Abstract
Thermostable Mn-dependent catalases are promising enzymes in biotechnological applications as H2O2-detoxifying systems. We cloned the genes encoding Mn-dependent catalases from Thermus thermophilus HB27 and HB8 and a less thermostable mutant carrying two amino acid replacements (M129V and E293G). When the wild-type and mutant genes were overexpressed in Escherichia coli, unmodified or six-His-tagged proteins of the expected size were overproduced as inactive proteins. Several attempts to obtain active forms or to activate the overproduced proteins were unsuccessful, even when soluble and thermostable proteins were used. Therefore, a requirement for a Thermus-specific activation factor was suggested. To overcome this problem, the Mn-dependent catalase genes were overexpressed directly in T. thermophilus under the control of the Pnar promoter. This promoter belongs to a respiratory nitrate reductase from of T. thermophilus HB8, whose transcription is activated by the combined action of nitrate and anoxia. Upon induction in T. thermophilus HB8, a 20- to 30-fold increase in catalase specific activity was observed, whereas a 90- to 110-fold increase was detected when the laboratory strain T. thermophilus HB27::nar was used as the host. The thermostability of the overproduced wild-type catalase was identical to that previously reported for the native enzyme, whereas decreased stability was detected for the mutant derivative. Therefore, our results validate the use of T. thermophilus as an alternative cell factory for the overproduction of thermophilic proteins that fail to be expressed in well-known mesophilic hosts.
Catalases (EC 1.11.1.6) are widely distributed enzymes in nature that decompose hydrogen peroxide to water and molecular oxygen as a detoxifying mechanism. In addition to its crucial biological relevance, this enzyme has many industrial applications, including (i) degradation of hydrogen peroxide after textile bleaching in order to prevent problems in subsequent dyeing (12); (ii) in the dairy industry, elimination of H2O2 added to sterilize milk (2, 44); (iii) coupled with oxidases, prevention of inactivation of the oxidases in the presence of high peroxide concentrations (15, 23, 43); and (iv) coupled with oxidases, prevention of the destruction by H2O2 of the target product in an oxidase-catalyzed reaction (19, 42).
Three phylogenetically distinct types of catalases have been described (29). Two of them contain a heme group, and the enzymes are defined as bifunctional and monofunctional catalases, depending on their abilities to function as peroxidases as well. The monofunctional heme catalases are by far the most widely distributed type and are present in Bacteria, Archaea, and Eukarya, whereas bifunctional catalases have been not found in higher eukaryotes yet. The third group of catalases is the nonheme or pseudocatalases, and these enzymes apparently have a much more restricted distribution. They contain Mn instead of ferric heme in the active site and therefore are also called Mn-dependent catalases. Until now, only five Mn-dependent catalases have been purified and biochemically characterized, and four of them are from thermophiles or hyperthermophiles, including Thermus sp. strain YS 8-13 (24), Thermus thermophilus (5-7), Thermoleophilum album (3), and Pyrobaculum calidifontis (4). The fifth Mn-dependent catalase is from the mesophile Lactobacillus plantarum (25).
Interestingly, the search for homologues of Mn-dependent catalases encoded in the available complete genomes has revealed their unsuspected presence in a wider range of bacteria, in which they are frequently found as hypothetical proteins of unknown function. In this sense the absence of Mn-dependent catalase from most nonpathogenic enterobacteria, including Escherichia coli (39), is especially relevant for this work. By contrast, very few of the sequenced archaeal genomes encode Mn-dependent catalases (4).
For some of the above-mentioned applications, the use of thermostable catalases would constitute a relevant advantage. The main source of such thermostable enzymes should be thermophilic bacteria, because they are adapted to function optimally at temperatures close to the temperatures at which the growth rate is maximal (1, 11). In addition to their exceptional thermal stability, enzymes from thermophiles are also intrinsically more resistant to other denaturing agents (cosolvents, chemical modification, extreme pH) (37), which allows the development of new industrial biotransformation processes (14). In addition to the Mn-dependent catalases, several thermophilic enzymes (thermozymes) with actual or potential biotechnological applications have been reported to be present in the great diversity of Thermus spp. isolates described so far (10, 16, 26).
Production of thermozymes of industrial interest is usually achieved through overexpression of the corresponding genes in model bacterial or eukaryotic hosts used as cell factories (1). When soluble thermozymes are successfully overproduced in these subrogate hosts, the mesophilic character of the host enzymes frequently allows a first purification step that involves simply heat denaturation of most of the host's proteins (1, 11). However, only a fraction of the thermozymes with biotechnological potential can be overproduced in an active form in such mesophilic hosts. In many instances, protein aggregates known as inclusion bodies accumulate during expression, probably because of domain misfolding during synthesis at temperatures that are 30 to 50°C below the natural growth temperature of the natural host (17, 32). Additionally, factors such as a requirement for a specific chaperone(s) implicated in the insertion of cofactors or metals or the lack of a genus- or species-specific posttranslational modification could also lead to the synthesis of inactive forms. Examples of the first type can be found in the synthesis of iron-sulfur-containing proteins whose activation depends on the presence of the so-called iscSUA genes (46) or in the synthesis of the respiratory nitrate reductase, which depends on the presence of a small protein (NarJ) to keep the structure partially folded, which allows insertion of the molybdopterin guanidine dinucleotide cofactor (9). Posttranslational modifications are especially relevant for secreted enzymes, which frequently suffer specific proteolytic cuts or disulfide bond formation (18).
