Abstract
Human immunodeficiency virus (HIV)-infected and viremic individuals exhibit elevated levels of plasma cytokines. Here we show that most cytokines are not in free form but appear associated with exosomes that are distinct from virions. Purified exosomes were analyzed to determine the levels of 21 cytokines and chemokines and compared with exosome-depleted plasma. Most cytokines were markedly enriched in exosomes from HIV-positive individuals relative to negative controls and to plasma. Moreover, exposure of naive peripheral blood mononuclear cells to exosomes purified from HIV-positive patients induced CD38 expression on naive and central memory CD4+ and CD8+ T cells, probably contributing to inflammation and viral propagation via bystander cell activation.
Keywords: exosomes, HIV, cytokines, chemokines, immune activation
Chronic immune activation is one of the strongest predictors of human immunodeficiency virus (HIV) disease progression, associated with chronic levels of detectable cytokines and elevated expression of activation markers on the surface of T lymphocytes [1–3]. Despite extensive studies highlighting direct and indirect viral induction of chronic immune activation, the mechanisms underlying the chronically activated immune state during HIV infection are not fully elucidated. Exosomes are small membrane delimited vesicles released both constitutively and on stimulation from a variety of cell types [4]. They are found in a number of biological fluids and are known to carry a variety of proteins and nucleic acid molecules [4]. Although they were originally thought to be little more than reservoirs for cellular debris, their role in regulating biological functions as well as disease is increasingly appreciated [4]. In this study, exosomes isolated from the plasma of HIV-infected individuals were analyzed for cytokine and chemokine content compared with exosomes from uninfected controls and assessed for immunomodulatory potential on bystander CD4+ and CD8+ T cells.
EXPERIMENTAL METHODS
Human Subjects
Samples were obtained from the Hope Clinic of Emory University (HIV-1 seronegative [n = 15]) and from the Infectious Disease Program of Grady Health System (antiretroviral naive HIV-1 seropositive [n = 10]). Plasma viral loads of the HIV-infected volunteers ranged from 1423 to 536 436 HIV-1 RNA copies/mL, with an average viral load of 205 957 HIV RNA copies/mL. All participants gave written informed consent, and the study was approved by the institutional review boards of Emory University and Morehouse School of Medicine.
Isolation of Exosomes From Human Plasma
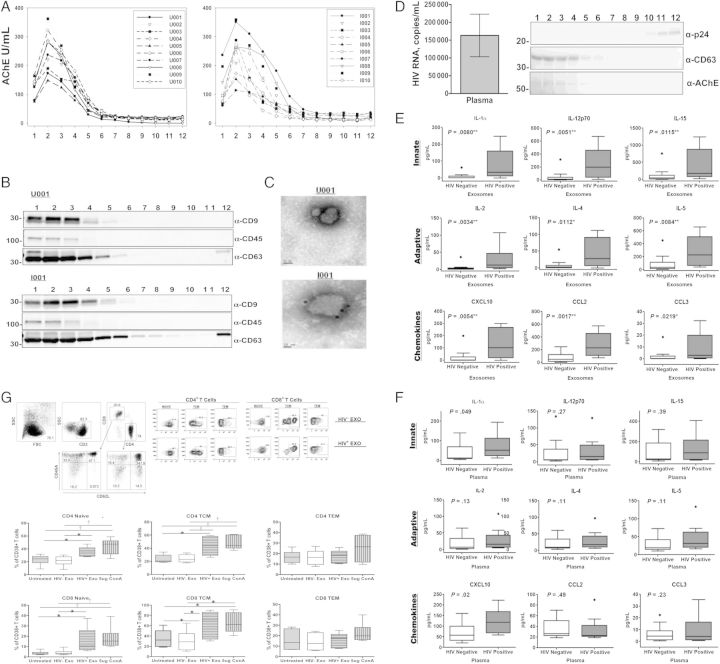
Data from our laboratory and others have shown Nef to be secreted from infected cells in exosomes (exNef), and it is present in the plasma of infected individuals at nanogram levels [5–8]. Optiprep (Sigma-Aldrich) velocity gradients were found to be efficient in purifying exosomes from infected human plasma [9] (Figure 1D). Exosomes from plasma of infected donors, including exNef, were found to segregate in the low-density/upper fractions of the iodixanol gradient, whereas virus particles segregated in the high-density/lower fractions. Subsequently, virion particles were identified by p24 analysis of gradient fractions, and exosomes were identified using multiple protein markers AChE, CD9, CD63, and CD45. The very upper low-density fractions were collected for exosomes, because they were found to have no p24 contamination. Plasma collected from whole blood in ethylenediaminetetraacetic acid tubes was subjected to differential centrifugation at 30 000 g for 30 min and at 100 000 g for 2 hours. The 100 000 g pellet was resuspended in 1 mL of ×1 phosphate-buffered saline, loaded onto Optiprep velocity gradients, and subjected to flotation centrifugation at 250 000 g for 2 hours. Eleven fractions were collected from each gradient and assayed for acetylcholinesterase (AChE) activity or CD63, markers for exosomes, and p24, a marker for viral particles (Figure 1D). Fractions with peak exosome content (fractions 2 and 3) were pooled, diluted 1:3 with phosphate-buffered saline, centrifuged for 2 hours at 400 000 g, and stored at 4°C.
