Abstract
Background
Rituximab has been used in antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) since 2003. Our objective was to describe outcomes and adverse events following rituximab since that time in an inception cohort.
Methods
Patients with AAV (diagnosed 1991–2012) who received rituximab (n = 120) were evaluated and incidence per person-year (PPY) with 95% confidence interval was calculated for relapse and infections. Time to remission and relapse by number of rituximab infusions given per treatment course (≤2 versus >2) and by ever having been exposed to cyclophosphamide were compared using Kaplan–Meier curves. Rituximab-treated patients were characterized in comparison with AAV patients treated with cyclophosphamide but not exposed to rituximab (n = 351) using Fisher's exact or rank tests.
Results
Rituximab resulted in 86% achieving remission and 41% having a subsequent relapse in a median of 19 months (range 9–29). Time to remission and relapse were similar between rituximab infusion courses (≤2 versus >2; remission P = 0.86 and relapse P = 0.78, respectively). Incidence of relapse was 0.22 PPY (0.14, 0.31) and of severe infection was 0.12 PPY (0.08, 0.24). Time to relapse was shorter in those never exposed to cyclophosphamide (n = 20): 50% by 8 months versus 50% by 24 and 30 months for those with prior or concurrent exposure to cyclophosphamide (n = 100). Compared with those who never received rituximab, rituximab-treated patients were younger (P < 0.001), more likely to have granulomatosis with polyangiitis (P = 0.001) and had more upper airway (P = 0.01) and less kidney involvement (P = 0.007).
Conclusions
Rituximab is beneficial when prescribed outside of a trial setting. Response to treatment and relapse is similar regardless of infusion number. Rituximab without cyclophosphamide may result in a shorter time to relapse supporting combination of these therapies.
Keywords: ANCA, glomerulonephritis, immunosuppression, outcomes, relapse
INTRODUCTION
In the 1970s, the addition of cyclophosphamide to glucocorticoid therapy for the treatment of small-vessel vasculitis resulted in a dramatic reduction in mortality in what was then an often fatal disease [1]. Cyclophosphamide has remained the mainstay of induction therapy [2–5] for what is now called antineutrophil cytoplasmic autoantibody (ANCA)-associated vasculitis (AAV). Because ANCA are pathogenic, there has been much interest in the use of anti-B-cell drugs in AAV. Studies reporting efficacy of rituximab in treatment-resistant AAV began over a decade ago [6–11]. More confidence in the effectiveness of rituximab came with two randomized control trials demonstrating the non-inferiority of rituximab to cyclophosphamide for induction therapy [12–14]. There are studies that support the use of rituximab with cyclophosphamide therapy for induction of remission [12, 15] and other literature that supports the use of rituximab and corticosteroids without cyclophosphamide [13, 14].
In 2003, our vasculitis center aimed to enroll at least 100 consecutive patients in whom rituximab was being administered into a cohort study. While randomized trials are essential for comparisons of treatment regimens, this type of inception cohort has the advantage of including those from the ‘real world’ and permits follow-up from the time of initial diagnosis until death. In this setting, we present data on the types of patients receiving rituximab, methods of administration and associated outcomes and adverse events in this cohort of patients with AAV.
MATERIALS AND METHODS
Study population
Patients from the Glomerular Disease Collaborative Network (GDCN) inception cohort [16–18] were included in this study if they had AAV and received rituximab at any point during their treatment course. The GDCN is comprised of private practice and tertiary medical center nephrologists primarily located in North Carolina and throughout the southeastern United States. We aimed to prospectively study at least 100 consecutive patients in whom rituximab was given beginning 15 March 2003. We enrolled and followed 120 patients through 9 October 2012. Entry criteria for this inception cohort have been previously described [16, 17]. Patients required diagnostic biopsy and/or a positive ANCA determination by immunofluorescence (IF) microscopy or antigen-specific, enzyme-linked immunosorbent assay (ELISA) and/or a strong clinical indication of AAV [19], as well as treatment at any time during their course with rituximab. Age and peak serum creatinine were collected at the time of initial diagnosis. Patients who had end-stage kidney disease (ESKD) at the time of presentation were excluded from this study.
Diagnostic disease categories were defined according to the modified Chapel Hill Consensus Conference nomenclature [20–23]. A diagnosis of granulomatosis with polyangiitis (GPA), microscopic polyangiitis (MPA), eosinophilic granulomatosis with polyangiitis, or pauci-immune necrotizing and/or crescentic glomerulonephritis (GN) without overt signs of systemic vasculitis were determined by previously described criteria [16, 17]. Definitions of remission, relapse and treatment resistance have been previously described [24]. Specifically, remission was defined as the absence of dysmorphic red blood cells and red blood cell casts on microscopic urinalysis or findings of focal sclerosing glomerulopathy without active crescents or necrosis on kidney biopsy and the absence of extra-renal manifestations of vasculitis. Proteinuria alone was not considered indicative of active GN. Severe infections were defined as any infection requiring intravenous antibiotics, hospitalization or resulting in an infection-related death. ‘Concurrent’ cyclophosphamide was defined as rituximab and cyclophosphamide both given for active disease prior to reaching a state of remission. ‘Previous’ cyclophosphamide was defined as administration of cyclophosphamide followed by achieving a state of remission. Rituximab administration was given only after achieving a state of remission in previous cyclophosphamide.
Patient participation was approved by the University of North Carolina Institutional Review Board (IRB), with informed consent provided by all patents for long-term follow-up.
