Abstract
Context:
Autosomal dominant hypocalcemia (ADH) types 1 and 2 are due to calcium-sensing receptor (CASR) and G-protein subunit-α11 (GNA11) gain-of-function mutations, respectively, whereas CASR and GNA11 loss-of-function mutations result in familial hypocalciuric hypercalcemia (FHH) types 1 and 2, respectively. Loss-of-function mutations of adaptor protein-2 sigma subunit (AP2σ 2), encoded by AP2S1, cause FHH3, and we therefore sought for gain-of-function AP2S1 mutations that may cause an additional form of ADH, which we designated ADH3.
Objective:
The objective of the study was to investigate the hypothesis that gain-of-function AP2S1 mutations may cause ADH3.
Design:
The sample size required for the detection of at least one mutation with a greater than 95% likelihood was determined by binomial probability analysis. Nineteen patients (including six familial cases) with hypocalcemia in association with low or normal serum PTH concentrations, consistent with ADH, but who did not have CASR or GNA11 mutations, were ascertained. Leukocyte DNA was used for sequence and copy number variation analysis of AP2S1.
Results:
Binomial probability analysis, using the assumption that AP2S1 mutations would occur in hypocalcemic patients at a prevalence of 20%, which is observed in FHH patients without CASR or GNA11 mutations, indicated that the likelihood of detecting at least one AP2S1 mutation was greater than 95% and greater than 98% in sample sizes of 14 and 19 hypocalcemic patients, respectively. AP2S1 mutations and copy number variations were not detected in the 19 hypocalcemic patients.
Conclusion:
The absence of AP2S1 abnormalities in hypocalcemic patients, suggests that ADH3 may not occur or otherwise represents a rare hypocalcemic disorder.
Hypocalcemia is usually associated with either undetectable serum PTH concentrations, as occurring in hypoparathyroidism, or elevated serum PTH concentrations, as occurring in secondary hyperparathyroidism, whose causes include vitamin D deficiency or chronic renal failure, or pseudohypoparathyroidism, which is due to PTH resistance (1). However, hypocalcemia may also occasionally occur in association with normal or low serum PTH concentrations, as in autosomal dominant hypocalcemia [ADH; online inheritance in man (OMIM) number 601198 and number 615361]. ADH may be associated with either of the following: 1) gain-of-function mutations of the calcium-sensing receptor (CaSR) (2–5), a guanine-nucleotide protein (G protein)-coupled receptor, which is encoded by CASR (6) and referred to as ADH type 1 (ADH1; OMIM number 601198); or 2) gain-of-function mutations of the G-protein subunit-α11 which is encoded by GNA11 and referred to as ADH2 (OMIM number 615361) (7). GNA11 mutations have also been reported in two families with isolated hypoparathyroidism, and these could be referred to as ADH2 because the hypocalcemia was associated with either serum PTH concentrations within the reference range (in ∼40% of patients) or below the lower limit of the reference range (in ∼60% of patients), similar to the situation in ADH patients (8). Thus, in ADH1 patients, the hypocalcemia may be mild (2.00–2.20 mmol/L) or severe (<1.90 mmol/L) and associated with serum PTH concentrations within the reference range in greater than 40% of ADH1 patients and below the lower limit of the reference range in the remaining less than 60% of ADH1 patients (3, 9).