All these reasons could make it extremely difficult or even impossible to overproduce in E. coli not just active forms of complex heterooligomeric thermozymes but even active forms of certain monomeric or homooligomeric thermozymes, as we show in this work with the Mn-dependent catalase. Therefore, development of genetic tools for alternate thermophilic hosts that could overcome these problems and be used as cell factories has been the goal of our research group in recent years (13, 34).
The genus Thermus includes an extremely wide diversity of thermophilic and extreme thermophilic bacteria, many of which are sources of thermozymes that have enormous potential (16, 36, 38). This fact and properties like the natural competence of many strains, the high growth rates, and the high culture yields make this thermophile one of the most promising models of potential subrogate cell factories for the production of thermozymes.
In previous work the stable and versatile nature of biocatalysts prepared with native Mn-dependent catalases from two strains of the extreme thermophilic bacterium T. thermophilus (21), which in this organism constitute the major H2O2-degrading activities, was reported. We describe here the cloning and controlled overproduction in T. thermophilus of such an Mn-dependent catalase, whose synthesis in an active form in E. coli has repeatedly failed. Our results demonstrate the high potential of T. thermophilus as an alternative cell factory for the overproduction of thermozymes with great biotechnological potential.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
T. thermophilus HB8 (= ATCC 27634) and HB27::nar (34) were used as hosts for expression of the Mn-dependent catalase-encoding genes. E. coli strains DH5α [supE44 ΔlacU169 (φ80lacZΔM15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1] and BL21(DE3)/pLysS [hsdS gal (λcIts857 ind1 Sam7 nin5 lacUV5-T7 gene 1)] were used as hosts for genetic manipulation of plasmids and for the overexpression of proteins, respectively. Plasmids pET-22b(+) and pET-28b(+) (Novagen) were used for cloning and for expression in E. coli BL21(DE3)/pLysS. Plasmid pMKE1 is a bifunctional E. coli-Thermus sp. vector with multiple cloning sites that allow directed cloning of genes to be expressed in T. thermophilus under the control of the Pnar promoter (34).
E. coli strains were grown in Luria-Bertani (LB) medium (28) at 37°C. Kanamycin (30 mg/liter) and/or ampicillin (100 mg/liter) was used when needed. Aerobic growth of T. thermophilus was carried out at 70°C with shaking (150 rpm) in TB medium (13). For plasmid selection, kanamycin (30 mg/liter) was added to TB medium plates. Transformation of T. thermophilus was achieved with naturally competent cells as described previously (13). Transformation of E. coli was performed as described previously (20).
Assays for catalase activity.
Catalase activity was assayed in vitro by monitoring the decomposition of 0.14% (wt/wt) hydrogen peroxide in 50 mM potassium phosphate buffer (pH 7.5) at 25°C with a Shimadzu UV-240 spectrophotometer. Aliquots (200 μl) of the cell fraction assayed were added to 2.9 ml of the reaction mixture, and the decrease in absorbance at 240 nm was measured. An extinction coefficient of 39.4 M−1 · cm−1 was used to calculate catalase activity (35). One unit of catalase activity was defined as the amount of enzyme required to transform 1 μmol of hydrogen peroxide to water and oxygen per min at 25°C. Catalase activity was also detected in nondenaturing acrylamide electrophoresis gels by using the method of Woodbury et al. (45).
Molecular biology protocols.
Isolation of T. thermophilus genomic DNA was performed as described by Marmur (31). Plasmid purification and restriction analysis were performed by using standard methods. DNA was sequenced by automatic methods (Applied Biosystems) by using the synthetic primers described below (Isogen Bioscience, Maarssen, The Netherlands). Amplification of the Mn-dependent catalase gene was performed with total DNA samples from T. thermophilus HB27 and HB8 by PCR by using oligonucleotides ONdecat (5′ GAG CAT ATG TTC CTG AGG A 3′) and OHindcat (5′ CTA AAG CTT ACT TGG CCT TCT 3′) and DNA polymerase from T. thermophilus (Biotools B & M, Madrid, Spain). The primers included NdeI and HindIII restriction sequences (underlined) for cloning purposes and were based on the sequence of the gene for the Mn-dependent catalase from Thermus sp. strain YS8-13 (accession number AB008786), whose N terminus was identical to that of the purified Mn-dependent catalase from T. thermophilus (21a).
PCR were carried out with purified total DNA from T. thermophilus HB8 and HB27. A single DNA fragment of the expected size (∼940 bp) was obtained from both strains and was subsequently inserted into plasmid pCR2.1 (Novagen) for further cloning steps. Five independently amplified products were sequenced from each strain.