Patient groups
Rituximab was used in 120 patients in a variety of situations including with or without cyclophosphamide or other immunosuppressive drugs at the time of induction or disease relapse, because of cyclophosphamide resistance, or with the aim to maintain disease remission (Figure 1). To understand differences in those who did and did not receive rituximab, we compared the rituximab-treated patients with 351 AAV patients enrolled in our inception cohort since 1991 who were treated with cyclophosphamide but who never received rituximab.
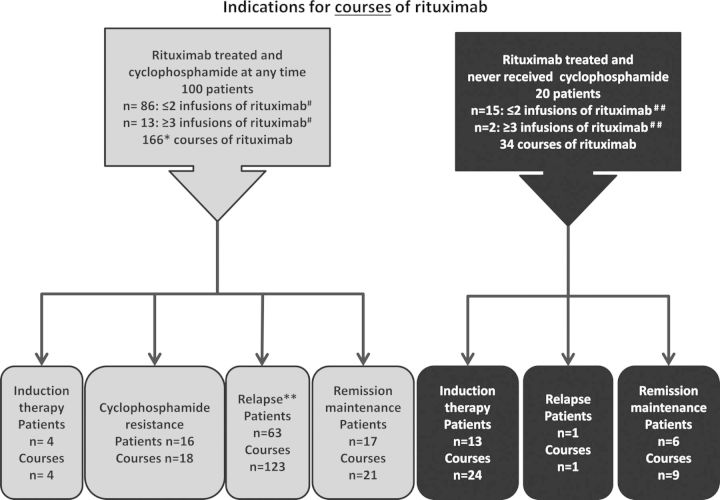
FIGURE 1:
Treatment groups. Single asterisk denotes 65 of the 166 courses of rituximab were given during the same flare as cyclophosphamide. Double asterisk denotes in 43 of the courses of rituximab, cyclophosphamide was co-administered for treatment of disease relapse. In 80 of the courses, rituximab was given for disease flare without concurrently giving cyclophosphamide. Hash denotes information included on first course of rituximab only. Data missing on one person in this group. Double hash denotes information included on first course of rituximab only. Data missing on three people in this group.
Therapy
All therapeutic immunosuppressive interventions were recorded. Patients who had received at least 1 infusion of rituximab were considered to have been treated with rituximab. Patients who received at least 1 intravenous (IV) dose of cyclophosphamide or 1 month of oral cyclophosphamide were considered to have been treated with cyclophosphamide.
Rituximab was administered according to two separate dosing regimens based on physician preference: 1 g of rituximab on Days 1 and 15, or 375 mg/m2 body surface area weekly for 4 weeks.
Statistical analysis
Descriptive statistics included the number (n), percentage (%), mean and standard deviation (SD) [or median and interquartile range (IQR)]. Comparisons between groups used Fisher's exact tests for categorical measures and Wilcoxon rank-sum tests or Kruskal–Wallis tests for continuous measures. Incidence rates were reported for relapse and infection as per patient-year of follow-up using all events, with a Poisson distribution used to calculate 95% confidence intervals (95% CI) and SDs. Kaplan–Meier estimators were used to plot the univariate probability of time to each outcome. Time ‘0’ was defined as the date of first rituximab infusion or start date of any therapy, as appropriate. Proportional hazards models were used for multivariable modeling of time to ESKD or death, with hazards ratio, 95% CI and P values presented. Analyses were conducted using SAS software (version 9.3 SAS Institute, Cary, NC). Exact P values were reported with a two-sided P value of <0.05 considered statistically significant.
RESULTS
Demographics
In the 120 patients who received rituximab, 166 courses were given to 100 patients who had previously or concurrently received cyclophosphamide [for a median of 6 months cyclophosphamide (range 1–45 months; IQR 4.5–11.1 months)], and 34 courses of rituximab were given in 20 patients who had no previous or concurrent exposure to cyclophosphamide (Figure 1).
Table 1 presents demographics of those who received rituximab without exposure to cyclophosphamide throughout treatment course (n = 20), those who were treated with rituximab who were also treated with cyclophosphamide at any time (n = 100) and those who were treated with cyclophosphamide and never received rituximab (n = 351). Compared with cyclophosphamide-treated patients who never received rituximab, patients who received rituximab were a decade younger, more likely to be diagnosed with GPA, and had more disease involvement of the upper airways but less of the kidney. Patients who received rituximab were followed almost twice as long as those who never received rituximab (almost 5 versus 2.5 years, P < 0.0001) and were also generally exposed to more months of any type of immunosuppressant. Although longer duration of therapy is expected with longer follow-up, in the rituximab-treated patients, more treatment likely also represents the need for management of ongoing or recurring disease activity. Because of the many differences between those who did and did not receive rituximab, further comparisons of the impact of treatment on outcomes between these non-randomized treatment groups was not informative and is, therefore, not presented.
Table 1.
