Abstract
Epidemiologic studies utilizing source apportionment (SA) of fine particulate matter have shown that particles from certain sources might be more detrimental to health than others; however, it is difficult to quantify the uncertainty associated with a given SA approach. In the present study, we examined associations between source contributions of fine particulate matter and emergency department visits for pediatric asthma in Atlanta, Georgia (2002–2010) using a novel ensemble-based SA technique. Six daily source contributions from 4 SA approaches were combined into an ensemble source contribution. To better account for exposure uncertainty, 10 source profiles were sampled from their posterior distributions, resulting in 10 time series with daily SA concentrations. For each of these time series, Poisson generalized linear models with varying lag structures were used to estimate the health associations for the 6 sources. The rate ratios for the source-specific health associations from the 10 imputed source contribution time series were combined, resulting in health associations with inflated confidence intervals to better account for exposure uncertainty. Adverse associations with pediatric asthma were observed for 8-day exposure to particles generated from diesel-fueled vehicles (rate ratio = 1.06, 95% confidence interval: 1.01, 1.10) and gasoline-fueled vehicles (rate ratio = 1.10, 95% confidence interval: 1.04, 1.17).
Keywords: air pollution, ensemble method, fine particulate matter, pediatric asthma, PM2.5, source apportionment, uncertainty
There is strong evidence that fine particulate matter with an aerodynamic diameter less than 2.5 µm (PM2.5) exacerbates pediatric asthma (1). However, PM2.5 is a heterogeneous mixture of particles, and some particles might be more harmful than others (2–10). Despite this, the current regulatory strategy treats all particles that contribute to particulate matter mass equally. Epidemiologic studies of source-apportioned PM2.5, rather than total PM2.5, might help identify the causal agents that precipitate acute asthmatic events and ultimately lead to more effective regulation.
Although source apportionment (SA) techniques offer much promise for epidemiologic studies, each approach has its own set of limitations when used in health studies (11). A challenge with SA is that an accepted gold standard for quantifying source concentrations does not exist (12), making quantification of the uncertainty associated with each technique difficult. This in turn might lead to biased health estimates and underestimated standard errors.
One approach to mitigating the limitations of individual SA models is to use an ensemble of SA estimates. Lee et al. (11) showed that using an ensemble average of 5 different SA models led to a predicted-to-observed PM2.5 ratio that was closer to 1, fewer observed zero-impact days, and a reduction in the reported day-to-day variability of the source contribution estimates compared with single SA models. Balachandran et al. (13) built on this approach by propagating the uncertainties of each SA approach in the ensemble average and then using Bayesian techniques to obtain multiple realizations of the source profiles to further capture the day-to-day uncertainties in the SA techniques. This Bayesian-based ensemble approach has been shown to provide results that are more consistent with independent observations and known emission sources than other single SA methods (13).
To our knowledge, this is the first epidemiologic study in which results from this novel Bayesian ensemble-based SA technique have been applied. We examined the association between ensemble-based PM2.5 source concentrations and emergency department (ED) visits for childhood asthma. By using daily data from an 8.5-year time series study in metropolitan Atlanta, we were also able to examine cumulative lagged associations.
METHODS
Exposure data: Bayesian-based ensemble averaging
The Bayesian-based ensemble approach combines 4 independent SA methods, 3 of which were receptor-based (chemical mass balance using molecular markers (14), chemical mass balance using gas-based constraints (15), and positive matrix factorization (16)) and 1 that was a chemical transport model (17). The receptor-based SA methods were conducted using measurements from the Jefferson Street monitoring site in downtown Atlanta (18). Ensemble averaging was conducted iteratively. In the first step, estimates of the source concentrations for each of the 4 SA methods were averaged with equal weighting, as follows:
| (1) |
and
| (2) |
where wjlk is the weight for source j from method l on day k and Sjlk is the source concentration for source j from method l on day k (13). The root mean square error (RMSE) was then calculated between each method and the ensemble average, as follows:
| (3) |
The RMSE can be viewed as an average estimate of uncertainty of the SA methods; therefore, the inverse of the RMSEs can be used as weights to calculate a weighted ensemble average (19). However, the RMSEs themselves also have uncertainty. To account for the uncertainties in the RMSEs, a Bayesian framework was used in which the weights (inverse of the uncertainties) were used as prior information and the RMSEs were used as the updated parameter information. It was assumed that each SA method's source concentration was normally distributed around some unknown “true” source concentration with a standard deviation of . Using a Bayesian framework, we obtained posterior samples of (i.e., the uncertainty of the RMSEs) and used these updated uncertainties as weights to calculate the ensemble-averaged source concentrations (equations 1 and 2). Greater detail is provided in the Web Appendix (available at http://aje.oxfordjournals.org/).
