Abstract
Background.
Although evidence indicates that Type II Diabetes is related to abnormal brain aging, the influence of elevated blood glucose on long-term cognitive change is unclear. In addition, the relationship between diet-based glycemic load and cognitive aging has not been extensively studied. The focus of this study was to investigate the influence of diet-based glycemic load and blood glucose on cognitive aging in older adults followed for up to 16 years.
Methods.
Eight-hundred and thirty-eight cognitively healthy adults aged ≥50 years (M = 63.1, SD = 8.3) from the Swedish Adoption/Twin Study of Aging were studied. Mixed effects growth models were utilized to assess overall performance and change in general cognitive functioning, perceptual speed, memory, verbal ability, and spatial ability as a function of baseline blood glucose and diet-based glycemic load.
Results.
High blood glucose was related to poorer overall performance on perceptual speed as well as greater rates of decline in general cognitive ability, perceptual speed, verbal ability, and spatial ability. Diet-based glycemic load was related to poorer overall performance in perceptual speed and spatial ability.
Conclusion.
Diet-based glycemic load and, in particular, elevated blood glucose appear important for cognitive performance/cognitive aging. Blood glucose control (perhaps through low glycemic load diets) may be an important target in the detection and prevention of age-related cognitive decline.
Key Words: Cognitive aging, Biomarkers, Nutrition.
Type II Diabetes Mellitus (T2DM) is a major contributor to morbidity and mortality worldwide and has become increasingly prevalent in the aging population, affecting up to 26% of those aged ≥65 years (1). Type II Diabetes Mellitus is also related to brain atrophy (2), cognitive decline (3,4), and increased risk of dementia (5). Although evidence indicates that T2DM is related to abnormal brain aging, there has been little focus on the specific influence of high blood glucose on long-term cognitive change. Diets rich in refined/simple carbohydrates may lead to high blood glucose (6), and have been associated with poorer memory (7,8) and reaction time (9). However, the influence of diet-based glycemic load on long-term cognitive change is less clear. Therefore, we investigated the hypothesis that high fasting blood glucose and diet-based glycemic load would be related to poorer cognitive performance and greater cognitive decline among cognitively healthy older adults.
Methods
Study Sample and Testing Procedure
Data collection procedures for the Swedish Adoption Twin Study of Aging (SATSA) have been detailed earlier (10). In brief, SATSA is a sample consisting of a subset of twins from the population-based Swedish Twin Registry (11). Questionnaire respondents aged ≥50 years (n = 2,018) were invited to participate in a series of in-person testing sessions (IPTs) consisting of a battery of cognitive tests and health assessments. Trained research nurses collected data during a 4-hour interview which included suitable breaks to minimize participant fatigue. After the first testing session (IPT1), the second (IPT2), and third (IPT3) testing sessions occurred at 3-year intervals. The next wave of testing (called IPT5) started 7 years later, because no in-person testing took place during the fourth wave (12). The sixth wave (IPT6) was initiated after another 3 years. Individuals could enter the study during any of the first four testing sessions. Over the study period, a total of 859 participants were enrolled in at least one wave. Of these, 840 had cognitive data and were absent of dementia at study entry, and 838 had valid data on blood glucose levels at their respective baseline cognitive testing point (see Figure 1). Information from 553 participants was available from the food frequency questionnaire which was mailed just prior to IPT1. We restricted our analyses to dementia-free testing occasions (ie, once diagnosed, an individual was excluded from further analysis). During the study period, 78 participants (9.4% of the sample) were excluded due to dementia diagnosis.
Figure 1.
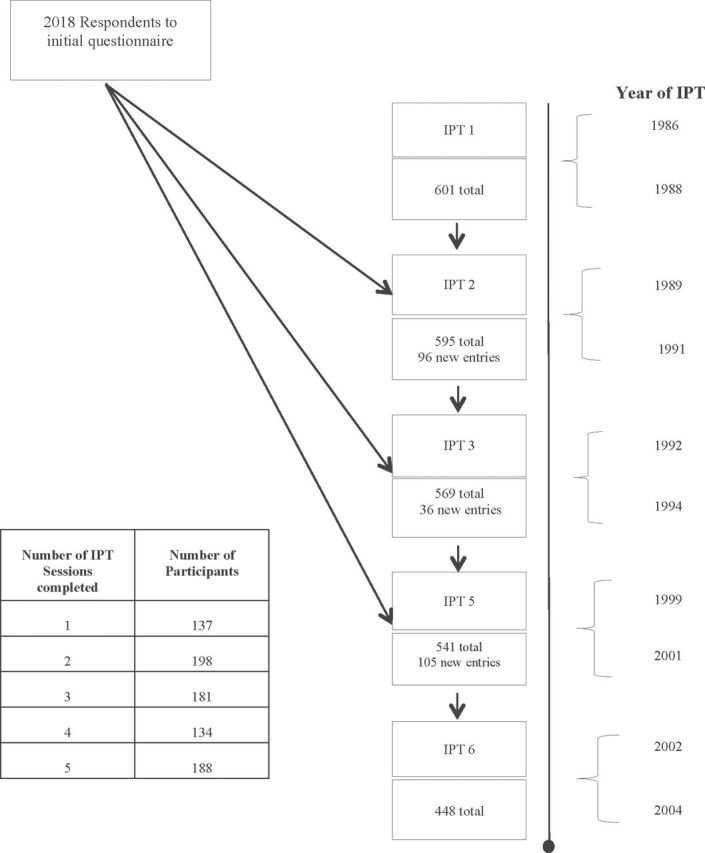
Flow chart of data collection procedures and timeline. Respondents to initial questionnaires were invited to participate in a series of five in-person testing sessions (IPTs), spanning up to 16 years. Participants aged ≥50 years were permitted to enter the study during the first four testing waves.
