Abstract
Background.
Results of prospective studies examining the association between cystatin C and incident cognitive impairment have been inconsistent. We tested the hypothesis that there is a U-shaped association in older women between cystatin C and risk of incident cognitive impairment 10 years later.
Methods.
We conducted a longitudinal analysis of a prospective cohort of 1,332 community-dwelling elderly women without dementia at baseline who had baseline cystatin C and serum creatinine measurements and completed an extended cognitive battery of neuropsychological tests with determination of cognitive status 10 years later. Incident cognitive impairment was defined as either new onset of adjudicated diagnosis of mild cognitive impairment or dementia.
Results.
Incident mild cognitive impairment or dementia was identified among 140 (26.0%) women in quartile 1 (Q1), 122 (22.6%) in Q2, 121 (22.5%) in Q3, and 156 (28.9%) in Q4 of cystatin C. In the fully adjusted model, compared to women in Q2–Q3 of cystatin C, adjusted odds ratios (95% CI) for incident cognitive impairment were 1.31 (0.98–1.75) for Q1, and 1.25 (0.94–1.66) for Q4 Compared to women in Q2–Q3 of estimated glomerular filtration rate (eGFRCysC), adjusted odds ratios (95% CI) for incident cognitive impairment after 10 years of follow-up were 1.18 (0.88–1.58) for Q4 (eGFRCysC 76.1–109.4mL/min/1.73 m2) and 1.26 (0.94–1.67) for Q1 (eGFRCysC 21.8–55.5mL/min/1.73 m2).
Conclusions.
These results support a U-shaped association between cystatin C concentration and risk of cognitive impairment or dementia 10 years later, but the association is not independent of potential confounding factors.
Key Words: Cystatin C, Cognitive impairment, Aging.
Adults aged 80 years and older comprise the fastest growing subset of the U.S. population (1). Cognitive impairment is one of the costliest disabilities, making successful aging without cognitive impairment a paramount goal for society (2). Kidney disease is also highly prevalent in the elderly (3), and reduced renal function in older adults has been associated with increased risk of adverse health outcomes (4–6).
Several, but not all, studies have reported the association between poorer kidney function, as defined by creatinine-based estimates, and cognitive impairment in older adults (7–13). However, creatinine-based indices or renal function are often misleading in the elderly because muscle mass declines and creatinine metabolism changes with aging; these changes have a proportionally greater effect in women (13–15). Cystatin C, an inhibitor of cysteine proteinase present in all nucleated cells and less dependent on muscle mass than creatinine, may be a more accurate measurement of renal function (16,17). Although higher cystatin C indicative of poorer kidney function might have an adverse effect on cognition, there is also growing experimental evidence that cystatin C may have a neuroprotective role (18–21).
Epidemiologic studies have examined the association between cystatin C and cognition reported inconsistent results. Two studies in longitudinal cohorts of elderly men and women in the United States reported that higher cystatin C levels were independently associated with poor cognitive function and a greater risk of cognitive decline (22) and an increased risk of unsuccessful aging as defined by a cardiovascular disease event, cancer, Chronic Obstructive Pulmonary Disease, incident cognitive impairment, or difficulty performing activities of daily living (23). In contrast, lower levels of cystatin C were independently associated with higher risk of Alzheimer’s disease in the Uppsala Longitudinal Study of Adult Men (24). To examine the association between kidney function as assessed by serum cystatin C levels or estimated glomerular filtration rate (eGFR) and risk of subsequent cognitive impairment or dementia 10 years later in older women without dementia at the initial assessment, we measured cystatin C using frozen serum specimens from the SOF Year 10 exam in 1,332 surviving women who completed extended cognitive battery of neuropsychological tests and had their cognitive status determined at the SOF Year 20 exam (average 9.8 years between exams).
Methods
Participants
From September 1986 to October 1988, 9,704 women who were 65 years or older and able to walk unassisted were recruited for participation in the baseline examination of the prospective Study of Osteoporotic Fractures. Women were recruited from population-based listings in four areas of the United States: Baltimore, Maryland, Minneapolis, Minnesota, Portland, Oregon, and Monongahela Valley, Pennsylvania. Women who had undergone bilateral hip replacement and those who were not able to walk without assistance were excluded. An additional 662 older African-American women (mean [SD] age, 75.4 [5.1] years) were enrolled in the study at the Year 10 exam using the same eligibility criteria increasing the total number of enrolled participants to 10,366. All participants provided informed consent and the protocol was approved by the Institutional Review Board.