Figure 1.
Exosomes are efficiently purified from human plasma, analyzed for cytokine content, and examined for immunomodulatory potential. Plasma collected from whole blood in ethylenediaminetetraacetic acid tubes was subjected to differential centrifugation at 30 000 g for 30 minutes and 100 000 g for 2 hours. The 100 000 g pellet was resuspended in 1 mL of ×1 phosphate-buffered saline, loaded onto Optiprep velocity gradients, and subjected to flotation centrifugation at 250 000 g for 2 hours. A–C, Fractions from individuals seropositive (n = 10) or seronegative (n = 10) for human immunodeficiency virus (HIV) were subjected to an enzymatic assay for acetylcholinesterase (AChE), a marker for exosomes (A) and Western blot analysis for exosomal markers CD9, CD45, and CD63 (B) and were immunolabeled with anti-CD63 and examined with electron microscopy to confirm preparation of purified exosomes (C). D, Plasma viral loads of HIV-infected volunteers ranged from 1423 to 536 436 HIV-1 RNA copies/mL, with an average viral load of 205 957 HIV RNA copies/mL. Representative fractions from an HIV-1–seropositive individual were assayed for exosomal markers and HIV-1 viral particles via Western blot analysis to confirm purification of exosomes from HIV-1 viral particles. Purified exosomes and whole plasma from individuals seropositive (n = 10) or seronegative (n = 15) for HIV-1 were analyzed for proinflammatory cytokine and chemokine expression using a 21-plex multiplex array. E, All 21 proinflammatory cytokines and chemokines measured (interleukin 1α [IL-1α], interleukin 2 [IL-2], interleukin 2Rα [IL-2Rα], interleukin 4 [IL-4], interleukin 5 [IL-5], interleukin 7 [IL-7], interleukin 9 [IL-9], interleukin 12p70 [IL-12p70], interleukin 15 [IL-15], interleukin 16 [IL-16], CD40L, granulocyte colony-stimulating factor (G-CSF), interferon [IFN] β and α2, CXCL10, CCL2, CCL3, CCL4, soluble Fas ligand [sFasL], soluble intracellular adhesion molecule 1 [sICAM], and tumor necrosis factor [TNF] α) were associated with and significantly elevated in the exosomes of HIV-1–seropositive individuals compared with seronegative controls (a selection of representative cytokines comparing HIV-1–seropositive and seronegative controls is displayed). F, Alternatively, IL-1α, IFN-α2, and CXCL10 were significantly elevated in the corresponding plasma of HIV-1–seropositive individuals compared with seronegative controls. Error bars represent mean and standard error of the mean (SEM) from independent donors. Difference between groups were tested for statistical significance with the Mann–Whitney U test. *P < .05; †P < .01; ‡P < .001. G, CD38 expression was increased on the surface of naive and central memory CD4+ and CD8+ T-cells. A total of 3.0 × 106 peripheral blood mononuclear cells (PBMCs) from 6 HIV-1–seronegative individuals were exposed to either pool exosomes isolated from the plasma of 3 HIV-1–seropositive (HIV+ Exo) or HIV-1–seronegative (HIV− Exo) individuals, left untreated, or treated with 5 µg/mL of concanavalin A (ConA) as a positive control. 48 hours after exposure, Naive (CD45RA+/CD62L+), central (TCM; CD45RA−/CD62L+) and effector (TEM; CD45RA−/CD62L−) memory CD4+ and CD8+ T cells were analyzed for CD38 expression using flow cytometry. Exosomes were normalized by total protein and added at a concentration of 1 μg/mL. Error bars represent mean and SEM values from 6 independent donors. Differences between groups were tested for statistical significance with 1-way analysis of variance. *P < .05; †P < .01; ‡P < .001.