The participants engaged in an average of 3.04 cognitive testing occasions (SD = 1.4) spanning an average of 7.9 years (SD = 6.1 years) with a maximum follow-up of 16 years, and 60% of the sample had cognitive data for at least three testing occasions. Dementia status was determined by established clinical diagnostic criteria (13). In brief, evaluation checklists corresponding to the Diagnostic and Statistical Manual of Mental Disorders (DSM; (III-R or IV) criteria for dementia guided final diagnoses (14,15). This study is in compliance with the Swedish Act concerning the ethical review of research involving humans, which includes informed consent of the participants.
Cognitive Assessment
The SATSA includes a comprehensive cognitive assessment (10) (see the Supplementary Appendix). A principal components analysis using preselected domains (ie, only verbal measures were used to create the verbal factor, and so on.) of the IPT1 measures, described earlier in detail (10,16), was used. In brief, digit symbol and figure identification loaded into the perceptual speed domain. Forward and backward digit span, picture memory, and immediate and delayed recall of names and faces loaded into a memory domain. Verbal abilities were tapped by information, synonyms, and analogies. Finally, figure logic, block design, and card rotations loaded together to create a spatial abilities domain. Reliabilities for these tests range between 0.82 and 0.96 (10).
Main Predictors: Blood Glucose and Diet-Based Glycemic Load
At study entry, participants were instructed to fast, and blood glucose was measured in millimoles per liter (mmol/L) using an enzymatic (glucose-oxidase) method (KODAK Ektachem). In addition to examining blood glucose as a continuous variable, we also used a dichotomy based on the American Diabetes Association guidelines (13), where fasting levels >7.0 mmol/L constituted high blood glucose. We considered this approach superior to classification by T2DM status because not all individuals with T2DM have high blood glucose, and not all individuals with high blood glucose have recorded T2DM diagnoses.
From the diet questionnaire, self-reported consumption of foods indicative of the participants’ glycemic load (foods high in refined carbohydrates or sugars) was examined (consumption of white bread, sweetened beverages, sugar in coffee, ice cream, cake/biscuits, and pastries). Participants were asked to rate their approximate daily frequency of consumption of white bread slices, sweetened beverages, and lumps/teaspoons of sugar in coffee. For ice cream, cake/biscuits, and pastry consumption, participants rated their normal consumption during a given year on a scale of: “>4 times/week,” “1–4 times/week,” “1–3 times/month,” “less than once/month,” or “never.” Because these variables were measured with different scales, a composite variable reflecting diet-based glycemic load was constructed by: (i) creating z-scores from each measure to put all items on an equal scale with mean = 0, SD = 1 and (ii) taking the sum of the z-score values.
Covariates
Baseline age, education (elementary school vs high school or above), gender, waist circumference, and depressive symptoms were entered as covariates in the statistical models described in what follows because they have been shown earlier to be associated with cognitive decline (17–20). Depressive symptoms were assessed using the mental health subscale from the Older Americans Resources and Services Depression Scale (21) which includes five yes/no items (M = 1.84, SD = 1.16), with reliability reported at .804 (21). We also examined if controlling for baseline cardiovascular disease (CVD) and T2DM would explain the results because these have been associated with cognitive decline (22). Cardiovascular disease was defined as self-reported symptoms of angina pectoris, heart attack, claudication, high blood pressure, stroke, or any other cardiovascular dysfunction (eg, thrombosis, tachycardia, circulation problems, heart valve problems, and phlebitis). Both CVD and T2DM were coded as self-reports of absence versus presence at baseline.
Statistical Analyses
Mixed effects growth modeling was used to examine the influence of diet-based glycemic load and blood glucose on cognitive aging. These models yield fixed effects (ie, fixed population parameters estimated by the overall performance of the entire sample), slope designating overall change in performance, and change conditional on a predictor (ie, time-by-predictor interaction), as well as random effects. This procedure provides flexibility by enabling the retention of individuals with missing data points by estimating their expected trajectories based on all available data (23). For all models, an unstructured error covariance specification was employed. Finally, a common twin pair identifier was used along with an individual identifier, thereby allowing the models to account for both the intraindividual and within-pair variance.
Individual models were analyzed for general cognitive ability and each of the four cognitive domains for both blood glucose and glycemic load levels. Initially, unconditional models with no predictors were estimated to provide a general picture of cognitive change over the period of observation. However, we were most interested in the fixed effects for overall cognitive performance, cognitive performance conditional on the value of the predictor (ie, whether performance would be better or worse as a function of diet-based glycemic load or blood glucose), change in cognitive scores over time, and change in cognitive scores conditional on the value of the predictor (ie, whether glycemic load or blood glucose affects change in cognitive performance over time). All variables were centered at the mean prior to analyses.
An age-based growth model was used, which allowed for the implementation of a cohort-sequential design (24). Here, the results reflect cognitive change, not just for the maximum of 16 years of follow-up, but for the entire age span (in this case, 37 years with ages ranging from 50 to 87 years). Age was divided by 10 so that the estimates reflect change (in T-score units) per decade. In addition to age, the models were adjusted for gender, education, waist circumference, and depressive symptoms (Model 2 in Tables 2 and 3). Then, T2DM and CVD were added to the models to see if they explained the results (Model 3 in Tables 2 and 3). The models were also adjusted for practice effects, coded as 0 (baseline only) versus 1 (at least one follow-up) as suggested by previous research with these cognitive data (25). Significant estimates in the models, as well as differences in baseline characteristics of the sample (using independent samples t tests), were taken at the p < .05 level. SAS (SAS Institute, Cary, NC) version 9.3 was utilized for all statistical analyses.