To determine whether higher cystatin C in older women is associated with a greater odds of subsequent cognitive impairment, we measured cystatin C using frozen serum specimens from the SOF Year 10 exam in 1,346 surviving women (mean age 77.7 years) who completed extended cognitive battery of neuropsychological tests and subsequently had their cognitive status determined at the SOF Year 20 Exam (average 9.8 years between exams). After women with cognitive impairment or dementia (Mini-Mental State Examination [MMSE] <20, self-reported dementia or Alzheimer’s disease, or on medications for Alzheimer’s dementia) at Year 10 exam (baseline exam for this analysis) were excluded, the cohort for this analysis was comprised of 1,332 women.
Assessment of Renal Function
Fasting morning blood was collected at the baseline examination and processed for serum which was stored at −70°C until thawed. Serum cystatin C assays were performed at the University of Minnesota Medical Center in 2010. Serum cystatin C concentrations were determined using a BN100 nephelometer (Dade Behring Inc., Deerfield, IL) using a particle-enhanced immunonepholometric assay (25) (assay range 0.23–8.00mg/L with inter-assay coefficient of variation [CV] of 4.0% at a level of 0.71mg/L and 3.1% at a level of 1.75mg/L [mean inter-assay CV 3.7%]) and then converted to standardized values traceable to a certified reference material (26). Serum creatinine was measured using the Roche 911 analyzer (Roche Diagnostic Corporation, Indianapolis, IN) using the Jaffe rate-blanked method calibrated with materials assayed by isotope-dilution mass spectrometry. Coefficient of variation was 4.0%. Cystatin-C based (eGFRcysC) and creatinine and cystatin C-based eGFR (eGFRCr+CysC) were computed using a Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation re-expressed for standardized cystatin C (27); eGFRCr was computed using a CKD-EPI equation (28). We used eGFRCysC as the primary measure of eGFR. In sensitivity analyses, we substituted eGFRCr+CysC (and eGFRCr) for eGFRCr+CysC (23).
Cognitive Testing
We used methods developed by Yaffe and colleagues (29) to define cognitive impairment. The MMSE (30), a test of global cognition, and a modified version of Trails B (31), a test of executive function, were administered at clinic visits including our study baseline (Year 10). At the Year 20 visit, an expanded neuropsychological test battery was administered to surviving women, which included Trails B, the Modified Mini-Mental State Examination (3MS), a 100-point extended version of the MMSE (32), the California Verbal Learning Test (CVLT) Short Form (33), Digit Span (from the Wechsler Adult Intelligence Scale-Revised) (34), and category and verbal fluency tests (35).
Cognitive impairment at the Year 20 exam was determined in a two-step process (29). First, women were screened for one or more of the following criteria: (i) score <88 on the 3MS; (ii) score <4 on the CVLT delayed recall; (iii) score ≥3.6 on the Informant Questionnaire on Cognitive Decline in the Elderly (36); (iv) previous dementia diagnosis; or (v) nursing home residence. The women who screened positive had their clinical cognitive status adjudicated and reviewed by a panel of clinical experts. The women who screened negative were considered normal. A diagnosis of dementia was made based on Diagnostic and Statistical Manual of Mental Disorders, 4th Edition criteria (37). Mild cognitive impairment (MCI) was diagnosed using a modified Petersen Criteria (38,39). Incident impairment was defined as either new onset of MCI or dementia.
Other Measures
At baseline (Year 10), information on education, age, health behaviors such as alcohol use, smoking, physical activity, and medical history, including self-reported history of physician diagnosis of stroke, diabetes mellitus, congestive heart failure, hypertension, cardiovascular disease, lung disease, kidney disease, and Alzheimer’s disease were collected. The 15-item Geriatric Depression Scale was administered (40). Functional status was determined by the modified version of the Stanford Health Assessment Questionnaire (41). Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters.
Statistical Analysis
Differences in baseline (Year 10 examination) characteristics according to quartiles of cystatin C were compared using chi-square tests for categorical variables, ANOVA for continuous variables with normal distributions, and Kruskal–Wallis tests for variables with skewed distributions.
We used logistic regression models to examine the association of baseline cystatin C with the odds of incident cognitive impairment. In primary analyses, we expressed the primary predictor using quartiles. Based on findings from earlier studies (24) suggesting the possibility of a nonlinear pattern between cystatin C and cognitive impairment, the referent group in these analyses was quartiles 2–3. We also performed a sensitivity analyses expressing cystatin C as a continuous variable and including a (cystatin C)2 quadratic term.
Similarly, estimated GFRCysC was expressed as quartiles with quartiles 2–3 serving as the referent group. We performed similar analyses for eGFRCr and eGFRCr+CysC.
