Abstract
Neurointervention is a rapidly evolving and complex field practiced by clinicians with backgrounds ranging from neurosurgery to radiology, neurology, cardiology, and vascular surgery. New devices, techniques, and clinical applications create exciting opportunities for impacting patient care, but also carry the potential for new iatrogenic injuries. Every step of every neurointerventional procedure carries risk, and a thorough appreciation of potential complications is fundamental to maximizing safety. This article presents the most frequent and dangerous iatrogenic injuries, their presentation, identification, prevention, and management.
Keywords: interventional radiology, neuroradiography, cerebral angiography, complications, iatrogenic disorders
Objectives: Upon completion of this article, the reader will be able to discuss the clinical presentation, imaging, and management of complications arising from neurointerventional procedures.
Accreditation: This activity has been planned and implemented in accordance with the Essential Areas and Policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint providership of Tufts University School of Medicine (TUSM) and Thieme Medical Publishers, New York. TUSM is accredited by the ACCME to provide continuing medical education for physicians.
Credit: Tufts University School of Medicine designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Adverse outcomes nearly always follow a cascade of minor deviations from standard practice, and seldom result from a single error.1 2 In most cases a series of events must occur, in the correct order, at the correct time, for an iatrogenic injury to occur. Conversely, disasters may be avoided by identifying components of this cascade early on and initiating the appropriate response. Each neurointerventional procedure carries unique risks which, when anticipated, can frequently be avoided. The most frequent and dangerous of these risks are discussed below. The reader is encouraged to read pertinent sections of this article immediately prior to neurointerventional procedures.
Complications of Diagnostic Angiography
Cerebral Angiography
Diagnostic cerebral angiography remains the gold standard for imaging cerebral vasculature and is also the first step in performing neurointerventional procedures. The most common complications are thromboembolism and air embolism from catheters and wires resulting in cerebral ischemia. Disruption of atherosclerotic plaques and arterial dissection are additional potential causes of ischemic events. Ischemic stroke occurring due to cerebral angiography may take the form of a large vessel occlusion or a small, distal arterial branch occlusion. Recent large series have reported transient neurological complications ranging from 0 to 0.7% and permanent neurological complications in 0 to 0.5% of cerebral angiograms.3 4 5 6 Quality improvement guidelines have recommended that transient neurologic deficits should occur in no more than 2.5% of patients and permanent neurologic deficits should occur in no more than 1% of patients.7
Brain magnetic resonance imaging (MRI) done after cerebral angiography often demonstrates scattered focal regions of restricted diffusion, which are usually asymptomatic.8 Symptomatic ischemic events occur with injury to eloquent cortex or critical deep structures, and may be observed on diffusion-weighted MRI imaging almost immediately following the event. Computed tomography (CT) angiography or repeat angiography may reveal disrupted atherosclerotic plaques or the classic “string sign” of an arterial dissection.9 Effective management of ischemic stroke in this setting begins with prompt recognition of the event, either by detection of a neurological change in the patient or by identifying a freshly occluded artery on the angiogram. Intra-arterial thrombectomy or thrombolysis can then be undertaken. In situations in which a neurological deficit occurs in the postprocedure period after an angiogram, rapid imaging with CT or MRI may be helpful, followed by a return to the angiography suite for intra-arterial treatment if necessary.
Spinal Angiography
Diagnostic spinal angiography carries risks similar to cerebral angiography, such as spinal cord ischemic events from thromboembolism or air embolism resulting in myelopathy, as well as vessel dissection or disruption of atherosclerotic plaques. Transient myelopathy has been reported to occur from 0 to 2.2% of spinal angiography procedures; irreversible myelopathy is extremely rare.10 11 12
Nonneurologic Complications of Diagnostic Angiography
Contrast nephrotoxicity, arterial occlusion requiring surgical thrombectomy or thrombolysis, and development of arteriovenous fistula (AVF) or pseudoaneurysm occurs in ∼0.2% diagnostic cerebral angiograms. Groin or retroperitoneal hematoma requiring either transfusion or surgical evacuation occurs in ∼0.5% of procedures.7
Patients with connective tissue disorders such as Ehlers–Danlos are at heightened risk of complications such as vessel dissection and retroperitoneal hematoma with any neurointerventional procedure.13 14
Radiation exposure during neurointerventional procedures averages 1.67 mSv, with an estimated risk of death by radiation-induced cancer of 1 per 6,000 procedures.15 Doses of 10 mSv or greater may occur in lengthy cases, with resultant hair loss.16 Radiation exposure may be controlled by minimizing fluoroscopy time and the number of images acquired during angiograms, the use of dynamic acquisition, and virtual collimation.
Complications of Endovascular Treatment of Intracranial Aneurysms
Overall complication rates for coiling of intracranial aneurysms range from 8.4 to 18.9%.17 18 19 20 Risk is increased in patients with subarachnoid hemorrhage, with the use of balloon- and stent-assisted coiling, treatment of very small and very large aneurysms,21 22 23 24 25 and performance by inexperienced operators.26
Aneurysm or Vessel Perforation
The overall risk of vessel perforation is 4.1% during treatment of ruptured aneurysms and 0.5% with treatment of unruptured aneurysms.27 Risk of perforation is higher during the treatment of aneurysms smaller than 4 mm.28 29 30
Aneurysm perforation during embolization may or may not be obvious on fluoroscopy or angiography. Other clues to the occurrence of a perforation are a sudden elevation in intracranial pressure or blood pressure, bradycardia, or a prolonged sinus pause. In awake patients, a sudden headache or a neurological change may signal a perforation. Whenever a perforation is identified or suspected, the perforating wire, catheter, or coil should be left in position and a prompt guide catheter angiogram should be done. Active extravasation of contrast may not be observed if the perforation is occluded by the microcatheter or microwire. A CT scan performed postperforation will appear to show more subarachnoid blood than is actually present due to the presence of contrast material in the subarachnoid space (Figs. 1 and 2).
Fig. 1.
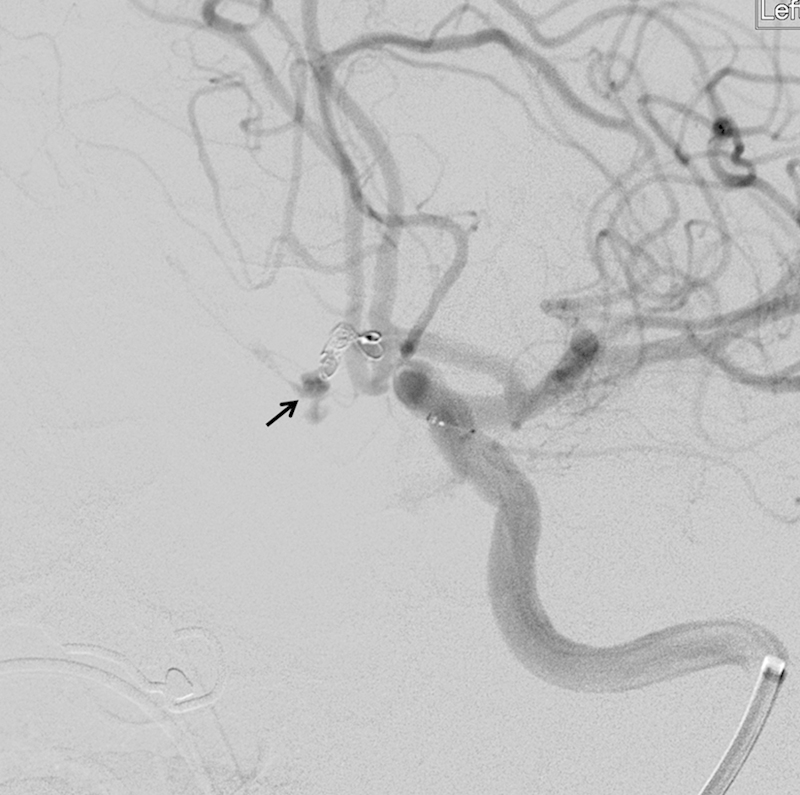
Digital subtraction angiogram during coiling of a ruptured anterior communicating artery aneurysm. Contrast extravasation into the subarachnoid space is noted from the aneurysm dome due to perforation by a coil (arrow).
Fig. 2.
