Abstract
A single intrathecal dose of adenosine 2A receptor (A2AR) agonist was previously reported to produce a multi-week reversal of allodynia in a chronic constriction injury (CCI) model of neuropathic pain. We aimed to determine if this long-term reversal was induced by A2AR agonism versus more generalized across adenosine receptor subtypes, and begin to explore the intracellular signaling cascades involved. In addition, we sought to identify whether the enduring effect could be extended to other models of neuropathic pain. We tested an A1R and A2BR agonist in CCI and found the same long duration effect with A2BR but not A1R agonism. An A2AR agonist (ATL313) produced a significant long-duration reversal of mechanical allodynia induced by long established CCI (administered 6 wk after surgery), spinal nerve ligation and sciatic inflammatory neuropathy. To determine if ATL313 had a direct effect on glia, ATL313 was coadministered with lipopolysaccharide to neonatal microglia and astrocytes in vitro. ATL313 significantly attenuated TNFα production in both microglia and astrocytes but had no effect on LPS induced IL-10. Protein kinase C significantly reversed the ATL313 effects on TNF in vitro in microglia and astrocytes, while a protein kinase A inhibitor only effected microglia. Both intrathecal PKA and PKC inhibitors significantly reversed the effect of the A2AR agonist on neuropathic allodynia. Therefore, A2AR agonists administered IT remain an exciting novel target for the treatment of neuropathic pain.
Keywords: mechanical allodynia, protein kinase, interleukin-10, ATL313, intrathecal, rats, microglia, astrocytes
Introduction
Neuropathic pain affects approximately 4% of the global population (Global Industry Analysts, 2011). Neuropathic pain remains intractable to available therapeutics as most available treatments target neurons (Global Industry Analysts, 2011). Glially-driven spinal neuroinflammation has received increasing attention as importantly contributing to such pain (Scholz and Woolf, 2007). Activated microglia and astrocytes release neuroexcitatory mediators such as pro-inflammatory cytokines and chemokines (Watkins et al., 2007). This contribution of glia in developing and maintaining neuropathic pain has led to the search for clinically relevant approaches to suppress glially-driven pain amplification.
There are four adenosine receptor subtypes, A1 and A3 receptors decrease cAMP, while A2A and A2B receptors increase cAMP (Fredholm et al., 2007). Adenosine receptors are found on all cell types within the CNS including neurons and glia (Dare et al., 2007). A2AR agonists recently attracted attention as potential glial inhibitors, based on their anti-inflammatory effects in peripheral immune cells. Remarkably, when A2AR agonists were administered intrathecally (IT), a fascinating phenomenon was revealed; namely, a single intrathecal (IT) injection of A2AR-selective agonists reversed neuropathic pain (mechanical allodynia, thermal hyperalgesia) for more than 4 weeks in a classic animal model of neuropathic pain, the sciatic chronic constriction injury (CCI) (Loram et al., 2009). While many drugs briefly suppress neuropathic pain, none other induces such enduring pain inhibition. Intriguingly, we demonstrated that A2AR agonism accounts for reversal of neuropathic pain initially, but continued A2AR activation cannot account for the enduring reversal (Loram et al., 2009). How a brief exposure to an A2AR agonist creates a strikingly persistent inhibition of neuropathic pain remains unknown.
Therefore, the aim of this study was to further explore the underlying mechanism for the remarkable long-acting effect of the A2AR agonist. We investigated whether the effects were unique to CCI, or if the long duration effect could be generalized to multiple neuropathic pain models. We further explored whether the effects were generalizable to other adenosine receptors. Our hypothesis, based on our previous findings, was that long-term reversal of allodynia was mediated by an attenuation of glial activation within the spinal cord, resulting in reduced pro-inflammatory cytokine production (Loram et al., 2009). We have demonstrated previously that IL-10 mRNA was significantly elevated following intrathecal A2AR agonist in CSF cells but not within the spinal cord tissue. It is possible that evaluating the whole spinal tissue diluted the potent change in IL-10 produced by glial cells alone. In order to further explore whether intrathecal A2AR agonism may affect glial cells within the spinal cord to produce IL-10, we tested an A2AR agonist on pure microglial cells, astrocytes and mixed astrocyte and microglial cells in vitro. Lastly, if the potential mechanism of the long duration of effect is via an increase in cAMP, then the intracellular kinases would be activated. Protein kinase (PK) A is the canonical kinase stimulated by cAMP, which is known to occur in peripheral immune cells following A2AR activation. In addition PKC is also activated by A2AR activation in peripheral immune cells (Cho et al., 2002; Goethe et al., 2007; Hucho et al., 2005; Tortora and Ciardiello, 2002a). Therefore, we aimed to explore whether PKA and/or PKC inhibitors administered intrathecally could reverse the effect of the A2AR agonist on neuropathic allodynia in vivo. And furthermore, whether PKA and/or PKC inhibitors would reverse the A2AR effect in glial cultures.
2. Materials and Methods
2.1 Subjects
Pathogen-free male Sprague Dawley rats (325-350 g, Harlan Laboratories) were housed two per cage with standard rat chow and water ad libitum. Housing was in a temperature-controlled environment (23 ± 2°C) with a 12 h light/dark cycle (lights on at 7:00 A.M.). All procedures occurred in the light phase. All rats were allowed 1 week of acclimation to the colony rooms before experimentation. The Institutional Animal Care and Use Committee of the University of Colorado at Boulder approved all procedures.
2.2 Drugs
The A2AR agonist 4-(3-(6-amino-9-(5-cyclopropylcarbamoyl-3,4-dihydroxytetrahydrofuran-2-yl)-9H-purin-2-yl)prop-2-ynyl)piperidine-1- carboxylic acid methyl ester (ATL313) was a gift from Dogwood Pharmaceuticals. The A2AR agonist 2-p-(2-carboxyethyl)phenethylamino-5′-N- ethylcarboxamido adenosine HCl (CGS21680) and CCPA were purchased from Tocris Bioscience. H-89 (PKA inhibitor) was purchased from Assay design (Ann Arbor, MI, USA). Lipopolysaccharide and chelerythrine (PKC inhibitor) were purchased from Sigma (St Louis, MO, USA). BAY606583 (A2BR agonist) was gifted by Bayer Healthcare (Wuppertal, Germany). All of the compounds except LPS were dissolved in DMSO to create 10 mM stock concentrations and stored at -20°C. LPS was diluted in sterile saline and stored at 1 mg/ml aliquots at -20°C. Fresh aliquots were diluted to the appropriate concentration in sterile endotoxin-free isotonic saline (Abbott Laboratories).
2.3 Von Frey test for mechanical allodynia
Rats were habituated to the testing apparatus for 4 consecutive days before testing. The von Frey test was performed on the plantar surface of each hindpaw within the region of sciatic nerve innervation, as described previously (Milligan et al., 2000). A logarithmic series of 10 calibrated Semmes-Weinstein monofilaments (Stoelting) were sequentially applied (from low- to high-intensity threshold) to the left and right hind- paws in random order, each for 8 s at constant pressure to determine the stimulus intensity threshold stiffness required to elicit a paw withdrawal response. Log stiffness of the hairs is determined by log10(milligrams × 10). The range of monofilaments used in these experiments (0.407–15.136 g) produces a logarithmically graded slope when interpolating a 50% response threshold of stimulus intensity [expressed as log10(milligrams × 10)] (Chaplan et al., 1994). The stimulus intensity threshold to elicit a paw withdrawal response was used to calculate the 50% paw withdrawal threshold (absolute threshold) using the maximum-likelihood fit method to fit a Gaussian integral psychometric function (Harvey, 1986). This method normalizes the withdrawal threshold for parametric analyses (Harvey, 1986). The behavioral testing was performed blind with respect to the drug administration.
2.4 Surgery
2.4.1 Chronic Constriction Injury
Chronic constriction injury (CCI) (Bennett and Xie, 1988) of the left sciatic nerve was aseptically performed under isoflurane anesthesia. Four ligatures of 4-0 chromic gut were tied loosely around the left sciatic nerve at the level of the midthigh, as described previously (Milligan et al., 2004b).
2.4.2 Spinal Nerve Ligation
Spinal nerve ligation of L5 and L6 left spinal nerve roots. was conducted as described by Kim and Chung (Kim and Chung, 1992). 125-150 g male Sprague-Dawley rats were used for this experiment. Under isoflurane anesthesia, the left L5 and L6 nerve roots were isolated and tightly ligated with 6-0 silk suture. The rats were allowed 2 wk recovery before drug delivery.