Demographics
| Presented as percent unless otherwise noted | Rituximab, no cyclophosphamide (n = 20) | Rituximab + cyclophosphamide (n = 100) | Cyclophosphamide (no rituximab) (n = 351) | P value* |
|---|---|---|---|---|
| Age (years), median (IQR) | 50 (38, 64) | 50 (35, 58) | 60 (47, 71) | <0.001 |
| Female | 75% | 49% | 46% | 0.81 |
| Race | 0.99 | |||
| White | 79% | 83% | 86% | |
| Black | 16% | 8% | 9% | |
| Other | 5% | 9% | 5% | |
| ANCA | 0.12 | |||
| MPO/pANCA | 15% | 42% | 56% | |
| PR3/cANCA | 40% | 56% | 42% | |
| Both PR3 and MPO | 5% | 1% | 0 | |
| ANCA positive (MPO) | 0% | 2% | 1% | |
| PR3 not known | ||||
| ANCA negative | 40% | 10% | 1% | |
| Disease category | 0.001 | |||
| MPA | 40% | 44% | 54% | |
| GPA | 55% | 45% | 22% | |
| GN | 5% | 9% | 23% | |
| EGPA | 0% | 2% | 1% | |
| Lung involvement | 10% | 57% | 51% | 0.23 |
| ENT involvement | 35% | 53% | 35% | 0.01 |
| Kidney involvement | 45% | 85% | 97% | 0.007 |
| Creatinine peak at diagnosis | 1.2 (1.0, 2.0) | 1.9 (1.1, 2.9) | 3.4 (2.0, 5.4) | 0.0001 |
| Ever treated with prednisone; Median months (IQR) |
95% 6 (4, 11) |
96% 12 (7, 25) |
96% 10 (5, 20) |
0.95 0.04 |
| Ever treated with IV CYC; Median months (IQR) |
0% NA |
83% 5 (4.7) |
66% 5 (2, 6) |
0.01 0.009 |
| Ever treated with Oral CYC; Median months (IQR) |
0% NA |
48% 7 (4, 12) |
54% 8 (3, 14) |
0.43 0.72 |
| Ever treated CYC (IV or oral); Median months (IQR) |
0% NA |
100% 7 (5, 13) |
100% 6 (3, 12) |
NA 0.09 |
| Ever treated with MMF; Median months (IQR) |
80% 20 (5, 30) |
72% 22 (4, 56) |
29% 11 (4,24) |
0.001 0.04 |
| Ever treated with AZA; Median months (IQR) |
50% 10 (2, 24) |
54% 12(2, 27) |
29% 7 (2, 18) |
0.01 0.39 |
| Ever treated with methylprednisolone; Median months (IQR) |
85% 4 (3, 6) |
87% 5 (3, 6) |
70% 3 (3, 3) |
0.01 0.0001 |
| Follow-up since first day of any immunosuppression median (IQR) (months) | 18 (7, 49) | 59 (40, 104) | 31 (12, 59) | 0.001 |
IQR, interquartile range; MPO, myeloperoxidase; ANCA, antineutrophil cytoplasmic antibody; PR3, proteinase 3; MPO, microscopic polyangiitis; GPA, granulomatosis with polyangiitis; GN, pauci-immune necrotizing and/or crescentic glomerulonephritis; EGPA, eosinophilic granulomatosis with polyangiitis; ENT, ear, nose and throat; CYC, cyclophosphamide; MMF, mycophenolate mofetil; AZA, azathioprine; CI, confidence interval; NA, not applicable.
*P values compare all rituximab treated (n = 120) to those who never received rituximab (n = 351) and were calculated by Fisher's exact test for categorical variables and Wilcoxon two-sample test for continuous variables.
Rituximab therapy administration
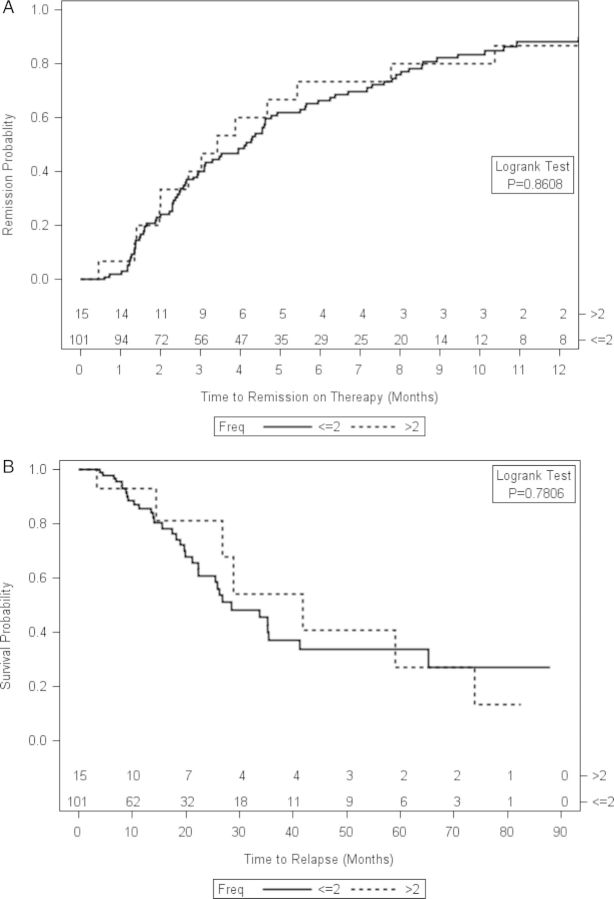
Of the 120 rituximab-treated patients, the first course was intended to be administered as 1 g on Days 1 and 15 in 101 patients, as 375 mg/m2 weekly for four doses in 15 patients, and the number of doses was undocumented in 5 patients. Infusion numbers of ≤2 infusions versus >2 infusions were compared with account for the few patients who did not receive all intended infusions. Time to remission and time to relapse were similar in the two dosing regimens (remission P = 0.8608, Figure 2A; relapse P = 0.7806, Figure 2B).
FIGURE 2:
Frequency of rituximab dosing does not affect time to remission or time to relapse. Shown are Kaplan–Meier curves depicting time to remission on therapy for patients who received 2 infusions of rituximab compared with patients who received >2 infusions of rituximab (A) or time to relapse for patients who received 2 infusions of rituximab compared with patients who received >2 infusions of rituximab (B). The number of doses of rituximab was undocumented in four patients.