For construction of the expression plasmids, the PCR products cloned in pCR2.1 were digested with NdeI and HindIII, purified, and ligated into plasmids pET22b(+), pET28b(+), and pMKE1, which were previously digested with the same endonucleases. The derivatives pET22bcat, pET28bcat, and pMKEcat were obtained. From these plasmids, either a wild-type cat gene or a mutant derivative (cat*) was overexpressed in E. coli (pET-derived plasmids) or in T. thermophilus (pMKEcat). In all instances, the cat genes were sequenced again directly from the expression plasmids before production of the enzyme.
Overexpression of Mn-dependent catalase.
Expression from the T7 RNA polymerase-dependent promoter of the pET-derived plasmids was induced by addition of IPTG (isopropyl-β-d-thiogalactopyranoside) to exponential cultures (50 ml) of the corresponding E. coli BL21/pLys/pET strains grown in LB medium to an optical density at 550 nm of 0.5. Cultures were incubated for an additional 3 h, and cells were then harvested by centrifugation, resuspended in 4 ml of 25 mM potassium phosphate buffer, and sonicated in 1-ml aliquots (three 30-s pulses; maximum power; high intensity; 0.5-s repeating duty cycle; Labsonic U; Braun). Particulate material (cell envelopes and inclusion bodies) was separated by centrifugation at 13,000 × g for 15 min at 4°C. The supernatant was heated at 70°C for 10 min and then centrifuged. The soluble and insoluble fractions obtained after heating and the particulate fractions were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis on 10% polyacrylamide gels as described by Laemmli (27) and stained with Coomassie blue.
Clones of T. thermophilus strains HB8 and HB27::nar harboring the pMKEcat and pMKEcat* plasmids were grown aerobically at 70°C with shaking (150 rpm) in kanamycin-containing TB medium. At an optical density at 550 nm of 0.2, transcription from the Pnar promoter was activated by adding KNO3 (40 mM) and simultaneously stopping the shaker, as described previously (34). After incubation for 4 h at 70°C, cell extracts were prepared as described above for E. coli strains. Expression was verified by assaying catalase activity both spectrophotometrically and in nondenaturing 7% polyacrylamide electrophoresis gels. The contents of these gels were revealed with ferricyanide (45). The thermal stability of overexpressed products was tested with the glyoxyl-immobilized derivatives of the Mn-dependent catalases (21) by incubating suspensions of the derivatives (with an approximate activity of 25 IU/ml) in phosphate buffer (pH 7) at 85°C and measuring the catalase activity at fixed time intervals.
Protein sequence comparison and analysis.
A computer analysis of the Cat protein was performed with the programs PROSITE (22), MAXHOM alignment (41), PHD (40), and TMHMM1.0 (E. L. L. Sonnhammer, G. Von Heijne, and A. Krogh, Sixth Int. Conf. Intelligent Syst. Mol. Biol., p. 175-182, 1998) by using remote access through the EXPASY molecular biology server (http://us.expasy.org/).
Nucleotide sequence accession number.
The sequence of the wild-type cat gene has been deposited in the EMBL/GenBank database under accession number AJ551423.
RESULTS
Sequence of the Mn-dependent catalase genes from T. thermophilus HB27 and HB8.
Single products of the size expected for the cat genes (∼940 bp) were amplified from total DNA of T. thermophilus strains HB27 and HB8 when oligonucleotides ONdecat and OHindcat were used as primers. Direct sequencing of independently amplified cat genes from the strains revealed identical sequences for the encoded Cat proteins. The amino acid sequences were identical to that described for Thermus sp. isolate YS8-13 (accession number AB008786), which revealed a high level of conservation of the enzyme despite minor differences in the DNA sequences. The predicted size of the Cat proteins (33.3 kDa) was in good agreement with the deduced electrophoretic mobility of the enzyme protein purified from T. thermophilus (Hidalgo et al., submitted).
Sequence alignments with Mn-dependent catalase apoproteins from P. calidifontis VA1 (4), Salmonella enterica serovar Typhimurium (39), and L. plantarum (25) revealed that there was complete conservation of residues E36, E70, H73, E155, and H188, which were identified as residues that coordinate two Mn2+ ions in the three-dimensional structure of the Mn-dependent catalases described so far (5-8).
Interestingly, one of five PCR products amplified from T. thermophilus HB8 had two differences in its DNA sequence which resulted in amino acid replacements (M129V and E293G). This mutant form of the catalase gene (cat*), along a wild-type form from strain HB27 (cat), was cloned into the expression vectors (see below) to assay enzyme production both in T. thermophilus and in E. coli.
Production of Mn-dependent catalase in E. coli.
The cat and cat* genes were cloned into the expression plasmids pET22b and pET28b. From the first plasmid, an unmodified protein was produced, but a polyhistidine tag (six-His tag) was fused at the N terminus when derivatives of the second plasmid were used.