The hypocalcemia in ADH1 patients may be associated with symptoms that include paraesthesia, carpopedal spasm, and seizures in approximately 50% of patients (3, 9), whereas the remaining 50% of ADH1 patients have asymptomatic hypocalcemia. Serum phosphate concentrations in ADH1 patients may be elevated or in the upper-normal range (2), and serum magnesium concentrations may be low or in the low-normal range (3). Approximately 10% of ADH1 patients have an absolute hypercalciuria, which may be associated with nephrocalcinosis or kidney stones (3, 10). Vitamin D preparations and calcium supplements to correct the hypocalcemia may worsen the hypercalciuria and lead to renal impairment (11). Ectopic calcifications in sites such as the basal ganglia may be found in greater than 35% of ADH1 patients (3). Approximately 20% of ADH1 probands may not have a family history because they have de novo CASR mutations (7). The clinical features of the reported hypocalcemic patients and families with GNA11 mutations (7, 8) seem to be similar to those of ADH1 patients, although this extrapolation requires cautious interpretation because only four such patients and families have been reported to date (7, 8). The CASR and GNA11 mutations that are associated with ADH1 and ADH2, respectively, result in an increased sensitivity of cells expressing CaSRs to extracellular calcium (Ca2+o) and are thus representations of gain-of-function mutations, in contrast to the loss-of-function mutations of the CASR and GNA11, which are associated with familial hypocalciuric hypercalcemia (FHH) type 1 (OMIM number 145980) and FHH2 (OMIM number 145981), respectively (5, 7, 12).
A recent study has shown that FHH3 is due to loss-of-function mutations of the AP2S1 gene, which encodes the adaptor protein-2 sigma subunit (AP2σ2) (13). Indeed, such AP2S1 mutations were found to occur in greater than 20% of FHH patients without CASR mutations and were shown to decrease the sensitivity of the CaSR-expressing cells to Ca2+o, consistent with a loss-of-function (13). Gain-of-function AP2S1 mutations have not been identified to date, and we postulated, by analogy to the situation in ADH1 and ADH2, that such gain-of-function mutations may result in an additional form of ADH, which we have designated ADH3. We sought to identify gain-of-function AP2S1 mutations in hypocalcemic patients who were considered to have ADH or isolated hypoparathyroidism but did not have CASR, GNA11, GCMB, or PTH mutations, which may occur in approximately 50% of such patients.
Materials and Methods
Patients
Informed consent was obtained from individuals, using protocols approved by the local and national ethics committees (MREC/02/2/93). Nineteen unrelated patients (13 males and six females) with hypocalcemia, likely due to isolated hypoparathyroidism, were ascertained, and six patients had a family history of hypocalcemia (Table 1). These patients did not have features of autoimmune disease, immunodeficiency, deafness, cardiofacial abnormalities, or a history of neck surgery, and because the patients had isolated (nonsyndromic) hypoparathyroidism, fluorescent in situ hybridization to detect deletions and translocations of chromosome 22q, which would be found in the DiGeorge syndrome, was not performed. Previous mutational analysis of CASR, GNA11, GCMB, and PTH genes by Sanger DNA sequencing, and alignment to the corresponding reference sequences, had not identified any abnormalities of the coding regions and exon-intron boundaries of these genes. All serum and urine estimations were undertaken at a time when the patients were not being treated with vitamin D preparations or calcium supplementation.
