Abstract
Aging impairs angiogenic capacity of cerebromicrovascular endothelial cells (CMVECs) promoting microvascular rarefaction, but the underlying mechanisms remain elusive. PACAP is an evolutionarily conserved neuropeptide secreted by endothelial cells and neurons, which confers important antiaging effects. To test the hypothesis that age-related changes in autocrine PACAP signaling contributes to dysregulation of endothelial angiogenic capacity, primary CMVECs were isolated from 3-month-old (young) and 24-month-old (aged) Fischer 344 x Brown Norway rats. In aged CMVECs, expression of PACAP was decreased, which was associated with impaired capacity to form capillary-like structures, impaired adhesiveness to collagen (assessed using electric cell-substrate impedance sensing [ECIS] technology), and increased apoptosis (caspase3 activity) when compared with young cells. Overexpression of PACAP in aged CMVECs resulted in increased formation of capillary-like structures, whereas it did not affect cell adhesion. Treatment with recombinant PACAP also significantly increased endothelial tube formation and inhibited apoptosis in aged CMVECs. In young CMVECs shRNA knockdown of autocrine PACAP expression significantly impaired tube formation capacity, mimicking the aging phenotype. Cellular and mitochondrial reactive oxygen species production (dihydroethidium and MitoSox fluorescence, respectively) were increased in aged CMVECs and were unaffected by PACAP. Collectively, PACAP exerts proangiogenic effects and age-related dysregulation of autocrine PACAP signaling may contribute to impaired angiogenic capacity of CMVECs in aging.
Key Words: Capillary density, Senescence, Vascular cognitive impairment, Vasculardementia, Ischemia
Several studies analyzing cerebrovascular architecture, assessing capillary density, and measuring cerebral blood flow in elderly humans and aged rodents suggest that angiogenesis in the brain is compromised by aging (1–3), which might contribute to a range of age-related central nervous system disorders with microvascular involvement, including vascular cognitive impairment and Alzheimer’s disease. The age-related loss of microvascular plasticity and rarefaction in the brain has significance for impaired metabolic support for the neuronal tissue and for impaired recovery after cerebral ischemia. Microvascular rarefaction is also related to increased inflammatory signals that may negatively regulate the stem cell population (4). Although age-related changes in expression of pro and antiangiogenic factors in the brain parenchyma likely have a role in dysregulation of brain capillarization in aging, there is increasing evidence that aging impairs the intrinsic angiogenic capacity of cerebromicrovascular endothelial cells (CMVECs) (5). Despite provocative evidence linking age-related microvascular rarefaction with diminished endothelial angiogenic capacity, the underlying mechanisms remain elusive.
PACAP is an evolutionarily conserved C-terminally α-amidated peptide that was first isolated 25 years ago from an ovine hypothalamic extract on the basis of its ability to stimulate cAMP formation in anterior pituitary cells (6). It exists in two forms, consisting of either 27 or 38 amino acids, the longer form being dominant in mammalian tissues (7). The PACAP belongs to the vasoactive intestinal polypeptide (VIP)-secretin-growth hormone-releasing hormone-glucagon superfamily. Although PACAP is a multifunctional peptide and shows wide tissue distribution (7), its expression is highest in the brain and several diverse functions of PACAP have been described in the central nervous system (8). Recent studies suggest that alterations in PACAP signaling may play an important role in age-related cognitive decline and development of age-related diseases of the CNS (9,10). Accordingly, PACAP has remarkable protective effects in animal models of age-related neurodegenerative diseases, including Parkinson’s disease and Alzheimer’s disease (9). The PACAP knockout mice exhibit symptoms of accelerated neurocognitive aging, including impairment of learning and memory (11–13), increased oxidative stress (14), and accelerated aging of the retina (15). The antiaging and neuroprotective effects of PACAP have been attributed, at least in part, to its antiapoptotic, anti-inflammatory, and antioxidant actions (9,10). Treatment with PACAP was also shown to exert anti-apoptotic effects (16) and reverse age-related learning impairment in molluscan models of aging (17) suggesting that the antiaging actions of PACAP are evolutionarily conserved. Recent studies demonstrate that endothelial cells are not only important sources of PACAP secretion (18) but also express receptors for PACAP (19) and their function is regulated by PACAP (20–23). Importantly, PACAP was also suggested to exert proangiogenic effects in tumor models (reviewed in Ref. (24)). Furthermore, a recent study demonstrated that secretion of PACAP by cerebral microvessels progressively declines with age in laboratory rodents (18). Nevertheless, the specific role of PACAP in age-related impairment of endothelial angiogenic capacity of CMVECs remains poorly understood.
The present study was designed to test the hypothesis that the age-related decline in autocrine PACAP expression contributes to age-related impairment of endothelial angiogenic capacity. A prediction based on this hypothesis is that increases in autocrine PACAP should restore a youthful endothelial phenotype improving endothelial angiogenic capacity. To test this hypothesis using cultured primary CMVECs derived from aged rats as a model system, we determined whether overexpression of PACAP or treatment with exogenous PACAP-38 elicits proangiogenic, antioxidative, and antiapoptotic changes in the endothelial phenotype. We also tested whether downregulation of PACAP (shRNA) in cultured primary CMVECs derived from young animals confers antiangiogenic effects, mimicking the aging phenotype. As endpoints, endothelial angiogenic capacity (tube formation, adhesion, and migration capacity), apoptosis, and cellular reactive oxygen species (ROS) production (dihydroethidium [DHE] and MitoSox fluorescence) were assessed.
Materials and Methods
Establishment and Characterization of Primary Cerebromicrovascular Endothelial Cell Cultures
Primary CMVEC cultures were established from the brains of 3-month old (“young”) and 24-month old (“aged”) male Fischer 344 x Brown Norway rats as described previously (5,25). All animals (n = 5 in each group) were disease free with no signs of systemic inflammation and/or neoplastic diseases. All rats were maintained according to National Institutes of Health guidelines and all animal use protocols were approved by the Institutional Lyses buffer Animal Care and Use Committees of the University of Oklahoma Health Sciences Center. The animals were euthanized with CO2. The brains were removed aseptically, rinsed in ice-cold PBS, and minced into ≈1 mm2. The tissue was washed twice in ice cold 1× PBS by low-speed centrifugation (50g, 2–3min). The diced tissue was digested in a solution of collagenase (800 U/g tissue), hyaluronidase (2.5 U/g tissue), and elastase (3 U/g tissue) in 1 mL PBS/100 mg tissue for 45 minutes at 37°C in rotating humid incubator. The digested tissue was passed through a 100-µm cell strainer to remove undigested blocks. The single cell lysate was centrifuged for 2min at 70g. After removing the supernatant carefully the pellet was washed twice in cold PBS supplemented with 2.5 % fetal calf serum and the suspension centrifuged at 300g, for 5min at 4°C.