Exosome Characterization
Purified exosomes were assayed by Western analysis for exosomal markers CD9, CD45, and CD63, and immunolabeled with anti-CD63 and examined via electron microscopy to confirm the quality of the purified exosome preparations. Purified exosomes and whole plasma were analyzed for proinflammatory cytokine and chemokine expression using a human cytokine/chemokine 21-plex magnetic bead kit (Affymetrix; Table 1). Data were acquired using a luminex-200 system and analyzed using Bio-Plex Manger software, version 6.0 (Figure 1B and 1C; Table 1).
Table 1.
Purified Exosomes and Whole Plasma From HIV-1–Seropositive (n = 10) and HIV-1–Seronegative (n = 15) Individuals Analyzed for Proinflammatory Cytokine and Chemokine Expression Using a 21-Plex Multiplex Arraya
| Cytokine | Exosome Cytokine Concentration, Median (IQR), pg/mL |
P Value | Plasma Cytokine Concentration, Median (IQR), pg/mL |
P Value | ||
|---|---|---|---|---|---|---|
| HIV Seronegative | HIV Seropositive | HIV Seronegative | HIV Seropositive | |||
| IL-1α | 0.333 (0.0–11.37) | 33.22 (10.03–158.7) | .008b | 14.60 (9.250–71.35) | 52.52 (24.70–114.5) | .05c |
| IL-2 | 1.838 (0.0–4.382) | 12.99 (4.424–47.22) | .003b | 7.040 (2.370–33.0) | 15.85 (6.028–43.23) | .13 |
| IL-2Rα | 21.49 (5.782–49.86) | 172.1 (46.21–373.9) | .006b | 83.67 (48.75–124.7) | 83.75 (72.25–108.8) | .60 |
| IL-4 | 2.930 (0.0–7.782) | 27.86 (6.355–91.18) | .01c | 9.270 (6.050–34.0) | 17.27 (8.965–42.99) | .11 |
| IL-5 | 34.19 (9.028–112.9) | 227.4 (57.89–509.3) | .008b | 19.81 (14.50–42.16) | 31.44 (20.02–63.13) | .11 |
| IL-7 | 26.85 (5.273–82.68) | 217.9 (48.84–619.1) | .01c | 9.530 (7.620–50.10) | 29.85 (7.458–84.45) | .31 |
| IL-9 | 71.79 (14.68–308.6) | 686.3 (240.0–1529) | .005b | 37.85 (30.81–122.1) | 68.24 (40.02–140.2) | .11 |
| IL-12p70 | 13.79 (2.812–41.35) | 197.9 (39.47–459.5) | .005b | 6.200 (4.520–37.07) | 16.02 (5.955–49.67) | .27 |
| IL-15 | 43.53 (6.533–121.0) | 173.8 (75.65–881.8) | .01c | 29.88 (20.21–185.1) | 90.27 (22.11–214.2) | .39 |
| IL-16 | 38.18 (5.550–72.10) | 142.6 (74.56–442.1) | .01b | 335.3 (146.1–1405) | 253.7 (151.0–450.5) | .33 |
| TNF-α | 2.211 (0.555–6.778) | 36.70 (6.687–95.20) | .002b | 24.81 (17.06–116.6) | 66.30 (17.90–180.3) | .42 |
| IFN-α2 | 0.0 (0.0–15.93) | 32.35 (14.76–122.6) | .005b | 18.02 (8.140–70.48) | 55.96 (34.91–146.8) | .01c |
| IFN-β | 0.0 (0.0–0.9324) | 44.14 (0.0–316.6) | .005b | 14.16 (5.400–55.91) | 7.885 (0.0–90.70) | .49 |
| CXCL10 | 0.0 (0.0–27.15) | 102.2 (17.91–268.9) | .005b | 57.82 (33.15–100.8) | 118.7 (67.38–168.5) | .02c |