2.4.3 Sciatic Inflammatory Neuropathy
Sciatic inflammatory neuropathy was performed as described previously (Milligan et al., 2004a). Chronic perisciatic catheters were constructed and implanted at mid-thigh level of the left hindleg as previously described (Chacur et al., 2001). This method allows multi-day recovery of the rats from anesthesia and surgery before the experiments.
2.4.4 Intrathecal drug delivery
Rats were lightly anesthetized with isoflurane. The lumbar region was shaved and cleaned. An 18 Gauge guide needle, with the hub removed, was inserted into the L5/6 intervertebral space. A PE-10 catheter was inserted into the guide needle, premarked such that the proximal end of the PE-10 tubing rested over the L4–L6 lumbar spinal cord. All drugs were administered over 20 s (1 μl of drug followed by 2 μl of sterile saline flush) with a 30 s delay before removing the catheter and guide needle. Each rat was anesthetized for a maximum of 5 min, and none incurred observable neurological damage from the procedure.
2.5 Glial cultures
Neonatal microglia and astrocytes were cultured from P0/1 day old male Sprague-Dawley pups as described previously (Hutchinson et al., 2010). While differences in responses to pro-inflammatory stimuli have been identified between neonatal and adult cortical glia cultures (Loram et al., 2012; Schwarz et al., 2011), these experiments provide information regarding the effect of adenosine receptor agonist and protein kinase inhibitors from CNS derived immunocompetent cells subsequent to LPS stimulation. At confluence, (7-10 days), the microglia were separated from the astrocytes by shaking the cells for 90 min on an orbital shaker. The microglia were rinsed and then plated at 40,000-50,000 cells per well in a 96-well plate in 100 μl MEM media with 100 U/ml penicillin, 100 μg/ml streptomycin and 10% FBS. Once the microglia were removed, DMEM (10 % FBS and 5 mM L-LME) was added to the flasks containing the astrocytes. The cells were incubated for 2 h to remove any residual microglia remaining in the flask. The cells were washed with DPBS twice and removed with trypsin (0.05%) for 5 min. The cells were centrifuged at 200 × g for 10 min at RT. The cells were resuspended in DMEM/F12 (100 U/ml penicillin, 100 μg/ml streptomycin, 10% FBS). The cells were counted with trypan exclusion and plated in 24-well tissue culture plates in 1 ml media at 100,000 cells/well. All cells were incubated for 48 h at 37°C and 5% CO2 until the experiment was conducted.
2.6 Enzyme linked immunosorbant assay (ELISA)
IL-10 protein in rat CSF was analyzed using a commercially available ELISA kit specific for rat IL-10 (R&D Systems, Minneapolis, MN, USA). TNFα and IL-10 protein were analyzed in the supernatant of the glial cultures using a commercially available ELISA kit specific for rat TNFα and rat IL-10 (R&D Systems, Minneapolis, MN, USA). The sensitivity of the TNFα assay is 5 pg/ml and for IL-10 is 10 pg/ml.
2.7 Statistical analysis
Behavioral measures were normalized as described above and analyzed using repeated measures 2-way ANOVA with time and treatment as main effects. ELISA data from the CSF were analysed using an unpaired t-test. Bonferroni post-hoc tests were used where appropriate and P < 0.05 was considered statistically significant.
2.8 Experimental procedures
2.8.1 Experiment 1: Effect of A1R and A2BR agonist on peripheral neuropathy-induced mechanical allodynia
Baseline behavioral measures were recorded after 4 days of 40 min/day habituation to the testing environment. CCI or sham surgery was then conducted and behavioral responses to mechanical stimuli or thermal stimuli were tested, in separate groups of rats, at 4 and 10 days after surgery. At 10-14 days after surgery, an acute IT administration of CCPA (A1R agonist) at 1 or 10 pmol, or BAY606583 (A2BR agonist) at 1 or 10 pmol or equivolume vehicle was given (n = 4-6 rats per group) in groups tested for mechanical allodynia. The behavioral responses were measured 1, 3, 24, 72 h and 1 wk after CCPA administration and 1, 3 d and weekly for 5 wk after BAY606583 administration.
2.8.2 Experiment 2: Effect of A2AR agonist on spinal nerve ligation, sciatic inflammatory neuropathy and established chronic constriction injury-induced mechanical allodynia
Baseline behavioral measures were recorded after 4 days of 40 min/day habituation to the testing environment.
CCI
CCI or sham surgery was then conducted and behavioral responses to mechanical stimuli were tested, 1, 2, 4 and 6 wk after surgery. The rats were then injected IT with ATL313 (0, 1 or 10 pmol in 1 μl) under brief isoflurane anesthesia. Behavioral testing was done 3 d and weekly for 6 wk after IT administration (n = 6-7 rats per group). We have demonstrated previously that ATL313 has no effect on sham-operated rats so only sham+vehicle were included.
SNL
SNL surgery was then conducted and behavioral responses to mechanical stimuli were tested 1 and 2 wk after surgery. The rats were then injected IT with ATL313 (0, 1 or 10 pmol in 1 μl) under brief isoflurane anesthesia. Behavioral testing was done 3 d and weekly for 6 wk after IT administration (n = 6-7 rats per group). Sham-operated rats received vehicle only.
SIN
Gel foam wraps were surgically implanted as described above. 4-5 days after surgery the rats were tested for behavioral responses. Injections of zymosan (160 μg) over the left sciatic nerve were performed in awake, unrestrained rats as previously described (Chacur et al., 2001; Milligan et al., 2003). Zymosan (160 μg in 50 μl incomplete Freund's adjuvant) was injected 4-5 days after surgery and administered every alternate day, four times. ATL313 (10 pmol in 1 μl) or vehicle (IT) was administered 24 h after the first zymosan injection, and immediately after behavioral testing, under isoflurane anesthesia.
2.8.3 Experiment 3: Effect of A2AR agonist on lipopolysaccharide (LPS) induced TNFα and IL-10 production in microglia and astrocyte cultures
Microglia and astrocytes were separately incubated with LPS (0, 100 ng/ml) and ATL313 (0, 0.01 μM, 0.1 μM, 1 μM) for 24, 48 or 72 h. The cells were centrifuged at 1,000 × g for 10 min at 4°C. The supernatant was processed for TNFα and IL-10 protein. In a separate experiment, microglia and astrocytes were either separately cultured or cocultured as a mixed glial population in 24 well plate or plated in transwell 24 well plates with the microglia plated on the insert and the astrocytes in the well. The cultures were incubated with 0 or 100 ng/ml LPS and 0 or 1 μM ATL313 for 24 h. The supernatant was collected and processed for TNFα and IL-10 protein. For the transwell incubation, the supernatant was collected from the media within the well rather than above the insert.
2.8.4 Experiment 4: Effect of A2AR agonist on IL-10 release into the CSF of neuropathic rats
Rats underwent CCI or SNL surgery as described above. For CCI rats 10-14 d after surgery they received ATL313 (10 pmol in 1 μl) or equivolume vehicle IT. For the SNL rats, 2 wk after surgery they received ATL313 (10 pmol in 1 μl) or equivolume vehicle IT. 1 wk after IT administration, lumbar CSF was collected using a similar protocol to that described for intrathecal injections. Under isoflurane anesthesia, an 18-g needle was inserted between L5 and L6 vertebrae for use as a guide cannula. PE-10 tubing was inserted into this guide and threaded rostrally to the lumbosacral enlargement as described for IT injections. A 3 ml syringe with attached 30-G needle was connected to the free end of the PE-10 tubing and CSF gently withdrawn into the catheter and syringe. Approximately 100 μl of CSF was obtained from each rat and placed into an eppendorf tube and frozen in liquid nitrogen for IL-10 protein analysis.
2.8.5 Experiment 5: Effect of inhibiting PKA and PKC in glial cultures
Using contained single cell cultures allows for the initial exploration of whether protein kinase A or C are involved in the reduction in TNFα following A2AR administration. H-89 is a PKA inhibitor, as it is a competitive inhibitor to the ATP binding site on the catalytic subunit of the PKA molecule. Chelerythrine, a PKC inhibitor, is a competitive inhibitor on the substrate but a non-competitive inhibitor with respect to ATP (Herbert et al., 1990). To explore their effects on primary glia, microglia cultures and astrocyte cultures were each incubated with LPS (0, 100 ng/ml) and ATL313 (0, 1 μM) for 24 h as described in experiment 3. In addition, PKA inhibitor (H-89, 0, 0.1 and 1 μM) or PKC inhibitor (chelerythrine, 0, 0.1 and 1 μM) were added to the cultures at the same time as LPS and ATL313. The cells were centrifuged at 1,000 × g for 10 min at 4°C. The supernatants were analyzed for TNFα and IL-10 protein.
