Key Points
Low doses of adoptively transferred third-party CD4+ iNKT cells protect from lethal GVHD while preserving graft-versus-tumor effects.
Third-party CD4+ iNKT cells are rejected early after transplantation yet protect from GVHD lethality through donor Tregs.
Abstract
Graft-versus-host disease (GVHD) is driven by extensive activation and proliferation of alloreactive donor T cells causing significant morbidity and mortality following allogeneic hematopoietic cell transplantation (HCT). Invariant natural killer T (iNKT) cells are a potent immunoregulatory T-cell subset in both humans and mice. Here, we explored the role of adoptively transferred third-party CD4+ iNKT cells for protection from lethal GVHD in a murine model of allogeneic HCT across major histocompatibility barriers. We found that low numbers of CD4+ iNKT cells from third-party mice resulted in a significant survival benefit with retained graft-versus-tumor effects. In vivo expansion of alloreactive T cells was diminished while displaying a T helper cell 2-biased phenotype. Notably, CD4+ iNKT cells from third-party mice were as protective as CD4+ iNKT cells from donor mice although third-party CD4+ iNKT cells were rejected early after allogeneic HCT. Adoptive transfer of third-party CD4+ iNKT cells resulted in a robust expansion of donor CD4+CD25+FoxP3+ regulatory T cells (Tregs) that were required for protection from lethal GVHD. However, in vivo depletion of myeloid-derived suppressor cells abrogated both Treg expansion and protection from lethal GVHD. Despite the fact that iNKT cells are a rare cell population, the almost unlimited third-party availability and feasibility of in vitro expansion provide the basis for clinical translation.
Introduction
Allogeneic hematopoietic cell transplantation (HCT) is a widely used curative therapy for patients with hematologic malignancies and congenital hematopoietic, immunologic, and metabolic disorders. Beneficial graft-versus-tumor (GVT) effects are mediated by donor T lymphocytes. However, alloreactive donor T cells cause graft-versus-host disease (GVHD) after stimulation by antigen-presenting dendritic cells in a proinflammatory environment resulting in tissue injury.1-3 Clinical manifestations of GVHD and immunosuppressive therapy are associated with significant morbidity, mortality, and a reduced quality of life outweighing some benefits of allogeneic HCT.4
Invariant natural killer T (iNKT) cells are a small but potent immunoregulatory T-lymphocyte subset characterized by the expression of a semi-invariant T-cell receptor (TCR) in humans (TCRα Vα24-Jα18) and mice (TCRα Vα14-Jα18).5,6 Preclinical animal models and clinical studies revealed that minimal intensity conditioning with total lymphoid irradiation (TLI) and antithymocyte globulin (ATG) results in a relative expansion of host iNKT cells which protect from GVHD and promote tolerance toward allogeneic solid organ transplants.7-13 Moreover, adoptively transferred donor CD4+ iNKT cells prevent lethal GVHD after myeloablative conditioning, followed by allogeneic HCT across major histocompatibility barriers.14 In contrast to conventional CD4+ and CD8+ T cells, the iNKT-cell receptor and glycolipid-presenting molecule CD1d interaction is highly conserved.15-20 Due to the paucity of cells, clinical translation may be accelerated through the availability of a ready source of iNKT cells from third-party donors. Therefore, we explored the role of adoptively transferred third-party CD4+ iNKT cells in a murine model of allogeneic HCT.
In the present study, we elucidate the immunoregulatory interplay between third-party CD4+ iNKT cells, donor CD4+CD25+FoxP3+ regulatory T cells (Tregs), donor myeloid-derived suppressor cells (MDSCs), and alloreactive T cells, to prevent lethal GVHD while preserving GVT effects. Moreover, we shed light on the survival of donor and third-party CD4+ iNKT cells after adoptive transfer. Our findings highlight potent immunoregulatory and tolerogenic properties of iNKT cells and provide the basis for clinical translation.
Methods
Mice
Gender-matched female or male mice between 10 and 14 weeks of age were used for all experiments. BALB/c (H-2Kd), C57BL/6 (H-2Kb), and FVB/N (H-2Kq) mice were purchased from The Jackson Laboratory. Luciferase (luc), Thy1.1, and CD45.1-expressing C57BL/6 mice, as well as luc and green fluorescent protein (GFP)-expressing FVB/N mice were generated as described previously.21 C57BL/6 albino FoxP3 mutant mice expressing diphtheria toxin (DT) receptor, GFP, and luc (FoxP3DTR/GFP/luc) were a kind gift from Dr Günter J. Hämmerling (Heidelberg, Germany) and bred in our animal facility. Animal protocols were approved by the Institutional Animal Care and Use Committee of Stanford University.
Cell isolation
For the isolation of donor and third-party CD4+ iNKT cells, spleens from the respective mice were dispersed in phosphate-buffered saline (PBS) (Life Technologies) with 2% fetal calf serum (Life Technologies) into single-cell suspensions, red blood cells were lysed with ammonium chloride buffer, and Fc receptor block (Miltenyi Biotec) was applied before B cells were depleted with CD45R (B220) MicroBeads (Miltenyi Biotec). iNKT cells were stained with PBS-57-CD1d tetramer phycoerythrin (National Institutes of Health) and enriched with anti-phycoerythrin MicroBeads (Miltenyi Biotec). After magnetic-activated cell sorting, cells were stained for TCR-β and CD4 and further purified on a FACSAria II cell sorter (BD Biosciences). Donor CD4+ and CD8+ conventional T cells (Tcons) were prepared from splenocytes and lymph nodes, and enriched with CD4 and CD8 MicroBeads (Miltenyi Biotec). T-cell–depleted bone marrow (TCD-BM) cells were prepared by flushing murine tibiae and femora with PBS supplemented with 2% fetal calf serum, followed by depleting T cells with CD4 and CD8 MicroBeads (Miltenyi Biotec). Lymphocytes were isolated from GVHD target tissues for analysis. Livers were dispersed and residual red blood cells were lysed with ammonium chloride buffer. Intrahepatic lymphocytes were isolated through density centrifugation with Percoll (GE Healthcare). Intestines were flushed with PBS and dissected into pieces before intestinal lymphocytes were isolated through tissue digestion with 1 mg⋅ml−1 collagenase IV (Life Technologies) for 30 minutes. Skin from tail and ears was cut into pieces and digested with 1 mg⋅ml−1 collagenase IV (Life Technologies) for 60 minutes.
Treg depletion from the BM graft
On days −2 and −1, FoxP3DTR/GFP/luc C57BL/6 albino mice were injected intraperitoneally (IP) with 50 µg⋅kg−1 DT (Sigma-Aldrich) dissolved in PBS to deplete FoxP3-expressing cells. Control mice were injected with PBS only. On day 0, secondary lymphoid organs were harvested and processed to isolate Tcons as described previously. TCD-BM and CD4+ iNKT cells were derived from untreated C57BL/6 or FVB/N mice.
Allogeneic BM transplantation
BALB/c recipient mice were treated with lethal total body irradiation (200 kV radiograph source; Kimtron) consisting of 2 doses of 4.0 Gy administered 4 hours apart. On the same day, 5.0 × 106 TCD-BM cells were injected via tail vein together with 1.0 × 106 Tcons from C57BL/6 or FVB/N mice. Donor or third-party CD4+ iNKT cells were co-injected on day 0. Transplanted animals were housed in autoclaved cages with antibiotic food (trimethoprim and sulfadiazine; Harlan). GVHD scores were assessed as described previously.22 Briefly, weight, fur, skin, activity, and posture were evaluated and a score from 0 to 2 was assigned to each characteristic, resulting in a maximal GVHD score of 10.
In vivo depletion of MDSCs
To deplete MDSCs, BALB/c recipient mice were injected IP with 10 µg⋅g−1 Ly-6G monoclonal antibody (clone RB8-8C5, Bio X Cell) dissolved in PBS on days 0 and +1. Control mice were injected with the respective isotype control (Bio X Cell).
Tumor model
To investigate GVT activity, we used A20 lymphoma cells and B-cell lymphoma (BCL)-1 cells with both expressing the luc gene (luc+). In the first model, 1.0 × 104 luc+ A20 lymphoma cells were injected IV into BALB/c recipients together with TCD-BM after lethal total body irradiation on day 0. In the second model, 3000 luc+ BCL-1 cells were injected IV into BALB/c recipients 7 days before allogeneic HCT. After transplantation, the tumor burden of recipient animals was assessed by bioluminescence imaging (BLI).