| CCL2 | 49.90 (15.38–124.0) | 232.6 (106.9–457.7) | .002b | 26.80 (22.47–51.15) | 22.85 (21.13–42.18) | .60 |
| CCL3 | 0.0 (0.0–2.248) | 2.928 (0.465–20.0) | .02c | 4.470 (1.220–8.940) | 4.355 (1.225–16.42) | .49 |
| CCL4 | 0.0 (0.0–0.0) | 11.76 (0.0–137.3) | .02c | 24.81 (17.06–116.6) | 66.30 (17.90–180.3) | .42 |
| CD40L | 1.877 (0.740–10.04) | 31.89 (6.800–114.5) | .01b | 187.2 (100.2–634.4) | 217.6 (123.1–342.5) | .80 |
| G-CSF | 8.170 (0.0–58.81) | 103.4 (20.90–435.4) | .02c | 9.760 (5.220–71.12) | 25.24 (8.748–74.59) | .21 |
| sICAM | 86.16 (51.02–167.5) | 446.4 (145.4–1363) | .01b | 2433 (985.9–4633) | 1489 (1288–1976) | .56 |
| sFasL | 1.998 (0.348–6.099) | 12.55 (6.914–52.34) | .006b | 5.370 (4.390–14.85) | 8.415 (5.600–16.31) | .26 |
Abbreviations: G-CSF, granulocyte colony-stimulating factor; HIV, human immunodeficiency virus; IFN, interferon; IL-1α, interleukin 1α; IL-2, interleukin 2; IL-2Rα, interleukin 2Rα; IL-4, interleukin 4; IL-5, interleukin 5; IL-7, interleukin 7; IL-9, interleukin 9; IL-12p70, interleukin 12p70; IL-15, interleukin 15; IL-16, interleukin 16; IQR, interquartile range; sFasL, soluble Fas ligand; sICAM, soluble intracellular adhesion molecule 1; TNF, tumor necrosis factor.
a All 21 proinflammatory cytokines and chemokines measured were associated with and significantly elevated in the exosomes of HIV-1–seropositive individuals compared with seronegative controls. Alternatively, IL-1α, IFN-α2, and CXCL10 were significantly elevated in the corresponding plasma of HIV-1–seropositive individuals compared with seronegative controls. The difference between groups was tested for statistical significance with the Mann–Whitney U test.
b P < .01.
c P < .05.
Assay for Immunomodulatory Potential
A total of 3.0 × 106 peripheral blood mononuclear cells (PBMCs) were cultured at 37°C in Roswell Park Memorial Institute 1640 medium supplemented with 20% heat-inactivated exosome-depleted fetal bovine serum and exposed to either pooled exosomes isolated from the plasma of 3 HIV-1–seropositive or HIV-1–seronegative individuals, while parallel untreated cultures and cultures treated with concanavalin A (ConA; 5 µg/mL) served as negative and positive controls, respectively. At 48 hours after exposure, naive (CD45RA+/CD62L+), central (TCM; CD45RA−/CD62L+), and effector (TEM; CD45RA−/CD62L−) memory CD4+ and CD8+ T-cells were analyzed for CD38 expression with flow cytometry. Exosome preparations were normalized by total protein and added at a concentration of 1 µg/mL. Statistical analysis was performed, and graphs were generated using SigmaPlot 10 or GraphPad Prism 6.0 software. All tests were set at a P level of < .05.