This Bayesian ensemble method was applied to estimate 2 seasonal source profiles (winter and summer), which in turn were used to estimate daily source concentrations for the 8.5-year time series (January 1, 2002–June 30, 2010) (13). Each day, 10 realizations of the source profiles were sampled from the seasonal source distribution and used in a chemical mass balance equation to estimate the daily concentrations of each source. As a result, for each source category that we identified, there were 10 separate time series with daily SA concentrations. Initially, 9 sources were identified, 5 of which were primary sources and 4 of which were secondary sources (11). Primary sources included biomass burning (BURN), primary PM2.5 from coal combustion, construction, and road dust (DUST), diesel-fueled vehicles and nonroad engines (DV), and gasoline-fueled vehicles and engine sources (GV). Secondary sources included ammonium bisulfate, ammonium sulfate, ammonium nitrate, and secondary organic carbon (SOC) not otherwise apportioned; however, only SOC was used in the present analysis because of concerns that the other secondary source concentrations might be biased (20). Thus, the epidemiologic analyses included only 6 sources. In addition to the source concentration estimates, daily concentrations of ambient ozone (8-hour maximum values) and total PM2.5 (24-hour average values) were obtained from the same Jefferson Street monitoring station.
Health data
Data on the number of daily ED visits were collected from all hospitals in Atlanta for the 8.5-year time series (January 1, 2002–June 30, 2010). Individual visits were restricted to pediatric patients (5–18 years of age) who lived in zip codes within the 5-county metropolitan Atlanta area. We defined ED visits for asthma as any visit with an International Classification of Diseases, Ninth Edition, code for asthma (493.0–493.9) or wheeze (786.07) (n = 121,162 visits).
Statistical methods
We estimated associations between the various PM2.5 sources and ED visits for pediatric asthma using Poisson generalized linear models that accounted for overdispersion. Exposure was modeled separately for each source (Sj), with individual terms for the source concentration on lag 0 (Sj0) through lag 7 (Sj7) in the model (i.e., an unconstrained distributed lag structure) (21). The following model was used to estimate the logarithm of the expected daily count of ED visits for pediatric asthma as a function of the PM2.5 source and covariates (for greater model detail, refer to the Web Appendix):
| (4) |
Two exposure windows were considered from these single-source models: 1) lags 0–2 (same day and previous 2 days' exposure), which was our a priori exposure based on previous analyses (22, 23), and 2) lags 0–7 (8-day exposure window including the same day and previous week), which was motivated by previous published findings that suggested that pollutant effects on asthma might be prolonged over a longer period (24, 25). To calculate rate ratios for these 3- and 8-day exposures, we exponentiated the sum of the betas for the relevant source exposures. For example, the rate ratio for a 3-day source exposure was calculated as RR0–2 = exp(β1 + β2 + β3), whereas the rate ratio for an 8-day source exposure was RR0–7 = exp(β1 + β2+ … + β8). Because the same model was used to calculate both rate ratios, the rate ratio for the 3-day source exposure is the lag 0–2 association after controlling for exposure to lags 3–7. Substantial correlation between day-to-day PM2.5 source concentrations for some sources (see Web Table 1) will cause the regression model in equation 4 to have a high degree of collinearity, leading to unstable estimates for βi. Nonetheless, the sum of β1 to β8 should be an unbiased estimate of the RR for cumulative exposure (21). Standard errors were calculated using the estimated covariance matrix from the genmod procedure in SAS (SAS Institute, Inc., Cary, North Carolina). All associations were estimated as the rate ratio for a cumulative 1-µg/m3 increase in the source concentration for each exposure day.
Covariate control included a cubic spline with 8 knots per year to account for long-term temporal trends. Separate cubic terms were included for average maximum temperature lag 0–2 and lag 3–7. Similarly, 2 cubic terms were included for average dew point lag 0–2 and lag 3–7. Indicators were included for season, day of the week, federal holidays, and the days after Thanksgiving and Christmas. To further control for temporal and meteorological trends, we included product terms between season and day of the week, as well as season and the maximum temperature cubic terms (for lag 0–2 and lag 3–7). Complete model formulation is available in the Web Appendix.
The single-source model analysis was conducted separately for each source (BURN, COAL, DUST, DV, GV, and SOC) and for total PM2.5. Because a strong association between ozone and pediatric asthma exacerbations has been found previous epidemiologic analyses in Atlanta (23, 25–27), we also ran the same models after controlling for ozone, using the same unconstrained 8-day distributed lag structure for ozone. To account for potential confounding by sources not included in the model, we created a multipollutant model that included the 8-day moving averages of all 6 sources. To incorporate the uncertainty in the ensemble-averaged SA concentrations, each analysis was performed 10 times, once for each of the 10 separate ensemble time series runs. Multiple imputation methods were used to estimate the combined point estimate and variance for each analysis. The summary regression coefficient Q was obtained by averaging the regression coefficients from each run, where m = 10, as follows:
| (5) |
Imputation-corrected variances were calculated according to the method described by Rubin (28). The first step required calculating the average variance from the ensemble runs (within imputation variance) (W) and the variance of the ensemble run coefficients (between imputation variance) (B), as follows:
| (6) |
and
| (7) |
With these 2 quantities, the total imputation-corrected variance (T) is calculated as
| (8) |
Confidence intervals were calculated based on a t-distribution with degrees of freedom (ν) equal to
| (9) |
Analyses were conducted using SAS, version 9.3.
