Abstract
Background. Meropenem plus levofloxacin treatment was shown to be a promising combination in our in vitro hollow fiber infection model. We strove to validate this finding in a murine Pseudomonas pneumonia model.
Methods. A dose-ranging study with meropenem and levofloxacin alone and in combination against Pseudomonas aeruginosa was performed in a granulocytopenic murine pneumonia model. Meropenem and levofloxacin were administered to partially humanize their pharmacokinetic profiles in mouse serum. Total and resistant bacterial populations were estimated after 24 hours of therapy. Pharmacokinetic profiling of both drugs was performed in plasma and epithelial lining fluid, using a population model.
Results. Meropenem and levofloxacin penetrations into epithelial lining fluid were 39.3% and 64.3%, respectively. Both monotherapies demonstrated good exposure responses. An innovative combination-therapy analytic approach demonstrated that the combination was statistically significantly synergistic (α = 2.475), as was shown in the hollow fiber infection model. Bacterial resistant to levofloxacin and meropenem was seen in the control arm. Levofloxacin monotherapy selected for resistance to itself. No resistant subpopulations were observed in any combination therapy arm.
Conclusions. The combination of meropenem plus levofloxacin was synergistic, producing good bacterial kill and resistance suppression. Given the track record of safety of each agent, this combination may be worthy of clinical trial.
Keywords: synergy, Pseudomonas aeruginosa, meropenem, levofloxacin, Mathematical Modeling
Combination chemotherapy is prudent in the face of ventilator-requiring, hospital-acquired pneumonia (VRHAP) due to Pseudomonas aeruginosa infection [1, 2]. There are 2 goals of therapy. The first is to obtain a rapid, multilog reduction in the bacterial burden. The second is to suppress the amplification of a resistant bacterial subpopulation.
Obtaining a rapid reduction in the organism load enhances the cell-killing capability of granulocytes. We recently demonstrated in a murine pneumonia model that early appropriate chemotherapy allows the bacterial burden to decline below the half-saturation point of neutrophils [2]. This allows the granulocytes to add 1–1.5 extra logs of bacterial killing in the second day. This leads to rapid bacterial clearance in the lungs of mice, which, for patients with pneumonia, hopefully translates to earlier extubation and transfer out of the intensive care unit.
Recently, Chastre et al examined the outcomes of people with ventilator-associated pneumonia who were treated with a carbapenem [3]. Of 28 patients that started with a fully susceptible isolate of P. aeruginosa, >50% had a ≥4-fold increase in the carbapenem minimum inhibitory concentration (MIC) during therapy. No differences were seen among imipenem, meropenem, or doripenem. Consequently, use of a regimen that also suppresses amplification of the less-susceptible populations is important.
In our previous in vitro hollow fiber infection model (HFIM) study, this combination had a more rapid attainment of multilog cell killing than the monotherapies and completely suppressed the emergence of resistance over 14 days [1]. We speculated that the mechanism was a saturation of the efflux pumps. We felt it was important to valid the benefit of this combination in a murine pneumonia model.
METHODS
Microorganism
P. aeruginosa strain PAO1 was a kind gift of Drs Bush and Queenan of Johnson & Johnson (Raritan, New York). Determination of MICs was performed using microbroth methods published by the Clinical and Laboratory Standards Institute [4]. Mutation frequencies for the antibiotics were determined to 3 times the MICs.