To create an endothelial cell enriched fraction the cell suspension was gradient centrifuged by using OptiPrep solution (Axi-Shield, PoC, Norway). Briefly, the cell pellet was resuspended in Hanks’ balanced salt solution and thoroughly mixed with 40% iodixanol (final concentration: 17% (w/v) iodixanol solution; ρ = 1.096g/mL). 2 mL of Hanks’ balanced salt solution was layered on top and centrifuged at 400g for 15min at 20°C. Endothelial cells, which banded at the interface between Hanks’ balanced salt solution and the 17% iodixanol layer, were collected. The endothelial cell enriched fraction was incubated for 30min at 4°C in the dark with anti-CD31/PE (BD BD Biosciences, San Jose, CA), anti-MCAM/FITC (BD Biosciences, San Jose, CA). After washing the cells twice with MACS Buffer (Milltenyi Biotech, Cambridge, MA), anti-FITC magnetic bead labeled and anti-PE magnetic bead labeled secondary antibodies were used for 15 minutes at room temperature. Endothelial cells were collected using the MACS LD magnetic separation columns according to the manufacturer’s guidelines (Milltenyi Biotech, Cambridge, MA). The endothelial fraction was cultured on fibronectin coated plates in endothelial growth medium (Cell Application, San Diego, CA) for 10 days. Endothelial cells were phenotypically characterized by flow cytometry (GUAVA 8HT, Merck Millipore, Billerica, MA). In brief, antibodies against five different endothelial specific markers were used (anti-CD31-PE, antierythropoietin receptor-APC, anti-VEGF R2-PerCP, anti-ICAM-fluorescein, and anti-CD146-PE) and isotype specific antibody labeled fractions served as negative controls. All antibodies were purchased from R&D Systems (R&D Systems, Minneapolis, MN). All other reagents used in this study were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise indicated. To study age-related changes in endothelial phenotype, primary CMVECs were initially cultured in MesoEndo Endothelial Cell Growth Medium (Cell Applications) followed by Endothelial Basal Medium supplemented with 10% fetal calf serum.
Quantitative Real-Time RT‒PCR
Quantitative real time RT‒PCR was performed to analyze the mRNA expression of PACAP, PAC1R (Adcyap1r1), VPAC1 (Vipr1), VPAC2R (Vipr2), VEGF and VEGFR2, as well as the reference genes Hprt, Ywhaz, and B2m, as reported previously (5,25,26). In brief, total RNA was isolated using a TaqMan Cells-to-CT Kit (Applied Biosystems, Foster City, California) and was reverse transcribed using Superscript III RT (Invitrogen, Carlsbad, California) as described previously (27,28). A real time RT‒PCR technique was used to analyze mRNA expression using a Stratagen MX3000, as reported (28). Amplification efficiencies were determined using a dilution series of a standard vascular sample. Quantification was performed using the efficiency-corrected ΔΔCq method. The relative quantities of the reference geneswere determined and a normalization factor was calculated based on the geometric mean for internal normalization.
Knockdown and Overexpression of PACAP in CMVECS
To investigate the role of autocrine PACAP signaling in regulation of the endothelial phenotype, downregulation of PACAP in young CMVECs was achieved by RNA interference using proprietary shRNA sequences (OriGene Technologies, Rockville, MD). Overexpression of PACAP in aged CMVECs was achieved by using a full-length PACAP cDNA encoding plasmid (OriGene). Transfection was performed using the electroporation-based LonzaNucleofector technology (Amaxa, Gaithersburg, Maryland), as described earlier (29–32). The success of transfection was confirmed by quantitative real-time RT‒PCR. Negative controls included transfection with scrambled shRNA (OriGene) or a GFP expressing vector (33).
Tube Formation Assay
To investigate the influence of autocrine PACAP signaling on tube formation ability, young CMVECs with or without shRNA knockdown of PACAP and aged CMVECs with or without overexpression of PACAP were plated on Geltrex Reduced Growth Factor Basement Membrane Matrix (Invitrogen, Carlsbad, California) in Medium 200PRF (Invitrogen). In separate experiments, the effect of treatment with PACAP-38 (10–7 mol/L) on endothelial tube formation was assessed. In brief, 150 µl/well of Geltrex was distributed in ice-cold 24-well plates. The gel was allowed to solidify while incubating the plates for 30 minutes at 37ºC. The CMVECs were then seeded at a density of 5 × 104 cells/well and placed in the incubator for 24 hours. Microscopic images were captured using a Nikon Eclipse Ti microscope equipped with a 10× phase-contrast objective (Nikon Instruments, Melville, New York). The extent of tube formation was quantified by measuring total tube length in five random fields per well using NIS-Elements microscope imaging software (Nikon Instruments Inc), as recently reported (5,25,33,34). The mean of the total tube length per total area imaged (µm tube/mm2) was calculated for each well. Experiments were run in quadruplicate. The experimenter was blinded to the experimental groups throughout the period of analysis.
Cell Adhesion Assay
Cell adhesion plays an important role in the multistep process of angiogenesis (35). To determine the effect of autocrine PACAP signaling on the adhesion capacity of endothelial cells, we used ECIS technology (Applied Biophysics, Troy, New York). Adhesion of young CMVECs (transfected with PACAP shRNA or scrambled shRNA) and aged CMVECs (transfected with PACAP cDNA or control plasmid) to collagen was monitored as reported (5,25,33). In a separate experiment, cells were treated with PACAP-38 (from 10–8 mol/l to 10–6 mol/l; for 48 h). Cells were seeded at a density of 2.5 × 105 cells/well in collagen coated (50 µg/mL) 96-well array culture dishes containing gold film surface electrodes (ECIS 96W1E; in each well one active electrode and a large counter electrode). The arrays were placed in an incubator and the time course for changes of capacitance (measured at 60kHz) due to the adhesion of cells to the active electrode was obtained. Time to reach 50% cell adhesion was used as an index of adhesiveness (100% change corresponds to the maximum level of cell coverage reached on the active electrode).
Assessment of Cell Migration by ECIS-based Wound-Healing Assay
The ECIS technology was used to monitor migration of young CMVECs (transfected with PACAP shRNA or scrambled shRNA) and aged CMVECs (transfected with PACAP cDNA or control plasmid), as reported (33). In a separate experiment, cells were treated with PACAP-38 (from 10–8mol/l to 10–6mol/l). In brief, CMVECs (2.5 × 105 cells/well) were seeded in 96-well array culture dishes (ECIS 96W1E), placed in an incubator (37oC), and changes in resistance and impedance were continuously monitored. When impedance reached a plateau, cells in each well were subjected to an elevated field pulse (“wounding”) of 5 mA applied for 20 seconds at 100kHz, which killed the cells present on the small active electrode due to severe electroporation. The detachment of the dead cells was immediately evident as a sudden drop in resistance (monitored at 4000 Hz) and a parallel increase in conductance. CMVECs surrounding the active electrode that had not been subjected to the wounding then migrated inward to replace the detached dead cells resulting in resistance recovery (continuously monitored at 4000 Hz for up to 24 h). Time to reach 50% resistance recovery (corresponding to 50% confluence on the active electrode) was determined and this parameter and the known physical dimensions of the electrode were used to calculate the migration rate (expressed as µm/h).
Apoptosis Assay
To determine whether PACAP exerts antiapoptotic effects in aged CMVECs, apoptotic cell death was assessed by measuring caspase activities using the Caspase-Glo3/7 assay kit (Promega, Madison, Wisconsin) as reported earlier (36, 37). Young and aged CMVECs were seeded at a density of 2 × 104 cells/well in white-walled 96-well plates. Aged CMVECs were treated with PACAP-38 (10–7mol/L). To measure caspase3/7 activity, 50 µL of sample was mixed for 30 seconds with of 50 µL Caspase-Glo3/7 reagent and incubated for 2 hours at room temperature. Lysis buffer with the reagent served as blank. Luminescence of the samples was measured using an Infinite M200 plate reader (Tecan, Research Triangle Park, North Carolina). Luminescent intensity values were normalized to the sample protein concentration.
