et al., 1998). Bili et al. (2001) found an age-related reduction in the number of antral follicles and a decrease in inhibin B in 472 PCOS patients and Elting, in 27 women found an age-related reduction in inhibin B increment during FSH-stimulated ovarian reserve testing (Elting et al., 2001). However, Piltonen et al. (2004) found unchanged age-related inhibin B levels in 42 women with PCOS. Inhibin B inhibits FSH release, and a lower inhibin B level may contribute to a higher FSH level and thus favor follicle growth with more regular and more frequent ovulatory cycles in older women with PCOS.
An alternative, though insufficiently corroborated, explanation for sustained fertility in PCOS is a reduced pace of ovarian aging, possibly involving pathways implicated in ovarian aging and aging in general. In experimental models with worms (Tissenbaum and Ruvkun, 1998; Mukhopadhyay and Tissenbaum, 2007; Angelo and Van Gilst, 2009) and mice (Baba et al., 2005), conditions that slow metabolism and confer metabolic efficiency also slow gamete aging, especially when the metabolic efficiency is not the direct result of undernutrition (Martin et al., 2007). Women with PCOS have been shown to be metabolically efficient proportional to the extent of insulin resistance (Robinson et al., 1992) which may be a manifestation of underlying metabolic efficiency (Georgopoulos et al., 2009). However, our study did not permit us to investigate whether this finding might be associated with or causally related to metabolic efficiency or insulin resistance.
We wish to highlight some limitations of our study. First, it is uncertain whether oocyte count in IVF is a suitable marker for sustained fertility or ovarian aging in PCOS patients. Indeed, there is a positive correlation between basal AMH levels and the number of retrieved oocytes in women undergoing ovarian stimulation (La Marca et al., 2010), but this is not the case for PCOS patients (Wang et al., 2007). Moreover, the longer lifeline of follicles in PCOS may make the correlation between the number of resting primordial follicles and growing follicles, i.e. AMH, less direct compared with controls. Second, the differences in endocrine and metabolic dysfunctions and thereby phenotype in PCOS women constitute a wide continuum. Minor fertility derangements may initially be successfully treated by simple treatment such as hormonal ovulation induction with clomiphene citrate, ovarian surgery or low-dose FSH stimulation therapies. The PCOS group in this study required more complex therapy like IVF and the results of this study may therefore constitute a subgroup of PCOS women. Third, collection of an increased number of oocytes in older women with PCOS may not imply a sustained oocyte quality.
It is uncertain how oocyte quality would be affected in aging polycystic ovaries. An increased density of follicles (Hughesdon, 1982; Webber et al., 2003; Maciel et al., 2004), lower apoptotic rate of granulosa cells (Das et al., 2008) and decreased follicle loss through atresia (Webber et al., 2007) may result in a larger resting follicle cohort, which may be associated with an improved oocyte quality. Indeed, in young women and also cattle, the antral follicle number and AMH levels correlate with the number of morphologically healthy follicles and oocytes, response to ovulation induction, number of high-quality transferable oocytes and blastocyst development (Scheffer et al., 2003; Ireland et al., 2008). Furthermore, production of androstenedione and testosterone by theca cells may be inherently related to the number of healthy growing follicles and low-circulation androgen levels reflect a low number of follicles (Mossa et al., 2010) and a poor ovarian response (Qin et al., 2011). As androgen levels are higher in older PCOS women than in controls, they may experience a better hormonal follicular function contributing to a relative better oocyte quality. In addition, circulating FSH concentration is inversely related to morphologically healthy follicles and oocytes (Ireland et al., 2007). FSH is a positive regulator of aromatase, eastadiol receptors and estradiol production by granulosa cells (Silva and Price, 2000). In normal women, FSH begins to increase at the age of 35 with a marked increase beyond 40 years, whereas women with PCOS at this age may achieve a normal menstrual cycle reflecting a sound hormonal constitution (Elting et al., 2000). Nonetheless, the ovarian microenvironment may still undergo age-related deterioration increasing the proportion of oocytes with meiotic abnormalities. Assessment of these defects, however, is challenging since routine morphological scoring of oocytes fails to reflect age-related biological changes (Stensen et al., 2010).
We conclude that our data indicate a better fertility with advanced age in PCOS women compared with controls and that future research may reveal the exact mechanisms for this phenomenon.
Authors’ roles
J.R. Mellembakken had the idea to the study and wrote the main part of the introduction and discussion; S.L. Berga had the overview and contributed significantly to writing the introduction and discussion; M.K. took the initial initiative of the study and wrote the initial abstract, introduction and discussion; T.T. took part in the general discussion and wrote some parts of the paper; T.Å. took part in the general discussion and wrote a part of ‘patients and methods’; P.F. did the statistics and wrote the main part of ‘patients and methods’ and results as well as parts of the discussion.