Murine Pneumonia Model
The model and detailed methods have been previously described [5]. All animal experimentation was approved by the University of Florida Institutional Animal Care and Use Committee. Female, 24–26-g, outbred Swiss-Webster mice (Taconic Farms, Taconic, NY) were rendered transiently neutropenic with 150 mg/kg of cyclophosphamide given intraperitoneally 4 days before infection plus 100 mg/kg given intraperitoneally 1 day before infection. Anesthetized mice were infected via the intranasal route with 5 × 107 colony-forming units (CFU) of P. aeruginosa. The bacterial inoculum was confirmed by quantitative cultures. Two hours after bacterial inoculation, and before therapy initiation, 5 mice were euthanized, and lungs were obtained for baseline quantitative cultures of lung homogenates. Seventeen animal cohorts were administered meropenem or levofloxacin alone or in combination. There was also an untreated control group. Twenty-four hours after treatment initiation, all mice were humanely euthanized, and lungs were aseptically collected, homogenized, and washed. The homogenates were quantitatively cultured onto drug-free agar to characterize the effect of each regimen on the total bacterial population. For groups that were treated with meropenem or levofloxacin alone, homogenates were also quantitatively cultured onto agar supplemented with 3 times the baseline MIC of the respective drug to define the extent to which the regimens selected for an antibiotic-resistant subpopulation. For mice that were treated with both antibiotics, tissue homogenates were quantitatively cultured onto agar plates supplemented with 3 times the MIC of meropenem and separate plates containing 3 times the MIC of levofloxacin. After incubation of the plates at 35°C for 48 hours, the colonies were enumerated.
Meropenem was administered every 4 hours to partially humanize its serum pharmacokinetic profiles. To humanize the serum pharmacokinetic profile of levofloxacin, this drug was administered twice daily, with 75% of the total dose administered at time 0 and the remaining 25% administered at hour 12. The levofloxacin humanization scheme was derived from earlier single-dose pharmacokinetic studies [6]. Meropenem dosing as a single agent was 5, 8, 12.5, and 50 mg/kg every 4 hours. For single-agent levofloxacin, total daily doses of 25, 63, 126, and 256 mg/kg/day were evaluated. In addition, 63 mg/kg/day of levofloxacin was administered in a nonhumanized fashion, as a single injection, to assess differences in outcomes with different dosing algorithms. For the drugs in combination, doses of 25 or 63 mg/kg/day of levofloxacin (humanized) were administered with 5, 8, or 12.5 mg/kg of meropenem every 4 hours. In another cohort, 12.5 mg/kg of meropenem was administered every 4 hours in combination with a single nonhumanized dose of levofloxacin. Finally, there was a no-treatment control.
Pharmacokinetic Studies
To correlate the doses of drug administered to mice with measures of exposure, pharmacokinetic studies for meropenem and levofloxacin were conducted in mice with P. aeruginosa pneumonia.
Briefly, neutropenic mice were infected under anesthesia with P. aeruginosa via the intranasal route. Two hours later, single doses of 50, 100, 200, and 400 mg/kg of meropenem and single doses of 2.39, 17.08, and 150 mg/kg of levofloxacin were given intraperitoneally to mice. Previous work had shown no drug interaction for the combination (data not shown). Plasma and bronchoalveolar (BAL) fluid were collected from 3 mice per time point (and dose) over 5–6 hours and were stored at −80°C until they were assayed for drug content by liquid chromatography/dual mass spectrometry (LC-MS/MS). The prediluted concentration of antibiotic in the BAL fluid (epithelial lining fluid [ELF]) was calculated by comparing the ratio of the amounts of urea measured in simultaneously collected plasma and BAL fluid.
Meropenem, Levofloxacin, and Urea Assays
For mouse plasma samples, an aliquot of 0.050 mL per sample was deproteinated with 0.150 mL acetonitrile. The resulting mixtures were centrifuged, and 0.100 mL of the supernatant was added to 1.00 mL of high-pressure liquid chromatography (HPLC)–grade water. Samples were analyzed by HPLC-MS/MS for meropenem and levofloxacin concentrations [6, 7]. For mouse BAL fluid samples, an aliquot of 0.050 mL was diluted with 0.100 mL of HPLC water and analyzed. The LC-MS/MS system was composed of a Shimadzu Prominence HPLC system and an ABSciex API5000 triple quadrupole mass spectrometer.