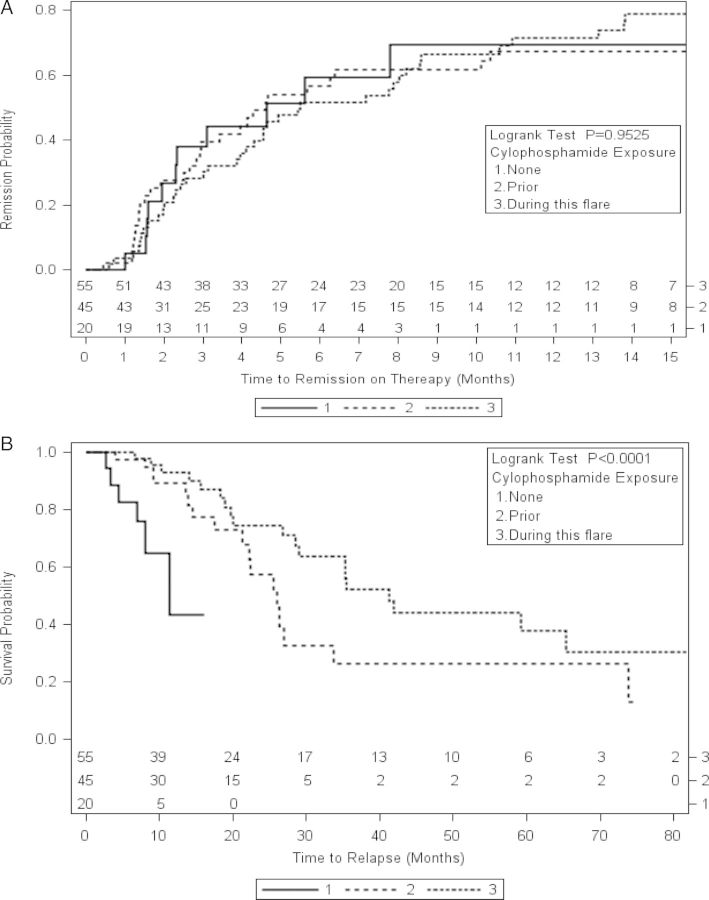
Sixty-five (33%) of the 200 treatments of rituximab were given concurrently with cyclophosphamide. Thirty-four (17%) were given without prior exposure to cyclophosphamide. Ninety-nine (50%) of the courses of rituximab were given with a patient history of prior cyclophosphamide exposure but without concurrent cyclophosphamide exposure. Time to relapse was shorter in patients who had never been exposed cyclophosphamide (Figure 3). Patients who received rituximab but did not receive cyclophosphamide included more female patients, more ANCA-negative patients and less PR3-ANCA patients, with less renal and more pulmonary involvement. The patients who only received rituximab had less of the traditional risk factors for relapse (PR3-ANCA and pulmonary involvement). There was no difference in the months of steroids preceding remission or relapse in the groups (Table 2). Twenty-one courses of rituximab were given to 17 patients for remission maintenance (Figure 1).
FIGURE 3:
Kaplan–Meier curve: time to relapse following first course of rituximab evaluating by exposure to cyclophosphamide.
Table 2.
Characteristics by exposure to cyclophosphamide (CTX) (n = 120)
| Variables | Exposure to CTX |
P values* | ||
|---|---|---|---|---|
| No CTX (n = 20) | Prior CTX (n = 43) | Concurrent CTX (n = 55) | ||
| Sex | 0.004 | |||
| Female | 75% | 60% | 38% | |
| ANCA Type | <0.0001 | |||
| MPO/P—ANCA | 15% | 56% | 31% | |
| PR3/C—ANCA | 40% | 44% | 67% | |
| ANCA negative | 40% | 0% | 2% | |
| PR3- and MPO-ANCA2 | 5% | 0% | 0% | |
| Organ | ||||
| Lung | 10% | 51% | 62% | 0.0002 |
| ENT | 35% | 49% | 55% | 0.34 |
| Kidney | 45% | 73% | 64% | 0.99 |
| Months of prednisone prior to remission, n/N | 13/20 | 27/43 | 34/55 | 0.11 |
| Median (IQR) | 4.8 (3, 8.9) | 10.9 (9, 30) | 10.5 (3.9, 19) | |
| Months of prednisone prior to first relapse, n/N | 6/20 | 16/43 | 19/55 | 0.54 |
| Median (IQR) | 6.4 (5.7, 18.5) | 14.6 (6.7, 38.5) | 15 (3.6, 26) | |
| Deaths | 5% | 5% | 9% | 0.63 |
CTX, cyclophosphamide; ANCA, antineutrophil cytoplasmic autoantibodies; MPO, myeloperoxidase; PR3, proteinase 3; P, perinuclear, cytoplasmic; C, cytoplasmic; IQR, interquartile range.
*P values were calculated by Fisher's exact test for categorical variables and Wilcoxon two-sample test for continuous variables.
Of the 200 courses of rituximab, 6 courses of rituximab did not result in B-cell depletion (rituximab dosed as 1 g given ×1 in one case, 1 g given ×2 in four cases and 375 mg/m2 weekly ×4 in one case). B cells were not tested for depletion in 24 cases.