Funding
The authors have no financial conflict of interest. No external funding was either sought or obtained for this study.
Acknowledgement
Clinical data used in this article were assessed according to the approval of the Data Protection Officer, Rikshospitalet Medical Centre, Norway. As imposed by the Officer, this article must hereby be marked ‘quality assurance’ solely to indicate this fact.
References
- Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Hum Reprod. 2004;19:41–47. doi: 10.1093/humrep/deh098. doi:10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- Alviggi C, Humaidan P, Howles CM, Tredway D, Hillier SG. Biological versus chronological ovarian age: implications for assisted reproductive technology. Reprod Biol Endocrinol. 2009;7:101. doi: 10.1186/1477-7827-7-101. doi:10.1186/1477-7827-7-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelo G, Van Gilst MR. Starvation protects germline stem cells and extends reproductive longevity in C. elegans. Science. 2009;326:954–958. doi: 10.1126/science.1178343. doi:10.1126/science.1178343. [DOI] [PubMed] [Google Scholar]
- Asamarai S, Adams JM, Murphy MK, Post MD, Hayden DL, Hall JE, Welt CK. Criteria for polycystic ovarian morphology in polycystic ovary syndrome as a function of age. J Clin Endocrinol Metab. 2009;94:4961–4970. doi: 10.1210/jc.2009-0839. doi:10.1210/jc.2009-0839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba T, Shimizu T, Suzuki Y, Ogawara M, Isono K, Koseki H, Kurosawa H, Shirasawa T. Estrogen, insulin, and dietary signals cooperatively regulate longevity signals to enhance resistance to oxidative stress in mice. J Biol Chem. 2005;280:16417–16426. doi: 10.1074/jbc.M500924200. doi:10.1074/jbc.M500924200. [DOI] [PubMed] [Google Scholar]
- Bili H, Laven J, Imani B, Eijkemans MJ, Fauser BC. Age-related differences in features associated with polycystic ovary syndrome in normogonadotrophic oligo-amenorrhoeic infertile women of reproductive years. Eur J Endocrinol. 2001;145:749–755. doi: 10.1530/eje.0.1450749. doi:10.1530/eje.0.1450749. [DOI] [PubMed] [Google Scholar]
- Bjercke S, Fedorcsak P, Abyholm T, Storeng R, Ertzeid G, Oldereid N, Omland A, Tanbo T. IVF/ICSI outcome and serum LH concentration on day 1 of ovarian stimulation with recombinant FSH under pituitary suppression. Hum Reprod. 2005;20:2441–2447. doi: 10.1093/humrep/dei101. doi:10.1093/humrep/dei101. [DOI] [PubMed] [Google Scholar]
- Catteau-Jonard S, Jamin SP, Leclerc A, Gonzales J, Dewailly D, di Clemente N. Anti-Mullerian hormone, its receptor, FSH receptor, and androgen receptor genes are overexpressed by granulosa cells from stimulated follicles in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93:4456–4461. doi: 10.1210/jc.2008-1231. doi:10.1210/jc.2008-1231. [DOI] [PubMed] [Google Scholar]
- Child TJ, Abdul-Jalil AK, Gulekli B, Tan SL. In vitro maturation and fertilization of oocytes from unstimulated normal ovaries, polycystic ovaries, and women with polycystic ovary syndrome. Fertil Steril. 2001;76:936–942. doi: 10.1016/s0015-0282(01)02853-9. doi:10.1016/S0015-0282(01)02853-9. [DOI] [PubMed] [Google Scholar]
- Dahlgren E, Johansson S, Lindstedt G, Knutsson F, Oden A, Janson PO, Mattson LA, Crona N, Lundberg PA. Women with polycystic ovary syndrome wedge resected in 1956 to 1965: a long-term follow-up focusing on natural history and circulating hormones. Fertil Steril. 1992;57:505–513. doi: 10.1016/s0015-0282(16)54892-4. [DOI] [PubMed] [Google Scholar]
- Danforth DR, Arbogast LK, Mroueh J, Kim MH, Kennard EA, Seifer DB, Friedman CI. Dimeric inhibin: a direct marker of ovarian aging. Fertil Steril. 1998;70:119–123. doi: 10.1016/s0015-0282(98)00127-7. doi:10.1016/S0015-0282(98)00127-7. [DOI] [PubMed] [Google Scholar]