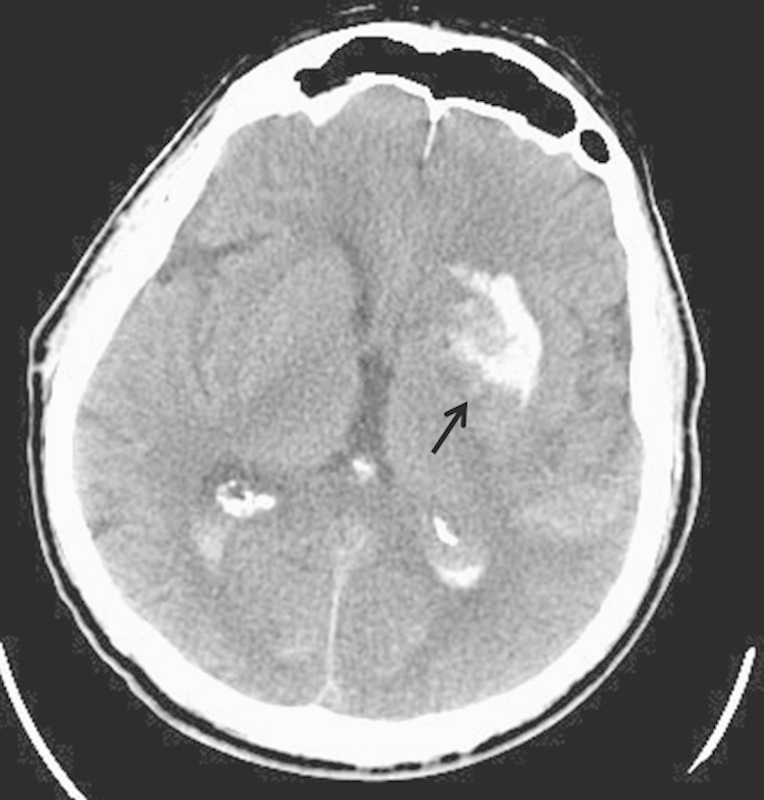
Axial noncontrast CT scan following thrombectomy for internal carotid artery occlusion and ischemic stroke. A “contrastoma” has appeared in the left thalamus due to extravasation of contrast material secondary to blood–brain barrier breakdown (arrow).
Following vessel perforation, heparin anticoagulation should be reversed with protamine (dose: 1 mg of protamine for every 100 units of heparin given). When possible, coils may be deployed to treat the perforation. Alternatively, a second microcatheter can be used to continue coiling the aneurysm while the first microcatheter is left in place.31 Another strategy is to use a balloon catheter to occlude the parent vessel to reduce the bleeding. In some cases, parent vessel sacrifice by embolization may be necessary to stop the hemorrhage.
Thromboembolism
Thrombus formation can result from manipulation of catheters, wires, coils, or balloons.32 Symptomatic thromboembolism occurs in 2 to 8% of patients during aneurysm coiling, but the majority of these are transient.32 33 34 35 36 37 38 Thrombi may be directly visualized with guide catheter angiogram as a filling defect within the parent vessel, or as a distal vessel occlusion. Adjustment of the angiogram image during the capillary phase by increasing the contrast and reducing the brightness at the monitor (a so-called stroke-o-gram) will make the ischemic defect more apparent and help identify the territory of affected arteries.
Thrombus formation during coiling is primarily mediated by platelet aggregation. Abciximab and other antiplatelet agents are the first step in management. Partial dosing of abciximab can have a paradoxical platelet activation effect, and therefore a full loading dose followed by intravenous (IV) infusion for 12 hours is recommended.39 40 Additionally, mechanical thrombectomy can be attempted using a 2- or 4-mm snare.41 Due to high rates of hemorrhagic complications, thrombolytic agents should be avoided, particularly in cases of ruptured aneurysms.42
Coil Dislodgement and Embolization
An appropriately sized initial framing coil is essential for successful embolization. An unstable or malpositioned coil can herniate into the parent vessel or embolize distally. Overpacking of an aneurysm can also dislodge previously released coils. To reduce this risk, shorter and softer coils are recommended in the final stages of coiling procedures.
Coil herniation places the patient at increased risk of flow compromise or thrombus formation in the parent vessel, while coil migration can result in distal ischemia. Wide-necked aneurysms are at greatest risk of coil dislodgement, with an overall incidence of 0.5%.20 A variety of devices are available for retrieving detached coils. Stent or balloon assistance may also be used to reinsert herniated coils into the aneurysm43 44; if the coil cannot be safely retrieved or repacked into the aneurysm, long-term antiplatelet therapy is recommended.
Coil Stretching and Unraveling
When the distal portion of a coil becomes trapped inside an aneurysm, attempted withdrawal of the coil can lead to stretching or unraveling. A slightly stretched coil, one that is only slightly lengthened, may still be deployed using stent or balloon assistance.44 45 A microsnare can also be used to withdraw a stretched coil.46 By contrast, a completely unraveled coil may elongate to over 1 m in length and can be difficult to control. A lengthy elongated coil may be withdrawn to the level of the femoral artery and secured to the vessel wall.47 Alternatively, the coil can be partially withdrawn, redirected, and released into an extracranial artery, such as a branch of the external carotid artery.
Vessel Injury
Arterial injury due to wire or guide catheter–induced intimal damage occurs in 0.6 to 3.6% of coiling cases.19 20 48 Intimal injury is likely underreported, as many operators do not routinely perform a surveillance angiogram of the access vessel after coiling. The vertebral artery is thought to be at greater risk of injury than the internal carotid.4 49 50 Vessel dissection is generally asymptomatic, but places the patient at increased risk of occlusive or thromboembolic complications.51 Imaging may directly reveal an injury in the form of a double lumen or intimal flap in arterial dissection, or indirectly in the form of arterial occlusion or stenosis, string sign, aneurysm, or pseudoaneurysm. Initial management of arterial dissection consists of dual antiplatelet therapy with aspirin 325 mg and clopidogrel 75 mg daily.52 For flow-limiting injury, anticoagulation with IV heparin or stenting may be necessary.53 Follow-up imaging should be done at 3 to 6 months, as 90% of dissections causing stenosis will be found to have interval resolution, and up to 50% of dissections causing occlusion will be found to have interval recanalization.54 Dual antiplatelet therapy may be discontinued at that time.
Rehemorrhage
Rehemorrhage after coiling of ruptured intracranial aneurysms occurs in some 0.9% of cases within 30 days after the procedure.55 Early rehemorrhage usually occurs in aneurysms that were incompletely occluded after initial treatment. Late rehemorrhage typically occurs in aneurysms with recanalization,56 57 underscoring the need for routine surveillance of coiled aneurysms.
Flow Diverters and Stent-Assisted Coiling
Flow diverters are wire mesh stents used to disrupt flow into an aneurysm, leading to thrombosis and endothelialization across the aneurysm neck. These devices are used for the management of aneurysms that are not amenable to traditional endosaccular approaches. The use of Pipeline flow diversion (ev3; Plymouth, MN) carried a 5.6% risk of major ipsilateral stroke or neurologic death.58 Risk of parent vessel or branch occlusion, as well as delayed aneurysmal hemorrhage, is increased in the setting of inadequate antiplatelet therapy.59 60 Ipsilateral intraparenchymal hemorrhage has been reported in 3% of cases,61 and delayed rupture of the treated aneurysm has been observed in 2.1% of cases in which the aneurysm is >10 mm in size.62 63 The occurrence of delayed hemorrhage in larger aneurysms treated with flow diversion has led to the hypothesis that an inflammatory response develops after acute thrombosis of the aneurysm, leading to erosion and rupture of the aneurysm in some cases. Because of this, coil embolization in addition to flow diversion has been recommended for larger aneurysms.60 61 62 63 64 65 66
Complications of Endovascular Treatment of Acute Ischemic Stroke
IV infusion of alteplase is considered to be first-line treatment for most patients with acute ischemic stroke presenting within 3 to 4.5 hours of onset of symptoms. Patients with large vessel occlusions and patients who are not candidates for IV alteplase are candidates for intra-arterial thrombectomy.
Mechanical Thrombectomy for Acute Ischemic Stroke
Intra-arterial treatment can be an effective alternative for patients who cannot be treated with IV alteplase, or do not respond to treatment with IV alteplase. The most commonly used intra-arterial techniques are suction thrombectomy and stent-retriever (stentriever) thrombectomy. Contemporary overall complication rates with thrombectomy for ischemic stroke include procedure-related mortality ranging from 0 to 2% and permanent neurological injury of 3 to 6.5%.67 68 69 The main threat after mechanical thrombectomy is intracerebral hemorrhage, which occurs in 4.9 to 10% of cases.67 68 69 It is common to see small regions of contrast extravasation in the affected territory of the brain after mechanical thrombectomy; this should not be confused with intracerebral hemorrhage. Tips for avoiding complications with intra-arterial treatment of ischemic stroke include meticulous attention to the patient's neurological status during and after the procedure, reversal of heparin for bleeding complications, and adequate control of blood pressure. Although specific guidelines for the management of blood pressure in this setting have not yet been published, adherence to the American Heart Association Guidelines recommendation to maintain blood pressure < 180/105 mm Hg (for patients treated with IV alteplase) seems reasonable.33
Because patients undergoing intra-arterial treatment of ischemic stroke have also often received IV alteplase, an uncommon but significant complication of alteplase merits consideration. Anaphylactoid reactions and angioedema are well-recognized reactions to alteplase and other thrombolytic agents, occurring in up to 2% of patients.70 Angioedema presents with localized swelling of the tongue, lips, or oropharynx, and typically occurs within 6 hours of exposure. Patients on angiotensin-converting-enzyme inhibitors appear to be at increased risk.71 While symptoms are typically mild, life-threatening airway obstruction may occur; elective oropharyngeal intubation for airway control may be preferred. CT scan of the face is also recommended to rule out tongue or oropharyngeal hemorrhage. High-dose dexamethasone (10 mg IV bolus, then 6 mg every 6 hours) and antihistamines may speed up resolution of the angioedema, while aerosolized epinephrine can reduce laryngeal edema.72
Complications of Extracranial Carotid Angioplasty and Stenting
While carotid endarterectomy (CEA) remains the gold standard for treatment of uncomplicated carotid stenosis, carotid angioplasty and stenting (CAS) is a viable option for select patients. The CREST trial found that both treatment options showed durable protection against ipsilateral stroke, with a higher rate of periprocedural stroke during CAS and a higher rate of myocardial infarction following CEA.73 Thromboembolism, dissection, intracranial hemorrhage (ICH), hyperperfusion syndrome, bradycardia, and hypotension are all potential complications of CAS.