Measurement of Cellular Reactive Oxygen Species Production
To assess the effect of PACAP on cellular production of ROS we used DHE (Invitrogen, Carlsbad, California), an oxidative fluorescent dye, as we reported earlier (38–40). In brief, 2 × 104 cells/well were seeded to a transparent 96-well plate and were treated with different concentrations of PACAP-38 (from 10–8 to 10–6 mol/l, for 24 h). Then the cells were washed with warm PBS and incubated with DHE (41,42) (3 × 10– 6 mol/L; at 37oC for 30 minutes). The DHE fluorescence was assessed by flow cytometry (38,39). In separate experiments, mitochondrial O2 – production in CMVECs was measured using MitoSOX Red (Invitrogen, Carlsbad, California), a mitochondrion-specific hydroethidine-derivative fluorescent dye, as reported earlier (25,38,43–45). Cell debris (low forward and side scatter) and dead cells (Sytox Green) were gated out for analysis.
Data Analysis
Statistical analyses were performed using one-way ANOVA followed by Tukey post hoc tests. p < .05 was considered statistically significant. Data are expressed as means ± S.E.M.
Results
Age-Related Changes in Expression of PACAP and PACAP Receptors in CMVECs
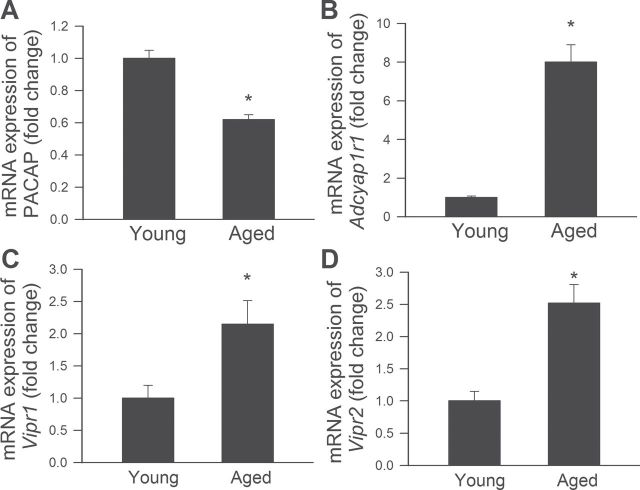
Expression of PACAP was significantly decreased in aged CMVECs when compared with that in young cells (Figure 1A). Compared to young CMVECs, mRNA expression of PAC1R, VPAC1, and VPAC2R was increased in aged cells (Figure 1A), perhaps as a partial compensation for the age-related decline in PACAP production.
Figure 1.
Downregulation of PACAP expression in aged cerebromicrovascular endothelial cells (CMVECs). (A‒ D) Quantitative real time RT‒PCR data showing mRNA expression of PACAP (Panel A) and the PACAP receptors PAC1R (Adcyap1r1, Panel B), VPAC1 (Vipr1, Panel C), and VPAC2R (Vipr2, Panel D) in young and aged CMVECs. Data are means ± S.E.M. (n = 5 in each group), *p < .05 vs. young control.
Autocrine PACAP Promotes Formation of Capillary-Like Structures by CMVECs
We performed an in vitro tube formation assay to model the reorganization stage of angiogenesis. When seeded onto Geltrex matrices, young CMVECs formed elaborated capillary networks and this response was significantly impaired in aged CMVECs (Figure 2C–D). We found that knockdown of PACAP impaired the ability of young CMVECs to form capillary-like structures (Figure 2D). Overexpression of PACAP in aged CMVECs (Figure 2D) and treatment with PACAP-38 (Figure 2E) increased tube formation by endothelial cells.
Figure 2.
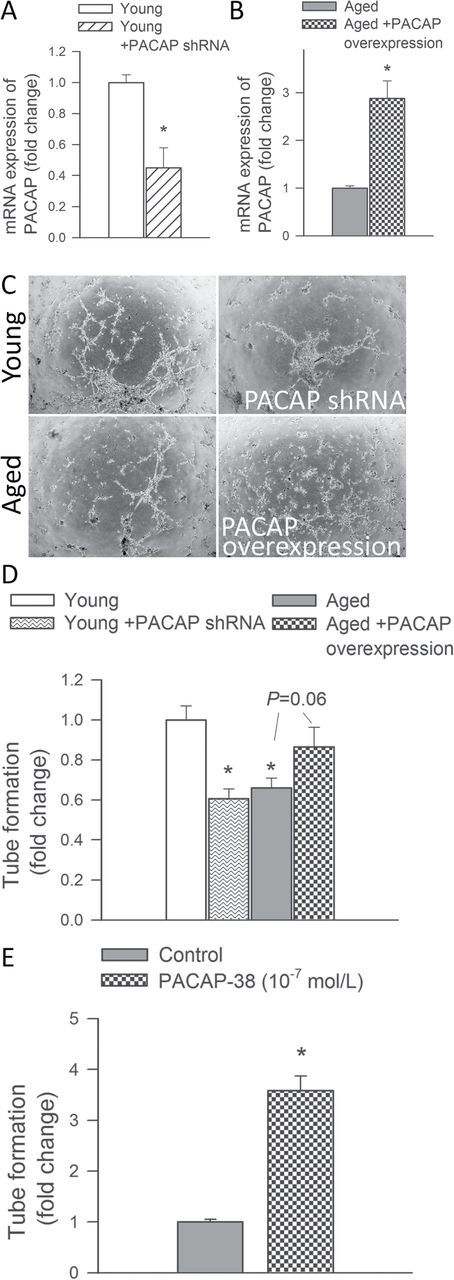
PACAP increases angiogenic capacity in cerebromicrovascular endothelial cells (CMVECs). (A‒B) Quantitative real time RT‒PCR data showing mRNA expression of PACAP in young CMVECs with or without transfection with PACAP shRNA and aged CMVECs with or without overexpression of PACAP. (C) The ability to form capillary-like structures by young CMVECs with or without shRNA knockdown of PACAP and aged CMVECs with or without overexpression of PACAP was assessed. Representative examples of capillary-like structures are shown on Panel C. (D) Summary data, expressed as relative changes in total tube length per area scanned, are shown in Panel D. (E) Changes in angiogenic capacity in CMVECs induced by PACAP-38 (10–7mol/l) treatment. Data are means ± standard error of the mean (n = 5 in each group), *p < .05 vs. respective control.
Effects of PACAP on Adhesion of CMVECs to Collagen
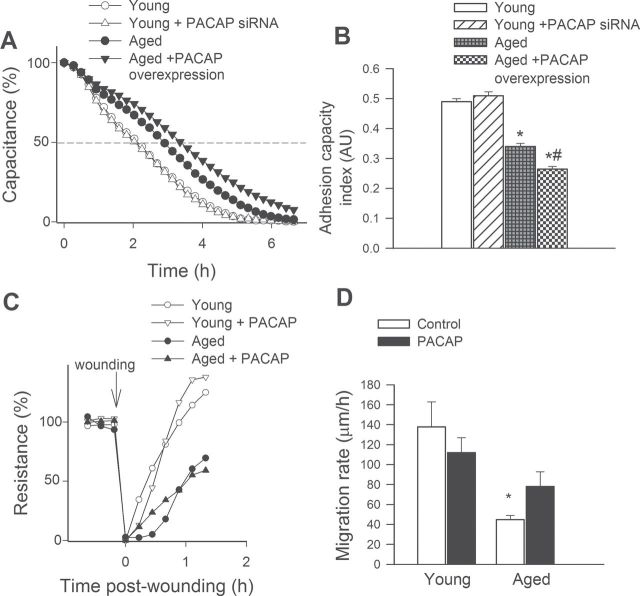
Endothelial cell adhesion events are known to have an important role in angiogenesis. We used ECIS technology to monitor changes of capacitance (at 60kHz) due to the adhesion of cells to the collagen-coated active electrode (Figure 3A). The inverse of the time constant (τ), calculated from an exponential curve fitting, was used as an index of adhesiveness. Aged CMVECs exhibited impaired adhesiveness to collagen (shown as an increase in the time needed to reach 50% cell adhesion) as compared to young cells. Knockdown of PACAP in young CMVECs was without effect on endothelial adhesiveness (Figure 3A and B). Overexpression of PACAP in aged CMVECs (Figure 3A and B) or treatment with PACAP-38 (not shown) decreased endothelial adhesiveness to collagen.