- Das M, Djahanbakhch O, Hacihanefioglu B, Saridogan E, Ikram M, Ghali L, Raveendran M, Storey A. Granulosa cell survival and proliferation are altered in polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93:881–887. doi: 10.1210/jc.2007-1650. doi:10.1210/jc.2007-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunaif A. Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev. 1997;18:774–800. doi: 10.1210/edrv.18.6.0318. doi:10.1210/er.18.6.774. [DOI] [PubMed] [Google Scholar]
- Durlinger AL, Gruijters MJ, Kramer P, Karels B, Kumar TR, Matzuk MM, Rose UM, de Jong FH, Uilenbroek JT, Grootegoed JA, et al. Anti-Mullerian hormone attenuates the effects of FSH on follicle development in the mouse ovary. Endocrinology. 2001;142:4891–4899. doi: 10.1210/endo.142.11.8486. doi:10.1210/en.142.11.4891. [DOI] [PubMed] [Google Scholar]
- Durlinger AL, Visser JA, Themmen AP. Regulation of ovarian function: the role of anti-Mullerian hormone. Reproduction. 2002;124:601–609. doi: 10.1530/rep.0.1240601. doi:10.1530/rep.0.1240601. [DOI] [PubMed] [Google Scholar]
- Elting MW, Korsen TJ, Rekers-Mombarg LT, Schoemaker J. Women with polycystic ovary syndrome gain regular menstrual cycles when ageing. Hum Reprod. 2000;15:24–28. doi: 10.1093/humrep/15.1.24. doi:10.1093/humrep/15.1.24. [DOI] [PubMed] [Google Scholar]
- Elting MW, Kwee J, Schats R, Rekers-Mombarg LT, Schoemaker J. The rise of estradiol and inhibin B after acute stimulation with follicle-stimulating hormone predict the follicle cohort size in women with polycystic ovary syndrome, regularly menstruating women with polycystic ovaries, and regularly menstruating women with normal ovaries. J Clin Endocrinol Metab. 2001;86:1589–1595. doi: 10.1210/jcem.86.4.7396. doi:10.1210/jc.86.4.1589. [DOI] [PubMed] [Google Scholar]
- Georgopoulos NA, Saltamavros AD, Vervita V, Karkoulias K, Adonakis G, Decavalas G, Kourounis G, Markou KB, Kyriazopoulou V. Basal metabolic rate is decreased in women with polycystic ovary syndrome and biochemical hyperandrogenemia and is associated with insulin resistance. Fertil Steril. 2009;92:250–255. doi: 10.1016/j.fertnstert.2008.04.067. doi:10.1016/j.fertnstert.2008.04.067. [DOI] [PubMed] [Google Scholar]
- Gougeon A. Regulation of ovarian follicular development in primates: facts and hypotheses. Endocr Rev. 1996;17:121–155. doi: 10.1210/edrv-17-2-121. [DOI] [PubMed] [Google Scholar]
- Heijnen EM, Eijkemans MJ, Hughes EG, Laven JS, Macklon NS, Fauser BC. A meta-analysis of outcomes of conventional IVF in women with polycystic ovary syndrome. Hum Reprod Update. 2006;12:13–21. doi: 10.1093/humupd/dmi036. doi:10.1093/humupd/dmi036. [DOI] [PubMed] [Google Scholar]
- Hirshfield AN. Granulosa cell proliferation in very small follicles of cycling rats studied by long-term continuous tritiated-thymidine infusion. Biol Reprod. 1989;41:309–316. doi: 10.1095/biolreprod41.2.309. doi:10.1095/biolreprod41.2.309. [DOI] [PubMed] [Google Scholar]
- Homburg R, Berkowitz D, Levy T, Feldberg D, Ashkenazi J, Ben-Rafael Z. In vitro fertilization and embryo transfer for the treatment of infertility associated with polycystic ovary syndrome. Fertil Steril. 1993;60:858–863. doi: 10.1016/s0015-0282(16)56287-6. [DOI] [PubMed] [Google Scholar]
- Hughesdon PE. Morphology and morphogenesis of the Stein-Leventhal ovary and of so-called ‘hyperthecosis. Obstet Gynecol Surv. 1982;37:59–77. doi: 10.1097/00006254-198202000-00001. doi:10.1097/00006254-198202000-00001. [DOI] [PubMed] [Google Scholar]
- Ireland JJ, Ward F, Jimenez-Krassel F, Ireland JL, Smith GW, Lonergan P, Evans AC. Follicle numbers are highly repeatable within individual animals but are inversely correlated with FSH concentrations and the proportion of good-quality embryos after ovarian stimulation in cattle. Hum Reprod. 2007;22:1687–1695. doi: 10.1093/humrep/dem071. doi:10.1093/humrep/dem071. [DOI] [PubMed] [Google Scholar]
