Abstract
Vascular interventional radiology procedures are relatively safe compared with analogous surgical procedures, with overall major complication rates of less than 1%. However, major vascular injuries resulting from these procedures may lead to significant morbidity and mortality. This review will discuss the etiology, clinical presentation, diagnosis, and management of vascular complications related to percutaneous vascular interventions. Early recognition of these complications and familiarity with treatment options are essential skills for the interventional radiologist.
Keywords: iatrogenic vascular injury, endovascular management of vascular injury, interventional radiology
Objectives: Upon completion of this article, the reader will be able to identify the common iatrogenic vascular complications for percutaneous arterial and venous interventions.
Accreditation: This activity has been planned and implemented in accordance with the Essential Areas and Policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint providership of Tufts University School of Medicine (TUSM) and Thieme Medical Publishers, New York. TUSM is accredited by the ACCME to provide continuing medical education for physicians.
Credit: Tufts University School of Medicine designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Interventional radiology (IR) encompasses a vast array of procedures, many of which include percutaneous arterial and venous access. Compared with analogous surgical procedures, IR procedures are relatively safe, with overall major complication rates of less than 1%.1 Despite their rarity, major vascular injuries occurring as a result of these procedures may lead to significant morbidity and mortality. Therefore, it is paramount that the interventional radiologist remains cognizant of the etiology, clinical presentation, diagnosis, and management of the various iatrogenic vascular complications.
Arterial Complications
Arterial interventions are relatively safe with an overall minor complication rate (e.g., bleeding or hematoma) of less than 10%, and major complications requiring transfusion or surgical intervention occurring at a rate of less than 1%.2 However, the risk depends on the site of access, type of procedure, sheath size, patient risk factors, and operator experience.
Arterial Access
The incidence of vascular access site–related complications ranges from 0.8 to 1.8% for diagnostic arteriography, but has been reported to be as high as 9% when intervention is concomitantly performed.3 The overall risk of major complications associated with retrograde common femoral arterial catheterization is less than 2%, regardless of a single wall or double wall technique4;conversely, the risk of complications from axillary or brachial artery catheterization varies widely, from 2 to 24%.5 6 The smaller caliber of these upper extremity vessels, which traditionally have been commonly punctured with a double wall technique, and difficulty in compressing these arteries due to suboptimal bony support result in an increased risk of thrombosis and pseudoaneurysm formation compared with the femoral approach. Radial artery catheterization is increasingly utilized by interventional cardiologists due to an associated major bleeding rate of 0.05%, which compares favorably to femoral artery access.7 For the majority of abdominal and lower extremity peripheral vascular interventions, however, femoral access remains the standard approach.
The arteriotomy site for femoral arterial access should be at the midpoint of the common femoral artery, which is usually just inferior to the equator of the femoral head situated between the inferior epigastric artery and the bifurcation of the superficial and profunda branches.8 9 Over the past several years, many operators have begun using ultrasound (US) guidance for femoral artery punctures; this may increase the likelihood of appropriate femoral artery puncture sites. A puncture that is too cephalad increases the risk for hematoma and pseudoaneurysm formation, as there is no underlying osseous structure to aid in manual compression upon femoral sheath removal. A puncture that is too distal increases the risk of arteriovenous fistulas (AVFs), as the femoral vein courses posterior to and directly underneath the artery at this location.8 9 Overall, the predominant complications associated with arterial access include hematoma and retroperitoneal hemorrhage, pseudoaneurysm, and AVF.
Hematoma and Retroperitoneal Hemorrhage
Postprocedural bleeding or hematoma occurs at a rate of 2 to 12%, but those that are significant enough to require transfusion occur in fewer than 1% of cases.10 11 Hematomas most often occur at the arteriotomy site, resulting in induration and discoloration. Treatment includes local compression to achieve hemostasis and reversal of precipitating factors (e.g., coagulopathy, hypertension).12 If the hematoma enlarges or the patient becomes hypotensive, further management should include serial hematocrit levels as well as administration of intravenous fluids, blood transfusion, or surgical consultation.
Retroperitoneal hemorrhage is a life-threatening complication of femoral arterial access, with an incidence of 0.15 to 0.5% but with an associated mortality rate of 6.6%.13 14 15 Risk factors for significant bleeding include anticoagulation, hypertension, high femoral arterial puncture (i.e., above the inguinal ligament), and use of larger sheath sizes. Fluoroscopy should be performed prior to obtaining femoral artery access to identify the femoral head and thereby reduce the risk of puncturing the artery in a more proximal location.
Retroperitoneal hemorrhage should be suspected when there is serial decline of hemoglobin and clinical findings associated with blood loss such as dizziness, orthostatic hypotension, or ipsilateral flank/abdominal pain. Doppler ultrasound (DUS) is less sensitive than computed tomography (CT) in the detection of retroperitoneal hemorrhage. If there is concern for active bleeding, CT angiography (CTA) of the abdomen and pelvis may identify the source.
Although the majority of cases respond to conservative management, including cessation of anticoagulation/antiplatelet agents, aggressive hydration, and potential blood transfusion, active bleeding may necessitate intervention. Contralateral femoral artery access with angiography to localize the site of bleeding with subsequent balloon tamponade or selective embolization utilizing coils, polyvinyl alcohol, or Gelfoam of the affected artery may be necessary.16 When abdominal compartment syndrome results from a large retroperitoneal hemorrhage, immediate surgical intervention is mandated.15
Pseudoaneurysm
Access site pseudoaneurysms represent a contained rupture at the arteriotomy site and are relatively uncommon with proper technique. This complication occurs following 0.1 to 0.2% of diagnostic angiograms and 3.5 to 5.5% of interventional procedures.17 Large sheath size (>7 French), difficult arterial access, inadequate postprocedural compression after sheath removal, and longer procedure times are risk factors for the development of pseudoaneurysms.
Most small (<2 cm) pseudoaneurysms are asymptomatic and thrombose spontaneously. However, large (>2 cm), symptomatic, or persistent lesions typically require treatment. Disproportionate and persistent pain, swelling, a palpable pulsatile mass, and a bruit on auscultation are indications to perform a targeted US evaluation.18 19 20
DUS has a high sensitivity (94%) and specificity (97%) for the detection of pseudoaneurysms and may demonstrate the classic “yin-yang” pattern of flow, representing to-and-fro flow signal, as well as a tract (“neck”) to the native artery.21 Additionally, simultaneous evaluation of the adjacent vein can be performed to assess for extrinsic compression and thrombosis, as well as the presence of a concomitant AVF. CTA may be beneficial in patients in whom there is high clinical suspicion but DUS fails to demonstrate a pseudoaneurysm, especially if there is concern for concomitant retroperitoneal hemorrhage.
Historically, US-guided compression, in which the transducer is used to compress the neck of the pseudoaneurysm while maintaining flow to the distal artery, served as the first-line treatment, with successful pseudoaneurysm obliteration in 75 to 85% of cases.22 23 24 However, this method is painful, time-consuming, and sometimes ineffective, particularly in anticoagulated patients (Fig. 1). Therefore, percutaneous US-guided thrombin injection has become the first-line treatment for iatrogenic pseudoaneurysms at many institutions.24 25 26 27
Fig. 1.
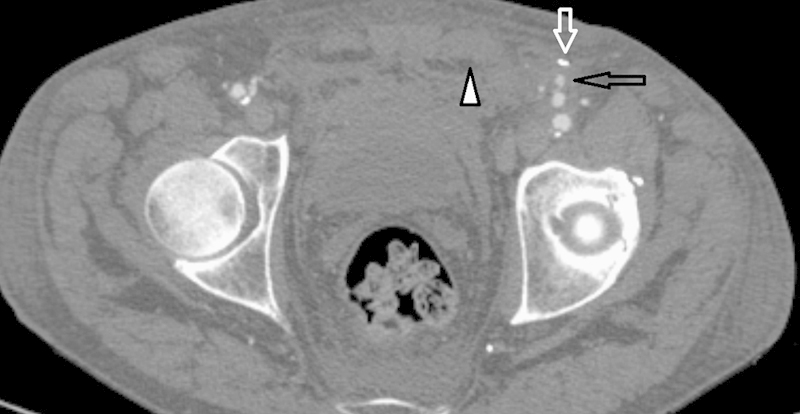
Left common femoral artery pseudoaneurysm (open black arrow) after antegrade common femoral artery puncture. Note the extraperitoneal hematoma (arrowhead). Also visible is the metallic streak artifact from failed closure device (open white arrow), likely secondary to the presence of heavily calcified atherosclerotic disease. This pseudoaneurysm resolved on its own on short-term follow-up.
Real-time US guidance is used and a 21- to 22-gauge needle is inserted into the pseudoaneurysm sac, directed away from the neck. US-guided aspiration into a saline-filled syringe with subsequent flushing to demonstrate aliasing under US can confirm the needle's position within the pseudoaneurysm sac. Bovine thrombin (100–1,000 units/mL) is then slowly injected into the lesion over 5 to 10 seconds and clot formation is monitored with color Doppler. It is important to recognize that injection is ceased once thrombus begins to form. Once coagulation is initiated, it can often be completed spontaneously without further thrombin injection. The success rate is greater than 90%, even in the face of anticoagulation,24 25 26 27 although treatment is more problematic with complex pseudoaneurysms. A second attempt should be made following a failed first attempt; however, the patient and operator should be aware that prior exposure to thrombin (topical or otherwise) may lead to antibody formation resulting in a small risk of an anaphylactic reaction. Although complications are rare, limb-threatening embolization and downstream thrombosis have been reported (Fig. 2).28 29 30
Fig. 2.