Chromatographic separation was performed in isocratic mode, using a Thermo Scientific Hypersil Gold column (150 × 4.6 mm; 5-µm particle size) and a mobile phase consisting of 85:15 0.1% formic acid in water:methanol at a flow rate of 0.75 mL/minute.
Meropenem concentrations were obtained using LC-MS/MS, monitoring the MS-MS transition m/z 384→ m/z 141. Analysis run time was 4.0 minutes. The assay was linear over a range of 0.005 to 10 mg/L (r2 = 0.993) for meropenem in mouse plasma and a range of 0.10 to 200 ng/mL (r2 = 0.994) for meropenem in mouse BAL fluid. The interday coefficients of variation for the quality control samples, which contained meropenem analyzed in replicates of 3 at 3 concentrations on each analysis day, ranged from 0.543% to 4.29% for meropenem in mouse plasma and from 1.87% to 5.08% for meropenem in BAL fluid. Accuracy (ie, percentage recovery) for these same quality control samples ranged between 92.7% and 103% for meropenem in mouse plasma and between 96.9% and 102% for meropenem in BAL fluid.
For levofloxacin, concentrations were obtained using the MS/MS transition m/z 362.2→ m/z 261.2. The interday coefficient of variation for the plasma quality control samples ranged from 3.65% to 6.22%, with the percentage recovery ranging from 102% to 108% at 3 levels (10 ng/mL, 100 ng/mL, and 500 ng/mL) in 3 replicates, and assay findings were linear over a range of 5.00 to 1000 ng/mL (r2 = 0.994). For BAL samples, the interday coefficient of variation ranged from 4.27% to 5.35%, and the percentage recovery ranged from 102.0% to 102.5%.
Analysis of urea in murine plasma and ELF was performed using the BioAssay Systems QuantiChrom urea assay kit (Hayward, California). The standard curve was linear, from 3.1 mg/dL to 50 mg/dL (r2 = 0.999). The range of the interday coefficient of variation was 3.63%–10.5% for plasma and 5.85%–9.77% for BAL fluid.
Population Pharmacokinetic Modeling Approach
The approach has been described previously [7]. We performed single-dose pharmacokinetic studies for meropenem and levofloxacin in infected mice. For the population modeling approach, the ELF was its own sampling compartment with its own volume of distribution. It required 8 parameters, 4 differential equations, and 2 system outputs (drug concentration in plasma and drug concentration in ELF) to define this system:
| (1) |
| (2) |
| (3) |
| (4) |
The plasma concentration is calculated as X2/[Vc/F], and the ELF concentration is calculated as X4/VELF.
The BigNPAG program [8] was used for all population modeling. The weighting used was the inverse of the observation variance. Bayesian estimates were obtained using the population-of-one utility in BigNPAG. Model evaluation was performed by predicted-observed plots. The mean weighted error served as the measure of bias. The bias-adjusted mean weighted squared error served as the measure of precision. Because only single plasma and ELF measurements were available for any animal, the Adaptive γ feature for weighting was not used.
Drug Interaction Analysis for Total Bacterial Burden Cell Kill
The approach embeds the Greco Universal Response Surface Approach (URSA) into the time-dependent framework, in which total bacterial cell killing is linked to drug concentrations through the URSA model. Because there was minimal resistance emergence and no resistant bacteria were recovered from the combination therapy arms, the full model, as published elsewhere [9], was not used. Full model details are found in the Supplementary Materials.
Simulation
We used the ADAPT V package [10] for simple simulation (concentration-time profile in plasma and ELF). For Monte Carlo simulation, Pmetrics [11] was used.
RESULTS
MICs and Mutational Frequency to Resistance
The meropenem and levofloxacin MICs for the PAO1 strain were both 0.5 mg/L.
The mutational frequency for isolates with ≥3 times the MIC to meropenem was −6.76 log CFU, and the mutational frequency for isolates with ≥3 times the MIC to levofloxacin was −6.77 log CFU.
