Abstract
Biofilms were grown on preconditioned voice prostheses with biosurfactants obtained from probiotic bacteria Lactococcus lactis 53 and Streptococcus thermophilus A in an artificial throat model. Both biosurfactants greatly reduced microbial numbers on prostheses and also induced a decrease in the airflow resistance that occurs on voice prostheses after biofilm formation. This study presents a promising strategy for prolonging the lifespan of voice prostheses.
Voice prostheses are used for speech rehabilitation of patients who have undergone a laryngectomy due to a malignant laryngeal tumor and need to breathe through a tracheostomy (1). The major drawback of voice prostheses involves their colonization within several weeks by a thick biofilm that consists of fungal and bacterial strains. This biofilm causes leakage of food and liquid, and it may block the valves and increase resistance to airflow (10), making it necessary to replace the prosthesis on average every 3 to 4 months. Prolonged administration of antifungal agents to patients is undesirable because of the danger of inducing resistance (7). The use of biosurfactants from probiotic bacteria as antimicrobial and/or antiadhesive agents is promising as a method of prolonging the lifetimes of voice prostheses, and their ability to inhibit adhesion of various microorganisms isolated from explanted voice prostheses has been demonstrated in a study using parallel-plate flow chambers (11).
The aim of the present study was to evaluate the extent of biofilm formation by a mixture of bacterial and fungal strains isolated from explanted voice prostheses and cultured on silicone rubber voice prostheses with an adsorbed biosurfactant layer. The two tested biosurfactants were obtained from the probiotic bacteria Lactococcus lactis 53 and Streptococcus thermophilus A. A microbial growth inhibition test was first performed in order to estimate the concentration of biosurfactant to be used in the artificial throat experiments. To this end, biofilms were grown on voice prostheses in an artificial throat model (9). In addition to biofilm evaluation, the effects of biosurfactant adsorption to voice prostheses on resistance to airflow were determined.
The biosurfactant was isolated as previously described (2; L. Rodrigues, J. Teixeira, and R. Oliveira, Abstr. 11th Eur. Congr. Biotechnol., p. 76, 2003) for the selected probiotic bacterial strains L. lactis 53 (biosurfactant 1) and S. thermophilus A (biosurfactant 2). The microorganisms used for the growth inhibition test are listed in Table 1. The inhibition test was performed as described by Elving et al. (8). Briefly, fungal strains and bacteria cultured overnight under appropriate conditions were harvested by centrifugation and diluted in reduced transport fluid [0.9 g of NaCl liter−1, 0.9 g of (NH4)2SO4 liter−1, 0.45 g of KH2PO4 liter−1, 0.19 g of MgSO4 liter−1, 0.45 g of K2HPO4 liter−1, 0.37 g of Na2EDTA liter−1, 0.2 g of l-cysteine HCl liter−1 (pH 6.8)] to a concentration allowing confluent growth when plated with a cotton swab on the agar. Fungal strains were plated on De Man, Rogosa and Sharpe (MRS) agar (Merck), whereas bacteria were plated on brain heart infusion agar (Oxoid, Basingstoke, England). Agar plates were dried for 20 min at room temperature, and 5-μl aliquots of biosurfactant 1 or 2 in several concentrations (3, 5, 10, 25, 50, and 100 mg ml−1) were spotted onto the surface of the agar plate. After overnight incubation, the agar plates were screened for growth inhibition zones around the biosurfactant spots.
TABLE 1.
Antimicrobial activities of biosurfactants with different concentrations against several bacterial and fungal strains isolated from explanted voice prostheses
| Microorganism | Growth inhibitiona observed at indicated concn (mg/ml) of biosurfactant:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 (from L. lactis 53)
|
2 (from S. ther- mophilus A)
|
|||||||||
| 5 | 10 | 25 | 50 | 100 | 3 | 5 | 10 | 50 | 100 | |
| Staphylococcus epidermidis GB 9/6 | ± | ± | + | + | + | ± | ± | ± | + | + |
| Streptococcus salivarius GB 24/9 | − | − | ± | ± | + | − | − | ± | ± | + |
| Staphylococcus aureus GB 2/1 | ± | ± | ± | + | + | − | − | ± | ± | + |
| Rothia dentocariosa GBJ 52/2B | − | − | ± | ± | ± | − | − | ± | ± | ± |
| Candida albicans GBJ 13/4A | − | ± | ± | + | + | − | − | ± | + | + |
| Candida tropicalis GB 9/9 | + | + | + | + | + | + | + | + | + | + |
+, growth inhibition observed (no colonies formed); ±, limited growth inhibition (some colonies formed); −, no growth inhibition (many colonies formed).
