Abstract
Objective. Syndesmophytes in AS typically grow slowly, but it is not known whether growth is uniform among syndesmophytes in the same intervertebral disc space (IDS) or among different IDSs in the same patient or if growth is heterogeneous. We examined the dynamics of syndesmophyte growth over 24 months using CT, with the main aim of determining if syndesmophytes in the same IDS or the same patient grow at similar rates.
Methods. We performed lumbar spine CT scans on 33 patients and measured syndesmophytes in four IDSs using a validated computer algorithm. Scans were done at baseline and 12 and 24 months. We compared absolute and percentage changes in volume from baseline to 12 months and to 24 months among syndesmophytes in the same IDS and among four IDSs of each patient. We also examined whether growth among all IDSs differed between study years.
Results. Among 60 IDSs with at least two syndesmophytes at baseline (range 2–6), there was substantial heterogeneity in both absolute (P < 0.0001) and percentage (P = 0.0002) volume increases among syndesmophytes in the same IDS. Several IDSs had both syndesmophytes with no growth and syndesmophytes that increased by >100 mm3. Similarly there was significant heterogeneity in syndesmophyte growth among IDSs of individual patients. Increases in total syndesmophyte volume for each patient also tended to differ between study years (P = 0.07).
Conclusion. Syndesmophytes in AS do not all grow continuously. Rates of growth over 24 months commonly differ between syndesmophytes in the same IDS and between different IDSs in the same patient, suggesting that local factors regulate syndesmophyte growth.
Keywords: ankylosing spondylitis, syndesmophyte, computed tomography
Introduction
Spinal fusion is a central feature of AS, so much so that it gives the condition its name [1]. Assessment of the presence and extent of spinal fusion in AS has focused on syndesmophytes and associated bridging, because these structures are generally more easily seen and quantified on radiographs than other vertebral structures susceptible to fusion. Spinal fusion in AS typically develops slowly [2]. Although most patients have radiographic signs of spine involvement after 15 years of AS, vertebral fusion is evident in <20% of patients with 10–20 years of AS, but in 60% of patients with ≥30 years of AS [3, 4]. Complete lumbar ankylosis and complete cervical ankylosis often develop later, affecting 23% and 34% of patients with AS for ≥40 years, respectively [5].
Slow progression of spinal fusion in AS has also been observed in longitudinal studies of radiographic change. In studies using the modified Stoke AS Spine Score (mSASSS; possible range 0–72), mean increases of ∼1 mSASSS unit/year have been consistently reported [6–9]. Scores for syndesmophytes and bridging comprise 67% of the possible mSASSS. At the group level, the mSASSS has been noted to increase linearly over time on serial radiographic examinations [3, 7, 9]. Although overall mean rates of progression are low, rates vary substantially among patients, with some patients having no progression over many years and up to one-third of patients having rapid progression, with increases of ≥5 mSASSS units/year [7, 9]. In addition to between-patient variation in progression, there is limited evidence of within-patient variation in progression, with periods of more rapid syndesmophyte growth preceding or following periods of stability in some patients [7, 9, 10]. However, it is not known whether syndesmophytes in different intervertebral disc spaces (IDSs) of a patient grow uniformly or if syndesmophyte development and growth is heterogeneous among IDSs. Similarly it is not known whether syndesmophytes in the same IDS grow uniformly. These questions have been difficult to address because of the limited visualization and precision provided by plain radiographs and semi-quantitative scoring methods [11].
Characterization of the dynamics of syndesmophyte growth within patients is important because it may provide insight into the processes that govern spinal fusion [12]. Uniform growth of syndesmophytes within the same IDS or among IDSs of a given patient would suggest that spinal fusion is governed by a systemic or endogenous process. In this model, once these processes are initiated, they proceed largely autonomously on a preprogrammed path. This model may apply in AS if syndesmophyte formation and growth are a constitutive part of the disease. Slow linear progression would be consistent with growth by accretion and a systemic process. Alternatively, heterogeneity of syndesmophyte growth within and among IDSs of the same patient would suggest that their growth is primarily, or at least partially, influenced by local factors. The aim of this study was to examine the uniformity of syndesmophyte growth in AS within individual IDSs, among IDSs within patients and within patients over time. We assessed syndesmophyte growth by examining changes in their volumes as measured on serial CT scans and quantified using a newly developed highly reliable computer algorithm [11].