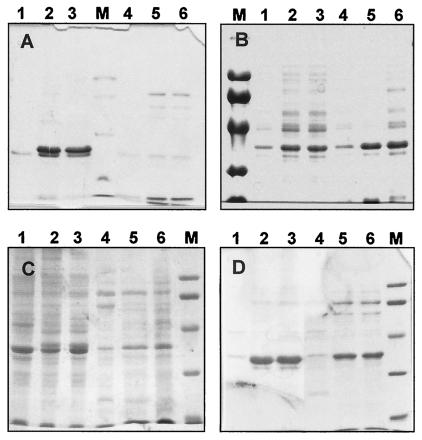
At 37°C, addition of IPTG (1 mM) to exponential cultures of E. coli BL21 harboring derivatives of the pET28b plasmids carrying the cat genes resulted in overproduction of six-His-tagged Cat and Cat* proteins of the expected sizes (∼37 kDa) (Fig. 1A). Most of these fusion proteins were found in the insoluble fraction, supporting the hypothesis that they formed aggregates (Fig. 1A, lanes 2 and 3). This was confirmed by the observation of inclusion bodies inside the induced cells (data not shown).
FIG. 1.
Overproduction of Mn-dependent catalases from T. thermophilus HB8 in E. coli. (A and C) Overproduction at 37°C (A) or 22°C (C) of His-tagged Cat and Cat* proteins from pET28b derivatives (1 mM IPTG). (B and D) Overproduction at 37°C (B) or 22°C (D) of unmodified Cat and Cat* proteins from pET22b derivatives (1 mM IPTG). Lane 1, insoluble fractions of cells harboring pET22b or pET28b (control); lane 2, insoluble fractions of cells harboring the cat* gene; lane 3, insoluble fractions of cells harboring the cat gene; lane 4, soluble thermostable (70°C for 10 min) fractions of cells harboring pET22 or pET28b (control); lane 5, soluble thermostable fractions of cells expressing the cat* mutant gene; lane 6, soluble thermostable fractions of cells expressing the wild-type cat gene; lane M, molecular weight markers.
In an attempt to increase the amount of soluble six-His-tagged Cat proteins and putatively the amount of active holoenzymes, we performed the induction experiments described above at 22°C. As shown in Fig. 1C, this resulted in a lower expression level concomitant with a moderate increase in the amount of six-His-tagged Cat proteins which remained soluble after heating at 70°C for 10 min. Despite this increase in the soluble and thermostable protein fraction, no catalase activity was detected either in the soluble fractions or in the particulate fractions. Further attempts to obtain active enzyme by induction with lower IPTG concentrations (0.1, 0.25, and 0.5 mM) in LB medium containing high concentrations of Mn2+ were unsuccessful (data not shown). Moreover, attempts were also made to activate the enzyme from the inclusion bodies through solubilization with 6 M guanidium chloride (32), followed by slow dialysis against Mn-containing reaction buffer. None of the assay protocols resulted in active enzyme.
In order to check if the inability to obtain active Mn-dependent catalases was related to the presence of the six-His tag fused at the N terminus, similar expression experiments were carried out with unmodified enzymes expressed from pET22 derivatives. As shown in Fig. 1B and D, a relevant fraction of the Cat and Cat* proteins (∼35 kDa) remained as the major soluble protein after heat treatment (70°C, 10 min), after induction at either 37 or 22°C. Therefore, native folding could be anticipated for the soluble Cat proteins of this fraction. Despite this, analysis of the catalase activity (at 70°C) revealed that in all instances the enzyme was not active. As with the six-His-tagged proteins, all attempts to activate the unmodified enzymes were unsuccessful.
Production of Mn-dependent catalases in T. thermophilus.
The results described above suggested that specific factors required for activation of the Cat apoproteins were absent from the E. coli cells used as the host for expression (see Discussion). Consequently, we decided to try overproduction in T. thermophilus by taking advantage of the recently described vector pMKE1 (34). Genes cloned in this vector are expressed from the Pnar promoter, which is induced by anoxia and nitrate in facultative anaerobic strains of T. thermophilus. Therefore, we assayed the expression of the cat and cat* genes from pMKE1 derivatives in T. thermophilus HB8 and HB27::nar; the latter strain is a derivative of aerobic strain HB27 which carries the nar operon (34).