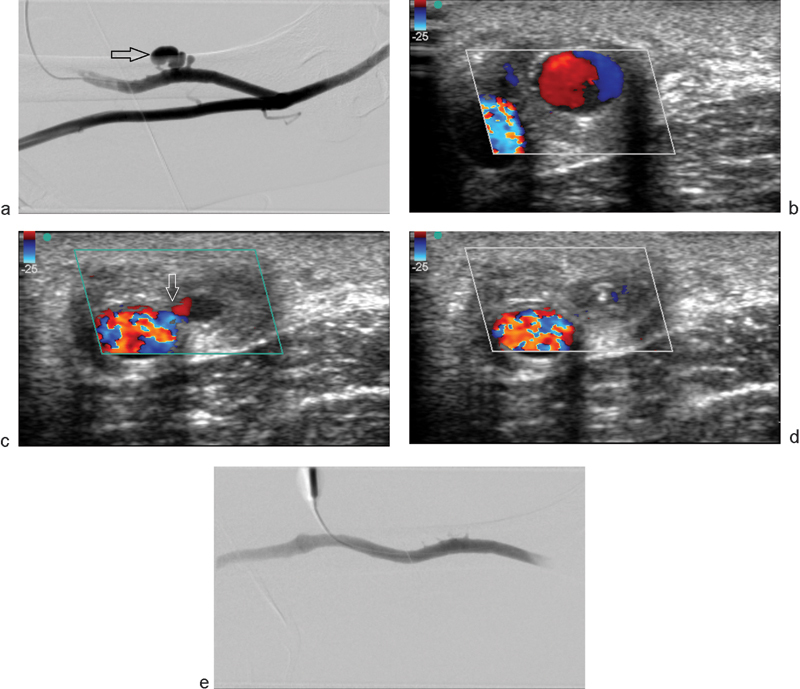
(a) Left upper arm dialysis fistulogram showing a multilobulated outpouching (arrow) arising from an arteriovenous dialysis access graft and representing a pseudoaneurysm. Clinically, this patient presented with an expansile, palpable, and painful mass. (b) Color Doppler ultrasound examination demonstrates the characteristic “yin-yang” of turbulent flow within the pseudoaneurysm. (c) A different obliquity shows a narrow neck (arrow) of the partially thrombosed pseudoaneurysm after injection of 100 units of thrombin. (d) Color Doppler image demonstrates completely thrombosed pseudoaneurysm after a total of 200 units of thrombin. (e) Postthrombin injection fistulogram demonstrates complete resolution of flow within the pseudoaneurysm.
Arteriovenous Fistula
AVFs are much less common than pseudoaneurysms following arterial catheterization, with a reported incidence of 0.017 to 0.86%.31 32 Approximately 33% of AVFs resolve spontaneously within 1 year.32 The clinical signs and symptoms are often similar to pseudoaneurysm, and include persistent pain and a pulsatile mass. Therefore, diagnosis typically requires imaging, and is usually confirmed with DUS.
The supplying artery typically demonstrates high-frequency, low-resistance flow and the vein shows a high-velocity, arterialized waveform pattern. The majority of access site AVFs can be managed conservatively; however, intervention is recommended for those AVFs persisting beyond 2 months, significantly increasing in size, or that become symptomatic (e.g., limb ischemia, arterial insufficiency, or congestive heart failure).19
Acute AVFs can be treated with US compression, but this method may be painful and is associated with high failure rates particularly in cases of short, broad fistulous tracts; AVFs more than 1 month old; AVFs with high flow rates; and large fistula sizes.
Coil embolization is an effective option for complex AVFs with easily accessible feeding and draining vessels. To prevent coil migration, the distal end of the coil may be anchored within a nonvital side branch.33 Contraindications to coil embolization includes high potential for nontarget embolization, the risk of end-organ ischemia, and the inability to reach the target site by an endovascular approach.
Vascular plugs provide another treatment option. A single vascular plug may suffice for the occlusion of large vessels. Vascular plugs also produce a greater cross-sectional coverage of the vessel lumen than do coils.
Endovascular covered stents are a popular choice as their use is associated with a high technical success rate and a low 1-year complication rate.34 However, subacute occlusion may occur as a result of increased thrombogenicity secondary to delayed endothelialization of the stent graft. Also, if the stent graft is placed near a joint involved with frequent flexion motion, kinking and compression of the stent graft may occur. Precise placement of the stent graft is crucial when adjacent to a vascular bifurcation so as to not occlude one of the branches.
Complications of Endovascular Interventions—Angioplasty and Stenting
Peripheral arterial disease (PAD) most commonly affects the lower extremities. Endovascular techniques such as angioplasty, stenting, and atherectomy are widely employed to treat patients with symptomatic PAD. Atherosclerosis is the predominant cause of lower extremity ischemia with smoking, diabetes, hypertension, and hyperlipidemia being the principal risk factors for development of atherosclerosis.
Revascularization is indicated in cases of limb-threatening ischemia or lifestyle-limiting claudication. Vascular access to the lower extremities is typically via retrograde contralateral common femoral artery access, although the retrograde high brachial artery may be preferred in patients with a history of cholesterol embolization during prior femoral artery access.35 Antegrade access is generally avoided due to a higher risk of dissection. It is paramount for the interventional radiologist to be familiar with both normal and variant anatomy of the peripheral arteries; preprocedural CT or MR angiography may help guide the intervention and limit complications.
Balloon angioplasty and stenting have demonstrated effectiveness in treating arterial stenoses. The iliac arteries are the most widely studied with complication rates ranging from 4 to 20%, but these rates are difficult to assess due to variations in reporting and grading classifications.36 37 38 Vessel size appears to be a major determinant of complication risk as the external iliac arteries have a higher complication rate than the common iliac arteries and women are at higher risk than men.39
Percutaneous transluminal angioplasty (PTA) of peripheral arteries is relatively safe, with the reported overall complication rate being less than 10%. Approximately 4% of patients will experience arteriotomy site bleeding or pseudoaneurysm formation, the majority of which are self-limited. Vasospasm is frequently encountered but the prophylactic local administration of vasodilators, such as 100 to 200 μg nitroglycerin, can often prevent spasms.35 If vasospasm persists or limits flow, a heparin bolus is recommended to prevent superimposed thrombosis.
PTA has demonstrated technical success rates of greater than 95% in revascularizing the superficial femoral artery (SFA), with a low risk of complications.40 However, Schillinger et al41 demonstrated primary stenting decreased the incidence of restenosis compared to balloon angioplasty with optional secondary stenting at both 6 and 12 months (24 vs. 43% and 37 vs. 63%, respectively) in the management of SFA stenosis. Endovascular stenting may be utilized in long, complex lesions as it evades some of the problems associated with balloon angioplasty, including flow-limiting dissection, residual stenosis, and elastic recoil. Late failure remains an important clinical problem following PTA with restenosis occurring in 40 to 60% of treated segments after 1 year.40 42
Restenosis
Restenosis is the most common complication following endovascular peripheral arterial interventions, particularly for long segments, occurring at a rate of up to 50% per year.43 Femoropopliteal angioplasty has a 5-year primary patency rate of 25 to 55% compared with up to 80% for surgical bypass grafting. Despite this, angioplasty continues to be commonly utilized because of its lower cost and complication rates relative to surgery, as well as the ability to retreat recurrent lesions.44
While drug-eluting stents have shown a reduction in restenosis of the coronary arteries, clinical trials have failed to demonstrate such a correlation within the lower extremities45. Self-expanding nitinol stents have yielded better results than those obtained with standard balloons at the femoropopliteal level.41 The use of paclitaxel-coated angioplasty balloons in the management of femoropopliteal disease has exhibited significant reductions in late lumen loss and target-lesion revascularization rates.46
Thrombosis
Up to 3% of patients will experience angioplasty-site thrombosis. PTA induces a prothrombotic state as it disrupts atherosclerotic plaques, leading to platelet aggregation. This prothrombotic state ultimately favors early (30 days) thrombotic occlusion.
DUS has demonstrated success in predicting early (30 days) and midterm (6 months) arterial thrombosis after femoropopliteal interventions. Specifically, a popliteal artery volume flow of less than 100 mL/min following angioplasty of a more proximal segment has proven to be a powerful predictor of subsequent arterial thrombosis.47
With regard to the treatment of arterial thrombosis, streptokinase, urokinase, recombinant tissue plasminogen activator alteplase (t-PA), and reteplase (r-PA) can all be utilized as thrombolytic agents. The duration of thrombolytic therapy is typically 12 to 36 hours. These agents are most effective in the treatment of acute or subacute thrombosis and can be delivered via drip infusion or pulse-spray techniques. In drip infusion, a catheter with multiple side holes spans the entire length of the thrombus and delivery of the thrombolytic agent is relatively slow. In pulse-spray thrombolysis, however, small aliquots of concentrated thrombolytic agent are forcibly injected through the catheter at short intervals, resulting in a more rapid lysis. Pharmacomechanical thrombolysis using one of multiple commercially available devices is also an option.
Contraindications to thrombolysis include irreversible limb ischemia, active hemorrhage, recent major surgery, stroke within the past 6 months, craniotomy or eye surgery within the past 2 months, and brain tumors. The vast majority of complications of thrombolysis are hemorrhagic and involve the access site.
Visonà et al48 determined that aspirin (75–100 mg/d), with or without dipyridamole, started prior to femoropopliteal endovascular treatment and continued lifelong, reduced the incidence of reocclusion at 6 and 12 months when compared with no therapy or vitamin K antagonists. Additionally, the use of low molecular weight heparin may be superior to unfractionated heparin in the prevention of early and midterm reocclusion after femoropopliteal angioplasty.48
Distal Embolization
Distal embolization occurs in ∼25% of patients undergoing angioplasty (with or without stenting) and up to 90% of patients undergoing atherectomy.49 However, only 2% of angioplasty patients and 4% of atherectomy patients experience clinically significant embolization.50 Embolic particles are mainly composed of thrombus and patients may benefit from thrombolytic therapy.37 51 52 Primary treatment strategies include mechanical aspiration, administration of antiplatelet agents, or catheter-directed thrombolysis with TPA (Fig. 3). Surgical consultation is warranted if endovascular methods fail.53 54 Embolic atherosclerotic plaque or cholesterol may require other techniques such as percutaneous or surgical embolectomy for definitive treatment.55
Fig. 3.
