Abstract
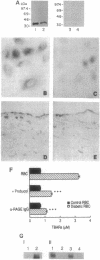
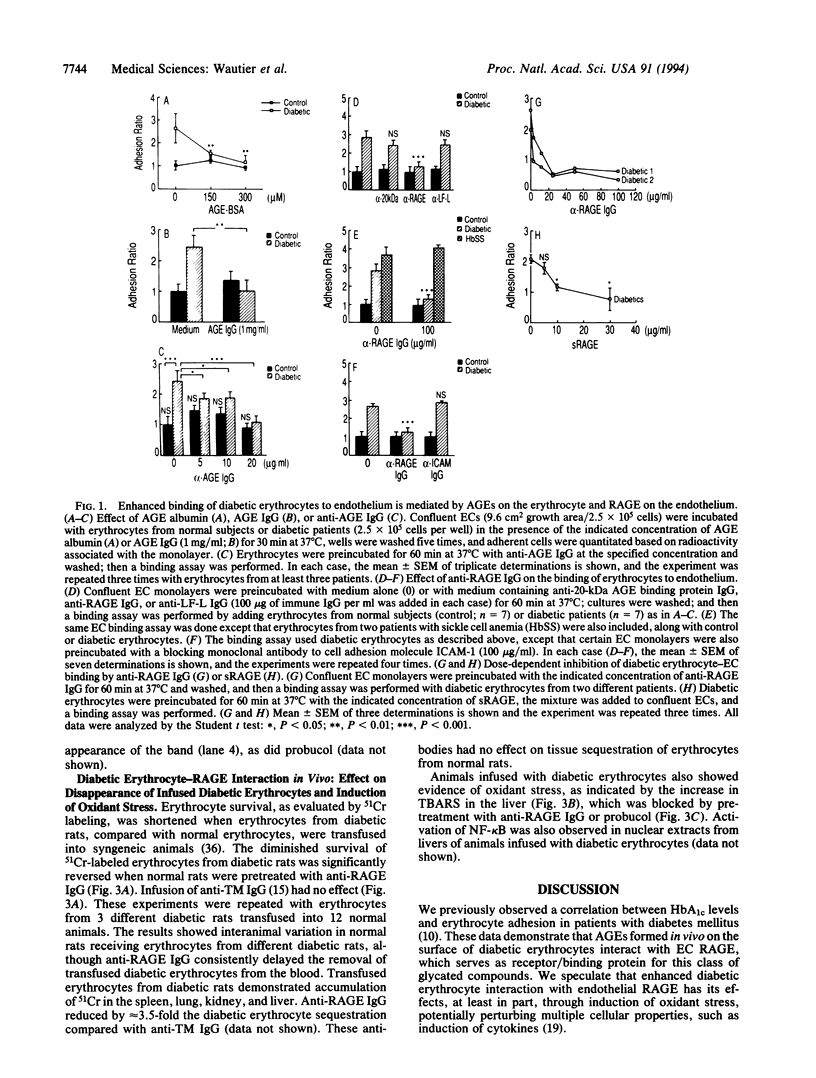
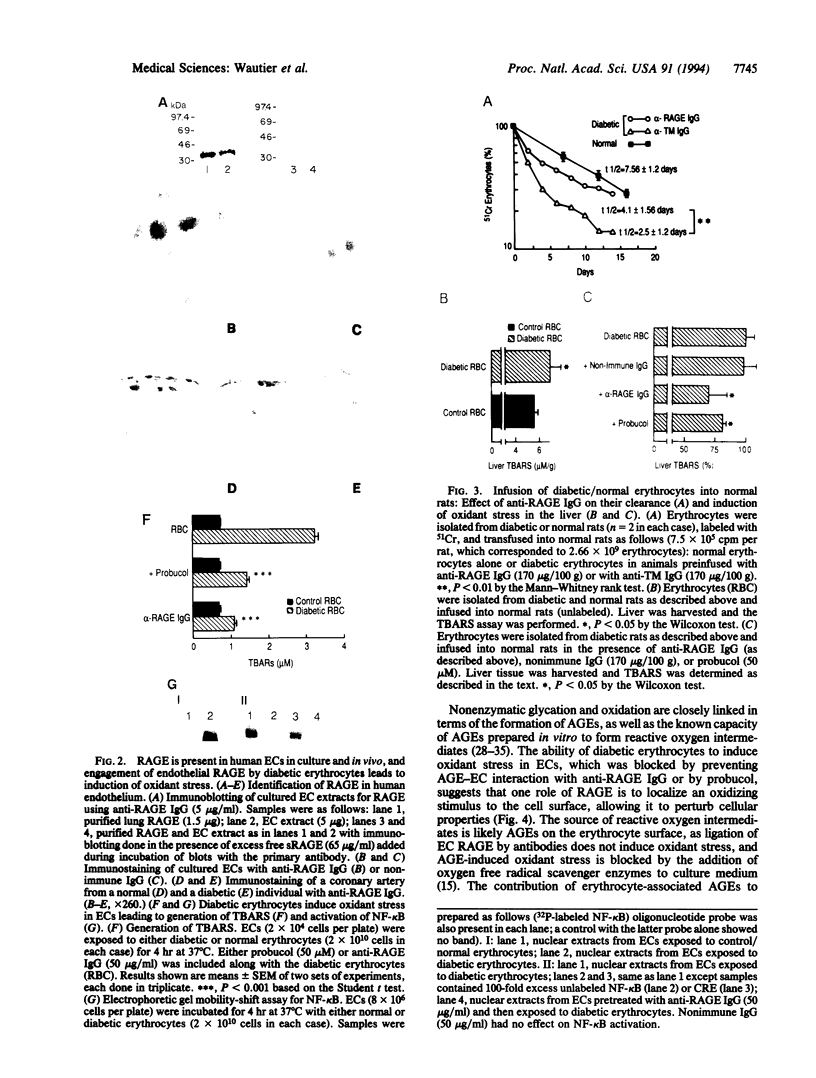
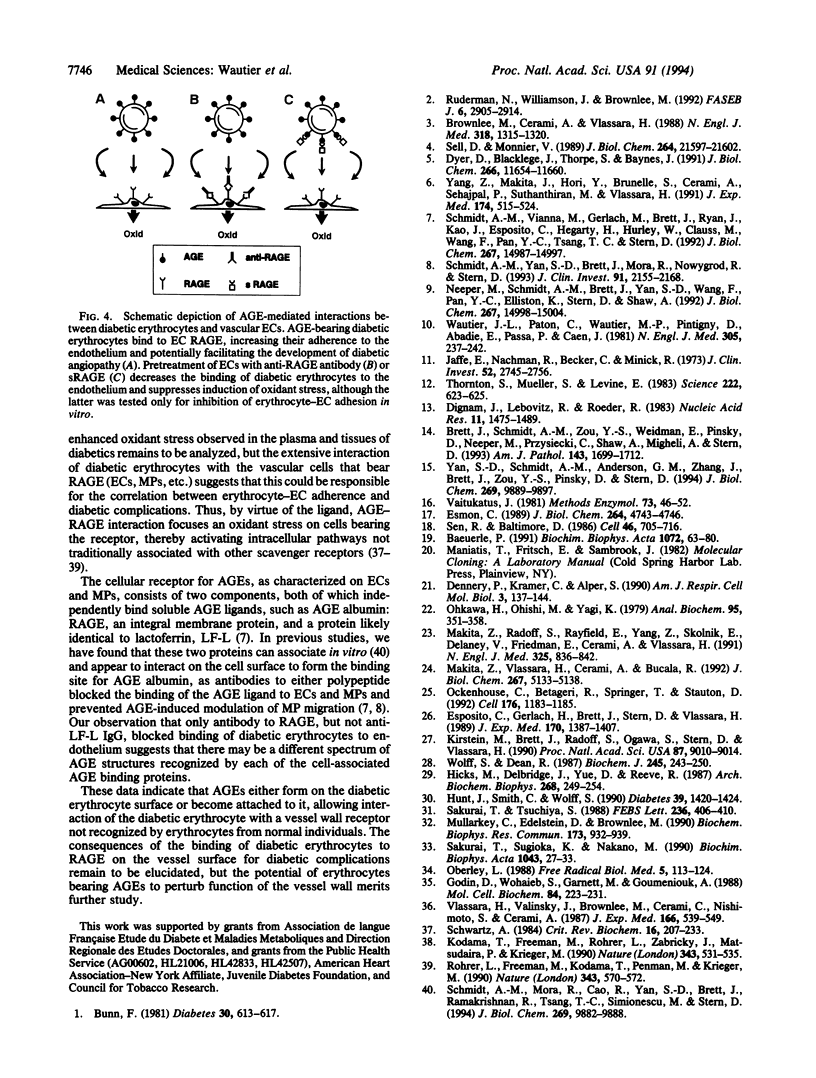
Vascular complications are an important cause of morbidity and mortality in patients with diabetes. The extent of vascular complications has been linked statistically to enhanced adherence of diabetic erythrocytes to endothelial cells (ECs) and to the accumulation of a class of glycated proteins termed advanced glycation end products (AGEs). We hypothesized that formation of AGEs on the surface of diabetic erythrocytes could mediate their interaction with ECs leading to binding and induction of vascular dysfunction. Enhanced binding of diabetic erythrocytes to ECs was blocked by preincubation of erythrocytes with anti-AGE IgG or preincubation of ECs with antibodies to the receptor for AGE (RAGE). Immunoblotting of cultured human ECs and immunostaining of normal/diabetic human tissue confirmed the presence of RAGE in the vessel wall. Binding of diabetic erythrocytes to endothelium generated an oxidant stress, as measured by production of thiobarbituric acid-reactive substances (TBARS) and activation of the transcription factor NF-kappa B, both of which were blocked by probucol or anti-RAGE IgG. Erythrocytes from diabetic rats infused into normal rats had an accelerated, early phase of clearance that was prevented, in part, by antibody to RAGE. Liver tissue from rats infused with diabetic erythrocytes showed elevated levels of TBARS, which was prevented by pretreatment with anti-RAGE IgG or probucol. Thus, erythrocyte surface AGEs can function as ligands that interact with RAGE on endothelium. The extensive contact of diabetic erythrocytes bearing surface-associated AGEs with vessel wall RAGE could be important in the development of vascular complications.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baeuerle P. A. The inducible transcription activator NF-kappa B: regulation by distinct protein subunits. Biochim Biophys Acta. 1991 Apr 16;1072(1):63–80. doi: 10.1016/0304-419x(91)90007-8. [DOI] [PubMed] [Google Scholar]
