Abstract
Emergency contraception (EC) prevents pregnancy after unprotected sex or contraceptive failure. Use of EC has increased markedly in countries where a product is available over the counter, yet barriers to availability and use remain. Although effective in clinical trials, it has not yet been possible to show a public health benefit of EC in terms of reduction of unintended pregnancy rates. Selective progesterone receptor modulators developed as emergency contraceptives offer better effectiveness than levonorgestrel, but still EC is less effective than use of ongoing regular contraception. Methods which inhibit ovulation whenever they are taken or which act after ovulation to prevent implantation and strategies to increase the uptake of effective ongoing contraception after EC use would prevent more pregnancies.
Keywords: contraception, emergency, review, effectiveness, future
Introduction
The majority of women of reproductive age (15–45 years) menstruate about every 28 ± 2 days and the dominant follicle will ovulate around Day 14. Although pregnancy will occur only if the freshly ovulated egg is fertilized within 24 h, sperm remain viable within the female reproductive tract for up to 7 days after intercourse. Thus, the ‘fertile window’ extends from the day of ovulation back to 6 days earlier. Pregnancy will occur only if the fertilized ovum reaches the uterus after it has been ‘prepared’ by secretion of progesterone from the corpus luteum. Around the time of implantation (Day 7–10 after ovulation), the developing embryo secretes a range of embryonic factors including hCG that prevent regression of the corpus luteum, which would otherwise occur by Day 12 post-ovulation.
In view of the complexity of these interactions between mother and embryo, it is hardly surprising that failure of synchrony between ovary and uterus is fairly common and the fecundity of our species is relatively low. The chance of conception following a single act of intercourse at a random time in the cycle is probably not more than 5% with the highest probability in the 48 h before ovulation (Table I). Despite this, unintended pregnancy is common and represents a significant public health problem.
Table I.
Probability of conception or clinical pregnancy after single intercourse (IC) taking place on days defined by their temporal relationship to ovulationa.
| Day of IC relative to ovulation | Conceptionb % | Pregnancyc % |
|---|---|---|
| −5 | 8 | 4 |
| −4 | 17 | 13 |
| −3 | 8 | 8 |
| −2 | 36 | 29 |
| −1 | 34 | 27 |
| 0 (ovulation) | 36 | 28 |
aData from Wilcox et al. (1995, 1998).
bProbability of detecting hCG followed or not by clinical pregnancy.
cProbability of clinical pregnancy.
Emergency contraception (EC) is defined as any drug or device used after intercourse to prevent unintended pregnancy when no contraceptive method has been used or following an error in contraceptive use. Described in the 1980s as a ‘well-kept secret’, in the 21st century, at least as measured by its availability worldwide and the number of publications on the subject in the medical literature, EC has become well recognized. This review discusses the available methods, their mode of actions and efficacy together with their safety and side effects. It goes further to discuss the availability and determinants of use of EC, the public health benefits, actions to be taken after EC has been used and discusses what research on the topic still needs to be done.
Materials and Methods
Searches were performed in Medline, Popline, EMBASE, Cochrane library and the Social Sciences Citation Index databases for relevant English language publications from 1970 to 2014. Summaries were discussed by the European Society of Human Reproduction and Embryology (ESHRE) Workshop Group.
Emergency contraceptive methods
A number of methods of EC exist. The first to be described was the so-called Yuzpe regimen comprising two doses of 100 μg ethinylestradiol with 0.5 mg levonorgestrel (LNG) licensed for use with the first dose taken within 72 h of unprotected intercourse and repeated after 12 h (Yuzpe and Lancee, 1977). Partly because of the demonstration of the superior efficacy of LNG (World Health Organization, 1998) and partly because the high dose of estrogen conferred a theoretical risk of venous thromboembolism and unpleasant side effects that affected compliance, use of the Yuzpe regimen has been discontinued in most countries. However, it remains on the WHO list of essential medicines (World Health Organization, 2013) and is usually mentioned in international guidelines because, if other methods are not available, the regimen can be mimicked by taking a number of combined oral contraceptive (COC) pills (8–10 depending on the brand) which are extremely widely and easily available. While other progestagens in COC pills seem to work as well as LNG, efficacy is reduced if the second, repeat dose of pills is not taken after a 12 h interval (Ellertson et al., 2003). The Yuzpe regimen will not be considered further in this review.
The most widely used EC is 1.5 mg LNG taken either as a single dose or in two 0.75 mg doses roughly 12 h apart. It has been marketed in Eastern Europe since 1979 by Gedeon Richter, Hungary (as Postinor®) and since 1999 by a number of different pharmaceutical companies worldwide.
Ulipristal acetate (UPA), a selective progesterone receptor modulator (SPRM), was first marketed for EC as a single dose of 30 mg in Europe in 2009 (as EllaOne®; HRA Pharma, Paris) and in the USA in 2010 (as Ella®; Watson Pharmaceuticals).
The SPRM mifepristone (10–25 mg) is used as an emergency contraceptive in China, Vietnam, Russia and in the Ukraine. It will not be considered further in this review.
The copper intrauterine device (IUD) is highly effective as EC. It is inserted up to 5 days after intercourse (or up to 5 days after the earliest estimated day of ovulation in some countries). The major disadvantage of the IUD for EC is that insertion requires technical expertise and clinical facilities beyond those available in pharmacies. A major advantage, however, is that the woman can choose to keep the device as ongoing, long-acting reversible contraception. The LNG-IUD (Mirena®) is presently not recommended for EC, however, studies are underway to determine its efficacy.
Mechanism of action
LNG and UPA oral emergency contraceptive preparations work by delaying or inhibiting ovulation but their efficacy in doing so varies according to the stage of the cycle when EC is used, as shown in a series of elegant experiments undertaken by Brache et al. (2013). In summary, and based on earlier studies (Gemzell-Danielsson and Marions, 2004), while LNG can disrupt or inhibit ovulation in 96% of cycles if it is given in the presence of an ovarian follicle measuring 12–17 mm in diameter, once the LH surge has started LNG has no effect on ovulation. When UPA is given before the start of the LH surge follicle rupture is delayed or inhibited in 100% of cycles (Brache et al., 2013). UPA remains reasonably effective even if given after the LH surge has started, delaying ovulation in 79% of cycles at this time while LNG delays ovulation in only 14% (and placebo in 10%). Once LH has reached its peak UPA, like LNG, no longer has any effect on ovulation. These differences are important since the risk of conception is at its highest in the 48 h before ovulation when LNG does not work and when UPA works less well or not at all (Glasier et al., 2011). Brache's findings help to explain the superior efficacy of UPA over LNG and also the need to take EC as soon as possible after intercourse (since if the woman has not yet ovulated, the longer she delays using EC the more likely she will be close to ovulation).