2.8.6 Effect of spinal protein kinase A and protein kinase C activation in CCI-induced allodynia reversed by A2AR agonist
Baseline behavioral measures were recorded after 4 d of 40 min/day habituation to the testing environment. CCI or sham surgery was then conducted and behavioral responses to mechanical stimuli or thermal stimuli we tested, in separate groups of rats, at 4 and 10 days after surgery. At 10-14 days after surgery, an acute IT administration of CGS21680 (A2AR agonist) at 10 pmol. CGS21680 is a commercially available A2AR agonist that is the conventional A2AR agonist used and has been tested previously against CCI and shown equal efficacy at 10 pmol to that of ATL313 at 1 pmol (Loram et al., 2009). At the time this experiment was conducted, ATL313 was not available. 1 wk after the CGS21680, a PKA inhibitor (H-89 1 fmol in 1 μl) or PKC inhibitor (chelerythrine, 60 fmol in 1μl) or equivolume vehicle was administered IT. The behavioral responses were measured before CGS21680, before PKA inhibitor and 1, 2, 4, 6, 24, 48 h, 4 d ad 7 d after PKA/vehicle administration. For the PKC inhibitor, behavioral responses were measured before CGS21680, before PKA inhibitor and 20 and 40 min, 1, 2, 3, 4, 6 and 24 h after PKC/vehicle administration. Both PKA and PKC inhibitors were tested in sham-operated rats with vehicle to ensure the drug alone induced no allodynia.
3. Results
3.1 Long duration of reversal of neuropathic allodynia extends to A2BR but not A1R agonism
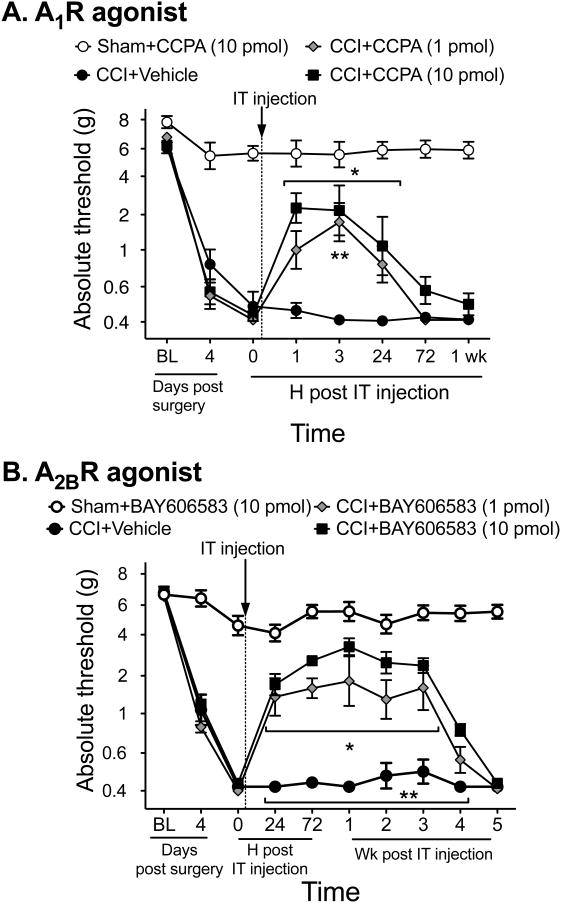
A first important question is whether this enduring effect or reversal of neuropathic allodynia only occurs with A2AR or does it extend to the other adenosine receptors more generally. Both A2AR and A2BR agonists increase cAMP while A1R and A3R agonists reduce cAMP. In order to determine if this long duration of effect on reversal of mechanical allodynia only occurs with A2AR or if it occurs via an increased cAMP such that the same effect would occur with an A2BR agonist, we tested A1R (CCPA) and A2BR (BAY 606583) agonists against CCI-induced allodynia, each at two doses equimolar or higher than ATL313, an A2AR agonist that produces more than 4 wk of pain reversal. The A1R agonist, which predominantly affects neurons (Cunha, 2008), transiently reversed allodynia for less than 3 d (Interaction: F21,119 = 7.19, P<0.001, n=4-6/gp, Fig. 1A). In contrast, the A2BR agonist produced a prolonged reversal of the allodynia for more than 3 wk (Interaction: F27,171 = 17.31, P<0.0001, n=5/6/gp, Fig. 1B) compared to CCI+vehicle. The results of the A2BR agonist are comparable to that seen by ATL313 (Loram et al., 2009). Thus, the phenomenon of the long-duration effect of A2AR agonism can be generalized to adenosine receptors elevating cAMP, thus beyond just A2AR.
Figure 1.
Mechanical allodynia is induced by CCI surgery. A single spinal (intrathecal, IT) administration of an A2AR agonist (ATL313) reduces neuropathic pain. (A) An A1R agonist (CCPA) transiently reverses neuropathic pain induced by CCI, lasting less than 72 h compared to vehicle controls. An A1R agonist has no effect on sham-operated rats. **P<0.05 at each time point for CCI+10 pmol CCPA compared to CCI+vehicle, *P<0.05 for CCI+1 pmol CCPA compared to CCI+vehicle; n=4-6 rats /group. (B) An A2BR agonist (BAY606583) administered at equimolar doses to ATL313 results in comparable reversal of CCI-induced neuropathic allodynia lasting 4 wk. There was no effect of the A2BR agonist on sham-operated rats. *P<0.01 CCI+1 pmol BAY606583 compared to CCI+vehicle, **P<0.01 CCI+10 pmol BAY606583 compared to CCI+vehicle; n=5-6 rats/group.
3.2 A2AR agonism produced long duration reversal of allodynia in multiple models of neuropathic pain
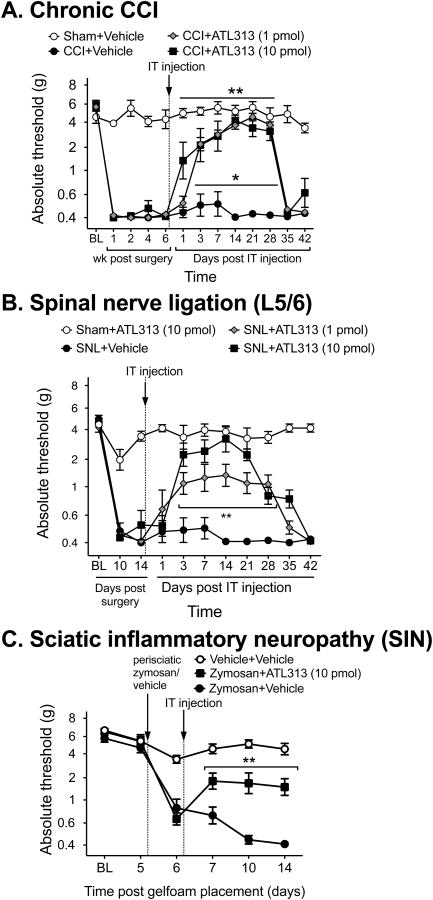
The results presented above demonstrate that the long duration reversal of mechanical allodynia occurs following agonism of adenosine receptors (A2AR and A2BR) that increase cAMP. The remainder of the experiments were designed to further explore the effects following A2AR agonism given its long duration of effect. If A2AR agonists have potential clinical relevance, their effects must generalize to other models with distinct underlying mechanisms (Kim et al., 1997), as well as to long-established neuropathic pain. To date, only an acute CCI model has been tested (Loram et al., 2009). Here, a single IT ATL313 injection was tested against long-established CCI (6 wk post-injury), spinal nerve ligation (SNL), and sciatic inflammatory neuropathy (Fig. 2). The A2AR agonist, ATL313, at both doses tested, produced a prolonged reversal of allodynia for at least 4 wk in the chronic CCI rats (Interaction: F36,252 = 16.06, P<0.0001, n=6/gp, Fig. 2A) compared to CCI+vehicle. The same duration of effect of both doses of ATl313 was identified in the SNL rats (Interaction: F30,240 = 7.86, P<0.001, n=5-8/gp, Fig. 2B). In the SIN model, ATL313 significantly reversed the mechanical allodynia for 1 wk after IT administration, which was the full duration of the experiment (Interaction: F10,95 = 14.14, P 0 0001, n=6-7 gp, Fig 2C)
Figure 2.