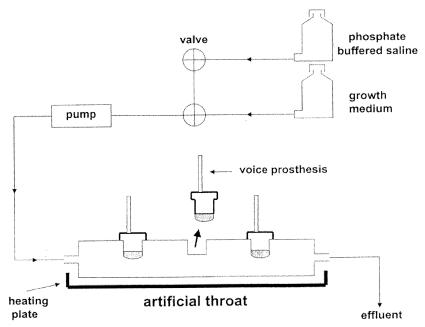
Voice prostheses (“Low Resistance” Groningen button; Médin Instruments and Supplies, Groningen, The Netherlands) made of implant grade silicone rubber were placed in artificial throats (9) (Fig. 1). For experiments with the selected biosurfactants at concentrations of up to 100 mg ml−1, artificial throats were autoclaved before use and equipped with one of three voice prostheses. One prosthesis was used as a control, and two prostheses were preconditioned overnight at 4°C with biosurfactant 1 or 2. To mimic the biofilms found in laryngectomized patients, the artificial throats were inoculated for 5 h with a combination of equal concentrations of the microbial strains listed in Table 1 (7). After inoculation, the voice prostheses were incubated at 37°C to grow a biofilm during 3 days by filling the devices with growth medium each day [the medium consisted of a mixture of 30% brain heart infusion broth and 70% defined yeast extract medium containing, per liter, 7.5 g of glucose, 3.5 g of (NH4)2SO4, 1.5 g of l-asparagine, 10 mg of l-histidine, 20 mg of dl-methionine, 20 mg of dl-tryptophan, 1 g of KH2PO4, 500 mg of MgSO4 · 7H2O, 500 mg of NaCl, 500 mg of CaCl2 · 2H2O, 100 mg of yeast extract, 500 μg of H3BO3, 400 μg of ZnSO4 · 7H2O, 120 μg of Fe(III)Cl3, 200 μg of Na2MoO4 · 2H2O, 100 μg of KI, and 40 μg of CuSO4 · 5H2O]. From day 4 to day 7 the artificial throats were perfused three times a day with 250 ml of phosphate-buffered saline (PBS) (10 mM KH2PO4 and K2HPO4 and 150 mM NaCl with pH adjusted to 7.0). At the end of each day, the prostheses were filled with growth medium for half an hour and then placed overnight in the moist environment of the drained artificial throats (i.e., without any medium inside). Previously, this cycle of nutrients and no nutrients had been demonstrated as being essential to the growth of biofilms with features similar to those found on explanted prostheses (9). All experiments were carried out in triplicate.
FIG. 1.
Schematic representation of the artificial throat equipped with three Groningen button voice prostheses.
To measure airflow resistances, compressed air was blown through each voice prosthesis prior to biofilm formation and after coverage with a 7-day-old biofilm (6). The airflow resistances of individual voice prostheses prior to biofilm formation differed due to manufacturing. Therefore, all airflow resistances measured were expressed relative to the airflow resistance of the same prosthesis prior to biofilm formation.
On the eighth day of an experiment, the airflow resistances and the numbers of CFU on the voice prostheses with a biofilm were determined. Biofilms were removed by scraping, and the collected material was sonicated on ice for 10 s (the material was suspended in reduced transport fluid) and subsequently serially diluted. The sonication procedure did not promote cell lysis. After plating onto blood agar (blood agar from Oxoid supplemented with 5% sheep blood, 0.5% hemin, and 0.1% menadione) for isolation of bacterial strains and onto MRS agar for isolation of fungal strains, plates were incubated at 37°C in an aerobic incubator for 3 days. The numbers of bacterial and fungal CFU on each prosthesis were determined and expressed as percentages of the numbers of the control.