Histopathology
Tissues were fixed in 10% neutral buffered formalin and processed routinely for microscopic examination after staining with hematoxylin and eosin (H&E). Stained tissue sections were evaluated for GVHD with an Olympus BX41 microscope (Olympus) by a board-certified veterinary pathologist who was blinded to the experimental groups. Representative digital photomicrographs were taken using an Axioskop 2 Plus microscope (Carl Zeiss) with a Nikon DS-Ri1 digital microscope camera and NIS-Elements imaging software (Nikon).
Flow cytometric analysis
PBS-57–loaded and unloaded murine CD1d tetramers were obtained from the National Institutes of Health Tetramer Facility. The following antibodies were purchased from BD Biosciences, eBioscience, or BioLegend: TCR-β (H57-597), CD3 (145-2C11), CD4 (GK1.5), CD19 (6D5), CD11b (M1/70), Gr-1 (RB6-8C5), CD49b (DX5), TER-119 (TER-119), CD45.1 (A20), CD45.2 (104), Thy-1.1 (OX-7), H-2Kd (AF6-88.5.5.3), H-2Kb (AF6-88.5), H-2Kq (KH114), CD25 (PC61), FoxP3 (FJK-16s), Helios (22F6), murine interferon γ (XMG1.2), murine tumor necrosis factor α (MP6-XT22), and murine interleukin (IL)-4 (11B11). Isotype controls were purchased from the respective vendors. To stain dead cells, LIVE/DEAD Fixable Dead Cell Stain (Life Technologies) was used. To gate on MDSCs, lineage negative was defined as CD3−CD19−CD49b−Ter119−. Data were acquired on a LSR II flow cytometer (BD Biosciences) and analysis was performed with FlowJo software 10.0.7 (Tree Star, Ashland, OR).
Cell proliferation assay
For analysis of cell proliferation, sorted GFP+CD4+ iNKT cells were stained with CellTrace Violet Cell Proliferation Kit (Life Technologies) according to the manufacturer’s instructions prior to injection into lethally irradiated BALB/c mice, together with TCD-BM and Tcons. Data were acquired on a LSR II flow cytometer (BD Biosciences) and analysis was performed with FlowJo software 10.0.7 (Tree Star).
BLI
BLI was performed as described previously.23 Briefly, firefly luciferin (Biosynth) was injected IP 10 minutes prior to image acquisition with an IVIS 100 Imaging System (Xenogen). Images were analyzed with Living Image software 4.2 (Xenogen).
Cytokine analysis
For intracellular cytokine staining (ICS), cells were stimulated with phorbol myristate acetate (Sigma-Aldrich) and ionomycin (Sigma-Aldrich) as described previously.14 Monensin (BD Biosciences) was used to block cellular protein transport. After staining surface antigens, cells were fixed and permeabilized (eBioscience) prior to staining of intracellular and intranuclear antigens. For quantitative measurement of serum cytokines, whole blood from recipient mice was obtained through puncture of the heart after euthanasia. Murine sera were stored at −20°C until they were analyzed with a multiplex assay (Luminex).
Statistical analysis
Differences in animal survival (Kaplan–Meier survival curves) were analyzed with the log-rank test. All other comparisons were performed with the Student t test. P < .05 was considered statistically significant.
Results
Third-party CD4+ iNKT cells protect from GVHD
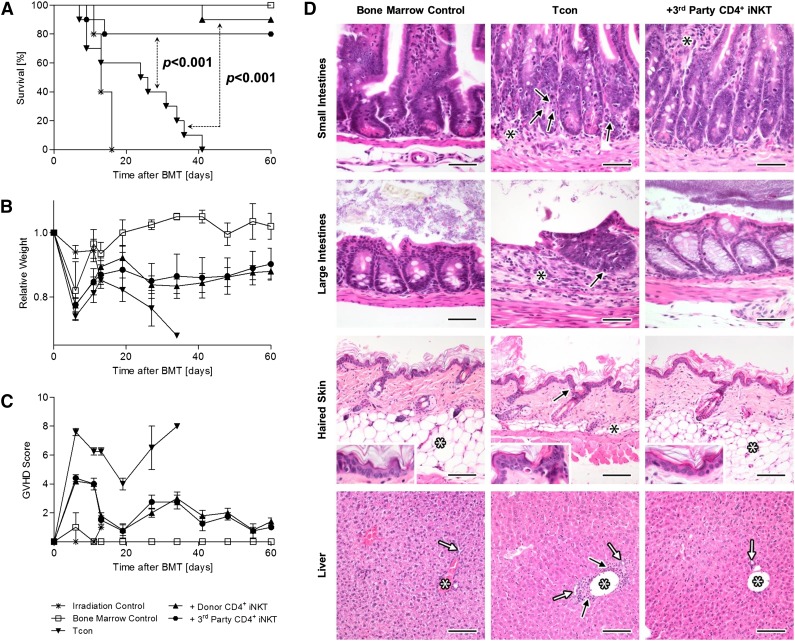
We investigated the role of third-party CD4+ iNKT cells in a murine model of allogeneic HCT across major histocompatibility barriers. BALB/c (H-2Kd) recipient mice were lethally irradiated with 2 × 4 Gy, followed by transplantation of 5.0 × 106 TCD-BM cells and 1.0 × 106 Tcons from C57BL/6 (H-2Kb) donor mice via tail vein injection which induced lethal GVHD. The adoptive transfer of 5.0 × 104 CD4+ iNKT cells (purity > 95%) from FVB/N (H-2Kq) third-party mice (median yield per spleen: 4.2 × 105 cells, 95% CI ± 0.5 × 105, n = 10) or C57BL/6 (H-2Kb) donor mice (median yield per spleen: 2.1 × 105 cells, 95% CI ± 0.2 × 105, n = 10) on day 0 resulted in a significant survival benefit compared with mice that received Tcons alone (both P < .001, Figure 1A). Likewise, recipient animals displayed higher body weights and improved GVHD scores (Figure 1B-C). Interestingly, we found that third-party CD4+ iNKT cells were as protective as CD4+ iNKT cells isolated from donor C57BL/6 mice, as no significant differences in survival (P = .50), body weight (P = .94), and GVHD score (P = .64) were observed between these 2 groups (Figure 1A-C). Our results were confirmed in a different mouse strain combination (see supplemental Figure 1 on the Blood Web site). Histopathologic examination of GVHD target tissues revealed reduced severity of GVHD in mice receiving adoptively transferred third-party CD4+ iNKT cells (Figure 1D). In conclusion, low numbers of third-party CD4+ iNKT cells protect from lethal GVHD with their effectiveness being comparable to donor CD4+ iNKT cells. Importantly, adoptive transfer of third-party CD4+ iNKT cells preserved GVT effects in 2 independent lymphoma models (supplemental Figure 2).
Figure 1.