Methods
Patients and study protocol
We enrolled patients ≥18 years of age with AS by the modified New York criteria in a prospective longitudinal study, the goal of which was to develop and test a quantitative method to measure syndesmophyte size using CT [11, 13]. We excluded patients who were pregnant, unsure of their ability to complete regular follow-up visits or unable to provide informed consent. We recruited patients from clinics at the National Institutes of Health (NIH) and Johns Hopkins Medical Institutions. The study was approved by the National Institute of Diabetes and Digestive and Kidney Diseases/National Institute of Arthritis and Musculoskeletal and Skin Diseases human subjects review committee and the Johns Hopkins Medical Institutions human subjects review board and all participants provided written informed consent.
Participants had clinical evaluations every 4 months and imaging (including CT scans) at baseline, 12 and 24 months. Clinical evaluations included patient-reported assessment of AS activity using the BASDAI and testing of serum CRP levels. Treatment was open and not determined by the study. Early participants who had syndesmophytes on their baseline scan were invited for a fourth CT scan at 48 months.
CT scanning and image analysis
We performed CT scans from T10–L4, which provided four IDSs for analysis: T11–T12, T12–L1, L1–L2 and L2–L3. The thoracolumbar region was chosen because syndesmophytes commonly occur there [14]. The estimated equivalent absorbed radiation dose of the CT scan was 8.01 mSv (0.801 rem).
We quantified syndesmophytes in each IDS using a semi-automated computer algorithm [15]. The algorithm measures voxels of bone density projecting from the vertebral rim and lying between the planes of the two vertebral endplates as a syndesmophyte. In this study we measured the volume of individual syndesmophytes, the combined volume of all syndesmophytes in a given IDS and the total volume of syndesmophytes in the four IDSs of each patient. Syndesmophytes arising from the same vertebral endplate were counted as individual if they were separated by areas of uninvolved vertebral rim. Bridged syndesmophytes were considered as a single syndesmophyte. A given IDS could have more than one bridged syndesmophyte if these were separated by areas of uninvolved vertebral rim.
Syndesmophyte volume measurements were highly reliable, as demonstrated by scans repeated on patients on the same day, with an intraclass correlation of 0.99 and coefficients of variation of 2.05% at the IDS level and 1.31% at the patient level [11]. Increases in syndesmophyte volume of >4% for individual IDSs and >3% for individual patients (i.e. sum of four IDSs) were values greater than those expected from measurement error [11]. The method has longitudinal construct validity for syndesmophyte growth over 2 years and is highly sensitive to change [16].
Statistical analysis
We examined syndesmophyte growth by the change in syndesmophyte volume, which could result from increases in either height or thickness. We examined changes from baseline to 12 months and baseline to 24 months separately. To examine heterogeneity of growth among syndesmophytes in the same IDS, we limited the analysis to IDSs with at least two syndesmophytes at baseline so that differences in growth could be tested. We computed both absolute and percentage changes in volume at 12 and 24 months. We used analysis of variance (ANOVA) to test whether changes in volume were similar among syndesmophytes in the same IDS, with the null hypothesis that changes were equal among all syndesmophytes. We used generalized estimating equations to account for clustering by IDS and patient. Also, we tested whether the growth of non-bridged syndesmophytes differed from one another when two or more occurred in the same IDS and separately tested bridged syndesmophytes.
To examine whether syndesmophyte growth was uniform among different IDSs of the same patient, we computed absolute and percentage changes in volume at 12 and 24 months and used ANOVA to test whether these changes differed among IDSs, accounting for clustering by patient.
Next, to examine heterogeneity in syndesmophyte growth over time in individual patients, we tested if changes in total syndesmophyte volume, summed for the four IDSs, over each study year (baseline–12 months, and 12–24 months) differed within patients, using the paired t-test. Lastly we tested whether AS activity, measured by the area under the curve (AUC) of the BASDAI and the AUC of CRP levels obtained every 4 months, as well as the percentage of time on treatment with either a TNF inhibitor or NSAIDs were associated with changes in syndesmophyte volume over 24 months at the group level. Changes over 48 months were examined descriptively. We used SAS software (SAS Institute, Cary, NC, USA) for analysis.
Results
Patients
We studied 33 patients (28 men and 5 women) who had a mean age of 45.5 years (s.d. 11.8) and mean duration of AS of 20.6 years (s.d. 12.4) at baseline. The mean BASDAI at baseline was 2.9 (s.d. 2.0), while the mean AUC for the BASDAI over the 2 years was 3.3 (s.d. 2.0). Mean CRP at baseline was 6.5 mg/l (s.d. 8.9) and the mean AUC of CRP was 6.8 mg/l (s.d. 7.8). Nine patients (27%) were treated with TNF inhibitors during the study, covering 17% of the person-years of observation. Twenty-nine patients (88%) were treated with NSAIDs, covering 61% of the person-years of observation. Mean mSASSS at baseline was 17.2 (s.d. 15.4). Twenty-four patients (73%) had syndesmophytes on the baseline CT scan in a total of 81 IDSs.