Murine Pharmacokinetics of Meropenem and Levofloxacin
As previously demonstrated, meropenem murine pharmacokinetics were best described by a 3-compartment model [7], and those for levofloxacin were best described by a 4-compartment model [6].
The point estimates of the mean and median parameter vectors and their standard deviations for each agent are displayed in Table 1. These were concordant with previous investigations [6, 7].
Table 1.
Population Pharmacokinetic Parameters of Meropenem and Levofloxacin in Infected Mice
| Drug, Parameter | Vc/F, L | CL/F, L/h | K23, h−1 | K32, h−1 | K24, h−1 | K42, h−1 | VELF, L | Ka, h−1 |
|---|---|---|---|---|---|---|---|---|
| Meropenem | ||||||||
| Mean | 0.00348 | 0.0169 | 17.30 | 11.98 | … | … | 0.0141 | 25.52 |
| Median | 0.00342 | 0.0169 | 16.57 | 11.04 | … | … | 0.0131 | 26.18 |
| SD | 0.0002214 | 0.00159 | 1.356 | 1.330 | … | … | 0.00253 | 3.459 |
| Levofloxacin | ||||||||
| Mean | 0.0229 | 0.0810 | 12.57 | 10.48 | 6.546 | 4.114 | 0.0628 | 4.823 |
| Median | 0.0201 | 0.0800 | 10.08 | 10.49 | 7.466 | 3.987 | 0.0663 | 4.718 |
| SD | 0.00405 | 0.0019 | 3.245 | 1.111 | 1.629 | 1.344 | 0.0138 | 1.131 |
Median values were used for Bayesian estimation.
Abbreviations: CL/F, apparent drug clearance; Ka, first-order absorption rate constant; K23, K32, K24, K42, first-order intercompartmental transfer rate constants; SD, standard deviation; Vc/F, apparent volume of the central compartment; VELF, volume of the epithelial lining fluid compartment.
The model fit the data well, with the following Bayesian (individual) observed-predicted regressions for meropenem for plasma and ELF:
For levofloxacin, the equations were as follows:
Measures of bias and precision were acceptable for all outputs.
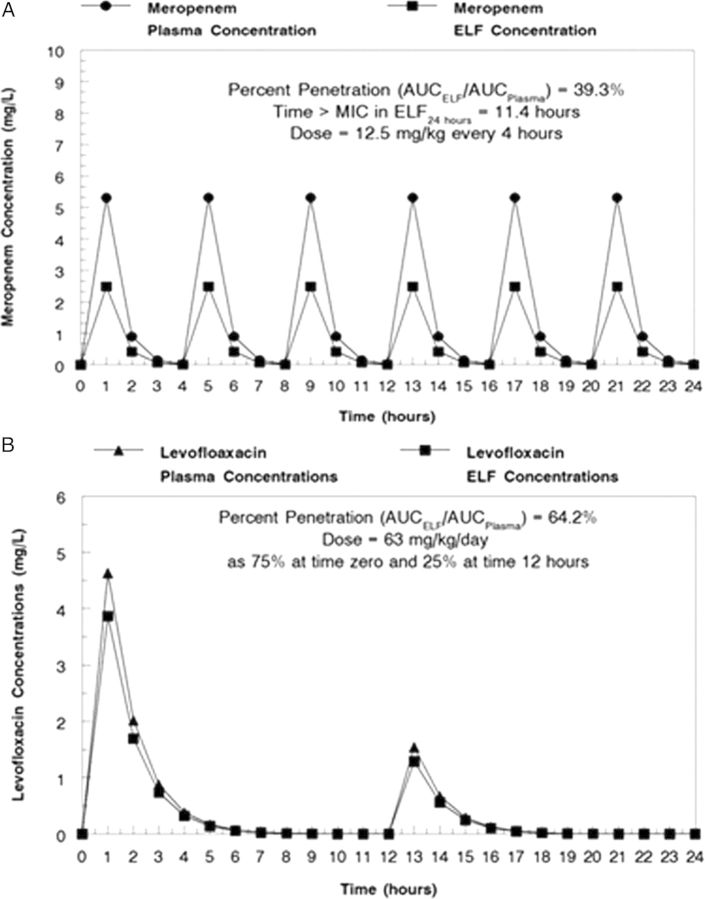
Simple simulations for both agents demonstrated a penetration into ELF of 39.3% for meropenem and 64.3% for levofloxacin. The duration of time the concentration of the drug remains above the MIC for the microbe (Time > MIC) for levofloxacin or the ratio of the AUC to the MIC (for levofloxacin) for the regimens used for therapy are displayed in Table 2. It should be noted that these values represent integrated measures. The analysis performed below is a completely different way of examining the exposure to drugs, in which time-dependent changes in drug concentrations and their interaction are accounted for.