Other possible mechanisms of action have been investigated—mainly for LNG and have been summarized by Lalitkumar et al. (2013). While one of the main actions of progestagens is on the cervical mucus, this effect is not seen until 9 h after intake of LNG. LNG appears to have no direct effects on sperm function in vitro and viable spermatozoa can be found in the genital tract 24–28 h after intake of LNG. LNG has no significant effect on the expression of steroid receptors or in vitro contractility of the Fallopian tube, and neither LNG nor UPA increase the rate of ectopic pregnancy (Cleland et al., 2010; Levy et al., 2014). Peri- and post-ovulatory administration of LNG does not significantly affect endometrial morphology or corpus luteum function and does not prevent an embryo attaching to the endometrium in vitro (Lalitkumar et al., 2007). While another SPRM (mifepristone) does have an effect on the endometrium and can both inhibit implantation and induce abortion, the dose of UPA used for EC does not exert significant effects on endometrial secretory development, suggesting that endometrial effects are unlikely to underlie the higher contraceptive efficacy of UPA for EC (Stratton et al., 2010). Finally, when implantation has already occurred LNG has no impact on the pregnancy or the newborn (Lalitkumar et al., 2013). There are far fewer data for UPA, but data from both clinical trials and post-marketing do not suggest a higher rate of subsequent miscarriage in women in whom pregnancy occurs despite using UPA for EC (Levy et al., 2014). UPA in a concentration relevant to EC does not prevent human embryo implantation in vitro (Berger et al., 2015).
The mechanism of action of an IUD used for EC also depends on the time in the cycle that it is used. It may prevent an oocyte from being fertilized if inserted before fertilization has occurred but will also prevent implantation if it is inserted later. This additional effect helps to explain the superior efficacy of the IUD for EC (Cleland et al., 2012).
Efficacy
There has never been a placebo-controlled trial of efficacy of EC. Efficacy is estimated by comparing the number of pregnancies that actually occur among a group of women who have used EC with the number of pregnancies that would have been expected to occur had EC not been used (Trussell et al., 2003). The expected number of pregnancies can be only an estimate because it is based on determining the risk of conception for each woman in the cohort on the reported cycle day when unprotected sex occurred related to her normal menstrual cycle duration and therefore to the risk of ovulation. However, the exact day of ovulation varies from cycle to cycle and many women cannot accurately recall the date of their last menstrual period. The data showing efficacy of both LNG and of UPA come from randomized trials comparing two different EC preparations, LNG versus Yuzpe or mifepristone and UPA versus LNG.
The two seminal studies of LNG for EC were undertaken by the WHO. In the first (World Health Organization, 1998), LNG was given in two doses of LNG 0.75 mg 12 h apart and compared with the Yuzpe regimen, while in the second (von Hertzen et al., 2002), the divided dose regimen of LNG was compared with a single dose of 1.5 mg and with mifepristone 10 mg. LNG was shown to prevent 74–93% of expected pregnancies (Table II). The single dose of 1.5 mg was as effective as the two dose regimen and this has become the preferred regimen in many countries, since it makes fewer demands on compliance.
Table II.
Number of pregnancies and estimated efficacy reported in the two World Health Organization (WHO) trials of LNG-EC.
| Reference | Treatment | Number of women | Number of observed pregnancies | Number of expecteda pregnancies | Efficacyb (%) | 95% CI |
|---|---|---|---|---|---|---|
| WHO (1998) | LNG | 976 | 11 | 76.3 | 86 | 74–93 |
| Yuzpe | 979 | 31 | 74.2 | 58 | 41–72 | |
| Von Hertzen et al. (2002) | LNG (2 groups combined) | 2712 | 44 | 216 | 80 | (71.2–85.6) |
aUsing Dixon's estimates of conception probabilities.
bPrevented fraction.
The efficacy of UPA is taken from data collected in two large randomized double blind trials undertaken by the manufacturers designed to demonstrate non-inferiority when UPA was compared with levonorgestrel emergency contraception (LNG-EC) (Creinin et al., 2006; Glasier et al., 2010a). In the first trial among over 770 women using LNG and 770 using UPA within 72 h of unprotected intercourse, LNG prevented 69% of expected pregnancies while UPA prevented 85% (Creinin et al., 2006). The second trial (Glasier et al., 2010a) included women presenting up to 5 days (120 h) after unprotected intercourse; the pregnancy rate among 852 women taking LNG was 2.6% [95% confidence interval (CI) 0.35–1.31] and 1.8% (95% CI 1.0–3.0) among 844 women taking UPA. A meta-analysis of the two studies (Table III) showed a significantly lower rate of pregnancy among women treated with UPA when EC was taken within 24, 72 or 120 h. Compared with LNG, UPA almost halved the risk of pregnancy among women treated within 5 days of intercourse. Among women taking UPA within 24 h of intercourse (one-third of women in the study) the pregnancy risk was reduced by almost two-thirds.
Table III.
The number of pregnancies/population treated with either LNG or UPA in the two large clinical trials and in the meta-analysis of both according to the interval between the time of unprotected sex and intake of EC, and corresponding ORs (from Glasier et al., 2010a).
| UPA | LNG | OR | |
|---|---|---|---|
| Creinin 0–72 h | 7/773 | 13/773 | 0.5 (0.18–1.24) |
| Glasier 0–120 h | 15/941 | 25/958 | 0.57 (0.29–1.09) |
| Meta-analysis 0–24 h | 5/548 | 15/600 | 0.35 (0.11–0.93) |
| Meta-analysis 0–72 h | 22/1617 | 35/1625 | 0.58 (0.33–0.99) |
| Meta-analysis 0–120 h | 22/1714 | 38/1731 | 0.55 (0.32–0.93) |
A Cochrane review updated in 2012 analysed data from 100 trials of EC (86 conducted in China) among 55 666 women (Cheng et al., 2012). Mid-dose mifepristone (25–50 mg) [20 trials; relative risk (RR) 0.64; 95% CI 0.45–0.92] or low-dose mifepristone (<25 mg) (11 trials; RR 0.70; 95% CI 0.50–0.97) were both significantly more effective than LNG, but the significance was marginal if only high-quality studies were considered (four trials; RR 0.70; 95% CI 0.49–1.01). Low-dose mifepristone was less effective than mid-dose mifepristone (25 trials; RR 0.73; 95% CI 0.55–0.97). This difference was not significant if only high-quality trials were included (six trials; RR 0.75; 95% CI 0.50–1.10). UPA appeared marginally more effective (two trials; RR 0.63) than LNG (P = 0.09) within 72 h of sexual intercourse.
The copper IUD is probably the most effective EC method. In a systematic review of 42 studies among 7034 emergency IUD insertions, 10 pregnancies occurred giving an overall failure rate of 0.14% (95% CI 0.08–0.25%; Cleland et al., 2012). Six pregnancies occurred among 5629 women in studies done in China (failure rate 0.11%; 95% CI 0.05–0.23%). Only one study (open to criticism) provided data from a prospective randomized comparison of post-coital copper-IUD insertion compared with no EC (Askalani et al., 1987). There was an almost 14-fold increased chance of a pregnancy in women in whom no emergency insertion of a copper IUD was performed (Table IV). The Cochrane review (Cheng et al., 2012) concluded that the copper IUD was the most effective EC method.
Table IV.