Third-party CD4+ iNKT cells protect from lethal GVHD. BALB/c recipient mice were irradiated with 2 × 4 Gy, followed by transplantation of 5.0 × 106 TCD-BM cells and 1.0 × 106 Tcons from C57BL/6 donor mice. In addition, 5.0 × 104 CD4+ iNKT cells from C57BL/6 donor or FVB/N third-party mice were transferred together with the graft. (A) Overall survival pooled from 2 independent experiments with 10 mice per group except irradiation control (n = 5). (B) Weight and (C) GVHD score from 1 of 2 independent experiments with 5 animals per group except irradiation control (n = 3). Error bars indicate standard error of the mean (SEM). (D) Photomicrographs of small intestines, large intestines, haired skin, and liver at day +25. Small intestinal histology is normal in BM mice with presence of regularly-shaped and -sized villi, regularly-spaced and -sized intestinal crypts, presence of a regular complement of enterocytes (including goblet cells), and absence of any significant inflammation in the lamina propria and submucosa. In Tcon mice, there are many apoptotic cells in the crypt epithelium (black arrows), which appear as shrunken, hypereosinophilic, round bodies with pyknotic nuclei. Mild-to-moderate lymphoplasmacytic inflammation (black asterisks) is also present in the lamina propria and/or submucosa of the same mice, causing mild separation of the intestinal crypts. Third-party CD4+ iNKT-cell–treated mice have essentially normal small intestines when compared with the BM control mice, except for the presence of mild inflammatory infiltrates in the lamina propria (black asterisks). H&E stain; original magnification ×400; bar = 50 µm. Large intestinal histology is normal in BM control and third-party CD4+ iNKT-cell–treated mice with presence of regularly-spaced and -sized intestinal glands, presence of a regular complement of enterocytes (including goblet cells), and absence of any significant inflammation in the lamina propria and submucosa. In Tcon mice, there is marked lymphoplasmacytic inflammation (black asterisks) in the lamina propria and/or submucosa associated with the loss of intestinal glands. The remaining intestinal glands are abnormal with loss of goblet cells and presence of many apoptotic cells (black arrow), which appear as shrunken, hypereosinophilic, round bodies with pyknotic nuclei. H&E stain; original magnification ×400; bar = 50 µm. The BM control and third-party CD4+ iNKT-cell–treated mice have normal histology of the skin with an intact, thin epidermis and a thick layer of subcutaneous adipose tissue (white asterisks). In the Tcon mice however, there is mild interface damage of the epidermis (as evidenced by apoptotic keratinocytes; black arrows) and mild-to-complete atrophy of the subcutaneous adipose tissue (black asterisks). A mild dermal inflammatory infiltrate is also noted within the Tcon mice. H&E stain; original magnification ×200 (inset magnification ×400); bar = 100 µm. The BM control and third-party CD4+ iNKT-cell–treated mice have normal liver histology, with portal triads containing a single portal vein (white asterisks), and one or two bile ductules (white arrows). The livers of Tcon mice also appear essentially normal, except for some minimal lymphoplasmacytic inflammation in periportal interstitial tissues (black arrows) and mild proliferation/hyperplasia of bile ductules. H&E stain; original magnification ×200; bar = 100 µm. BMT, bone marrow transplantation.
Third-party CD4+ iNKT cells inhibit Tcon proliferation and promote T helper 2 (Th2)-biased immune responses in vivo
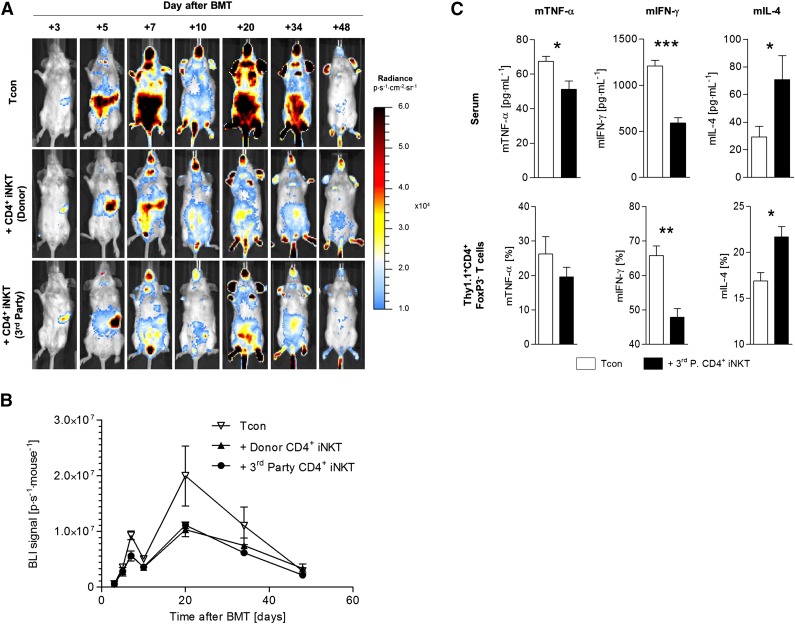
GVHD pathophysiology is characterized by activation and extensive proliferation of alloreactive donor T cells in secondary lymphoid organs, followed by infiltration and destruction of GVHD target tissues.24,25 The release of proinflammatory Th1 cytokines (eg, IFN-γ) by T cells perpetuates the cycle of alloreactivity. Using BLI, we assessed the proliferation of alloreactive Tcons derived from luc+ C57BL/6 donor mice with and without adoptive transfer of 5.0 × 104 CD4+ iNKT cells from donor C57BL/6 mice and third-party FVB/N mice, respectively. We found significantly lower bioluminescence signals in animals treated with CD4+ iNKT cells from donor (day +7: P = .002; day +10: P = .006; day +20: P = .04) and third-party (day +7: P = .008; day +10: P = .03; day +20: P = .05) mice compared with animals that received Tcons only (Figure 2A-B). Therefore, adoptively transferred third-party CD4+ iNKT cells inhibit expansion of alloreactive T cells in vivo comparable to donor CD4+ iNKT cells. In addition, we analyzed murine sera and re-isolated Thy1.1+CD4+FoxP3- donor Tcons 10 days after allogeneic HCT and analyzed cytokine production with a multiplex assay and ICS, respectively. Animals that were treated with third-party CD4+ iNKT cells showed significantly lower levels of murine tumor necrosis factor α (multiplex: P = .01; ICS: P = .40) and murine interferon γ (multiplex: P < .001; ICS: P = .009) but significantly higher levels of murine IL-4 (multiplex: P = .04; ICS: P = .04) compared with animals that received Tcons only (Figure 2C). Consequently, third-party CD4+ iNKT cells bias donor T cells toward a tolerogenic Th2 phenotype while inhibiting their in vivo expansion.
Figure 2.
Third-party CD4+ iNKT cells inhibit Tcon proliferation and induce a Th2 immune phenotype. Photon emission of Tcons from luc+ C57BL/6 donor mice was measured by BLI to assess the in vivo expansion capacity of alloreactive T cells in donor and third-party CD4+ iNKT-cell–treated BALB/c recipient mice. (A) Representative serial bioluminescence images. (B) Bioluminescence signal intensity time course. Error bars indicate SEM. Shown is 1 of 2 independent experiments with 5 mice per group. (C) To determine alterations in immune polarization in third-party CD4+ iNKT-cell–treated BALB/c recipient mice, murine sera, and Thy1.1+CD4+FoxP3− T cells from spleens were analyzed at day +10 by multiplex assay and ICS, respectively. Error bars indicate SEM. Pooled data from 2 independent experiments with 6 mice per group are shown. *P ≤ .05; **P ≤ .01; ***P ≤ .001.
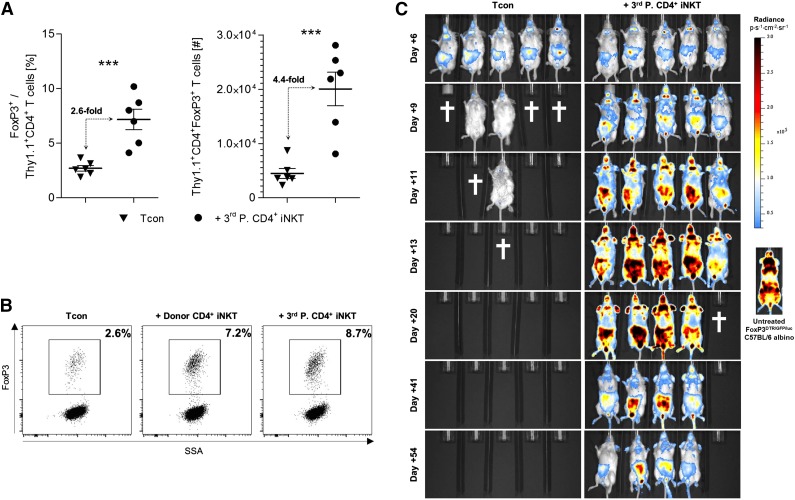
Third-party CD4+ iNKT cells promote an expansion of donor Tregs
At day +10, we found a significant relative (P < .001) and absolute (P < .001) increase of Thy1.1+ donor Tregs in BALB/c recipient mice treated with FVB/N third-party CD4+ iNKT cells in peripheral lymph nodes, mesenteric lymph nodes, spleen, liver, gut, and skin, compared with animals that received Tcons alone (Figure 3A). Interestingly, this expansion was similar to BALB/c mice that received CD4+ iNKT cells derived from C57BL/6 donor mice (Figure 3B). We prepared Tcons from FoxP3DTR/GFP/luc C57BL/6 albino mice in order to study the temporal and spatial characteristics of donor Treg expansion. In this particular mouse strain, the expression of luc is regulated by the FoxP3 promoter enabling the visualization of donor Tregs through BLI. Bioluminescence intensity deriving from donor Tregs was steadily increasing for the first 3 weeks in secondary lymphoid organs and GVHD target sites in animals that were treated with third-party CD4+ iNKT cells (Figure 3C). The temporal and spatial distribution of these Tregs resembles alloreactive donor Tcons as they can be first found in the lymph nodes and spleen, followed by the liver, gut, and skin. The bioluminescence pattern is similar to that of untreated FoxP3DTR/GFP/luc C57BL/6 albino mice. We were able to follow these Tregs for at least 8 weeks after allogeneic HCT (Figure 3C). In contrast, we found a steadily decreasing BLI signal in animals that received Tcons only, finally resulting in death from GVHD of all BALB/c mice.