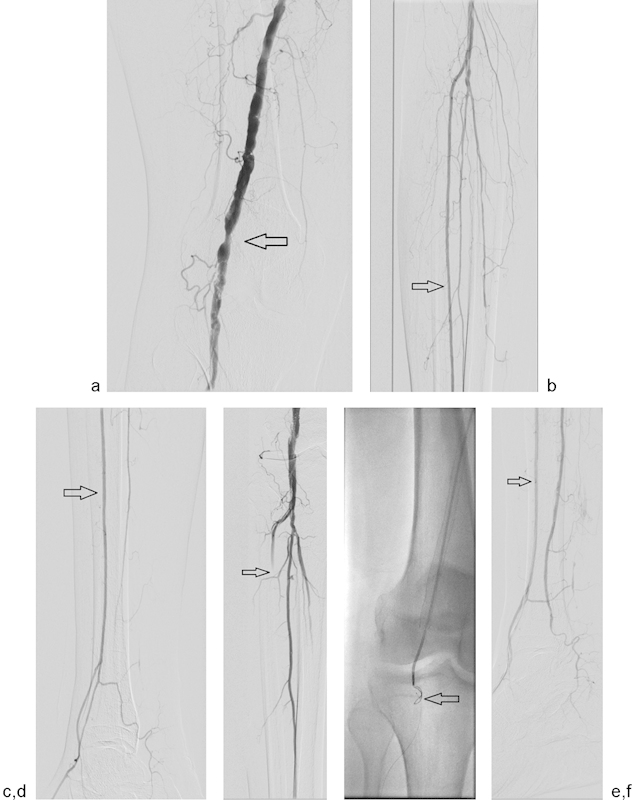
(a) Initial superficial femoral artery and popliteal runoff image from a 62-year-old man with lifestyle-limiting calf claudication. Note the severe stenosis above the knee joint (arrow). (b and c) Two-vessel runoff with dominant supply by the anterior tibial artery (arrow). (d) After stent placement in the distal SFA, the anterior tibial artery is occluded by distal embolization or in situ thrombosis (arrow). (e) A guidewire could not cross the occlusion, so retrograde access through the dorsalis pedis artery was achieved. A 0.014-inch wire was placed in retrograde fashion through the occlusion and snared from above (arrow). Once wire access was achieved, Angiojet (Boston Scientific, Marlborough, MA) was used for thrombectomy. (f) Postthrombectomy digital subtraction angiography demonstrated a patent anterior tibial artery (arrow).
Dissection
The incidence of dissection secondary to endovascular intervention is uncertain as many cases are asymptomatic. During initial access, coiling of the wire under fluoroscopy or resistance to passage may indicate subintimal passage with resulting retrograde arterial dissection. In such instances, careful withdrawal of the wire, repositioning of the access needle, or a new puncture is recommended. The anterograde flow of blood is usually sufficient to tack down these small dissection flaps without further complication.
When angioplasty is performed in significantly diseased arteries or with oversized balloons, hemodynamically significant dissection may occur. The external iliac artery is particularly prone to such dissection. Subintimal angioplasty techniques, most often performed in the iliac or SFA, carry a risk of inadvertent dissection. In this technique, a dissection is intentionally created utilizing a wire and catheter to cross a total occlusion. The true lumen is reentered at the distal end of the occlusion; however, extension of the dissection further distally than originally intended may be necessary in complicated cases.
The majority of non–flow-limiting dissections can be clinically observed and will spontaneously resolve. Flow-limiting dissections, or those associated with limb ischemia, typically mandate intervention. If there is a localized femoral artery dissection, patch angioplasty and endarterectomy are treatment options. In proximal common or external iliac artery dissection, a self-expanding stent can be placed from a contralateral femoral approach (Fig. 4).
Fig. 4.
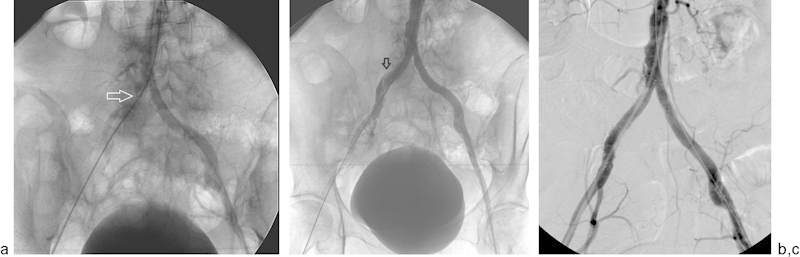
A 67-year-old woman presented with acute lower gastrointestinal bleeding and underwent visceral angiography via a right common femoral artery approach. After multiple catheter exchanges, it was difficult to advance the wire at the level of right common iliac artery and the left common femoral artery was accessed. (a) Abdominal aortogram demonstrates lack of flow distal to the right common iliac artery suggesting dissection (arrow). (b) After angioplasty, flow was restored in the right common iliac artery. However, flow was relatively slow and a dissection flap is visible involving right common iliac artery (arrow). (c) Bilateral common iliac artery 10-mm diameter self-expanding kissing stents were deployed. Contrast injection revealed widely patent bilateral common iliac artery stents.
Rupture
The incidence of arterial rupture during aortoiliac angioplasty and stenting is extremely rare (less than 0.1%), but it is among the most dreaded complications.51 56 Risk factors include variables that influence the strength and integrity of the arterial wall such as chronic steroid use, overinflation during angioplasty, calcified plaque that penetrates the arterial wall, fibromuscular dysplasia, and vasculitis/arteritis.36 37 Once the rupture is recognized, it is critical to maintain wire access across the angioplasty site so that bleeding can be controlled with balloon tamponade.57 Definitive therapy, such as endovascular stent grafting, can be performed after the patient is hemodynamically stable (Fig. 5).
Fig. 5.
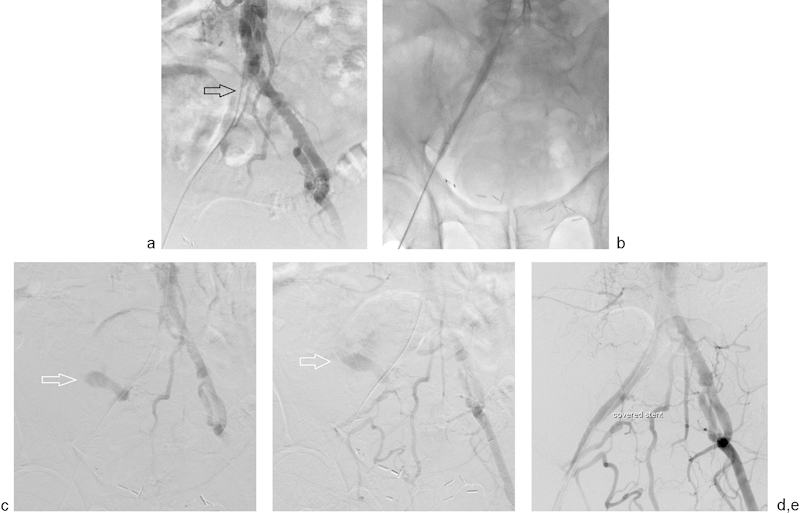
A 68-year-old man presents for treatment of his longstanding right common and external iliac artery occlusions. (a) Digital subtraction angiogram performed via right common femoral artery approach confirms occlusion of right common and external iliac arteries (arrow). (b) After accessing the vessels from the right common femoral artery, the iliac artery was angioplastied to 8 mm. (c and d) Postangioplasty digital subtraction angiography demonstrates extravasation of contrast due to external iliac artery rupture (arrow). The patient experienced immediate hypotension and hemodynamic instability. (e) The angioplasty balloon was immediately reinflated and a 9-mm stent graft was placed to cover the rupture. Poststent graft images demonstrated resolution of the extravasation. The patient regained hemodynamic stability immediately with the help of IV fluid resuscitation. He was discharged uneventfully the following morning.
Venous Complications
Venous Access
Central venous catheters (CVCs) are commonly placed for the administration of drugs, fluids, parenteral nutrition, procedures of dialysis/apheresis, monitoring of central venous pressures, and cardiac pacing. CVCs include both peripherally inserted (PICC) and centrally inserted (CICC) CVCs.58 Specific CVC types are often indicated based on a given medication's pH, rates of infusion, and estimated length of use.58
Approximately 5 million CVCs are placed annually, and complications have substantial impact on the health care system.59 Various factors influence the incidence of CVC complications, which can be separated into administrative policies (routine training of health care providers in aseptic technique), insertion techniques (blind vs. US-guided), stabilization (with or without sutures), type of lumen (single or multiple), tip position, device lumen-to-vein ratio, site of insertion, device selection, and care of the venous access device after placement (dressing type, access technique, flushing/locking, replacement time).58 Randomized controlled trials designed to determine the relationship between various sites of insertion (femoral, internal jugular, or subclavian) and complications have been inconclusive.60
Thrombosis
Thrombosis is a commonly encountered CVC complication. A recent study of over 7,000 CVC placements demonstrated a 2.1% incidence of catheter-associated deep venous thrombosis. This rate was dramatically influenced by individual patient factors. For example, the incidence of catheter-associated deep venous thrombosis increased to 4.8% in the elderly or malnourished, and up to 6.8% in patients with inflammatory bowel disease.61 Current guidelines on the prevention of catheter thrombosis recommend the use of US-guided insertion.62 A prospective nonrandomized study reported that US reduces intimal damage compared with blind insertion, explaining the reduction in catheter-associated thrombosis.63 Thrombosis is also less frequently noted with appropriate vein/catheter diameters and “central” positions of the catheter tip.64 Patients with hypercoagulable states often have indications for CVC insertion, such as cancer (requiring chemotherapy), renal failure (requiring dialysis), infection (requiring IV antibiotics), or malnutrition (requiring total parenteral nutrition). The activated partial thromboplastin time is an independent predictor of catheter-associated thrombosis in cancer patients with hypercoagulable states.65 An understanding of the variables that predispose to catheter-associated thrombosis can aid in its prevention.