Antibodies
The following antibodies were used in this study: rabbit polyclonal anti-CD63 (Santa Cruz), rabbit polyclonal anti-CD9 (Santa Cruz), rabbit polyclonal anti-CD45 (Abcam), rabbit polyclonal anti-p24 (ImmunoDiagnostic), murine monoclonal anti-AChE (EMD Millipore), and goat anti-mouse or anti-rabbit immunoglobulin G (IgG) (H+L) labeled with horseradish peroxidase (Thermo Fisher Scientific). The following fluorochrome-conjugated monoclonal antibodies were used for flow cytometry analyses: Alexa Fluor 700–labeled anti-CD3 (UCHT1; BD Bioscience), allophycocyanin (APC)/cyanine 7 (Cy7)–labeled anti-CD4 (OKT4; Biolegend), peridinin chlorophyll protein complex-labeled anti-CD4 (RPA-T4; BD Bioscience), V450-labeled anti-CD8 (RPA-T8; BD Bioscience), biotin-labeled anti-CD45RA (HI100; BD Bioscience), phycoerythrin (PE)/Cy7–labeled anti-CD62L (DREG-56; Biolegend), PE/cyanine 5 (Cy5)–labeled anti-CD38 (HIT2; Biolegend), APC/CY7-labeled anti-HLADR (L243; Biolegend), PE-Texas Red–labeled anti-streptavidin (BD Bioscience), PE/Cy5-labeled mouse IgG1K isotype control (MOPC-21; Biolegend), and APC-Cy7–labeled mouse IgG2aK isotype control (MOPC-173; Biolegend).
RESULTS
Exosomes purified from the plasma of HIV-1–seropositive and seronegative individuals were characterized for AChE activity, the presence of CD45, CD9, and CD63 (Figure 1A–C), and the absence of HIV p24 (Figure 1D) to ascertain their purity. They were then compared for levels of 21 cytokines/chemokines by multiplex assay (Table 1). In the exosomes isolated from HIV-positive individuals (n = 10; Table 1), all 21 cytokines/ chemokines were detected. In addition, their levels were also found to be significantly elevated (Figure 1E) relative to both their corresponding plasma levels and when compared with exosomes isolated from HIV-negative controls (n = 15; Table 1, Figure 1E). Only interleukin 1α, interferon α, and CXCL10 were elevated in the plasma of HIV-1–viremic individuals compared with controls (Figure 1F; Table 1).
To test the potential clinical relevance of exosome cytokine content, PBMCs from uninfected human donors were exposed to pooled exosomes isolated from 3 HIV-positive individuals or HIV-negative controls for 48 hours and assessed for induction of CD38 and HLA-DR, markers for activation, on CD4+ and CD8+ T cells via flow cytometry. PBMCs exposed to ConA and untreated PBMCs were used as positive and negative controls, respectively. A significant increase in cell surface expression of CD38 was observed on both naive and central memory CD4+ and CD8+ T cells exposed to HIV positive exosomes compared with cells exposed to HIV-negative exosomes and untreated controls (Figure 1G), but, interestingly, HLA-DR was not significantly up-regulated by coculture with exosomes (data not shown).
DISCUSSION
In this report we describe evidence of active and selective enrichment of most screened cytokine and chemokines in plasma exosomes of HIV-seropositive individuals. The levels of these factors were significantly increased compared with both the soluble plasma levels, and the levels in exosomes in the plasma of HIV-seronegative individuals. We used AChE normalization to get a real comparison of cytokine levels between the exosomal vs the soluble plasma fraction, confirming that the increase of cytokine/chemokines levels in exosomes is real and significant. Finally, we found that the exosomes from HIV-seropositive individuals was biologically active inducing increases in the activation marker, CD38, on the surface of naive and central memory CD4+ and CD8+ lymphocytes. There was no comparable effect on the same cell subtypes due to exosomes from HIV-seronegative individuals. Although HLA-DR was up-regulated with ConA stimulation, expression of this activation marker surprisingly was not significantly up-regulated during coculture with the HIV-seropositive exosomes (data not shown). Although both activation markers are often associated, they also have been shown to undergo distinct regulation in HIV-infected patients [10].