Figure 3.
Third-party CD4+ iNKT cells promote an expansion of donor Tregs. (A) Relative and absolute numbers of donor Tregs from spleens of BALB/c recipient mice at day +10. Error bars indicate SEM. Pooled data from 2 independent experiments with 6 mice per group are shown. (B) Representative dot plots gated on live Thy1.1+CD4+ donor T cells re-isolated at day +10 from BALB/c recipient spleens. Shown is 1 of 2 independent experiments. (C) BALB/c recipient mice received Tcons from FoxP3DTR/GFP/luc C57BL/6 albino mice with and without CD4+ iNKT cells from FVB/N third-party mice. Bioluminescence signals derive from luc+FoxP3+ donor T cells. Shown is 1 of 2 independent experiments with 5 mice per group, as well as a representative bioluminescence image from one untreated FoxP3DTR/GFP/luc C57BL/6 albino mouse. The † indicates that all animals from the respective group died or needed to be euthanized. ***P ≤ .001. SSA, side scatter area.
Donor Tregs expand from the graft and are required for protection from GVHD lethality through third-party CD4+ iNKT cells
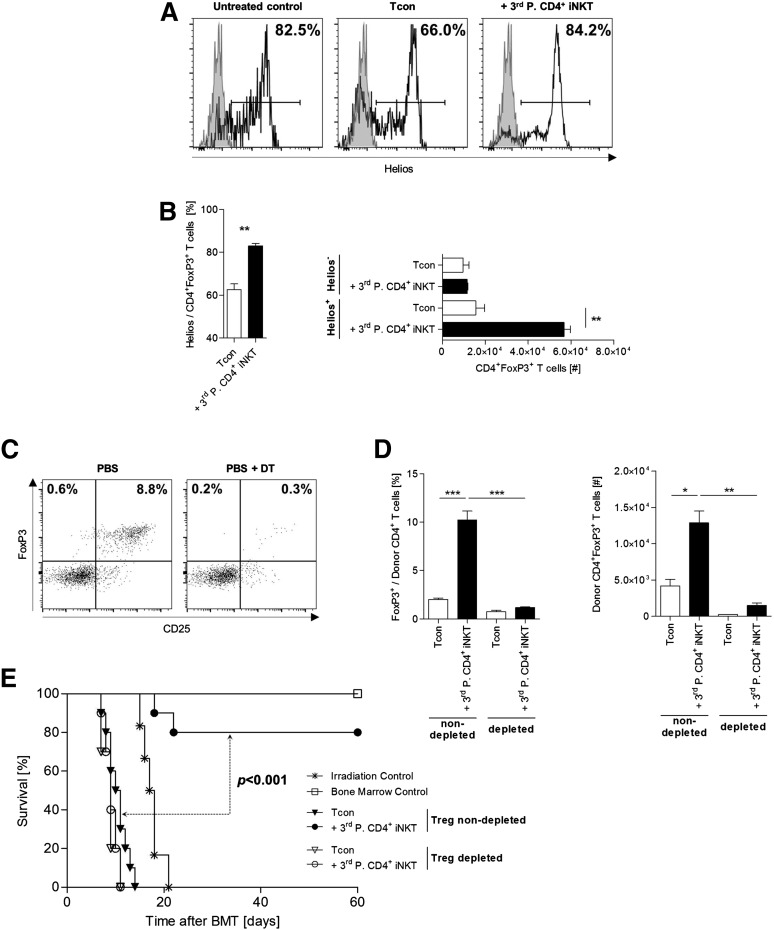
Expression of the Ikaros nuclear transcription factor Helios is widely recognized as a marker to distinguish thymus-derived natural Tregs (nTregs) from peripherally induced Tregs.26,27 In our animal model, third-party CD4+ iNKT cells promoted the expansion of Helios+ donor Tregs (P = .006), whereas no increase could be found within the Helios- donor Treg subset (P = .63, Figure 4A-B). These findings indicate that the presence of donor Tregs within the graft is required for Treg expansion. To test this hypothesis, we used FoxP3DTR/GFP/luc C57BL/6 albino donor mice. Thereby, we generated Tcons that were efficiently depleted of Tregs through IP injection of DT on days −2 and −1 before allogeneic HCT (Figure 4C). TCD-BM was derived from untreated wild-type (WT) C57BL/6 mice. BALB/c mice that were treated with CD4+ iNKT cells from third-party mice failed to efficiently expand donor Tregs when they received a graft that was depleted of Tregs compared with mice that received a Treg nondepleted graft (Figure 4D). Therefore, these Tregs derived from the nTreg pool contained within the graft rather than being induced from the naïve T-cell compartment. Moreover, the adoptive transfer of third-party CD4+ iNKT cells did not provide protection from lethal GVHD in mice that received a Treg-depleted graft. Only animals that received a Treg nondepleted graft showed a significantly improved survival through adoptively transferred third-party CD4+ iNKT cells (P < .001, Figure 4E). In conclusion, third-party CD4+ iNKT cells mediate protection from GVHD lethality through expansion of donor Tregs that are contained within the graft.
Figure 4.
Donor Tregs expand from the graft and are required for protection from GVHD lethality. (A) Representative histograms and (B) relative and absolute numbers of Helios-expressing donor Tregs re-isolated from recipient livers at day +10. Error bars indicate SEM. Shown are 3 mice per group from 1 of 3 independent experiments. (C) FoxP3DTR/GFP/luc C57BL/6 albino donor mice were injected IP with DT to deplete these mice of Tregs. Dot plots are gated on live TCR-β+CD4+ T cells and show representative examples of Tcons prepared from mice treated with PBS only or DT dissolved in PBS. (D) Relative and absolute numbers of donor Tregs re-isolated from spleens of BALB/c mice at day +10. Mice received either a Treg-nondepleted or Treg-depleted BM graft with or without adoptive transfer of 5.0 × 104 FVB/N third-party CD4+ iNKT cells. TCD-BM and third-party CD4+ iNKT cells derive from untreated WT C57BL/6 mice. Error bars indicate SEM. Three animals per group from 1 of 3 independent experiments are shown. (E) Overall survival of BALB/c recipient mice receiving either a Treg-nondepleted or Treg-depleted graft from C57BL/6 donor mice with or without adoptive transfer of 5.0 × 104 FVB/N third-party CD4+ iNKT cells. TCD-BM was derived from untreated WT C57BL/6 mice. Ten mice per group except irradiation control (n = 6). Pooled data from 2 independent experiments are shown. *P ≤ .05; **P ≤ .01; ***P ≤ .001.