The occurrence of an acute neurological change during the procedure suggests thromboembolism, and should be followed by an intracranial angiogram. The angiogram may demonstrate intraluminal thrombus or delayed contrast passage through distal intracranial vessels, indicating a shower of emboli. When thromboemboli are identified, options include mechanical thrombectomy or infusion of an IV glycoprotein IIb/IIIa inhibitors such as abciximab. Abciximab has an advantage over other glycoprotein IIb/IIIa inhibitors in that it can be reversed with platelet transfusion.74 Intra-arterial thrombolytics can also be used, but may be less effective against the platelet-rich thrombi, and also carry a risk of ICH.
Angioplasty is the maneuver during CAS procedures most associated with thromboembolic complications75; therefore, minimal angioplasty is often prudent, and excessive dilation should be avoided as it also places the patient at risk of bradycardia and hypotension.76 The operator should be prepared for the possibility of bradycardia and hypotension by having IV atropine and dopamine prepared and ready to inject immediately after angioplasty, particularly when dilating at the level of the carotid sinus.
ICH can also complicate CAS. A new neurologic deficit, headache, and a Cushing response (hypertension and bradycardia) should raise suspicion for ICH. Immediate head CT should be performed, and the sheath should be left in place. If ICH is identified, heparin anticoagulation is reversed with protamine, and strict blood pressure control is maintained.
Hyperperfusion syndrome can occur in up to 5% of CAS cases,77 and is associated with ICH in 0.7%.78 Patients present with ipsilateral headache, nausea, seizures, or focal neurologic deficit without radiographic evidence of ischemia from 6 hours to 4 days following CAS. The condition is theorized to result from an abrupt increase in cerebral blood flow following CAS in the setting of previous chronic hypoperfusion and impaired autoregulation. Treatment consists of strict blood pressure control, and typically resolves without permanent deficit in the absence of associated ICH. Hyperperfusion syndrome must be distinguished from transient contrast encephalopathy, another complication rarely seen after CAS.79 In transient contrast encephalopathy, CT will show cortical enhancement and cerebral edema. The syndrome is typically self-limited and most patients have no long-term deficits.
Approximately 30% of cerebral ischemic events occur in a delayed fashion, 2 to 14 days following the procedure.80 81 For these patients, initial evaluation begins with noncontrast head CT and carotid duplex exam. When neurologic deficits suggest a large-vessel occlusion, emergent return to the angiography suite for thrombectomy or intra-arterial thrombolysis should be considered.81 Recurrent focal neurologic deficit months or years after CAS suggests in-stent restenosis, which may be treated using a cutting balloon with distal embolic protection.82 83
Complications of Intracranial Angioplasty and Stenting
This section concerns procedures for the treatment of intracranial atherosclerotic stenosis and vasospasm. Stent-assisted treatment of intracranial aneurysms is also discussed in the section Complications of endovascular treatment of intracranial aneurysms.
Patients with symptomatic intracranial stenosis greater than 70% who have failed medical therapy may be candidates for balloon angioplasty with or without stenting. No Class I evidence yet exists that shows a benefit of intracranial angioplasty and stenting over medical management, and as such, patient selection is paramount. The overall perioperative stroke rate has been estimated at 7.9%, with a perioperative death rate of 3.4%.84 Vessel perforation, intraluminal thrombus, shower emboli, and dissection are also potential complications, and angioplasty without stenting carries a 30% rate of restenosis at 3 and 12 months.85 Tips for the avoidance of complications during intracranial stenosis include minimalistic angioplasty to avoid artery rupture and dissection, and using angioplasty alone without placement of a stent if the postangioplasty angiogram shows significant improvement in the stenosis.
Treatment of Vasospasm
Cerebral vasospasm presents most commonly following aneurysmal subarachnoid hemorrhage, but may also complicate neurointerventional procedures. Hyperdynamic therapy, intra-arterial injection of pharmacologic agents, and balloon angioplasty are all potential therapeutic interventions. Some investigators recommend angioplasty as first-line treatment for vasospasm on an emergent basis,86 while others advocate a trial of hyperdynamic therapy prior to proceeding with angioplasty.87 88 Balloon angioplasty is used for symptomatic vasospasm affecting intracranial arteries >1.5 mm in diameter,89 which includes the intracranial internal carotid artery (ICA), vertebral and basilar arteries, and the M1, A1, and P1 segments. For vessels that are not easily accessible or safely treated with a balloon, intra-arterial injection of nicardipine or verapamil can be used.90
In subarachnoid hemorrhage patients who have undergone craniotomy, procedural systemic heparinization poses a 1.8% risk of major hemorrhage.91 By contrast, use of systemic heparinization is thought generally safe in subarachnoid hemorrhage patients with a ventriculostomy.92 93 The major complication rate with endovascular treatment for vasospasm was recently reported at 5%, with a 1.1% incidence of vessel rupture.94 Other complications are discussed in greater detail above, and include thromboembolism, arterial dissection, reperfusion hemorrhage, branch occlusion, bleeding from untreated aneurysms, retroperitoneal hemorrhage, and groin hematoma.95 Tips for the avoidance of complications with intracranial angioplasty for vasospasm include limiting treatment to only arteries that are relatively easy to access with a balloon catheter and undersizing the balloon diameter to minimize risk of rupture.
Complications of Embolization Procedures
Endovascular embolization is a versatile technique used in the management of intracranial and spinal tumors, arteriovenous malformations (AVMs), and AVFs. A wide range of embolic materials and techniques are used, each of which carries its own set of potential complications.
Retained Microcatheter
Microcatheters may become trapped by both adhesive and nonadhesive liquid embolic material. A retained microcatheter may be retrieved by administering continuous, gentle traction on the device for 5 to 10 minutes or more. Additional techniques for the retrieval of a trapped microcatheter include using a monorail snare technique to grasp the trapped catheter96 or advancing an intermediate catheter over the trapped microcatheter to apply countertraction.97 Firmly glued-in catheters may need to be permanently implanted by cutting the catheter at the level of the femoral artery.98 Blood flow down the aorta will press the microcatheter against the arterial wall, allowing it to become endothelialized over time.99 Patients should be kept on aspirin indefinitely if a retained microcatheter is left in place. Both femoral artery pseudoaneurysm and popliteal artery occlusion have been reported after cutting and leaving in place a glued-in catheter.100 101 If the catheter impairs cerebral blood flow, surgical extraction may be required.
Microcatheter fractures above the level of the aorta may result in distal embolization and infarction.102 In these cases, a microsnare can be used to grasp the fragment, or a self-expanding stent can be used to trap the microcatheter against the parent artery wall. If a stent is used, dual antiplatelet therapy is required.
Hemorrhage
Hemorrhage of tumors can occur during or after embolization, and is usually due to infarction of the tumor with hemorrhagic transformation. Risk of tumor hemorrhage can be minimized by avoiding overly ambitious embolization of large tumors. Operators should be on the alert for the possibility of hemorrhage, particularly after the procedure, and prepare for the possibility of urgent craniotomy and resection of the tumor if necessary. AVM rupture can occur during or after embolization due to occlusion of draining veins without adequate disruption of feeding arterial flow,103 with reported rates varying from 1.6 to 10.6%.104 105 106 Importantly, medium and large brain AVMs should not be completely embolized in a single session, as complete occlusion of these lesions carries a higher risk of hemorrhage.105
Postembolization Edema or Hemorrhage
Significant cerebral edema may occur following occlusion of high-flow fistulas, AVMs, pial AVFs, or tumors; this is the so-called normal perfusion breakthrough syndrome.107 Abrupt occlusion of a longstanding arteriovenous shunt exposes vessels with disturbed autoregulation to increased flow, resulting in hyperemic cerebral edema and the potential for hemorrhage. Patients who are thought to be at risk for postprocedural cerebral edema may be treated with dexamethasone before and after the procedure; in addition, indomethacin may be used to induce cerebral vasoconstriction.108 Strict control of blood pressure is essential, as is aggressive treatment of intracranial hypertension when a ventriculostomy is in place.