Growth of individual syndesmophytes
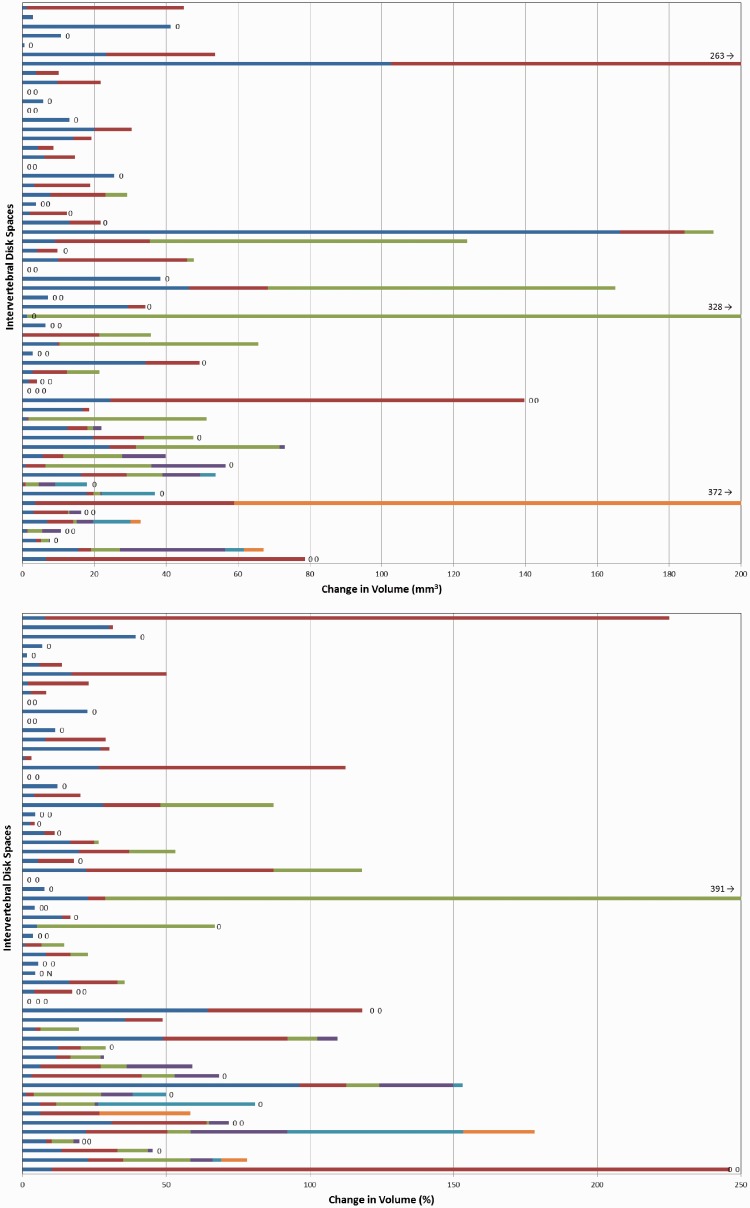
For this analysis we included 60 IDSs (of 24 patients) that had two or more syndesmophytes on the baseline scan. These IDSs included 2–6 syndesmophytes each (a total 187 syndesmophytes). Mean growth from baseline to 12 months was 12 mm3 (range 0–326) and from baseline to 24 months was 29 mm3 (range 0–830). Growth was correlated with baseline volume, with larger syndesmophytes growing more (Spearman’s r = 0.39 for association at 12 months; P < 0.0001). There was remarkable variation in growth between syndesmophytes in the same IDS. While some IDSs had little growth of all syndesmophytes and others had similar growth among syndesmophytes, there were several IDSs in which one syndesmophyte added >100 mm3 while other syndesmophytes did not grow (Fig. 1). Results were similar for absolute increase and percentage increase. Similar heterogeneity was evident over 24 months (supplementary Fig. S1, available at Rheumatology Online). Statistical comparisons confirmed significant differences in growth among syndesmophytes in the same IDS (Table 1).
Fig. 1.