The antimicrobial activities of the biosurfactants, at several concentrations, to a variety of bacterial and fungal strains isolated from explanted voice prostheses were evaluated and compared (Table 1). The two biosurfactants are antimicrobial agents but, depending on the microorganism, have different effective concentrations. It was found that both biosurfactants show a high antimicrobial activity against Candida tropicalis GB 9/9 even at low concentrations. At the highest concentration tested (100 mg ml−1), both biosurfactants were active against all bacterial and fungal strains involved in this study, except for Rothia dentocariosa GBJ52/2B, which formed some colonies within the biosurfactant spots. Elving et al. (5) recently demonstrated that R. dentocariosa was the most frequently isolated bacterial strain for a group of patients whose prostheses failed after a short time of use, making replacement necessary, which suggests that the organism may be associated with prosthetic failure. Therefore, the exclusion of this bacterial strain from the oral microflora by selected antibiotics or salivary peptides might well be more effective than the currently applied antimycotic regime, which has no proven clinical efficacy. From the results of the microbial growth inhibition test, a concentration of 100 mg ml−1 for each biosurfactant was chosen for preconditioning the voice prostheses.
Biosurfactants 1 and 2 decreased significantly the amount of bacteria in the biofilm, to 4 and 13% of the control, respectively (Table 2). Biosurfactant 1 reduced the amount of fungal organisms to 15% of the control, whereas biosurfactant 2 reduced them to 26% of the control. According to the literature, the consumption of buttermilk, which contains antimycotic-agent-releasing L. lactis, positively affects the lifetime of voice prostheses (3). Moreover, the biosurfactants released by S. thermophilus interfere with the initial deposition of fungal strains onto silicone rubber (2), as well as on the formation of a mixed fungal-bacterial biofilm on silicone rubber voice prostheses in the modified Robbins device (1).
TABLE 2.
Percentages of culturable bacterial and fungal strains isolated from voice prostheses after biofilm formation in the artificial throat and decreases in airflow resistance caused by biofilms influenced by biosurfactants compared with the effects of PBS (control)a
| Agent tested | % of control CFU (mean ± SD)
|
Decrease in airflow resistance (cm H2O · s liter−1) | |
|---|---|---|---|
| Bacteria | Fungal strains | ||
| Biosurfactant 1 | 4 ± 2 | 15 ± 3 | −16 ± 6 |
| Biosurfactant 2 | 13 ± 2 | 26 ± 1 | −22 ± 6 |
| PBSb | 100 | 100 | 0 |
Differences from the control were significant (paired Student's t test, P < 0.05). Biosurfactant 1 was obtained from L. lactis 53, and biosurfactant 2 was obtained from S. thermophilus A. All experiments were carried out in triplicate with separately cultured strains.
The numbers of bacterial and fungal CFU observed with the control were 6.7 × 106 and 1.4 × 105 per cm2, respectively, on the esophageal surface of the “Low Resistance” Groningen button voice prosthesis; these numbers were set as 100%. PBS causes an increase in airflow resistance of 31 ± 9 cm H2O·s liter−1; this was set as 0.
The influence of the biosurfactants tested in the airflow resistance of biofilms is summarized in Table 2. The airflow resistance of the Groningen button voice prostheses used prior to biofilm formation was 66 ± 7 cm H2O · s liter−1 (average ± standard deviation) as averaged over all nine prostheses involved in this study. The airflow resistances of prostheses increased on average by 31 ± 9 cm H2O · s liter−1 after 7 days of biofilm formation and perfusion of the artificial throats with PBS (control). Biosurfactants 1 and 2 both caused significant decreases (Student's t test, P < 0.05) in airflow resistance, 16 and 22 cm H2O · s liter−1, respectively, compared with the values observed for the control. The decrease in airflow resistance also suggests that the integrity of the biofilm was affected. The density of biofilms on voice prostheses causes unwanted increases in airflow resistance, impeding speech, and is caused by extracellular polymeric substances (EPS) (4). It has been suggested that it is not the thickness of biofilms that is the most important factor in valve failure but rather the combined presence of EPS-producing bacterial strains and fungal species (7). Elving et al. concluded that the persistence of biofilms on voice prostheses is determined not so much by the number of organisms in the biofilm as by the production of EPS that glue the biofilm together and increase the airflow resistance of voice prostheses. Apparently, it is more effective to decrease the viability of biofilms by acting directly on the EPS than to reduce the number of organisms on the esophageal surface of voice prostheses (8).