Remission and relapse following rituximab therapy
Of the 120 patients who received rituximab, 103 (86%) achieved remission with 42 (41%) having a subsequent relapse in a median of 19 (9, 25) months. Of the patients who achieved remission with rituximab therapy 3% required an additional course of rituximab to finally achieve adequate remission. There was no difference in time to remission or time to relapse between MPO-ANCA, PR3-ANCA and ANCA-negative patients (remission P = 0.43; relapse P < 0.08), suggesting a trend for the ANCA-negative patients to relapse sooner. In ANCA-positive patients, ANCA titers increased more than 5 units/mL at the time of relapse from prior levels in 64/130 (49%) cases. ANCA titers were stable or decreased from prior levels at the time of relapse in 66/130 (51%) cases.
Rituximab was originally given for four indications: 17 (14%) were treated for new onset disease, 18 (15%) were given rituximab for new onset disease that was treatment resistant to cyclophosphamide, 70 (58%) received rituximab for relapse and 14 (12%) were given rituximab for relapse treatment resistant to cyclophosphamide (Table 3). One patient received a first course of rituximab for remission maintenance after having been given cyclophosphamide for induction therapy and then found to be unable to tolerate azathioprine or mycophenolate mofetil (MMF) for remission maintenance (excluded from Table 3). When comparing the groups of patients who received their first course of rituximab for differing indications, there was a higher proportion of males who received rituximab for new diagnosis of AAV than for treatment resistance to cyclophosphamide (Table 3). Race and disease category were not different across the groups receiving rituximab for differing reasons (Table 3) but there was a greater proportion of patients with PR3-ANCA and lung involvement who received rituximab for a relapse than for treatment resistance to cyclophosphamide (Table 3). There was no significant difference across groups in the percentage of patients achieving remission on therapy or complete remission off therapy, time to remission on therapy or complete remission off therapy, or the percentage who had disease relapse (Table 3). In the three patients who received rituximab for induction therapy and subsequently relapsed, there was a significantly shorter time to relapse than the time to relapse for patients who received rituximab for other indications (mean 4 ± 1 months, P = 0.012) (Table 3).
Table 3.
Indication and outcome for first treatment with rituximab (n = 119a)
| New diagnosis, rituximab induction (n = 17) | New diagnosis, CYC resistance (n = 18) | Relapse, rituximab (n = 70) | Relapse, CYC resistance (n = 14) | *P value | |
|---|---|---|---|---|---|
| Female | 10 (59%) | 4 (22%) | 42 (60%) | 8 (57%) | 0.03 |
| MPO/pANCA | 1 (6%) | 7 (39%) | 31 (44%) | 5 (36%) | 0.0006 |
| PR3/cANCA | 8 (50%) | 9 (50%) | 37 (53%) | 9 (64%) | |
| ANCA negative | 6 (38%) | 1 (6%) | 2 (3%) | 0 | |
| Both PR3 and MPO | 1 (6%) | 1 (6%) | 0 | 0 | |
| Organ | |||||
| Kidney | 10 (59%) | 16 (89%) | 57 (81%) | 10 (71%) | 0.13 |
| Lung | 3 (18%) | 9 (50%) | 37 (53%) | 10 (71%) | 0.02 |
| ENT | 6 (35%) | 5 (28%) | 40 (57%) | 8 (57%) | 0.08 |
| Remission off therapy | 3 (18%) | 2 (11%) | 15 (21%) | 1 (7%) | 0.62 |
| Time to remission off therapy |
n = 3 7 ± 5 9 (1, 10) |
n = 2 9 ± 5 9 (6, 13) |
n = 15 13 ± 13 8 (4, 22) |
n = 1 16 16 |
0.68 |
| Relapse | 3 (18%) | 4 (22%) | 30 (43%) | 6 (43%) | 0.14 |
| Time to relapse |
n = 3 4 ± 1 3 (3, 5) |
n = 4 21 ± 10 18 (15, 28) |
n = 30 23 ± 17 20 (9, 27) |
n = 6 31 ± 10 32 (20, 41) |
0.01 |
| Time to last follow-up without relapse, death, ESKD |
n = 11 9 ± 10 5 (3, 12) |
n = 13 28 ± 24 21 (16, 30) |
n = 36 18 ± 31 15 (7, 24) |
n = 6 31 ± 37 16 (2, 60) |
0.08 |
MPO, myeloperoxidase; ANCA, antineutrophil cytoplasmic antibody; PR3, proteinase 3; ENT, ear, nose and throat; ESKD, end-stage kidney disease.
*P values were calculated by Fisher's exact test for categorical variables and Wilcoxon two-sample test for continuous variables.
aOne patient was given first course of rituximab as remission maintenance, so the table includes 119 instead of 120 patients.
Twenty-one patients went into complete remission and came off all therapy; of these, all had documented B-cell return. There were no distinguishing features among these patients in terms of type of ANCA or organ involvement.
Multiple courses of rituximab for treatment of relapse
Of the 45 patients who had a second treatment with rituximab, 38 (84%) achieved remission again and 12 (32%) subsequently relapsed in a median of 20 (12, 24) months. The median time between the first and second rituximab courses was 19 months (IQR 11, 31). Thirty-three (73%) had no further relapse over the median follow-up time of 9 (5, 16) months. Five (11%) of the 45 achieved complete remission.
A third course of rituximab was given to 18 patients. Fourteen (78%) achieved remission on therapy and 3 (17%) achieved complete remission and stopped all therapy. Six patients relapsed following the third rituximab course in a median of 22 (16, 33) months. A fourth course of rituximab was given to 10 patients, a fifth course to 3 patients, a sixth course to 2 patients and 1 person had 8 courses of rituximab. The median time between rituximab courses over all was 20 months (IQR 11, 29).