Base models were adjusted for age and race. Multivariable models were further adjusted for variables that were associated with cystatin C quartiles at p <.05, and variables that were known or suspected confounders of the association between cystatin C and cognition (smoking, history of diabetes, history of cardiovascular disease, history of stroke). Analyses were performed using SAS version 9.2 (SAS Inc., Cary, NC).
Results
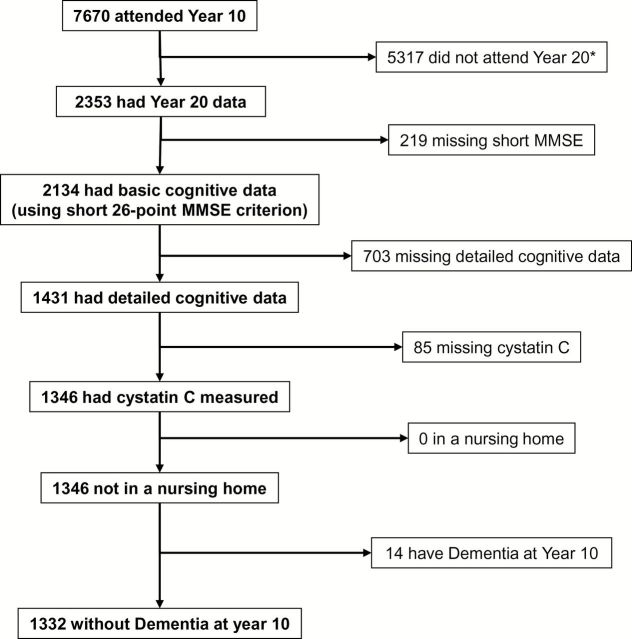
Of the 7,670 women who came to the Year 10 examination at the Minnesota, Oregon, and Pennsylvania sites, 2,353 completed at least the questionnaire component of the Year 20 examination. Of these women, 1,431 had detailed cognitive examination data at Year 20 examination, of whom 1,346 had serum available at the Year 10 examination for measurement of cystatin C. After 14 women were excluded because of prevalent cognitive impairment at Year 10, defined as MMSE score <20, self-reported dementia, or taking medications indicated for Alzheimer’s dementia, 1,332 women were included in the prospective analytical cohort (Figure 1).
Figure 1.
Cohort flow. *1,889 of these were from the Baltimore site, who did not participate in Visit 9.
At baseline (Year 10), the women were elderly with a mean age (SD) of 77.7 (3.4) years, with 12.9 (2.6) years of education on average, 11.7% were African American. Other characteristics of the cohort are displayed in Table 1. On average the baseline creatinine was 0.77 (0.18) mg/dL, standardized cystatin C was 1.06 (0.21) mg/L.
Table 1.
Baseline Characteristics of the Study Participants by Quartiles of Cystatin C
| Variable | Entire Cohort (n = 1,332) | Cystatin C Quartiles, mg/L | p Value | |||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | |||
| 0.61–0.91 (n = 334) | 0.92–1.01 (n = 311) | 1.02–1.14 (n = 343) | 1.15–2.36 (n = 344) | |||
| Age, years, mean (SD) | 77.73 (3.4) | 76.8 (3.6) | 77.5 (3.4) | 78.1 (3.3) | 78.4 (3.1) | <.001 |
| African American, n (%) | 156 (11.7) | 72 (21.6) | 32 (10.3) | 34 (9.9) | 18 (5.2) | <.001 |
| Education, years, mean (SD) | 12.9 (2.6) | 13.1 (2.6) | 13.0 (2.6) | 12.8 (2.5) | 12.6 (2.5) | .042 |
| Geriatric Depression Scale score, mean (SD) | 1.6 (2.1) | 1.5 (2.0) | 1.5 (1.8) | 1.6 (2.0) | 1.9 (2.3) | .003 |
| Fair or poor self-reported health, n (%) | 179 (13.4) | 28 (8.4) | 45 (14.5) | 48 (13.9) | 58 (16.9) | .011 |
| ≥1 IADL impairment, n (%) | 489 (36.7) | 93 (27.8) | 107 (34.5) | 132 (38.5) | 157 (45.6) | <.001 |
| Current smoker, n (%) | 39 (2.9) | 10 (3) | 7 (2.3) | 7 (2.1) | 15 (4.4) | .27 |
| Alcohol use within previous 30 d, n (%) | 602 (45.2) | 158 (47.5) | 142 (45.7) | 168 (48.9) | 134 (38.9) | .044 |
| History of stroke, n (%) | 39 (2.9) | 14 (4.2) | 9 (2.9) | 9 (2.6) | 7 (2.0) | .399 |
| History of hypertension, n (%) | 478 (35.9) | 101 (30.2) | 100 (32.2) | 120 (34.9) | 157 (45.6) | <.001 |