Table 2.
Time > Minimum Inhibitory Concentration (MIC) in Epithelial Lining Fluid (ELF) for Meropenem and Ratio of the Area Under the Receiver Operating Characteristic Curve (AUC) to the MIC in ELF for Levofloxacin, by Dose, for Treatment of Murine P. aeruginosa Pneumonia
| Variable, Dose in mg/kg | Value |
|---|---|
| Meropenem time > MICa | |
| 5 | 8.22/24 h |
| 8 | 9.84/24 h |
| 12.5 | 11.4/24 h |
| 50 | 16.1/24 h |
| Levofloxacin AUC:MICa ratio | |
| 25 | 10.03 |
| 63 | 25.28 |
| 126 | 50.55 |
| 256 | 102.7 |
For meropenem, doses were administered every 4 hours. For levofloxacin, doses indicated are daily doses.
a MIC = 0.5 mg/L
The simulated concentration-time profiles over 24 hours for 12.5 mg/kg of meropenem every 4 hours and 63 mg/kg/day of levofloxacin, fractionated as 75% at time 0 and 25% at hour 12, are displayed in Figure 1A and 1B from simulation.
Figure 1.
A, Simulated murine plasma and epithelial lining fluid (ELF) concentration-time profiles for meropenem (A) and levofloxacin (B). Abbreviations: AUC, area under the curve; MIC, minimum inhibitory concentration.
P. aeruginosa Killing by Meropenem and Levofloxacin Alone and in Combination
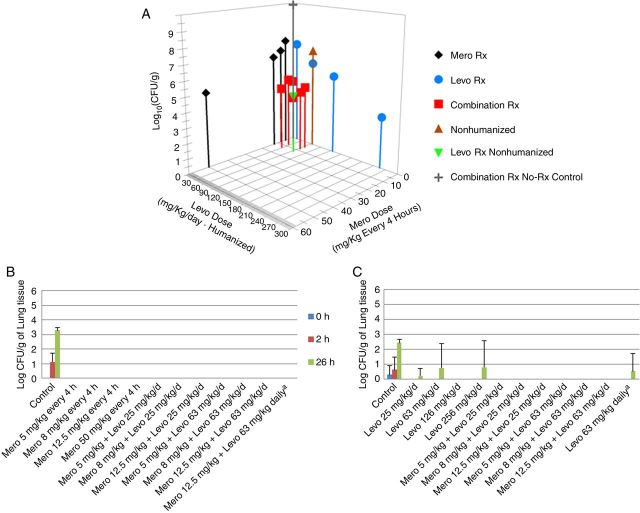
Cell killing data for the total population are displayed in Figure 2A. Clear exposure responses were demonstrated for both meropenem alone and levofloxacin alone. The combination therapy arms demonstrated good effect. All combination therapy arms generated near-maximal bacterial killing at less than maximal drug doses.
Figure 2.
A, Total bacterial cell killing by meropenem (Mero) alone, levofloxacin (Levo) alone, or with Mero plus Levo in combination. B, Bacterial population resistant to Mero. C, Bacterial population resistant to Levo. aLevo was administered daily and not in a humanized fashion. Abbreviation: CFU, colony-forming units.