Table 1.
Clinical and Biochemical Findings in the Hypocalcemic Patients, Considered as Having ADH but Without CASR or GNA11 Mutations
| Patient | Sex | Family History | Age | Clinical Features at Presentation/Diagnosis | Serum |
Urine |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Calcium, mmol/La | Phosphate, mmol/Lb,c | ALP, U/Ld,e | Mg, mmol/Lf | Creatinine, μmol/Lg | PTHh,i | 25OHD, nmol/Lj | CCCRk,l,m | |||||
| 15/01 | M | No | 55 y | Asymptomatic | 1.99 | 1.28b | 95d | 0.8 | 180 | 2.0h | — | 0.01k |
| 07/02 | M | Sons affected | 46 y | Cataracts | 1.35 | 1.9b | 49d | — | 49 | 5.0h | — | — |
| 08/03 | M | No | 60 y | Asymptomatic | 1.63 | 1.38b | 104e | 0.93 | — | 1.6i | — | 0.003k |
| 05/04 | F | Yesn | Neonate | Seizures | 1.62 | 3.10c | 228e | 0.65 | 49 | 0.8i | — | 0.004k |
| 13/04 | F | Son affected | 53 y | Asymptomatic | 1.93 | 0.87b | 53d | 0.86 | 81 | 4.7i | 77.5 | 0.018k |
| 05/05 | M | Mother and sister affected | 3 y | Asymptomatic | 2.03 | 1.91c | 129e | 0.97 | 49 | 1.1i | 85 | 0.05k |
| 16/07 | M | No | 9 y | Seizures | 1.86 | 2.77b | 336e | 0.7 | 49 | 5.6i | 105 | 0.04k |
| 01/08 | M | Noo | 2 y | Seizures | 0.94 | 2.20c | 432e | 0.73 | 47 | 2.6i | <18 | 0.02k |
| 19/09 | F | No | 68 y | Asymptomatic | 1.91 | 1.21b | 91d | 0.68 | 75 | 18h | 64 | 0.004k |
| 19/10 | M | — | 1 mo | Seizures | 1.63 | 3.29c | 104d | 0.68 | 30 | 3.6i | 43 | — |
| 01/12 | F | Noo | 2 mo | Seizures | 1.25 | 2.52c | 388e | 0.57 | <18 | 1.4i | 101 | 1.25l |
| 09/12 | M | Noo | 2 mo | Seizures | 1.30 | 2.35c | — | — | 25 | <0.3i | 17 | 0.001k |
| 16/12 | M | Father affected | 31 y | Carpopedal spasm | 1.88 | 1.73b | 52d | — | 88 | 0.7i | 35 | 0.008k |
| 04/13 | M | No | 62 y | Paraesthesia | 1.64 | 1.43b | 53d | 0.76 | 104 | 22h | 29 | 13.5m |
| 07/13 | M | No | 2 y | Seizures | 1.37 | 3.74c | 125e | 1.03 | 38 | 1.6i | 36 | 0.006k |
| 10/13 | F | No | 25 y | Tetany | 1.53 | 1.55b | 131e | 0.70 | 61 | <0.3i | 50 | — |
| 15/13 | M | Father affected | 57 y | Polyuria | 1.94 | 0.96b | 74d | 0.93 | 84 | 26h | 86 | 8.94m |
| 19/13 | F | No | 9 y | Seizures | 1.35 | 3.32c | 166e | 0.64 | 52 | <0.3i | 18 | 0.05k |
| 25/13 | M | No | 31 y | Tetany, cataracts | 1.31 | 1.20b | 155e | 0.73 | 80 | 0.3 | 27 | 0.03m |
Abbreviations: ALP, alkaline phosphatase activity; CCCR, calcium to creatinine clearance ratio; F, female; M, male; —, not known; 25OHD, 25-hydroxyvitamin D. All serum and urine estimations were undertaken at a time when the patients were not being treated with vitamin D preparations or calcium supplementation. Thirteen patients were white Europeans and six patients (01/08, 19/10, 01/12, 9/12, 16/12, 25/13) were of Asian or Middle Eastern origin. The age at presentation or diagnosis is provided. Fifty percent of ADH patients are asymptomatic, and hence, the age of presentation is the same as that at diagnosis; however, if the age at presentation and diagnosis are not the same, then the age at diagnosis is provided. Other family members were unavailable for studies.
Normal serum ranges listed below are reported elsewhere (12).
Albumin-adjusted calcium, 2.10–2.60 mmol/L.
Phosphate, 0.70–1.40 mmol/L.
Phosphate, 1.20–2.10 mmol/L.
ALP activity, 30–130 U/L.
ALP activity, 70–330 U/L.
Magnesium (Mg), 0.70–1.05 mmol/L.
Creatinine, 54–145 μmol/L.
PTH, 10–65 ng/L.
PTH, 1.3–7.6 pmol/L.
25OHD, greater than 50 nmol/L.
CCCR, greater than 0.02.
Urine calcium to creatinine ratio, 0.1–0.7 per 24 hours.
Urine calcium excretion, Greater than 4.0 mmol per 24 hours.