The approach developed in this study is a promising strategy, as it was demonstrated that the adsorbed biosurfactants inhibit biofilm formation and the occurrence of increased airflow resistance. As a consequence, the useful lifespan of voice prostheses may be lengthened, an effect which would directly benefit laryngectomized patients.
Acknowledgments
The FCT provided financial support for L. Rodrigues through doctoral research grant SFRH/BD/4700/2001.
REFERENCES
- 1.Busscher, H. J., B. van de Belt-Gritter, M. Westerhof, R. van Weissenbruch, F. W. Albers, and H. C. van der Mei. 1999. Microbial interference in the colonization of silicone rubber implant surfaces in the oropharynx: Streptococcus thermophilus against a mixed fungal/bacterial biofilm, p. 66-74. In E. Rosenberg (ed.), Microbial ecology and infectious disease. American Society for Microbiology, Washington, D.C.
- 2.Busscher, H. J., C. G. van Hoogmoed, G. I. Geertsema-Doornbusch, M. van der Kuijl-Booij, and H. C. van der Mei. 1997. Streptococcus thermophilus and its biosurfactants inhibit adhesion by Candida spp. on silicone rubber. Appl. Environ. Microbiol. 10:3810-3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Busscher, H. J., G. Bruinsma, R. van Weissenbruch, C. Leunisse, H. C. van der Mei, F. Dijk, and F. W. Albers. 1998. The effect of buttermilk consumption on biofilm formation on silicone rubber voice prostheses in an artificial throat. Eur. Arch. Otorhinolaryngol. 255:410-413. [DOI] [PubMed] [Google Scholar]
- 4.Decho, A. W., and T. Kawaguchi. 1999. Confocal imaging of in situ natural microbial communities and their extracellular polymeric secretions using Nanoplast resin. BioTechniques 27:1246-1252. [PubMed] [Google Scholar]
- 5.Elving, G. J., H. C. van der Mei, H. J. Busscher, R. van Weissenbruch, and F. W. Albers. 2002. A comparison of the microbial composition of voice prosthetic biofilms from patients requiring frequent versus infrequent replacements. Ann. Otol. Rhinol. Laryngol. 111:200-203. [DOI] [PubMed] [Google Scholar]
- 6.Elving, G. J., H. C. van der Mei, H. J. Busscher, R. van Weissenbruch, and F. W. Albers. 2003. Influence of different combinations of bacteria and yeasts in voice prosthetic biofilms on the airflow resistances of prostheses. Antonie van Leeuwenhoek 83:45-55. [DOI] [PubMed] [Google Scholar]
- 7.Elving, G. J., H. C. van der Mei, R. van Weissenbruch, F. W. Albers, and H. J. Busscher. 2000. Effect of antifungal agents on indwelling voice prosthetic biofilms. Curr. Opin. Otolaryngol. Head Neck Surg. 8:165-168. [Google Scholar]
- 8.Elving, G. J., H. C. van der Mei, H. J. Busscher, A. N. Amerongen, E. C. Veerman, R. van Weissenbruch, and F. W. Albers. 2000. Antimicrobial activity of synthetic salivary peptides against voice prosthetic microorganisms. Laryngoscope 110:321-324. [DOI] [PubMed] [Google Scholar]
- 9.Leunisse, C., R. van Weissenbruch, H. J. Busscher, H. C. van der Mei, and F. W. Albers. 1999. The artificial throat: a new method for standardization of in vitro experiments with tracheo-esophageal voice prostheses. Acta Otolaryngol. 119:604-608. [PubMed] [Google Scholar]
- 10.Mahieu, H. F., J. J. van Saene, J. Den Besten, and H. K. van Saene. 1986. Oropharynx decontamination preventing Candida vegetation on voice prostheses. Arch. Otolaryngol. Head Neck Surg. 112:1090-1092. [DOI] [PubMed] [Google Scholar]
- 11.Rodrigues, L., H. C. van der Mai, J. Teixeira, and R. Oliveira. Biosurfactant from Lactococcus lactis 53 inhibits microbial adhesion on silicone rubber. Appl. Microbiol. Biotechnol., in press. [DOI] [PubMed]