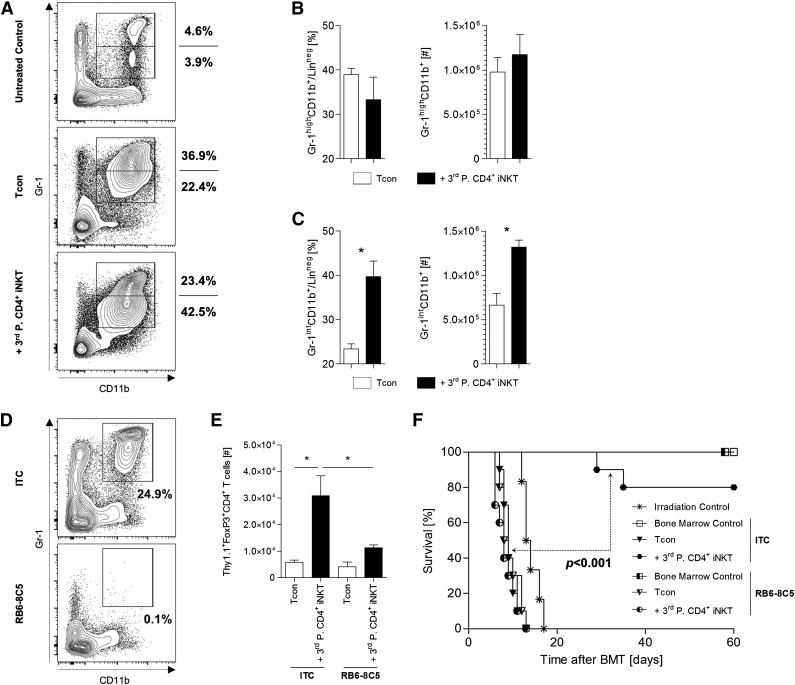
MDSCs are required for Treg expansion and protection from lethal GVHD
MDSCs are immature cells of myeloid origin that harbor a variety of immunoregulatory properties in the context of allogeneic HCT.28,29 Hongo et al recently described the importance of MDSCs for tolerance induction after TLI/ATG conditioning.30 In our model, the adoptive transfer of third-party CD4+ iNKT cells resulted in a relative and absolute expansion of donor CD11b+Gr-1int MDSCs at day +10 (Figure 5A-C). To elucidate the relevance of MDSCs for Treg expansion and GVHD amelioration, we depleted MDSCs by IP injection of the monoclonal antibody RB8-8C5 into recipient animals at days 0 and +1 (Figure 5D). Depletion of MDSCs abrogated both the expansion of donor Tregs (Figure 5E) and the protection from lethal GVHD (P < .001, Figure 5F). Similar results were found for adoptively transferred donor CD4+ iNKT cells (data not shown). We conclude that MDSCs are crucial for Treg expansion and protection from GVHD lethality through CD4+ iNKT cells. Our findings suggest a cascade of several distinct cellular interactions that are required in order to result in immune tolerance after allogeneic HCT.
Figure 5.
MDSCs are required for Treg expansion and protection from lethal GVHD. (A) Representative dot plots gated on live donor (CD45.1+) and lineage negative (CD3−CD19−CD49b−TER-119−) cells from spleens of BALB/c recipient mice at day +10 after allogeneic HCT. Absolute and relative cell numbers of donor (B) CD11b+Gr-1high and (C) CD11b+Gr-1int MDSCs. Error bars indicate SEM. Three animals per group from 1 of 3 independent experiments are shown. (D) Representative dot plots gated on live and lineage negative (CD3-CD19-CD49b-TER-119-) cells from spleens of BALB/c mice injected with ITC or RB6-8C5. (E) Absolute number of donor Tregs re-isolated from spleens of BALB/c mice at day +10. Error bars indicate SEM. Three animals per group from 1 of 2 independent experiments are shown. (F) Overall survival of BALB/c recipient mice either depleted (RB6-8C5) or nondepleted (ITC) of MDSCs. Ten mice per group except irradiation control (n = 6). Pooled data from 2 independent experiments are shown. *P ≤ .05. ITC, isotype control antibody.
Adoptively transferred CD4+ iNKT cells home to the recipient liver and expand in vivo
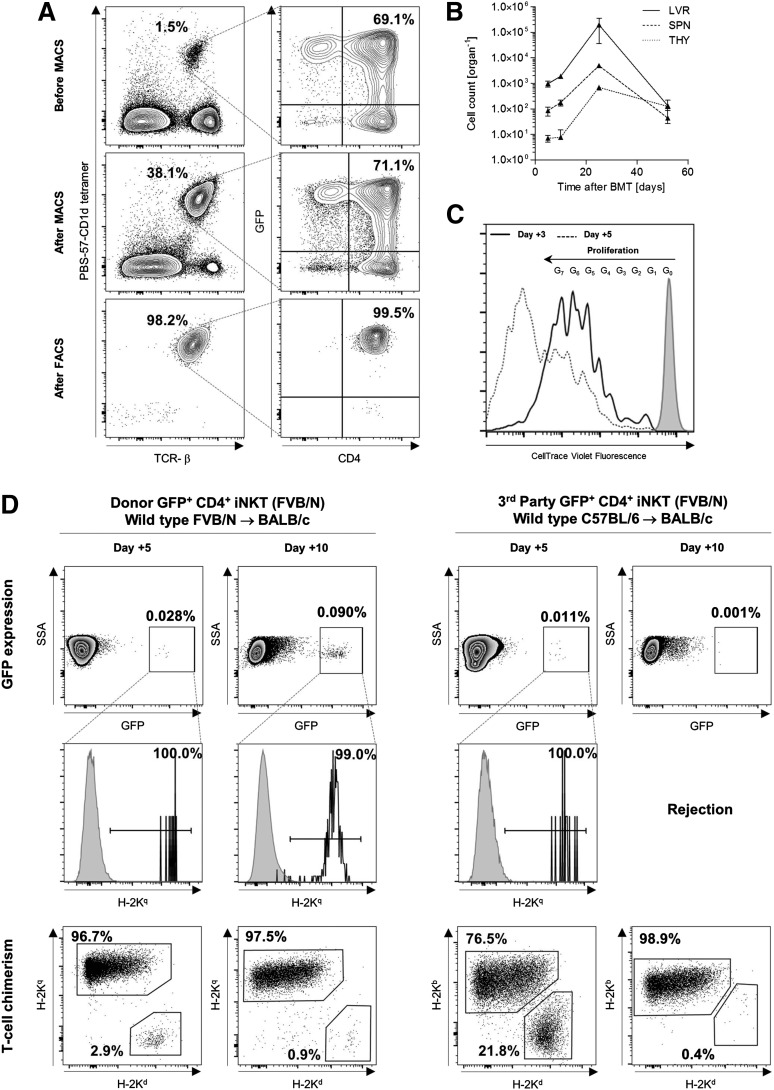
In order to shed light on the trafficking pattern of CD4+ iNKT cells after adoptive transfer, we purified these cells from the spleens of GFP+ FVB/N mice (Figure 6A). The expression of GFP allows for the re-isolation of adoptively transferred CD4+ iNKT cells from BALB/c recipient mice independent of cellular activation that can be associated with TCR downregulation.31 We injected 1.0 × 105 GFP+ CD4+ iNKT cells from FVB/N mice into BALB/c recipients, together with 5.0 × 106 TCD-BM cells and 1.0 × 106 Tcons both from FVB/N mice. At day +5, we were able to re-isolate GFP+ cells via flow cytometry from recipient livers and to a lesser extent from spleens. Twenty days later, we found an expanding population of donor GFP+ CD4+ iNKT in these two organs. At day +25, GFP+ CD4+ iNKT cells could be detected in recipient thymi as well (Figure 6B). However, we could not re-isolate GFP+ cells from lymph nodes, gut, or skin at any time point. In addition, we labeled 1.5 × 106 GFP+ CD4+ iNKT cells with CellTrace Violet prior to tail vein injection in order to illustrate cell divisions through decreasing fluorescence intensity measured by flow cytometry. Different cell generations could be identified on days +3 and +5, confirming in vivo proliferation of donor GFP+ CD4+ iNKT cells (Figure 6C).
Figure 6.
Third-party CD4+ iNKT cells are rejected early after allogeneic HCT. (A) GFP+CD4+ iNKT cells were enriched (magnetic-activated cell sorting) and sorted (fluorescence-activated cell sorting) from whole splenocytes of GFP-expressing FVB/N mice enabling distinct re-isolation from BALB/c recipient mice after adoptive transfer. Representative dot plots gated on live cells. (B) Absolute cell numbers of adoptively transferred FVB/N donor GFP+CD4+ iNKT cells re-isolated at different time points from various organs of BALB/c mice that received TCD-BM and Tcons from WT FVB/N donor mice. No GFP+CD4+ iNKT cells could be re-isolated from lymph nodes, gut, and skin (not shown). Error bars indicate SEM. Shown are cell numbers from 3 mice per time point from 1 of 2 independent experiments. (C) Fluorescence intensity deriving from 1.5 × 106 adoptively transferred FVB/N donor GFP+CD4+ iNKT cells labeled with CellTrace Violet and re-isolated at days +3 and +5. Histogram gated on GFP+ cells pooled from the spleen and liver of one BALB/c mouse that received TCD-BM and Tcons from one WT FVB/N donor mouse. Shown is 1 of 3 independent experiments. (D) Donor and third-party model of allogeneic HCT with BALB/c (H-2Kd) mice receiving TCD-BM and Tcons from WT FVB/N (H-2Kq) and C57BL/6 (H-2Kb) mice, respectively. Adoptively transferred CD4+ iNKT cells derived from GFP-expressing FVB/N (H-2Kq) mice and were re-isolated from recipient livers at days +5 and +10. Shown are representative dot plots gated on live cells from 1 of 2 independent experiments. The third row shows T-cell chimerism from the same sample. Comparable results were obtained for re-isolation of GFP+ cells from recipient spleens (not shown). LVR, liver; SPN, spleen; THY, thymus.