Table 2.
Blood Glucose in Relation to Cognitive Performance and Change per 10 Years of Age (n = 838)
| General Ability | Speed | Memory | Verbal | Spatial | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Est. | SE | p | Est. | SE | p | Est. | SE | p | Est. | SE | p | Est. | SE | P | |
| Blood glucose (continuous) | |||||||||||||||
| Model 1* | |||||||||||||||
| Intercept | 51.50 | 0.35 | <.001 | 49.59 | 0.29 | <.001 | 51.65 | 0.34 | <.001 | 51.33 | 0.33 | <.001 | 50.32 | 0.32 | <.001 |
| Change | −3.45 | 0.18 | <.001 | −6.25 | 0.20 | <.001 | −2.93 | 0.23 | <.001 | −1.21 | 0.17 | <.001 | −4.26 | 0.19 | <.001 |
| Predictor | −0.70 | 0.38 | .060 | −1.02 | 0.31 | .001 | −0.82 | 0.35 | .020 | −0.42 | 0.35 | .220 | −0.59 | 0.35 | .100 |
| Change × predictor | −0.92 | 0.23 | <.001 | −0.80 | 0.26 | .002 | −0.30 | 0.31 | .330 | −0.64 | 0.22 | .004 | −0.73 | 0.25 | .004 |
| Model 2† | |||||||||||||||
| Intercept | 51.69 | 0.35 | <.001 | 50.28 | 0.35 | <.001 | 51.50 | 0.40 | <.001 | 51.68 | 0.34 | <.001 | 50.89 | 0.37 | <.001 |
| Change | −2.64 | 0.40 | <.001 | −4.59 | 0.43 | <.001 | −1.17 | 0.52 | .024 | −0.94 | 0.36 | .009 | −2.97 | 0.46 | <.001 |
| Predictor | −0.31 | 0.34 | .354 | −0.82 | 0.30 | .006 | −0.33 | 0.33 | .326 | 0.00 | 0.31 | .993 | −0.41 | 0.33 | .219 |
| Change × predictor | −0.71 | 0.22 | .002 | −0.65 | 0.25 | .010 | −0.15 | 0.29 | .606 | −0.42 | 0.21 | .048 | −0.51 | 0.25 | .046 |
| Model 3‡ | |||||||||||||||
| Intercept | 51.72 | 0.35 | <.001 | 50.28 | 0.35 | <.001 | 51.48 | 0.40 | <.001 | 51.68 | 0.34 | <.001 | 50.84 | 0.37 | <.001 |
| Change | −2.56 | 0.40 | <.001 | −4.60 | 0.43 | <.001 | −1.13 | 0.52 | .040 | −0.92 | 0.36 | .010 | −3.04 | 0.45 | <.001 |
| Predictor | −0.15 | 0.36 | .670 | −0.82 | 0.32 | .010 | −0.26 | 0.34 | .440 | −0.03 | 0.33 | .930 | −0.39 | 0.35 | .260 |
| Change × predictor | −0.64 | 0.24 | .010 | −0.67 | 0.26 | .010 | −0.28 | 0.31 | .360 | −0.30 | 0.22 | .180 | −0.48 | 0.26 | .070 |
| Blood glucose (dichotomous) | |||||||||||||||
| Model 1* | |||||||||||||||
| Intercept | 51.59 | 0.36 | <.001 | 49.71 | 0.30 | <.001 | 51.72 | 0.35 | <.001 | 51.41 | 0.34 | <.001 | 50.42 | 0.33 | <.001 |
| Change | −3.20 | 0.18 | <.001 | −6.08 | 0.20 | <.001 | −2.83 | 0.23 | <.001 | −1.07 | 0.17 | <.001 | −4.13 | 0.20 | <.001 |
| Predictor | −1.38 | 2.06 | .500 | −2.49 | 1.74 | .150 | −1.08 | 1.99 | .590 | −1.85 | 1.90 | .330 | −0.85 | 1.92 | .660 |
| Change × predictor | −5.72 | 1.21 | <.001 | −4.32 | 1.39 | .002 | −3.20 | 1.62 | .050 | −2.87 | 1.18 | .020 | −3.74 | 1.38 | .007 |
| Model 2† | |||||||||||||||
| Intercept | 51.71 | 0.35 | <.001 | 50.37 | 0.35 | <.001 | 51.47 | 0.41 | <.001 | 51.67 | 0.34 | <.001 | 50.91 | 0.37 | <.001 |
| Change | −2.44 | 0.40 | <.001 | −4.43 | 0.43 | <.001 | −1.10 | 0.52 | .034 | −0.85 | 0.36 | .019 | −2.84 | 0.45 | <.001 |
| Predictor | 0.93 | 1.81 | .608 | −1.64 | 1.65 | .323 | 1.38 | 1.87 | .460 | 0.98 | 1.74 | .574 | 0.62 | 1.79 | .728 |
| Change × predictor | −4.94 | 1.13 | <.001 | −3.48 | 1.32 | .009 | −2.75 | 1.54 | .075 | −2.19 | 1.11 | .048 | −3.02 | 1.32 | .023 |
| Model 3‡ | |||||||||||||||
| Intercept | 51.68 | 0.35 | <.001 | 50.36 | 0.35 | <.001 | 51.42 | 0.41 | <.001 | 51.65 | 0.34 | <.001 | 50.90 | 0.37 | <.001 |
| Change | −2.49 | 0.40 | <.001 | −4.44 | 0.43 | <.001 | −1.11 | 0.52 | .030 | −0.85 | 0.36 | .020 | −2.85 | 0.45 | <.001 |
| Predictor | 1.90 | 1.89 | .320 | −1.26 | 1.74 | .470 | 2.63 | 1.95 | .180 | 1.44 | 1.81 | .430 | 0.97 | 1.88 | .610 |
| Change × predictor | −4.71 | 1.20 | <.001 | −3.60 | 1.38 | .010 | −3.62 | 1.61 | .200 | −1.56 | 1.15 | .180 | −2.87 | 1.38 | .040 |
Note s: Est. = estimate or unstandardized regression coefficient; SE = standard error of measurement.