RESULTS
There were 2,170 days for which we had SA estimates for lags 0–7 for all sources and for PM2.5. Table 1 summarizes the pollutant, meteorological, and ED data during the study period. There were 121,162 ED visits for acute asthma or wheezing among children 5–18 years of age. The mean 24-hour average concentration of fine particulate matter was 14.51 µg/m3, and the mean 8-hour maximum concentration of ozone was 40.61 ppb.
Table 1.
Summary Statistics for Fine Particulate Matter, Ozone, Meteorological Data, and Emergency Department Visits for Pediatric Asthma in 5 Counties, Atlanta, Georgia, January 2002–June 2010
| Variable | No. of Daysa | Median | Mean (SD) | Minimum | Maximum | IQR |
|---|---|---|---|---|---|---|
| Pollutant | ||||||
| 24-Hour max fine particulate matter, µg/m3 | 2,170 | 13.18 | 14.51 (7.33) | 1.06 | 72.56 | 9.16 |
| 8-Hour max ozone, ppb, | 2,090 | 39.34 | 40.61 (19.16) | 0.52 | 116.37 | 28.09 |
| Meteorology | ||||||
| Maximum temperature, °C | 2,170 | 23 | 21.96 (8.37) | −1 | 40 | 13 |
| Dew point, °C | 2,163 | 11 | 9.64 (9.34) | −20 | 24 | 16 |
| Health outcome | ||||||
| ED visits for asthma/wheezing | 2,170 | 37 | 39.40 (18.78) | 3 | 157 | 23 |
Abbreviations: ED, emergency department; IQR, interquartile range; SD, standard deviation.
a The analysis was restricted to days when information on all sources and fine particulate matter were nonmissing for the 8-day lag.
Summary statistics of the daily source concentrations from each of the 10 ensemble runs are presented in Table 2. When averaged across all ensemble runs, BURN had the highest mean concentration (2.72 µg/m3) and greatest standard deviation (2.64), whereas primary coal combustion had the lowest (0.13 (standard deviation, 0.12) µg/m3). The mean concentration of DV was greater than that of GV (1.04 vs. 0.80 µg/m3), with DV having greater average standard deviations within each ensemble run (0.97 vs. 0.69). The last column in Table 2 shows the average day-to-day correlation between each of the 10 ensemble runs by source. DUST had the highest correlation between the runs (r = 0.98), whereas the other 5 sources had lower correlations, ranging from 0.74 to 0.76. Between-source correlations ranged between −0.46 (SOC and BURN) and 0.49 (SOC and PM2.5, SOC and ozone, and DV and PM2.5) (Table 3). On average across all days, the ensemble-averaged concentrations for the 6 sources constituted 49% of the total PM2.5 mass.
Table 2.
Mean and Standard Deviation Summary Statistics for the Pollutant Source Concentrations, Atlanta, Georgia, January 2002–June 2010a
| Source | Minimum | Median | Mean | Maximum | SD | IQR | Correlationb Between Ensemble Runs |
|---|---|---|---|---|---|---|---|
| Biomass burning | 0.000 (0.000) | 1.894 (0.023) | 2.723 (0.024) | 32.103 (5.933) | 2.643 (0.085) | 2.600 (0.070) | 0.752 |
| Primary coal combustion | 0.000 (0.000) | 0.092 (0.001) | 0.126 (0.001) | 1.228 (0.173) | 0.124 (0.001) | 0.134 (0.003) | 0.737 |
| Dust/resuspended soil | 0.000 (0.000) | 0.252 (0.001) | 0.378 (0.001) | 7.045 (1.244) | 0.463 (0.010) | 0.273 (0.004) | 0.981 |
| Diesel-fueled vehicles | 0.000 (0.000) | 0.810 (0.014) | 1.038 (0.005) | 9.856 (0.916) | 0.970 (0.015) | 0.995 (0.033) | 0.744 |
| Gasoline-fueled vehicles | 0.013 (0.009) | 0.654 (0.011) | 0.797 (0.006) | 6.843 (0.675) | 0.689 (0.010) | 0.647 (0.009) | 0.764 |
| Secondary organic carbon | 0.000 (0.000) | 1.352 (0.009) | 1.620 (0.013) | 27.578 (1.071) | 1.619 (0.013) | 1.991 (0.055) | 0.747 |
Abbreviations: IQR, interquartile range; SD, standard deviation.
a Averaged across 10 ensemble runs. All results are reported in µg/m3.
b Mean Spearman correlation calculated from all pairwise runs.
Table 3.