Details of affected members are not available.
Patient is the offspring of consanguineous unaffected parents.
Statistical analysis
It is unclear whether gain-of-function AP2S1 mutations may be associated with ADH. To establish whether the study was sufficiently powered to detect at least one AP2S1 mutation with a greater than 95% likelihood, the sample size required was determined by binomial probability analysis (Microsoft Excel), as previously reported (14). Because FHH1 and ADH1 are associated with a similar prevalence of CASR mutations, approximately 65% and greater than 70%, respectively (2, 3, 5, 9, 15), the binomial analysis was based on a 20% prevalence for AP2S1 mutations in hypocalcemic patients without CASR, GNA11, GCMB, or PTH mutations, which would be similar to that observed for AP2S1 loss-of-function mutations in FHH patients (13) and less than the prevalence of greater than 70% for gain-of-function CASR mutations observed in ADH patients (2, 3, 9, 15). The binomial distribution probability was calculated using the following formula:
where n = sample size, x = number of probands harboring mutations; n-x = number of probands with no mutation; and p = prevalence of AP2S1 mutations in the cohort.
Mutational analysis
Leukocyte DNA was extracted from venous blood samples (14) and quantified using the high-sensitivity Qubit system (Invitrogen). AP2S1-specific primers were used to perform PCR amplification of the five exons and eight exon-intron boundaries of the AP2S1 gene, as previously reported (13). DNA sequence analysis of the PCR products was performed using the BigDye Terminator version 3.1 cycle sequencing kit (Life Technologies) and an ABI automated capillary sequencer (Applied Biosystems), as reported (5). The DNA sequences were aligned to the corresponding genomic sequence (ENST00000263270) from Ensembl (www.ensembl.org) to detect any mutations. An assessment of single-nucleotide polymorphisms (SNPs) was undertaken using the NCBI database single nucleotide polymorphism (dbSNP) (http://ncbi.nlm.nih.gov/SNP/) and Exome Variant Server (http://evs.gs.washington.edu/EVS/) databases (16). The occurrence of AP2S1 deletions or duplications was assessed by estimating the AP2S1 copy number variations (CNVs) in DNA from hypocalcemic patients and normocalcemic controls using quantitative PCR and the Rotorgene SYBR green kit (QIAGEN) (17). Samples were assessed in triplicate using primers specific for AP2S1 and TATA-box binding protein 1 (TBP1), as a reference gene. Threshold cycle (CT) values were obtained and analyzed, as described (13, 17). AP2S1 Ct values were expressed relative to TBP1 Ct values and normalized to the mean AP2S1/TBPI Ct value from four normocalcemic controls.
Results
Patients
The 19 hypocalcemic patients comprised 13 males and six females with ages at diagnosis or presentation that ranged from the neonatal period to 68 years (Table 1), and greater than 65% of the patients had presented with hypocalcemic symptoms. More than 70% of the patients had severe hypocalcemia; serum PTH concentrations were either below the lower limit of normal, in the low normal range, or in the high normal range in 40%, greater than 40%, and greater than 15% of the patients, respectively (n = 19); more than 60% of the patients (n = 19) had elevated serum phosphate concentrations and 25% had serum phosphate concentrations in the high normal range. Hypomagnesemia was found in 30% of 16 patients and serum alkaline phosphatase activity was within the normal range in 80% of 18 patients. The urinary calcium to creatinine clearance ratio was high in 30% of 12 patients. These findings are similar to those reported in ADH1 patients (3, 9).