*Unadjusted model.
†Results are adjusted for baseline age, gender, education, waist circumference, and depressive symptoms.
‡Results are adjusted for baseline age, gender, education, waist circumference, depressive symptoms, cardiovascular disease, and type II diabetes.
Table 3.
Diet-Based Glycemic Load in Relation to Cognitive Performance and Change per 10 Years of Age (n = 553)
| General Ability | Speed | Memory | Verbal | Spatial | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Est. | SE | p | Est. | SE | p | Est. | SE | p | Est. | SE | p | Est. | SE | p | |
| Model 1* | |||||||||||||||
| Intercept | 50.01 | 0.39 | <.001 | 48.97 | 0.35 | <.001 | 50.28 | 0.40 | <.001 | 50.23 | 0.40 | <.001 | 49.62 | 0.38 | <.001 |
| Change | −3.57 | 0.21 | <.001 | −6.31 | 0.21 | <.001 | −2.51 | 0.27 | <.001 | −1.28 | 0.19 | <.001 | −4.11 | 0.22 | <.001 |
| Predictor | −0.53 | 0.15 | .000 | −0.50 | 0.13 | .000 | −0.42 | 0.15 | .004 | −0.31 | 0.15 | .040 | −0.36 | 0.14 | .010 |
| Change × predictor | −0.01 | 0.08 | .230 | −0.04 | 0.08 | .580 | 0.08 | 0.10 | .450 | 0.02 | 0.08 | .760 | −0.07 | 0.08 | .403 |
| Model 2† | |||||||||||||||
| Intercept | 50.74 | 0.39 | <.001 | 49.94 | 0.41 | <.001 | 50.84 | 0.45 | <.001 | 50.93 | 0.39 | <.001 | 50.26 | 0.42 | <.001 |
| Change | −2.63 | 0.44 | <.001 | −4.41 | 0.50 | <.001 | −1.08 | 0.59 | .068 | −1.04 | 0.42 | .014 | −2.85 | 0.53 | <.001 |
| Predictor | −0.24 | 0.13 | .064 | −0.29 | 0.13 | .023 | −0.08 | 0.14 | .544 | −0.11 | 0.13 | .422 | −0.29 | 0.13 | .029 |
| Change × predictor | 0.05 | 0.07 | .457 | 0.03 | 0.08 | .714 | 0.18 | 0.10 | .078 | 0.13 | 0.07 | .075 | 0.05 | 0.08 | .573 |
| Model 3‡ | |||||||||||||||
| Intercept | 50.75 | 0.39 | <.001 | 49.94 | 0.41 | <.001 | 50.85 | 0.45 | <.001 | 50.98 | 0.39 | <.001 | 50.18 | 0.43 | <.001 |
| Change | −2.69 | 0.44 | <.001 | −4.42 | 0.50 | <.001 | −0.98 | 0.59 | .100 | −1.01 | 0.42 | .020 | −2.93 | 0.53 | <.001 |
| Predictor | −0.22 | 0.13 | .090 | −0.28 | 0.13 | .030 | −0.07 | 0.14 | .620 | −0.08 | 0.13 | .520 | −0.29 | 0.13 | .030 |
| Change × predictor | 0.06 | 0.07 | .370 | 0.03 | 0.08 | .740 | 0.19 | 0.11 | .080 | 0.15 | 0.07 | .040 | 0.07 | 0.08 | .400 |
Notes : Est. = estimate or unstandardized regression coefficient; SE = standard error of measurement.
*Unadjusted model.
†Results are adjusted for baseline age, gender, education, waist circumference, and depressive symptoms.
‡Results are adjusted for baseline age, gender, education, waist circumference, depressive symptoms, cardiovascular disease and type II diabetes.
Results
Baseline Sample Characteristics
A total of 838 individuals were included in the analyses (see Table 1). The participants averaged 63.1 years at baseline (SD = 8.3 years, range 50–87 years). Approximately 60% were female and 75% attained no more than elementary education. The average waist circumference was 92cm (SD = 8cm). In all, 30.9% of the sample reported CVD at baseline and 3.3% reported T2DM. The mean blood glucose level was 4.8 (SD = 1.8) mmol/L. When examined as a dichotomous variable, blood samples of 30 participants (3.6% of the sample) were in excess of 7.0 mmol/L, which constitute clinically high blood glucose (22). Finally, individuals with high blood glucose were more likely to be older and have CVD and T2DM.
Table 1.