- Ireland JL, Scheetz D, Jimenez-Krassel F, Themmen AP, Ward F, Lonergan P, Smith GW, Perez GI, Evans AC, Ireland JJ. Antral follicle count reliably predicts number of morphologically healthy oocytes and follicles in ovaries of young adult cattle. Biol Reprod. 2008;79:1219–1225. doi: 10.1095/biolreprod.108.071670. doi:10.1095/biolreprod.108.071670. [DOI] [PubMed] [Google Scholar]
- La Marca A, Sighinolfi G, Radi D, Argento C, Baraldi E, Artenisio AC, Stabile G, Volpe A. Anti-Mullerian hormone (AMH) as a predictive marker in assisted reproductive technology (ART) Hum Reprod Update. 2010;16:113–130. doi: 10.1093/humupd/dmp036. doi:10.1093/humupd/dmp036. [DOI] [PubMed] [Google Scholar]
- Ledger WL. Clinical utility of measurement of anti-mullerian hormone in reproductive endocrinology. J Clin Endocrinol Metab. 2010;95:5144–5154. doi: 10.1210/jc.2010-0701. doi:10.1210/jc.2010-0701. [DOI] [PubMed] [Google Scholar]
- Lunde O, Djoseland O, Grottum P. Polycystic ovarian syndrome: a follow-up study on fertility and menstrual pattern in 149 patients 15–25 years after ovarian wedge resection. Hum Reprod. 2001;16:1479–1485. doi: 10.1093/humrep/16.7.1479. doi:10.1093/humrep/16.7.1479. [DOI] [PubMed] [Google Scholar]
- Maciel GA, Baracat EC, Benda JA, Markham SM, Hensinger K, Chang RJ, Erickson GF. Stockpiling of transitional and classic primary follicles in ovaries of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2004;89:5321–5327. doi: 10.1210/jc.2004-0643. doi:10.1210/jc.2004-0643. [DOI] [PubMed] [Google Scholar]
- Malizia BA, Hacker MR, Penzias AS. Cumulative live-birth rates after in vitro fertilization. N Engl J Med. 2009;360:236–243. doi: 10.1056/NEJMoa0803072. doi:10.1056/NEJMoa0803072. [DOI] [PubMed] [Google Scholar]
- Martin B, Pearson M, Kebejian L, Golden E, Keselman A, Bender M, Carlson O, Egan J, Ladenheim B, Cadet JL, et al. Sex-dependent metabolic, neuroendocrine, and cognitive responses to dietary energy restriction and excess. Endocrinology. 2007;148:4318–4333. doi: 10.1210/en.2007-0161. doi:10.1210/en.2007-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossa F, Jimenez-Krassel F, Folger JK, Ireland JL, Smith GW, Lonergan P, Evans AC, Ireland JJ. Evidence that high variation in antral follicle count during follicular waves is linked to alterations in ovarian androgen production in cattle. Reproduction. 2010;140:713–720. doi: 10.1530/REP-10-0214. doi:10.1530/REP-10-0214. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay A, Tissenbaum HA. Reproduction and longevity: secrets revealed by C. elegans. Trends Cell Biol. 2007;17:65–71. doi: 10.1016/j.tcb.2006.12.004. doi:10.1016/j.tcb.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Mulders AG, Laven JS, Eijkemans MJ, de Jong FH, Themmen AP, Fauser BC. Changes in anti-Mullerian hormone serum concentrations over time suggest delayed ovarian ageing in normogonadotrophic anovulatory infertility. Hum Reprod. 2004;19:2036–2042. doi: 10.1093/humrep/deh373. doi:10.1093/humrep/deh373. [DOI] [PubMed] [Google Scholar]
- Piltonen T, Koivunen R, Perheentupa A, Morin-Papunen L, Ruokonen A, Tapanainen JS. Ovarian age-related responsiveness to human chorionic gonadotropin in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2004;89:3769–3775. doi: 10.1210/jc.2003-031851. doi:10.1210/jc.2003-031851. [DOI] [PubMed] [Google Scholar]
- Qin Y, Zhao Z, Sun M, Geng L, Che L, Chen ZJ. Association of basal serum testosterone levels with ovarian response and in vitro fertilization outcome. Reprod Biol Endocrinol. 2011;9:9. doi: 10.1186/1477-7827-9-9. doi:10.1186/1477-7827-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S, Chan SP, Spacey S, Anyaoku V, Johnston DG, Franks S. Postprandial thermogenesis is reduced in polycystic ovary syndrome and is associated with increased insulin resistance. Clin Endocrinol. 1992;36:535–536. doi: 10.1111/j.1365-2265.1992.tb02262.x. doi:10.1111/j.1365-2265.1992.tb02261.x. [DOI] [PubMed] [Google Scholar]