Thromboembolism
Rates of thromboembolic complications vary with the embolic agent used. A recent study demonstrated a 3.8% risk of stroke in AVM embolization using n-BCA, versus a 5.8% risk of stroke following PVA embolization.109 Identification and management of thromboembolism with endovascular procedures was discussed above in the Complications of endovascular treatment of intracranial aneurysms section.
Embolization of Unintended Targets
Inadvertent embolization of the brain, retina, or cranial nerves can occur through several different mechanisms. First, meticulous effort must be made to identify potential dangerous collaterals prior to embolization; a superselective, high-magnification angiogram to identify collaterals to the brain or eye is essential, and provocative testing should be done whenever possible. Reflux of embolic material in a retrograde direction may occur during embolization, causing the embolic material to spill into collateral vessels. The risk of reflux can be minimized by mixing the embolic material with iodinated contrast material and doing the injection under fluoroscopic guidance; the injection should be stopped and aspiration through the microcatheter should be performed whenever reflux of contrast material is observed. Inadvertent embolization may also occur during treatment of high-flow lesions, due to the embolic agent traveling more distally than intended. Smaller particles may penetrate more deeply into tumors and cause greater devascularization, but are also associated with higher hemorrhage rates.110 111 Intratumoral shunting also increases the risk of unintended distal embolization. For extracranial embolizations, close attention must be paid to known extracranial–intracranial anastomoses to prevent embolic stroke. Cranial nerve palsies may occur via inadvertent embolization of the vasa nervorum, and are a risk with both intracranial and extracranial embolization procedures.112 Embolic agents may travel to the pulmonary vasculature,113 114 115 and embolization of superficial vessels in the head and neck can result in ischemic necrosis of skin and mucosa. Retinal embolization has also been reported after embolization of meningiomas.116 In general, when using particle embolic material, the use of larger particles (>300-μm diameter) may help minimize risk of inadvertent embolization because the particles are too large to pass via collaterals into many vital end-organ arteries.
Complications of Testing Procedures
Balloon Test Occlusion
Balloon test occlusion is a commonly used technique to assess the risk of cerebral ischemia prior to arterial sacrifice. When performed without prior test occlusion, sacrifice of the ICA carries a 26% stroke rate and a mortality rate of 12%117; by contrast, internal carotid occlusion after test occlusion carried a stroke rate of 16.2% in a recent series.118 The high stroke rate following a negative balloon occlusion test indicates the need for adjunctive testing prior to vessel sacrifice. Neurologic complications following balloon test occlusion include thromboembolic stroke in 0.4% of cases, arterial dissection in 1.2%, and aneurysm dissection in 0.2%.119 Very rarely, overinflation of the balloon can rupture intracranial vessels or result in development of a carotid-cavernous fistula. Balloon inflation in the carotid bulb or basilar artery may also cause a vasovagal response with bradycardia and hypotension, or rarely, cardiac arrest. The vast majority of vasovagal responses respond quickly to balloon deflation, and do not require IV fluid bolus or vasopressor administration.
WADA Testing
Intra-arterial injection of amobarbital or methohexital is a standard component of the preoperative work-up for epilepsy in an attempt to lateralize speech and memory function. Thromboembolism and arterial dissection with subsequent occlusion or pseudoaneurysm occur with similar frequencies as diagnostic cerebral angiograms. Inadvertent Amytal injection into the basilar artery can occur in patients with caroticobasilar anastomoses such as persistent trigeminal artery; posterior circulation injection of Amytal typically results in apnea and unconsciousness. Seizures and cerebral edema are rare complications of WADA testing.120
Complications of Venous Procedures
Venous Sampling
The most common indication for intracranial venous sampling is in the evaluation of Cushing disease. Inferior petrosal sinus sampling (IPSS) may provide confirmation and lateralization of an adrenocorticotropic hormone–secreting pituitary adenoma when biochemical testing and MRI do not clearly identify the source. Permanent neurologic complications at experienced centers are very rare, occurring in fewer than 0.1% of cases.121 Reported complications include brainstem ischemia or hemorrhage,122 subarachnoid hemorrhage,123 and transient sixth cranial nerve palsy.124 In one series, 2 out of 34 (5.9%) patients undergoing IPSS had deep venous thrombosis, and 1 patient died from resultant pulmonary embolism.125
Transvenous Embolization
Transvenous embolization is a useful technique for selected carotid-cavernous fistulas, dural AVFs, vein of Galen malformations, and vertebral-venous fistulas. Risks include perforation or rupture of intracranial vessels, venous infarction, and worsening of venous hypertension and are discussed above. Additionally, transvenous liquid embolics can reflux into arterial feeders and further reflux into normal arterial territories, causing ischemia of normal tissue. Intracranial abscess has also been reported after transvenous embolization.126 Transient neurologic complications may be noted in up to 10% of patients, although permanent deficits are rare.127 Even after successful transvenous embolization of a dural fistula, delayed development of a second fistula at a different site can still occur.128 129
Venous Thrombolysis and Thrombectomy
Cerebral venous sinus thrombosis has a high associated mortality rate. Patients with progressive neurologic deterioration, despite systemic anticoagulation, may benefit from endovascular therapy.130 131 Mechanical thrombectomy and infusion of thrombolytics are standard techniques. Accurate complication rates surrounding venous thrombolysis and thrombectomy are lacking due to the small sizes of reported series. Worsening of cerebral edema or hemorrhage, venous perforation and ICH, and rethrombosis of revascularized venous structures have all been reported. Additionally, mechanical clot disruption in the larger dural venous sinuses and jugular veins may cause symptomatic pulmonary emboli.
Conclusion
New and rapidly changing tools and techniques allow for neurointerventional management of a wide range of neurologic conditions. Embedded within this impressive flexibility are new risks which, in many cases, are still poorly characterized. Full appreciation for potential iatrogenic injury is essential in identifying and modifying dangerous situations and maximizing patient safety.
References
- 1.Mold J W, Stein H F. The cascade effect in the clinical care of patients. N Engl J Med. 1986;314(8):512–514. doi: 10.1056/NEJM198602203140809. [DOI] [PubMed] [Google Scholar]
- 2.Woolf S H, Kuzel A J, Dovey S M, Phillips R L Jr. A string of mistakes: the importance of cascade analysis in describing, counting, and preventing medical errors. Ann Fam Med. 2004;2(4):317–326. doi: 10.1370/afm.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Willinsky R A, Taylor S M, TerBrugge K, Farb R I, Tomlinson G, Montanera W. Neurologic complications of cerebral angiography: prospective analysis of 2,899 procedures and review of the literature. Radiology. 2003;227(2):522–528. doi: 10.1148/radiol.2272012071. [DOI] [PubMed] [Google Scholar]