Figure 3.
Effects of PACAP on adhesion capacity and migration capacity of cerebromicrovascular endothelial cells (CMVECs). (A) In young CMVECs with or without transfection with PACAP shRNA and aged CMVECs with or without overexpression of PACAP adhesion to collagen was monitored by electric cell-substrate impedance sensing (ECIS) technology (see Methods section). Time course of changes of capacitance (at 32kHz) after addition of CMVECs to collagen-coated wells is depicted in Panel A. An 100% change corresponds to the maximum level of cell coverage reached on the active electrode. We calculated the time constant from each individual dataset, the inverse of which was used as an index of adhesiveness. (B) The summary data for cell adhesion index in CMVECs from each experimental group. Data are means ± standard error of the mean (n = 5 in each group). *p < .05 vs. young control, # p < .05 vs. aged control. (C) Cell migration was monitored by electric cell-substrate impedance sensing (ECIS) technology in a wound-healing assay (see Methods section). The time course of resistance recovery after wounding (electric pulse of 5 mA for 20 seconds at 60kHz; 100% represents prewounding levels) in cultures of young and aged CMVECs with or without treatment with PACAP-38 is cleary shown. Time to reach 50% resistance recovery (corresponding to 50% confluence on the active electrode) was determined for each group and this parameter and the known physical dimensions of the electrode were used to calculate the migration rate (expressed as µm/h). (D) The summary data for migration rate in each group. Data are means ± S.E.M. (n = 5 in each group), *p < .05 vs. young control.
Effect of PACAP on the Migratory Capability of CMVECs
The migratory capability of vascular endothelial cells has a pivotal role in the maintenance of microvascular integrity and angiogenesis. An ECIS-based wound-healing assay was used to assess the effect of PACAP on migratory capability of CMVECs. We found that aged CMVECs exhibited impaired migratory capability as compared to young CMVECs (Figure 3C and D). The PACAP treatment was without effect on migratory capability of young CMVECs (Figure 3C and D). Treatment with PACAP tended to decrease the time for aged CMVECs to reach 50% of the maximum confluence (Figure 3C). Figure 3D indicates that the increase in the calculated migration rate in aged CMVECs with PACAP treatment did not reach statistical significance.
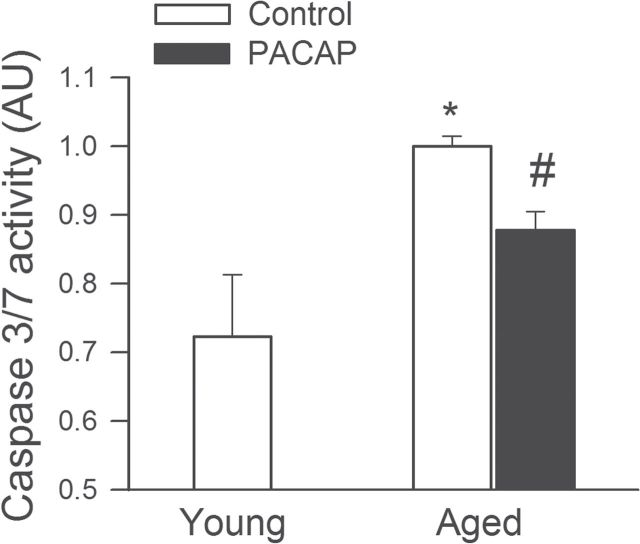
PACAP Inhibits Programmed Cell Death in Aged CMVECs
Induction of endothelial apoptosis is an important mechanism that inhibits angiogenesis promoting microvascular rarefaction. We found that in CMVECs derived from aged rats apoptosis was increased (Figure 4). The PACAP-38 significantly inhibited endothelial apoptosis as shown by the decreased caspase3/7 activity, restoring it to levels observed in young cells (Figure 4).
Figure 4.
PACAP significantly inhibits apoptosis in aged cerebromicrovascular endothelial cells (CMVECs). Apoptotic cell death was assessed by measuring caspase 3/7 activity in cell lysates. *p < .05 vs. control, # p < .05 vs. aged. Data are mean ± S.E.M. (n = 5 for each group).
Effect of PACAP on Expression of VEGF and VEGFR2
Quantitative real time RT‒PCR technique was performed to elucidate the effect of PACAP on the expression of factors involved in VEGF signaling. Neither overexpression of PACAP in CMVECs derived from aged rats (Figure 5A) nor downregulation of the peptide in young cells influenced the expression of VEGF (not shown). The mRNA expression of VEGFR2, the receptor which is mainly responsible for mediating the proangiogenic effects of VEGF, was upregulated by increased expression of PACAP in aged cells (Figure 5B).
Figure 5.
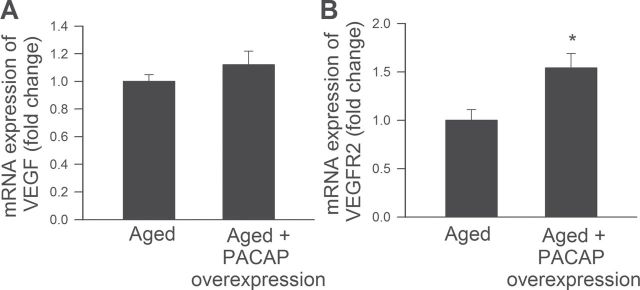
Quantitative real time RT‒PCR data showing the effect of overexpression of PACAP on mRNA expression of VEGF (A) and VEGFR2 (B) in aged cerebromicrovascular endothelial cells (CMVECs). Data are means ± standard error of the mean (n = 5 in each group), *p < .05 vs. control.
Effect of PACAP on Cellular and Mitochondrial ROS Production in CMVECs
Aging is associated with increased cellular ROS production (46), which contributes to cerebromicrovascular endothelial dysfunction (47). As shown by the DHE and MitoSox fluorescence-based measurements, PACAP-38 treatment exerted no significant effect on cellular and mitochondrial ROS production in CMVECs (Figure 6).
Figure 6.
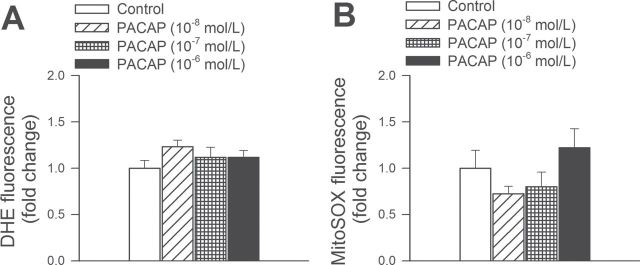
PACAP decreases oxidative stress in aged cerebromicrovascular endothelial cells (CMVECs). (A and B) Flow cytometric analysis of DHE- (A) and MitoSox- (B) fluorescence (indicating cellular and mitochondrial ROS production, respectively) in primary CMVECs derived from aged Fischer 344 x Brown Norway rats. The effect of PACAP treatment (10–8 to 10–6 mol/l, for 24h) on ROS production by aged CMVECs. Data are mean ± standard error of the mean (n = 8 in each group).