Spearman Correlations Coefficients for the Associations Among the Pollutant Sources, Fine Particulate Matter, and Ozone, Atlanta, Georgia, January 2002–June 2010a
| Pollutant | BURN | COAL | DUST | DV | GV | SOC | PM2.5 | Ozone |
|---|---|---|---|---|---|---|---|---|
| BURN | 1.00 | |||||||
| COAL | 0.24 | 1.00 | ||||||
| DUST | −0.05 | 0.17 | 1.00 | |||||
| DV | 0.04 | 0.21 | 0.25 | 1.00 | ||||
| GV | 0.38 | 0.06 | 0.12 | 0.22 | 1.00 | |||
| SOC | −0.46 | 0.02 | 0.31 | 0.43 | −0.10 | 1.00 | ||
| PM2.5 | 0.15 | 0.21 | 0.41 | 0.49 | 0.29 | 0.49 | 1.00 | |
| Ozone | −0.32 | 0.06 | 0.50 | 0.12 | −0.11 | 0.49 | 0.47 | 1.00 |
Abbreviations: BURN, biomass burning; DUST, construction and road dust; DV, diesel-fueled vehicles and nonroad engines; GV, gasoline-fueled vehicles and other engine sources; PM2.5, fine particulate matter with an aerodynamic diameter less than 2.5 µm; SOC, secondary organic carbon not otherwise apportioned.
a Averaged across 10 ensemble runs.
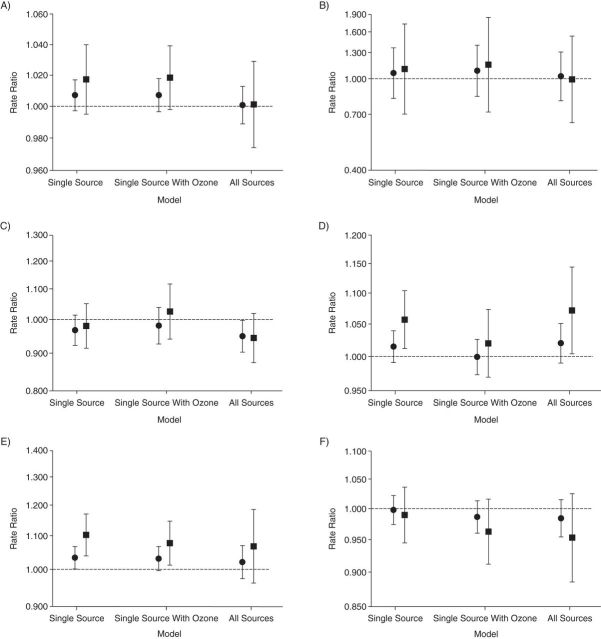
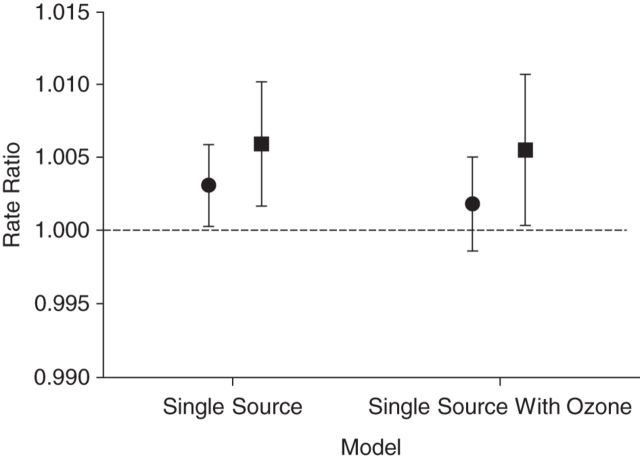
Figure 1 shows associations with pediatric asthma for 3 separate models: the single-source model (with the exposure modeled using an unconstrained distributed lag), the single-source model with the addition of ozone control, and the all-sources model with simultaneous control for the other sources. Results for exposure to total PM2.5 concentration are presented for the single-source model and single-source model with ozone control in Figure 2. For each model (Figures 1 and 2), 2 separate exposures were considered: cumulative exposure to lag 0–2 (controlling for lag 3–7) and cumulative exposure to lag 0–7.
Figure 1.
Rate ratios, plotted on the natural log scale, for the association of a 1-µg/m3 increase in source concentration with emergency department visits for pediatric asthma, Atlanta, Georgia, 2002–2010. Results are for lags 0–2 (circles) and lags 0–7 (squares) for the following sources: A) biomass burning, B) primary coal combustion, C) dust/resuspended soil, D) diesel-fueled vehicles, E) gasoline-fueled vehicles, and F) secondary organic carbons not otherwise apportioned. The rate ratios and 95% confidence intervals corresponding to this figure are listed in Web Table 2. Bars, 95% confidence intervals.
Figure 2.
Rate ratios, plotted on the natural log scale, for the association of a 1-µg/m3 increase in the total concentration of particulate matter with an aerodynamic diameter less than 2.5 µm with emergency department visits for pediatric asthma, Atlanta, Georgia, 2002–2010. Results for lags 0–2 are indicated by circles and lags 0–7 are indicated by squares. The rate ratios and 95% confidence intervals corresponding to this figure are listed in Web Table 2. Bars, 95% confidence intervals.