| History of cardiovascular disease,* n (%) | 134 (10.1) | 32 (9.6) | 26 (8.4) | 32 (9.3) | 44 (12.8) | .249 |
| History of diabetes, n (%) | 66 (4.9) | 17 (5.1) | 14 (4.5) | 20 (5.8) | 15 (4.4) | .813 |
| Body mass index, kg/m2, mean (SD) | 27.6 (4.9) | 26.4 (4.4) | 27.2 (4.6) | 28.2 (5.1) | 28.7 (5.1) | <.001 |
| Creatinine, mg/dL, mean (SD) | 0.77 (0.2) | 0.65 (0.1) | 0.71 (0.1) | 0.77 (0.1) | 0.93 (0.2) | <.001 |
| Estimated GFR,† mL/min/1.73 m2, mean (SD) | 71.1 (14.6) | 88.1 (7.7) | 76.2 (5.7) | 67.9 (5.7) | 53.4 (9.3) | <.001 |
Notes: Age, education, GDS score and creatinine use Kruskal–Wallis tests for skewed distributions. Other continuous variables report p values from ANOVA tests. IADL = Instrumental Activities of Daily Living.
*History of cardiovascular disease includes self-reported history of angina, myocardial infarction, and congestive heart failure.
†Calculated by the CKD-EPI creatinine–cystatin C equation (27).
Women in higher cystatin C quartiles were older, less likely to be African American, had fewer years of education, higher Geriatric Depression Score, were more likely to report fair or poor health, more likely to have IADL impairments, more likely to have history of hypertension, and had higher BMI. As expected, women in higher cystatin C quartiles had higher serum creatinine and lower eGFRCr+CysC (Table 1).
Association of Cystatin C With Incident Cognitive Impairment
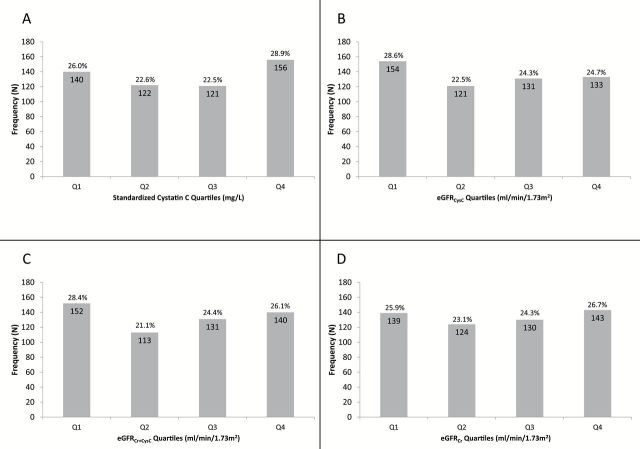
After 10 years of follow-up, in cystatin C quartile 1 (Q1), 140 (26.0%); Q2, 122 (22.6%); Q3, 121 (22.5%); and Q4, 156 (28.9%) of women developed incident cognitive impairment or dementia (Figure 2A–D). In the unadjusted model and model adjusted for age and race, there appeared to be a U-shaped association between cystatin C and incident impairment/dementia with a higher risk among women with higher and those with lower cystatin C concentrations. Only the association between higher cystatin C (Q4) and risk of impairment/dementia reached the level of significance compared with referent group (Q2–Q3) in the unadjusted analysis: odds ratios (OR) (95% CI) were 1.22 (0.93–1.60) in Q1 and 1.40 (1.08–1.83) in Q4 (Table 2). This association became not statistically significant in the fully adjusted model: adjusted OR (95% CI) for incident cognitive impairment were 1.31 (0.98–1.75), p = .067 for Q1, and 1.25 (0.94–1.66) for Q4 of cystatin C (Table 2).
Figure 2.
(A–D) Frequency of incident cognitive impairment by quartiles of cystatin C and eGFR as calculated by cystatin C and creatinine-based equations. Quartile cut points: (cystatin C) 0.918, 1.019, 1.154mg/L; (eGFRCysC) 55.597, 65.740, 76.098mL/min/1.73 m2; (eGFRCr+CysC) 61.863, 71.867, 81.166mL/min/1.73 m2; (eGFRCr) 66.385, 78.946, 86.082mL/min/1.73 m2.
Table 2.