Power calculation and sample size
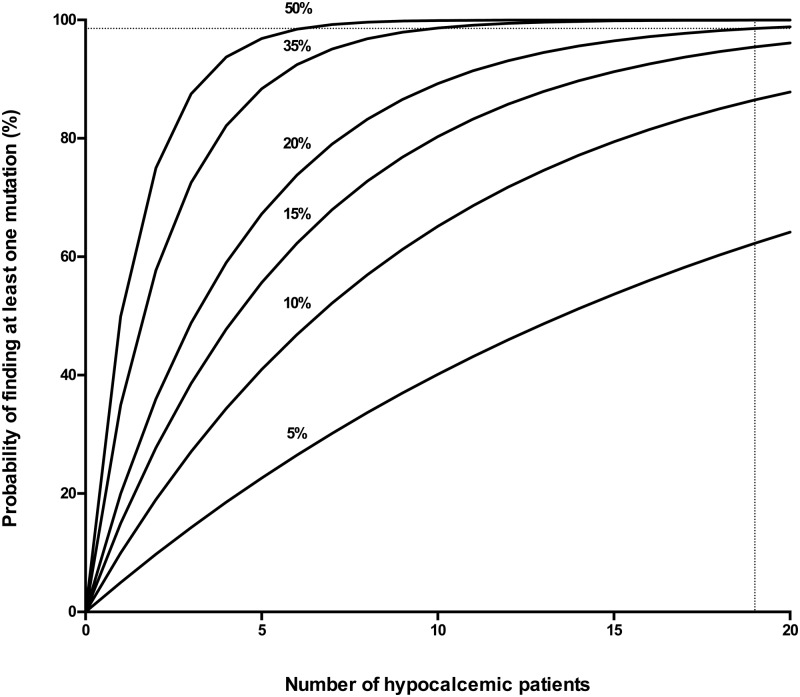
The prevalence of AP2S1 mutations in hypocalcemic patients is not known, and a binomial probability analysis in which the prevalence of AP2S1 mutations was varied was performed (Figure 1). This showed that if the prevalences of AP2S1 mutations in hypocalcemic patients were 5%, 10%, 15%, or 20%, then the sample sizes to detect at least one AP2S1 mutation in a hypocalcemic patient with a greater than 95% probability, who had been previously shown to not have CASR, GNA11, GCMB, or PTH mutations, were n = 59, n = 29, n = 19, and n = 14, respectively. Thus, if the prevalence of AP2S1 mutations in ADH was similar to that observed for AP2S1 mutations in FHH (ie, 20%), then a sample size of 14 hypocalcemic patients would have a greater than 95% probability of detecting at least one AP2S1 mutation, and the sample size of 19 hypocalcemic patients, used in this study, would have a greater than 98% and greater than 95% likelihood of detecting at least one AP2S1 mutation, assuming a mutation prevalence of 20% and 15%, respectively.
Figure 1.
Binomial probability analysis to predict the sample size required to detect one or more AP2S1 mutations for different mutation prevalences in hypocalcemic patients. The number of patients is shown on the x-axis, and the probability of detecting at least one AP2S1 mutation, at different prevalences ranging from 5% to 50%, is shown on the y-axis. The analysis indicated that a sample size of 19 hypocalcemic patients (represented by the dotted line) is required to detect at least one AP2S1 mutation at a 20% prevalence, which is observed in FHH3, with a probability of greater than 98%.
AP2S1 mutational analysis
DNA sequence analysis of the 429-bp coding region of the AP2S1 gene and the eight intron-exon boundaries in the 19 hypocalcemic patients (Table 1) did not detect any point mutations, deletions, insertions, or unreported SNPs. Furthermore, an examination of the four reported synonymous AP2S1 SNPs (rs201984742 (I5I), rs150080027 (D25D), rs151335841 (F87F), and rs143384246 (N92N)), the nonsynonymous AP2S1 SNP (rs11550738 (C68S), and the splice donor variant (rs112326072) revealed the presence of only the major (ie, more frequent) alleles in the hypocalcemic patients. AP2S1 CNVs were not detected, indicating the absence of deletions, insertions, or duplications (Supplemental Figure 1). Finally, 11 probands were found to have known CASR and/or GNA11 SNPs (Supplemental Table 1). Thus, AP2S1 mutations, deletions, or duplications were not identified in any of the hypocalcemic patients analyzed in this study.