Studies on the use of the IUD for EC (adapted from Cleland et al., 2012).
| Country (number of studies) | Population at risk | Pregnancies | PR (%) (95% CI) |
|---|---|---|---|
| China (30) | 5629 | 6 | 0.11 (0.05–0.23) |
| UK (4) | 496 | 0 | 0.00 (0.00–0.70) |
| USA (3) | 401 | 0 | 0.00 (0.00–0.85) |
| Italy (3) | 253 | 0 | 0.00 (0.00–1.38) |
| Egypt (1) | 200 | 4 | 2.00 (0.00–5.93) |
| Netherlands (1) | 55 | 0 | 0.00 (0.00–5.93) |
| Total (42) | 7034 | 10 | 0.14 (0.08–0.26) |
PR, pregnancy rate.
Side effects and safety
In most clinical trials of a new drug, users are asked to keep a daily diary of side effects. Thus, a number of mild but common conditions which occur during the course of everyday life (such as headache or backache) are listed as side effects of EC. In addition, many women are anxious about pregnancy and stressed by the need to seek out EC urgently, and this too has an influence on the incidence of common complaints in the days following EC use. The incidence of side effects is virtually identical when UPA is compared with LNG (Glasier et al., 2010a), however, the effects on menses are different in that UPA tends to delay menses, while menstruation tends to occur earlier than expected after LNG treatment. Around one in five women complain of headaches in the days following EC use, 10–15% record nausea and dysmenorrhoea (at next menses), one in 20 note fatigue, dizziness or abdominal pain and still fewer complain of back ache (Glasier et al., 2010a).
LNG has been licensed for many years as a contraceptive given continuously in the form of an implant or an IUD and an oral contraceptive ‘mini pill’. There is a large amount of data on its safety and there is no evidence that use of LNG for EC is associated with any serious or life-threatening adverse events. There are fewer data for UPA, but data from the clinical trials plus those from post-marketing surveillance up to the end of May 2013 are available, accounting for over 1.4 million exposures to UPA (Levy et al., 2014). No serious or life-threatening events have been attributed to the use of UPA for EC. Also reassuring is the fact that millions of women have been exposed to a single dose of 200–600 mg of mifepristone when undergoing medical abortion, and this too has proved to be extremely safe (Sitruk-Ware, 2006).
Most drugs are contraindicated if a woman may already be pregnant or at risk of getting pregnant. Emergency contraceptive users are all at risk of conception and some are already pregnant when they use EC so data on the safety of EC in the event of pregnancy are important. Data on teratogenicity are difficult to collect because, as demonstrated in the clinical trials, the great majority of women who conceive despite using EC choose to have an induced abortion (Levy et al., 2014). Moreover, existing data give a false impression of the incidence of fetal anomaly and complications of pregnancy, since most clinicians do not regard failure of EC and subsequent pregnancy as an adverse event and so only pregnancies which are complicated or associated with fetal malformation tend to be reported (Levy et al., 2014). There is no evidence that either LNG or UPA result in an increased risk of miscarriage, ectopic pregnancy, fetal anomaly or complications during pregnancy or delivery (Levy et al., 2014). While fewer than 30 births have been reported in which there may have been inadvertent exposure of the infant to UPA, the data concerning fetal anomaly among ongoing pregnancies after failed medical abortion using mifepristone are again reassuring (Sitruk-Ware, 2006).
The side effects and safety of the IUD are the same for emergency as for routine use. Since women who have had unprotected sex may be at risk of sexually transmitted infections (STIs) the approach with regard to prevention of pelvic infection associated with IUD insertion should be the same as for interval insertion. Thus, in settings where the background risk of chlamydia or cervical gonococcus is high, or in individuals with recognized risk factors for STI, consideration should be given to simultaneous administration of appropriate antibiotics. The alternative is to screen women presenting for emergency IUD insertion for STI and treat those who are found to be infected, however, the default rate for follow-up review is often high. There is a risk of perforation which is not greater than that associated with routine insertion. If the device is kept for ongoing contraception women should be warned about the risks of both expulsion of the device and heavy or prolonged menstrual bleeding. The risk of ectopic pregnancy is not increased among IUD users. It is likely, however, that if an IUD is inserted when a woman has very recently conceived it will induce abortion—it is for this reason that the insertion of an IUD as an emergency is not indicated after the time in the cycle when implantation should have occurred. There is no evidence of fetal anomaly in the event of a pregnancy continuing after emergency IUD insertion (Lalitkumar et al., 2013).
Availability
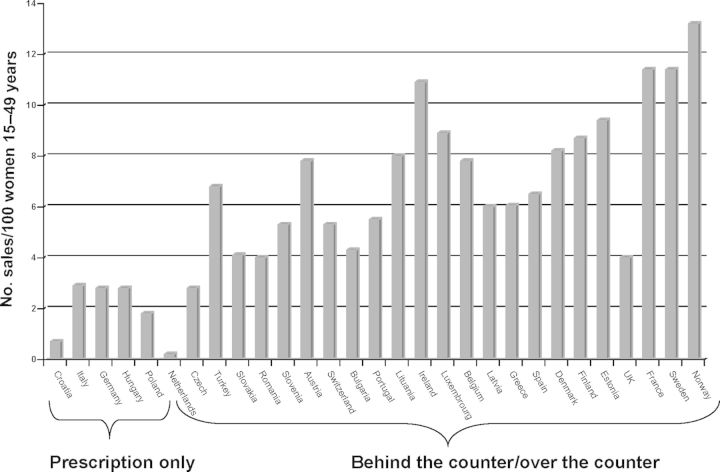
In 2014, at least one hormonal EC preparation was available in 148 countries worldwide. In 56 of these countries, EC was available without the need for a prescription from a doctor and in 17 it is available over the counter. Where there are no licensed methods (e.g. in Afghanistan and Western Sahara) it is unlikely that women are aware that they can use the COC pill as EC. In Europe EC is illegal in Malta. In eight countries (Albania, Croatia, Germany, Hungary, Italy, Kazakhstan, Macedonia and Poland), EC is not available without a doctor's prescription. In 11 European countries, EC can be bought over the counter in pharmacies (it can be taken off the shelf without the need for discussion with a pharmacist) while in the rest of Europe it is available without a prescription but only in consultation with a pharmacist (so-called ‘behind-the-counter’) (European Consortium for Emergency Contraception, 2014). In Europe, there is clear evidence that access to EC influences its use (Fig. 1). In November 2014, the European Medicines Agency's Committee for Medicinal Products for Human Use (CHMP) recommended a change in classification status from prescription to non-prescription for ellaOne (UPA). Based on the assessment of available information, the CHMP found that ellaOne can be used safely and effectively without medical prescription. This means that ellaOne could be available without prescription in all European Union (EU) countries except Malta. It is possible that countries which have chosen to keep LNG available only on prescription may relax their position. Unusually, this CHMP recommendation has been sent to the European Commission for a legally binding decision (European Medicines Agency, 2014).
Figure 1.
EC use as a function of access policies in Europe [sales data Intercontinental Marketing Services (IMS Health), 2013].
Determinants of use
The likelihood of using EC depends on availability and accessibility as well as on knowledge about EC and recognition of the need to use it.