In addition, we administered levofloxacin as a single nonhumanized dose of 63 mg/kg/day alone, as well as in combination with 12.5 mg/kg of meropenem every 4 hours. The fractionated, humanized regimen made a significant difference when levofloxacin was administered alone (P = .007), but this difference was lost when levofloxacin was administered in combination with meropenem (P = .648).
Mutants resistant to meropenem or levofloxacin were recovered at 24 hours in the no-treatment control cohort. Small numbers of less susceptible isolates were recovered from levofloxacin-treated animals. Mutants were recovered from the levofloxacin single-drug arm when the drug was administered in a nonhumanized fashion but not when administered in a humanized fashion. No less susceptible mutants were recovered from the meropenem monotherapy arm or combination therapy arms. These data are displayed in Figure 2B and 2C.
Drug-Effect Interaction Analysis
All groups were analyzed simultaneously. Because the pharmacokinetic and pharmacodynamics groups were studied separately, we fixed the pharmacokinetic parameters for meropenem and levofloxacin to the values of their median parameter vectors. The mean, median, and standard deviation of the pharmacodynamic parameter values are displayed in Table 3. The model fit the data well. For the Bayesian (individual) analysis r2 was 0.990; observed was equal to 0.999 × predicted + 0.012; bias was −0.110; and precision was 0.209. The maximal kill rate constant was 3.398 h−1. The drug concentrations at which the kill rate was half-maximal were 1.763 mg/L and 0.338 mg/L for meropenem and levofloxacin, respectively. Perhaps most importantly, the value of α (the drug interaction parameter, which determines whether the interaction is synergistic, additive, or antagonistic) was 2.475, with a 95% confidence interval of 2.014–2.710. Because the lower bound of the confidence interval does not cross 0, the drug interaction is significantly synergistic. It is likely that the synergistic interaction was responsible for the ability to administer levofloxacin without fractionation (ie, the nonhumanized fashion) without loss of microbiological activity and without resistance emergence.
Table 3.
Parameters for Bacterial Cell Killing for the Interaction of Meropenem and Levofloxacin
| Parameter | Popmax, CFU/g | Kg, h−1 | Kk, h−1 | EC50, mg/L |
α | Initial Burden,a CFU/g | Hill's Constant |
||
|---|---|---|---|---|---|---|---|---|---|
| Meropenem | Levofloxacin | Meropenem | Levofloxacin | ||||||
| Mean | 7.70 × 109 | 0.464 | 3.398 | 1.763 | 0.338 | 2.475 | 5.01 × 107 | 18.96 | 19.71 |
| Median | 7.68 × 109 | 0.462 | 3.384 | 1.755 | 0.321 | 2.522 | 5.02 × 107 | 19.65 | 19.57 |
| SDb | … | … | … | 0.135 | … | 0.120 | 1.84 × 105 | 0.964 | 0.139 |
Mean values were used for Bayesian estimation.
Abbreviations: α, drug interaction constant; CFU, colony-forming units; EC , concentration for which killing is 50% maximal; Kg, growth rate constant; Kk, kill rate constant; Popmax, maximal population density; SD, standard deviation.
a Initial bacterial burden in the lung.
b Ellipses indicate that all support points had the same point estimates of the parameter values.
Our group has previously reported [2] that a minimum cell-killing level of 2 logs is required for obtaining a high likelihood of reducing the bacterial burden below the half-saturation point for granulocytes, which enhances cell killing and the rate of bacterial clearance in pneumonia. The largest licensed dose of levofloxacin is 750 mg daily, and the maximal licensed adult dose of meropenem is 2 g administered every 8 hours. Using human pharmacokinetic parameter values in which the ELF was measured [12, 13], we performed Monte Carlo simulation (3 hours after meropenem infusion) to determine the probability of obtaining 2-log cell killing. This target was attained 95.7% of the time.