In all patients who received repeated rituximab courses, the dose given was 1 g ×2 separated by 14 days except for the following: two instances of the second course of rituximab given 1 g ×1, one instance in a third course of rituximab when 375 mg/m2 was given weekly ×4 and once when 1 g was given ×1, one instance in a fourth course of rituximab when 1 g was given ×1 and one instance in a sixth course of rituximab when 1 g was given ×1.
B-cell repopulation data were available in 50 patients and 66 courses of rituximab used following previous rituximab administration. In 58/66 courses, B-cell repopulation was documented. In all of these courses, relapse occurred in a median of 10.5 (IQR 5, 19.5) months following B-cell repopulation date. Relapses occurred in 8/66 instances when B cells were documented as being depleted.
Immunosuppression in addition to rituximab
Median months of total prednisone treatment in the cohort was 11 (5, 25) months which included prednisone given with induction therapy and for treatment of relapse. One hundred and four (87%) patients were at some point treated with methylprednisolone with a median of 5 (3, 6) infusions. One hundred (83%) patients were exposed to cyclophosphamide for a median of 7 (5, 13) months. Ninety (75%) patients were treated for a median of 21 (5, 55) months with MMF and 64 (53%) patients received a median of 8 (2, 26) months of azathioprine.
In the time period following a patient's first rituximab infusion, 72 (60%) patients were treated for a median of 5 (3, 13) months with prednisone; 33 (28%) patients were treated for a median of 3 (1, 5) months with cyclophosphamide; 38 (32%) patients were treated for a median of 20 (4, 48) months with MMF; and 34 (28%) patients were treated for a median of 6 (1, 26) months with azathioprine.
Adverse events
Adverse events following immunosuppression with rituximab or rituximab and cyclophosphamide are listed in Table 4. Follow-up was different between these two groups (Table 1). Infectious prophylaxis therapy was given in conjunction with rituximab in 52/120 (43%) of patients and trimethoprim sulfamethaxazole was used almost exclusively. Of the 120 rituximab-treated patients, 18 (15%) had no infections and 102 (85%) patients experienced a total of 410 infections. Most common infections included upper respiratory (29% of all infections) and pulmonary (27%), followed by urinary tract/pyelonephritis (11%), systemic or organ threatening (7%) and viral infections (5%).
Table 4.
Adverse events
| Rituximab, no cyclophosphamide (n = 20) | Rituximab + cyclophosphamide (n = 100) | P value | |
|---|---|---|---|
| Incidence of relapse (per person-year) 95% CI | 0.22 (0.14, 0.31) | 0.21 (0.18, 0.24) | 0.89 |
| Incidence of infection (per person-year) 95% CI | 0.21 (0.15, 0.28) | 0.13 (0.12, 0.15) | 0.08 |
| Incidence of severe infection (per person-year) 95% CI | 0.12 (0.08, 0.24) | 0.17 (0.13, 0.22) | 0.63 |
| Cancera, n (%) | 0 | 18 (18%) | 0.04 |
| MI, n (%) | 0 | 0 | 0.99 |
| CVA, n (%) | 0 | 1 (1%) | 0.99 |
| ESKD, n (%) | 1 (5%) | 9 (9%) | 0.99 |
| Death, n (%) | 1 (5%) | 7 (7%) | 0.99 |
| ESKD or death (%) | 1 (5%) | 14 (14%) | 0.46 |
*P values were calculated by Fisher's exact test for categorical variables and Wilcoxon two-sample test for continuous variables.
CI, confidence interval; MI, myocardial infarction; CVA, cerebrovascular accident, ESKD, end-stage kidney disease.
aIncludes non-melanoma skin cancers.
Of the 120 rituximab-treated patients, there were 18 cancers following immunosuppression: 7 non-melanoma skin cancers, 1 gynecologic, 2 colon, 2 bladder, 2 hematologic, 1 prostate and 3 renal cell carcinomas (Table 4).
Among the 120 patients who received rituximab, late-onset neutropenia, defined as an absolute neutrophil count ≤1.5 × 109 [26] was noted in 16 (13%) patients with a mean onset of 226 days following first rituximab infusion and a range of 60–1149 days. Only 2 of these 16 patients had neutropenia >1 year following first rituximab dose.
DISCUSSION
Over 10 years of experience prescribing rituximab in a variety of clinical circumstances for treatment and management of AAV shows us that the addition of rituximab to the armamentarium of therapies aimed at controlling disease is associated with reasonable safety profiles and beneficial effects. Our experience highlights the multiple uses of rituximab in AAV including as primary immunosuppressant for induction, in combination with cyclophosphamide as induction, as primary immunosuppressant for relapse, in combination with cyclophosphamide for relapse and as rescue therapy in cyclophosphamide-resistant disease.
This cohort is unique in its inclusion of such varying patient presentations. Our data reflect no difference in time to remission and time to relapse regardless of whether clinicians used ≤2 infusions of rituximab or >2 infusions of rituximab for any given course. This has been an area of debate, specifically regarding whether greater number of infusions allow for improved B-cell depletion and longer time to relapse but, in our population, this does not appear to be the case. Presence of MPO-ANCA, PR3-ANCA or being ANCA-negative did not impact time to remission or relapse.
Rituximab therapy in this study was administered in a variety of circumstances during a time when clinicians were still understanding how best to use this drug for AAV. It is now known that rituximab is effective for induction therapy [12–14] and relapse treatment [13] in AAV. However, relapse is common following rituximab regardless of whether other immunosuppression is withdrawn or maintained [11]. Due to having only 20 patients who received rituximab without any exposure to cyclophosphamide, this study has limited ability to compare the impact of rituximab treatment in the absence of cyclophosphamide therapy to treatment with both. Of note, the cohort who never received cyclophosphamide had less PR3-ANCA, more ANCA-negative patients and less lung involvement but similar mortality.