Change in volume of individual syndesmophytes from baseline to 12 months
Each bar represents a single intervertebral disc space, with individual syndesmophytes represented by different colours. Syndesmophytes that had no or negative change in volume are represented by zeros (negative changes result from slight variations in where syndesmophytes are segmented from the vertebral rim). There were two to six syndesmophytes per disc space. The upper panel shows absolute increase in volume and the lower panel shows percentage increase in volume from baseline. In the percentage panel, N denotes new syndesmophytes, for which no growth rate can be computed.
Table 1.
Heterogeneity in absolute and percentage growth in individual syndesmophytes within the same intervertebral disc space over 12 and 24 months
| Baseline–12 months |
Baseline–24 months |
|||||
|---|---|---|---|---|---|---|
| All syndesmophytes | Non-bridging syndesmophytes onlya | Bridging syndesmophytes onlya | All syndesmophytes | Non-bridging syndesmophytes onlya | Bridging syndesmophytes onlya | |
| Syndesmophytes, n | 187 | 130 | 57 | 187 | 130 | 57 |
| Intervertebral disc spaces, n | 60 | 36 | 25 | 60 | 40 | 25 |
| Change in absolute volume, χ2 | 40.9 (P < 0.0001) | 25.1 (P = 0.009) | 18.5 (P = 0.003) | 39.7 (P = 0.0002) | 25.5 (P = 0.008) | 9.2 (P = 0.10) |
| Change in percentage volume, χ2 | 38.7 (P = 0.0002) | 23.1 (P = 0.02) | 15.4 (P = 0.009) | 28.8 (P = 0.007) | 19.0 (P = 0.06) | 20.0 (P = 0.002) |
The chi-square results indicate whether growth of all syndesmophytes was equal, with significant results indicating that the growth of at least one syndesmophyte differed from that of other syndesmophytes in the same disc space. aIncluding intervertebral disc spaces with at least two non-bridging or at least two bridging syndesmophytes.
Because bridged syndesmophytes may grow differently from non-bridged syndesmophytes, we examined these types of syndesmophytes separately. Comparing non-bridged syndesmophytes with other non-bridged syndesmophytes in the same IDS, there was evidence of heterogeneity in both absolute and percentage growth over 12 and 24 months (Table 1). There was also evidence of heterogeneity in growth among bridged syndesmophytes within the same IDS in all measures except absolute growth at 24 months.
Syndesmophyte volume changes in individual IDSs
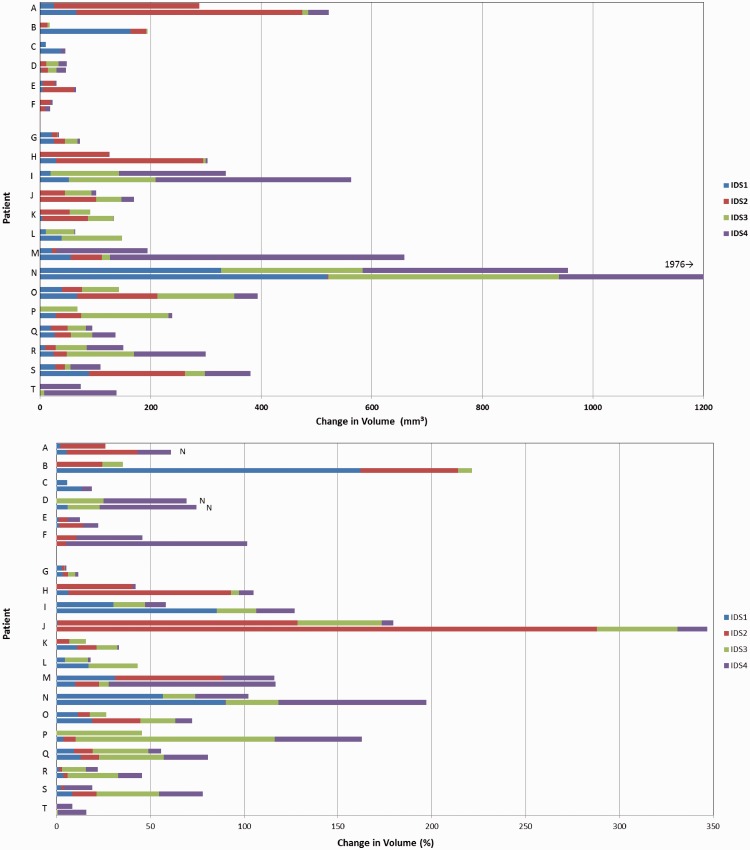
In this analysis we included all IDSs of all patients. Mean syndesmophyte growth per IDS from baseline to 12 months was 22 mm3 (range 0–372) and from baseline to 24 months was 50 mm3 (range 0–1038). Growth was larger in IDSs with higher baseline syndesmophyte volumes (Spearman’s r = 0.42 for association at 12 months; P < 0.0001). For depiction of heterogeneity in growth among IDSs of individual patients, we focused on the 20 patients who had syndesmophytes in at least three of the four IDSs at baseline. Fig. 2 shows the absolute and percentage increases in syndesmophyte volume in each IDS over 12 and 24 months for these patients. There was substantial heterogeneity in syndesmophyte growth among different IDSs of the same patient. For example, patient A had minor growth in IDS1, large growth in IDS2 and no growth in IDS4 during the first 12 months; over 24 months, small growth occurred in IDS3 and IDS4. Among the six patients with three involved IDSs, unequal growth was present in one or two IDSs in five patients (all but patient D). Among the 14 patients with four involved IDSs at baseline, unequal growth among all IDSs was evident for most patients (Fig. 2). IDSs with small volume increases (<50 mm3) over 24 months had smaller median syndesmophyte volumes at baseline than IDSs with larger volume increases (178 vs 571 mm3, P < 0.0001) and had much additional room to grow, indicating that the small volume changes in these IDSs were not limited by ceiling effects. Increases in syndesmophyte volume showed different patterns among IDSs (supplementary Fig. S2, available at Rheumatology Online).