ATL313 reverses well-established neuropathic allodynia from chronic constriction injury performed 6 wk before administration (A). **P<0.0001 CCI+ATL313 10 pmol compared to CCI+vehicle, * P<0.05 CCI+ATL313 1 pmol compared to CCI+vehicle; n=6-7 rats/group. ATL313 dose-dependently reverses acute neuropathic allodynia from spinal nerve ligation (B). P< 0.01 for both doses of ATL313 compared to SNL+vehicle; n=5-8 rats/group. ATL313 reverses neuropathic allodynia induced by sciatic inflammatory neuropathy (C, 160 μg zymosan applied to the sciatic nerve on alternate days). P<0.001 compared to zymosan+vehicle; n=6-7 rats/group. All data are mean±SEM.
3.3 A2AR agonism decreases TNFα release from glial cells in vitro
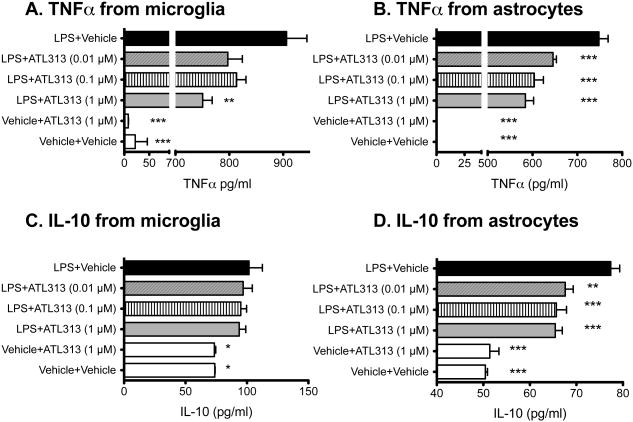
While diverse CNS cell types express A2AR (Fredholm et al., 2007; Palmer and Trevethick, 2008), we have shown previously that IT A2AR agonism reduced glial activation in the spinal cord of rats with CCI (Loram et al., 2009). A2AR agonists can attenuate pro-inflammatory cytokine production induced in microglial cells but it is not known if there is an upregulation of IL-10 (Chen and Pedata, 2008). This may be important as glial pro-inflammatory cytokines serve a critical role in the development and maintenance of neuropathic pain (Watkins et al., 2007) and intrathecal IL-10 is effective in attenuating neuropathic pain (Milligan et al., 2006). Therefore, we tested the effects of an A2AR agonist on proinflammatory cytokine release in microglia and astrocyte cultures in vitro. When microglia and astrocytes were incubated separately for 24 h, 1 μM ATL313 significantly attenuated LPS-stimulated TNFα release on the microglia (F5,11=379.2, P<0.0001, Fig. 3A) and all doses of ATL313 significantly attenuated TNFα release from the astrocytes (F5,18=573.2, P<0.0001, Fig. 3B). LPS significantly increased IL-10 release (F5,12=4.03, P<0.05, Fig. 3C). There was no significant effect of ATL313 on IL-10 release in the presence or absence of LPS (P>0.05). In astrocytes, LPS again induced IL-10 release (F5,18=36.12, P<0.0001, Fig. 3D). However, IL-10 release was significantly reduced by ATL313 at all 3 doses (P<0.05). Therefore, A2AR agonism attenuates TNFα release by both microglia and astrocytes in vitro but does not increase IL-10 release in microglia and decreases IL-10 in astrocytes.
Figure 3.
An A2AR agonist downregulates TNFα in central immune cells. (A) TNFα release (pg/ml) from neonatal cortical microglia and (B) astrocytes incubated for 24 h with LPS is attenuated by co-administration of ATL313. n=3/4 wells/group and the experiment was replicated at least twice. *P<0.05, ** P<0.01. *** P<0.001 compared to LPS+vehicle. IL-10 release (pg/ml) from neonatal cortical microglia (C) and astrocytes (D) incubated for 24 h with LPS is upregulated and maintained by ATL313 but not upregulated by co-administration of ATL313. *P<0.05, ** P<0.01. *** P<0.001 compared to LPS+vehicle. n=3/4 wells/group and the experiment was replicated at least twice.
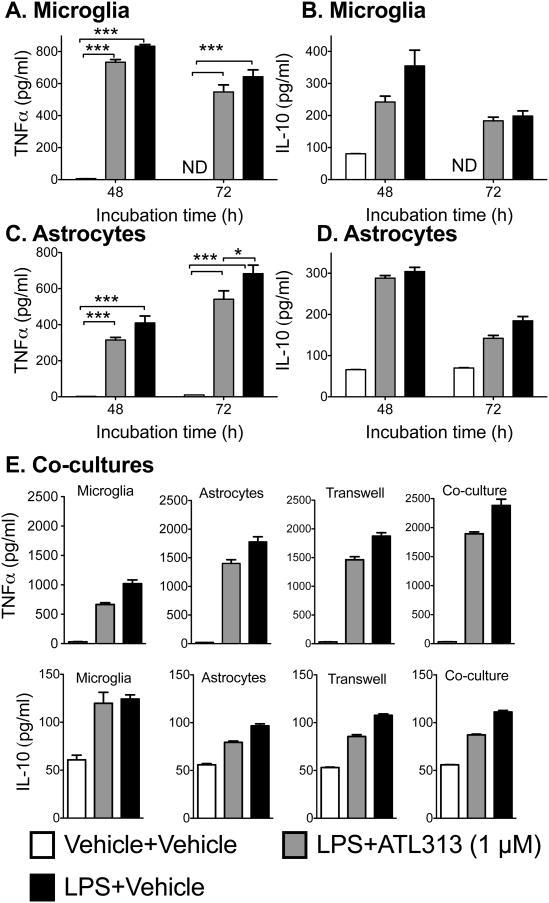
In order to determine if ATL313 required a longer incubation period to upregulate IL-10 release, we repeated the above experiment with separate glial cultures but incubated the cells for 48 h and 72 h (Fig. 4). Only the high dose of ATL313 was used. As with the 24 h incubation, there was significant attenuation of TNFα release from both microglia (P<0.05, Fig. 4A) and astrocytes (P<0.05, Fig. 4C) but no significant change in effect of ATL313 on IL-10 release in either cell population (P>0.05, Fig. 4B & D).
Figure 4.
TNFα and IL-10 produced by glial cells in vitro in response to LPS +/- ATL313 are unaffected by duration of incubation or locality of other glial cells. TNFα release (pg/ml) and IL-10 release (pg/ml) from neonatal cortical microglia incubated for 48 h and 72 h produced comparable results as a 24 h incubation with IL-10 not being elevated by ATL313 beyond that induced by LPS alone (A, B). TNFα release (pg/ml) and IL-10 release (pg/ml) from neonatal cortical astrocytes incubated for 48 h and 72 h with LPS+ATL313 produced comparable results as a 24 h incubation with IL-10 not being elated beyond that induced by LPS alone (C, D). TNFα and IL-10 release (pg/ml) from neonatal cortical microglia and astrocytes incubated for 24 h either in isolated cell types, co-incubated or with transwell inserts allowing non-contact communication (E). None of the above incubation conditions altered the TNFα or IL-10 release profiles following LPS +/- ATL313 coadministration. *P<0.05, ** P<0.01. *** P<0.001 compared to LPS+vehicle. n=3/4 wells/group and the experiment was replicated at least twice. All data are mean±SEM.
We then aimed to determine if the glia required a co-culture environment in order for ATL313 to upregulate IL-10 release. Therefore, microglia and astrocytes were cocultured as a mixed glial population, allowing for cell-cell contact and in a transwell environment, which allows for paracrine communication without direct cellular contact between cell populations (Fig. 4E). In both these conditions, ATL313 produced a significant attenuation of LPS induced TNFα release (P<0.05) but no significant change in IL-10 production from what was seen in the previous in vitro cell culture conditions.
3.4 A2AR agonism increases IL-10 release into the CSF in neuropathic rats
We have demonstrated previously that immune cells within the CSF have elevated IL-10 mRNA in CCI rats injected IT with ATL313. Here, IL-10 protein was measured in the CSF to define whether, in vivo, ATL313 induced IL-10 release in both rats with CCI and SNL. IL-10 in the CSF of SNL rats was significantly elevated at 1 wk after IT administration of ATL313 (t10=2.61, P<0.05) and there was a strong trend for elevated IL-10 in CCI rats receiving ATL313 IT 1 wk before CSF collection (t12=1.99, P=0.07, Table 1).
Table 1.