With the exception of the rate ratios for SOC, the rate ratios for lag 0–7 were larger than the rate ratios for lag 0–2 in all models (Figures 1 and 2). The single-source model resulted in significant associations with ED visits for DV lag 0–7 (RR = 1.06, 95% confidence interval (CI): 1.01, 1.10), GV lag 0–2 (RR = 1.03, 95% CI: 1.00, 1.07) and lag 0–7 (RR = 1.10, 95% CI: 1.04, 1.17), and PM2.5 lag 0–2 (RR = 1.00, 95% CI: 1.00, 1.01) and lag 0–7 (RR = 1.01, 95% CI: 1.00, 1.01). Some associations were attenuated when ozone was included in the model (e.g., DV lag 0–7 and PM2.5 lag 0–2), whereas the lag 0–7 rate ratios for GV and PM2.5 remained significant. Controlling for all sources in the same model resulted in a decrease in most point estimates, and only DV lag 0–7 was significant (RR = 1.07, 95% CI: 1.00, 1.14). Results from both the single-source model and the single-source model with ozone control are suggestive of a higher association with BURN; however, the confidence intervals included the null. The rate ratios for the ozone associations from the single-source models with ozone control are presented in Figure 3. Ozone alone was strongly associated with ED visits for pediatric asthma (for each 25-ppb increase, RR = 1.11, 95% CI: 1.03, 1.20). The association with ozone was little changed when the models included control for a single source (Figure 3), with the exception of control for PM2.5, which attenuated the ozone association. Tables containing the estimates shown in Figures 1–3 are provided in the Web Appendix.
Figure 3.

Rate ratios for the cumulative association of a 25-ppb increase in ozone for lags 0–7, controlling for individual sources one at a time, Atlanta, Georgia, 2002–2010. For comparison, the results of an “ozone only” model (i.e., with no sources or particulate matter with an aerodynamic diameter less than 2.5 µm (PM2.5)) are shown. The rate ratios and 95% confidence intervals corresponding to this figure are listed in Web Table 2. Bars, 95% confidence intervals.
In Table 4, we used the rate ratio estimates from the single-source models for lag 0–7 cumulative exposure to demonstrate how the 10 ensemble runs contributed to the combined point estimates and their standard errors. The ratio of the imputation-corrected standard error to the average standard error shows the degree to which the confidence intervals were inflated because of the propagation of error from the ensemble runs. The degree of inflation ranged from 3% (DUST) to 76% (BURN). The individual unconstrained distributed lag results from the single-source model are shown for the sources and total PM2.5 in Web Figures 1 and 2, respectively, and in tabular form in Web Table 3.
Table 4.
Summary Statistics for the Mean and Standard Error of the Point Estimate From the Ensemble Runsa, Atlanta, Georgia, January 2002–June 2010
| Source | Mean Point Estimate | SE of Point Estimates | Mean SE | Imputation-Corrected SE | Ratio of Imputation-Corrected SE/Average SE |
|---|---|---|---|---|---|
| Biomass burning | 0.017 | 0.009 | 0.006 | 0.011 | 1.758 |
| Primary coal combustion | 0.098 | 0.162 | 0.147 | 0.225 | 1.528 |
| Dust/resuspended soil | −0.020 | 0.008 | 0.034 | 0.035 | 1.027 |
| Diesel-fueled vehicles | 0.055 | 0.013 | 0.017 | 0.022 | 1.281 |
| Gasoline-fueled vehicles | 0.098 | 0.017 | 0.024 | 0.023 | 1.259 |
| Secondary organic carbon | −0.023 | 0.012 | 0.011 | 0.017 | 1.478 |
Abbreviation: SE, standard error.
a Each measure was computed from the mean of 10 ensemble runs using the single-source model to calculate the rate ratio of a combined increase of 1 µg/m3 over lags 0–7.
DISCUSSION
Our results show variability in the extent to which specific sources of fine particulate matter are associated with ED visits for pediatric asthma. Traffic-related sources (DV and GV) were associated with ED visits for pediatric asthma when the cumulative 8-day exposure was considered, whereas sources of biomass burning were suggestive of an association, though it was not statistically significant. Exposure to gasoline-fueled vehicles remained statistically significant after controlling for ozone in the model. When all sources were included in the same model, only the association with 8-day exposure to diesel vehicles was statistically significant.
An important contribution of the present study is the use of ensemble-based SA data in a health analysis. A concern when applying SA outputs to epidemiologic analyses, rather than directly measured pollutants, is the unaccounted for uncertainty in exposures. Generally, the use of ensemble-averaged results is expected to reduce measurement error compared with individual SA methods, though this cannot be easily validated because of the lack of a gold standard. Because the ensemble averages that we used were estimated from a statistical model, it was important to incorporate this additional uncertainty, which led to higher standard errors around the measures of association. To propagate the uncertainty, all reported model results for the sources were the result of a combination of 10 separate ensemble runs, with the net result of inflating the summary confidence intervals by 3%–76%. The relatively small increase in the confidence intervals for DUST (3%) can be attributed to the strong correlation between the ensemble runs (r = 0.98, Table 2), which in turn is a reflection of the relative agreement between SA methods.