Central Venous Catheter Misplacement
Insertion failure rates vary from 5.0 to 8.9% for nonimage-guided placement but decrease to less than 2.0% when radiologic guidance is used.66 67 68 69 Misplacement ranges in severity from the common malpositioned tip to the rarer canalization of adjacent vessels, mediastinal structures, or pleura resulting in important complications such as hematoma, unintentional arteriotomy, and pneumothorax.70 When using real-time US guidance, however, randomized studies report decreased risks of cannulation failure (RR of 0.18, 95% CI: 0.10–0.32), arterial puncture (RR of 0.25, 95% CI: 0.15–0.42), hematoma (RR of 0.30, 95% CI: 0.19–0.46), pneumothorax (RR of 0.21, 95% CI: 0.06–0.73), and hemothorax (RR of 0.10, 95% CI: 0.02–0.54).71
Hematomas typically occur at the venotomy site, subcutaneous tunnel, or pocket, and are more common in patients with bleeding diatheses. Most hematomas occur from minor vein wall trauma during CVC placement and go unnoticed. These hematomas resolve spontaneously because the low venous pressures allow adjacent structures to tamponade the bleed, and typically do not require treatment.72
Occasionally, inadvertent puncture of an adjacent artery results in an enlarging hematoma. With right internal jugular vein access, iatrogenic arterial puncture rates range from 1 to 11%.72 73 If arterial puncture is suspected, careful inspection of the guidewire course should allow the operator to determine if the wire is following a venous or arterial course.73 If arterial puncture is still uncertain, injection of contrast through the puncture site needle or smallest catheter possible (usually 3 French) will determine its position.74 In the event an inadvertent arterial puncture is discovered, treatment usually consists of removing the needle followed by local pressure at the arteriotomy site. However, if the inadvertent arteriotomy is dilated or the anatomy precludes local compression, a closure device may be considered for hemostasis.75 76 77 For this reason, it is essential that wire access be maintained. Surgical consultation is recommended if bleeding persists despite local pressure or application of a closure device. It should be noted that the use of US guidance has virtually eliminated significant unintentional arterial punctures.
Finally, iatrogenic malposition into the pericardium or pleural space can occur either from direct puncture at the time of placement or from catheter tip erosion. The worst outcome is the uncontrolled hemorrhage into the low pressure pericardium or pleural space, both of which can rapidly accumulate large volumes of blood resulting in major morbidity and mortality.66 72 74 Additionally, infusion of fluid or drugs into a malpositioned catheter may cause a rapidly developing pericardial or pleural effusion.77 Pericardial placement or erosion of the catheter tip may result in cardiac tamponade physiology or hemopericardium, which can be detected on imaging studies. The catheter should be left in place and aspiration of the fluid attempted.72 78 Depending on the stability of the patient, urgent pericardiocentesis with endovascular stenting or thoracic surgical intervention may be performed.
The pleural space is in close proximity to the upper extremity central veins and pneumothoraces occur more often with subclavian vein (2.2%) than with jugular vein catheterization (0.6%).79 Placement of catheters directly into the pleural space occurs far less frequently. Most pneumothoraces resolve spontaneously with line removal but some patients may require a chest tube (Fig. 6).80 A surgical consult is rarely needed.
Fig. 6.
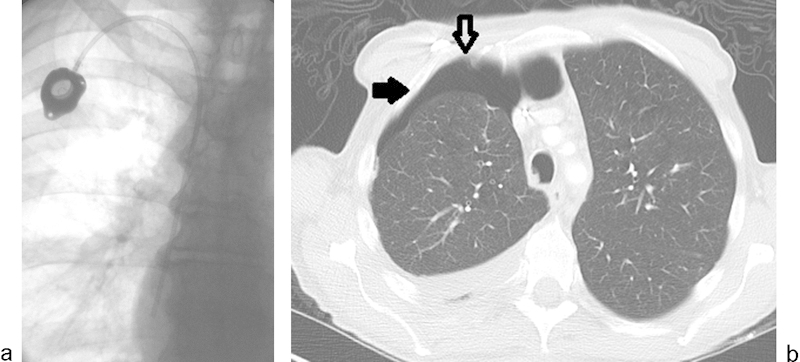
(a) Postprocedure image from an uneventful right internal jugular vein chest port placement. Real-time ultrasound guidance was used. (b) Asymptomatic moderate right hydropneumothorax noted on electively scheduled restaging CT 4 days later (solid arrow). Chest port is visible within the right chest wall (open arrow).
Venous Perforation
A rare but serious complication of venous catheter placement is venous perforation. This may occur when advancing a stiff catheter, dilator, or peel-away sheath over a kinked wire. The kink causes the wire and catheter to move in unison, which results in a slice through the wall of the vein.74 Fluoroscopic visualization and gentle back-tension on the guidewire will help avoid kinking. If venous perforation does occur, it is imperative to maintain wire access. A venogram can be performed to assess and characterize the perforation. Treatment options include balloon tamponade or covered stent placement; a surgical consultation is required if endovascular treatment methods fail.
Venous Angioplasty
Balloon angioplasty has demonstrated mixed results in the treatment of central upper extremity venous and dialysis access stenoses. The technical success rate for angioplasty of central upper extremity venous stenosis is estimated at 75%, with 6-month primary patency falling to less than 30% and most treated stenosis failing within 2 years without reintervention.81 82 83 Endovascular techniques such as thrombolysis and angioplasty are preferred over surgery in the treatment of dialysis graft/fistula stenosis, and primary patency rates of at least 50% can be expected at 1 year.84 85 86 Restenosis often occurs, but because balloon angioplasty is minimally invasive and well tolerated, serial dilatations can often improve patency rates without the need for surgery. Venous angioplasty complications are often minor (e.g., hematomas, postprocedural bleeding) and occur in fewer than 10% of cases.82
Occasionally, vein rupture may occur during angioplasty for which the significance depends on the extent of rupture. Vascular rupture occurs in 2 to 6% of cases, more often in fistulas than grafts, but complicated rupture requiring blood transfusion or emergent surgery, and limb-threatening ischemia occur in fewer than 0.5% of cases.87 88 Small ruptures present as mild irregularities of the vessel lumen during the postangioplasty fistulogram and are often clinically insignificant, resolving without treatment. Large ruptures appear as extravascular contrast pooling with a rapidly expanding subcutaneous hematoma. Because the angioplasty balloon should already be in place, reinflation of the balloon often seals the leak. If the rupture persists despite prolonged balloon inflation, a covered stent can be used. If stenting is not an option, the graft may be embolized or compressed until clotting occurs (Fig. 7).89 90 91
Fig. 7.
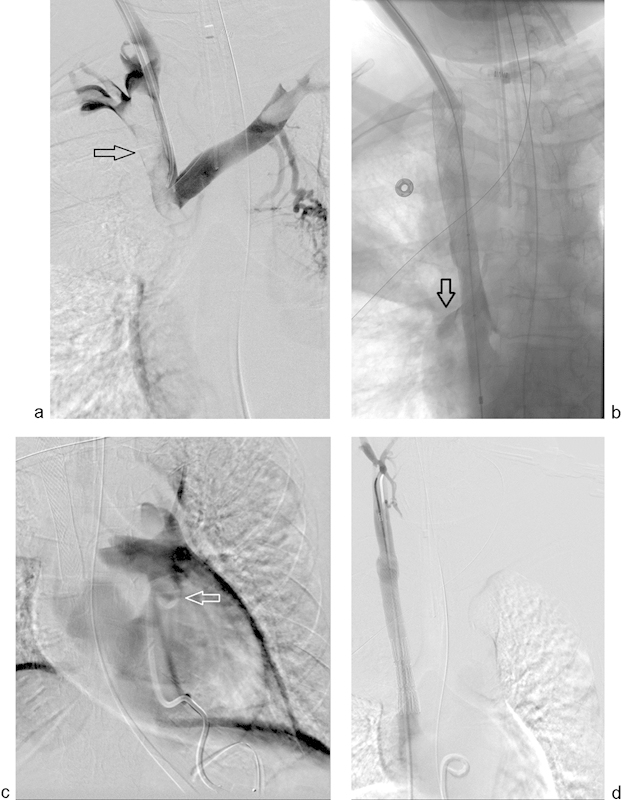
(a) Initial venogram from a 51-year-old woman with mediastinal malignancy and acute SVC syndrome, demonstrating complete superior vena cava (SVC) occlusion and likely superimposed thrombus (arrow). (b) After recanalizing and stenting of the SVC, the patient became acutely hypotensive. Venography demonstrates contrast extravasation into the pericardium (open arrow). (c) Contrast injected after emergent pericardiocentesis through a 10 French locking loop drainage catheter (arrow) confirms placement in pericardium. Eight hundred milliliter of blood was withdrawn. (d) SVC rupture was repaired with a covered endograft and the patient stabilized.