Most interventions have concentrated on ameliorating the supply side (i.e. making sure EC is readily available), while efforts to bolster demand have been modest. In Europe, over a third of women—those that live in Germany, Poland and Italy—need a prescription for EC, despite evidence demonstrating that easier access leads to greater use with no deleterious health consequences. In a Cochrane review, evaluating the impact of advance provision of EC pills as opposed to prescription-only access across 11 RCTs the odds ratio (OR) of using EC was more than twice as high in women given advanced supplies [OR = 2.47 (1.80–3.40)] (Polis et al., 2007a). A review assessing the effect of advance provision by women's age corroborated the benefits of unrestricted access as an important step in promoting use for women of all ages, including adolescents (Rodriguez et al., 2013). Yet, discriminatory policies imposing an age cut-off for non-prescription access still prevail in many settings. In general, national interventions in support of EC have proved effective resulting in substantial increases in EC use at the population level. In France, women experienced a 72% increase in lifetime use of EC in the 5 years following the implementation of non-prescription access in pharmacies in 1999 (Moreau et al., 2006). Implementation barriers in service provision of EC still persist, due to inadequate knowledge or ethical objections on the part of providers. A study of pharmacists in Florida, conducted 2 years after EC deregulation, revealed widespread misperceptions, which were significantly related to intentions to dispense EC and self-reported dispensing practises (Richman et al., 2012). Cost too may hinder access, and pricing and reimbursement policies vary widely by country. Little is known about the effects of cost on actual use levels, although the recent experience of Scotland, where emergency contraception methods (ECs) were made free of charge in pharmacies in 2008, seems to have increased EC use, though data on women requiring abortion are inconsistent. Even in countries where EC is available without prescription simple logistical issues, such as the lack of availability, during the lunch hour, of a pharmacist approved to issue EC, can prevent women from accessing the pills (Glasier et al., 2010b).
Despite improved access, EC is still underutilized. Nine out of 10 women requesting an abortion, whether in Scotland, France and Denmark have not used EC to try to prevent the pregnancy (Sørensen et al., 2000; Moreau et al., 2005; Cameron et al., 2012). In Scotland making EC free of charge has made no measurable difference to EC use among women who are requesting abortion (Cameron et al., 2012). Lack of knowledge about both when and how to use EC remains important and underexplored as barriers. While an overwhelming majority of women are aware of EC in high-income countries, the awareness gap is profound in low- and middle-income countries (Westley et al., 2013) and within countries, the wealthiest and most educated women are more likely to have heard about EC (Westley et al., 2013). Beyond method awareness, widespread misperceptions about safety and mode of action, as well as misinformation about accessibility and the timeframe for use, also hinder women's ability to use EC (Moreau et al., 2005). While awareness is a prerequisite for use, awareness alone rarely translates into action among women in need of EC.
Research conducted in the UK, France and Denmark consistently shows that the most common barrier to EC use is the lack of recognition of pregnancy risk following an unprotected intercourse (Sørensen et al., 2000; Moreau et al., 2005; Lakha Glasier, 2006). As is true for any method, the use of EC depends on women's motivation to prevent a pregnancy (Lakha Glasier, 2006) and on their socio-economic status, with lower usage among women living in more deprived areas (Cameron et al., 2012). Further insights from qualitative research also suggest that women's perceptions of pregnancy risk and their chance of acting on it depend on the social and contextual circumstances in which unprotected intercourse occurs (Williamson et al., 2009). EC is more likely to be used when unprotected intercourse is an isolated event, resulting from contraceptive failure for an unexpected sexual act, as opposed to situations in which unprotected intercourse represents a normative behaviour.
Contraception following EC
When EC is obtained direct from pharmacies, in most high-income countries there is no opportunity to provide an ongoing effective method of contraception. Pharmacists should advise women of the need to start an effective ongoing contraception after using EC, but this does not always happen. In a mystery shopper study undertaken in Edinburgh, Scotland, less than half of pharmacists (43%) providing EC gave advice about the next contraceptive method (Glasier et al., 2010b). Since over 90% of women who use EC do not get pregnant, most of them remain at risk of pregnancy after using EC and some get pregnant through a further act of sexual intercourse occurring in the same cycle in which EC was used. In a meta-analysis of 11 trials of EC involving almost 5000 women, the risk of pregnancy among women who admitted to having unprotected sex after using EC was almost 3-fold that of women who did not have further unprotected sex (Cheng et al., 2012).
Most modern guidance recommends that women start an effective method of contraception immediately after using EC (so-called ‘quick starting’) but pharmacies can provide only condoms. In the absence of hormonal contraception being available without a prescription, there is an urgent need for strategies to facilitate the uptake of effective contraception after use of EC obtained from a pharmacy.
One randomized trial among almost 1000 women in Jamaica offered women presenting for EC a coupon which they could redeem for a supply of oral contraceptives at a modestly discounted price (Chin-Quee et al., 2010). The intervention had no effect on use of effective contraception; <1% of coupons were redeemed and only 13% of women in the intervention group and 11% of the control group had used the pill for 2 months or more after getting EC from a pharmacy. The authors explained the failure of the intervention on the fact that condom use is strongly encouraged as a means of STI protection and is the norm in Jamaica. More than two-third of the women in the intervention group said they did not want to use the pill. In a recent pilot study done in Edinburgh, 168 women recruited in pharmacies were randomized to receive standard care (verbal advice about going to see a doctor to discuss contraception after EC), a 1 month supply of progestogen-only oral contraceptive pill (POP) or an invitation to a ‘drop-in’ consultation with a local specialist family planning service (Michie et al., 2014). Telephone follow-up recorded contraceptive use 6–8 weeks after EC was used. The proportion of women using effective contraception at follow-up was significantly greater in both the POP arm of the study [56% (22/39), P ≤ 0.001] and the ‘drop-in’ arm [52% (13/25), P = 0.006] compared with standard care [16% (5/31)].
EC as a public health intervention
Of 15 studies (Raymond et al., 2007; Polis et al., 2007b) about increasing access to EC, only one (Shaaban et al., 2013) has demonstrated any reduction in unintended pregnancy or abortion rates at the population level. One possible reason for this may be that women do not use EC often enough after the most risky acts of unprotected intercourse to cause a measurable population level effect—even if they have a supply to keep at home. In one trial among the women who received ECs in advance of need, 45% who had unprotected sex did not use the pills (Raine et al., 2005). In another trial, one-third of women who received ECs in advance had at least one episode of unprotected sex without using EC (Raymond et al., 2006). The only study in which advance provision of EC did show a significant effect was undertaken among Egyptian women using the lactational amenorrhoea method of contraception (Shaaban et al., 2013). Women who received EC to keep at home had a lower pregnancy rate (0.8 versus 5.0%, P = 0.0002) within the first 6 months after childbirth and they were far more likely to start using an ongoing contraceptive, perhaps because advanced access to ECs allowed them the opportunity to obtain ongoing contraception at a clinic.
One common objection to making EC more accessible is the concern that women may be less likely to use a regular contraceptive method. EC is substantially less effective than most other contraceptives; if the average woman used the Yuzpe regimen whenever she had sex for 1 year, her pregnancy risk would be >35% and if she used progestin-only EC, her risk of pregnancy would be 20% compared with 8% for regular use of oral contraception and <1% for intrauterine or implantable contraception. Increased access to ECs does not increase risk-taking or reduce ongoing contraceptive use (Raymond et al., 2007; Schwarz et al., 2008; Ekstrand et al., 2013; Shaaban et al., 2013).