The finding that diesel- and gasoline-fueled vehicle sources were associated with childhood asthma is well supported in the literature. Studies have found that residential proximity to roadways is associated with both incident asthma (29) and asthma exacerbation (30). In a study in which the associations between source apportioned PM2.5 and asthmatic children were examined, Gent et al. (31) found that traffic-related exposures were the most harmful and lead to a statistically significant increase in symptoms of asthma. In particular, investigators in previous studies have found indicators of diesel exhaust to be associated with hospital admissions for asthma (32) and airway inflammation (33) in asthmatic subjects.
Although we typically conceptualize lagged exposures as the cumulative association of an increase of 1 µg/m3 in each of the lagged days examined (e.g., previous 8 days) with the last days' (e.g., lag 0) ED visits, for some sources such an exposure might be unlikely. For example, the cumulative association of a 1-µg/m3 increase in biomass burning over 8 days might not be realistic, given that most burn events occur sporadically and over short time intervals. An equivalent way to conceptualize the rate ratio is the association of a single-day 1-µg/m3 increase in biomass burning sustained over 8 days of ED visits. In the first conceptualization, the lag applies to the cumulative exposure and a single-day health association, whereas in the latter, a single-day exposure is associated with cumulative lagged health association; for many source exposures, this latter interpretation of the rate ratio might be more realistic.
Our decision to control for ozone was driven by a concern for confounding, given that ozone was found to be strongly associated with pediatric asthma in previous studies in Atlanta (23, 25–27, 34). The slight attenuation of rate ratios in Figure 1 for the single-source models with ozone control, as well as the similarity between the results of the ozone-only model and the single-source model that controlled for ozone shown in Figure 3, suggested that confounding by ozone was unlikely to be a major concern. There did, however, appear to be some confounding of the association between ozone and PM2.5 that might have been caused by secondary PM2.5 sources not examined in this analysis that were likely to be well-correlated with ozone (35).
One should exercise caution when interpreting the results from the single-source model, because these estimates might be confounded by other pollutants. We created a model that included a term for each source to account for potential between-source confounding. Although multicollinearity was a potential concern in the all-sources model, the confidence intervals did not increase greatly relative to the single-source results, suggesting that this was not a major issue. However, the results in Figure 1 do suggest that there might be confounding or measurement error present in the single-source results. This is particularly evident in the lag 0–2 and lag 0–7 associations with GV, which were significant in the single-source model but nonsignificant in the model in which we controlled for all sources. Measurement error due to spatial misalignment is a concern for this (and any) study in which SA results are extrapolated from a single monitoring site to a greater metropolitan area. Some sources of particular matter are likely to be more spatially homogenous than others (e.g., secondary organic carbons will be more homogenous than local vehicle emissions), and these differences in spatial variation will lead to differing degrees of spatial misalignment and will potentially bias health estimates (36).
The availability of daily source concentrations over an 8.5-year period enabled us to examine different extended lag structures. Many past studies have been limited in their ability to examine the long lag structure of PM2.5 sources and constituents because monitoring data were only available for every third or sixth day (5, 8, 37, 38). Studies in which investigators have looked at the temporal patterns of PM2.5 exposure and acute asthma exacerbations have consistently found evidence of a lagged effect (24, 25, 32). In particular, the lag pattern that we observed for total PM2.5 (Web Figure 2) is consistent with that from an earlier Atlanta-based study by Peel et al. (25) in which they found PM2.5 lags 0 and 6 to have the strongest association with ED visits for asthma. There is biological plausibility behind these findings of a delayed association because particles have been shown to penetrate deep into the lung (39), particularly in persons who suffer from asthma (40), which over time could lead to inflammation in the alveolar region of the lungs (41).
With the exception of DUST and SOC, both of which were found to have a null association, all sources and total PM2.5 showed greater associations of ED visits with lag 0–7 than with lag 0–2. We chose to include lag 0–2 because it is commonly reported in both the asthma and SA literature (3, 22, 23, 31, 42, 43); however, we did so after controlling for lags 3–7, which is not a common approach. If the true effect of pollution from a particular source is distributed over 8 days, then failing to account for the effects at longer lags (e.g., lag 3–7) will result in confounding of the estimated effects at shorter lags (e.g., lag 0–2) if the source concentrations during lag 3–7 are correlated with the source concentrations during lag 0–2. Although it would have been interesting to consider even longer lags, our power to do so was limited because of days with missing values for the ensemble-based source concentration results.
We found that fine particulate matter generated from diesel- and gasoline-fueled vehicle sources was associated with a significant increase in the number of ED visits for acute asthma-related events among children 5–18 years of age. Our results, which corroborate previous findings, suggest that for children, the harmful effects from a single day's exposure to these sources are sustained throughout the week. The present study takes advantage of a novel Bayesian ensemble-based SA technique that helps to minimize the potential for bias that results from relying on any single SA method and provides a means for inflating the confidence intervals around the point estimates to account for the uncertainty in SA methods. As a result of this latter feature, our results may be more conservative than those from single SA studies. Nonetheless, we found associations with traffic sources, which adds to previous findings regarding the differential toxicity of sources and provides evidence to support integrated air quality strategies focused on regulating source emissions.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Epidemiology, Rollins School of Public Health, Emory University, Atlanta, Georgia (Katherine Gass); School of Civil and Environmental Engineering, Georgia Institute of Technology, Atlanta, Georgia (Sivaraman Balachandran, Armistead G. Russell); Biostatistics and Bioinformatics, Rollins School of Public Health, Emory University, Atlanta, Georgia (Howard H. Chang); and Department of Environmental Health, Rollins School of Public Health, Emory University, Atlanta, Georgia (Matthew J. Strickland).