IL-10 protein (pg/ml) in the CSF 1 wk after ATL313/vehicle in CCI or SNL rats.
| Saline | ATL313 | ||
|---|---|---|---|
| SNL | Mean | 93 | 105.4* |
| SEM | 3.2 | 3.5 | |
| CCI | Mean | 89.3 | 98.7 |
| SEM | 1.6 | 4.4 |
P<0.05 between groups
3.5 A2AR agonism attenuates TNFα release partially via PKA and PKC
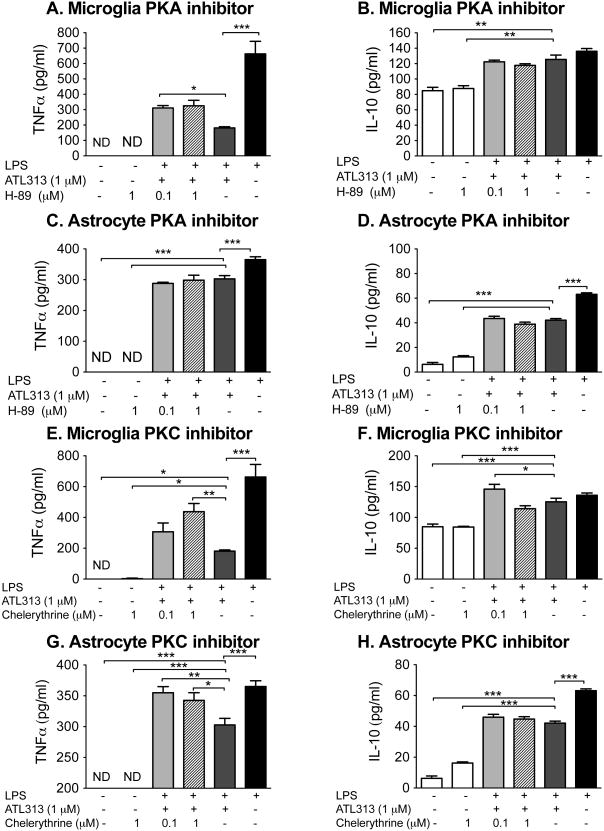
In order to confirm that the A2AR agonist attenuated TNFα in the glia cultures via PKA and/or PKC, the PKA and PKC inhibitors were co-incubated with LPS and ATL313 (Fig. 5). The intracellular signaling through cAMP is complex and unique to the substrates available that can be subsequently phosphorylated by the protein kinases activated by cAMP. These intracellular signaling cascades are cell specific and dependent on the microenvironment of the cells in situ (Scott et al., 2013). This cell and microenvironment specificity thus greatly complicates the interpretation of data resulting from in vivo study of drugs targeting cAMP signaling cascades (Scott et al., 2013). As a consequence, we began to explore in the intracellular cascades involved using in vitro glial cultures before use in in vivo behavioral models. The PKA inhibitor at 0.1 μM significantly reversed the ATL313 mediated TNFα suppression in microglia (P<0.05) but had no effect on ATL313 mediated suppression of TNFα in astrocytes. The PKA inhibitor had no effect on IL-10 either alone or in the presence of ATL313 in either the microglia or astrocytes (P>0.05). The PKC inhibitor at the high dose, significantly reversed the ATL313 mediated TNFα suppression (P<0.05) in both microglia and astrocytes. The PKC inhibitor, like the PKA inhibitor, had no effect on IL-10 either alone or in the presence of ATL313 in either the microglia or astrocytes (P>0.05).
Figure 5.
TNFα release from neonatal microglia (b) and neonatal astrocytes (c) incubated with LPS (100 ng/ml) for 24 h was attenuated by ATL313 (1 μM). Administration of H-89 (PKA inhibitor) partially reversed the effects of ATL313 on TNFα production in microglia (A) but not astrocytes (B). IL-10 production induced by LPS was not affected by ATL313 or H-89 in microglia (C, D). Administration of chelerythrine (PKC inhibitor) had no effect on ATL313 mediated effects of TNFα production in microglia (E) but reversed the ATL313 effect in astrocytes (F). IL-10 production induced by LPS was not affected by ATL313 in microglia (G). However, chelerythrine+LPS+ATl313 increased IL-10 compared to LPS+ATL313+vehicle in microglia (G). Chelerythrine had no effect on IL-10 responses in astrocytes (H). Protein measured by rat-specific ELISA. *P<0.05, **P<0.01, ***P<0.001 compared to LPS+ATL313+veh; n=4-5 wells/group. All data are mean±SEM.
3.6 A2AR agonism reverses neuropathic allodynia via PKA and PKC
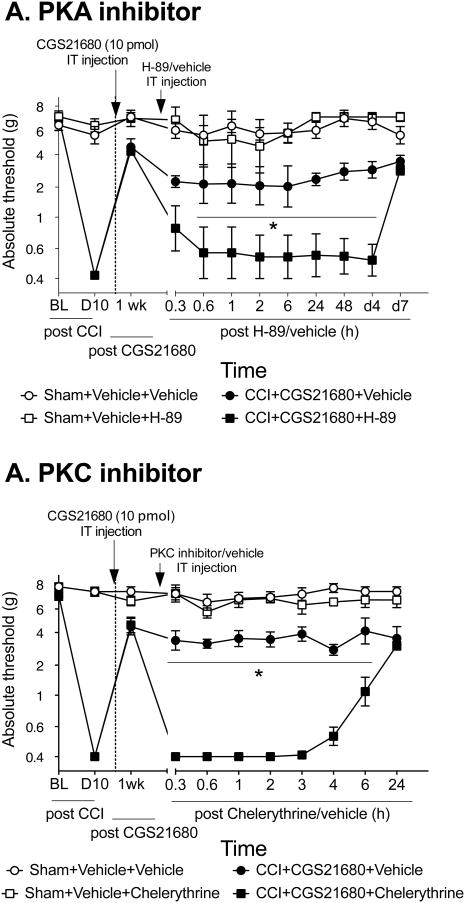
Studies have identified that A2AR agonism signals intracellularly with PKA and possibly through PKC (Kreckler et al., 2009; Leghmari et al., 2008). In order to determine if these intracellular signaling kinases contribute to the reversal of mechanical allodynia induced by IT A2AR agonist, PKA and PKC inhibitors were separately administered in allodynic rats that had undergone CCI surgery. 1 wk after IT A2AR, rats received one IT injection of a PKA inhibitor (H-89) (Fig. 6). H-89 significantly reversed the effect of CGS21680 in rats with CCI, such that the rats became fully allodynia from 6 h- 4 d after PKA inhibitor was administered (Interaction: F33,220=0.25, P<0.0001). The PKA inhibitor had no effect on sham-operated rats (P>0.05). The PKC inhibitor also significantly reversed the effect of CGS21680 on mechanical allodynia, but the effects lasted less than 24 h (Interaction: F30,200=0.39, P<0.0001).
Figure 6.
An A2AR agonist attenuates neuropathic allodynia via PKA and PKC. Mechanical allodynia induced by CCI was reversed by CGS21680 (10 pmol administered IT). 1 wk after CGS21680, a PKA inhibitor (H-89, 1 fmol) was administered IT. H-89 reversed the anti-allodynic effects of CGS21680 back to full allodynia for at least 4 days (A). 1 wk after CGS21680, a PKC inhibitor (chelerythrine, 60 fmol) was administered IT. Chelerythrine transiently reversed the anti-allodynic effects of CGS21680 back to full allodynia for 6 h (B). *P<0.05 compared to CCI+ATL+vehicle group; n=6 rats/group.
4. Discussion
The role of adenosine 2A receptor (A2AR) agonists as peripheral immune modulators is well recognized. The effect of A2AR agonists in the central nervous system is more controversial. In this series of studies, we provide evidence that intrathecal delivery of A2AR agonists very broadly influence pain dysregulation as they suppress mechanical allodynia in numerous models of neuropathic pain. This long duration effect from a single intrathecal administration occurs not only with A2AR agonism but also with A2BR agonism. While A2BR agonists also increase intracellular cAMP signaling, an A1R agonist, which decreases intracellular cAMP signaling, only produced transient reversal of mechanical allodynia lasting less than 3 d. ATL313 effectively attenuated LPS-induced TNFα in neonatal glia in vitro, but ATL313, at the doses and conditions we tested, had no effect on IL-10 in microglia and even decreased LPS-induced IL-10 production. However, IL-10 is released and upregulated following ATL313 IT in rats with SNL and potentially in CCI also. We provide preliminary evidence that the A2AR agonist may act via protein kinase A (PKA) and PKC as inhibitors to PKA and PKC reversed the A2AR mediated reduction in TNFα release in glial cell in vitro. In addition, PKA and PKC inhibitors reversed the effects of the A2AR agonist when administered intrathecally in rats with CCI induced neuropathic allodynia.