Fig. 2.
Change in syndesmophyte volume per intervertebral disc space from baseline to 12 and 24 months
Patients A–F: patients with syndesmophytes in three disc spaces at baseline; patients G–T: patients with syndesmophytes in four disc spaces at baseline. Each colour represents the change in syndesmophyte volume in a disc space. The top bar of each pair shows the 12 month increases and the bottom bar of each pair shows the 24 month increases for each patient. The upper panel shows absolute increase and the lower panel shows percentage increase. In the percentage panel, N denotes new syndesmophytes, for which no growth rate can be computed.
In an analysis that included data of all 33 patients, there were significant differences in syndesmophyte growth among IDSs of the same patient at both 12 and 24 months, measured as absolute or per cent changes (Table 2). Results were similar in analyses limited to the 20 patients with syndesmophytes in at least three IDSs (data not shown).
Table 2.
Heterogeneity in absolute and percentage change in syndesmophyte volume among intervertebral disc spaces of the same patient over 12 and 24 months
| Baseline–12 months | Baseline–24 months | |
|---|---|---|
| Intervertebral disc spaces, n | 132 | 132 |
| Change in absolute volume, χ2 | 11.8 (P = 0.008) | 10.6 (P = 0.02) |
| Change in percentage volume, χ2 | 11.5 (P = 0.01) | 11.2 (P = 0.01) |
The chi-square results indicate whether growth in syndesmophyte volume among disc spaces was equal, with significant results indicating that growth in at least one disc space differed from that of other disc spaces in the same patient.
In the six patients who had an additional CT scan at 48 months, heterogeneity in syndesmophyte growth among IDSs persisted over time (supplementary Fig. S3, available at Rheumatology Online).
Syndesmophyte volume increases in individual patients
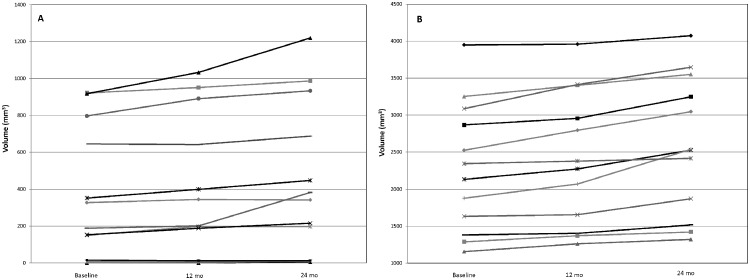
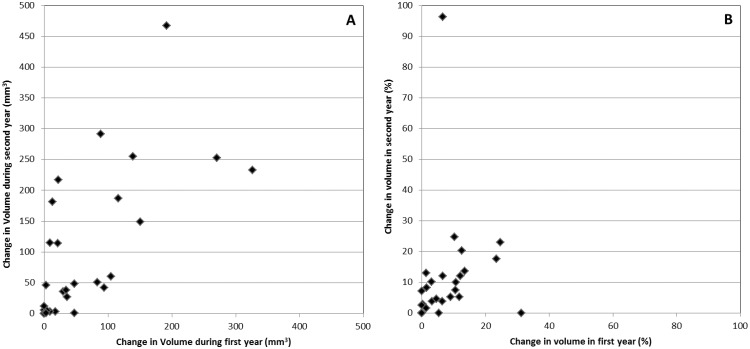
Within patients we examined whether changes in total syndesmophyte volume across the four IDSs were similar in the first 12 months and the second 12 months of the study. Most patients had generally similar increases in syndesmophyte volume in both periods, with a few noticeable exceptions (Fig. 3). However, plots of changes in the first year against changes in the second year revealed striking differences in some patients, with little growth in one year and substantial growth in the other year (Fig. 4). Overall, there was limited evidence supporting differences in growth between years within patients (paired t = 1.89, P = 0.07). Differences were also apparent when percentage changes were examined (Fig. 4). Results were similar in patients examined over 48 months (supplementary Fig. S4, available at Rheumatology Online).