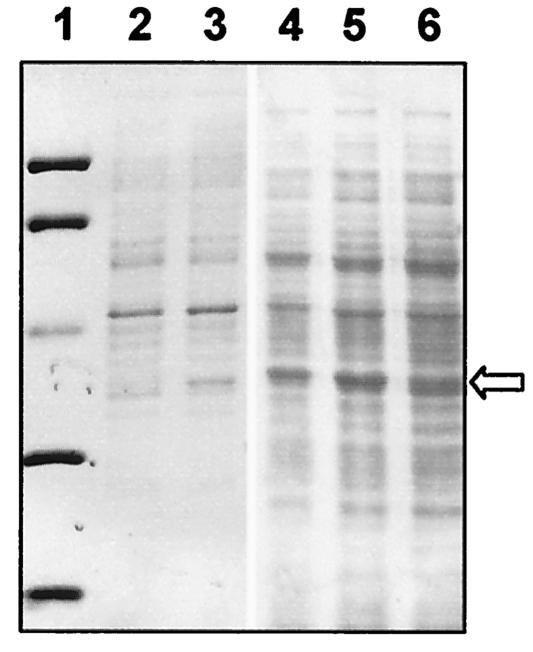
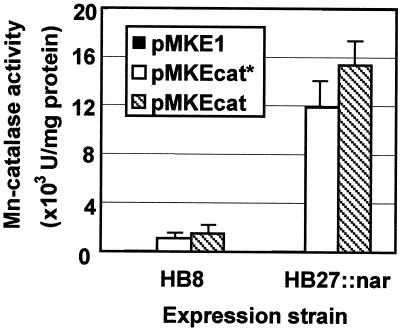
As shown in Fig. 2, induction of the Pnar promoter in exponential cultures of both strains of T. thermophilus transformed with pMKEcat or pMKEcat* resulted in the production of proteins of the expected sizes in amounts that were just large enough to be detected by Coomassie blue staining. When the specific activities of induced and control cultures (transformed with pMKE1) were compared, moderate 20- and 27-fold increases were detected in the HB8 strain for induction of the cat* and cat genes, respectively (Fig. 3). By contrast, the increase in specific activity obtained was approximately 2 orders of magnitude for both enzymes when strain HB27::nar was used for expression (Fig. 3).
FIG. 2.
Overproduction of Mn-dependent catalases in T. thermophilus. Lane 1, molecular mass markers (94, 67, 43, 30, and 20.1 kDa); lanes 2 and 3, soluble extract from T. thermophilus HB8 harboring pMKE1 (control) or pMKEcat; lanes 4 to 6, soluble extracts from T. thermophilus HB27::nar harboring pMKE1 (control), pMKEcat*, and pMKEcat, respectively. The position corresponding to the overexpressed Mn-dependent catalase is indicated by an arrow.
FIG. 3.
Specific activity of overproduced Mn-dependent catalase. The specific activities of catalases in soluble extracts from T. thermophilus HB8 and HB27::nar expressing pMKE1 (control), pMKEcat, and pMKEcat* were determined. Measurements were carried out with triplicate samples. The error bars represent standard deviations.
Stability assays.
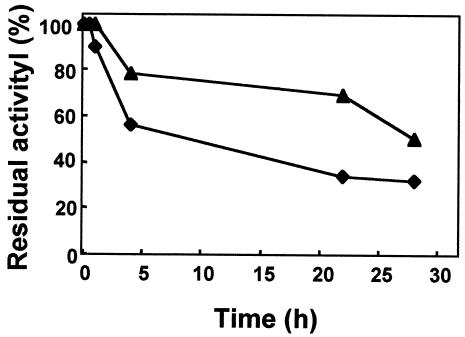
As the Cat and Cat* proteins had two differences in their amino acid sequences, we checked their temperature stabilities, with the perspective of future biotechnological applications of these enzymes. As expected, the catalase activity of a glyoxyl agarose-immobilized derivative from soluble fractions of cells overexpressing the wild-type Cat protein proved to be more stable than its counterpart expressing the Cat* mutant (Fig. 4). The difference was not dependent on which of the strains (HB8 or HB27::nar) was used as the host for overexpression (data not shown).
FIG. 4.
Thermostability of the Cat and Cat* proteins. Thermal inactivation of glyoxyl derivatives of the unmodified wild-type Cat (▴) and mutant Cat* (⧫) Mn-dependent catalases overproduced in T. thermophilus HB27::nar was determined. The experimental conditions were as follows: 85°C and 25 mM potassium phosphate buffer (pH 7.0). The amount of incubated enzyme was 25 IU/ml.
DISCUSSION
The use of thermophilic enzymes for biotechnological purposes has been limited very often by the difficulty of overproducing the enzymes in an appropriate host, which could allow synthesis in an active form. This was clearly the case in the experiments that we describe here involving a thermophilic Mn-dependent catalase with high biotechnological potential whose expression in E. coli results in accumulation of inactive forms.
The reasons that underlie the absence of activity in the products of the cat genes expressed in E. coli cannot be ascertained with the present data, but two arguments suggest that there is a requirement for a genus-specific mechanism of activation of the Mn-dependent catalase. First, Mizobata et al. (33) described the overproduction in E. coli of inactive forms of the apocatalase from Thermus sp. strain YS8-13 and partial activation of the catalase activity by a protocol which included chaotropic agents and high temperatures in the presence of elevated concentrations of manganese (33). Therefore, partial unfolding and high temperature seem to be required for incorporation of Mn2+ in the active center (33), suggesting that there is an in vitro requirement for specific factors to keep the protein in the unfolded state before activation. This fact is reminiscent of the requirement for the Isc proteins in the synthesis and repair of iron-sulfur clusters (46) or the requirement for the NarJ chaperone in the insertion of the molybdopterin guanidine dinucleotide cofactor into the alpha subunit of nitrate reductase A of E. coli (9).