Third-party CD4+ iNKT cells are rejected early after allogeneic HCT
Although third-party CD4+ iNKT cells mediate protection from lethal GVHD through donor Tregs, we were interested in the survival of this potent immunoregulatory T-cell subset either of donor or third-party origin. Therefore, we assessed the number of CD4+ iNKT cells from GFP+ FVB/N mice (H-2Kq) in BALB/c (H-2Kd) recipient mice by flow cytometry at different time points posttransplant; 5.0 × 106 TCD-BM cells and 1.0 × 106 Tcons derived from WT FVB/N (H-2Kq) mice and WT C57BL/6 (H-2Kb) mice in a donor and third-party model, respectively. At day +5 following allogeneic HCT, both donor and third-party CD4+ iNKT cells could be identified as a rare yet distinct GFP+H-2Kq+ population in recipient livers and spleens. Meanwhile, recipient mice were mixed T-lymphocyte chimera, but converted to full donor chimerism on subsequent days. Donor GFP+H-2Kq+ CD4+ iNKT cells expanded in recipient livers and spleens, whereas third-party CD4+ iNKT cells were rejected by day +10 (Figure 6D).
Discussion
iNKT cells play a critical role within the cellular and humoral immune network bridging the gap between innate and adaptive immunity.32 The instant release of immunoregulatory cytokines upon stimulation is the functional hallmark of this rare T-cell subpopulation in both humans and mice. The importance of host iNKT cells for tolerance induction after TLI/ATG conditioning has been studied extensively in animal models, and could be applied successfully to the clinic resulting in a significantly reduced incidence of acute GVHD and enabling combined allogeneic HCT and kidney transplantation without lifelong pharmacologic immunosuppression.7-13 Moreover, recent clinical studies revealed that iNKT-cell numbers of the graft and peripheral blood posttransplant were both associated with protection against acute GVHD in humans.33,34
In our present study, we explored the impact of third-party CD4+ iNKT cells in a murine model of allogeneic HCT across major histocompatibility barriers following myeloablative conditioning. We found that low numbers of adoptively transferred CD4+ iNKT cells from third-party mice provided robust protection from lethal GVHD, while retaining Tcon-mediated GVT effects. Protection from alloimmunity was conferred through expanded donor Tregs as depletion of Tregs from the graft completely abrogated any survival benefit. We could show that these Tregs expand from the nTreg pool, rather than being induced from the naïve T-cell compartment. Importantly, we were able to demonstrate that these Tregs persist for at least 8 weeks after allogeneic HCT. Interestingly, adoptive transfer of CD4+ iNKT cells resulted in an expansion of donor CD11b+Gr-1int MDSCs and in vivo depletion of this cell population abrogated both Treg expansion and protection from GVHD lethality.
Treatment with third-party CD4+ iNKT cells resulted in a significantly decreased expansion of donor Tcons that showed a Th2-biased cytokine pattern. The paradigm of humoral immune homeostasis comprises a well-balanced but delicate system of distinct cytokine patterns. Immune polarization toward a Th2 phenotype is critical for GVHD prevention35-40 and minimal intensity conditioning with TLI/ATG is considered a tolerizing regimen due to the relative radioresistance of host iNKT cells upregulating anti-apoptotic genes like BCL-2.41,42 In particular, donor T cells from combined kidney and HCT recipients produced more IL-4 and showed a decreased expansion capacity when challenged with alloantigens in a mixed lymphocyte reaction.9 Analogously, adoptive transfer of both donor and third-party CD4+ iNKT cells induces Th2 polarization which was associated with GVHD amelioration after myeloablative conditioning in murine models of allogeneic HCT.14
Strikingly, adoptively transferred donor and third-party CD4+ iNKT cells both resulted in an expansion of donor MDSCs and Tregs, Th2-biased donor T cells, inhibition of Tcon proliferation, and protection from lethal GVHD. Given the fact that third-party CD4+ iNKT cells are rejected early after allogeneic HCT but still mediate sustained protection from GVHD through donor Tregs, CD4+ iNKT cells polarize the immune system toward a tolerogenic phenotype shortly after adoptive transfer. This is remarkable considering the low CD4+ iNKT-cell numbers that were co-injected resulting in a CD4+ iNKT:Tcon ratio of only 1:20. Our previous studies showed that adoptive transfer of 1.0 × 106 freshly isolated donor Tregs (Treg:Tcon = 1:1) is required to provide robust protection from GVHD lethality, although delayed Tcon administration allowed for the use of lower Treg numbers due to an advantageous in vivo proliferation.43 Consequently, the tolerogenic potency of donor and third-party CD4+ iNKT cells is amplified through the expansion of donor Treg. We recently demonstrated in a similar model of allogeneic HCT across major histocompatibility barriers that the adoptive transfer of 5.0 × 105 third-party Tregs, 2 days prior to the injection of 1.0 × 106 donor Tcons, resulted in an inferior survival benefit compared with the same number of donor Tregs.44 In our present study, this limitation could not be noticed with adoptively transferred third-party CD4+ iNKT cells. We believe that the early expansion of donor Tregs through third-party CD4+ iNKT cells overcomes this obstacle caused by MHC disparities and rejection of third-party Tregs.
Donor CD4+ iNKT cells proliferate in vivo upon adoptive transfer, although the total expansion capacity was too low to be assessed by BLI of CD4+ iNKT cells from luc+ mice (not shown). Interestingly, CD4+ iNKT cells home preferentially to the recipient liver unlike adoptively transferred Tregs and CD49b+CD4+ NKT cells, which migrate to secondary lymphoid organs prior to infiltrating GVHD target tissues comparable to the trafficking pattern of Tcons.45 In contrast to naïve CD62LhighCD44low T cells, CD4+ iNKT cells show a memory-like CD62LlowCD44high phenotype.46,47 CD62L interacts with glycosylation-dependent cell adhesion molecule 1 of high endothelial venules and mucosal vascular addressin cell adhesion molecule 1 of endothelial cells of gut-associated lymphoid tissue, enabling entry of naïve T cells into lymph nodes.48-51 Moreover, CD62L expression on adoptively transferred Tregs is required for Treg function in vivo.52 Surprisingly, CD4+ iNKT cells could not be re-isolated from recipient lymph nodes, gut, or skin, but flow cytometric and BLI studies revealed that donor Tregs infiltrate these tissues and expand. Therefore, our present study implies a central role of the recipient liver and thymus for alloimmunity, immune homeostasis, and immunologic tolerance. In fact, roughly half of the lymphocytes residing in murine livers express the semi-invariant TCR, TCRα Vα14-Jα18, under steady state conditions. Besides, the liver hosts different NK cell subsets, Kupffer cells, and stellate macrophages providing an immunologic environment.53 Hepatic endothelial cells upregulate chemokines and adhesion molecules upon tissue injury allowing recruitment of lymphocyte subsets to the liver.54 Further research beyond the scope of this study is required to determine the immunologic relevance of the liver for GVHD immunopathology, and to characterize the molecules and mechanisms responsible for the hepatotropism of adoptively transferred CD4+ iNKT cells.
Our findings from murine studies have important clinical implications as there are a variety of protocols describing the in vitro expansion of human iNKT cells from whole peripheral blood.18,55-57 Noteworthy, a much lower number of iNKT cells is required in order to provide protection from GVHD as compared with adoptively transferred Tregs. Hence, peripheral blood apheresis from healthy donors may not be required. In addition, with third-party CD4+ iNKT cells being fully functional, an almost unlimited source of cells could be available. However, dose-finding studies in human beings are indispensable to determine the most appropriate cell numbers for clinical translation. Importantly, we could show that Tcon-mediated GVT effects are preserved after adoptive transfer of iNKT cells.
Our studies outline the potency of third-party iNKT cells within the cellular and humoral immunoregulatory network. Because third-party iNKT cells prevent alloimmunity in our murine GVHD model, these results may be of particular importance for other clinical fields such as solid organ transplantation and autoimmunity.