This work was funded by grants R834799 and RD-83386601 from the US Environmental Protection Agency and grants R21ES022795 and K01ES019877 from the National Institute of Environmental Health Sciences.
We thank Atmospheric Research and Analysis, Inc., for the use of Southeastern Aerosol Research and Characterization (SEARCH) data.
The contents of this article are solely the responsibility of the authors and do not necessary represent the official views of the US Environmental Protection Agency. Further, the US Environmental Protection Agency does not endorse the purchase of any commercial products or serves mentioned in the publication.
Conflict of interest: none declared.
REFERENCES
- 1.US Environmental Protection Agency. Integrated Science Assessment for Particulate Matter (Final Report). Washington, DC: U.S. Environmental Protection Agency; 2009. (EPA/600/R-08/139F). [PubMed] [Google Scholar]
- 2.Andersen ZJ, Wahlin P, Raaschou-Nielsen O, et al. Ambient particle source apportionment and daily hospital admissions among children and elderly in Copenhagen. J Expo Sci Environ Epidemiol. 2007;177:625–636. [DOI] [PubMed] [Google Scholar]
- 3.Bell ML, Ebisu K, Leaderer BP, et al. Associations of PM2.5 constituents and sources with hospital admissions: analysis of four counties in Connecticut and Massachusetts (USA) for persons ≥ 65 years of age. Environ Health Perspect. 2014;1222:138–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bell ML, Ebisu K, Peng RD, et al. Hospital admissions and chemical composition of fine particle air pollution. Am J Respir Crit Care Med. 2009;17912:1115–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ito K, Mathes R, Ross Z, et al. Fine particulate matter constituents associated with cardiovascular hospitalizations and mortality in New York City. Environ Health Perspect. 2011;1194:467–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mar TF, Norris GA, Koenig JQ, et al. Associations between air pollution and mortality in Phoenix, 1995–1997. Environ Health Perspect. 2000;1084:347–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ostro B, Feng WY, Broadwin R, et al. The effects of components of fine particulate air pollution on mortality in California: results from CALFINE. Environ Health Perspect. 2007;1151:13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peng RD, Bell ML, Geyh AS, et al. Emergency admissions for cardiovascular and respiratory diseases and the chemical composition of fine particle air pollution. Environ Health Perspect. 2009;1176:957–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zanobetti A, Franklin M, Koutrakis P, et al. Fine particulate air pollution and its components in association with cause-specific emergency admissions. Environ Health. 2009;8:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ozkaynak H, Thurston GD. Associations between 1980 U.S. mortality rates and alternative measures of airborne particle concentration. Risk Anal. 1987;74:449–461. [DOI] [PubMed] [Google Scholar]
- 11.Lee D, Balachandran S, Pachon J, et al. Ensemble-trained PM2.5 source apportionment approach for health studies. Environ Sci Technol. 2009;4318:7023–7031. [DOI] [PubMed] [Google Scholar]
- 12.Thurston GD, Ito K, Mar T, et al. Workgroup report: workshop on source apportionment of particulate matter health effects—intercomparison of results and implications. Environ Health Perspect. 2005;11312:1768–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balachandran S, Chang HH, Pachon JE, et al. Bayesian-based ensemble source apportionment of PM2.5. Environ Sci Technol. 2013;4723:13511–13518. [DOI] [PubMed] [Google Scholar]
- 14.Zheng M, Cass GR, Schauer JJ, et al. Source apportionment of PM2.5 in the Southeastern United States using solvent-extractable organic compounds as tracers. Environ Sci Technol. 2002;3611:2361–2371. [DOI] [PubMed] [Google Scholar]
- 15.Marmur A, Unal A, Mulholland JA, et al. Optimization-based source apportionment of PM2.5 incorporating gas-to-particle ratios. Environ Sci Technol. 2005;399:3245–3254. [DOI] [PubMed] [Google Scholar]
- 16.Paatero P, Tapper U. Positive matrix factorization: a non-negative factor model with optimal utilization of error estimates of data values. Environmetrics. 1994;52:111–126. [Google Scholar]
- 17.Byun D, Schere KL. Review of the governing equations, computational algorithms, and other components of the Models-3 Community Multiscale Air Quality (CMAQ) modeling system. Appl Mech Rev. 2006;592:51–77. [Google Scholar]
- 18.Hansen DA, Edgerton ES, Hartsell BE, et al. The Southeastern Aerosol Research and Characterization Study: part 1—overview. J Air Waste Manag Assoc. 2003;5312:1460–1471. [DOI] [PubMed] [Google Scholar]
- 19.Balachandran S, Pachon JE, Hu Y, et al. Ensemble-trained source apportionment of fine particulate matter and method uncertainty analysis. Atmos Environ. 2012;61:387–394. [Google Scholar]