Four studies have also explored whether easier access to EC may affect rates of STIs. In these studies, women were randomly assigned to advanced provision of EC or to a control group, who obtained EC from a clinic when needed (Gold et al., 2004; Raine et al., 2005; Raymond et al., 2006; Ekstrand et al., 2008). In one trial adolescents who received EC in advance were more likely to use EC pills when needed but did not report more frequent unprotected sex, did not reduce their use of condoms or hormonal contraception, and did not have higher rates of STI (Gold et al., 2004). In another study providing education and information to teenagers about EC did not increase sexual activity or use of EC, but did increase knowledge about how and when to use EC (Graham et al., 2002).
What more needs to be done?
Since the 1990s EC has been the subject of a multitude of publications, and in 2014 it is widely available throughout much of the world. Although not the ‘holy grail’ for reducing unintended pregnancy rates that many enthusiasts had hoped, nonetheless EC has an important role to play in contraceptive choice. There is, however, still much that can be done to improve its availability, uptake and effective use and to encourage uptake of effective ongoing contraception after EC. Importantly, complacency should not deter us from developing more effective methods of EC.
Availability
UPA, the most effective oral emergency contraceptive available in Europe is, at the time of writing, still not available as a pharmacy medicine. If ratified by the EU, approval of UPA as a pharmacy medicine and the provision of both UPA and LNG as true over-the-counter contraceptive methods would do much to increase effective use. The copper IUD is by far the most effective EC method, with a failure rate of about 1 per 1000 (Cleland et al., 2012) and provides excellent ongoing contraceptive protection for at least 5 or 10 years after insertion. Clearly, increased use of the copper IUD as EC has the potential to substantially reduce rates of unintended pregnancy at the population level. However, in many countries the IUD is expensive (e.g. in the USA) and not many women know to ask for it. Moreover, since most EC users present to pharmacies where the IUD is not available and since many family doctors do not feel competent to insert emergency IUDs or are uncertain of medical eligibility for the method, strategies to increase the use of IUDs for EC need to be tested.
Quality of care
Much could be done to improve the quality of provision of EC in countries where it is available only after consultation with a health provider (including pharmacists). For example, women are not infrequently denied EC by providers who are ill-informed about the medical eligibility criteria for EC use (Glasier et al., 2010b). In some countries pharmacists either refuse to provide the service or else covertly raise barriers to access because they have moral objections to EC. Many doctors deny nulliparous women the IUD. A mandatory pregnancy test before EC is not necessary and further impairs access by, at the very least, increasing the cost. While for some of these barriers political will and even legislation is required, better training of health care providers would be a step in the right direction. Both the International and the European Consortia for Emergency Contraception (ICEC and ECEC) were established to expand knowledge about and access to EC and the ECEC aims to promote the standardization of service delivery in Europe. With this in mind both consortia have developed and widely disseminated evidence-based clinical guidance (European Consortium for Emergency Contraception, 2014).
Research on existing methods
A recent publication comparing the efficacy of LNG-EC with that of UPA (Glasier et al., 2010a) demonstrated an effect of weight and BMI on efficacy of EC. Using data collected for the two non-inferiority studies (Creinin et al., 2006, Glasier et al., 2010a), the risk of pregnancy was more than 3-fold greater for obese women (BMI 30 kg/m2 or more) compared with women with a normal BMI (OR, 3.60; 95% CI 1.96–6.53; P < 0.0001), whichever EC was taken. However, for women with a BMI ≥ 30 kg/m2, the risk was greater for those taking LNG (OR, 4.41; 95% CI 2.05–9.44, P = 0.0002) than for UPA users (OR, 2.62; 95% CI 0.89–7.00; ns). As a result of this, in March 2014 Health Canada asked pharmaceutical companies marketing LNG-EC to add new warnings to product packages advising that the pills ‘are less effective in women over 165–176 lb (75–80 g) and are not effective in women who weigh over 176 pounds (80 kg). The European Medicines Agency (EMA) had issued similar advice in relation to one LNG-EC product (NorLevo HRA Pharma Paris). However, perhaps as a result of huge media interest and heated discussion among health providers the EMA reviewed all the available data on LNG and UPA and concluded in July 2014 that the evidence was insufficient to draw any conclusions about the effect of weight on effectiveness of EC and advised that both LNG and UPA could be used by women of any weight, since the benefits outweighed the risks. Many argue that more research is needed to determine whether there is truly an effect of weight on the efficacy of EC.
Quick-starting of an ongoing method of contraception
Once EC has been used ongoing contraception is required if further pregnancies are to be prevented.
An obvious solution to encourage quick-starting of effective contraception after EC obtained from pharmacies would be to deregulate hormonal contraception. Many have argued cogently for this over the years (American College of Obstetricians and Gynecologists, 2012) but in Europe at least it seems unlikely to happen, at least in the near future. In London, England, initiatives allowing pharmacists routinely to provide supplies of oral contraceptives to young women attending for EC have not been evaluated for effectiveness (Parsons et al., 2013).
However, there is concern that antiprogestins might alter the effectiveness of progestogen containing contraception and the requirement for extra contraceptive precautions is unknown. A recent RCT of UPA or placebo (taken when the dominant follicle was >13 mm), followed by quick start of the COC pill, showed that ovarian quiescence after either UPA or placebo was achieved by 14 days of COC pills (Cameron et al., 2013). This suggests that no more than 14 days of extra contraceptive precautions may be necessary when quick starting COC after UPA. There are as yet no data on the effect of quick starting the POP immediately after using UPA for EC. Of equal concern is the possibility that quick starting either a POP or a COC may jeopardize the efficacy of UPA, since the immediate intake of a progestin may reverse the effects of the anti-progestogen on ovulation.
Development of new methods
Currently, available oral emergency contraceptives work by delaying or inhibiting ovulation and are effective for only part of the fertile window. While UPA continues to be effective even after the onset of the LH surge, once LH has reached its peak neither can stop ovulation (Brache et al., 2013). Adding the cyclo-oxygenase-2 inhibitor Meloxicam, which inhibits follicle rupture, has been shown to increase effectiveness of LNG (Massai et al., 2007) and would probably do the same if given in combination with UPA although this has not been tested. Other non-steroidal anti-inflammatory drugs may also be effective.