Association Between Cystatin C and Odds of Cognitive Impairment
| Odds Ratio of Cognitive Impairment (95% CI) | ||||||
|---|---|---|---|---|---|---|
| Unadjusted Model | p Value | Base Model* | p Value | MV Model† | p Value | |
| Cystatin C quartiles, mg/L | ||||||
| Q1 (0.61–0.91) | 1.22 (0.93–1.60) | .147 | 1.24 (0.94–1.64) | .125 | 1.31 (0.98–1.75) | .067 |
| Q2–Q3 (0.92–1.14) | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) | |||
| Q4 (1.15–2.37) | 1.40 (1.08–1.83) | .012 | 1.38 (1.05–1.81) | .020 | 1.25 (0.94–1.66) | .121 |
Notes: *Adjusted for age and race.
†Adjusted for multiple covariates, including age, race, education, self-reported health status, Geriatric Depression scale, Instrumental Activities of Daily Living, smoking, alcohol intake, body mass index, history of coronary vascular disease, history of diabetes, history of hypertension, and history of stroke.
Results of sensitivity analyses in which cystatin C was expressed as a continuous variable and quadratic term was included suggested the possibility of a nonlinear association, but p values for cystatin C (p = .06) and (cystatin C)2 (p = .08) did not reach significance.
Association of eGFR With Incident Cognitive Impairment
After 10 years of follow-up, in eGFRCysC Q1, 154 (28.6%); Q2, 121 (22.5%); Q3, 131 (24.3%); and Q4, 133 (24.7%) of women developed incident cognitive impairment or dementia (Figure 2B). In the unadjusted model and model adjusted for age and race, there appeared to be a U-shaped association between eGFRCysC and incident impairment/dementia with a higher risk among women with lower eGFRCysC and those with higher eGFRCysC compared with women in Q2–Q3 of eGFRCysC, although only latter association reached statistical significance. After adjustment for other covariates these associations lost statistical significance: compared with referent group (Q2–Q3), adjusted OR (95% CI) 1.26 (0.94–1.67) in Q1 and 1.18 (0.88–1.58) in Q4 (Table 3). In general, findings were similar in analyses where eGFRCr+CysC or eGFRCr was substituted for eGFRcysC, although the association between higher (Q4) eGFRCr+CysC and greater risk of cognitive impairment remained significant after multivariate adjustment: compared with referent group (Q2–Q3), adjusted OR (95% CI) 1.29 (0.97–1.71) in Q1 and 1.35 (1.01–1.82) in Q4 (Figure 2C and D, Table 3).
Table 3.
Association Between eGFR and Odds of Cognitive Impairment
| Odds Ratio of Cognitive Impairment (95% CI) | ||||||
|---|---|---|---|---|---|---|
| Unadjusted Model | p Value | Base Model* | p Value | MV Model† | p Value | |
| eGFRCysC ‡ quartiles, mL/min/1.73 m2 | ||||||
| Q4 (76.1–109.4) | 1.08 (0.82–1.41) | .580 | 1.13 (0.85–1.49) | .403 | 1.18 (0.88–1.58) | .276 |
| Q2–Q3 (55.6–76.0) | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) | |||
| Q1 (21.8–55.5) | 1.42 (1.09, 1.85) | .010 | 1.37 (1.05–1.80) | .022 | 1.26 (0.94–1.67) | .117 |
| eGFRCr+CysC § quartiles, mL/min/1.73 m2 | ||||||
| Q4 (81.2–116.4) | 1.24 (0.95–1.62) | .121 | 1.25 (0.94–1.66) | .124 | 1.35 (1.01–1.82) | .046 |
| Q2–Q3 (61.9–81.1) | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) | |||
| Q1 (23.5–61.8) | 1.45 (1.11–1.90) | .007 | 1.36 (1.03–1.79) | .029 | 1.29 (0.97–1.71) | .080 |
| eGFRCr || quartiles, mL/min/1.73 m2 | ||||||
| Q4 (86.1–115.1) | 1.15 (0.88–1.50) | .317 | 1.27 (0.95–1.69) | .106 | 1.32 (0.98–1.77) | .068 |
| Q2–Q3 (66.5–86.0) | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) | |||
| Q1 (21.6–66.4) | 1.13 (0.86–1.48) | .368 | 1.13 (0.86–1.49) | .379 | 1.09 (0.82–1.45) | .535 |
Notes: eGFR = estimated glomerular filtration rate.
*Adjusted for age and race.