- 4.Fifi J T, Meyers P M, Lavine S D. et al. Complications of modern diagnostic cerebral angiography in an academic medical center. J Vasc Interv Radiol. 2009;20(4):442–447. doi: 10.1016/j.jvir.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 5.Thiex R, Norbash A M, Frerichs K U. The safety of dedicated-team catheter-based diagnostic cerebral angiography in the era of advanced noninvasive imaging. AJNR Am J Neuroradiol. 2010;31(2):230–234. doi: 10.3174/ajnr.A1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dawkins A A, Evans A L, Wattam J. et al. Complications of cerebral angiography: a prospective analysis of 2,924 consecutive procedures. Neuroradiology. 2007;49(9):753–759. doi: 10.1007/s00234-007-0252-y. [DOI] [PubMed] [Google Scholar]
- 7.Citron S J, Wallace R C, Lewis C A. et al. Quality improvement guidelines for adult diagnostic neuroangiography. Cooperative study between ASITN, ASNR, and SIR. J Vasc Interv Radiol. 2003;14(9 Pt 2):S257–S262. [PubMed] [Google Scholar]
- 8.Bendszus M, Koltzenburg M, Burger R, Warmuth-Metz M, Hofmann E, Solymosi L. Silent embolism in diagnostic cerebral angiography and neurointerventional procedures: a prospective study. Lancet. 1999;354(9190):1594–1597. doi: 10.1016/S0140-6736(99)07083-X. [DOI] [PubMed] [Google Scholar]
- 9.Fredericks R K, Thomas T D, Lefkowitz D S, Troost B T. Implications of the angiographic string sign in carotid atherosclerosis. Stroke. 1990;21(3):476–479. doi: 10.1161/01.str.21.3.476. [DOI] [PubMed] [Google Scholar]
- 10.Forbes G, Nichols D A, Jack C R Jr. et al. Complications of spinal cord arteriography: prospective assessment of risk for diagnostic procedures. Radiology. 1988;169(2):479–484. doi: 10.1148/radiology.169.2.3174997. [DOI] [PubMed] [Google Scholar]
- 11.Niimi Y, Sala F, Deletis V, Setton A, de Camargo A B, Berenstein A. Neurophysiologic monitoring and pharmacologic provocative testing for embolization of spinal cord arteriovenous malformations. AJNR Am J Neuroradiol. 2004;25(7):1131–1138. [PMC free article] [PubMed] [Google Scholar]
- 12.Chen J, Gailloud P. Safety of spinal angiography: complication rate analysis in 302 diagnostic angiograms. Neurology. 2011;77(13):1235–1240. doi: 10.1212/WNL.0b013e3182302068. [DOI] [PubMed] [Google Scholar]
- 13.Chuman H, Trobe J D, Petty E M. et al. Spontaneous direct carotid-cavernous fistula in Ehlers-Danlos syndrome type IV: two case reports and a review of the literature. J Neuroophthalmol. 2002;22(2):75–81. doi: 10.1097/00041327-200206000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Horowitz M B, Purdy P D, Valentine R J, Morrill K. Remote vascular catastrophes after neurovascular interventional therapy for type 4 Ehlers-Danlos Syndrome. AJNR Am J Neuroradiol. 2000;21(5):974–976. [PMC free article] [PubMed] [Google Scholar]
- 15.Bergeron P, Carrier R, Roy D, Blais N, Raymond J. Radiation doses to patients in neurointerventional procedures. AJNR Am J Neuroradiol. 1994;15(10):1809–1812. [PMC free article] [PubMed] [Google Scholar]
- 16.Hayakawa M, Moritake T, Kataoka F. et al. Direct measurement of patient's entrance skin dose during neurointerventional procedure to avoid further radiation-induced skin injuries. Clin Neurol Neurosurg. 2010;112(6):530–536. doi: 10.1016/j.clineuro.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 17.Henkes H Fischer S Weber W et al. Endovascular coil occlusion of 1811 intracranial aneurysms: early angiographic and clinical results Neurosurgery 2004542268–280., discussion 280–285 [DOI] [PubMed] [Google Scholar]
- 18.Brilstra E H, Rinkel G J, van der Graaf Y, van Rooij W J, Algra A. Treatment of intracranial aneurysms by embolization with coils: a systematic review. Stroke. 1999;30(2):470–476. doi: 10.1161/01.str.30.2.470. [DOI] [PubMed] [Google Scholar]
- 19.Lozier A P, Connolly E S Jr, Lavine S D, Solomon R A. Guglielmi detachable coil embolization of posterior circulation aneurysms: a systematic review of the literature. Stroke. 2002;33(10):2509–2518. doi: 10.1161/01.str.0000031928.71695.a9. [DOI] [PubMed] [Google Scholar]
- 20.Murayama Y, Nien Y L, Duckwiler G. et al. Guglielmi detachable coil embolization of cerebral aneurysms: 11 years' experience. J Neurosurg. 2003;98(5):959–966. doi: 10.3171/jns.2003.98.5.0959. [DOI] [PubMed] [Google Scholar]
- 21.Golshani K, Ferrel A, Lessne M. et al. Stent-assisted coil embolization of ruptured intracranial aneurysms: A retrospective multicenter review. Surg Neurol Int. 2012;3:84. doi: 10.4103/2152-7806.99174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bodily K D, Cloft H J, Lanzino G, Fiorella D J, White P M, Kallmes D F. Stent-assisted coiling in acutely ruptured intracranial aneurysms: a qualitative, systematic review of the literature. AJNR Am J Neuroradiol. 2011;32(7):1232–1236. doi: 10.3174/ajnr.A2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ding D, Raper D, Starke R. et al. Thromboembolic and haemorrhagic complications associated with endovascular coil embolization of ruptured basilar apex aneurysms. J Neurointerv Surg. 2014;6 01:A59. [Google Scholar]
- 24.Brinjikji W, Cloft H J, Kallmes D F. Difficult aneurysms for endovascular treatment: overwide or undertall? AJNR Am J Neuroradiol. 2009;30(8):1513–1517. doi: 10.3174/ajnr.A1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chalouhi N, Jabbour P, Singhal S. et al. Stent-assisted coiling of intracranial aneurysms: predictors of complications, recanalization, and outcome in 508 cases. Stroke. 2013;44(5):1348–1353. doi: 10.1161/STROKEAHA.111.000641. [DOI] [PubMed] [Google Scholar]
- 26.Singh V, Gress D R, Higashida R T, Dowd C F, Halbach V V, Johnston S C. The learning curve for coil embolization of unruptured intracranial aneurysms. AJNR Am J Neuroradiol. 2002;23(5):768–771. [PMC free article] [PubMed] [Google Scholar]
- 27.Cloft H J, Kallmes D F. Cerebral aneurysm perforations complicating therapy with Guglielmi detachable coils: a meta-analysis. AJNR Am J Neuroradiol. 2002;23(10):1706–1709. [PMC free article] [PubMed] [Google Scholar]
- 28.Ricolfi F, Le Guerinel C, Blustajn J. et al. Rupture during treatment of recently ruptured aneurysms with Guglielmi electrodetachable coils. AJNR Am J Neuroradiol. 1998;19(9):1653–1658. [PMC free article] [PubMed] [Google Scholar]
- 29.Doerfler A, Wanke I, Egelhof T. et al. Aneurysmal rupture during embolization with Guglielmi detachable coils: causes, management, and outcome. AJNR Am J Neuroradiol. 2001;22(10):1825–1832. [PMC free article] [PubMed] [Google Scholar]
- 30.Sluzewski M, Bosch J A, van Rooij W J, Nijssen P C, Wijnalda D. Rupture of intracranial aneurysms during treatment with Guglielmi detachable coils: incidence, outcome, and risk factors. J Neurosurg. 2001;94(2):238–240. doi: 10.3171/jns.2001.94.2.0238. [DOI] [PubMed] [Google Scholar]
- 31.Willinsky R, terBrugge K. Use of a second microcatheter in the management of a perforation during endovascular treatment of a cerebral aneurysm. AJNR Am J Neuroradiol. 2000;21(8):1537–1539. [PMC free article] [PubMed] [Google Scholar]
- 32.Soeda A, Sakai N, Sakai H. et al. Thromboembolic events associated with Guglielmi detachable coil embolization of asymptomatic cerebral aneurysms: evaluation of 66 consecutive cases with use of diffusion-weighted MR imaging. AJNR Am J Neuroradiol. 2003;24(1):127–132. [PMC free article] [PubMed] [Google Scholar]
- 33.Qureshi A I Luft A R Sharma M Guterman L R Hopkins L N Prevention and treatment of thromboembolic and ischemic complications associated with endovascular procedures: Part II—Clinical aspects and recommendations Neurosurgery 20004661360–1375., discussion 1375–1376 [DOI] [PubMed] [Google Scholar]
- 34.Viñuela F, Duckwiler G, Mawad M. Guglielmi detachable coil embolization of acute intracranial aneurysm: perioperative anatomical and clinical outcome in 403 patients. J Neurosurg. 1997;86(3):475–482. doi: 10.3171/jns.1997.86.3.0475. [DOI] [PubMed] [Google Scholar]
- 35.Pelz D M, Lownie S P, Fox A J. Thromboembolic events associated with the treatment of cerebral aneurysms with Guglielmi detachable coils. AJNR Am J Neuroradiol. 1998;19(8):1541–1547. [PMC free article] [PubMed] [Google Scholar]