- Brett J., Schmidt A. M., Yan S. D., Zou Y. S., Weidman E., Pinsky D., Nowygrod R., Neeper M., Przysiecki C., Shaw A. Survey of the distribution of a newly characterized receptor for advanced glycation end products in tissues. Am J Pathol. 1993 Dec;143(6):1699–1712. [PMC free article] [PubMed] [Google Scholar]
- Brownlee M., Cerami A., Vlassara H. Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. N Engl J Med. 1988 May 19;318(20):1315–1321. doi: 10.1056/NEJM198805193182007. [DOI] [PubMed] [Google Scholar]
- Bunn H. F. Evaluation of glycosylated hemoglobin diabetic patients. Diabetes. 1981 Jul;30(7):613–617. doi: 10.2337/diab.30.7.613. [DOI] [PubMed] [Google Scholar]
- Dennery P. A., Kramer C. M., Alpert S. E. Effect of fatty acid profiles on the susceptibility of cultured rabbit tracheal epithelial cells to hyperoxic injury. Am J Respir Cell Mol Biol. 1990 Aug;3(2):137–144. doi: 10.1165/ajrcmb/3.2.137. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer D. G., Blackledge J. A., Thorpe S. R., Baynes J. W. Formation of pentosidine during nonenzymatic browning of proteins by glucose. Identification of glucose and other carbohydrates as possible precursors of pentosidine in vivo. J Biol Chem. 1991 Jun 25;266(18):11654–11660. [PubMed] [Google Scholar]
- Esmon C. T. The roles of protein C and thrombomodulin in the regulation of blood coagulation. J Biol Chem. 1989 Mar 25;264(9):4743–4746. [PubMed] [Google Scholar]
- Esposito C., Gerlach H., Brett J., Stern D., Vlassara H. Endothelial receptor-mediated binding of glucose-modified albumin is associated with increased monolayer permeability and modulation of cell surface coagulant properties. J Exp Med. 1989 Oct 1;170(4):1387–1407. doi: 10.1084/jem.170.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godin D. V., Wohaieb S. A., Garnett M. E., Goumeniouk A. D. Antioxidant enzyme alterations in experimental and clinical diabetes. Mol Cell Biochem. 1988 Dec;84(2):223–231. doi: 10.1007/BF00421057. [DOI] [PubMed] [Google Scholar]
- Hicks M., Delbridge L., Yue D. K., Reeve T. S. Increase in crosslinking of nonenzymatically glycosylated collagen induced by products of lipid peroxidation. Arch Biochem Biophys. 1989 Jan;268(1):249–254. doi: 10.1016/0003-9861(89)90586-9. [DOI] [PubMed] [Google Scholar]