The goal of treatment of AAV is to improve patient outcomes while limiting therapy-related adverse events. There are treatment regimens published which suggest effective use of rituximab and cyclophosphamide together for achieving induction of remission [12, 15]. Our data support this concept although this study was not designed to compare treatment strategies, and there are important differences in our studied groups. Although we do not have an established protocol for the co-administration of cyclophosphamide and rituximab, the results of our study suggest the possibility of a therapeutic approach in patients with AAV in which cyclophosphamide and rituximab could be combined to broaden the disease modifying immunosuppression while limiting toxicity by dosage reduction of each given therapy. This concept has been supported previously in the literature in a report of a rituximab based low-dose cyclophosphamide regimen which was effective in achieving long-term remission in AAV [27].
Although reports have been made in other retrospective studies and case reports of the effectiveness and safety of rituximab in AAV [15, 25, 28, 29], our study was unique in that it has close to 10 years of follow-up on a diverse patient group (MPA and GPA, PR3- and MPO-ANCA-positive as well as ANCA-negative patients). The strength of an inception cohort study design is that a real-world clinical setting is utilized without exclusion criteria, as is needed in a conventional treatment trial. This can also be interpreted as a weakness, given the heterogeneous population with a variety of disease presentations, levels of severity and differences in exposure to immunosuppression outside of rituximab. For example, rituximab is becoming accepted by a growing number of clinicians as a means of lowering glucocorticoid exposure and speeding complete glucocorticoid discontinuation, but this was difficult to study in our inception cohort as we do not have total glucocorticoid dose in all patients. Likewise, we could not evaluate the impact of rituximab administration on lowering dosage of other immunosuppressants.
This study supports future development of multi-drug treatment strategies in patients with vasculitis and randomized control trials to evaluate the effectiveness and adverse event profiles of these multi-drug regimens compared with rituximab only or cyclophosphamide only immunosuppressant strategies. This study adds to mounting evidence that rituximab and cyclophosphamide can be used together for successful treatment of AAV in patients with all varieties of disease categories, ANCA specificities and disease presentations, including severe renal injury. In this heterogenous population, use of cyclophosphamide and rituximab in sequence or in combination as multi-drug strategies had reasonable safety profiles and was therapeutically efficacious. Randomized control trials are required to prove if multi-drug strategies are more or less beneficial than single-drug strategies.
In conclusion, rituximab has been used effectively in a real-world population of AAV with MPO-ANCA, PR3-ANCA and ANCA-negative disease for over 10 years. It has been shown to be effective for new disease induction therapy both by itself (along with corticosteroids) and in combination with cyclophosphamide and corticosteroids, as relapse therapy in similar combinations, for cyclophosphamide-resistant disease and for remission maintenance therapy. The use of 375 mg/m2 gives no advantage with respect to achieving remission or preventing relapse compared with 1 g ×2 separated by 14 days. Rituximab and cyclophosphamide given during active disease prior to attaining remission may allow for more sustained remission than use of rituximab without cyclophosphamide. There is a population of patients who do not relapse following rituximab administration and achieve remission off of immunosuppressive therapy.
CONFLICT OF INTEREST STATEMENT
The results presented in this paper have not been published previously in whole or part, except in abstract format.
REFERENCES
- 1.Fauci AS, Haynes B, Katz P. The spectrum of vasculitis: clinical, pathologic, immunologic and therapeutic considerations. Ann Intern Med 1978; 89: 660–676 [DOI] [PubMed] [Google Scholar]
- 2.Harper L, Morgan MD, Walsh M, et al. Pulse versus daily oral cyclophosphamide for induction of remission in ANCA-associated vasculitis: long-term follow-up. Ann Rheum Dis 2012; 71: 955–960 [DOI] [PubMed] [Google Scholar]
- 3.de Groot K, Harper L, Jayne DR, et al. Pulse versus daily oral cyclophosphamide for induction of remission in antineutrophil cytoplasmic antibody-associated vasculitis: a randomized trial. Ann Intern Med 2009; 150: 670–680 [DOI] [PubMed] [Google Scholar]
- 4.Rihova Z, Jancova E, Merta M, et al. ANCA-associated renal vasculitis—epidemiology, diagnostics and treatment. Prague Med Rep 2004; 105: 237–260 [PubMed] [Google Scholar]
- 5.Haubitz M, Schellong S, Gobel U, et al. Intravenous pulse administration of cyclophosphamide versus daily oral treatment in patients with antineutrophil cytoplasmic antibody-associated vasculitis and renal involvement: a prospective, randomized study. Arthritis Rheum 1998; 41: 1835–1844 [DOI] [PubMed] [Google Scholar]