Discussion
This is the first study to demonstrate the protective effects of PACAP on function and phenotype of CMVECs derived from a rodent model of aging that recapitulates cerebrovascular alterations and deficits present in elderly humans at risk for vascular coginitive impairment. There is increasing evidence in support of the hypothesis that the composition of the age-associated vascular secretory phenotype is a key determinant in the development of age-related diseases. In support of this hypothesis previous studies demonstrate that aging in endothelial cells is associated with a proinflammatory shift in cytokine expression profile (48) and alterations in the secretion of trophic factors and autocrine/paracrine growth factors (for example, BDNF, TGFβ). Recent studies demonstrate that CMVECs are an important source of the trophic factor PACAP (18), which is known to regulate the function of a range of cell types in the brain. Here, we demonstrate that aging leads to downregulation of PACAP in CMVECs (Figure 1), extending earlier findings showing that secretion of PACAP by cerebral microvessels significantly declines with age (18).
We found that CMVECs express both PACAP and PACAP receptors (Figure 1). The findings that knockdown of PACAP in young CMVECs impairs capillary morphogenesis and overexpression of PACAP or treatment with PACAP improves the ability of aged CMVECs to form capillary-like structures (Figure 2) suggest that dysfunction of a PACAP/PACAP receptor autocrine system contributes to the age-related impairment of endothelial angiogenic capacity. Although the proangiogenic effects of PACAP are strongly supported by observations in tumor models [reviewed in ref. (24)], further studies are needed to determine whether genetic depletion of PACAP results in cerebromicrovascular rarefaction in mice, mimicking the aging phenotype. Future studies should also determine whether restoration of PACAP signaling in aging can improve endothelial angiogenic capacity in vivo, increasing cerebromicrovascular density. Interestingly, not every step (for example, adhesion and migration) in the multistep process of angiogenesis appears to be regulated by PACAP (Figure 3), suggesting that its regulatory role is different from that of VEGF and other proangiogenic growth factors.
Several lines of evidence indicate that endothelial cell apoptosis may play a major role in regulation of angiogenesis [reviewed in Ref. (49)]. Previously aging was shown to be associated with significantly increased endothelial apoptosis (50–52). Counteracting proliferation, age-related excessive apoptosis likely limits angiogenesis and may actively lead to regression of cerebral microvessels (49). Growth factors, which confer antiaging vascular effects, not only stimulate endothelial cell proliferation and migration but also concomitantly inhibit endothelial cell apoptosis (49). In agreement with its putative role as a proangiogenic and antiaging autocrine mediator, PACAP was found to attenuate apoptotic cell death in aged CMVECs (Figure 4). Previous studies also demonstrate that PACAP inhibits H2O2-induced apoptosis in young endothelial cells (23). The antiapoptotic action of PACAP has also been demonstrated in several other cell types, including human hemangioendothelioma cells, kidney cells, and retinal pigment epithelial cells, and attributed to phosphorylation of the antiapoptotic ERK1/2 and/or decreases the proapoptotic activation of JNK and p38MAPK (23,53‒55). In the present study, we show that overexpression of PACAP upregulates the expression of VEGFR2 in aged CMVECs, suggesting that a crosstalk between autocrine PACAP and VEGF signaling may contribute to the proangiogenic and antiapoptotic effects of PACAP (Figure 5). This concept is supported by previous studies in a variety of cell types showing that PACAP/VIP and VEGF signaling are functionally linked (56–59). Further studies are warranted to experimentally test this hypothesis in CMVECs. Increased oxidative stress was suggested to contribute to impairment of endothelial angiogenic capacity, endothelial apoptosis, and thereby to capillary rarefaction (60). Although previous studies suggested that PACAP may increase cellular resistance against oxidative stress (14,55), in this study PACAP treatment had no effect on increased ROS production in aged CMVECs (Figure 6).
Findings from previous investigations suggest that in addition to regulation of angiogenesis PACAP also regulates other aspects of cerebromicrovascular endothelial function. Accordingly, we have recently provided evidence that PACAP increases transendothelial electrical resistance and reduces disassembly of tight and adherens junctions in pathological conditions (20). Because there is evidence that aging is associated with dysregulation of tight junctions and disruption of the blood–brain barrier (61–63), further studies are warranted to elucidate the role of PACAP deficiency in these alterations. In addition to its autocrine endothelial effect, endothelium-derived PACAP is also likely to cross the blood–brain barrier and regulate the function of neurons (9,10,12,13), pericytes (64), astrocytes (65,66), and microglia (67) as well. The PACAP receptors are widely distributed in the central nervous system, including the hypothalamus, cerebral cortex, hippocampus, amygdala, substantia nigra, dentate gyrus, cerebellum, and pons (7,68‒70) and its neuromodulator and neurotrophic actions have been well characterized [reviewed in Ref. (9)]. Because PACAP was shown to exert multifaceted neuroprotective actions in several neuropathological disorders (9,71‒74), further studies are warranted to elucidate the role of impaired microvascular production of PACAP in development of age-related neurodegenerative diseases.
The PACAP and VIP belong to the same superfamily of neuropeptides which exert their effects by activating G-protein-coupled receptors. Importantly, VIP participates in the pathophysiology of several neurological disorders, inhibiting cell death and confering anti-inflammatory and neuroprotective effects. Because microvascular endothelial cells express PACAP, VIP, and PACAP receptors (PAC1R, VPACR-1, and VPACR-2), the existence of a complex microvascular autocrine regulatory mechanism seems to be very likely. Because there are data suggesting that aging may suppress VIP expression in the brain, age-related decline in PACAP and VIP may exert synergistic effects (75–77).
In conclusion, autocrine PACAP activates endogenous proangiogenic and antiapoptotic mechanisms in CMVECs. We propose that dysregulation of PACAP signaling contributes to aging-induced impairment of angiogenic capacity of CMVECs, which may contribute to cerebromicrovascular rarefaction in aging. Aging-induced phenotypic alterations of CMVECs were also shown to contribute to neurovascular uncoupling (78), disruption of the blood–brain barrier (79), and neuroinflammation (62), which are thought to contribute to the development of both vascular cognitive impairment and Alzheimer’s disease (79–81). Further studies are warranted to determine whether autocrine PACAP signaling also exerts protective effects against the aforementioned aging-induced phenotypic and functional endothelial alterations as well.
Funding
This work was supported by grants from the American Heart Association (to ZT, PT, ST, AC, and ZU), the National Center for Complementary and Alternative Medicine (R01-AT006526 to ZU), the National Institute on Aging (AG031085 to AC; AG038747 to WES), the National Institute of Neurological Disorders and Stroke (R01- NS056218 to AC, and WES), the Oklahoma Center for the Advancement of Science and Technology (to AC, ZU, WES), Hungarian Scientific Research Fund (OTKA- K104984 to DR), and the NemzetiFejlesztésiÜgynökség (Developing Competitiveness of Universities in the South Transdanubian Region, “Identification of new biomarkers, ” and “Comparative studies on Helix pomatia, Myrmecophaga tridactyla, and Chelonoidis nigra ” (SROP-4.2.2.A-11/1/KONV-2012-0017), and “Complex examination of neuropeptides” (SROP-4.2.2.A-11/1/KONV-2012-0024 to AK and DR), “PTE-MTA Lendület Program,” and Hungarian Brain Research Program (KTIA_13_NAP-A-III/4 to BE, TA, and DR), the Arimura Foundation (to AT and DR), and the Ellison Medical Foundation (to WES).