- Hunt J. V., Smith C. C., Wolff S. P. Autoxidative glycosylation and possible involvement of peroxides and free radicals in LDL modification by glucose. Diabetes. 1990 Nov;39(11):1420–1424. doi: 10.2337/diab.39.11.1420. [DOI] [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973 Nov;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirstein M., Brett J., Radoff S., Ogawa S., Stern D., Vlassara H. Advanced protein glycosylation induces transendothelial human monocyte chemotaxis and secretion of platelet-derived growth factor: role in vascular disease of diabetes and aging. Proc Natl Acad Sci U S A. 1990 Nov;87(22):9010–9014. doi: 10.1073/pnas.87.22.9010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama T., Freeman M., Rohrer L., Zabrecky J., Matsudaira P., Krieger M. Type I macrophage scavenger receptor contains alpha-helical and collagen-like coiled coils. Nature. 1990 Feb 8;343(6258):531–535. doi: 10.1038/343531a0. [DOI] [PubMed] [Google Scholar]
- Makita Z., Radoff S., Rayfield E. J., Yang Z., Skolnik E., Delaney V., Friedman E. A., Cerami A., Vlassara H. Advanced glycosylation end products in patients with diabetic nephropathy. N Engl J Med. 1991 Sep 19;325(12):836–842. doi: 10.1056/NEJM199109193251202. [DOI] [PubMed] [Google Scholar]
- Makita Z., Vlassara H., Cerami A., Bucala R. Immunochemical detection of advanced glycosylation end products in vivo. J Biol Chem. 1992 Mar 15;267(8):5133–5138. [PubMed] [Google Scholar]
- Mullarkey C. J., Edelstein D., Brownlee M. Free radical generation by early glycation products: a mechanism for accelerated atherogenesis in diabetes. Biochem Biophys Res Commun. 1990 Dec 31;173(3):932–939. doi: 10.1016/s0006-291x(05)80875-7. [DOI] [PubMed] [Google Scholar]
- Neeper M., Schmidt A. M., Brett J., Yan S. D., Wang F., Pan Y. C., Elliston K., Stern D., Shaw A. Cloning and expression of a cell surface receptor for advanced glycosylation end products of proteins. J Biol Chem. 1992 Jul 25;267(21):14998–15004. [PubMed] [Google Scholar]
- Oberley L. W. Free radicals and diabetes. Free Radic Biol Med. 1988;5(2):113–124. doi: 10.1016/0891-5849(88)90036-6. [DOI] [PubMed] [Google Scholar]
- Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979 Jun;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Rohrer L., Freeman M., Kodama T., Penman M., Krieger M. Coiled-coil fibrous domains mediate ligand binding by macrophage scavenger receptor type II. Nature. 1990 Feb 8;343(6258):570–572. doi: 10.1038/343570a0. [DOI] [PubMed] [Google Scholar]
- Ruderman N. B., Williamson J. R., Brownlee M. Glucose and diabetic vascular disease. FASEB J. 1992 Aug;6(11):2905–2914. doi: 10.1096/fasebj.6.11.1644256. [DOI] [PubMed] [Google Scholar]
- Sakurai T., Sugioka K., Nakano M. O2- generation and lipid peroxidation during the oxidation of a glycated polypeptide, glycated polylysine, in the presence of iron-ADP. Biochim Biophys Acta. 1990 Mar 12;1043(1):27–33. doi: 10.1016/0005-2760(90)90106-8. [DOI] [PubMed] [Google Scholar]
- Sakurai T., Tsuchiya S. Superoxide production from nonenzymatically glycated protein. FEBS Lett. 1988 Aug 29;236(2):406–410. doi: 10.1016/0014-5793(88)80066-8. [DOI] [PubMed] [Google Scholar]
- Schmidt A. M., Mora R., Cao R., Yan S. D., Brett J., Ramakrishnan R., Tsang T. C., Simionescu M., Stern D. The endothelial cell binding site for advanced glycation end products consists of a complex: an integral membrane protein and a lactoferrin-like polypeptide. J Biol Chem. 1994 Apr 1;269(13):9882–9888. [PubMed] [Google Scholar]
- Schmidt A. M., Vianna M., Gerlach M., Brett J., Ryan J., Kao J., Esposito C., Hegarty H., Hurley W., Clauss M. Isolation and characterization of two binding proteins for advanced glycosylation end products from bovine lung which are present on the endothelial cell surface. J Biol Chem. 1992 Jul 25;267(21):14987–14997. [PubMed] [Google Scholar]
- Schmidt A. M., Yan S. D., Brett J., Mora R., Nowygrod R., Stern D. Regulation of human mononuclear phagocyte migration by cell surface-binding proteins for advanced glycation end products. J Clin Invest. 1993 May;91(5):2155–2168. doi: 10.1172/JCI116442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz A. L. The hepatic asialoglycoprotein receptor. CRC Crit Rev Biochem. 1984;16(3):207–233. doi: 10.3109/10409238409108716. [DOI] [PubMed] [Google Scholar]
- Sell D. R., Monnier V. M. Structure elucidation of a senescence cross-link from human extracellular matrix. Implication of pentoses in the aging process. J Biol Chem. 1989 Dec 25;264(36):21597–21602. [PubMed] [Google Scholar]