UPA delays ovulation by at least 5 days after intercourse (the theoretical lifetime of a sperm), but ovulation does eventually occur and women who have repeated acts of intercourse after using UPA are at risk of pregnancy. Mifepristone 25 mg used as EC almost certainly has an effect on the endometrium to impair implantation of the fertilized oocyte. Researchers, however, have deliberately shied away from developing methods of EC intentionally to inhibit implantation although this would likely result in a highly effective method. There appears to be general distaste for contraceptive methods which affect implantation—a mechanism which many anti-abortion activists and the Roman Catholic Church (among others) consider to be unethical; indeed a great deal of effort has been devoted to trying to demonstrate that, for the IUD for example, it simply does not happen. There is no good biological reason for this reluctance. Moreover, the majority of women who are desperate to avoid an unwanted pregnancy are unlikely to mind if implantation is inhibited as long as the method is effective. In two international surveys of attitudes to the idea of a once-a-month pill in every country more than half the women asked found the concept of a pill which inhibited implantation acceptable (Rimmer et al., 1992; Glasier et al., 1999). There are plenty of drugs available which would inhibit implantation and be specific to the reproductive process (high dose mifepristone for example) which could be developed for EC (Raymond et al., 2013). Recent advances in the biology of fertilization may provide ideas for the development of new methods. Folate receptor 4 (folr 4, now called Juno) on the oolemma is conserved across species and interacts with Izumo1 on sperm preventing fertilization by further sperm (Bianchi et al., 2014). Folr4 knock-out mice are infertile. However, contraceptives based on these principles are at least 20 years away and fraught with the problems of demonstrating safety and would pose considerable regulatory issues. Although unlikely to be approved by countries that are predominantly Roman Catholic, there is no reason to deny the rest of the world access to methods of EC which would reliably prevent more unwanted pregnancies.
Conclusions
The spread of EC across the world had been surprisingly successful, given the moral objections to its availability and use that have been expressed by many opponents. While there has never been a RCT of the efficacy of EC, nevertheless reasonable comparative data demonstrate effectiveness at preventing pregnancy for individual women. EC is safe and has few side effects, and in most settings, its use is not associated with abandonment of more effective methods of contraception, or with an increase in risky sexual behaviour. Access to EC, including the cost, has a major influence on its use. If EC is available without a doctor's prescription its use is more widespread. The likelihood of using EC depends also on knowledge about EC, the recognition of the need to use it and the motivation to do so.
While EC can prevent pregnancy for individual women, it has not been shown to be effective at a public health level perhaps because many women who could use it do not because they do not recognize or acknowledge the need to do so. Still fewer than 15% of women presenting for induced abortion (and in many countries considerably less than that) say they used EC to try to prevent an unintended pregnancy. Interventions to facilitate the start of effective ongoing contraception (especially long-acting methods) very soon after EC use should help to reduce unintended pregnancies.
Despite great enthusiasm for EC among the research community, there is still much to be done. Availability, access and quality of care need to be improved in many countries worldwide. We really do need to know whether obesity jeopardizes the efficacy of the standard doses of EC and whether quick starting hormonal contraception has any detrimental effect. Finally, there is scope for the development of new, more effective methods including those which work by inhibiting implantation.
Authors' roles
All lecturers and discussants contributed to the preparation of the final manuscript.
Funding
The meeting was organized by the European Society of Human Reproduction and Embryology with an unrestricted educational grant from Institut Biochimique S.A. (Switzerland).
Conflict of interest
Anna Glasier acts as a consultant for HRA-Pharma. Sharon Cameron and Anna Glasier have received funding for research from HRA Pharma. Kristina Gemzell-Danielsson acts occasionally as consultant or invited speaker supported by HRA-Pharma, Bayer AG and Gedeon Richter on matters related to emergency contraception
Acknowledgements
The secretarial assistance of Mrs Simonetta Vassallo is gratefully acknowledged. J. Trussell's presentation was partially supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development grant for Infrastructure for Population Research at Princeton University, Grant R24HD047879 (JT).
Appendix
A meeting was organized by ESHRE (29–30 August 2014) to discuss the above subjects. The contributors included: D.T. Baird (Simpson Centre for Reproductive Health, University of Edinburgh, UK), S. Cameron (Consultant Gynaecologist, Chalmers Sexual and Reproductive Health Service, Obstetrics and Gynaecology, University of Edinburgh, Royal Infirmary of Edinburgh, Edinburgh, UK), J.L.H. Evers (Dept. Obstet. Gynecol., Maastricht University Medical Centre, Maastricht, The Netherlands), K. Gemzell-Danielsson (Chair Div. of Obstetrics and Gynecology Dept. of Women's and Children's Health, Karolinska Institutet, Karolinska University Hospital, Stockholm, Sweden), A. Glasier (Simpson Centre for Reproductive Health, University of Edinburgh, UK), C. Moreau (Population Family and reproductive health, Bloomberg school of public health, Hopkins university, Baltimore MD USA and Inserm U1018, CESP-‘Gender, Sexual and Reproductive Health’, Hopital du Kremlin Bicetre, Le Kremlin Bicetre, France), J. Trussell (Professor of Economics and Public Affairs, Faculty Associate, Office of Population Research, Princeton University, Princeton, NJ, USA), H. von Hertzen (World Health Organization, Geneva, Switzerland). The discussants included: P.G. Crosignani (Scientific Direction, IRCCS Ca' Granda Foundation, Maggiore Policlinico Hospital, Milano, Italy), C. La Vecchia (Istituto di Ricerche Farmacologiche ‘Mario Negri’ and Department of Clinical Sciences and Community Health, Università degli Studi di Milano, Milano, Italy), A. Volpe (Dipartimento Integrato Materno Infantile, Università di Modena, Italy). The report was prepared by A. Glasier and P.G. Crosignani.
References
- American College of Obstetricians and Gynecologists. Over-the-Counter Access to Oral Contraceptives. 2012. Committee Opinion Number 544, December.