Acknowledgments
The authors acknowledge the inspiration from early studies by Mr. Artúr Görgey (82). The authors would like to express their gratitude for the support of the Donald W. Reynolds Foundation, which funds aging research at the University of Oklahoma Health Sciences Center under its Aging and Quality of Life Program.
References
- 1. Murugesan N, Demarest TG, Madri JA, Pachter JS. Brain regional angiogenic potential at the neurovascular unit during normal aging. Neurobiol Aging. 2012;33:1004.e1–1004.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Riddle DR, Sonntag WE, Lichtenwalner RJ. Microvascular plasticity in aging. Ageing Res Rev. 2003;2:149–168. [DOI] [PubMed] [Google Scholar]
- 3. Sonntag WE, Deak F, Ashpole N, et al. Insulin-like growth factor-1 in CNS and cerebrovascular aging. Front Aging Neurosci. 2013;5:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shen Q, Wang Y, Kokovay E, et al. Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions. Cell Stem Cell. 2008;3:289–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ungvari Z, Tucsek Z, Sosnowska D, et al. Aging-induced dysregulation of dicer1-dependent microRNA expression impairs angiogenic capacity of rat cerebromicrovascular endothelial cells. J Gerontol A BiolSci Med Sci. 2013;68:877–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Miyata A, Arimura A, Dahl RR, et al. Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylatecyclase in pituitary cells. Biochem Biophys Res Commun.1989;164:567–574. [DOI] [PubMed] [Google Scholar]
- 7. Arimura A, Somogyvári-Vigh A, Miyata A, Mizuno K, Coy DH, Kitada C.Tissue distribution of PACAP as determined by RIA: highly abundant in the rat brain and testes. Endocrinology. 1991;129:2787–2789. [DOI] [PubMed] [Google Scholar]
- 8. Vaudry D, Falluel-Morel A, Bourgault S, et al. Pituitary adenylatecyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol Rev. 2009;61:283–357. [DOI] [PubMed] [Google Scholar]
- 9. Reglodi D, Kiss P, Lubics A, Tamas A.Review on the protective effects of PACAP in models of neurodegenerative diseases in vitro and in vivo. Curr Pharm Des. 2011;17:962–972. [DOI] [PubMed] [Google Scholar]
- 10. Reglodi D, Kiss P, Szabadfi K, et al. PACAP is an endogenous protective factor-insights from PACAP-deficient mice. J Mol Neurosci. 2012;48:482–492. [DOI] [PubMed] [Google Scholar]
- 11. Sauvage M, Brabet P, Holsboer F, Bockaert J, Steckler T. Mild deficits in mice lacking pituitary adenylatecyclase-activating polypeptide receptor type 1 (PAC1) performing on memory tasks. Brain Res Mol Brain Res. 2000;84:79–89. [DOI] [PubMed] [Google Scholar]
- 12. Otto C, Kovalchuk Y, Wolfer DP, et al. Impairment of mossy fiber long-term potentiation and associative learning in pituitary adenylatecyclase activating polypeptide type I receptor-deficient mice. J Neurosci. 2001;21:5520–5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Matsuyama S, Matsumoto A, Hashimoto H, Shintani N, Baba A. Impaired long-term potentiation in vivo in the dentate gyrus of pituitary adenylatecyclase-activating polypeptide (PACAP) or PACAP type 1 receptor-mutant mice. Neuroreport. 2003;14:2095–2098. [DOI] [PubMed] [Google Scholar]
- 14. Ohtaki H, Satoh A, Nakamachi T, et al. Regulation of oxidative stress by pituitary adenylatecyclase-activating polypeptide (PACAP) mediated by PACAP receptor. J Mol Neurosci. 2010;42:397–403. [DOI] [PubMed] [Google Scholar]
- 15. Szabadfi K, Kiss P, Reglodi D, et al. Differences between wild type and PACAP KO mice in retinal aging. J Mol Neurosci. 2013;51(Suppl 1):225–226.23606220 [Google Scholar]
- 16. Pirger Z, Nemeth J, Hiripi L, et al. PACAP has anti-apoptotic effect in the salivary gland of an invertebrate species, Helix pomatia. J Mol Neurosci. 2008;36:105–114. [DOI] [PubMed] [Google Scholar]
- 17. Pirger Z, Naskar S, László Z, Kemenes G, Reglődi D, Kemenes I.Reversal of age related learning deficiency by the vertebrate pituitary adenylatecyclase activating polypeptide (PACAP) and insulin-like growth factor-1 (IGF-1) in a novel invertebrate model of aging: the pond snail (Lymnaea stagnalis). J Gerontol Biol Med Sci. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tripathy D, Sanchez A, Yin X, Martinez J, Grammas P.Age-related decrease in cerebrovascular-derived neuroprotective proteins: effect of acetaminophen. Microvasc Res. 2012;84:278–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Baun M, Hay-Schmidt A, Edvinsson L, Olesen J, Jansen-Olesen I. Pharmacological characterization and expression of VIP and PACAP receptors in isolated cranial arteries of the rat. Eur J Pharmacol. 2011;670:186–194. [DOI] [PubMed] [Google Scholar]
- 20. Wilhelm I, Fazakas C, Tamas A, Toth G, Reglodi D, Krizbai IA.PACAP enhances barrier properties of cerebral microvessels. J MolNeurosci. In press. PMID: 24614973. [DOI] [PubMed] [Google Scholar]
- 21. Lenti L, Zimmermann A, Kis D, et al. PACAP and VIP differentially preserve neurovascular reactivity after global cerebral ischemia in newborn pigs. Brain Res. 2009;1283:50–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Warren JB, Donnelly LE, Cullen S, et al. Pituitary adenylatecyclase-activating polypeptide: a novel, long-lasting, endothelium-independent vasorelaxant. Eur J Pharmacol. 1991;197:131–134. [DOI] [PubMed] [Google Scholar]
- 23. Rácz B, Gasz B, Borsiczky B, et al. Protective effects of pituitary adenylatecyclase activating polypeptide in endothelial cells against oxidative stress-induced apoptosis. Gen Comp Endocrinol. 2007;153:115–123. [DOI] [PubMed] [Google Scholar]
- 24. Ribatti D, Conconi MT, Nussdorfer GG. Nonclassic endogenous novel [corrected] regulators of angiogenesis. Pharmacol Rev. 2007;59:185–205. [DOI] [PubMed] [Google Scholar]
- 25. Ungvari Z, Podlutsky A, Sosnowska D, et al. Ionizing radiation promotes the acquisition of a senescence-associated secretory phenotype and impairs angiogenic capacity in cerebromicrovascular endothelial cells: role of increased DNA damage and decreased DNA repair capacity in microvascularradiosensitivity. J Gerontol A BiolSci Med Sci. 2013;68:1443–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Toth P, Csiszar A, Tucsek Z, et al. Role of 20-HETE, TRPC channels, and BKCa in dysregulation of pressure-induced Ca2+ signaling and myogenic constriction of cerebral arteries in aged hypertensive mice. Am J Physiol Heart Circ Physiol. 2013;305:H1698–H1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Csiszar A, Ungvari Z, Edwards JG, et al. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res. 2002;90:1159–1166. [DOI] [PubMed] [Google Scholar]
- 28. Csiszar A, Smith K, Labinskyy N, Orosz Z, Rivera A, Ungvari Z.Resveratrol attenuates TNF-{alpha}-induced activation of coronary arterial endothelial cells: role of NF-{kappa}B inhibition. Am J Physiol. 2006;291:H1694–1699. [DOI] [PubMed] [Google Scholar]