Acknowledgments
The authors thank Dr Kent P. Jensen from the Strober Laboratory for assistance with the Luminex multiplex assay. PBS-57–loaded mCD1d tetramer was provided by the National Institutes of Health Tetramer Facility.
This study was supported in part by Program Project grants from the National Institutes of Health, National Cancer Institute (CA49605) and the National Heart, Lung, and Blood Institute (HL075462), the Dr Mildred Scheel Foundation for Cancer Research (D.S. and C.B.), the Stanford Institute for Immunity, Transplantation and Infection Young Investigator Award (D.S.), and the Fondazione Italiana per la Ricerca sul Cancro (A.P.).
Footnotes
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: D.S. designed and performed research, analyzed data, and wrote the manuscript; J.B., A.P., and C.B. performed research; R.H.L. evaluated histopathology and took photomicrographs; E.H.M. and R.S.N. provided overall guidance and all authors edited the manuscript for content.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Robert S. Negrin, Center for Clinical Sciences Research Building, Room 2205, 269 Campus Dr, Stanford, CA 94305; e-mail: negrs@stanford.edu.
References
- 1.Ferrara JL, Deeg HJ. Graft-versus-host disease. N Engl J Med. 1991;324(10):667–674. doi: 10.1056/NEJM199103073241005. [DOI] [PubMed] [Google Scholar]
- 2.Shlomchik WD, Couzens MS, Tang CB, et al. Prevention of graft versus host disease by inactivation of host antigen-presenting cells. Science. 1999;285(5426):412–415. doi: 10.1126/science.285.5426.412. [DOI] [PubMed] [Google Scholar]
- 3.Blazar BR, Murphy WJ, Abedi M. Advances in graft-versus-host disease biology and therapy. Nat Rev Immunol. 2012;12(6):443–458. doi: 10.1038/nri3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Magenau J, Reddy P. Next generation treatment of acute graft-versus-host disease. Leukemia. 2014;28(12):2283–2291. doi: 10.1038/leu.2014.195. [DOI] [PubMed] [Google Scholar]
- 5.Lantz O, Bendelac A. An invariant T cell receptor alpha chain is used by a unique subset of major histocompatibility complex class I-specific CD4+ and CD4-8- T cells in mice and humans. J Exp Med. 1994;180(3):1097–1106. doi: 10.1084/jem.180.3.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schneidawind D, Pierini A, Negrin RS. Regulatory T cells and natural killer T cells for modulation of GVHD following allogeneic hematopoietic cell transplantation. Blood. 2013;122(18):3116–3121. doi: 10.1182/blood-2013-08-453126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strober S, Modry DL, Hoppe RT, et al. Induction of specific unresponsiveness to heart allografts in mongrel dogs treated with total lymphoid irradiation and antithymocyte globulin. J Immunol. 1984;132(2):1013–1018. [PubMed] [Google Scholar]
- 8.Lan F, Zeng D, Higuchi M, Higgins JP, Strober S. Host conditioning with total lymphoid irradiation and antithymocyte globulin prevents graft-versus-host disease: the role of CD1-reactive natural killer T cells. Biol Blood Marrow Transplant. 2003;9(6):355–363. doi: 10.1016/s1083-8791(03)00108-3. [DOI] [PubMed] [Google Scholar]
- 9.Lowsky R, Takahashi T, Liu YP, et al. Protective conditioning for acute graft-versus-host disease. N Engl J Med. 2005;353(13):1321–1331. doi: 10.1056/NEJMoa050642. [DOI] [PubMed] [Google Scholar]
- 10.Scandling JD, Busque S, Dejbakhsh-Jones S, et al. Tolerance and chimerism after renal and hematopoietic-cell transplantation. N Engl J Med. 2008;358(4):362–368. doi: 10.1056/NEJMoa074191. [DOI] [PubMed] [Google Scholar]
- 11.Kohrt HE, Turnbull BB, Heydari K, et al. TLI and ATG conditioning with low risk of graft-versus-host disease retains antitumor reactions after allogeneic hematopoietic cell transplantation from related and unrelated donors. Blood. 2009;114(5):1099–1109. doi: 10.1182/blood-2009-03-211441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scandling JD, Busque S, Shizuru JA, Engleman EG, Strober S. Induced immune tolerance for kidney transplantation. N Engl J Med. 2011;365(14):1359–1360. doi: 10.1056/NEJMc1107841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scandling JD, Busque S, Dejbakhsh-Jones S, et al. Tolerance and withdrawal of immunosuppressive drugs in patients given kidney and hematopoietic cell transplants. Am J Transplant. 2012;12(5):1133–1145. doi: 10.1111/j.1600-6143.2012.03992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schneidawind D, Pierini A, Alvarez M, et al. CD4+ invariant natural killer T cells protect from murine GVHD lethality through expansion of donor CD4+CD25+FoxP3+ regulatory T cells. Blood. 2014;124(22):3320–3328. doi: 10.1182/blood-2014-05-576017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murphy GF, Bronstein BR, Knowles RW, Bhan AK. Ultrastructural documentation of M241 glycoprotein on dendritic and endothelial cells in normal human skin. Lab Invest. 1985;52(3):264–269. [PubMed] [Google Scholar]
- 16.Balk SP, Bleicher PA, Terhorst C. Isolation and characterization of a cDNA and gene coding for a fourth CD1 molecule. Proc Natl Acad Sci USA. 1989;86(1):252–256. doi: 10.1073/pnas.86.1.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blumberg RS, Terhorst C, Bleicher P, et al. Expression of a nonpolymorphic MHC class I-like molecule, CD1D, by human intestinal epithelial cells. J Immunol. 1991;147(8):2518–2524. [PubMed] [Google Scholar]
- 18.Brossay L, Chioda M, Burdin N, et al. CD1d-mediated recognition of an alpha-galactosylceramide by natural killer T cells is highly conserved through mammalian evolution. J Exp Med. 1998;188(8):1521–1528. doi: 10.1084/jem.188.8.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Exley M, Garcia J, Wilson SB, et al. CD1d structure and regulation on human thymocytes, peripheral blood T cells, B cells and monocytes. Immunology. 2000;100(1):37–47. doi: 10.1046/j.1365-2567.2000.00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borg NA, Wun KS, Kjer-Nielsen L, et al. CD1d-lipid-antigen recognition by the semi-invariant NKT T-cell receptor. Nature. 2007;448(7149):44–49. doi: 10.1038/nature05907. [DOI] [PubMed] [Google Scholar]
- 21.Zeiser R, Leveson-Gower DB, Zambricki EA, et al. Differential impact of mammalian target of rapamycin inhibition on CD4+CD25+Foxp3+ regulatory T cells compared with conventional CD4+ T cells. Blood. 2008;111(1):453–462. doi: 10.1182/blood-2007-06-094482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cooke KR, Kobzik L, Martin TR, et al. An experimental model of idiopathic pneumonia syndrome after bone marrow transplantation: I. The roles of minor H antigens and endotoxin. Blood. 1996;88(8):3230–3239. [PubMed] [Google Scholar]
- 23.Edinger M, Cao YA, Verneris MR, Bachmann MH, Contag CH, Negrin RS. Revealing lymphoma growth and the efficacy of immune cell therapies using in vivo bioluminescence imaging. Blood. 2003;101(2):640–648. doi: 10.1182/blood-2002-06-1751. [DOI] [PubMed] [Google Scholar]