Baseline Characteristics of Sample Based on Blood Glucose and Diet-Based Glycemic Load
| Total Cohort (N = 838) | Normal Glucose (n = 808) | High Glucose (n = 30) | p | |
|---|---|---|---|---|
| Age | ||||
| Mean years (SD) | 63.1 (8.3) | 63.0 (8.3) | 67.0 (8.0) | .010 |
| Gender | ||||
| %Women | 59.6 | 59.3 | 66.7 | .410 |
| Education | ||||
| % Elementary school | 74.5 | 74.4 | 76.7 | .260 |
| % High school or above | 25.5 | 18.1 | 16.7 | |
| Waist circumference* | ||||
| Mean (SD) | 92 (8) | 91 (8) | 93 (7) | .340 |
| Blood glucose level† | ||||
| Mean (SD) | 4.82 (1.8) | 4.5 (0.9) | 12.2 (3.4) | <.001 |
| Depressive symptoms‡, mean (SD) | 1.84 (1.16) | 1.83 (1.17) | 2.10 (1.12) | .269 |
| % Cardiovascular disease§ | 30.9 | 28.8 | 86.7 | <.001 |
| % Type II Diabetes§ | 3.3 | 1.0 | 66.7 | <.001 |
Notes: SD = standard deviation.
*Waist circumference measured in cm.
†Baseline blood glucose measured in mmol/L; high blood glucose = values > 7.0.
‡The Older Americans Resources and Services Depression Scale with five yes/no items was used.
§ Assessed as absence vs. presence at baseline
Diet data were available for 553 of the 838 participants. The 285 participants without diet information did not differ from the others with respect to gender (p = .582) or education (p = .136), but they were on average younger at baseline (mean = 59.2, SD = 8.0, vs mean = 65.2, SD = 7.8, p < .01), and had a smaller mean waist circumference (mean = 81cm, SD = 7cm, vs mean = 92cm, SD = 8cm, p < .01). Diet-based glycemic load and blood glucose were correlated (r = .16, p < .001).
Unconditional Growth Models
Initial unconditional growth models indicated a significant decline in terms of general cognitive ability (β = −3.36, SE = 0.18, p < .001), perceptual speed (β = −6.18, SE = 0.02, p < .001), memory (β = −2.91, SE = 0.23, p < .001), verbal ability (β = −1.13, SE = 0.17, p < .001), and spatial ability (β = −4.17, SE = 0.18, p < .001). In all five models, we found significant random effects for the variance in the intercept and slope (p’s < .05), indicating that overall cognitive scores and change in the score varied significantly across the participants. There was a significant covariance of the intercept with the slope for two of the five models—general cognitive ability and perceptual speed—indicating that having a higher initial score was associated with greater decline. This may have been due to a floor effect (ie, lower initial scores showed smaller slope estimates because there was no “room” for change). Finally, we found significant residual variance across all models (p’s < .001), indicating substantial unexplained variance after accounting for all the included fixed and random effects.
Conditional Growth Models
Blood glucose (continuous).—
Results where blood glucose was entered as a continuous predictor variable are presented in the top section of Table 2. The predictor (cross-sectional) estimate shown in Model 2 indicates that there was a significant relationship between higher blood glucose and poorer overall perceptual speed performance (β = −0.82, SE = 0.25, p < .01), meaning that for each additional mmol/L of blood glucose, perceptual speed scores were lower by 0.82 T-score units. Controlling for CVD and T2DM (Model 3) did not explain this result, as indicated by the fact that predictor estimates significant in Model 2 remained significant in Model 3. There were no significant effects of blood glucose levels on overall general cognitive ability or any of the remaining cognitive domains, indicated by nonsignificant predictor estimates for these outcomes (p’s > .05).
With respect to the change × predictor (longitudinal) estimates, higher blood glucose was related to greater rates of decline in general cognitive ability (β = −0.71, SE = 0.22, p < .01), perceptual speed (β = −0.65, SE = 0.25, p < .05), verbal abilities (β = −0.42, SE = 0.21, p < .05), and spatial abilities (β = −0.51, SE = 0.25, p < .05). This means that, for example, each additional mmol/L of blood glucose was associated with a decline in general cognitive ability of 0.71 T-score units per decade. After the addition of CVD and T2DM in Model 3, the significant change × predictor estimates reflecting declines in general cognitive ability and perceptual speed were retained, whereas those for verbal and spatial performance were explained (reduced to nonsignificant).
Blood glucose (dichotomous).—
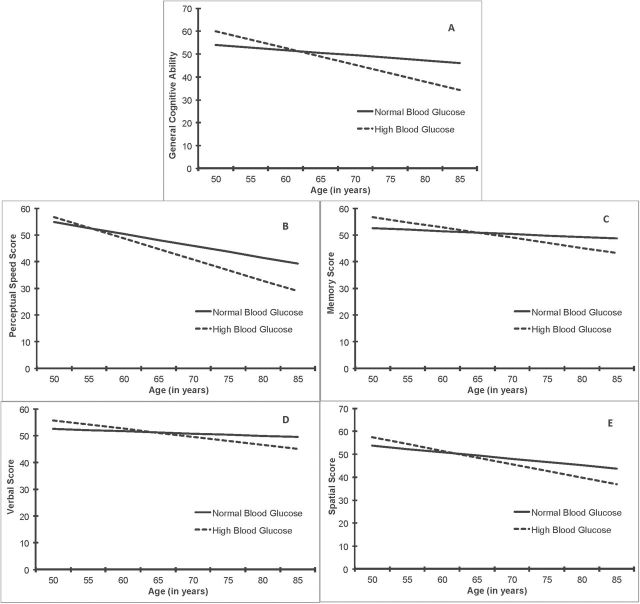
Results for normal versus high blood glucose (above the clinical T2DM threshold) are presented in the bottom section of Table 2 and in Figure 2A–E. For overall cognitive performance (predictor estimates), we found no significant relationships between high blood glucose and overall scores on any cognitive measure (p’s > .05) when controlling for basic covariates (Model 2). However, high blood glucose was significantly related to greater rates of decline (as indicated by significant change × predictor estimates) in general cognitive ability (β = −4.71, SE = 1.20, p < .001), indicating almost a half SD greater decline in those with high compared with normal blood glucose. High blood glucose was also related to steeper declines in perceptual speed (β = −3.60, SE = 1.38, p < .05), verbal ability (β = −2.19, SE = 1.11, p < .05), and spatial ability (β = −3.02, SE = 1.32, p < .05). When T2DM and CVD were also controlled for (Model 3), the results for general cognitive ability and perceptual speed remained essentially unchanged, but the significant associations between high blood glucose and decline in verbal and spatial performance were reduced to nonsignificant. Finally, we examined the same associations separately for individuals with and without T2DM. These analyses yielded no significant findings perhaps because of reduced power, or the possibility that our results only apply to the full spectrum of blood glucose levels.