- 29. Csiszar A, Ahmad M, Smith KE, et al. Bone morphogenetic protein-2 induces proinflammatory endothelial phenotype. Am J Pathol. 2006;168:629–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Csiszar A, Labinskyy N, Jimenez R, et al. Anti-oxidative and anti-inflammatory vasoprotective effects of caloric restriction in aging: role of circulating factors and SIRT1. Mech Ageing Dev. 2009;130:518–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ungvari Z, Bagi Z, Feher A, et al. Resveratrol confers endothelial protection via activation of the antioxidant transcription factor Nrf2. Am J Physiol Heart Circ Physiol. 2010;299:H18–H24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ungvari Z, Bailey-Downs L, Gautam T, et al. Adaptive induction of NF-E2-related factor-2-driven antioxidant genes in endothelial cells in response to hyperglycemia. Am J Physiol Heart Circ Physiol. 2011;300:H1133–H1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Valcarcel-Ares MN, Gautam T, Warrington JP, et al. Disruption of Nrf2 signaling impairs angiogenic capacity of endothelial cells: implications for microvascular aging. J Gerontol A Biol Sci Med Sci. 2012;67:821–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Csiszar A, Sosnowska D, Tucsek Z, et al. Circulating factors induced by caloric restriction in the nonhuman primate Macacamulatta activate angiogenic processes in endothelial cells. J Gerontol A Biol Sci Med Sci. 2013;68:235–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Clapp C, Thebault S, Jeziorski MC, Martínez De La Escalera G. Peptide hormone regulation of angiogenesis. Physiol Rev. 2009;89:1177–1215. [DOI] [PubMed] [Google Scholar]
- 36. Ungvari Z, Ridgway I, Philipp EE, et al. Extreme longevity Is associated with increased resistance to oxidative stress in Arctica islandica, the longest-living non-colonial animal. J Gerontol A Biol Sci Med Sci. 2011;66:741–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bailey-Downs LC, Sosnowska D, Toth P, et al. Growth hormone and IGF-1 deficiency exacerbate high-fat diet-induced endothelial impairment in obese Lewis dwarf rats: implications for vascular aging. J Gerontol A Biol Sci Med Sci. 2012;67:553–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Csiszar A, Podlutsky A, Podlutskaya N, et al. Testing the oxidative stress hypothesis of aging in primate fibroblasts: is there a correlation between species longevity and cellular ROS production? J Gerontol A Biol Sci Med Sci. 2012;67:841–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ungvari Z, Sosnowska D, Podlutsky A, Koncz P, Sonntag WE, Csiszar A.Free radical production, antioxidant capacity, and oxidative stress response signatures in fibroblasts from lewis dwarf rats: effects of life span-extending peripubertal GH treatment. J Gerontol A Biol Sci Med Sci. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Csiszar A, Pinto JT, Gautam T, et al. Resveratrol encapsulated in novel fusogenic liposomes activates Nrf2 and attenuates oxidative stress in cerebromicrovascular endothelial cells from aged rats. J Gerontol A Biol Sci Med Sci. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bailey-Downs LC, Mitschelen M, Sosnowska D, et al. Liver-specific knockdown of IGF-1 decreases vascular oxidative stress resistance by impairing the Nrf2-dependent antioxidant response: a novel model of vascular aging. J Gerontol A Biol Sci Med Sci. 2012;67:313–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bailey-Downs LC, Tucsek Z, Toth P, et al. Aging exacerbates obesity-induced oxidative stress and inflammation in perivascular adipose tissue in mice: a paracrine mechanism contributing to vascular redox dysregulation and inflammation. J Gerontol A Biol Sci Med Sci. 2013;68:780–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ungvari Z, Orosz Z, Labinskyy N, et al. Increased mitochondrial H2O2 production promotes endothelial NF-kappaB activation in aged rat arteries. Am J Physiol Heart Circ Physiol. 2007;293:H37–H47. [DOI] [PubMed] [Google Scholar]
- 44. Ungvari Z, Labinskyy N, Mukhopadhyay P, et al. Resveratrol attenuates mitochondrial oxidative stress in coronary arterial endothelial cells. Am J Physiol Heart Circ Physiol. 2009;297:H1876–H1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Csiszar A, Sosnowska D, Wang M, Lakatta EG, Sonntag WE, Ungvari Z. Age-associated proinflammatory secretory phenotype in vascular smooth muscle cells from the non-human primate Macaca mulatta: reversal by resveratrol treatment. J Gerontol A Biol Sci Med Sci. 2012;67:811–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Marzetti E, Csiszar A, Dutta D, Balagopal G, Calvani R, Leeuwenburgh C. Role of mitochondrial dysfunction and altered autophagy in cardiovascular aging and disease: from mechanisms to therapeutics. Am J Physiol Heart Circ Physiol. 2013;305:H459–H476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Toth P, Tarantini S, Tucsek Z, et al. Resveratrol treatment rescues neurovascular coupling in aged mice: role of improved cerebromicrovascular endothelial function and downregulation of NADPH oxidase. Am J Physiol Heart Circ Physiol. 2014;306:H299–H308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G. Aging-induced proinflammatory shift in cytokine expression profile in coronary arteries. FASEB J. 2003;17:1183–1185. [DOI] [PubMed] [Google Scholar]
- 49. Dimmeler S, Zeiher AM. Endothelial cell apoptosis in angiogenesis and vessel regression. Circ Res. 2000;87:434–439. [DOI] [PubMed] [Google Scholar]
- 50. Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G. Proinflammatory phenotype of coronary arteries promotes endothelial apoptosis in aging. Physiol Genomics. 2004;17:21–30. [DOI] [PubMed] [Google Scholar]
- 51. Hoffmann J, Haendeler J, Aicher A, et al. Aging enhances the sensitivity of endothelial cells toward apoptotic stimuli: important role of nitric oxide. Circ Res. 2001;89:709–715. [DOI] [PubMed] [Google Scholar]
- 52. Tucsek Z, Gautam T, Sonntag WE, et al. Aging exacerbates microvascular endothelial damage induced by circulating factors present in the serum of septic patients. J Gerontol A Biol Sci Med Sci. 2013;68:652–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rácz B, Gallyas F, Jr, Kiss P, et al. The neuroprotective effects of PACAP in monosodium glutamate-induced retinal lesion involve inhibition of proapoptotic signaling pathways. Regul Pept. 2006;137:20–26. [DOI] [PubMed] [Google Scholar]
- 54. Horvath G, Brubel R, Kovacs K, et al. Effects of PACAP on oxidative stress-induced cell death in rat kidney and human hepatocyte cells. J MolNeurosci. 2011;43:67–75. [DOI] [PubMed] [Google Scholar]
- 55. Mester L, Kovacs K, Racz B, et al. Pituitary adenylate cyclase-activating polypeptide is protective against oxidative stress in human retinal pigment epithelial cells. J Mol Neurosci. 2011;43:35–43. [DOI] [PubMed] [Google Scholar]
- 56. Collado B, Gutiérrez-Cañas I, Rodríguez-Henche N, Prieto JC, Carmena MJ.Vasoactive intestinal peptide increases vascular endothelial growth factor expression and neuroendocrine differentiation in human prostate cancer LNCaP cells. Regul Pept. 2004;119:69–75. [DOI] [PubMed] [Google Scholar]