Fig. 3.
Total syndesmophyte volumes in all four intervertebral disc spaces at baseline, 12 and 24 months in each patient
Patients were sorted into (A) those with baseline volumes <1000 mm3 and (B) those with baseline volumes >1000 mm3 for ease of presentation.
Fig. 4.
Association of changes in absolute syndesmophyte volume and percentage change from baseline to 12 months and from 12 to 24 months
(A) Absolute syndesmophyte volume and (B) percentage change.
Syndesmophyte volume increases for the group
Summary results for all patients indicated that increases in syndesmophyte volume were largely linear, but with a slight acceleration over time (supplementary Fig. S5, available at Rheumatology Online). Changes in syndesmophyte volume over 24 months did not differ between men and women (F = 0.80, P = 0.38) or between those with and without syndesmophytes at baseline (F = 0.14, P = 0.71). Changes in syndesmophyte volume were also not associated with the AUC of the BASDAI (t = −1.13, P = 0.27), the AUC of CRP (t = −0.29, P = 0.78) or the proportion of time treated with NSAIDs (t = 0.06, P = 0.95) or TNF inhibitors (t = −0.24, P = 0.82). Results were similar for percentage changes over 24 months.
Discussion
This study provides evidence of substantial heterogeneity in growth among syndesmophytes in individual IDSs and among IDSs in individual patients. Within the same IDS, some syndesmophytes grew substantially while others did not grow. Similarly, syndesmophytes in some IDSs grew, while those in adjacent IDSs were stable. Although larger syndesmophytes tended to grow more quickly, heterogeneity was also observed for percentage increases in volume, demonstrating that the findings were independent of baseline volume. Heterogeneity in growth was present among both bridged and non-bridged syndesmophytes, indicating that the results were not confounded by different types of syndesmophyte in an IDS.
CT scanning allowed us to quantify changes in syndesmophyte volume and investigate the dynamics of syndesmophyte growth in ways not possible with plain radiography. For example, it is difficult to investigate heterogeneity in growth among IDSs of the same patient using radiographs because individual syndesmophytes in all areas of the IDS cannot be identified with this method. Prior studies of the development of new syndesmophytes or bridging tended to focus on the number of patients who demonstrated progression rather than on whether progression occurred coordinately across IDSs. However, inferences supporting heterogeneity in growth among IDSs can be drawn from studies that reported that new syndesmophytes or bridging develop in a minority of disc spaces over 2 years, with no changes in most other IDSs [10, 17, 18]. Because the mSASSS scores only the anterior portion of the vertebral body, studies using this measure could examine heterogeneity in the same IDS by testing whether new syndesmophytes developed simultaneously at one or both anterior vertebral corners of an IDS (i.e. the relative occurrence of new scores of 4 vs new scores of 2). We are not aware of studies addressing this question, but in our experience, new syndesmophytes more commonly develop on one vertebral corner than simultaneously on both vertebral corners of an IDS.
Heterogeneity resulted not only from growth at different rates, but from growth in some syndesmophytes and no growth in neighbouring syndesmophytes. This observation suggests that local factors influence growth. This may be due to the presence (or absence) of growth-enhancing factors, the presence (or absence) of growth-inhibiting factors, the balance between these or the susceptibility of the local annulus fibrosis and intervertebral ligaments to these factors. The nature of these factors is unknown, although IL-23-mediated inflammatory responses have received much attention recently [19, 20]. The pattern of growth is not consistent with systemic effects, because these would be expected to influence all syndesmophytes similarly. However, it is possible that patterns of growth result from complex interactions among pro- and anti-proliferative factors, the responsiveness of the substrate, biomechanics, treatment and the relative dominance of these factors at particular times.