Figure 2.
Fully adjusted growth models of cognitive aging as a function of normal vs high blood glucose. Compared with normal blood glucose, high blood glucose was significantly related to greater rates of decline in general cognitive ability (A), perceptual speed (B), as well as verbal (D) and spatial (E) scores. The differences between normal vs high blood glucose in terms of change in memory scores over time were not statistically significant (C).
Diet-based glycemic load.—
Diet-based glycemic load was significantly related to poorer overall perceptual speed (β = −0.29, SE = 0.13, p < .05) and spatial ability (β = −0.29, SE = 0.13, p < .05) when controlling for basic covariates in Model 2. These relationships were unaffected when CVD and T2DM were added (Model 3). No significant relationships between diet-based glycemic load and rates of cognitive decline were observed (p’s > .05), except that the relationship between diet-based glycemic load and less verbal decline reached significance (β = 0.15, SE = 0.07, p < .05) when CVD and T2DM were entered into the model (Table 3).
Discussion
Previous research indicates that T2DM is related to declines in verbal fluency (22), attention, executive functioning, memory, information processing speed, and brain atrophy (26). Type II Diabetes Mellitus also increases the risk for dementia and cognitive decline (27–29). Our findings expand upon previous research by suggesting that elevated blood glucose (not just T2DM) exerts a specific detrimental influence on cognitive decline in dementia-free older adults. In addition, our study indicates that diet-based glycemic load is a predictor of poorer cognitive performance.
Because our results for perceptual speed could not be fully explained by underlying cardiovascular pathology, we can speculate that the influence of blood glucose on perceptual speed may exist above and beyond cardiovascular abnormalities, although more research is needed to confirm or refute this assumption. Nonetheless, our results indicating significant associations between high blood glucose and poorer performance as well as steeper decline in perceptual speed are particularly salient because changes in speed of processing may help to explain cognitive aging in general (30). Our finding that high blood glucose was related to declines in verbal (questions of general knowledge, which possibly tap into long-term memory) and spatial abilities are not completely surprising given that high blood glucose is related to atrophy in the hippocampus (31), a brain structure important for long-term and spatial memory (32). Specifically, high blood glucose has been shown to impair hippocampal synaptic plasticity (eg, reductions in brain-derived neurotropic factor, long-term potentiation (33,34)) in rats. In older individuals, microvascular disruption may accompany T2DM and is linked with smaller brain volume, reduced blood flow, and impaired vasoreactivity, which regulates optimal blood vessel performance and delivery of glucose to the brain (35). Somewhat unexpected was the lack of an association between blood glucose and memory. In the present study, the memory domain consisted of both short-term (eg, digit span) and long-term (eg, name & face recall) memory tests. It is possible that blood glucose affects short-term and long-term memory differently, although further investigation is required to test this assumption.
Relationships among glycemic load, poorer overall perceptual speed, and spatial ability scores are consistent with previous results showing associations between high dietary sugar and deficits in processing speed (36) in people, and dietary sugar-induced spatial memory impairment in rats (37). We found diet-based glycemic load significantly related to slower decline in verbal ability. We can speculate that diet-based glycemic load may reduce stress-induced cognitive deficits via a “comfort food” effect (38). Further, socialization, which may increase dessert consumption (39), may have also contributed to this result. These notions may deserve consideration in future studies.
The covariates utilized in our models (age, gender, education, waist circumference, depressive symptoms, CVD, and T2DM) exerted little influence on the results with one exception. Cardiovascular disease reduced the association between higher blood glucose and greater decline in verbal and spatial abilities to nonsignificant, indicating that only cardiovascular risk factors may offer a potential underlying mechanism. One possibility is that high blood glucose affects cardiovascular factors via inflammation, leading to impaired synaptic plasticity (40).
We found no evidence of glycemic load-related cognitive change with age. One possibility is that testing may have begun at a point after some cognitive decline had already occurred (mean baseline age was 63.1 years). The food frequency questionnaire, based on standard methods used in Sweden at that time, was not validated and did not measure all foods indicative of glycemic load (eg, candy, chocolate, and white rice). Further, the actual amount (grams/ounces) consumed was not available. Therefore, our use of the term “glycemic load” does not adhere fully to convention. The lack of detail in the diet data collection stems partly from the fact that baseline data were collected in the mid 1980s before more detailed measures were widely available. However, this also offered the advantage of a uniquely long follow-up period. In addition, because diet and blood glucose were only assessed at a single time point, potential changes over time could not be captured. However, our main goal was to assess cognitive change subsequent to baseline assessment, thereby shedding light on possible risk factors for poor age-related cognitive change.
Metabolic factors related to blood glucose that we did not have access to (eg, insulin, inflammatory markers, and HbAlc), should be investigated in the future. Other limitations include our inability to adjust for potentially important confounders such as alcohol and smoking status due to extensive missing data, and our measure of depressive symptoms was relatively crude. We were also unable to effectively consider adherence to fasting due to missing information for a substantial portion of the sample. However, a systematic bias is unlikely in this cognitively healthy sample, and the participants were specifically instructed to fast prior to the testing session.