An additional argument is based in the results shown in Fig. 1B and D, which show how a relevant amount of the protein could be synthesized in a soluble and thermostable form (it remained soluble after heating at 70°C), which supports the hypothesis that the backbone of the protein is folded in a way similar to the way in which the wild-type holoenzyme is folded. Therefore, the absence of activity suggests that the active center of the enzyme is not functional, probably because of the absence of Mn2+ from its active center.
Previously published data also support the existence of specific factors implicated in the insertion of Mn2+ cofactors in other enzymes. In this sense, it has been proposed that a biochemically uncharacterized protein designated MTM1 functions in the mitochondrial activation of superoxide dismutase 2 (SOD) from the yeast Saccharomyces cerevisiae, specifically by facilitating insertion of the essential manganese cofactor (30). With the relevant function of SOD in aerobic organisms in mind, we believe that SOD-specific Mn insertion factors should also be widely distributed. Moreover, some similarities between these cofactors and those putatively implicated in the insertion of Mn2+ in Mn-dependent catalases could be expected. In fact, a manganese-dependent catalase (katN) from S. enterica serovar Typhimurium is encoded by the last gene of a four-gene operon, and the upstream genes (yciGFE) could play a role in the activation of the enzyme (39). Homologues of the yciGFE genes are organized as an operon in the genome of E. coli and could be the genes required for activation of SOD in this organism. These YciGFE homologues could substitute for their Salmonella orthologues during the production in E. coli of the active KatN Mn-dependent catalase (39). In this scenario, it could be expected that such YciGFE putative activators could not recognize an apocatalase from a phylogenetically distant organism, such as T. thermophilus. In fact, homologues of the Salmonella YciGFE proteins are not encoded in the genome of T. thermophilus HB27 (accession numbers AE017221 and AE017222), suggesting that these proteins are not well conserved.
Whatever the origin of inactive forms was, our results clearly demonstrate that use of the producer organism as a cell factory overcomes the problems of enzyme activation and allows overproduction of the enzyme in an active form. In this sense, the overproduction levels depend dramatically on the strain used as the host; the levels are much lower in T. thermophilus HB8 than in HB27::nar. This difference has been observed also for a reporter gene encoding a thermostable β-galactosidase expressed from pMKE1 and seems to be related to some leakiness of the anaerobic control of the Pnar promoter in the HB8 strain (34). When specific activities were compared, the expression in HB27::nar was roughly 10-fold higher than that in HB8, making the HB27::nar strain an excellent system for overproduction of this enzyme and most likely for future expression of other thermozymes, which, like the Mn-dependent catalase, cannot be synthesized in more conventional hosts. Additional applications in the production or selection of modified forms of the enzyme, such as the Cat* protein, can be predicted.
Acknowledgments
The financial support of project BIO2001-1627 from the Spanish Ministerio de Ciencia y Tecnología to J. Berenguer is acknowledged. A. Hidalgo and R. Moreno are recipients of fellowships from Gobierno Vasco and Biotools B & M, respectively. An institutional grant from Fundación Ramón Areces to the Centro de Biología Molecular “Severo Ochoa” is also acknowledged.
REFERENCES
- 1.Adams, M. W., and R. M. Kelly. 1998. Finding and using hyperthermophilic enzymes. Trends Biotechnol. 16:329-332. [DOI] [PubMed] [Google Scholar]
- 2.Akertek, E., and L. Tarhan. 1995. Characterization of immobilized catalases and their application in pasteurization of milk with H2O2. Appl. Biochem. Biotechnol. 50:291-303. [Google Scholar]
- 3.Allgood, G. S., and J. J. Perry. 1986. Characterization of a manganese-containing catalase from the obligate thermophile Thermoleophileum album. J. Bacteriol. 168:563-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amo, T., H. Atomi, and T. Imanaka. 2002. Unique presence of a manganese catalase in a hyperthermophilic archaeon, Pyrobaculum calidifontis VA1. J. Bacteriol. 184:3305-3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antonyuk, S. V., W. R. Melik-Adamyan, A. N. Popov, V. S. Lamzin, P. D. Hempstead, P. M. Harrison, P. J. Artymiuk, and S. M. Barynin. 2000. Three-dimensional structure of the enzyme dimanganese catalase from Thermus thermophilus at 1 Å resolution. Crystallogr. Rep. 45:105-116. [Google Scholar]