†Adjusted for multiple covariates, including age, race, education, self-reported health status, Geriatric Depression Scale, Instrumental Activities of Daily Living, smoking, alcohol intake, body mass index, history of coronary vascular disease, history of diabetes, history of hypertension, and history of stroke.
‡Calculated by the CKD-EPI cystatin C equation (27).
§Calculated by the CKD-EPI creatinine–cystatin C equation (27).
||Calculated by the CKD-EPI creatinine equation (27).
Discussion
In this prospective cohort of community dwelling elderly women, our results support a U-shaped association between cystatin C concentration and eGFRCr+CysC and risk of cognitive impairment or dementia 10 years later, but this association is explained, in part, by confounding factors.
A cross-sectional study in participants of Chronic Renal Insufficiency Cohort Cognitive Study revealed an association between higher cystatin C and poor performance on cognitive testing in adults with chronic kidney disease (42). In addition, several prospective studies reported the association between higher levels of cystatin C and greater risk of cognitive impairment. The Health ABC study reported an independent association between high level of cystatin C (>1.25mg/L) and worse cognitive performance at baseline and greater cognitive decline as measured by the 3MS and Digit Symbol Substitution Test in a cohort of 3,030 elderly individuals (22). The Cardiovascular Health Study evaluated the relationship between cystain C and aging success (defined as aging free of cardiovascular disease, cancer, chronic obstructive lung disease, and having intact physical and cognitive function) during a 6-year follow-up in 2,140 elderly participants without these conditions at baseline (23). They reported reduction in successful life years in the highest quartile of cystatin C compared with the lowest. In addition, higher quartiles of cystatin C (Q3 [1.02–1.15mg/L] and Q4 [≥1.16mg/L]) were independently associated with incident cognitive or physical disability (hazard ratio [HR] 1.56 [95% CI: 1.09–2.23] for Q3 and HR 1.39 [1.00–1.98] for Q4) compared with the lowest quartile (23). In this study, we also found greater risk of cognitive impairment or dementia in women in the highest quartile of cystatin C, but the association was modest in magnitude and explained at least in part by confounding factors. Our study may have had insufficient power to determine the association because of low prevalence of high cystatin C (eg, cystatin C >1.25mg/L). To be in the cohort, the women had to survive and undergo cognitive testing 10 years after measurement of kidney function. Because chronic kidney disease is a risk factor for death, this selected group of women who survived into very old age had better kidney function at baseline.
Our findings also suggest a trend for a higher risk of cognitive impairment or dementia among older women with lower cystatin C levels. Experiments in animal models have demonstrated that cystatin C plays neuro-protective role via pathways that are dependent on inhibition of cysteine proteases, induction of autophagy, and induction of proliferation in acute and chronic neurodegenerative conditions (18,19). Epidemiologic evidence supports these experimental findings. Serum cystatin C levels were measured at two visits 7 years apart in the Uppsala Longitudinal Study of Adult Men, a community-based study of elderly men who were followed for 11.3 years for development of Alzheimer’s disease (24). Lower levels of cystatin C were associated with higher risk of Alzheimer’s disease independently of other covariates (HR for lowest [<1.12 µmol/L] vs highest [>1.30 µmol/L] tertile = 2.67, 95% CI: 1.22–5.83, p < .02) (24). In another study, cystatin C level below the median (<1,067ng/mL) in patients with MCI was associated with greater incidence of Alzheimer’s dementia at 2.5 years follow-up (43).
Both lower eGFRCr+CysC and higher eGFRCr+CysC appeared to be associated with greater odds of incident cognitive impairment in our cohort, although only higher eGFRCr+CysC was independently associated with higher incidence of cognitive impairment after multivariable adjustment. This finding may reflect the neuroprotective effect of higher concentrations of cystatin C (eg, lower eGFRCysC) and slightly higher creatinine concentrations (eg, lower eGFRCr) as a surrogate for higher muscle mass. Prior studies that did not include cystatin C in the eGFR estimation have reported an association between lower eGFRCr and greater risk of cognitive decline (8,11), but in this study there is a trend for an association between higher eGFRCr as well as lower eGFRCr with higher risk of cognitive decline. It is possible that in our healthy “survivor” cohort of elderly individuals, mildly decreased kidney function is not a strong risk factor for poor cognitive outcomes.
This study has a number of strengths including its prospective design, the well characterized cohort, use of cystatin C and creatinine-based measures of renal function, as well as rigorous methods to diagnose clinically relevant cognitive impairment. However, this study has several limitations. The cohort was comprised of very elderly women and findings might not apply to other population groups. We had no direct measure of GFR, and very few women in our study had significant renal impairment. Finally, because women with significant kidney disease or cognitive impairment were less likely to survive into their 90s, our results might represent survivor bias.