A2AR agonists are effective both in acute neuropathic pain and in well-established neuropathic pain for an extended period of time (several weeks) after a single intrathecal injection (Loram et al., 2009). We have also demonstrated previously that A2AR agonism effectively attenuated both mechanical allodynia and thermal hyperalgesia from CCI (Loram et al., 2009). However, each model of neuropathic pain presents differently in terms of time from surgery to presentation of stable allodynia, intensity of allodynia and whether thermal hyperalgesia is present. As such, subtle but possibly crucial differences in mechanism may underlie the differences in allodynia and hyperalgesia between models (Kim et al., 1997). Therefore, the ability of A2AR agonism providing comparable results in multiple neuropathic pain models provides further strength to A2AR agonists being a good candidate for future development.
Our data confirm previous reports that intrathecally administered A1R agonists transiently reverse neuropathic allodynia (Lee and Yaksh, 1996; Yamamoto et al., 2003; Zahn et al., 2007). A2AR and A2BR, but not A1R, activation enduringly reverses neuropathic pain. As A2BR and A2AR, but not A1R, activation increases cAMP (Hasko and Cronstein, 2004), this suggests that multi-week reversal of pain may potentially be linked to elevated cAMP. Adenosine receptors are found on all cells within the CNS including on neurons. A2AR on neurons are pleiotropic with complex interactions with other neuropeptide receptors both at the presynaptic and postsynaptic sites (Sebastiao and Ribeiro, 2009a, b). Adenosine receptors have been identified in the spinal cord with the dominant receptor being A1 receptor in the dorsal horn of the spinal cord (Sebastiao et al., 2011). In addition, A2AR have been demonstrated to be upregulated in the spinal cord following spinal cord injury (Paterniti et al., 2011). In the spinal cord injury model, the A2AR was colocalized to some neurons, but not all (Paterniti et al., 2011). These studies extend to the underlying mechanism by which these A2AR agonists work within the spinal cord as dogma dictates that elevated cAMP, as seen following A2AR and A2BR activation, results in increased neuronal excitability (Momin and McNaughton, 2009). The studies examining adenosine receptors on neurons have been done from cells or tissue from the brain. Therefore, it is not known if the same results occur from neuronal cells in the spinal cord. Activation of A2AR on neurons enhances the release of presynaptic glutamate and the subsequent increase in calcium influx. Both can enhance neuronal cell death (Dai and Zhou, 2011). There is also evidence of an increase in noradrenaline release following A2AR activation and thus an impact on sympathetic nervous activity in the spinal cord (Brooke et al., 2004). However, when looking at in vivo models of brain injury or ischemia, there is bidirectional effect with some studies showing protection and some showing enhancement of injury (Dai and Zhou, 2011). If A2AR activation had an effect on neurons in the spinal cord, the effect would most likely increase excitatory neurotransmitter release. However, the present evidence suggests that the elevated cAMP within glial cells produces the opposite effect, with an attenuation of pro-inflammatory cytokine production and subsequent reduction in pain.
The studies here demonstrate that A2AR agonism in single glial populations or cocultured glia, where functional interactions may occur (Schubert et al., 2000), significantly attenuated TNFα. Both stimulation of peripheral immune cells with cAMP in vitro and induction of intracellular cAMP by a non-selective adenosine agonist (NECA), attenuated LPS induced pro-inflammatory mediators (Kreckler et al., 2009; Kreckler et al., 2006). In addition, studies with peripheral immune cell in vitro indicate that elevated cAMP can reduce pro-inflammatory cytokines and upregulate IL-10 through PKA (Kreckler et al., 2009; Palmer and Trevethick, 2008) and PKC (Diniz et al., 2008) both of which activate the cAMP response element binding protein (CREB) amongst other intracellular mediators (Avni et al., 2010; Wen et al., 2010). The protein kinase inhibitors used in this studies have predominantly protein kinase A or C inhibition, but do have some non selectivity for other intracellular kinases (Davies et al., 2000). Therefore, the signaling cascade initiated by cAMP may be important to attenuate pro-inflammatory cytokines in immune cells, but further studies would further elucidate the associated intracellular signaling cascades.
Numerous studies have identified that in vitro cAMP induced PKA (Cho et al., 2002; Tortora and Ciardiello, 2002a, b) and to some extent PKC (Goethe et al., 2007; Gold et al., 1998; Hucho et al., 2005) and that PKA induction reduces pro-inflammatory mediator production in a model of traumatic brain injury (Atkins et al., 2007). However, we are the first to demonstrate that the reversal of allodynia by A2AR agonist is in part via PKA activation in vivo. While the interpretation of the in vivo kinase inhibitor data need to be made within the context of the complex interaction of the protein kinases with numerous substrates (Scott et al., 2013), which is further complicated by the microenvironment in which it is activated (Scott et al., 2013). The results remain intriguing, as even though the PKA inhibitor did indeed reverse the effects of the A2AR agonist on neuropathic pain, even after 4 days of reversal, the allodynic reversal effects of the A2AR agonist returned. cAMP is also known to increase PKC phosphorylation and the PKC inhibitor was effective in reversing the A2AR mediated reversal of allodynia in vivo, though with shorter duration than that of the PKA inhibitor. It is possible that inhibiting both kinases may result in a persistent resetting of the immune cells as has been identified to occur in peripheral macrophages (Csoka et al., 2012). It is interesting to note that at 1 wk after the A2AR agonist was administered, the A2AR antagonist had no effect on the mechanical allodynia (Loram et al., 2009) but the PKA and PKC inhibitor effectively abolished the effect of the A2AR agonist. These results do suggest that subsequent to A2AR binding, some enduring intracellular changes occur downstream of A2AR, but independent of A2AR activation, that induce the long term reversal of the allodynia. Future studies to explore whether these intracellular kinases, activated by numerous cAMP mediated receptors and not just A2AR, are key in inducing long-term reversal of allodynia (Antoni, 2012).
The PKA and PKC inhibitors significantly suppressed ATL313-induced TNFα attenuation in both microglia and astrocytes in vitro, but had no effect on IL-10. Together, these studies suggest that ATL313 may, via PKA and PKC in glial cells in the spinal cord dorsal horn of neuropathic rats, enduringly suppress pro-inflammatory cytokines. The glial in vitro results presented here contrast with data from peripheral immune cells where IL-10 protein was upregulated by an A2AR agonist (Csoka et al., 2007; Link et al., 2000) and that it was mediated by PKA (Kreckler et al., 2009; Palmer and Trevethick, 2008).
Previous studies have demonstrated that microglia stimulated in vitro with higher doses of adenosine or with an A2BR agonist both upregulated IL-10 production when coadministered with LPS (Koscso et al., 2012). We have shown that A2AR agonism upregulates IL-10 mRNA in cerebrospinal fluid (CSF) cells (Loram et al., 2009) and here that IL-10 protein was moderately elevated in the CSF 1 wk after ATL313 IT in SNL rats. Therefore, elevated CSF IL-10 following ATL313 may not be produced by glia, as we identified in spinal cord mRNA previously, but rather from immunocompetent cells, largely macrophages, of the intrathecal space (Loram et al., 2009). The in vitro experiments were done in neonatal cortical glia. The literature has identified differences in both morphology and responses to inflammatory stimuli between neonatal and adult cortical glia (Loram et al., 2012; Schwarz et al., 2011), with neonatal cortical glia demonstrating a more pro-inflammatory phenotype. In rodent models, neonates are less susceptible to neuropathic pain induction compared to adult rodents, with less spinal glial activation in the neonatal spinal cord (Moss et al., 2007). However, this does imply that neonatal spinal glia are not excitable were they to have received the proper activation signals (Costigan et al., 2009). Nonetheless, the neonatal cortical glia, used in culture conditions were used to mimic a pro-inflammatory environment to evaluate the effect of A2AR agonist and the subsequent protein kinases on TNF and IL-10 levels. Therefore, we cannot exclude the possibility of adult spinal glia producing IL-10 in vivo. However, the data do demonstrate the effect of A2AR agonism attenuating LPS-induced TNFα production, and that the PKA and PKC inhibitors had the ability to reverse the effects of A2AR agonism. The potential of exploring the effect of A2AR agonists on spinal glia ex vivo is worth future investigation.
Together, these data demonstrate that single IT A2AR agonist doses enduringly attenuate neuropathic pain across multiple models. The presence of an A2AR agonist does not alter normal cellular responses when the cells are in a homeostatic surveying state. Thus, the potent effect seen by A2AR agonism requires prior neuroinflammation. The proposed underlying mechanism involves a significant downregulation of TNFα, by glia, as demonstrated here, and CSF immune cells (Loram et al., 2009). A modest elevation in IL-10 in the CSF may be produced by immune cells within the CSF but may not be produced by glial cells. This unique effect has implications in understanding the role of A2AR in modulating neuropathic pain.
Research Highlights.