Our finding of differences in growth among patients over time support previous radiographic studies [7, 9, 10]. In addition, we found that total syndesmophyte volume increased in a largely linear pattern over 2 years in this group [7, 9]. It is interesting that in moving from the level of individual syndesmophytes, to the IDS, to the patient and finally to the group, the progressively more summarized data masked the heterogeneity present at the more fundamental levels. While the linear increase at group level might imply uniform growth among all syndesmophytes, our results indicate that this is not the case.
The strengths of our study include quantification of syndesmophyte volume on three CT scans over 2 years and examination of growth at multiple levels of organization. The high reliability of the method ensures that the changes represent true growth rather than measurement error. However, our study also has limitations. Two years represents only a small portion of a chronic disease such as AS, and growth dynamics may be different in early or very late AS. However, patterns were similar when examined over 4 years in a subset of patients. We examined four thoracolumbar IDSs, and growth patterns may be different in other spine regions. We also examined only 33 patients, most of whom had well-controlled AS. We do not know if findings would be similar among patients with more active AS. However, we found no association between syndesmophyte growth at the group level and measures of AS activity, but the power of these analyses was limited. Improvements in scanner technology may help to reduce the radiation exposure of future examinations [21].
Our findings suggest that new insights into spinal fusion in AS may come from examination of the growth of individual syndesmophytes and comparing the local environments of growing syndesmophytes with those of stable syndesmophytes. Given the limitations in tissue sampling, this may be facilitated by novel imaging techniques, including PET, which may be able to distinguish growing from senescent syndesmophytes in real time [22]. Our results also have implications for testing of biomarkers of spinal fusion in AS. Spurious or false-negative associations may result if studies measure growth in only a few syndesmophytes, which may not be representative of all syndesmophytes in a given patient. Biomarker associations will be more valid if tested in a subset of patients in whom most syndesmophytes demonstrate growth rather than in patients with growth in a minority of syndesmophytes. Null associations at the patient level may not preclude an important role for the biomarker at the local level.
Rheumatology key messages.
In patients with AS, syndesmophytes in the same disc space often grow at different rates.
In patients with AS, syndesmophytes in different disc spaces of the same patient also grow at different rates.
These findings suggest that factors local to the disc space influence syndesmophyte growth in AS.
Supplementary Material
Acknowledgements
We thank Lori Guthrie, RN and Amanda Bertram for assistance.
Funding: This work was supported by the Intramural Research Program, National Institute of Arthritis and Musculoskeletal and Skin Diseases and by the Clinical Center, NIH, and the Johns Hopkins University School of Medicine General Clinical Research Center (grant number M01-RR00052 from the National Center for Research Resources/NIH).
Disclosure statement: The authors have declared no conflicts of interest.
Supplementary data
Supplementary data are available at Rheumatology Online.
References
- 1.Helliwell PS, Hickling P, Wright V. Do the radiological changes of classic ankylosing spondylitis differ from the changes found in the spondylitis associated with inflammatory bowel disease, psoriasis, and reactive arthritis? Ann Rheum Dis. 1998;57:135–40. doi: 10.1136/ard.57.3.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carette S, Graham D, Little H, Rubenstein J, Rosen P. The natural disease course of ankylosing spondylitis. Arthritis Rheum. 1983;26:186–90. doi: 10.1002/art.1780260210. [DOI] [PubMed] [Google Scholar]
- 3.Brophy S, Mackay K, Al-Saidi A, Taylor G, Calin A. The natural history of ankylosing spondylitis as defined by radiological progression. J Rheumatol. 2002;29:1236–43. [PubMed] [Google Scholar]
- 4.Boonen A, vander Cruyssen B, de Vlam K, et al. Spinal radiographic changes in ankylosing spondylitis: association with clinical characteristics and functional outcome. J Rheumatol. 2009;36:1249–55. doi: 10.3899/jrheum.080831. [DOI] [PubMed] [Google Scholar]