In conclusion, our findings support a U-shaped association between cystatin C concentration (and eGFR) with risk of cognitive impairment or dementia 10 years later, but it is uncertain whether this association is independent or explained by confounding factors. Both high and low cystatin C concentrations might be associated with poor cognitive outcomes in the very old. Because of the confusing state of the evidence in the very elderly, providers might need to shift their focus away from laboratory biomarkers to more functional estimates of performance that might allow for identification of more relevant barriers to healthy aging in this population. Whether cystatin C can become a target or therapeutic agent used for prevention of cognitive impairment and dementia in the elderly remains to be determined.
Funding
The Study of Osteoporotic Fractures is supported by National Institutes of Health funding. The National Institute on Aging provides support under the following grant numbers: R01 AG005407, R01 AR35582, R01 AR35583, R01 AR35584, R01 AG005394, R01 AG027574, and R01 AG027576.
References
- 1. Garrett N, Martini EM. The boomers are coming: a total cost of care model of the impact of population aging on the cost of chronic conditions in the United States. Dis Manag. 2007;10:51–60. [DOI] [PubMed] [Google Scholar]
- 2. Quentin W, Riedel-Heller SG, Luppa M, Rudolph A, König HH. Cost-of-illness studies of dementia: a systematic review focusing on stage dependency of costs. Acta Psychiatr Scand. 2010;121:243–259. [DOI] [PubMed] [Google Scholar]
- 3. Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. [DOI] [PubMed] [Google Scholar]
- 4. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. [DOI] [PubMed] [Google Scholar]
- 5. Shlipak MG, Katz R, Sarnak MJ, et al. Cystatin C and prognosis for cardiovascular and kidney outcomes in elderly persons without chronic kidney disease. Ann Intern Med. 2006;145:237–246. [DOI] [PubMed] [Google Scholar]
- 6. Bowling CB, Muntner P. Epidemiology of chronic kidney disease among older adults: a focus on the oldest old. J Gerontol A Biol Sci Med Sci. 2012;67:1379–1386. [DOI] [PubMed] [Google Scholar]
- 7. Kurella M, Chertow GM, Luan J, Yaffe K. Cognitive impairment in chronic kidney disease. J Am Geriatr Soc. 2004;52:1863–1869. [DOI] [PubMed] [Google Scholar]
- 8. Kurella M, Chertow GM, Fried LF, et al. Chronic kidney disease and cognitive impairment in the elderly: the health, aging, and body composition study. J Am Soc Nephrol. 2005;16:2127–2133. [DOI] [PubMed] [Google Scholar]
- 9. Kurella M, Yaffe K, Shlipak MG, Wenger NK, Chertow GM. Chronic kidney disease and cognitive impairment in menopausal women. Am J Kidney Dis. 2005;45:66–76. [DOI] [PubMed] [Google Scholar]
- 10. O’Hare AM, Walker R, Haneuse S, et al. Relationship between longitudinal measures of renal function and onset of dementia in a community cohort of older adults. J Am Geriatr Soc. 2012;60:2215–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Seliger SL, Siscovick DS, Stehman-Breen CO, et al. Moderate renal impairment and risk of dementia among older adults: the Cardiovascular Health Cognition Study. J Am Soc Nephrol. 2004;15:1904–1911. [DOI] [PubMed] [Google Scholar]
- 12. Slinin Y, Paudel ML, Ishani A, et al. ; Osteoporotic Fractures in Men Study Group. Kidney function and cognitive performance and decline in older men. J Am Geriatr Soc. 2008;56:2082–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Anand S, Johansen KL, Kurella Tamura M. Aging and chronic kidney disease: the impact on physical function and cognition. J Gerontol A Biol Sci Med Sci. 2014;69:315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Davison AM. Renal disease in the elderly. Nephron. 1998;80:6–16. [DOI] [PubMed] [Google Scholar]
- 15. Giannelli SV, Patel KV, Windham BG, Pizzarelli F, Ferrucci L, Guralnik JM. Magnitude of underascertainment of impaired kidney function in older adults with normal serum creatinine. J Am Geriatr Soc. 2007;55:816–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fliser D, Ritz E. Serum cystatin C concentration as a marker of renal dysfunction in the elderly. Am J Kidney Dis. 2001;37:79–83. [DOI] [PubMed] [Google Scholar]
- 17. Shlipak MG, Sarnak MJ, Katz R, et al. Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med. 2005;352:2049–2060. [DOI] [PubMed] [Google Scholar]