- 36.Derdeyn C P, Cross D T III, Moran C J. et al. Postprocedure ischemic events after treatment of intracranial aneurysms with Guglielmi detachable coils. J Neurosurg. 2002;96(5):837–843. doi: 10.3171/jns.2002.96.5.0837. [DOI] [PubMed] [Google Scholar]
- 37.Ross I B Dhillon G S Complications of endovascular treatment of cerebral aneurysms Surg Neurol 200564112–18., discussion 18–19 [DOI] [PubMed] [Google Scholar]
- 38.Kanaan H Jankowitz B Aleu A et al. In-stent thrombosis and stenosis after neck-remodeling device-assisted coil embolization of intracranial aneurysms Neurosurgery 20106761523–1532., discussion 1532–1533 [DOI] [PubMed] [Google Scholar]
- 39.Steinhubl S R, Talley J D, Braden G A. et al. Point-of-care measured platelet inhibition correlates with a reduced risk of an adverse cardiac event after percutaneous coronary intervention: results of the GOLD (AU-Assessing Ultegra) multicenter study. Circulation. 2001;103(21):2572–2578. doi: 10.1161/01.cir.103.21.2572. [DOI] [PubMed] [Google Scholar]
- 40.Quinn M J, Plow E F, Topol E J. Platelet glycoprotein IIb/IIIa inhibitors: recognition of a two-edged sword? Circulation. 2002;106(3):379–385. doi: 10.1161/01.cir.0000019581.22812.b2. [DOI] [PubMed] [Google Scholar]
- 41.Fourie P, Duncan I C. Microsnare-assisted mechanical removal of intraprocedural distal middle cerebral arterial thromboembolism. AJNR Am J Neuroradiol. 2003;24(4):630–632. [PMC free article] [PubMed] [Google Scholar]
- 42.Cronqvist M, Pierot L, Boulin A, Cognard C, Castaings L, Moret J. Local intraarterial fibrinolysis of thromboemboli occurring during endovascular treatment of intracerebral aneurysm: a comparison of anatomic results and clinical outcome. AJNR Am J Neuroradiol. 1998;19(1):157–165. [PMC free article] [PubMed] [Google Scholar]
- 43.Luo C B, Chang F C, Teng M MH, Guo W Y, Chang C Y. Stent management of coil herniation in embolization of internal carotid aneurysms. AJNR Am J Neuroradiol. 2008;29(10):1951–1955. doi: 10.3174/ajnr.A1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sugiu K, Martin J-B, Jean B, Rüfenacht D A. Rescue balloon procedure for an emergency situation during coil embolization for cerebral aneurysms. Technical note. J Neurosurg. 2002;96(2):373–376. doi: 10.3171/jns.2002.96.2.0373. [DOI] [PubMed] [Google Scholar]
- 45.Fessler R D Ringer A J Qureshi A I Guterman L R Hopkins L N Intracranial stent placement to trap an extruded coil during endovascular aneurysm treatment: technical note Neurosurgery 2000461248–251., discussion 251–253 [PubMed] [Google Scholar]
- 46.Fiorella D Albuquerque F C Deshmukh V R McDougall C G Monorail snare technique for the recovery of stretched platinum coils: technical case report Neurosurgery 200557(1, Suppl):E210, discussion E210 [DOI] [PubMed] [Google Scholar]
- 47.Nii K, Onizuka M, Kaneko Y, Aikawa H, Tsutsumi M, Kazekawa K. Irretrievable unraveled coil remaining in the vascular lumen between the cerebral aneurysm and puncture site. J Neuroendovasc Therapy. 2009;3:42–46. [Google Scholar]
- 48.Friedman J A, Nichols D A, Meyer F B. et al. Guglielmi detachable coil treatment of ruptured saccular cerebral aneurysms: retrospective review of a 10-year single-center experience. AJNR Am J Neuroradiol. 2003;24(3):526–533. [PMC free article] [PubMed] [Google Scholar]
- 49.Cloft H J, Jensen M E, Kallmes D F, Dion J E. Arterial dissections complicating cerebral angiography and cerebrovascular interventions. AJNR Am J Neuroradiol. 2000;21(3):541–545. [PMC free article] [PubMed] [Google Scholar]
- 50.Inamasu J, Guiot B H. Iatrogenic vertebral artery injury. Acta Neurol Scand. 2005;112(6):349–357. doi: 10.1111/j.1600-0404.2005.00497.x. [DOI] [PubMed] [Google Scholar]
- 51.Kim Y K, Schulman S. Cervical artery dissection: pathology, epidemiology and management. Thromb Res. 2009;123(6):810–821. doi: 10.1016/j.thromres.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 52.Engelter S T, Brandt T, Debette S. et al. Antiplatelets versus anticoagulation in cervical artery dissection. Stroke. 2007;38(9):2605–2611. doi: 10.1161/STROKEAHA.107.489666. [DOI] [PubMed] [Google Scholar]
- 53.Pham M H Rahme R J Arnaout O et al. Endovascular stenting of extracranial carotid and vertebral artery dissections: a systematic review of the literature Neurosurgery 2011684856–866., discussion 866 [DOI] [PubMed] [Google Scholar]
- 54.Redekop G J. Extracranial carotid and vertebral artery dissection: a review. Can J Neurol Sci. 2008;35(2):146–152. doi: 10.1017/s0317167100008556. [DOI] [PubMed] [Google Scholar]
- 55.Fleming J B, Hoh B L, Simon S D. et al. Rebleeding risk after treatment of ruptured intracranial aneurysms. J Neurosurg. 2011;114(6):1778–1784. doi: 10.3171/2011.1.JNS101232. [DOI] [PubMed] [Google Scholar]
- 56.Byrne J V, Sohn M J, Molyneux A J, Chir B. Five-year experience in using coil embolization for ruptured intracranial aneurysms: outcomes and incidence of late rebleeding. J Neurosurg. 1999;90(4):656–663. doi: 10.3171/jns.1999.90.4.0656. [DOI] [PubMed] [Google Scholar]
- 57.Uda K Goto K Ogata N Izumi N Nagata S Matsuno H Embolization of cerebral aneurysms using Guglielmi detachable coils—problems and treatment plans in the acute stage after subarachnoid hemorrhage and long-term efficiency Neurol Med Chir (Tokyo) 1998383143–152., discussion 152–154 [DOI] [PubMed] [Google Scholar]
- 58.Becske T, Kallmes D F, Saatci I. et al. Pipeline for uncoilable or failed aneurysms: results from a multicenter clinical trial. Radiology. 2013;267(3):858–868. doi: 10.1148/radiol.13120099. [DOI] [PubMed] [Google Scholar]
- 59.Fischer S, Vajda Z, Aguilar Perez M. et al. Pipeline embolization device (PED) for neurovascular reconstruction: initial experience in the treatment of 101 intracranial aneurysms and dissections. Neuroradiology. 2012;54(4):369–382. doi: 10.1007/s00234-011-0948-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kulcsár Z, Houdart E, Bonafé A. et al. Intra-aneurysmal thrombosis as a possible cause of delayed aneurysm rupture after flow-diversion treatment. AJNR Am J Neuroradiol. 2011;32(1):20–25. doi: 10.3174/ajnr.A2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brinjikji W, Murad M H, Lanzino G, Cloft H J, Kallmes D F. Endovascular treatment of intracranial aneurysms with flow diverters: a meta-analysis. Stroke. 2013;44(2):442–447. doi: 10.1161/STROKEAHA.112.678151. [DOI] [PubMed] [Google Scholar]
- 62.Kulcsár Z, Augsburger L, Reymond P. et al. Flow diversion treatment: intra-aneurismal blood flow velocity and WSS reduction are parameters to predict aneurysm thrombosis. Acta Neurochir (Wien) 2012;154(10):1827–1834. doi: 10.1007/s00701-012-1482-2. [DOI] [PubMed] [Google Scholar]
- 63.Pierot L, Wakhloo A K. Endovascular treatment of intracranial aneurysms: current status. Stroke. 2013;44(7):2046–2054. doi: 10.1161/STROKEAHA.113.000733. [DOI] [PubMed] [Google Scholar]
- 64.Cebral J R, Mut F, Raschi M. et al. Aneurysm rupture following treatment with flow-diverting stents: computational hemodynamics analysis of treatment. AJNR Am J Neuroradiol. 2011;32(1):27–33. doi: 10.3174/ajnr.A2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Darsaut T E, Rayner-Hartley E, Makoyeva A, Salazkin I, Berthelet F, Raymond J. Aneurysm rupture after endovascular flow diversion: the possible role of persistent flows through the transition zone associated with device deformation. Interv Neuroradiol. 2013;19(2):180–185. doi: 10.1177/159101991301900206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Siddiqui A H Kan P Abla A A Hopkins L N Levy E I Complications after treatment with pipeline embolization for giant distal intracranial aneurysms with or without coil embolization Neurosurgery 2012712E509–E513., discussion E513 [DOI] [PubMed] [Google Scholar]
- 67.Jansen O Macho J M Killer-Oberpfalzer M Liebeskind D Wahlgren N; TREVO Study Group. Neurothrombectomy for the treatment of acute ischemic stroke: results from the TREVO study Cerebrovasc Dis 2013363218–225. [DOI] [PubMed] [Google Scholar]