- 6.Barynin, V. V., and A. I. Grebenko. 1986. T-catalase in nonheme catalase of extremely thermophilic bacterium Thermus thermophilus HB-8. Dolk. Akad. Nauk. SSSR 286:461-464. [Google Scholar]
- 7.Barynin, V. V., P. D. Hempstead, A. A. Vagin, S. V. Antonyuk, W. R. Melik-Adamyan, V. S. Lamzin, P. M. Harrison, and P. J. Artymiuk. 1997. The three-dimensional structure of the di-Mn catalase and the environment of the di-Mn sites in different redox states. J. Ind. Biochem. 67:196. [Google Scholar]
- 8.Barynin, V. V., M. M. Whittaker, S. Antonyuk, V. S. Lamzin, P. M. Harrison, P. J. Artymiuk, and J. W. Whittaker. 2001. Crystal structure of manganese catalase from Lactobacillus plantarum. Structure 9:725-738. [DOI] [PubMed] [Google Scholar]
- 9.Blasco, F., J. P. Dos Santos, A. Magalon, C. Frixon, B. Guigliarelli, C. L. Santini, and G. Giordano. 1998. NarJ is a specific chaperone required for molybdenum cofactor assembly in nitrate reductase A of Escherichia coli. Mol. Microbiol. 28:435-447. [DOI] [PubMed] [Google Scholar]
- 10.Bruins, M. E., A. E. M. Janssen, and R. M. Boom. 2001. Thermozymes and their applications. Appl. Biochem. Biotechnol. 90:155-186. [DOI] [PubMed] [Google Scholar]
- 11.Coolbear, T., R. M. Daniel, and H. W. Morgan. 1992. The enzymes from extreme thermophiles: bacterial sources, thermostabilities and industrial relevance. Adv. Biochem. Eng. Biotechnol. 45:58-98. [DOI] [PubMed] [Google Scholar]
- 12.Costa, S. A., T. Tzanov, A. Paar, M. Gudelj, G. M. Gübitz, and A. Cavaco-Paulo. 2001. Immobilization of catalases from Bacillus SF on alumina for the treatment of textile bleaching effluents. Enzyme Microb. Technol. 28:815-819. [DOI] [PubMed] [Google Scholar]
- 13.de Grado, M., P. Castán, and J. Berenguer. 1999. A high-transformation efficiency cloning vector for Thermus. Plasmid 42:241-245. [DOI] [PubMed] [Google Scholar]
- 14.Demirjian, D. C., F. Moris-Varas, and C. S. Cassidy. 2001. Enzymes from extremophiles. Curr. Opin. Chem. Biol. 5:144-151. [DOI] [PubMed] [Google Scholar]
- 15.Dey, E. S., J. R. Miller, S. Kovacevic, and K. Mosbach. 1991. Stabilization of d-amino acid oxidase from yeast Trigonopsis variabilis used for production of glutaryl-7-aminocephalosporanic acid from cephalosporin C. Appl. Biochem. Biotechnol. 2:239-250. [Google Scholar]
- 16.Eichler, J. 2001. Biotechnological uses of archaeal extremozymes. Biotechnol. Adv. 19:261-278. [DOI] [PubMed] [Google Scholar]
- 17.Fernández-Herrero, L. A., M. A. Badet-Denisot, B. Badet, and J. Berenguer. 1995. glmS of Thermus thermophilus HB8: an essential gene for cell-wall synthesis identified immediately upstream of the S-layer gene. Mol. Microbiol. 17:1-12. [DOI] [PubMed] [Google Scholar]
- 18.Fernández-Herrero, L. A., and V. de Lorenzo. 2001. Formulation of disulphide bonds during secretion of proteins through the periplasmic-independent type I pathway. Mol. Microbiol. 40:332-346. [DOI] [PubMed] [Google Scholar]
- 19.Fernández-Lafuente, R., V. Rodríguez, and J. M. Guisan. 1998. The coimmobilization of d-amino acid oxidase and catalase enables the quantitative transformation of d-amino acids (d-phenylalanine) into α-ketoacids (phenypyruvic acid). Enzyme Microb. Technol. 23:28-33. [Google Scholar]
- 20.Hanahan, D. 1983. Studies on transformation of E. coli with plasmids. J. Mol. Biol. 166:557-580. [DOI] [PubMed] [Google Scholar]
- 21.Hidalgo, A., L. Betancor, F. López-Gallego, R. Moreno, J. Berenguer, R. Fernández-Lafuente, and J. M. Guisán. 2003. Preparation of a versatile biocatalyst of immobilized and stabilized catalase from Thermus thermophilus. Enzyme Microb. Technol. 33:278-285. [Google Scholar]
- 21a.Hidalgo, A., L. Betancor, C. Mateo, F. López-Gallego, R. Moreno, J. Berenguer, R. Fernández-Lafuente, and J. M. Guisán. Biotechnol. Progr., in press. [DOI] [PubMed]
- 22.Hofmann, K., P. Bucher, L. Falquet, and A. Bairoch. 1999. The PROSITE database, its status in 1999. Nucleic Acids Res. 27:215-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hwang, S. O., D. J. Trantolo, and D. L. Wise. 1990. Gas phase acetaldehyde production in a continuous bioreactor. Biotechnol. Bioeng. 36:834-838. [DOI] [PubMed] [Google Scholar]