- Askalani AH, Al-Senity AM, Al-Agizy HM, Salam HI, Al-Masry GI, El-Sadek SM. Evaluation of copper T-200 as a post-coital contraceptive. Egyptian J Obstet Gynaecol. 1987;13:63–66. [Google Scholar]
- Berger C, Boggavarapu NR, Meneziz J, Lalitkumar PGL, Gemzell-Danielsson K. Effects of ulipristal acetate on human embryo implantation in a three-dimensional human endometrial in vitro co-culture system. Hum Reprod. 2015;30:800–811. doi: 10.1093/humrep/dev030. [DOI] [PubMed] [Google Scholar]
- Bianchi E, Doe B, Goulding D, Wright GJ. Juno is the egg Izumo receptor and is essential for mammalian fertilization. Nature. 2014;508:483–487. doi: 10.1038/nature13203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brache V, Cochon L, Deniaud M, Croxatto HB. Ulipristal acetate prevents ovulation more effectively than levonorgestrel: analysis of pooled data from three randomized trials of emergency contraception regimens. Contraception. 2013;88:611–618. doi: 10.1016/j.contraception.2013.05.010. [DOI] [PubMed] [Google Scholar]
- Cameron ST, Gordon R, Glasier A. The effect on use of making emergency contraception available free of charge. Contraception. 2012;86:366–369. doi: 10.1016/j.contraception.2012.02.018. [DOI] [PubMed] [Google Scholar]
- Cameron ST, Gemzell–Danielsson K, Klipping C, Michie L, Berger C, Levy D, Abitol JL. Ulipristal acetate (UPA) vs levonorgestrel (LNG) and quick start of contraception. First Global Conference on Contraception, Reproductive and Sexual Health. Eur J Contracept Reprod Health. 2013;18(Suppl 1):s45. [Google Scholar]
- Cheng L, Che Y, Gülmezoglu AM. Interventions for emergency contraception. Cochrane Database Syst Rev. 2012;8:CD001324. doi: 10.1002/14651858.CD001324.pub4. [DOI] [PubMed] [Google Scholar]
- Chin-Quee DS, Wedderburn M, Otterness C, Janowitz B, Chen-Mok M. Bridging emergency contraceptive pill users to regular contraception: results from a randomized trial in Jamaica. Contraception. 2010;81:133–139. doi: 10.1016/j.contraception.2009.08.015. [DOI] [PubMed] [Google Scholar]
- Cleland K, Raymond E, Trussell J, Cheng L, Zhu H. Ectopic pregnancy and emergency contraceptive pills: a systematic review. Obstet Gynecol. 2010;115:1263–1266. doi: 10.1097/AOG.0b013e3181dd22ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland K, Zhu H, Goldstuck N, Cheng L, Trussell J. The efficacy of intrauterine devices for emergency contraception: a systematic review of 35 years of experience. Hum Reprod. 2012;27:1994–2000. doi: 10.1093/humrep/des140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creinin MD, Schlaff W, Archer DF, Wan L, Frezieres R, Thomas M, Rosenberg M, Higgins J. Progesterone receptor modulator for emergency contraception: a randomized controlled trial. Obstet Gynecol. 2006;108:1089–1097. doi: 10.1097/01.AOG.0000239440.02284.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrand M, Larsson M, Darj E, Tydén T. Advance provision of emergency contraceptive pills reduces treatment delay: a randomised controlled trial among Swedish teenage girls. Acta Obstet Gynecol Scand. 2008;87:354–359. doi: 10.1080/00016340801936024. [DOI] [PubMed] [Google Scholar]
- Ekstrand M, Tydén T, Darj E, Larsson M. Twelve-month follow-up of advance provision of emergency contraception among teenage girls in Sweden-a randomized controlled trial. Ups J Med Sci. 2013;118:271–275. doi: 10.3109/03009734.2013.841308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellertson C, Webb A, Blanchard K, Bigrigg A, Haskell S, Shochet T, Trussell J. Modifying the Yuzpe regimen of emergency contraception: a multicenter randomized controlled trial. Obstet Gynecol. 2003;101:1160–1167. doi: 10.1016/s0029-7844(03)00353-3. [DOI] [PubMed] [Google Scholar]
- European Consortium for Emergency Contraception. Emergency Contraception Availability in Europe. http://www.ec-ec.org/emergency-contraception-in-europe/emergency-contraception-availability-in-europe/ (18 May 2014, date last accessed)
- European Medicines Agency. EMA recommends availability of ellaOne emergency contraceptive without prescription. EMA Press Office EMA/710568/2014.
- Gemzell-Danielsson K, Marions L. Mechanisms of action of mifepristone and levonorgestrel when used for emergency contraception. Hum Reprod Update. 2004;10:341–348. doi: 10.1093/humupd/dmh027. [DOI] [PubMed] [Google Scholar]
- Glasier AF, Smith KB, Cheng L, Ho PC, van der Spuy Z, Baird DT. An international study on the acceptability of a once-a-month pill. Hum Reprod. 1999;14:3018–3022. doi: 10.1093/humrep/14.12.3018. [DOI] [PubMed] [Google Scholar]
- Glasier A, Cameron ST, Fine PM, Logan SJS, Casale W, Van Horn J, Sogor L, Blithe DL, Scherrer B, Mathe H, et al. Ulipristal acetate versus levonorgestrel for emergency contraception: a randomized non-inferiority trial and meta-analysis. Lancet. 2010a;375:555–562. doi: 10.1016/S0140-6736(10)60101-8. [DOI] [PubMed] [Google Scholar]
- Glasier A, Manners R, Loudon JC, Muir A. Community pharmacists providing emergency contraception give little advice about future contraceptive use: a Mystery Shopper Study. Contraception. 2010b;82:538–542. doi: 10.1016/j.contraception.2010.05.008. [DOI] [PubMed] [Google Scholar]
- Glasier A, Cameron ST, Blithe D, Scherrer B, Mathe H, Levy D, Gainer E, Ulmann A. Can we identify women at risk of pregnancy despite using emergency contraception? Data from randomized trials of ulipristal acetate and levonorgestrel. Contraception. 2011;84:363–367. doi: 10.1016/j.contraception.2011.02.009. [DOI] [PubMed] [Google Scholar]
- Gold MA, Wolford JE, Smith KA, Parker AM. The effects of advance provision of emergency contraception on adolescent women's sexual and contraceptive behaviors. J Pediatr Adolesc Gynecol. 2004;17:87–96. doi: 10.1016/j.jpag.2003.11.018. [DOI] [PubMed] [Google Scholar]
- Graham A, Moore L, Sharp D, Diamond I. Improving teenagers' knowledge of emergency contraception: cluster randomized controlled trial of a teacher led intervention. Br Med J. 2002;234:1179–1184. doi: 10.1136/bmj.324.7347.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakha F, Glasier A. Unintended pregnancy and use of emergency contraception among a large cohort of women attending for antenatal care or abortion in Scotland. Lancet. 2006;368:1782–1787. doi: 10.1016/S0140-6736(06)69737-7. [DOI] [PubMed] [Google Scholar]
- Lalitkumar PGL, Lalitkumar S, Meng CX, Stavreus-Evers A, Hambiliki F, Bentin-Ley U, Gemzell-Danielsson K. Mifepristone but not levonorgestrel inhibits human blastocyst attachment to an in vitro endometrial three-dimensional cell culture model. Hum Reprod. 2007;22:3031–3037. doi: 10.1093/humrep/dem297. [DOI] [PubMed] [Google Scholar]
- Lalitkumar PG, Berger C, Gemzell-Danielsson K. Emergency contraception. Best Pract Res Clin Endocrinol Metab. 2013;27:91–101. doi: 10.1016/j.beem.2012.09.003. [DOI] [PubMed] [Google Scholar]
- Levy DP, Jager M, Kapp N, Abitbol JL. Ulipristal acetate for emergency contraception: post-marketing experience after use by more than 1 million women. Contraception. 2014;89:431–433. doi: 10.1016/j.contraception.2014.01.003. [DOI] [PubMed] [Google Scholar]