- 68.Hussain S I, Zaidat O O, Fitzsimmons B-FM. The Penumbra system for mechanical thrombectomy in endovascular acute ischemic stroke therapy. Neurology. 2012;79(13) 01:S135–S141. doi: 10.1212/WNL.0b013e31826958a8. [DOI] [PubMed] [Google Scholar]
- 69.Akins P T Amar A P Pakbaz R S Fields J D; SWIFT Investigators. Complications of endovascular treatment for acute stroke in the SWIFT trial with solitaire and Merci devices AJNR Am J Neuroradiol 2014353524–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hill M D, Barber P A, Takahashi J, Demchuk A M, Feasby T E, Buchan A M. Anaphylactoid reactions and angioedema during alteplase treatment of acute ischemic stroke. CMAJ. 2000;162(9):1281–1284. [PMC free article] [PubMed] [Google Scholar]
- 71.Engelter S T, Fluri F, Buitrago-Téllez C. et al. Life-threatening orolingual angioedema during thrombolysis in acute ischemic stroke. J Neurol. 2005;252(10):1167–1170. doi: 10.1007/s00415-005-0789-9. [DOI] [PubMed] [Google Scholar]
- 72.Temiño V M, Peebles R S Jr. The spectrum and treatment of angioedema. Am J Med. 2008;121(4):282–286. doi: 10.1016/j.amjmed.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 73.Mantese V A Timaran C H Chiu D Begg R J Brott T G; CREST Investigators. The Carotid Revascularization Endarterectomy versus Stenting Trial (CREST): stenting versus carotid endarterectomy for carotid disease Stroke 201041(10, Suppl):S31–S34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Qureshi A I, Saad M, Zaidat O O. et al. Intracerebral hemorrhages associated with neurointerventional procedures using a combination of antithrombotic agents including abciximab. Stroke. 2002;33(7):1916–1919. doi: 10.1161/01.str.0000019423.08947.43. [DOI] [PubMed] [Google Scholar]
- 75.Jin S-C Kwon O-K Oh C W et al. A technical strategy for carotid artery stenting: suboptimal prestent balloon angioplasty without poststenting balloon dilatation Neurosurgery 20106751438–1442., discussion 1442–1443 [DOI] [PubMed] [Google Scholar]
- 76.Sadato A Satow T Ishii A Ohta T Hashimoto N Use of a large angioplasty balloon for predilation is a risk factor for embolic complications in protected carotid stenting Neurol Med Chir (Tokyo) 2004447337–342., discussion 343 [DOI] [PubMed] [Google Scholar]
- 77.Meyers P M Higashida R T Phatouros C C et al. Cerebral hyperperfusion syndrome after percutaneous transluminal stenting of the craniocervical arteries Neurosurgery 2000472335–343., discussion 343–345 [DOI] [PubMed] [Google Scholar]
- 78.Abou-Chebl A, Yadav J S, Reginelli J P, Bajzer C, Bhatt D, Krieger D W. Intracranial hemorrhage and hyperperfusion syndrome following carotid artery stenting: risk factors, prevention, and treatment. J Am Coll Cardiol. 2004;43(9):1596–1601. doi: 10.1016/j.jacc.2003.12.039. [DOI] [PubMed] [Google Scholar]
- 79.Dangas G, Monsein L H, Laureno R. et al. Transient contrast encephalopathy after carotid artery stenting. J Endovasc Ther. 2001;8(2):111–113. doi: 10.1177/152660280100800202. [DOI] [PubMed] [Google Scholar]
- 80.Qureshi A I, Luft A R, Janardhan V. et al. Identification of patients at risk for periprocedural neurological deficits associated with carotid angioplasty and stenting. Stroke. 2000;31(2):376–382. doi: 10.1161/01.str.31.2.376. [DOI] [PubMed] [Google Scholar]
- 81.Wholey M H, Wholey M H, Tan W A. et al. Management of neurological complications of carotid artery stenting. J Endovasc Ther. 2001;8(4):341–353. doi: 10.1177/152660280100800403. [DOI] [PubMed] [Google Scholar]
- 82.Tamberella M R, Yadav J S, Bajzer C T, Bhatt D L, Abou-Chebl A. Cutting balloon angioplasty to treat carotid in-stent restenosis. J Invasive Cardiol. 2004;16(3):133–135. [PubMed] [Google Scholar]
- 83.Heck D. Results of cutting balloon angioplasty for carotid artery in-stent restenosis in six patients: description of the technique, long-term outcomes, and review of the literature. J Neurointerv Surg. 2009;1(1):48–50. doi: 10.1136/jnis.2009.000323. [DOI] [PubMed] [Google Scholar]
- 84.Cruz-Flores S, Diamond A L. Angioplasty for intracranial artery stenosis. Cochrane Database Syst Rev. 2006;(3):CD004133. doi: 10.1002/14651858.CD004133.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mori T, Mori K, Fukuoka M, Arisawa M, Honda S. Percutaneous transluminal cerebral angioplasty: serial angiographic follow-up after successful dilatation. Neuroradiology. 1997;39(2):111–116. doi: 10.1007/s002340050376. [DOI] [PubMed] [Google Scholar]
- 86.Rosenwasser R H Armonda R A Thomas J E Benitez R P Gannon P M Harrop J Therapeutic modalities for the management of cerebral vasospasm: timing of endovascular options Neurosurgery 1999445975–979., discussion 979–980 [DOI] [PubMed] [Google Scholar]
- 87.Weyer G W, Nolan C P, Macdonald R L. Evidence-based cerebral vasospasm management. Neurosurg Focus. 2006;21(3):E8. doi: 10.3171/foc.2006.21.3.8. [DOI] [PubMed] [Google Scholar]
- 88.Harrigan M R. Hypertension may be the most important component of hyperdynamic therapy in cerebral vasospasm. Crit Care. 2010;14(3):151. doi: 10.1186/cc8983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.American Society of Interventional and Therapeutic Neuroradiology Mechanical and pharmacologic treatment of vasospasm AJNR Am J Neuroradiol 200122(8, Suppl):S26–S27. [PMC free article] [PubMed] [Google Scholar]
- 90.Bauer A M, Rasmussen P A. Treatment of intracranial vasospasm following subarachnoid hemorrhage. Front Neurol. 2014;5:72. doi: 10.3389/fneur.2014.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Raabe A, Gerlach R, Zimmermann M, Seifert V. The risk of haemorrhage associated with early postoperative heparin administration after intracranial surgery. Acta Neurochir (Wien) 2001;143(1):1–7. doi: 10.1007/s007010170131. [DOI] [PubMed] [Google Scholar]
- 92.Bernardini G L, Mayer S A, Kossoff S B, Hacein-Bey L, Solomon R A, Pile-Spellman J. Anticoagulation and induced hypertension after endovascular treatment for ruptured intracranial aneurysms. Crit Care Med. 2001;29(3):641–644. doi: 10.1097/00003246-200103000-00033. [DOI] [PubMed] [Google Scholar]
- 93.Hoh B L Nogueira R G Ledezma C J Pryor J C Ogilvy C S Safety of heparinization for cerebral aneurysm coiling soon after external ventriculostomy drain placement Neurosurgery 2005575845–849., discussion 845–849 [DOI] [PubMed] [Google Scholar]
- 94.Hoh B L Ogilvy C S Endovascular treatment of cerebral vasospasm: transluminal balloon angioplasty, intra-arterial papaverine, and intra-arterial nicardipine Neurosurg Clin N Am 2005163501–516., vi [DOI] [PubMed] [Google Scholar]
- 95.Sayama C M, Liu J K, Couldwell W T. Update on endovascular therapies for cerebral vasospasm induced by aneurysmal subarachnoid hemorrhage. Neurosurg Focus. 2006;21(3):E12. doi: 10.3171/foc.2006.21.3.12. [DOI] [PubMed] [Google Scholar]
- 96.Kelly M E Turner R IV Gonugunta V Rasmussen P A Woo H H Fiorella D Monorail snare technique for the retrieval of an adherent microcatheter from an onyx cast: technical case report Neurosurgery 200863101E89, discussion E89 [DOI] [PubMed] [Google Scholar]
- 97.Binning M J, Yashar P, Orion D. et al. Use of the Outreach Distal Access Catheter for microcatheter stabilization during intracranial arteriovenous malformation embolization. AJNR Am J Neuroradiol. 2012;33(9):E117–E119. doi: 10.3174/ajnr.A2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Weber W Kis B Siekmann R Jans P Laumer R Kühne D Preoperative embolization of intracranial arteriovenous malformations with Onyx Neurosurgery 2007612244–252., discussion 252–254 [DOI] [PubMed] [Google Scholar]
- 99.Zoarski G H, Lilly M P, Sperling J S, Mathis J M. Surgically confirmed incorporation of a chronically retained neurointerventional microcatheter in the carotid artery. AJNR Am J Neuroradiol. 1999;20(1):177–178. [PubMed] [Google Scholar]
- 100.Bingöl H, Sirin G, Akay H T, Iyem H, Demirkilic U, Tatar H. Management of a retained catheter in an arteriovenous malformation. Case report. J Neurosurg. 2007;106(3):481–483. doi: 10.3171/jns.2007.106.3.481. [DOI] [PubMed] [Google Scholar]