- Sen R., Baltimore D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell. 1986 Aug 29;46(5):705–716. doi: 10.1016/0092-8674(86)90346-6. [DOI] [PubMed] [Google Scholar]
- Thornton S. C., Mueller S. N., Levine E. M. Human endothelial cells: use of heparin in cloning and long-term serial cultivation. Science. 1983 Nov 11;222(4624):623–625. doi: 10.1126/science.6635659. [DOI] [PubMed] [Google Scholar]
- Vaitukaitis J. L. Production of antisera with small doses of immunogen: multiple intradermal injections. Methods Enzymol. 1981;73(Pt B):46–52. doi: 10.1016/0076-6879(81)73055-6. [DOI] [PubMed] [Google Scholar]
- Vlassara H., Valinsky J., Brownlee M., Cerami C., Nishimoto S., Cerami A. Advanced glycosylation endproducts on erythrocyte cell surface induce receptor-mediated phagocytosis by macrophages. A model for turnover of aging cells. J Exp Med. 1987 Aug 1;166(2):539–549. doi: 10.1084/jem.166.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wautier J. L., Paton R. C., Wautier M. P., Pintigny D., Abadie E., Passa P., Caen J. P. Increased adhesion of erythrocytes to endothelial cells in diabetes mellitus and its relation to vascular complications. N Engl J Med. 1981 Jul 30;305(5):237–242. doi: 10.1056/NEJM198107303050501. [DOI] [PubMed] [Google Scholar]
- Wolff S. P., Dean R. T. Glucose autoxidation and protein modification. The potential role of 'autoxidative glycosylation' in diabetes. Biochem J. 1987 Jul 1;245(1):243–250. doi: 10.1042/bj2450243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan S. D., Schmidt A. M., Anderson G. M., Zhang J., Brett J., Zou Y. S., Pinsky D., Stern D. Enhanced cellular oxidant stress by the interaction of advanced glycation end products with their receptors/binding proteins. J Biol Chem. 1994 Apr 1;269(13):9889–9897. [PubMed] [Google Scholar]
- Yang Z., Makita Z., Horii Y., Brunelle S., Cerami A., Sehajpal P., Suthanthiran M., Vlassara H. Two novel rat liver membrane proteins that bind advanced glycosylation endproducts: relationship to macrophage receptor for glucose-modified proteins. J Exp Med. 1991 Sep 1;174(3):515–524. doi: 10.1084/jem.174.3.515. [DOI] [PMC free article] [PubMed] [Google Scholar]