- 24.Kagawa, M., N. Murakoshi, Y. Nishikawa, G. Matsumoto, Y. Kurata, T. Mizobata, Y. Kawata, and J. Nagai. 1999. Purification and cloning of a thermostable manganese catalase from a thermophilic bacterium. Arch. Biochem. Biophys. 362:346-355. [DOI] [PubMed] [Google Scholar]
- 25.Kono, Y., and I. Fridovich. 1983. Isolation and characterization of the pseudocatalase of Lactobacillus plantarum. J. Biol. Chem. 258:6015-6019. [PubMed] [Google Scholar]
- 26.Kristjansson, J. K. 1989. Thermophilic organisms as sources of thermostable enzymes. Trends Biotechnol. 7:349-353. [Google Scholar]
- 27.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of the bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 28.Lennox, E. X. 1955. Transduction of linked genetic characters of the host by bacteriophage P1. Virology 1:190-206. [DOI] [PubMed] [Google Scholar]
- 29.Loewen, P. C., M. G. Klotz, and D. J. Hassett. 2000. Catalase—an “old” enzyme that continues to surprise us. ASM News 66:76-82. [Google Scholar]
- 30.Luk, E., M. Carroll, M. Baker, and V. Cizewski-Culotta. 2003. Manganese activation of superoxide dismutase 2 in Saccharomyces cerevisiae requires MTM1, a member of the mitochondrial carrier family. Proc. Natl. Acad. Sci. USA 100:10353-10357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marmur, J. 1961. A procedure for the isolation of deoxyribonucleic acid from microorganisms. J. Mol. Biol. 3:208-218. [Google Scholar]
- 32.Marston, F. A. O., and D. L. Hartley. 1990. Solubilization of protein aggregates. Methods Enzymol. 182:264-276. [DOI] [PubMed] [Google Scholar]
- 33.Mizobata, T., M. Kagawa, N. Murakoshi, E. Kusaka, K. Kameo, Y. Kawata, and J. Nagai. 2000. Overproduction of Thermus sp. YS 8-13 manganese catalase in Escherichia coli. Production of soluble apoenzyme and in vitro formation of active holoenzyme. Eur. J. Biochem. 267:4264-4271. [DOI] [PubMed] [Google Scholar]
- 34.Moreno, R., O. Zafra, F. Cava, and J. Berenguer. 2003. Development of a gene expression vector for Thermus thermophilus based on the promoter of the respiratory nitrate reductase. Plasmid 49:2-8. [DOI] [PubMed] [Google Scholar]
- 35.Nelson, D. P., and L. A. Kiesow. 1972. Enthalpy of decomposition of hydrogen peroxide by catalase at 25°C (with molar extinction coefficients of H2O2 solutions in the UV). Anal. Biochem. 49:474-478. [DOI] [PubMed] [Google Scholar]
- 36.Niehaus, F., C. Bertoldo, M. Kähler, and G. Antranikian. 1999. Extremophiles as a source of novel enzymes for industrial application. Appl. Microbiol. Biotechnol. 51:711-729. [DOI] [PubMed] [Google Scholar]
- 37.Owusu, R. K., and D. A. Cowan. 1989. Correlation between microbial protein thermostability and resistance to denaturation in aqueous organic solvent two-phase systems. Enzyme Microb. Technol. 11:568-574. [Google Scholar]
- 38.Persidis, A. 1998. Extremophiles. Nat. Biotechnol. 16:593-594. [DOI] [PubMed] [Google Scholar]
- 39.Robbe-Saule, V., C. Coynault, M. Ibañez-Ruiz, D. Hermant, and F. Norel. 2001. Identification of a non-haem catalase in Salmonella and its regulation by RpoS (σs). Mol. Microbiol. 39:1533-1545. [DOI] [PubMed] [Google Scholar]
- 40.Rost, B. 1996. PHD: predicting one-dimensional protein structure by profile-based neural networks. Methods Enzymol. 1996:525-539. [DOI] [PubMed] [Google Scholar]
- 41.Sander, C., and R. Schneider. 1991. Database of homology-derived protein structures and the structural meaning of sequence alignment. Proteins 9:56-68. [DOI] [PubMed] [Google Scholar]
- 42.Schoevaart, R., and T. Kieboom. 2001. Combined catalytic reactions—nature's way. Chem. Innovat. 2001(December):33-38.
- 43.Schussel, L. J., and J. E. Atwater. 1996. A continuous alcohol oxidase bioreactor for regenerative life support. Enzyme Microb. Technol. 18:229-235. [Google Scholar]
- 44.Tarhan, L. 1990. Enzymatic properties of immobilized catalase on protein coated supports. Biomed. Biochim. Acta 49:307-316. [PubMed] [Google Scholar]
- 45.Woodbury, W., A. K. Spencer, and M. A. Stahmann. 1971. An improved procedure using ferricyanide for detecting catalase isozymes. Anal. Biochem. 44:301-305. [DOI] [PubMed] [Google Scholar]
- 46.Zheng, L., V. L. Cash, D. H. Flint, and D. R. Dean. 1998. Assembly of iron-sulfur clusters. Identification of an iscSUA-hscBA-fdx gene cluster from Azotobacter vinelandii. J. Biol. Chem. 273:13264-13272. [DOI] [PubMed] [Google Scholar]