- 6.Brihaye B, Aouba A, Pagnoux C, et al. Adjunction of rituximab to steroids and immunosuppressants for refractory/relapsing Wegener's granulomatosis: a study on 8 patients. Clin Exp Rheumatol 2007; 25: S23–S27 [PubMed] [Google Scholar]
- 7.Eriksson P. Nine patients with anti-neutrophil cytoplasmic antibody-positive vasculitis successfully treated with rituximab. J Intern Med 2005; 257: 540–548 [DOI] [PubMed] [Google Scholar]
- 8.Roccatello D, Baldovino S, Alpa M, et al. Effects of anti-CD20 monoclonal antibody as a rescue treatment for ANCA-associated idiopathic systemic vasculitis with or without overt renal involvement. Clin Exp Rheumatol 2008; 26: S67–S71 [PubMed] [Google Scholar]
- 9.Stasi R, Stipa E, Poeta GD, et al. Long-term observation of patients with anti-neutrophil cytoplasmic antibody-associated vasculitis treated with rituximab. Rheumatology (Oxford) 2006; 45: 1432–1436 [DOI] [PubMed] [Google Scholar]
- 10.Lovric S, Erdbruegger U, Kumpers P, et al. Rituximab as rescue therapy in anti-neutrophil cytoplasmic antibody-associated vasculitis: a single-centre experience with 15 patients. Nephrol Dial Transplant 2009; 24: 179–185 [DOI] [PubMed] [Google Scholar]
- 11.Jones RB, Ferraro AJ, Chaudhry AN, et al. A multicenter survey of rituximab therapy for refractory antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum 2009; 60: 2156–2168 [DOI] [PubMed] [Google Scholar]
- 12.Jones RB, Tervaert JW, Hauser T, et al. Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis. N Engl J Med 2010; 363: 211–220 [DOI] [PubMed] [Google Scholar]
- 13.Stone JH, Merkel PA, Spiera R, et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med 2010; 363: 221–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Specks U, Merkel PA, Seo P, et al. Efficacy of remission-induction regimens for ANCA-associated vasculitis. N Engl J Med 2013; 369: 417–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pendergraft WF, III, Cortazar FB, Wenger J, et al. Long-term maintenance therapy using rituximab-induced continuous B-cell depletion in patients with ANCA vasculitis. Clin J Am Soc Nephrol 2014; 9: 736–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nachman PH, Hogan SL, Jennette JC, et al. Treatment response and relapse in antineutrophil cytoplasmic autoantibody-associated microscopic polyangiitis and glomerulonephritis. J Am Soc Nephrol 1996; 7: 33–39 [DOI] [PubMed] [Google Scholar]
- 17.Hogan SL, Nachman PH, Wilkman AS, et al. Prognostic markers in patients with antineutrophil cytoplasmic autoantibody-associated microscopic polyangiitis and glomerulonephritis. J Am Soc Nephrol 1996; 7: 23–32 [DOI] [PubMed] [Google Scholar]
- 18.Hogan SL, Falk RJ, Chin H, et al. Predictors of relapse and treatment resistance in antineutrophil cytoplasmic antibody-associated small-vessel vasculitis. Ann Intern Med 2005; 143: 621–631 [DOI] [PubMed] [Google Scholar]
- 19.Hagen EC, Andrassy K, Chernok E, et al. The value of indirect immunofluorescence and solid phase techniques for ANCA detection. A report on the first phase of an international cooperative study on the standardization of ANCA assays. EEC/BCR Group for ANCA Assay Standardization. J Immunol Methods 1993; 159: 1–16 [DOI] [PubMed] [Google Scholar]
- 20.Jennette JC, Falk RJ, Andrassy K, et al. Nomenclature of systemic vasculitides. Proposal of an international consensus conference. Arthritis Rheum 1994; 37: 187–192 [DOI] [PubMed] [Google Scholar]
- 21.Falk RJ, Gross WL, Guillevin L, et al. Granulomatosis with Polyangiitis (Wegener's): an alternative name for Wegener's Granulomatosis. Arthritis Rheum 2011; 63: 863–864 [DOI] [PubMed] [Google Scholar]
- 22.Falk RJ, Gross WL, Guillevin L, et al. Granulomatosis with polyangiitis (Wegener's): an alternative name for Wegener's granulomatosis. Ann Rheum Dis 2011; 70: 704. [DOI] [PubMed] [Google Scholar]
- 23.Falk RJ, Gross WL, Guillevin L, et al. Granulomatosis with polyangiitis (Wegener's): an alternative name for Wegener's granulomatosis. J Am Soc Nephrol 2011; 22: 587–588 [DOI] [PubMed] [Google Scholar]
- 24.McGregor JG, Falk RJ. Vasculitis: the elusive optimal induction strategy for vasculitis. Nat Rev Nephrol 2012; 8: 195–196 [DOI] [PubMed] [Google Scholar]
- 25.Rhee EP, Laliberte KA, Niles JL. Rituximab as maintenance therapy for anti-neutrophil cytoplasmic antibody-associated vasculitis. Clin J Am Soc Nephrol 2010; 5: 1394–1400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tesfa D, Ajeganova S, Hagglund H, et al. Late-onset neutropenia following rituximab therapy in rheumatic diseases: association with B lymphocyte depletion and infections. Arthritis Rheum 2011; 63: 2209–2214 [DOI] [PubMed] [Google Scholar]
- 27.Mansfield N, Hamour S, Habib AM, et al. Prolonged disease-free remission following rituximab and low-dose cyclophosphamide therapy for renal ANCA-associated vasculitis. Nephrol Dial Transplant 2011; 26: 3280–3286 [DOI] [PubMed] [Google Scholar]
- 28.Roll P, Ostermeier E, Haubitz M, et al. Efficacy and safety of rituximab treatment in patients with antineutrophil cytoplasmic antibody-associated vasculitides: results from a German registry (GRAID). J Rheumatol 2012; 39: 2153–2156 [DOI] [PubMed] [Google Scholar]
- 29.Cartin-Ceba R, Golbin JM, Keogh KA, et al. Rituximab for remission induction and maintenance in refractory granulomatosis with polyangiitis (Wegener's): ten-year experience at a single center. Arthritis Rheum 2012; 64: 3770–3778 [DOI] [PubMed] [Google Scholar]