Discussion
Our study has not identified AP2S1 mutations in hypocalcemic patients who had clinical features consistent with ADH and did not have CASR, GNA11, GCMB, or PTH mutations. These results suggest that AP2S1 gain-of-function mutations are unlikely to be a major cause of ADH. In addition, CNVs such as gene duplications that may lead to overexpression of AP2S1 were not identified. However, our study analyzed both familial and nonfamilial hypocalcemic cases because ADH has been reported to occur in both familial and sporadic forms (7), and it is possible that the likelihood of detecting an AP2S1 mutation may have been higher if a group of solely familial hypocalcemic patients were analyzed. Moreover, our study analyzed the coding region and exon-intron boundaries only, and it remains possible that these patients may harbor mutations in the noncoding regulatory regions of the AP2S1 gene that lead to an increased expression of the AP2σ2 subunit and enhance the sensitivity of cells expressing CaSR to Ca2+o, thereby resulting in a gain of CaSR function and hypocalcemia. However, the noncoding regulatory AP2S1 regions remain to be defined, thereby precluding their current analysis, and of note, noncoding mutations of the CASR and GNA11 genes have not been reported in ADH1 or ADH2, respectively (4, 5, 7).
Adaptor proteins are heterotetrameric complexes, and a mutation in one of the constituent subunits may lead to loss of function, but it is less clear how it would lead to a gain-of-function mutation, and it seems possible that AP2S1 gain-of-function mutations may not occur. Indeed, to date only loss-of-function mutations of other adaptor protein complexes have been reported (18, 19). These comprise a homozygous splice site mutation of the AP1S1 gene, which is predicted to disrupt the sigma subunit of the adaptor protein-1 complex and is associated with the mental retardation, enteropathy, deafness, peripheral neuropathy, ichthyosis, and keratoderma syndrome (19), and loss-of-function mutations of the AP3B1 gene, which encodes the β3A-subunit of the adaptor protein-3 complex, have been identified in patients with Hermansky-Pudlak syndrome, which is associated with oculocutaneous albinism, platelet dysfunction, and abnormalities of lysosomal storage (18).
In summary, we did not find any AP2S1 abnormalities in hypocalcemic patients. We conclude that AP2S1 abnormalities are unlikely to be a cause of ADH and that ADH3, caused by gain-of-function AP2S1 mutations, may not occur or otherwise represents a rare hypocalcemic disorder.
Acknowledgments
This work was supported by United Kingdom Medical Research Council Program Grants G9825289 and G1000467 (to M.A.N., F.M.H., and R.V.T.) and National Institute for Health Research Oxford Biomedical Research Centre Programme (to M.A.N. and R.V.T.). A.R. and S.A.H. are Wellcome Trust Clinical Training Fellows.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ADH
- autosomal dominant hypocalcemia
- ADH1
- ADH type 1
- ADH2
- ADH type 2
- ADH3
- ADH type 3
- AP2σ2
- adaptor protein-2 sigma subunit
- Ca2+o
- extracellular calcium
- CaSR
- calcium-sensing receptor
- CNV
- copy number variation
- CT
- threshold cycle
- FHH
- familial hypocalciuric hypercalcemia
- GNA11
- gene encoding G-protein-α11 subunit
- OMIM
- Online Mendelian Inheritance in Man
- SNP
- single-nucleotide polymorphism.