- 20.Dennis R, Fox T, Fuentes M, et al. A framework for evaluating regional-scale numerical photochemical modeling systems. Environ Fluid Mech (Dordr). 2010;104:471–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwartz J. The distributed lag between air pollution and daily deaths. Epidemiology. 2000;113:320–326. [DOI] [PubMed] [Google Scholar]
- 22.Darrow LA, Hess J, Rogers CA, et al. Ambient pollen concentrations and emergency department visits for asthma and wheeze. J Allergy Clin Immunol. 2012;1303:630–638.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strickland MJ, Darrow LA, Klein M, et al. Short-term associations between ambient air pollutants and pediatric asthma emergency department visits. Am J Respir Crit Care Med. 2010;1823:307–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Halonen JI, Lanki T, Yli-Tuomi T, et al. Urban air pollution, and asthma and COPD hospital emergency room visits. Thorax. 2008;637:635–641. [DOI] [PubMed] [Google Scholar]
- 25.Peel JL, Tolbert PE, Klein M, et al. Ambient air pollution and respiratory emergency department visits. Epidemiology. 2005;162:164–174. [DOI] [PubMed] [Google Scholar]
- 26.Tolbert PE, Mulholland JA, MacIntosh DL, et al. Air quality and pediatric emergency room visits for asthma in Atlanta, Georgia, USA. Am J Epidemiol. 2000;1518:798–810. [DOI] [PubMed] [Google Scholar]
- 27.Friedman MS, Powell KE, Hutwagner L, et al. Impact of changes in transportation and commuting behaviors during the 1996 Summer Olympic Games in Atlanta on air quality and childhood asthma. JAMA. 2001;2857:897–905. [DOI] [PubMed] [Google Scholar]
- 28.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, NY: J. Wiley & Sons; 1987. [Google Scholar]
- 29.McConnell R, Islam T, Shankardass K, et al. Childhood incident asthma and traffic-related air pollution at home and school. Environ Health Perspect. 2010;1187:1021–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boehmer TK, Foster SL, Henry JR, et al. Residential proximity to major highways—United States, 2010. MMWR Surveill Summ. 2013;62(Suppl 3):46–50. [PubMed] [Google Scholar]
- 31.Gent JF, Koutrakis P, Belanger K, et al. Symptoms and medication use in children with asthma and traffic-related sources of fine particle pollution. Environ Health Perspect. 2009;1177:1168–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim SY, Peel JL, Hannigan MP, et al. The temporal lag structure of short-term associations of fine particulate matter chemical constituents and cardiovascular and respiratory hospitalizations. Environ Health Perspect. 2012;1208:1094–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patel MM, Chillrud SN, Deepti KC, et al. Traffic-related air pollutants and exhaled markers of airway inflammation and oxidative stress in New York City adolescents. Environ Res. 2013;121:71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.White MC, Etzel RA, Wilcox WD, et al. Exacerbations of childhood asthma and ozone pollution in Atlanta. Environ Res. 1994;651:56–68. [DOI] [PubMed] [Google Scholar]
- 35.Franklin M, Schwartz J. The impact of secondary particles on the association between ambient ozone and mortality. Environ Health Perspect. 2008;1164:453–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bell ML, Ebisu K, Peng RD. Community-level spatial heterogeneity of chemical constituent levels of fine particulates and implications for epidemiological research. J Expo Sci Environ Epidemiol. 2011;214:372–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ito K, Christensen WF, Eatough DJ, et al. PM source apportionment and health effects: 2. An investigation of intermethod variability in associations between source-apportioned fine particle mass and daily mortality in Washington, DC. J Expo Sci Environ Epidemiol. 2006;164:300–310. [DOI] [PubMed] [Google Scholar]
- 38.Ostro B, Roth L, Malig B, et al. The effects of fine particle components on respiratory hospital admissions in children. Environ Health Perspect. 2009;1173:475–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carvalho TC, Peters JI, Williams RO., 3rd Influence of particle size on regional lung deposition—what evidence is there? Int J Pharm. 2011;406(1-2):1–10. [DOI] [PubMed] [Google Scholar]
- 40.Anderson PJ, Wilson JD, Hiller FC. Respiratory tract deposition of ultrafine particles in subjects with obstructive or restrictive lung disease. Chest. 1990;975:1115–1120. [DOI] [PubMed] [Google Scholar]
- 41.Peters A, Wichmann HE, Tuch T, et al. Respiratory effects are associated with the number of ultrafine particles. Am J Respir Crit Care Med. 1997;1554:1376–1383. [DOI] [PubMed] [Google Scholar]
- 42.Sacks JD, Rappold AG, Davis JA, Jr, et al. Influence of urbanicity and county characteristics on the association between ozone and asthma emergency department visits in North Carolina. Environ Health Perspect. 2014;1225:506–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Delfino RJ, Zeiger RS, Seltzer JM, et al. Association of asthma symptoms with peak particulate air pollution and effect modification by anti-inflammatory medication use. Environ Health Perspect. 2002;11010:A607–A617. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.