- 5.Ward MM, Learch TJ, Gensler LS, et al. Regional radiographic damage and functional limitations in patients with ankylosing spondylitis: differences in early and late disease. Arthritis Care Res. 2013;65:257–65. doi: 10.1002/acr.21821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wanders AJ, Landewé RB, Spoorenberg A, et al. What is the most appropriate radiologic scoring method for ankylosing spondylitis? A comparison of the available methods based on the Outcome Measures in Rheumatology Clinical Trials filter. Arthritis Rheum. 2004;50:2622–32. doi: 10.1002/art.20446. [DOI] [PubMed] [Google Scholar]
- 7.Baraliakos X, Listing J, von der Recke A, Braun J. The natural course of radiographic progression in ankylosing spondylitis—evidence for major individual variations in a large proportion of patients. J Rheumatol. 2000;36:997–1002. doi: 10.3899/jrheum.080871. [DOI] [PubMed] [Google Scholar]
- 8.Poddubnyy D, Haibel H, Listing J, et al. Baseline radiographic damage, elevated acute-phase reactant levels, and cigarette smoking status predict spinal radiographic progression in early axial spondylarthritis. Arthritis Rheum. 2012;64:1388–98. doi: 10.1002/art.33465. [DOI] [PubMed] [Google Scholar]
- 9.Ramiro S, Stolwijk C, van Tubergen A, et al. Evolution of radiographic damage in ankylosing spondylitis: a 12 year prospective follow-up of the OASIS study. Ann Rheum Dis. 2013 doi: 10.1136/annrheumdis-2013-204055. Aug 16. doi:10.1136/annrheumdis-2013-204055 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 10.van Tubergen A, Ramiro S, van der Heijde D, et al. Development of new syndesmophytes and bridges in ankylosing spondylitis and their predictors: a longitudinal study. Ann Rheum Dis. 2012;71:518–23. doi: 10.1136/annrheumdis-2011-200411. [DOI] [PubMed] [Google Scholar]
- 11.Tan S, Yao J, Flynn JA, Yao L, Ward MM. Quantitative measurement of syndesmophyte volume and height in ankylosing spondylitis using CT. Ann Rheum Dis. 2014;73:544–50. doi: 10.1136/annrheumdis-2012-202661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gliozzi AS, Guiot C, Delsanto PP, Iordache DA. A novel approach to the analysis of human growth. Theor Biol Med Model. 2012;9:17. doi: 10.1186/1742-4682-9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984;27:361–8. doi: 10.1002/art.1780270401. [DOI] [PubMed] [Google Scholar]
- 14.Spencer DG, Park WM, Dick HM, et al. Radiological manifestations in 200 patients with ankylosing spondylitis: correlation with clinical features and HLA B27. J Rheumatol. 1979;6:305–15. [PubMed] [Google Scholar]
- 15.Tan S, Yao J, Yao L, et al. Improved precision of syndesmophyte measurement for the evaluation of ankylosing spondylitis using CT: a phantom and patient study. Phys Med Biol. 2012;57:4683–704. doi: 10.1088/0031-9155/57/14/4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tan S, Yao J, Flynn JA, Yao L, Ward MM. Quantitative syndesmophyte measurement in ankylosing spondylitis using CT: longitudinal validity and sensitivity to change over 2 years. Ann Rheum Dis. 2013 doi: 10.1136/annrheumdis-2013-203946. Dec 2. doi:10.1136/annrheumdis-2012-203946 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiowchanwisawakit P, Lambert RGW, Conner-Spady B, Maksymowych WP. Focal fat lesions at vertebral corners on magnetic resonance imaging predict the development of new syndesmophytes in ankylosing spondylitis. Arthritis Rheum. 2011;63:2215–25. doi: 10.1002/art.30393. [DOI] [PubMed] [Google Scholar]
- 18.Baraliakos X, Listing J, Rudwaleit M, Sieper J, Braun J. The relationship between inflammation and new bone formation in patients with ankylosing spondylitis. Arthritis Res Ther. 2008;10:R104. doi: 10.1186/ar2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chambler A, Rawlinson S, Emery R, Pitsillides A. Quantitative cytochemical evidence for local increases in bone turnover at the acromial enthesis of the human coracoacromial ligament. J Rheumatol. 2004;31:2216–25. [PubMed] [Google Scholar]
- 20.Sherlock JP, Joyce-Shaikh B, Turner SP, et al. IL-23 induces spondyloarthropathy by acting on ROR-γt+ CD3+CD4−CD8− entheseal resident T cells. Nat Med. 2012;18:1069–76. doi: 10.1038/nm.2817. [DOI] [PubMed] [Google Scholar]
- 21.Kalra MK, Woisetschläger M, Dahlström N, et al. Radiation dose reduction with sinogram affirmed iterative reconstruction technique for abdominal computed tomography. J Comput Assist Tomogr. 2012;36:339–46. doi: 10.1097/RCT.0b013e31825586c0. [DOI] [PubMed] [Google Scholar]
- 22.Bruijnen ST, van der Weijden MA, Klein JP, et al. Bone formation rather than inflammation reflects ankylosing spondylitis activity on PET-CT: a pilot study. Arthritis Res Ther. 2012;14:R71. doi: 10.1186/ar3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.