Intrathecal A2AR agonist induced long duration reversal of mechanical allodynia in a variety of neuropathic models
Acknowledgments
Financial support for these studies was provided by Dogwood Pharmaceuticals, LLC, Bayer Pharmaceuticals and by NIH grants DA02422 and RC1-NS067807 and Department of Defense grant DM102108.
Footnotes
Conflict of interest: LCL, JR and LRW are named a patent regarding ATL313. JR was employed by Dogwood Pharmaceuticals. This work was funded by the Department of Defense, National Institute of Health and Dogwood Pharmaceuticals.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Antoni FA. New paradigms in cAMP signalling. Mol Cell Endocrinol. 2012;353:3–9. doi: 10.1016/j.mce.2011.10.034. [DOI] [PubMed] [Google Scholar]
- Atkins CM, Oliva AA, Jr, Alonso OF, Pearse DD, Bramlett HM, Dietrich WD. Modulation of the cAMP signaling pathway after traumatic brain injury. Experimental Neurology. 2007;208:145–158. doi: 10.1016/j.expneurol.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avni D, Ernst O, Philosoph A, Zor T. Role of CREB in modulation of TNFalpha and IL-10 expression in LPS-stimulated RAW264.7 macrophages. Mol Immunol. 2010;47:1396–1403. doi: 10.1016/j.molimm.2010.02.015. [DOI] [PubMed] [Google Scholar]
- Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- Brooke RE, Deuchars J, Deuchars SA. Input-specific modulation of neurotransmitter release in the lateral horn of the spinal cord via adenosine receptors. J Neurosci. 2004;24:127–137. doi: 10.1523/JNEUROSCI.4591-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacur M, Milligan ED, Gazda LS, Armstrong C, Wang H, Tracey KJ, Maier SF, Watkins LR. A new model of sciatic inflammatory neuritis (SIN): induction of unilateral and bilateral mechanical allodynia following acute unilateral peri-sciatic immune activation in rats. Pain. 2001;94:231–244. doi: 10.1016/S0304-3959(01)00354-2. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Pogrel JW, Yaksh TL. Role of voltage-dependent calcium channel subtypes in experimental tactile allodynia. J Pharmacol Exp Ther. 1994;269:1117–1123. [PubMed] [Google Scholar]
- Chen JF, Pedata F. Modulation of ischemic brain injury and neuroinflammation by adenosine A2A receptors. Current pharmaceutical design. 2008;14:1490–1499. doi: 10.2174/138161208784480126. [DOI] [PubMed] [Google Scholar]
- Cho YS, Kim MK, Tan L, Srivastava R, Agrawal S, Cho-Chung YS. Protein kinase A RIalpha antisense inhibition of PC3M prostate cancer cell growth: Bcl-2 hyperphosphorylation, Bax up-regulation, and Bad-hypophosphorylation. Clinical cancer research : an official journal of the American Association for Cancer Research. 2002;8:607–614. [PubMed] [Google Scholar]
- Costigan M, Moss A, Latremoliere A, Johnston C, Verma-Gandhu M, Herbert TA, Barrett L, Brenner GJ, Vardeh D, Woolf CJ, Fitzgerald M. T-cell infiltration and signaling in the adult dorsal spinal cord is a major contributor to neuropathic pain-like hypersensitivity. J Neurosci. 2009;29:14415–14422. doi: 10.1523/JNEUROSCI.4569-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csoka B, Nemeth ZH, Virag L, Gergely P, Leibovich SJ, Pacher P, Sun CX, Blackburn MR, Vizi ES, Deitch EA, Hasko G. A2A adenosine receptors and C/EBPbeta are crucially required for IL-10 production by macrophages exposed to Escherichia coli. Blood. 2007;110:2685–2695. doi: 10.1182/blood-2007-01-065870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csoka B, Selmeczy Z, Koscso B, Nemeth ZH, Pacher P, Murray PJ, Kepka-Lenhart D, Morris SM, Jr, Gause WC, Leibovich SJ, Hasko G. Adenosine promotes alternative macrophage activation via A2A and A2B receptors. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2012;26:376–386. doi: 10.1096/fj.11-190934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha RA. Different cellular sources and different roles of adenosine: A1 receptor-mediated inhibition through astrocytic-driven volume transmission and synapse-restricted A2A receptor-mediated facilitation of plasticity. Neurochem Int. 2008;52:65–72. doi: 10.1016/j.neuint.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Dai SS, Zhou YG. Adenosine 2A receptor: a crucial neuromodulator with bidirectional effect in neuroinflammation and brain injury. Reviews in the neurosciences. 2011;22:231–239. doi: 10.1515/RNS.2011.020. [DOI] [PubMed] [Google Scholar]
- Dare E, Schulte G, Karovic O, Hammarberg C, Fredholm BB. Modulation of glial cell functions by adenosine receptors. Physiology & behavior. 2007;92:15–20. doi: 10.1016/j.physbeh.2007.05.031. [DOI] [PubMed] [Google Scholar]
- Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diniz C, Borges F, Santana L, Uriarte E, Oliveira JM, Goncalves J, Fresco P. Ligands and therapeutic perspectives of adenosine A(2A) receptors. Curr Pharm Des. 2008;14:1698–1722. doi: 10.2174/138161208784746842. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Chern Y, Franco R, Sitkovsky M. Aspects of the general biology of adenosine A2A signaling. Prog Neurobiol. 2007;83:263–276. doi: 10.1016/j.pneurobio.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Global Industry Analysts. Global Pain Management Market to Reach US$60 Billion by 2015 2011 [Google Scholar]
- Goethe R, Basler T, Phi-van L. Regulation of C/EBPbeta mRNA expression and C/EBPbeta promoter activity by protein kinases A and C in a myelomonocytic cell line (HD11) In: et al., editors. Inflammation research : official journal of the European Histamine Research Society. Vol. 56. 2007. pp. 274–281. [DOI] [PubMed] [Google Scholar]
- Gold MS, Levine JD, Correa AM. Modulation of TTX-R INa by PKC and PKA and their role in PGE2-induced sensitization of rat sensory neurons in vitro. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1998;18:10345–10355. doi: 10.1523/JNEUROSCI.18-24-10345.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey LO. Efficient estimation of sensory thresholds. Behav Res Methods Instrum Comput. 1986;18:623–632. [Google Scholar]
- Hasko G, Cronstein BN. Adenosine: an endogenous regulator of innate immunity. Trends Immunol. 2004;25:33–39. doi: 10.1016/j.it.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Herbert JM, Augereau JM, Gleye J, Maffrand JP. Chelerythrine is a potent and specific inhibitor of protein kinase C. Biochem Biophys Res Commun. 1990;172:993–999. doi: 10.1016/0006-291x(90)91544-3. [DOI] [PubMed] [Google Scholar]
- Hucho TB, Dina OA, Levine JD. Epac mediates a cAMP-to-PKC signaling in inflammatory pain: an isolectin B4(+) neuron-specific mechanism. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:6119–6126. doi: 10.1523/JNEUROSCI.0285-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Loram LC, Zhang Y, Shridhar M, Rezvani N, Berkelhammer D, Phipps S, Foster PS, Landgraf K, Falke JJ, Rice KC, Maier SF, Yin H, Watkins LR. Evidence that tricyclic small molecules may possess toll-like receptor and myeloid differentiation protein 2 activity. Neuroscience. 2010;168:551–563. doi: 10.1016/j.neuroscience.2010.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KJ, Yoon YW, Chung JM. Comparison of three rodent neuropathic pain models. Exp Brain Res. 1997;113:200–206. doi: 10.1007/BF02450318. [DOI] [PubMed] [Google Scholar]
- Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- Koscso B, Csoka B, Selmeczy Z, Himer L, Pacher P, Virag L, Hasko G. Adenosine augments IL-10 production by microglial cells through an A2B adenosine receptor-mediated process. Journal of Immunology. 2012;188:445–453. doi: 10.4049/jimmunol.1101224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreckler LM, Gizewski E, Wan TC, Auchampach JA. Adenosine suppresses lipopolysaccharide-induced tumor necrosis factor-alpha production by murine macrophages through a protein kinase A- and exchange protein activated by cAMP-independent signaling pathway. J Pharmacol Exp Ther. 2009;331:1051–1061. doi: 10.1124/jpet.109.157651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreckler LM, Wan TC, Ge ZD, Auchampach JA. Adenosine inhibits tumor necrosis factor-alpha release from mouse peritoneal macrophages via A2A and A2B but not the A3 adenosine receptor. J Pharmacol Exp Ther. 2006;317:172–180. doi: 10.1124/jpet.105.096016. [DOI] [PubMed] [Google Scholar]