- Massai MR, Forcelledo ML, Brache V, Tejada AS, Salvatierra AM, Reyes MV, Alvarez F, Faúndes A, Croxatto HB. Does meloxicam increase the incidence of anovulation induced by single administration of levonorgestrel in emergency contraception? A Pilot Study. Hum Reprod. 2007;22:434–439. doi: 10.1093/humrep/del369. [DOI] [PubMed] [Google Scholar]
- Michie L, Cameron ST, Glasier A, Larke N, Muir A, Lorimer A. Pharmacy-based interventions for initiating effective contraception following the use of emergency contraception: a pilot study. Contraception. 2014;90:447–453. doi: 10.1016/j.contraception.2014.05.004. [DOI] [PubMed] [Google Scholar]
- Moreau C, Bouyer J, Goulard H, Bajos N. The remaining barriers to the use of emergency contraception: perception of pregnancy risk by women undergoing induced abortions. Contraception. 2005;71:202–207. doi: 10.1016/j.contraception.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Moreau C, Bajos N, Trussell J. The impact of pharmacy access to emergency contraceptive pills in France. Contraception. 2006;73:602–608. doi: 10.1016/j.contraception.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Parsons J, Adams C, Aziz N, Holmes J, Jawad R, Whittlesea C. Evaluation of a community pharmacy delivered oral contraception service. J Fam Plann Reprod Health Care. 2013;39:97–101. doi: 10.1136/jfprhc-2012-100304. [DOI] [PubMed] [Google Scholar]
- Polis CB, Schaffer K, Blanchard K, Glasier A, Harper CC, Grimes DA. Advance provision of emergency contraception for pregnancy prevention: a meta-analysis. Obstet Gynecol. 2007a;110:1379–1388. doi: 10.1097/01.AOG.0000295603.84568.f6. [DOI] [PubMed] [Google Scholar]
- Polis CB, Schaffer K, Blanchard K, Glasier A, Harper CC, Grimes DA. Advance provision of emergency contraception for pregnancy prevention (full review) Cochrane Database Syst Rev. 2007b;2:CD005497. doi: 10.1002/14651858.CD005497.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raine TR, Harper CC, Rocca CH, Fischer R, Padian N, Klausner JD, Darney PD. Direct access to emergency contraception through pharmacies and effect on unintended pregnancy and STIs: a randomized controlled trial. J Am Med Assoc. 2005;293:54–62. doi: 10.1001/jama.293.1.54. [DOI] [PubMed] [Google Scholar]
- Raymond EG, Stewart F, Weaver M, Monteith C, Van Der Pol B. Impact of increased access to emergency contraceptive pills: a randomized controlled trial. Obstet Gynecol. 2006;108:1098–1106. doi: 10.1097/01.AOG.0000235708.91572.db. [DOI] [PubMed] [Google Scholar]
- Raymond E, Trussell J, Polis C. Population effect of increased access to emergency contraceptive pills. Obstet Gynecol. 2007;109:181–188. doi: 10.1097/01.AOG.0000250904.06923.4a. [DOI] [PubMed] [Google Scholar]
- Raymond EG, Coeytaux F, Gemzell-Danielsson K, Moore K, Trussell J, Winikoff B. Embracing post-fertilisation methods of family planning: a call to action. J Fam Plann Reprod Health Care. 2013;39:244–246. doi: 10.1136/jfprhc-2013-100702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richman A, Daley E, Baldwin J, Kromrey J, O'Rourke K, Perrin K. The role of pharmacists and emergency contraception: are pharmacists' perceptions of emergency contraception predictive of their dispensing practices? Contraception. 2012;86:370–375. doi: 10.1016/j.contraception.2012.01.014. [DOI] [PubMed] [Google Scholar]
- Rimmer C, Horga M, Cerar V, Alder EM, Baird DT, Glasier A. Do women want a once a month pill? Hum Reprod. 1992;7:608–611. doi: 10.1093/oxfordjournals.humrep.a137705. [DOI] [PubMed] [Google Scholar]
- Rodriguez M, Curtis K, Mary Gaffield L, Jackson E, Kapp N. Advance supply of emergency contraception: a systematic review. Contraception. 2013;97:590–601. doi: 10.1016/j.contraception.2012.09.011. [DOI] [PubMed] [Google Scholar]
- Schwarz EB, Gerbert B, Gonzales R. Computer-assisted provision of emergency contraception: a randomized controlled trial. J Gen Intern Med. 2008;23:794–799. doi: 10.1007/s11606-008-0609-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaaban OM, Hassen SG, Nour SA, Kames MA, Yones EM. Emergency contraceptive pills as a backup for lactational amenorrhea method (LAM) of contraception: a randomized controlled trial. Contraception. 2013;87:363–369. doi: 10.1016/j.contraception.2012.07.013. [DOI] [PubMed] [Google Scholar]
- Sitruk-Ware R. Mifepristone and misoprostol sequential regimen side effects, compliactions and safety. Contraception. 2006;74:48–55. doi: 10.1016/j.contraception.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Sørensen M, Pedersen B, Nyrnberg L. Differences between users and non-users of emergency contraception after a recognized unprotected intercourse. Contraception. 2000;62:1–3. doi: 10.1016/s0010-7824(00)00128-1. [DOI] [PubMed] [Google Scholar]
- Stratton P, Levens ED, Hartog B, Piquion J, Wei Q, Merino M, Nieman LK. Endometrial effects of a single early luteal dose of the selective progesterone receptor modulator CDB-2914. Fertil Steril. 2010;93:2035–2041. doi: 10.1016/j.fertnstert.2008.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trussell J, Ellertson C, Von Hertzen H, Bigrigg A, Webb A, Evans M, Ferden S, Leadbetter C. Estimating the effectiveness of emergency contraceptive pills. Contraception. 2003;67:259–265. doi: 10.1016/s0010-7824(02)00535-8. [DOI] [PubMed] [Google Scholar]
- Von Hertzen H, Piaggio G, Peregoudov A, Ding J, Chen J, Song S, Bártfai G, Ng E, Gemzell-Danielsson K, Oyunbileg A, et al. for the WHO Research Group on Post-ovulatory Methods of Fertility Regulation. Low dose mifepristone and two regimens of levonorgestrel for emergency contraception: a WHO multicentre randomised trial. Lancet. 2002;360:1803–1810. doi: 10.1016/S0140-6736(02)11767-3. [DOI] [PubMed] [Google Scholar]
- Westley E, Kapp N, Palermo T, Bleck J. A review of global access to emergency contraception. Int J Gynecol Obstet. 2013;123:4–6. doi: 10.1016/j.ijgo.2013.04.019. [DOI] [PubMed] [Google Scholar]
- Wilcox AJ, Weinberg CR, Baird DD. Timing of sexual intercourse in relation to ovulation: effects on the probability of conception, survival of the pregnancy and sex of the baby. N Engl J Med. 1995;333:1517–1521. doi: 10.1056/NEJM199512073332301. [DOI] [PubMed] [Google Scholar]
- Wilcox AJ, Weinberg CR, Baird DD. Post-ovulatory ageing of the human oocyte and embryo failure. Hum Reprod. 1998;13:394–397. doi: 10.1093/humrep/13.2.394. [DOI] [PubMed] [Google Scholar]
- Williamson LM, Buston K, Sweeting H. Young women's perceptions of pregnancy risk and use of emergency contraception: findings from a qualitative study. Contraception. 2009;79:310–315. doi: 10.1016/j.contraception.2008.10.014. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Methods for fertility regulation. Randomized controlled trial of levonorgestrel versus the Yuzpe regimen of combined oral contraceptives for emergency contraception. Lancet. 1998;352:428–433. [PubMed] [Google Scholar]
- World Health Organization. WHO Model List of Essential Medicines. 18th edn. 2013. April http://www.who.int/medicines/publications/essentialmedicines/en/index.htm. (2 February 2015, date last accessed)
- Yuzpe AA, Lancee WJ. Ethinylestradiol and dl-norgestrel as a post-coital contraceptive. Fertil Steril. 1977;28:932–936. [PubMed] [Google Scholar]