These data suggest a potentially interesting mechanism by which HIV infection could indirectly recruit novel targets through activation induction of naive CD4+ T cells and contribute to the continuous chronic viral replication in vivo. In addition, generalized activation of naive and memory CD8+ T cells could contribute to dysfunction of this cell pool as a whole, resulting in poor responses to HIV and other pathogens, exhaustion of antigen-specific T cells, and induction of chronic immune activation.
Interestingly, this mechanism has remarkable similarity to a host mechanism leading to immune privilege during pregnancy. Multiple roles of extracellular vesicles have been examined in the complex process of a successful pregnancy. Furthermore, their involvement in the pathology of preeclampsia has been examined, where elevated circulating extracellular vesicles have been implicated in contributing to exacerbated maternal systemic innate immune cell activation and vascular dysfunction [11]. This same process has also been found to be hijacked during carcinogenesis, leading to immunomodulation/inflammation and ultimately allowing tumor growth [12]. It is clear from the literature in this area that knowledge of the role played by these extracellular vesicles would allow harnessing their ability for immunotherapy [13].
One of the characteristics of chronic immune activation is chronically elevated cytokine levels [3]. While cytokine levels in the plasma of HIV-seropositive individuals have been the subject of intense investigation, the results presented here demonstrate that a significant amount of cytokines/chemokines is not released in free form but is associated with and seemingly enriched within exosomes. Given the increased stability of molecules carried by exosomes [14], it is plausible that association of cytokines and chemokines within exosomes would imply their increased half-life, as well as their wider distribution to specific target cells distal from the producer cells. Although there are several reports by our group and others of increased exosome release from HIV-infected cells and Nef-transduced cell lines [6–8, 15], the origin of cytokine-laden exosomes in HIV-infected individuals remains to be fully elucidated. Consequently, further investigations will be needed to ascertain the respective contribution of infection, HIV proteins, and host cell responses in the release of cytokine-laden exosomes into the circulation. In summary, our results suggest a potentially important mechanism that may contribute to chronic viral replication and chronic immune activation during HIV infection, leading to exciting avenues for future inquiry.
Notes
Acknowledgments. We thank the following persons: Cameron Tran, William Roth, James Lillard, Deborah Lyn, Cindy Tran, Eduardo Silveira, Kenneth Rogers, Martin Shelton, Mafuz Khan, and Jung Joo Hong.
Author contributions. K. A. K. was responsible for the concept and design of the study, acquisition of in vitro data, and cowriting the manuscript with V. C. B.; J. C. was responsible for acquisition of multiplex cytokine/chemokine data; M. B. H. was responsible for isolating exosomes from human plasma; P. K. A. contributed to the statistical analysis and experimental design. M. D. P. provided scientific input and assisted in manuscript revision. W. A., F. V., and V. C. B. provided substantial scientific input, assisted in the design and concept of the study and participated in writing and revising the manuscript. The manuscript was approved by all contributing authors.
Financial support. This work was supported by a United Negro College Fund/Merck Graduate Research Fellowship (to K. A. K.), the American Medical Association Foundation (K. A. K.), Clinical Research Education and Career Development (CRECD; grant 8R25MD007589-10 to K. A. K.), Minority Biomedical Research Support, National Institute of General Medical Sciences, National Institutes of Health (NIH) (grant R25 GM058268 to K. A. K.), National Institute on Minority Health and Health Disparities (grants 8G12MD007602 and 8U54MD007588), National Institute of Allergy and Infectious Diseases (grant 1R21AI095150-01A1), Georgia Research Alliance (grant GRA.VAC08.W), and Emory Center for AIDS Research (grant P30 A1050409).