- Lee YW, Yaksh TL. Pharmacology of the spinal adenosine receptor which mediates the antiallodynic action of intrathecal adenosine agonists. J Pharmacol Exp Ther. 1996;277:1642–1648. [PubMed] [Google Scholar]
- Leghmari K, Bennasser Y, Tkaczuk J, Bahraoui E. HIV-1 Tat protein induces IL-10 production by an alternative TNF-alpha-independent pathway in monocytes: Role of PKC-delta and p38 MAP kinase. Cellular immunology. 2008 doi: 10.1016/j.cellimm.2008.04.015. [DOI] [PubMed] [Google Scholar]
- Link AA, Kino T, Worth JA, McGuire JL, Crane ML, Chrousos GP, Wilder RL, Elenkov IJ. Ligand-activation of the adenosine A2a receptors inhibits IL-12 production by human monocytes. J Immunol. 2000;164:436–442. doi: 10.4049/jimmunol.164.1.436. [DOI] [PubMed] [Google Scholar]
- Loram LC, Harrison JA, Sloane EM, Hutchinson MR, Sholar P, Taylor FR, Berkelhammer D, Coats BD, Poole S, Milligan ED, Maier SF, Rieger J, Watkins LR. Enduring reversal of neuropathic pain by a single intrathec a l injection of adenosine 2A receptor agonists: a novel therapy for neuropathic pain. J Neurosci. 2009;29:14015–14025. doi: 10.1523/JNEUROSCI.3447-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loram LC, Sholar PW, Taylor FR, Wiesler JL, Babb JA, Strand KA, Berkelhammer D, Day HE, Maier SF, Watkins LR. Sex and estradiol influence glial pro-inflammatory responses to lipopolysaccharide in rats. Psychoneuroendocrinology. 2012;37:1688–1699. doi: 10.1016/j.psyneuen.2012.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan ED, Maier SF, Watkins LR. Sciatic inflammatory neuropathy in the rat: surgical procedures, induction of inflammation, and behavioral testing. Methods Mol Med. 2004a;99:67–89. doi: 10.1385/1-59259-770-X:067. [DOI] [PubMed] [Google Scholar]
- Milligan ED, Mehmert KK, Hinde JL, Harvey LO, Martin D, Tracey KJ, Maier SF, Watkins LR. Thermal hyperalgesia and mechanical allodynia produced by intrathecal administration of the human immunodeficiency virus-1 (HIV-1) envelope glycoprotein, gp120. Brain Res. 2000;861:105–116. doi: 10.1016/s0006-8993(00)02050-3. [DOI] [PubMed] [Google Scholar]
- Milligan ED, Sloane EM, Langer SJ, Hughes TS, Jekich BM, Frank MG, Mahoney JH, Levkoff LH, Maier SF, Cruz PE, Flotte TR, Johnson KW, Mahoney MM, Chavez RA, Leinwand LA, Watkins LR. Repeated intrathecal injections of plasmid DNA encoding interleukin-10 produce prolonged reversal of neuropathic pain. Pain. 2006;126:294–308. doi: 10.1016/j.pain.2006.07.009. [DOI] [PubMed] [Google Scholar]
- Milligan ED, Twining C, Chacur M, Biedenkapp J, O'Connor K, Poole S, Tracey K, Martin D, Maier SF, Watkins LR. Spinal glia and proinflammatory cytokines mediate mirror-image neuropathic pain in rats. J Neurosci. 2003;23:1026–1040. doi: 10.1523/JNEUROSCI.23-03-01026.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan ED, Zapata V, Chacur M, Schoeniger D, Biedenkapp J, O'Connor KA, Verge GM, Chapman G, Green P, Foster AC, Naeve GS, Maier SF, Watkins LR. Evidence that exogenous and endogenous fractalkine can induce spinal nociceptive facilitation in rats. Eur J Neurosci. 2004b;20:2294–2302. doi: 10.1111/j.1460-9568.2004.03709.x. [DOI] [PubMed] [Google Scholar]
- Momin A, McNaughton PA. Regulation of firing frequency in nociceptive neurons by pro-inflammatory mediators. Exp Brain Res. 2009;196:45–52. doi: 10.1007/s00221-009-1744-2. [DOI] [PubMed] [Google Scholar]
- Moss A, Beggs S, Vega-Avelaira D, Costigan M, Hathway GJ, Salter MW, Fitzgerald M. Spinal microglia and neuropathic pain in young rats. Pain. 2007;128:215–224. doi: 10.1016/j.pain.2006.09.018. [DOI] [PubMed] [Google Scholar]
- Palmer TM, Trevethick MA. Suppression of inflammatory and immune responses by the A(2A) adenosine receptor: an introduction. Br J Pharmacol. 2008;153(1):S27–34. doi: 10.1038/sj.bjp.0707524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterniti I, Melani A, Cipriani S, Corti F, Mello T, Mazzon E, Esposito E, Bramanti P, Cuzzocrea S, Pedata F. Selective adenosine A2A receptor agonists and antagonists protect against spinal cord injury through peripheral and central effects. J Neuroinflammation. 2011;8:31. doi: 10.1186/1742-2094-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz J, Woolf CJ. The neuropathic pain triad: neurons, immune cells and glia. Nature Neurosci. 2007;10:1361–1368. doi: 10.1038/nn1992. [DOI] [PubMed] [Google Scholar]
- Schubert P, Morino T, Miyazaki H, Ogata T, Nakamura Y, Marchini C, Ferroni S. Cascading glia reactions: a common pathomechanism and its differentiated control by cyclic nucleotide signaling. Ann N Y Acad Sci. 2000;903:24–33. doi: 10.1111/j.1749-6632.2000.tb06346.x. [DOI] [PubMed] [Google Scholar]
- Schwarz JM, Sholar PW, Bilbo SD. Sex differences in microglial colonization of the developing rat brain. J Neurochem. 2011 doi: 10.1111/j.1471-4159.2011.07630.x. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JD, Dessauer CW, Tasken K. Creating order from chaos: cellular regulation by kinase anchoring. Annu Rev Pharmacol Toxicol. 2013;53:187–210. doi: 10.1146/annurev-pharmtox-011112-140204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastiao AM, Assaife-Lopes N, Diogenes MJ, Vaz SH, Ribeiro JA. Modulation of brain-derived neurotrophic factor (BDNF) actions in the nervous system by adenosine A(2A) receptors and the role of lipid rafts. Biochim Biophys Acta. 2011;1808:1340–1349. doi: 10.1016/j.bbamem.2010.06.028. [DOI] [PubMed] [Google Scholar]
- Sebastiao AM, Ribeiro JA. Adenosine receptors and the central nervous system. Handbook of experimental pharmacology. 2009a:471–534. doi: 10.1007/978-3-540-89615-9_16. [DOI] [PubMed] [Google Scholar]
- Sebastiao AM, Ribeiro JA. Tuning and fine-tuning of synapses with adenosine. Current neuropharmacology. 2009b;7:180–194. doi: 10.2174/157015909789152128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortora G, Ciardiello F. Protein kinase A as target for novel integrated strategies of cancer therapy. Annals of the New York Academy of Sciences. 2002a;968:139–147. doi: 10.1111/j.1749-6632.2002.tb04332.x. [DOI] [PubMed] [Google Scholar]
- Tortora G, Ciardiello F. Protein kinase A type I: a target for cancer therapy. Clin Cancer Res. 2002b;8:303–304. [PubMed] [Google Scholar]
- Watkins LR, Hutchinson MR, Milligan ED, Maier SF. “Listening” and “talking” to neurons: implications of immune activation for pain control and increasing the efficacy of opioids. Brain Research Reviews. 2007;56:148–169. doi: 10.1016/j.brainresrev.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen AY, Sakamoto KM, Miller LS. The role of the transcription factor CREB in immune function. Journal of Immunology. 2010;185:6413–6419. doi: 10.4049/jimmunol.1001829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S, Nakanishi O, Matsui T, Shinohara N, Kinoshita H, Lambert C, Ishikawa T. Intrathecal adenosine A1 receptor agonist attenuates hyperalgesia without inhibiting spinal glutamate release in the rat. Cell Mol Neurobiol. 2003;23:175–185. doi: 10.1023/A:1022997805525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn PK, Straub H, Wenk M, Pogatzki-Zahn EM. Adenosine A1 but not A2a receptor agonist reduces hyperalgesia caused by a surgical incision in rats: a pertussis toxin-sensitive G protein-dependent process. Anesthesiol. 2007;107:797–806. doi: 10.1097/01.anes.0000286982.36342.3f. [DOI] [PubMed] [Google Scholar]