DISCUSSION
We had previously studied the combination of meropenem and levofloxacin in our in vitro HFIM [1], where the regimen demonstrated great promise. The combination demonstrated significantly improved cell killing and full suppression of resistance for the PAO1 isolate of P. aeruginosa and its MexAB-overexpressed isogenic daughter isolate. We felt it important to validate these findings in an animal model of Pseudomonas pneumonia.
We used a neutropenic model, to mimic as closely as possible the HFIM, which lacks an immune system. The findings from this investigation and our previous investigation are conservative because granulocytes can contribute to bacterial clearance when the bacterial burden is below the half-saturation breakpoint concentration [2, 5].
The pharmacokinetics of both meropenem and levofloxacin were concordant with our earlier murine pneumonia studies (Table 1 and elsewhere [6, 7]). The pharmacokinetic model included an ELF compartment, so the actual effect-site concentrations could be directly linked to the antibacterial effect observed.
Meropenem penetrated into the ELF at approximately 39.3% of the plasma AUC, while levofloxacin penetrated into the ELF at approximately 64.3% of the plasma AUC. The ELF concentrations over time were the drivers for cell killing.
The ability of the drugs alone and in combination to effect cell killing and suppress resistance amplification is shown in Figure 2. Near-maximal cell killing is obtained by combination therapy at less than maximal doses. Also, no resistant clones were identified from any combination therapy group. On the basis of the raw data, the findings previously seen with the HFIM [5] were recapitulated in this murine system.
Greco et al had previously identified the URSA model for drug interaction [14]. This model analyzed static concentrations in multiwell plates. We have extended this model to allow time-dependent analysis of combination therapy. We estimated the maximal kill rate constant, the parameters of a sigmoid-Emax effect model for each drug, and identify a parameter, α, that quantifies drug interaction. When α is positive and the lower bound of the 95% confidence interval does not cross 0, the drug interaction is statistically significant (at the .05 level). When α is negative and the upper bound of the 95% confidence interval does not cross 0, the interaction is statistically significantly antagonistic. All other interactions are accorded an interaction of additivity.
In the case of meropenem and levofloxacin, the α was substantial, at 2.475, with the lower bound of the confidence interval at 2.014, which is interpreted as being significantly synergistic. This explains the performance of the combinations even at relatively modest doses. In addition, we partially humanized the administration of levofloxacin, which we have previously shown enhances its activity when murine pharmacokinetics are used [15]. In this study, with levofloxacin alone, there was significantly less activity when the drug was administered without humanization. However, when administered in combination with meropenem, the lack of humanization did not have a significant effect on cell killing. This is likely because of the synergistic interaction.
A Monte Carlo simulation was performed for meropenem (2 g intravenously as a 3-h infusion every 8 hours [7]) in combination with levofloxacin (750 mg intravenously daily [13]). This combination attained 2-log killing 95.7% of the time in a 5000-subject simulation. This is critically important, as such cell killing will have a high likelihood of reducing the bacterial burden below the half-saturation point (ie, the bacterial burden in which granulocyte-mediated bacterial cell killing is half saturated; reducing the burden below this point allows the granulocytes to effect net bacterial killing) in patients with VRHABP. These patients have bacterial burdens exceeding 7.5 logs approximately 25% of the time [2]. The rapid cell killing also has the added benefit of reducing the burden to values less than the inverse of the mutational frequency to resistance, thus decreasing the probability of amplification of less susceptible bacteria.
Meropenem and levofloxacin have a good safety track record. This regimen is safe, produces excellent bacterial killing, and suppresses resistance. For sites where meropenem and levofloxacin have good susceptibility profiles, this combination may represent a reasonable choice. It should be emphasized this is a single isolate evaluation with modest MICs for the agents in question. It should also be noted that the combination also performed well in the HFIM with an isogenic pump–overexpressed mutant (the MIC increased 4-fold for meropenem and 2-fold for levofloxacin relative to the parent strain). Meropenem plus levofloxacin may represent a regimen worthy of clinical evaluation.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases (NIAID) or the National Institutes of Health.