Inferior Vena Cava Filters
Inferior vena cava (IVC) filters and their associated complications are a significant consideration when discussing vascular complications of IR procedures. IVC filters are placed for the prevention of life-threatening pulmonary emboli (PE). A randomized controlled trial studying IVC filters (PREPIC trial) reported an initial reduction of symptomatic PE with IVC filters compared with anticoagulation. However, after 8 years of follow-up, a statistically significant difference between the IVC filter and anticoagulation was no longer maintained and IVC filters were found to increase symptomatic deep vein thromboses (DVTs). It is important to note that patients in the PREPIC trial received anticoagulation, so its findings are not translatable to patients who cannot receive anticoagulation.92 Because IVC filters are increasingly utilized as a supplement to anticoagulation for the prevention of PE, it is important to consider their associated complications.93
Many recent trials separate IVC filter complications into periprocedural or long term.94 As adapted from the SIR practice guidelines, periprocedural complications include death (0.12%), filter embolization (0.1%), malposition (1 to 9%) (Figs. 8 and 9), filter migration >2 cm (1.3–4.5%), and access site occlusive thrombosis (3–10%).95 Based on manufacturer experience (MAUDE database), most complications are associated with retrievable filters.96 Symptomatic DVTs are one of the most common types of periprocedural complication and could be related to a hypercoagulable patient population compounded by preexisting IVC stenosis or thrombosis.97 This may also result in recurrent PE as the thrombus extends cranial to the filter.98 It remains uncertain if a given filter thrombosis is caused by a trapped thrombus or by thrombus forming in situ.99 Propagating IVC thrombosis has been shown to be manageable with mechanical thrombectomy, catheter-directed thrombolysis, or balloon angioplasty.100
Fig. 8.
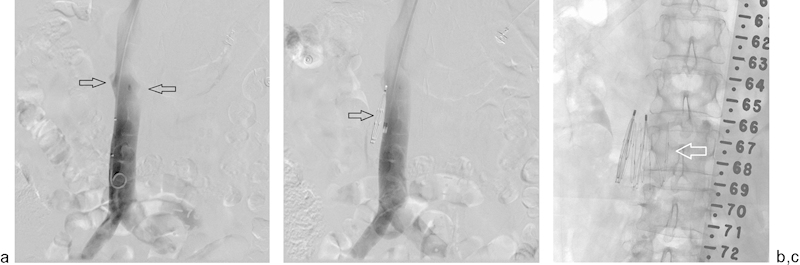
A 65-year-old woman with a glioblastoma multiforme and acute pulmonary embolism with iliofemoral deep vein thrombosis was referred for an emergent IVC filter placement. (a) Cavagram via the right internal jugular vein demonstrates the location of the renal veins (arrows). (b) A VenaTech IVC filter was deployed, but failed to open. Repeat cavagram demonstrates the filter to have been deployed alongside the IVC (arrow), suggestive of gonadal vein placement. No attempts were made to retrieve this filter. (c) Another VenaTech LP filter was placed appropriately below the renal veins within the IVC (arrow).
Fig. 9.
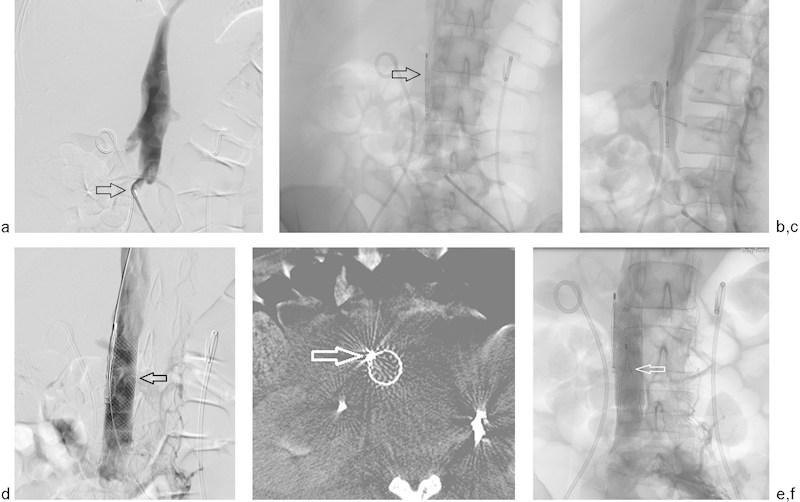
(a) Cavagram before filter placement on a 46-year-old woman with a right-sided iliofemoral DVT from pelvic malignancy. Access was gained from the left common femoral vein. Note the sharp angle taken by the left iliac vein as it joins the IVC (arrow). (b) Retrievable filter was deployed within the wall of the inferior vena cava (arrow). (c) This is demonstrated by contrast injection through the cava. (d) An 18-mm self-expanding stent (Wallstent, Boston Scientific Inc., Marlborough, MA) was deployed (arrow) to secure the undeployed filter to the IVC wall. (e) Cone-beam CT was performed to further confirm the position of the filter (arrow). (f) A second filter (Denali, Bard Inc., Tempe, AZ) (arrow) was placed lower in the IVC within the stent.
Mechanical complications related to IVC filters are a rare but significant long-term complication and include strut fracture, filter tilt, filter migration, and IVC penetration.101 The overall mechanical complication rates for IVC filters are difficult to determine because of their rarity, large variability of reported complications per manufacturer, and lack of randomized controlled trials. For example, IVC penetration, defined as filter elements >3 mm beyond the lumen or within an adjacent structure, has not been systematically reported.96 However, according to the MAUDE database, IVC filter perforations account for up to 20% of reported complications, with clinically significant perforation occurring 0.4% of the time.102
Studies have reported higher complication rates with retrievable filters compared with permanent ones, with one study attributing 87% of filter complications to retrievable filters.103 IVC filters are commonly assessed with contrast-enhanced CT or CT venograms to evaluate filter integrity, tilt, and caval penetration. If a filter strut is determined to be missing, CT of the chest should be performed to evaluate for strut embolization.
Standard retrieval techniques can be used if the IVC filter tip remains centered in the caval lumen. However, ∼15% of filters cannot be removed with standard retrieval techniques because of tip or wall embedment. Advanced techniques using endobronchial forceps or laser sheaths may be employed by experienced practitioners.104 105 In limited series, the endobronchial forceps and laser sheath techniques were able to retrieve 95 and 96% of IVC filter bodies, respectively.104 106 Embolized fractured fragments of the IVC filter can also be retrieved with the endobronchial forceps technique.
Fractured Catheters
The incidence of dislodgement or fracture of subcutaneous implanted CVCs is between 0.2 and 1.0%.107 The majority of patients with this complication are asymptomatic and detected incidentally on imaging. Fractured catheter tips are most commonly located within the right atrium, right ventricle, and pulmonary artery.107 Immediate removal of the migrated fragment is recommended since subsequent embolization, myocardial rupture, valvular perforation, and infection may ensue.108
A percutaneous central venous access approach is the most commonly utilized technique for removal of intravascular foreign bodies, and retrieval can be accomplished with hooked guidewires, snares, Fogarty balloon catheters, or Dormia baskets (Fig. 10).109 While central access is the most common approach, there is a case report of percutaneous retrieval of an intravascular foreign body utilizing peripheral forearm venous access.110 Guide catheter kinking and fracture within the femoral arterial sheath with subsequent contralateral arterial access and snare removal has also been reported.111
Fig. 10.
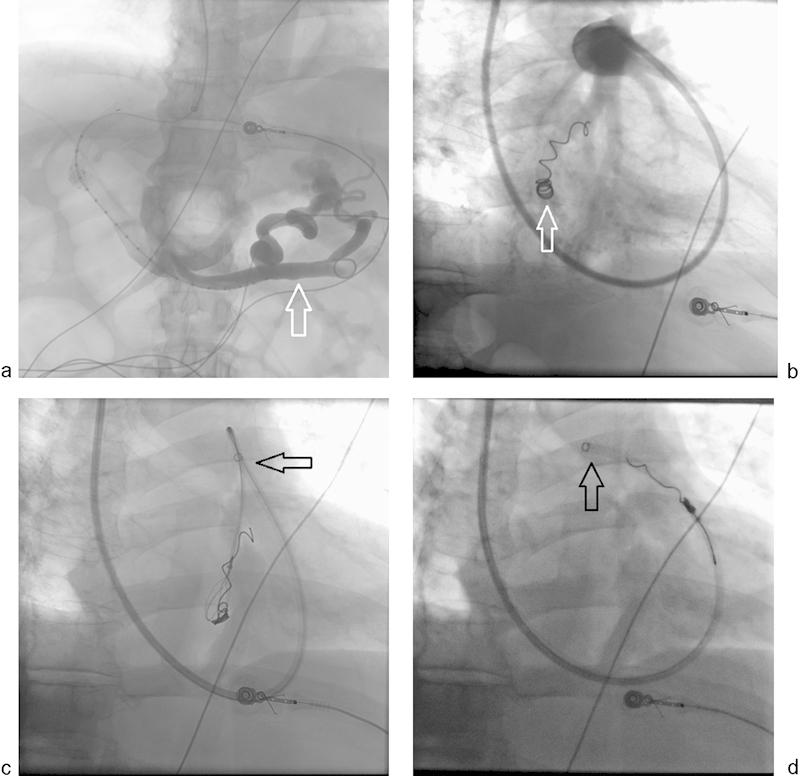
A 52-year-old man with portal hypertension became acutely unstable due to massive upper gastrointestinal variceal bleed. (a) An emergency transjugular intrahepatic portosystemic shunt from right hepatic vein to right portal vein was performed with demonstration of massive residual esophageal varices (arrow). (b) While attempting to embolize the varices, an undersized 6-mm coil that was deployed in the varix migrated quickly to the heart and lodged in a branch of the right pulmonary artery (arrow). (c and d) The TIPS sheath was relocated into the right pulmonary artery (arrow) and a 10-mm gooseneck snare was used to retrieve the coil.
Conclusion
Most iatrogenic percutaneous vascular injuries are self-limited and of minimal clinical significance. Despite radiologic guidance, life-threatening vascular complications remain an important consideration during arterial and venous interventions. These complications can generally be managed with endovascular techniques with a select subpopulation of patients requiring surgical intervention. In these cases, early recognition and familiarity with treatment strategies are invaluable skills for anyone performing even simple interventions.