- Scheffer GJ, Broekmans FJ, Looman CW, Blankenstein M, Fauser BC, teJong FH, teVelde ER. The number of antral follicles in normal women with proven fertility is the best reflection of reproductive age. Hum Reprod. 2003;18:700–706. doi: 10.1093/humrep/deg135. doi:10.1093/humrep/deg135. [DOI] [PubMed] [Google Scholar]
- Silva JM, Price CA. Effect of follicle-stimulating hormone on steroid secretion and messenger ribonucleic acids encoding cytochromes P450 aromatase and cholesterol side-chain cleavage in bovine granulosa cells in vitro. Biol Reprod. 2000;62:186–191. doi: 10.1095/biolreprod62.1.186. doi:10.1095/biolreprod62.1.186. [DOI] [PubMed] [Google Scholar]
- Stensen MH, Tanbo T, Storeng R, Åbyholm T, Fedorcsak P. Routine morphological scoring systems in assisted reproduction treatment fail to reflect age-related impairment of oocyte and embryo quality. Reprod Biomed Online. 2010;21:118–125. doi: 10.1016/j.rbmo.2010.03.018. doi:10.1016/j.rbmo.2010.03.018. [DOI] [PubMed] [Google Scholar]
- Stubbs SA, Hardy K, Da Silva-Buttkus P, Stark J, Webber LJ, Flanagan AM, Themmen AP, Visser JA, Groome NP, Franks S. Anti-mullerian hormone protein expression is reduced during the initial stages of follicle development in human polycystic ovaries. J Clin Endocrinol Metab. 2005;90:5536–5543. doi: 10.1210/jc.2005-0907. doi:10.1210/jc.2005-0907. [DOI] [PubMed] [Google Scholar]
- te Velde ER, Pearson PL. The variability of female reproductive ageing. Hum Reprod Update. 2002;8:141–154. doi: 10.1093/humupd/8.2.141. doi:10.1093/humupd/8.2.141. [DOI] [PubMed] [Google Scholar]
- Tehrani FR, Solaymani-Dodaran M, Hedayati M, Azizi F. Is polycystic ovary syndrome an exception for reproductive aging? Hum Reprod. 2010;25:1775–1781. doi: 10.1093/humrep/deq088. doi:10.1093/humrep/deq088. [DOI] [PubMed] [Google Scholar]
- Themmen AP. Anti-Mullerian hormone: its role in follicular growth initiation and survival and as an ovarian reserve marker. J Natl Cancer Inst Monogr. 2005;34:18–21. doi: 10.1093/jncimonographs/lgi026. doi:10.1093/jncimonographs/lgi026. [DOI] [PubMed] [Google Scholar]
- The Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic and long-term health risks related to polycystic ovary syndrome (PCOS) Hum Reprod. 2004;19:41–47. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- Tissenbaum HA, Ruvkun G. An insulin-like signaling pathway affects both longevity and reproduction in Caenorhabditis elegans. Genetics. 1998;148:703–717. doi: 10.1093/genetics/148.2.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vulpoi C, Lecomte C, Guilloteau D, Lecomte P. Ageing and reproduction: is polycystic ovary syndrome an exception? Ann Endocrinol (Paris) 2007;68:45–50. doi: 10.1016/j.ando.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Wang JG, Nakhuda GS, Guarnaccia MM, Sauer MV, Lobo RA. Mullerian inhibiting substance and disrupted folliculogenesis in polycystic ovary syndrome. Am J Obstet Gynecol. 2007;196:77.e1–77.e5. doi: 10.1016/j.ajog.2006.07.046. doi:10.1016/j.ajog.2006.07.046. [DOI] [PubMed] [Google Scholar]
- Webber LJ, Stubbs S, Stark J, Trew GH, Margara R, Hardy K, Franks S. Formation and early development of follicles in the polycystic ovary. Lancet. 2003;362:1017–1021. doi: 10.1016/s0140-6736(03)14410-8. doi:10.1016/S0140-6736(03)14410-8. [DOI] [PubMed] [Google Scholar]
- Webber LJ, Stubbs SA, Stark J, Margara RA, Trew GH, Lavery SA, Hardy K, Franks S. Prolonged survival in culture of preantral follicles from polycystic ovaries. J Clin Endocrinol Metab. 2007;92:1975–1978. doi: 10.1210/jc.2006-1422. doi:10.1210/jc.2006-1422. [DOI] [PubMed] [Google Scholar]