Financial support. This work was supported by the NIAID (grants R01AI079578 and RO1AI079729 to the Institute for Therapeutic Innovation).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Louie A, Grasso C, Bahniuk N, et al. The combination of meropenem and levofloxacin is synergistic with respect to both Pseudomonas aeruginosa kill rate and resistance suppression. Antimicrob Agents Chemother 2010; 54:2646–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drusano GL, Liu W, Fikes S, et al. Interaction of drug- and granulocyte-mediated killing of Pseudomonas aeruginosa in a murine pneumonia model. J Infect Dis 2014; 210:1319–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luyt C-E, Aubry A, Lu Q, et al. Imipenem, meropenem or doripenem to treat patients with Pseudomonas aeruginosa ventilator-associated pneumonia. Antimicrob Agents Chemother 2014; 58:1372–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clinical and Laboratory Standards Institute. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard. 9th ed . M07-A9. Wayne, PA: Clinical and Laboratory Standards Institute, 2012. [Google Scholar]
- 5.Drusano GL, Vanscoy B, Liu W, Fikes S, Brown D, Louie A. Saturability of granulocyte kill of Pseudomonas aeruginosa in a murine model of pneumonia. Antimicrob Agents Chemother 2011; 55:2693–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Louie A, Fregeau C, Liu W, Kulawy R, Drusano GL. Pharmacodynamics of levofloxacin in a murine pneumonia model of Pseudomonas aeruginosa infection: determination of epithelial lining fluid (ELF) targets. Antimicrob Agents Chemother 2009; 53:3325–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drusano GL, Lodise TP, Melnick D, et al. Meropenem penetration into epithelial lining fluid in mice and men and delineation of exposure targets. Antimicrob Agents Chemother 2011; 55:3406–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leary R, Jelliffe R, Schumitzky A, Van Guilder M. An adaptive grid non-parametric approach to pharmacokinetic and dynamic (PK/PD) models.. In: Proceedings of the 14th IEEE Symposium on Computer-Based Medical Systems Bethesda, MD: IEEE Computer Society, 2001:389–394. [Google Scholar]
- 9.Drusano GL, Neely M, Van Guilder M, et al. Analysis of combination drug therapy to develop regimens with shortened duration of treatment for tuberculosis. PLoS One 2014; 9:e101311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D'Argenio DZ, Schumitzky A, Wang X. ADAPT 5 User's Guide: Pharmacokinetic/Pharmacodynamic Systems Analysis Software. Los Angeles: Biomedical Simulations Resource, 2009. [Google Scholar]
- 11.Neely MN, van Guilder MG, Yamada WM, Schumitzk A, Jelliffe RW. Accurate detection of outliers and subpopulations with pmetrics, a nonparametric and parametric pharmacometric modeling and simulation package for R. Ther Drug Monit 2012; 34: 467–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lodise TP, Sörgel F, Mason B, Melnick D, Kinzig M, Drusano GL. Penetration of meropenem into epithelial lining fluid in intubated patients with nosocomial pneumonia. Antimicrob Agents Chemother 2011; 55:1606–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drusano GL, Preston SL, Gottfried MH, Danziger LH, Rodvold KA. Levofloxacin penetration into epithelial lining fluid as determined by population pharmacokinetic modeling and Monte Carlo simulation. Antimicrob Agents Chemother 2002; 46:586–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greco WR, Bravo G, Parsons JC. The search for synergy: a critical review from a response surface perspective. Pharmacol Rev 1995; 47:331–85. [PubMed] [Google Scholar]
- 15.Deziel M, Heine H, Louie A, et al. Identification of effective antimicrobial regimens for use in humans for the therapy of Bacillus anthracis infections and post-exposure prophylaxis. Antimicrob Agents Chemother 2005; 49:5099–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.