Acknowledgment
The authors thank Megan Griffiths, scientific writer for the Imaging Institute, Cleveland Clinic, Cleveland, Ohio, for her help in revising the article.
References
- 1.Sacks D, McClenny T E, Cardella J F, Lewis C A. Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol. 2003;14(9, Pt 2):S199–S202. doi: 10.1097/01.rvi.0000094584.83406.3e. [DOI] [PubMed] [Google Scholar]
- 2.Singh H, Cardella J F, Cole P E. et al. Quality improvement guidelines for diagnostic arteriography. J Vasc Interv Radiol. 2002;13(1):1–6. doi: 10.1016/s1051-0443(07)60001-3. [DOI] [PubMed] [Google Scholar]
- 3.Arora N, Matheny M E, Sepke C, Resnic F S. A propensity analysis of the risk of vascular complications after cardiac catheterization procedures with the use of vascular closure devices. Am Heart J. 2007;153(4):606–611. doi: 10.1016/j.ahj.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 4.Egglin T K, O'Moore P V, Feinstein A R, Waltman A C. Complications of peripheral arteriography: a new system to identify patients at increased risk. J Vasc Surg. 1995;22(6):787–794. doi: 10.1016/s0741-5214(95)70070-6. [DOI] [PubMed] [Google Scholar]
- 5.Lipchik E O, Sugimoto H. Percutaneous brachial artery catheterization. Radiology. 1986;160(3):842–843. doi: 10.1148/radiology.160.3.3737927. [DOI] [PubMed] [Google Scholar]
- 6.Chitwood R W Shepard A D Shetty P C et al. Surgical complications of transaxillary arteriography: a case-control study J Vasc Surg 1996235844–849., discussion 849–850 [DOI] [PubMed] [Google Scholar]
- 7.Jolly S S, Amlani S, Hamon M, Yusuf S, Mehta S R. Radial versus femoral access for coronary angiography or intervention and the impact on major bleeding and ischemic events: a systematic review and meta-analysis of randomized trials. Am Heart J. 2009;157(1):132–140. doi: 10.1016/j.ahj.2008.08.023. [DOI] [PubMed] [Google Scholar]
- 8.Sherev D A, Shaw R E, Brent B N. Angiographic predictors of femoral access site complications: implication for planned percutaneous coronary intervention. Catheter Cardiovasc Interv. 2005;65(2):196–202. doi: 10.1002/ccd.20354. [DOI] [PubMed] [Google Scholar]
- 9.Turi Z G. Optimizing vascular access: routine femoral angiography keeps the vascular complication away. Catheter Cardiovasc Interv. 2005;65(2):203–204. doi: 10.1002/ccd.20412. [DOI] [PubMed] [Google Scholar]
- 10.Tsetis D. Endovascular treatment of complications of femoral arterial access. Cardiovasc Intervent Radiol. 2010;33(3):457–468. doi: 10.1007/s00270-010-9820-3. [DOI] [PubMed] [Google Scholar]
- 11.Yatskar L, Selzer F, Feit F. et al. Access site hematoma requiring blood transfusion predicts mortality in patients undergoing percutaneous coronary intervention: data from the National Heart, Lung, and Blood Institute Dynamic Registry. Catheter Cardiovasc Interv. 2007;69(7):961–966. doi: 10.1002/ccd.21087. [DOI] [PubMed] [Google Scholar]
- 12.Valji K. Philadelphia, PA: Saunders Elsevier; 2006. Standard angiographic and interventional techniques; pp. 15–48. [Google Scholar]
- 13.Sreeram S, Lumsden A B, Miller J S, Salam A A, Dodson T F, Smith R B. Retroperitoneal hematoma following femoral arterial catheterization: a serious and often fatal complication. Am Surg. 1993;59(2):94–98. [PubMed] [Google Scholar]
- 14.Kent K C Moscucci M Mansour K A et al. Retroperitoneal hematoma after cardiac catheterization: prevalence, risk factors, and optimal management J Vasc Surg 1994206905–910., discussion 910–913 [DOI] [PubMed] [Google Scholar]
- 15.Bhatty S, Cooke R, Shetty R, Jovin I S. Femoral vascular access-site complications in the cardiac catheterization laboratory: diagnosis and management. Interv Cardiol. 2011;3(4):503–514. [Google Scholar]
- 16.Levine G N, Kern M J, Berger P B. et al. Management of patients undergoing percutaneous coronary revascularization. Ann Intern Med. 2003;139(2):123–136. doi: 10.7326/0003-4819-139-2-200307150-00012. [DOI] [PubMed] [Google Scholar]
- 17.Hirsch A T, Haskal Z J, Hertzer N R. et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation. 2006;113(11):e463–e654. doi: 10.1161/CIRCULATIONAHA.106.174526. [DOI] [PubMed] [Google Scholar]
- 18.Webber G W, Jang J, Gustavson S, Olin J W. Contemporary management of postcatheterization pseudoaneurysms. Circulation. 2007;115(20):2666–2674. doi: 10.1161/CIRCULATIONAHA.106.681973. [DOI] [PubMed] [Google Scholar]
- 19.Toursarkissian B Allen B T Petrinec D et al. Spontaneous closure of selected iatrogenic pseudoaneurysms and arteriovenous fistulae J Vasc Surg 1997255803–808., discussion 808–809 [DOI] [PubMed] [Google Scholar]
- 20.Graham A N, Wilson C M, Hood J M, Barros D'Sa A A. Risk of rupture of postangiographic femoral false aneurysm. Br J Surg. 1992;79(10):1022–1025. doi: 10.1002/bjs.1800791012. [DOI] [PubMed] [Google Scholar]
- 21.Coughlin B F, Paushter D M. Peripheral pseudoaneurysms: evaluation with duplex US. Radiology. 1988;168(2):339–342. doi: 10.1148/radiology.168.2.3293107. [DOI] [PubMed] [Google Scholar]
- 22.Eisenberg L, Paulson E K, Kliewer M A, Hudson M P, DeLong D M, Carroll B A. Sonographically guided compression repair of pseudoaneurysms: further experience from a single institution. AJR Am J Roentgenol. 1999;173(6):1567–1573. doi: 10.2214/ajr.173.6.10584803. [DOI] [PubMed] [Google Scholar]
- 23.Coley B D, Roberts A C, Fellmeth B D, Valji K, Bookstein J J, Hye R J. Postangiographic femoral artery pseudoaneurysms: further experience with US-guided compression repair. Radiology. 1995;194(2):307–311. doi: 10.1148/radiology.194.2.7824703. [DOI] [PubMed] [Google Scholar]
- 24.Morgan R, Belli A M. Current treatment methods for postcatheterization pseudoaneurysms. J Vasc Interv Radiol. 2003;14(6):697–710. doi: 10.1097/01.rvi.0000071089.76348.6a. [DOI] [PubMed] [Google Scholar]
- 25.Sánchez-Enciso M A, Nuño-Escobar C, González-Ojeda A, Llamas-Macias F J, Ramos-López C R, Fuentes-Orozco C. Treatment of arterial pseudoaneurysms with percutaneous ultrasound-guided thrombin injection. Cir Cir. 2012;80(2):134–139. [PubMed] [Google Scholar]
- 26.Heis H A, Bani-Hani K E, Elheis M A, Yaghan R J, Bani-Hani B K. Postcatheterization femoral artery pseudoaneurysms: therapeutic options. A case-controlled study. Int J Surg. 2008;6(3):214–219. doi: 10.1016/j.ijsu.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 27.Krüger K, Zähringer M, Söhngen F D. et al. Femoral pseudoaneurysms: management with percutaneous thrombin injections—success rates and effects on systemic coagulation. Radiology. 2003;226(2):452–458. doi: 10.1148/radiol.2262012107. [DOI] [PubMed] [Google Scholar]
- 28.Ohlow M A, Secknus M A, von Korn H, Weiss R, Lauer B. Percutaneous thrombin injection for treatment of iatrogenic femoral artery pseudoaneurysms: a case for caution. Angiology. 2008;59(3):372–375. doi: 10.1177/0003319707304575. [DOI] [PubMed] [Google Scholar]
- 29.Sadiq S, Ibrahim W. Thromboembolism complicating thrombin injection of femoral artery pseudoaneurysm: management with intraarterial thrombolysis. J Vasc Interv Radiol. 2001;12(5):633–636. doi: 10.1016/s1051-0443(07)61490-0. [DOI] [PubMed] [Google Scholar]
- 30.Ahmad F, Turner S A, Torrie P, Gibson M. Iatrogenic femoral artery pseudoaneurysms—a review of current methods of diagnosis and treatment. Clin Radiol. 2008;63(12):1310–1316. doi: 10.1016/j.crad.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 31.Glaser R L, McKellar D, Scher K S. Arteriovenous fistulas after cardiac catheterization. Arch Surg. 1989;124(11):1313–1315. doi: 10.1001/archsurg.1989.01410110071014. [DOI] [PubMed] [Google Scholar]
- 32.Kelm M, Perings S M, Jax T. et al. Incidence and clinical outcome of iatrogenic femoral arteriovenous fistulas: implications for risk stratification and treatment. J Am Coll Cardiol. 2002;40(2):291–297. doi: 10.1016/s0735-1097(02)01966-6. [DOI] [PubMed] [Google Scholar]
- 33.Jackson J E, Mansfield A O, Allison D J. Treatment of high-flow vascular malformations by venous embolization aided by flow occlusion techniques. Cardiovasc Intervent Radiol. 1996;19(5):323–328. doi: 10.1007/BF02570183. [DOI] [PubMed] [Google Scholar]
- 34.Thalhammer C, Kirchherr A S, Uhlich F, Waigand J, Gross C M. Postcatheterization pseudoaneurysms and arteriovenous fistulas: repair with percutaneous implantation of endovascular covered stents. Radiology. 2000;214(1):127–131. doi: 10.1148/radiology.214.1.r00ja04127. [DOI] [PubMed] [Google Scholar]