- 18. Gauthier S, Kaur G, Mi W, Tizon B, Levy E. Protective mechanisms by cystatin C in neurodegenerative diseases. Front Biosci (Schol Ed). 2011;3:541–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gauthier SA, Tizon B, Sahoo S, Levy E. In vitro assays measuring protection by proteins such as cystatin C of primary cortical neuronal and smooth muscle cells. Methods Mol Biol. 2012;849:275–287. [DOI] [PubMed] [Google Scholar]
- 20. Kaeser SA, Herzig MC, Coomaraswamy J, et al. Cystatin C modulates cerebral beta-amyloidosis. Nat Genet. 2007;39:1437–1439. [DOI] [PubMed] [Google Scholar]
- 21. Sastre M, Calero M, Pawlik M, et al. Binding of cystatin C to Alzheimer’s amyloid beta inhibits in vitro amyloid fibril formation. Neurobiol Aging. 2004;25:1033–1043. [DOI] [PubMed] [Google Scholar]
- 22. Yaffe K, Lindquist K, Shlipak MG, et al. Cystatin C as a marker of cognitive function in elders: findings from the health ABC study. Ann Neurol. 2008;63:798–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sarnak MJ, Katz R, Fried LF, et al. ; Cardiovascular Health Study. Cystatin C and aging success. Arch Intern Med. 2008;168:147–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sundelöf J, Arnlöv J, Ingelsson E, et al. Serum cystatin C and the risk of Alzheimer disease in elderly men. Neurology. 2008;71:1072–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Erlandsen EJ, Randers E, Kristensen JH. Evaluation of the Dade Behring N Latex Cystatin C assay on the Dade Behring Nephelometer II System. Scand J Clin Lab Invest. 1999;59:1–8. [DOI] [PubMed] [Google Scholar]
- 26. Inker LA, Eckfeldt J, Levey AS, et al. Expressing the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) cystatin C equations for estimating GFR with standardized serum cystatin C values. Am J Kidney Dis. 2011;58:682–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Inker LA, Schmid CH, Tighiouart H, et al. ; CKD-EPI Investigators. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Levey AS, Stevens LA, Schmid CH, et al. ; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yaffe K, Middleton LE, Lui LY, et al. Mild cognitive impairment, dementia, and their subtypes in oldest old women. Arch Neurol. 2011;68:631–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 31. Reitan RM, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery: Theory and Clinical Interpretation. Tucson, AZ: Neuropsychology Press; 1985. [Google Scholar]
- 32. McDowell I, Kristjansson B, Hill GB, Hébert R. Community screening for dementia: the Mini Mental State Exam (MMSE) and Modified Mini-Mental State Exam (3MS) compared. J Clin Epidemiol. 1997;50:377–383. [DOI] [PubMed] [Google Scholar]
- 33. Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test—Second Edition (CVLT-II). San Antonio, TX: Psychological Corporation; 2000. [Google Scholar]
- 34. Wechsler D. Wechsler Adult Intelligence Scale—Revised. New York, NY: The Psychological Corporation; 1988. [Google Scholar]
- 35. Spreen O, Strauss E. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. New York, NY: Oxford University Press; 1991. [Google Scholar]
- 36. Jorm AF, Jacomb PA. The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): socio-demographic correlates, reliability, validity and some norms. Psychol Med. 1989;19:1015–1022. [DOI] [PubMed] [Google Scholar]
- 37. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 38. Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. [DOI] [PubMed] [Google Scholar]
- 39. Petersen RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58:1985–1992. [DOI] [PubMed] [Google Scholar]
- 40. Yesavage JA. Geriatric Depression Scale. Psychopharmacol Bull. 1988;24:709–711. [PubMed] [Google Scholar]
- 41. Pincus T, Summey JA, Soraci SA, Jr, Wallston KA, Hummon NP. Assessment of patient satisfaction in activities of daily living using a modified Stanford Health Assessment Questionnaire. Arthritis Rheum. 1983;26:1346–1353. [DOI] [PubMed] [Google Scholar]
- 42. Yaffe K, Kurella-Tamura M, Ackerson L, et al. Higher levels of cystatin c are associated with worse cognitive function in older adults with chronic kidney disease: The Chronic Renal Insufficiency Cohort Cognitive Study. J Am Geriatr Soc. 2014 Sep;62:1623–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ghidoni R, Benussi L, Glionna M, et al. Plasma cystatin C and risk of developing Alzheimer’s disease in subjects with mild cognitive impairment. J Alzheimers Dis. 2010;22:985–991. [DOI] [PubMed] [Google Scholar]