In conclusion, this study provides a unique and novel look at midlife risk factors for metabolic dysfunction, which may underlie accelerated cognitive aging. Blood glucose control (perhaps through reductions in diet-based glycemic load) may be an important target in the prevention of age-related cognitive decline.
Supplementary Material
Supplementary material can be found at: http://biomedgerontology.oxfordjournals.org/
Funding
This study is supported by the National Institute of Aging (AG04563, AG10175), the MacArthur Foundation Research Network on Successful Aging, the Swedish Research Council for Health, Working Life and Welfare (FORTE) (97:0147:1B, 2009-0795), and the Swedish Research Council (825-2007-7460, 825-2009-6141) and by a Future Leaders of Aging Research in Europe (FLARE) postdoctoral grant (FORTE 2010-1852) to A.K.D.A.
Supplementary Material
Acknowledgment
The authors thank Megan Eli, BA, and Lakshmi Seetharaman, CPA, for contributing to the editing of this manuscript.
References
- 1. Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–223. 10.1016/S0140-6736(05)17741-1 [DOI] [PubMed] [Google Scholar]
- 2. den Heijer T, Vermeer SE, van Dijk EJ, et al. Type 2 diabetes and atrophy of medial temporal lobe structures on brain MRI. Diabetologia. 2003;46:1604–1610. 10.1007/s00125-003-1235-0 [DOI] [PubMed] [Google Scholar]
- 3. Hassing LB, Grant MD, Hofer SM, et al. Type 2 diabetes mellitus contributes to cognitive decline in old age: a longitudinal population-based study. J Int Neuropsychol Soc. 2004;10:599–607. doi:10.1093/ageing/afh100 [DOI] [PubMed] [Google Scholar]
- 4. Messier C. Impact of impaired glucose tolerance and type 2 diabetes on cognitive aging. Neurobiol Aging. 2005;26(suppl 1):26–30. 10.1016/j.neurobiolaging.2005.09.014 [DOI] [PubMed] [Google Scholar]
- 5. Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol. 2006;5:64–74. doi:10.1016/S1474-4422(05)70284-2 [DOI] [PubMed] [Google Scholar]
- 6. Popkin BM, Nielsen SJ. The sweetening of the world’s diet. Obes Res. 2003;11:1325–1332. doi:10.1038/oby.2003.179 [DOI] [PubMed] [Google Scholar]
- 7. Benton D, Ruffin MP, Lassel T, et al. The delivery rate of dietary carbohydrates affects cognitive performance in both rats and humans. Psychopharmacology (Berl). 2003;166:86–90. 10.1007/s00213-002-1334-5 [DOI] [PubMed] [Google Scholar]
- 8. Greenwood CE, Kaplan RJ, Hebblethwaite S, Jenkins DJ. Carbohydrate-induced memory impairment in adults with type 2 diabetes. Diabetes Care. 2003;26:1961–1966. :10.2337/diacare.26.7.1961 [DOI] [PubMed] [Google Scholar]
- 9. Lowden A, Holmbäck U, Akerstedt T, Forslund J, Lennernäs M, Forslund A. Performance and sleepiness during a 24h wake in constant conditions are affected by diet. Biol Psychol. 2004;65:251–263. 10.1016/S0301-0511(03)00114-5 [DOI] [PubMed] [Google Scholar]
- 10. Pedersen NL, Plomin R, Nesselroade J. A quantitative genetic analysis of cognitive abilities during the second half of the lifespan. Psychol Sci 1992;3:346–353. 10.1111/j.1467-9280.1992.tb00045.x [Google Scholar]
- 11. Lichtenstein P, De Faire U, Floderus B, Svartengren M, Svedberg P, Pedersen NL. The Swedish Twin Registry: a unique resource for clinical, epidemiological and genetic studies. J Intern Med. 2002;252:184–205. doi:10.1046/j.1365-2796.2002.01032.x [DOI] [PubMed] [Google Scholar]
- 12. Finkel DP, Pedersen NL. Processing speed and longitudinal trajectories of change for cognitive abilities: The Swedish adoption/twin study of aging. Aging Neuropsychol C. 2004;11:325–345. 10.1080/13825580490511152 [Google Scholar]
- 13. American Diabetes Association. Standards of medical care in diabetes--2011. Diabetes Care. 2011;34(suppl 1):S11–S61. doi:10.2337/dc11-S011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3rd ed. Washington, DC: American Psychatric Association; 1987. [Google Scholar]
- 15. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychatric Association; 1994. [Google Scholar]
- 16. Finkel D, Reynolds CA, McArdle JJ, Gatz M, Pedersen NL. Latent growth curve analyses of accelerating decline in cognitive abilities in late adulthood. Dev Psychol. 2003;39:535–550. 10.1037/0012-1649.39.3.535 [DOI] [PubMed] [Google Scholar]
- 17. Jorm AF. Is depression a risk factor for dementia or cognitive decline? A review. Gerontology. 2000;46:219–227. [DOI] [PubMed] [Google Scholar]
- 18. Verhaeghen P, Salthouse TA. Meta-analyses of age-cognition relations in adulthood: estimates of linear and nonlinear age effects and structural models. Psychol Bull. 1997;122:231–249. 10.1037/0033-2909.122.3.231 [DOI] [PubMed] [Google Scholar]
- 19. Geary DC, Saults SJ, Liu F, Hoard MK. Sex differences in spatial cognition, computational fluency, and arithmetical reasoning. J Exp Child Psychol. 2000;77:337–353. 10.1006/jecp.2000.2594 [DOI] [PubMed] [Google Scholar]