- 35.Valji K. Philadelphia, PA: Saunders Elsevier; 2006. Pelvic and lower extremity arteries; pp. 127–181. [Google Scholar]
- 36.Bosch J L, Hunink M G. Meta-analysis of the results of percutaneous transluminal angioplasty and stent placement for aortoiliac occlusive disease. Radiology. 1997;204(1):87–96. doi: 10.1148/radiology.204.1.9205227. [DOI] [PubMed] [Google Scholar]
- 37.Dyet J F, Gaines P A, Nicholson A A. et al. Treatment of chronic iliac artery occlusions by means of percutaneous endovascular stent placement. J Vasc Interv Radiol. 1997;8(3):349–353. doi: 10.1016/s1051-0443(97)70570-0. [DOI] [PubMed] [Google Scholar]
- 38.St Goar F G, Joye J D, Laird J R. Percutaneous arterial aortoiliac intervention. J Interv Cardiol. 2001;14(5):533–537. doi: 10.1111/j.1540-8183.2001.tb00369.x. [DOI] [PubMed] [Google Scholar]
- 39.Ballard J L Sparks S R Taylor F C et al. Complications of iliac artery stent deployment J Vasc Surg 1996244545–553., discussion 553–555 [DOI] [PubMed] [Google Scholar]
- 40.Dormandy J A Rutherford R B Management of peripheral arterial disease (PAD). TASC Working Group. TransAtlantic Inter-Society Consensus (TASC) J Vasc Surg 200031(1, Pt 2):S1–S296. [PubMed] [Google Scholar]
- 41.Schillinger M, Sabeti S, Loewe C. et al. Balloon angioplasty versus implantation of nitinol stents in the superficial femoral artery. N Engl J Med. 2006;354(18):1879–1888. doi: 10.1056/NEJMoa051303. [DOI] [PubMed] [Google Scholar]
- 42.Johnston K W. Femoral and popliteal arteries: reanalysis of results of balloon angioplasty. Radiology. 1992;183(3):767–771. doi: 10.1148/radiology.183.3.1294068. [DOI] [PubMed] [Google Scholar]
- 43.Buechel R, Stirnimann A, Zimmer R, Keo H, Groechenig E. Drug-eluting stents and drug-coated balloons in peripheral artery disease. Vasa. 2012;41(4):248–261. doi: 10.1024/0301-1526/a000200. [DOI] [PubMed] [Google Scholar]
- 44.Pentecost M J Criqui M H Dorros G et al. Guidelines for peripheral percutaneous transluminal angioplasty of the abdominal aorta and lower extremity vessels. A statement for health professionals from a Special Writing Group of the Councils on Cardiovascular Radiology, Arteriosclerosis, Cardio-Thoracic and Vascular Surgery, Clinical Cardiology, and Epidemiology and Prevention, the American Heart Association J Vasc Interv Radiol 200314(9, Pt 2):S495–S515. [DOI] [PubMed] [Google Scholar]
- 45.Cejna M, Thurnher S, Illiasch H. et al. PTA versus Palmaz stent placement in femoropopliteal artery obstructions: a multicenter prospective randomized study. J Vasc Interv Radiol. 2001;12(1):23–31. doi: 10.1016/s1051-0443(07)61397-9. [DOI] [PubMed] [Google Scholar]
- 46.Tepe G, Zeller T, Albrecht T. et al. Local delivery of paclitaxel to inhibit restenosis during angioplasty of the leg. N Engl J Med. 2008;358(7):689–699. doi: 10.1056/NEJMoa0706356. [DOI] [PubMed] [Google Scholar]
- 47.Ascher E Hingorani A P Marks N A Popliteal artery volume flow measurement: a new and reliable predictor of early patency after infrainguinal balloon angioplasty and subintimal dissection J Vasc Surg 200745117–23., discussion 23–24 [DOI] [PubMed] [Google Scholar]
- 48.Visonà A, Tonello D, Zalunardo B. et al. Antithrombotic treatment before and after peripheral artery percutaneous angioplasty. Blood Transfus. 2009;7(1):18–23. doi: 10.2450/2008.0008-08b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Müller-Hülsbeck S, Schäfer P J, Hümme T H. et al. Embolic protection devices for peripheral application: wasteful or useful? J Endovasc Ther. 2009;16 01:I163–I169. doi: 10.1583/08-2596.1. [DOI] [PubMed] [Google Scholar]
- 50.McKinsey J F Zeller T Rocha-Singh K J Jaff M R Garcia L A; DEFINITIVE LE Investigators. Lower extremity revascularization using directional atherectomy: 12-month prospective results of the DEFINITIVE LE study JACC Cardiovasc Interv 201478923–933. [DOI] [PubMed] [Google Scholar]
- 51.Strecker E P, Boos I B, Hagen B. Flexible tantalum stents for the treatment of iliac artery lesions: long-term patency, complications, and risk factors. Radiology. 1996;199(3):641–647. doi: 10.1148/radiology.199.3.8637980. [DOI] [PubMed] [Google Scholar]
- 52.Carnevale F C, De Blas M, Merino S, Egaña J M, Caldas J G. Percutaneous endovascular treatment of chronic iliac artery occlusion. Cardiovasc Intervent Radiol. 2004;27(5):447–452. doi: 10.1007/s00270-004-0086-5. [DOI] [PubMed] [Google Scholar]
- 53.Lam R C, Shah S, Faries P L, McKinsey J F, Kent K C, Morrissey N J. Incidence and clinical significance of distal embolization during percutaneous interventions involving the superficial femoral artery. J Vasc Surg. 2007;46(6):1155–1159. doi: 10.1016/j.jvs.2007.07.058. [DOI] [PubMed] [Google Scholar]
- 54.Zafar N, Prasad A, Mahmud E. Utilization of an aspiration thrombectomy catheter (Pronto) to treat acute atherothrombotic embolization during percutaneous revascularization of the lower extremity. Catheter Cardiovasc Interv. 2008;71(7):972–975. doi: 10.1002/ccd.21561. [DOI] [PubMed] [Google Scholar]
- 55.Reekers J A, Kromhout J G, Spithoven H G, Jacobs M J, Mali W M, Schultz-Kool L J. Arterial thrombosis below the inguinal ligament: percutaneous treatment with a thrombosuction catheter. Radiology. 1996;198(1):49–53. doi: 10.1148/radiology.198.1.8539405. [DOI] [PubMed] [Google Scholar]
- 56.Hamer O W, Borisch I, Finkenzeller T. et al. Iliac artery stent placement: clinical experience and short-term follow-up regarding a self-expanding nitinol stent. J Vasc Interv Radiol. 2004;15(11):1231–1238. doi: 10.1097/01.RVI.0000134499.74025.84. [DOI] [PubMed] [Google Scholar]
- 57.Smith T P, Cragg A H. Non-surgical treatment of iliac artery rupture following angioplasty. J Vasc Interv Radiol. 1989;4:16–18. [Google Scholar]
- 58.Cotogni P, Pittiruti M. Focus on peripherally inserted central catheters in critically ill patients. World J Crit Care Med. 2014;3(4):80–94. doi: 10.5492/wjccm.v3.i4.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ryder M A. Peripheral access options. Surg Oncol Clin N Am. 1995;4(3):395–427. [PubMed] [Google Scholar]
- 60.Hamilton H C, Foxcroft D R. Central venous access sites for the prevention of venous thrombosis, stenosis and infection in patients requiring long-term intravenous therapy. Cochrane Database Syst Rev. 2007;(3):CD004084. doi: 10.1002/14651858.CD004084.pub2. [DOI] [PubMed] [Google Scholar]
- 61.Bhakta A, Tafen M, Ahmed M. et al. Risk of catheter-associated deep venous thrombosis in inflammatory bowel disease. Dis Colon Rectum. 2014;57(12):1379–1383. doi: 10.1097/DCR.0000000000000257. [DOI] [PubMed] [Google Scholar]
- 62.Debourdeau P, Kassab Chahmi D, Le Gal G. et al. 2008 SOR guidelines for the prevention and treatment of thrombosis associated with central venous catheters in patients with cancer: report from the working group. Ann Oncol. 2009;20(9):1459–1471. doi: 10.1093/annonc/mdp052. [DOI] [PubMed] [Google Scholar]
- 63.Pittiruti M Hamilton H Biffi R MacFie J Pertkiewicz M; ESPEN. ESPEN guidelines on parenteral nutrition: central venous catheters (access, care, diagnosis and therapy of complications) Clin Nutr 2009284365–377. [DOI] [PubMed] [Google Scholar]
- 64.Gallieni M, Pittiruti M, Biffi R. Vascular access in oncology patients. CA Cancer J Clin. 2008;58(6):323–346. doi: 10.3322/CA.2008.0015. [DOI] [PubMed] [Google Scholar]
- 65.Senthil M, Chaudhary P, Smith D D. et al. A shortened activated partial thromboplastin time predicts the risk of catheter-associated venous thrombosis in cancer patients. Thromb Res. 2014;134(1):165–168. doi: 10.1016/j.thromres.2014.04.022. [DOI] [PubMed] [Google Scholar]
- 66.Laméris J S, Post P J, Zonderland H M, Gerritsen P G, Kappers-Klunne M C, Schütte H E. Percutaneous placement of Hickman catheters: comparison of sonographically guided and blind techniques. AJR Am J Roentgenol. 1990;155(5):1097–1099. doi: 10.2214/ajr.155.5.2120941. [DOI] [PubMed] [Google Scholar]
- 67.Takasugi J K, O'Connell T X. Prevention of complications in permanent central venous catheters. Surg Gynecol Obstet. 1988;167(1):6–11. [PubMed] [Google Scholar]
- 68.Hull J E, Hunter C S, Luiken G A. The Groshong catheter: initial experience and early results of imaging-guided placement. Radiology. 1992;185(3):803–807. doi: 10.1148/radiology.185.3.1438766. [DOI] [PubMed] [Google Scholar]