- 24.Beilhack A, Schulz S, Baker J, et al. Prevention of acute graft-versus-host disease by blocking T-cell entry to secondary lymphoid organs. Blood. 2008;111(5):2919–2928. doi: 10.1182/blood-2007-09-112789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beilhack A, Schulz S, Baker J, et al. In vivo analyses of early events in acute graft-versus-host disease reveal sequential infiltration of T-cell subsets. Blood. 2005;106(3):1113–1122. doi: 10.1182/blood-2005-02-0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hill JA, Feuerer M, Tash K, et al. Foxp3 transcription-factor-dependent and -independent regulation of the regulatory T cell transcriptional signature. Immunity. 2007;27(5):786–800. doi: 10.1016/j.immuni.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 27.Thornton AM, Korty PE, Tran DQ, et al. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol. 2010;184(7):3433–3441. doi: 10.4049/jimmunol.0904028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Highfill SL, Rodriguez PC, Zhou Q, et al. Bone marrow myeloid-derived suppressor cells (MDSCs) inhibit graft-versus-host disease (GVHD) via an arginase-1-dependent mechanism that is up-regulated by interleukin-13. Blood. 2010;116(25):5738–5747. doi: 10.1182/blood-2010-06-287839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van der Merwe M, Abdelsamed HA, Seth A, Ong T, Vogel P, Pillai AB. Recipient myeloid-derived immunomodulatory cells induce PD-1 ligand-dependent donor CD4+Foxp3+ regulatory T cell proliferation and donor-recipient immune tolerance after murine nonmyeloablative bone marrow transplantation. J Immunol. 2013;191(11):5764–5776. doi: 10.4049/jimmunol.1302191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hongo D, Tang X, Baker J, Engleman EG, Strober S. Requirement for interactions of natural killer T cells and myeloid-derived suppressor cells for transplantation tolerance. Am J Transplant. 2014;14(11):2467–2477. doi: 10.1111/ajt.12914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilson MT, Johansson C, Olivares-Villagómez D, et al. The response of natural killer T cells to glycolipid antigens is characterized by surface receptor down-modulation and expansion. Proc Natl Acad Sci USA. 2003;100(19):10913–10918. doi: 10.1073/pnas.1833166100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brennan PJ, Brigl M, Brenner MB. Invariant natural killer T cells: an innate activation scheme linked to diverse effector functions. Nat Rev Immunol. 2013;13(2):101–117. doi: 10.1038/nri3369. [DOI] [PubMed] [Google Scholar]
- 33.Chaidos A, Patterson S, Szydlo R, et al. Graft invariant natural killer T-cell dose predicts risk of acute graft-versus-host disease in allogeneic hematopoietic stem cell transplantation. Blood. 2012;119(21):5030–5036. doi: 10.1182/blood-2011-11-389304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rubio MT, Moreira-Teixeira L, Bachy E, et al. Early posttransplantation donor-derived invariant natural killer T-cell recovery predicts the occurrence of acute graft-versus-host disease and overall survival. Blood. 2012;120(10):2144–2154. doi: 10.1182/blood-2012-01-404673. [DOI] [PubMed] [Google Scholar]
- 35.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136(7):2348–2357. [PubMed] [Google Scholar]
- 36.Cherwinski HM, Schumacher JH, Brown KD, Mosmann TR. Two types of mouse helper T cell clone. III. Further differences in lymphokine synthesis between Th1 and Th2 clones revealed by RNA hybridization, functionally monospecific bioassays, and monoclonal antibodies. J Exp Med. 1987;166(5):1229–1244. doi: 10.1084/jem.166.5.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fowler DH, Kurasawa K, Smith R, Eckhaus MA, Gress RE. Donor CD4-enriched cells of Th2 cytokine phenotype regulate graft-versus-host disease without impairing allogeneic engraftment in sublethally irradiated mice. Blood. 1994;84(10):3540–3549. [PubMed] [Google Scholar]
- 38.Krenger W, Snyder KM, Byon JC, Falzarano G, Ferrara JL. Polarized type 2 alloreactive CD4+ and CD8+ donor T cells fail to induce experimental acute graft-versus-host disease. J Immunol. 1995;155(2):585–593. [PubMed] [Google Scholar]
- 39.Tawara I, Maeda Y, Sun Y, et al. Combined Th2 cytokine deficiency in donor T cells aggravates experimental acute graft-vs-host disease. Exp Hematol. 2008;36(8):988–996. doi: 10.1016/j.exphem.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yi T, Chen Y, Wang L, et al. Reciprocal differentiation and tissue-specific pathogenesis of Th1, Th2, and Th17 cells in graft-versus-host disease. Blood. 2009;114(14):3101–3112. doi: 10.1182/blood-2009-05-219402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yao Z, Liu Y, Jones J, Strober S. Differences in Bcl-2 expression by T-cell subsets alter their balance after in vivo irradiation to favor CD4+Bcl-2hi NKT cells. Eur J Immunol. 2009;39(3):763–775. doi: 10.1002/eji.200838657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seino K, Harada M, Taniguchi M. NKT cells are relatively resistant to apoptosis. Trends Immunol. 2004;25(5):219–221. doi: 10.1016/j.it.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 43.Nguyen VH, Zeiser R, Dasilva DL, et al. In vivo dynamics of regulatory T-cell trafficking and survival predict effective strategies to control graft-versus-host disease following allogeneic transplantation. Blood. 2007;109(6):2649–2656. doi: 10.1182/blood-2006-08-044529. [DOI] [PubMed] [Google Scholar]
- 44.Pierini A, Colonna L, Alvarez M, et al. Donor requirements for CD4+ CD25+ FoxP3+ regulatory T cells capable of suppressing CD4+ and CD8+ conventional T cell proliferation and graft versus host disease. Blood. 2013;122(21):4484. [Google Scholar]
- 45.Leveson-Gower DB, Olson JA, Sega EI. et al. Low doses of natural killer T cells provide protection from acute graft-versus-host disease via an IL-4-dependent mechanism. Blood. 2011;117(11):3220–3229. doi: 10.1182/blood-2010-08-303008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Godfrey DI, Berzins SP. Control points in NKT-cell development. Nat Rev Immunol. 2007;7(7):505–518. doi: 10.1038/nri2116. [DOI] [PubMed] [Google Scholar]
- 47.Raberger J, Schebesta A, Sakaguchi S, et al. The transcriptional regulator PLZF induces the development of CD44 high memory phenotype T cells. Proc Natl Acad Sci USA. 2008;105(46):17919–17924. doi: 10.1073/pnas.0805733105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gallatin WM, Weissman IL, Butcher EC. A cell-surface molecule involved in organ-specific homing of lymphocytes. Nature. 1983;304(5921):30–34. doi: 10.1038/304030a0. [DOI] [PubMed] [Google Scholar]
- 49.Siegelman MH, van de Rijn M, Weissman IL. Mouse lymph node homing receptor cDNA clone encodes a glycoprotein revealing tandem interaction domains. Science. 1989;243(4895):1165–1172. doi: 10.1126/science.2646713. [DOI] [PubMed] [Google Scholar]
- 50.Lasky LA, Singer MS, Dowbenko D, et al. An endothelial ligand for L-selectin is a novel mucin-like molecule. Cell. 1992;69(6):927–938. doi: 10.1016/0092-8674(92)90612-g. [DOI] [PubMed] [Google Scholar]
- 51.Briskin MJ, McEvoy LM, Butcher EC. MAdCAM-1 has homology to immunoglobulin and mucin-like adhesion receptors and to IgA1. Nature. 1993;363(6428):461–464. doi: 10.1038/363461a0. [DOI] [PubMed] [Google Scholar]
- 52.Ermann J, Hoffmann P, Edinger M, et al. Only the CD62L+ subpopulation of CD4+CD25+ regulatory T cells protects from lethal acute GVHD. Blood. 2005;105(5):2220–2226. doi: 10.1182/blood-2004-05-2044. [DOI] [PubMed] [Google Scholar]
- 53.Racanelli V, Rehermann B. The liver as an immunological organ. Hepatology. 2006;43(2 suppl 1):S54–S62. doi: 10.1002/hep.21060. [DOI] [PubMed] [Google Scholar]
- 54.Lalor PF, Shields P, Grant A, Adams DH. Recruitment of lymphocytes to the human liver. Immunol Cell Biol. 2002;80(1):52–64. doi: 10.1046/j.1440-1711.2002.01062.x. [DOI] [PubMed] [Google Scholar]
- 55.van der Vliet HJ, Nishi N, Koezuka Y, et al. Potent expansion of human natural killer T cells using alpha-galactosylceramide (KRN7000)-loaded monocyte-derived dendritic cells, cultured in the presence of IL-7 and IL-15. J Immunol Methods. 2001;247(1-2):61–72. doi: 10.1016/s0022-1759(00)00272-6. [DOI] [PubMed] [Google Scholar]
- 56.Watarai H, Nakagawa R, Omori-Miyake M, Dashtsoodol N, Taniguchi M. Methods for detection, isolation and culture of mouse and human invariant NKT cells. Nat Protoc. 2008;3(1):70–78. doi: 10.1038/nprot.2007.515. [DOI] [PubMed] [Google Scholar]
- 57.Exley MA, Wilson B, Balk SP. Curr Protoc Immunol. 2010. Isolation and functional use of human NKT cells. Chapter 14:Unit 14.11. [DOI] [PubMed] [Google Scholar]