- 101.Rückert R I, Bender A, Rogalla P. Popliteal artery occlusion as a late complication of liquid acrylate embolization for cerebral vascular malformation. J Vasc Surg. 1999;29(3):561–565. doi: 10.1016/s0741-5214(99)70287-9. [DOI] [PubMed] [Google Scholar]
- 102.Mizoue T, Arita K, Nakahara T, Kawamoto H, Kurisu K. [A case of cerebral arteriovenous malformation with accidental migration of a microcatheter during endovascular procedure] No Shinkei Geka. 1997;25(5):443–446. [PubMed] [Google Scholar]
- 103.Hademenos G J, Massoud T F. Risk of intracranial arteriovenous malformation rupture due to venous drainage impairment. A theoretical analysis. Stroke. 1996;27(6):1072–1083. doi: 10.1161/01.str.27.6.1072. [DOI] [PubMed] [Google Scholar]
- 104.Jayaraman M V, Marcellus M L, Hamilton S. et al. Neurologic complications of arteriovenous malformation embolization using liquid embolic agents. AJNR Am J Neuroradiol. 2008;29(2):242–246. doi: 10.3174/ajnr.A0793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Heidenreich J O, Hartlieb S, Stendel R. et al. Bleeding complications after endovascular therapy of cerebral arteriovenous malformations. AJNR Am J Neuroradiol. 2006;27(2):313–316. [PMC free article] [PubMed] [Google Scholar]
- 106.Carvi y Nievas M, Haas E, Höllerhage H-G. Severe intracranial bleedings during endovascular procedures: outcome of surgically treated patients. Neurol Res. 2007;29(1):81–90. doi: 10.1179/174313206X152492. [DOI] [PubMed] [Google Scholar]
- 107.Spetzler R F, Wilson C B, Weinstein P, Mehdorn M, Townsend J, Telles D. Normal perfusion pressure breakthrough theory. Clin Neurosurg. 1978;25:651–672. doi: 10.1093/neurosurgery/25.cn_suppl_1.651. [DOI] [PubMed] [Google Scholar]
- 108.Hansen P A, Knudsen F, Jacobsen M, Haase J, Bartholdy N. Indomethacin in controlling “normal perfusion pressure breakthrough” in a case of large cerebral arteriovenous malformation. J Neurosurg Anesthesiol. 1995;7(2):117–120. doi: 10.1097/00008506-199504000-00007. [DOI] [PubMed] [Google Scholar]
- 109.n-BCA Trail Investigators . N-butyl cyanoacrylate embolization of cerebral arteriovenous malformations: results of a prospective, randomized, multi-center trial. AJNR Am J Neuroradiol. 2002;23(5):748–755. [PMC free article] [PubMed] [Google Scholar]
- 110.Wakhloo A K, Perlow A, Linfante I. et al. Transvenous n-butyl-cyanoacrylate infusion for complex dural carotid cavernous fistulas: technical considerations and clinical outcome. AJNR Am J Neuroradiol. 2005;26(8):1888–1897. [PMC free article] [PubMed] [Google Scholar]
- 111.Kallmes D F, McGraw J K, Evans A J. et al. Thrombogenicity of hydrophilic and nonhydrophilic microcatheters and guiding catheters. AJNR Am J Neuroradiol. 1997;18(7):1243–1251. [PMC free article] [PubMed] [Google Scholar]
- 112.Gaynor B G, Elhammady M S, Jethanamest D, Angeli S I, Aziz-Sultan M A. Incidence of cranial nerve palsy after preoperative embolization of glomus jugulare tumors using Onyx. J Neurosurg. 2014;120(2):377–381. doi: 10.3171/2013.10.JNS13354. [DOI] [PubMed] [Google Scholar]
- 113.Pelz D M, Lownie S P, Fox A J, Hutton L C. Symptomatic pulmonary complications from liquid acrylate embolization of brain arteriovenous malformations. AJNR Am J Neuroradiol. 1995;16(1):19–26. [PMC free article] [PubMed] [Google Scholar]
- 114.Kline J N, Ryals T J, Galvin J R, Loftus C M, Hunter J H. Pulmonary embolization and infarction. An iatrogenic complication of transcatheter embolization of a cerebral arteriovenous malformation with polyvinyl alcohol sponge. Chest. 1993;103(4):1293–1295. doi: 10.1378/chest.103.4.1293. [DOI] [PubMed] [Google Scholar]
- 115.Pukenas B A Satti S R Bailey R Weigele J B Hurst R W Stiefel M F Onyx pulmonary artery embolization after treatment of a low-flow dural arteriovenous fistula: case report Neurosurgery 2011685E1497–E1500., discussion E1500 [DOI] [PubMed] [Google Scholar]
- 116.Chan R C, Thompson G B. Ischemic necrosis of the scalp after preoperative embolization of meningeal tumors. Neurosurgery. 1984;15(1):76–81. doi: 10.1227/00006123-198407000-00014. [DOI] [PubMed] [Google Scholar]
- 117.Linskey M E, Jungreis C A, Yonas H. et al. Stroke risk after abrupt internal carotid artery sacrifice: accuracy of preoperative assessment with balloon test occlusion and stable xenon-enhanced CT. AJNR Am J Neuroradiol. 1994;15(5):829–843. [PMC free article] [PubMed] [Google Scholar]
- 118.Whisenant J T Kadkhodayan Y Cross D T Moran C J Derdeyn C P Incidence and mechanisms of stroke after permanent carotid artery occlusion following temporary occlusion testing J Neurointerv Surg 2014. (e-pub ahead of print). doi:10.1136/neurintsurg-2014-011207 [DOI] [PubMed] [Google Scholar]
- 119.Mathis J M, Barr J D, Jungreis C A. et al. Temporary balloon test occlusion of the internal carotid artery: experience in 500 cases. AJNR Am J Neuroradiol. 1995;16(4):749–754. [PMC free article] [PubMed] [Google Scholar]
- 120.Loddenkemper T, Morris H H, Möddel G. Complications during the Wada test. Epilepsy Behav. 2008;13(3):551–553. doi: 10.1016/j.yebeh.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 121.Doppman J L. There is no simple answer to a rare complication of inferior petrosal sinus sampling. AJNR Am J Neuroradiol. 1999;20(2):191–192. [PMC free article] [PubMed] [Google Scholar]
- 122.Miller D L, Doppman J L, Peterman S B, Nieman L K, Oldfield E H, Chang R. Neurologic complications of petrosal sinus sampling. Radiology. 1992;185(1):143–147. doi: 10.1148/radiology.185.1.1523298. [DOI] [PubMed] [Google Scholar]
- 123.Bonelli F S, Huston J III, Meyer F B, Carpenter P C. Venous subarachnoid hemorrhage after inferior petrosal sinus sampling for adrenocorticotropic hormone. AJNR Am J Neuroradiol. 1999;20(2):306–307. [PMC free article] [PubMed] [Google Scholar]
- 124.Lefournier V, Gatta B, Martinie M. et al. One transient neurological complication (sixth nerve palsy) in 166 consecutive inferior petrosal sinus samplings for the etiological diagnosis of Cushing's syndrome. J Clin Endocrinol Metab. 1999;84(9):3401–3402. doi: 10.1210/jcem.84.9.6011-3. [DOI] [PubMed] [Google Scholar]
- 125.Blevins L S Jr, Clark R V, Owens D S. Thromboembolic complications after inferior petrosal sinus sampling in patients with Cushing's syndrome. Endocr Pract. 1998;4(6):365–367. doi: 10.4158/EP.4.6.365. [DOI] [PubMed] [Google Scholar]
- 126.Zurin A A, Ushikoshi S, Houkin K, Kikuchi Y, Abe H, Saitoh H. Cerebral abscess as an unusual complication of coil embolization in a dural arteriovenous fistula. Case report. J Neurosurg. 1997;87(1):109–112. doi: 10.3171/jns.1997.87.1.0109. [DOI] [PubMed] [Google Scholar]
- 127.Klisch J Huppertz H J Spetzger U Hetzel A Seeger W Schumacher M Transvenous treatment of carotid cavernous and dural arteriovenous fistulae: results for 31 patients and review of the literature Neurosurgery 2003534836–856., discussion 856–857 [DOI] [PubMed] [Google Scholar]
- 128.Nakagawa H, Kubo S, Nakajima Y, Izumoto S, Fujita T. Shifting of dural arteriovenous malformation from the cavernous sinus to the sigmoid sinus to the transverse sinus after transvenous embolization. A case of left spontaneous carotid-cavernous sinus fistula. Surg Neurol. 1992;37(1):30–38. doi: 10.1016/0090-3019(92)90062-r. [DOI] [PubMed] [Google Scholar]
- 129.Yamashita K, Taki W, Nakahara I, Nishi S, Sadato A, Kikuchi H. Development of sigmoid dural arteriovenous fistulas after transvenous embolization of cavernous dural arteriovenous fistulas. AJNR Am J Neuroradiol. 1993;14(5):1106–1108. [PMC free article] [PubMed] [Google Scholar]
- 130.Einhäupl K M, Villringer A, Meister W. et al. Heparin treatment in sinus venous thrombosis. Lancet. 1991;338(8767):597–600. doi: 10.1016/0140-6736(91)90607-q. [DOI] [PubMed] [Google Scholar]
- 131.Ferro J M Canhão P Stam J Bousser M-G Barinagarrementeria F; ISCVT Investigators. Prognosis of cerebral vein and dural sinus thrombosis: results of the International Study on Cerebral Vein and Dural Sinus Thrombosis (ISCVT) Stroke 2004353664–670. [DOI] [PubMed] [Google Scholar]