Disclaimer. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Potential conflicts of interest. All authors: No potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Bofill M, Mocroft A, Lipman M, et al. Increased numbers of primed activated CD8+CD38+CD45RO+ T cells predict the decline of CD4+ T cells in HIV-1-infected patients. AIDS 1996; 10:827–34. [DOI] [PubMed] [Google Scholar]
- 2.Liu Z, Cumberland WG, Hultin LE, Prince HE, Detels R, Giorgi JV. Elevated CD38 antigen expression on CD8+ T cells is a stronger marker for the risk of chronic HIV disease progression to AIDS and death in the Multicenter AIDS Cohort Study than CD4+ cell count, soluble immune activation markers, or combinations of HLA-DR and CD38 expression. J Acquir Immune Defic Syndr Hum Retrovirol 1997; 16:83–92. [DOI] [PubMed] [Google Scholar]
- 3.Katsikis PD, Mueller YM, Villinger F. The cytokine network of acute HIV infection: a promising target for vaccines and therapy to reduce viral set-point? PLoS Pathog 2011; 7:e1002055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meckes DG, Jr, Raab-Traub N. Microvesicles and viral infection. J Virol 2011; 85:12844–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell TD, Khan M, Huang MB, Bond VC, Powell MD. HIV-1 Nef protein is secreted into vesicles that can fuse with target cells and virions. Ethn Dis 2008; 18(2 suppl 2):S2–9. [PMC free article] [PubMed] [Google Scholar]
- 6.Muratori C, Cavallin LE, Kratzel K, et al. Massive secretion by T cells is caused by HIV Nef in infected cells and by Nef transfer to bystander cells. Cell Host Microbe 2009; 6:218–30. [DOI] [PubMed] [Google Scholar]
- 7.Lenassi M, Cagney G, Liao M, et al. HIV Nef is secreted in exosomes and triggers apoptosis in bystander CD4 T cells. Traffic 2009; 11:110–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raymond AD, Campbell-Sims TC, Khan M, et al. HIV type 1 Nef is released from infected cells in CD45+ microvesicles and is present in the plasma of HIV-infected individuals. AIDS Res Hum Retroviruses 2011; 27:167–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cantin R, Diou J, Belanger D, Tremblay AM, Gilbert C. Discrimination between exosomes and HIV-1: purification of both vesicles from cell-free supernatants. J Immunol Methods 2008; 338:21–30. [DOI] [PubMed] [Google Scholar]
- 10.Hua S, Lecuroux C, Saez-Cirion A, et al. Potential role for HIV-Specific CD38-/HLA-DR+ CD8+ T cells in viral suppression and cytotoxicity in HIV controllers . PLoS One 2014; 9:e101920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tannetta D, Dragovic R, Alyahyaei Z, Southcombe J. Extracellular vesicles and reproduction-promotion of successful pregnancy. Cell Mol Immunol 2014; 11:548–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Altevogt P, Bretz NP, Ridinger J, Utikal J, Umansky V. Novel insights into exosome-induced, tumor-associated inflammation and immunomodulation. Semin Cancer Biol 2014; 28C:51–7. [DOI] [PubMed] [Google Scholar]
- 13.Gehrmann U, Naslund TI, Hiltbrunner S, Larssen P, Gabrielsson S. Harnessing the exosome-induced immune response for cancer immunotherapy. Semin Cancer Biol 2014; 28C:58–67. [DOI] [PubMed] [Google Scholar]
- 14.Pant S, Hilton H, Burczynski ME. The multifaceted exosome: biogenesis, role in normal and aberrant cellular function, and frontiers for pharmacological and biomarker opportunities. Biochem Pharmacol 2012; 83:1484–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ali SA, Huang MB, Campbell PE, et al. Genetic characterization of HIV type 1 Nef-induced vesicle secretion. AIDS Res Hum Retroviruses 2010; 26:173–92. [DOI] [PMC free article] [PubMed] [Google Scholar]