References
- 1. Hannan FM, Thakker RV. Investigating hypocalcaemia. BMJ. 2013;346:f2213. [DOI] [PubMed] [Google Scholar]
- 2. Pollak MR, Brown EM, Estep HL, et al. Autosomal dominant hypocalcaemia caused by a Ca(2+)-sensing receptor gene mutation. Nat Genet. 1994;8:303–307. [DOI] [PubMed] [Google Scholar]
- 3. Pearce SH, Williamson C, Kifor O, et al. A familial syndrome of hypocalcemia with hypercalciuria due to mutations in the calcium-sensing receptor. N Engl J Med. 1996;335:1115–1122. [DOI] [PubMed] [Google Scholar]
- 4. Hannan FM, Thakker RV. Calcium-sensing receptor (CaSR) mutations and disorders of calcium, electrolyte and water metabolism. Best Pract Res Clin Endocrinol Metab. 2013;27:359–371. [DOI] [PubMed] [Google Scholar]
- 5. Hannan FM, Nesbit MA, Zhang C, et al. Identification of 70 calcium-sensing receptor mutations in hyper- and hypo-calcaemic patients: evidence for clustering of extracellular domain mutations at calcium-binding sites. Hum Mol Genet. 2012;21:2768–2778. [DOI] [PubMed] [Google Scholar]
- 6. Brown EM, Gamba G, Riccardi D, et al. Cloning and characterization of an extracellular Ca(2+)-sensing receptor from bovine parathyroid. Nature. 1993;366:575–580. [DOI] [PubMed] [Google Scholar]
- 7. Nesbit MA, Hannan FM, Howles SA, et al. Mutations affecting G-protein subunit α11 in hypercalcemia and hypocalcemia. N Engl J Med. 2013;368:2476–2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mannstadt M, Harris M, Bravenboer B, et al. Germline mutations affecting Gα11 in hypoparathyroidism. N Engl J Med. 2013;368:2532–2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Raue F, Pichl J, Dorr HG, et al. Activating mutations in the calcium-sensing receptor: genetic and clinical spectrum in 25 patients with autosomal dominant hypocalcaemia—a German survey. Clin Endocrinol (Oxf). 2011;75:760–765. [DOI] [PubMed] [Google Scholar]
- 10. Yamamoto M, Akatsu T, Nagase T, Ogata E. Comparison of hypocalcemic hypercalciuria between patients with idiopathic hypoparathyroidism and those with gain-of-function mutations in the calcium-sensing receptor: is it possible to differentiate the two disorders? J Clin Endocrinol Metab. 2000;85:4583–4591. [DOI] [PubMed] [Google Scholar]
- 11. Thakker RV. Genetic developments in hypoparathyroidism. Lancet. 2001;357:974–976. [DOI] [PubMed] [Google Scholar]
- 12. Pearce SH, Trump D, Wooding C, et al. Calcium-sensing receptor mutations in familial benign hypercalcemia and neonatal hyperparathyroidism. J Clin Invest. 1995;96:2683–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nesbit MA, Hannan FM, Howles SA, et al. Mutations in AP2S1 cause familial hypocalciuric hypercalcemia type 3. Nat Genet. 2013;45:93–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Newey PJ, Nesbit MA, Rimmer AJ, et al. Whole-exome sequencing studies of nonhereditary (sporadic) parathyroid adenomas. J Clin Endocrinol Metab. 2012;97:E1995–E2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. D'Souza-Li L, Yang B, Canaff L, et al. Identification and functional characterization of novel calcium-sensing receptor mutations in familial hypocalciuric hypercalcemia and autosomal dominant hypocalcemia. J Clin Endocrinol Metab. 2002;87:1309–1318. [DOI] [PubMed] [Google Scholar]
- 16. Fu W, O'Connor TD, Jun G, et al. Analysis of 6,515 exomes reveals the recent origin of most human protein-coding variants. Nature. 2013;493:216–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dell'Angelica EC, Shotelersuk V, Aguilar RC, Gahl WA, Bonifacino JS. Altered trafficking of lysosomal proteins in Hermansky-Pudlak syndrome due to mutations in the β3A subunit of the AP-3 adaptor. Mol Cell. 1999;3:11–21. [DOI] [PubMed] [Google Scholar]
- 19. Montpetit A, Cote S, Brustein E, et al. Disruption of AP1S1, causing a novel neurocutaneous syndrome, perturbs development of the skin and spinal cord. PLoS Genet. 2008;4:e1000296. [DOI] [PMC free article] [PubMed] [Google Scholar]