- 57. Casibang M, Purdom S, Jakowlew S, et al. Prostaglandin E2 and vasoactive intestinal peptide increase vascular endothelial cell growth factor mRNAs in lung cancer cells. Lung Cancer. 2001;31:203–212. [DOI] [PubMed] [Google Scholar]
- 58. Moody TW, Leyton J, Casibang M, Pisegna J, Jensen RT. PACAP-27 tyrosine phosphorylates mitogen activated protein kinase and increases VEGF mRNAs in human lung cancer cells. Regul Pept. 2002;109:135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gloddek J, Pagotto U, PaezPereda M, Arzt E, Stalla GK, Renner U. Pituitary adenylate cyclase-activating polypeptide, interleukin-6 and glucocorticoids regulate the release of vascular endothelial growth factor in pituitary folliculostellate cells. J Endocrinol. 1999;160:483–490. [DOI] [PubMed] [Google Scholar]
- 60. Benndorf RA, Schwedhelm E, Gnann A, et al. Isoprostanes inhibit vascular endothelial growth factor-induced endothelial cell migration, tube formation, and cardiac vessel sprouting in vitro, as well as angiogenesis in vivo via activation of the thromboxane A(2) receptor: a potential link between oxidative stress and impaired angiogenesis. Circ Res. 2008;103:1037–1046. [DOI] [PubMed] [Google Scholar]
- 61. Zlokovic BV.The blood–brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. [DOI] [PubMed] [Google Scholar]
- 62. Toth P, Tucsek Z, Sosnowska D, et al. Age-related autoregulatory dysfunction and cerebromicrovascular injury in mice with angiotensin II-induced hypertension. J Cereb Blood Flow Metab. 2013;33:1732–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tucsek Z, Toth P, Sosnowska D, et al. Obesity in aging exacerbates blood brain barrier disruption, neuroinflammation and oxidative stress in the mouse hippocampus: effects on expression of genes involved in beta-amyloid generation and Alzheimer’s disease. J Gerontol Biol Med Sci. In press. PMID: 24269929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Markhotina N, Liu GJ, Martin DK.Contractility of retinal pericytes grown on silicone elastomer substrates is through a protein kinase A-mediated intracellular pathway in response to vasoactive peptides. IET Nanobiotechnol. 2007;1:44–51. [DOI] [PubMed] [Google Scholar]
- 65. Tatsuno I, Morio H, Tanaka T, et al. Astrocytes are one of the main target cells for pituitary adenylate cyclase-activating polypeptide in the central nervous system. Astrocytes are very heterogeneous regarding both basal movement of intracellular free calcium ([Ca2+]i) and the [Ca2+]i response to PACAP at a single cell level. Ann N Y Acad Sci. 1996;805:613–619. [DOI] [PubMed] [Google Scholar]
- 66. Masmoudi-Kouki O, Douiri S, Hamdi Y, et al. Pituitary adenylate cyclase-activating polypeptide protects astroglial cells against oxidative stress-induced apoptosis. J Neurochem. 2011;117:403–411. [DOI] [PubMed] [Google Scholar]
- 67. Yang S, Yang J, Yang Z, et al. Pituitary adenylate cyclase-activating polypeptide (PACAP) 38 and PACAP4-6 are neuroprotective through inhibition of NADPH oxidase: potent regulators of microglia-mediated oxidative stress. J Pharmacol Exp Ther. 2006;319:595–603. [DOI] [PubMed] [Google Scholar]
- 68. Köves K, Arimura A, Görcs TG, Somogyvári-Vigh A. Comparative distribution of immunoreactive pituitary adenylatecyclase activating polypeptide and vasoactive intestinal polypeptide in rat forebrain. Neuroendocrinology. 1991;54:159–169. [DOI] [PubMed] [Google Scholar]
- 69. Vigh S, Arimura A, Köves K, Somogyvári-Vigh A, Sitton J, Fermin CD. Immunohistochemical localization of the neuropeptide, pituitary adenylatecyclase activating polypeptide (PACAP), in human and primate hypothalamus. Peptides. 1991;12:313–318. [DOI] [PubMed] [Google Scholar]
- 70. Ghatei MA, Takahashi K, Suzuki Y, Gardiner J, Jones PM, Bloom SR. Distribution, molecular characterization of pituitary adenylatecyclase-activating polypeptide and its precursor encoding messenger RNA in human and rat tissues. J Endocrinol. 1993;136:159–166. [DOI] [PubMed] [Google Scholar]
- 71. Atlasz T, Babai N, Kiss P, et al. Pituitary adenylatecyclase activating polypeptide is protective in bilateral carotid occlusion-induced retinal lesion in rats. Gen Comp Endocrinol. 2007;153:108–114. [DOI] [PubMed] [Google Scholar]
- 72. Rat D, Schmitt U, Tippmann F, et al. Neuropeptide pituitary adenylatecyclase-activating polypeptide (PACAP) slows down Alzheimer’s disease-like pathology in amyloid precursor protein-transgenic mice. FASEB J. 2011;25:3208–3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Brown D, Tamas A, Reglödi D, Tizabi Y. PACAP protects against salsolinol-induced toxicity in dopaminergic SH-SY5Y cells: implication for Parkinson’s disease. J Mol Neurosci. 2013;50:600–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Dogrukol-Ak D, Kumar VB, Ryerse JS, et al. Isolation of peptide transport system-6 from brain endothelial cells: therapeutic effects with antisense inhibition in Alzheimer and stroke models. J Cereb Blood Flow Metab. 2009;29:411–422. [DOI] [PubMed] [Google Scholar]
- 75. Duncan MJ, Herron JM, Hill SA. Aging selectively suppresses vasoactive intestinal peptide messenger RNA expression in the suprachiasmatic nucleus of the Syrian hamster. Brain Res Mol Brain Res. 2001;87:196–203. [DOI] [PubMed] [Google Scholar]
- 76. Krajnak K, Kashon ML, Rosewell KL, Wise PM. Aging alters the rhythmic expression of vasoactive intestinal polypeptide mRNA but not arginine vasopressin mRNA in the suprachiasmatic nuclei of female rats. J Neurosci. 1998;18:4767–4774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Gozes I, Schächter P, Shani Y, Giladi E. Vasoactive intestinal peptide gene expression from embryos to aging rats. Neuroendocrinology. 1988;47:27–31. [DOI] [PubMed] [Google Scholar]
- 78. Park L, Anrather J, Girouard H, Zhou P, Iadecola C. Nox2-derived reactive oxygen species mediate neurovascular dysregulation in the aging mouse brain. J Cereb Blood Flow Metab. 2007;27:1908–1918. [DOI] [PubMed] [Google Scholar]
- 79. Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci. 2011;12:723–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Lin AL, Zheng W, Halloran JJ, et al. Chronic rapamycin restores brain vascular integrity and function through NO synthase activation and improves memory in symptomatic mice modeling Alzheimer’s disease. J Cereb Blood Flow Metab. 2013;33:1412–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Gorelick PB, Scuteri A, Black SE, et al. American Heart Association Stroke Council, Council on Epidemiology and Prevention, Council on Cardiovascular Nursing, Council on Cardiovascular Radiology and Intervention, and Council on Cardiovascular Surgery and Anesthesia.Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:2672–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Görgey A. Uber die festen, fluchtigen, fetten Saueren des Cocusnussoles.Sitzungsberichte der mathematisch-naturwissenschaftlichen Classe der k Akademie der Wissenschaften in Wien Vienna: Akademie der Wissenschaften in Wien; 1848:208–227.