- 20. Anstey K, Christensen H. Education, activity, health, blood pressure and apolipoprotein E as predictors of cognitive change in old age: a review. Gerontology. 2000;46:163–177. [DOI] [PubMed] [Google Scholar]
- 21. Blazer D, Williams CD. Epidemiology of dysphoria and depression in an elderly population. Am J Psychiatr. 1980;137:439–444. [DOI] [PubMed] [Google Scholar]
- 22. Kanaya AM, Barrett-Connor E, Gildengorin G, Yaffe K. Change in cognitive function by glucose tolerance status in older adults: a 4-year prospective study of the Rancho Bernardo study cohort. Arch Intern Med. 2004;164:1327–1333. [DOI] [PubMed] [Google Scholar]
- 23. Singer JD, Willett JB. Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. New York: Oxford Press, 2003:266–299. doi:10.1001/archinte.164.12.1327 [Google Scholar]
- 24. McArdle JJ, Bell RQ. An introduction to latent growth models for developmental analysis In: Little TD, Schnabel KU, & Baumert J, eds. Modeling Longitudinal and Multi-group Data: Practical Issues, Applied Approaches, and Scientific Examples. Mahwah, NJ: Erlbaum; 2000:69–108. [Google Scholar]
- 25. Finkel D, Reynolds CA, McArdle JJ, Pedersen NL. The longitudinal relationship between processing speed and cognitive ability: genetic and environmental influences. Behav Genet. 2005;35:535–549. doi:10.1007/s10519-005-3281-5 [DOI] [PubMed] [Google Scholar]
- 26. Manschot SM, Brands AM, van der Grond J, et al. ; Utrecht Diabetic Encephalopathy Study Group. Brain magnetic resonance imaging correlates of impaired cognition in patients with type 2 diabetes. Diabetes. 2006;55:1106–1113. 10.2337/diabetes.55.04.06.db05-1323a [DOI] [PubMed] [Google Scholar]
- 27. Xu W, Qiu C, Gatz M, Pedersen NL, Johansson B, Fratiglioni L. Mid- and late-life diabetes in relation to the risk of dementia: a population-based twin study. Diabetes. 2009;58:71–77. :10.2337/db08-0586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Craft S. Alzheimer disease: Insulin resistance and AD—extending the translational path. Nat Rev Neurol. 2012;8:360–362. :10.1038/nrneurol.2012.112 [DOI] [PubMed] [Google Scholar]
- 29. Craft S, Cholerton B, Baker LD. Insulin and Alzheimer’s disease: untangling the web. J Alzheim Dis. 2013;33(suppl 1):S263–S275. 10.3233/JAD-2012-129042 [DOI] [PubMed] [Google Scholar]
- 30. Finkel D, Reynolds CA, McArdle JJ, Pedersen NL. Age changes in processing speed as a leading indicator of cognitive aging. Psychol Aging. 2007;22:558–568. 10.1037/0882-7974.22.3.558 [DOI] [PubMed] [Google Scholar]
- 31. Cherbuin N, Sachdev P, Anstey KJ. Higher normal fasting plasma glucose is associated with hippocampal atrophy: The PATH Study. Neurology. 2012;79:1019–1026. 10.1212/WNL.0b013e31826846de [DOI] [PubMed] [Google Scholar]
- 32. Burgess N, Maguire EA, O’Keefe J. The human hippocampus and spatial and episodic memory. Neuron. 2002;35:625–641. 10.1016/S0896-6273(02)00830-9 [DOI] [PubMed] [Google Scholar]
- 33. Molteni R, Wu A, Vaynman S, Ying Z, Barnard RJ, Gómez-Pinilla F. Exercise reverses the harmful effects of consumption of a high-fat diet on synaptic and behavioral plasticity associated to the action of brain-derived neurotrophic factor. Neuroscience. 2004;123:429–440. doi:10.1016/j.neuroscience.2003.09.020 [DOI] [PubMed] [Google Scholar]
- 34. Stranahan AM, Norman ED, Lee K, et al. Diet-induced insulin resistance impairs hippocampal synaptic plasticity and cognition in middle-aged rats. Hippocampus. 2008;18:1085–1088. 10.1002/hipo.20470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Last D, Alsop DC, Abduljalil AM, et al. Global and regional effects of type 2 diabetes on brain tissue volumes and cerebral vasoreactivity. Diabetes Care. 2007;30:1193–1199. doi:10.2337/dc06-2052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cukierman T, Gerstein HC, Williamson JD. Cognitive decline and dementia in diabetes—systematic overview of prospective observational studies. Diabetologia. 2005;48:2460–2469. doi:10.1007/s00125-005-0023-4 [DOI] [PubMed] [Google Scholar]
- 37. Jurdak N, Lichtenstein AH, Kanarek RB. Diet-induced obesity and spatial cognition in young male rats. Nutr Neurosci. 2008;11:48–54. 10.1179/147683008X301333 [DOI] [PubMed] [Google Scholar]
- 38. Dallman MF, Pecoraro N, Akana SF, et al. Chronic stress and obesity: a new view of “comfort food”. Proc Natl Acad Sci U S A. 2003;100:11696–11701. 10.1073/pnas.1934666100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wansink B. Environmental factors that increase the food intake and consumption volume of unknowing consumers. Annu Rev Nutr. 2004;24:455–479. doi:10.1146/annurev.nutr.24.012003.132140 [DOI] [PubMed] [Google Scholar]
- 40. Esposito K, Nappo F, Marfella R, et al. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation. 2002;106:2067–2072. 10.1161/01.CIR.0000034509.14906.AE [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.