- 69.Morris S L, Jaques P F, Mauro M A. Radiology-assisted placement of implantable subcutaneous infusion ports for long-term venous access. Radiology. 1992;184(1):149–151. doi: 10.1148/radiology.184.1.1609072. [DOI] [PubMed] [Google Scholar]
- 70.McGee D C, Gould M K. Preventing complications of central venous catheterization. N Engl J Med. 2003;348(12):1123–1133. doi: 10.1056/NEJMra011883. [DOI] [PubMed] [Google Scholar]
- 71.Wu S Y, Ling Q, Cao L H, Wang J, Xu M X, Zeng W A. Real-time two-dimensional ultrasound guidance for central venous cannulation: a meta-analysis. Anesthesiology. 2013;118(2):361–375. doi: 10.1097/ALN.0b013e31827bd172. [DOI] [PubMed] [Google Scholar]
- 72.Gibson F, Bodenham A. Misplaced central venous catheters: applied anatomy and practical management. Br J Anaesth. 2013;110(3):333–346. doi: 10.1093/bja/aes497. [DOI] [PubMed] [Google Scholar]
- 73.Jobes D R, Schwartz A J, Greenhow D E, Stephenson L W, Ellison N. Safer jugular vein cannulation: recognition of arterial puncture and preferential use of the external jugular route. Anesthesiology. 1983;59(4):353–355. [PubMed] [Google Scholar]
- 74.Mauro M A, Weeks S M. Philadelphia, PA: Lippincott Williams & Wilkins; 2005. Venous access; pp. 1142–1156. [Google Scholar]
- 75.Wallace M J, Ahrar K. Percutaneous closure of a subclavian artery injury after inadvertent catheterization. J Vasc Interv Radiol. 2001;12(10):1227–1230. doi: 10.1016/s1051-0443(07)61685-6. [DOI] [PubMed] [Google Scholar]
- 76.Nicholson T, Ettles D, Robinson G. Managing inadvertent arterial catheterization during central venous access procedures. Cardiovasc Intervent Radiol. 2004;27(1):21–25. doi: 10.1007/s00270-003-0043-8. [DOI] [PubMed] [Google Scholar]
- 77.Bodenham A. Reducing major procedural complications from central venous catheterisation. Anaesthesia. 2011;66(1):6–9. doi: 10.1111/j.1365-2044.2010.06583.x. [DOI] [PubMed] [Google Scholar]
- 78.Orme R M, McSwiney M M, Chamberlain-Webber R F. Fatal cardiac tamponade as a result of a peripherally inserted central venous catheter: a case report and review of the literature. Br J Anaesth. 2007;99(3):384–388. doi: 10.1093/bja/aem181. [DOI] [PubMed] [Google Scholar]
- 79.Celik B, Sahin E, Nadir A, Kaptanoglu M. Iatrogenic pneumothorax: etiology, incidence and risk factors. Thorac Cardiovasc Surg. 2009;57(5):286–290. doi: 10.1055/s-0029-1185365. [DOI] [PubMed] [Google Scholar]
- 80.Despars J A, Sassoon C S, Light R W. Significance of iatrogenic pneumothoraces. Chest. 1994;105(4):1147–1150. doi: 10.1378/chest.105.4.1147. [DOI] [PubMed] [Google Scholar]
- 81.Criado E, Marston W A, Jaques P F, Mauro M A, Keagy B A. Proximal venous outflow obstruction in patients with upper extremity arteriovenous dialysis access. Ann Vasc Surg. 1994;8(6):530–535. doi: 10.1007/BF02017408. [DOI] [PubMed] [Google Scholar]
- 82.Wisselink W Money S R Becker M O et al. Comparison of operative reconstruction and percutaneous balloon dilatation for central venous obstruction Am J Surg 19931662200–204., discussion 204–205 [DOI] [PubMed] [Google Scholar]
- 83.Agarwal A K. Central vein stenosis. Am J Kidney Dis. 2013;61(6):1001–1015. doi: 10.1053/j.ajkd.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 84.III. NKF-K/DOQI Clinical Practice Guidelines for Vascular Access: update 2000. Am J Kidney Dis. 2001;37(1) 01:S137–S181. doi: 10.1016/s0272-6386(01)70007-8. [DOI] [PubMed] [Google Scholar]
- 85.Kanterman R Y, Vesely T M, Pilgram T K, Guy B W, Windus D W, Picus D. Dialysis access grafts: anatomic location of venous stenosis and results of angioplasty. Radiology. 1995;195(1):135–139. doi: 10.1148/radiology.195.1.7892454. [DOI] [PubMed] [Google Scholar]
- 86.Manninen H I, Kaukanen E T, Ikäheimo R. et al. Brachial arterial access: endovascular treatment of failing Brescia-Cimino hemodialysis fistulas—initial success and long-term results. Radiology. 2001;218(3):711–718. doi: 10.1148/radiology.218.3.r01mr38711. [DOI] [PubMed] [Google Scholar]
- 87.Aruny J E Lewis C A Cardella J F et al. Quality improvement guidelines for percutaneous management of the thrombosed or dysfunctional dialysis access J Vasc Interv Radiol 200314(9, Pt 2):S247–S253. [PubMed] [Google Scholar]
- 88.Kornfield Z N, Kwak A, Soulen M C. et al. Incidence and management of percutaneous transluminal angioplasty-induced venous rupture in the “fistula first” era. J Vasc Interv Radiol. 2009;20(6):744–751. doi: 10.1016/j.jvir.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 89.Pappas J N, Vesely T M. Vascular rupture during angioplasty of hemodialysis raft-related stenoses. J Vasc Access. 2002;3(3):120–126. doi: 10.1177/112972980200300307. [DOI] [PubMed] [Google Scholar]
- 90.Raynaud A C, Angel C Y, Sapoval M R, Beyssen B, Pagny J Y, Auguste M. Treatment of hemodialysis access rupture during PTA with Wallstent implantation. J Vasc Interv Radiol. 1998;9(3):437–442. doi: 10.1016/s1051-0443(98)70295-7. [DOI] [PubMed] [Google Scholar]
- 91.Vesely T M. Role of stents and stent grafts in management of hemodialysis access complications. Semin Vasc Surg. 2007;20(3):175–183. doi: 10.1053/j.semvascsurg.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 92.Decousus H, Leizorovicz A, Parent F. et al. A clinical trial of vena caval filters in the prevention of pulmonary embolism in patients with proximal deep-vein thrombosis. Prévention du Risque d'Embolie Pulmonaire par Interruption Cave Study Group. N Engl J Med. 1998;338(7):409–415. doi: 10.1056/NEJM199802123380701. [DOI] [PubMed] [Google Scholar]
- 93.Hammond C J, Bakshi D R, Currie R J. et al. Audit of the use of IVC filters in the UK: experience from three centres over 12 years. Clin Radiol. 2009;64(5):502–510. doi: 10.1016/j.crad.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 94.Molvar C. Inferior vena cava filtration in the management of venous thromboembolism: filtering the data. Semin Intervent Radiol. 2012;29(3):204–217. doi: 10.1055/s-0032-1326931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kaufman J A, Kinney T B, Streiff M B. et al. Guidelines for the use of retrievable and convertible vena cava filters: report from the Society of Interventional Radiology multidisciplinary consensus conference. J Vasc Interv Radiol. 2006;17(3):449–459. doi: 10.1097/01.rvi.0000203418-39769.0d. [DOI] [PubMed] [Google Scholar]
- 96.Angel L F, Tapson V, Galgon R E, Restrepo M I, Kaufman J. Systematic review of the use of retrievable inferior vena cava filters. J Vasc Interv Radiol. 2011;22(11):1522–1.53E6. doi: 10.1016/j.jvir.2011.08.024. [DOI] [PubMed] [Google Scholar]
- 97.Streiff M B. Vena caval filters: a review for intensive care specialists. J Intensive Care Med. 2003;18(2):59–79. doi: 10.1177/0885066602250372. [DOI] [PubMed] [Google Scholar]
- 98.Ray C E Jr, Prochazka A. The need for anticoagulation following inferior vena cava filter placement: systematic review. Cardiovasc Intervent Radiol. 2008;31(2):316–324. doi: 10.1007/s00270-007-9244-x. [DOI] [PubMed] [Google Scholar]
- 99.Hann C L, Streiff M B. The role of vena caval filters in the management of venous thromboembolism. Blood Rev. 2005;19(4):179–202. doi: 10.1016/j.blre.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 100.Sildiroglu O, Ozer H, Turba U C. Management of the thrombosed filter-bearing inferior vena cava. Semin Intervent Radiol. 2012;29(1):57–63. doi: 10.1055/s-0032-1302453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Singh S, Haut E, Brotman D, Rockville, MD: Agency for Healthcare Research & Quality; 2013. Comparative Effectiveness of Pharmacologic and Mechanical Prophylaxis of Venous Thromboembolism Among Special Populations: Evidence Report/Technology Assessment. [PubMed] [Google Scholar]
- 102.Stawicki S P, Sims C A, Sharma R. et al. Vena cava filters: a synopsis of complications and related topics. J Vasc Access. 2008;9(2):102–110. [PubMed] [Google Scholar]
- 103.Andreoli J M, Lewandowski R J, Vogelzang R L, Ryu R K. Comparison of complication rates associated with permanent and retrievable inferior vena cava filters: a review of the MAUDE database. J Vasc Interv Radiol. 2014;25(8):1181–1185. doi: 10.1016/j.jvir.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 104.Stavropoulos S W, Dixon R G, Burke C T. et al. Embedded inferior vena cava filter removal: use of endobronchial forceps. J Vasc Interv Radiol. 2008;19(9):1297–1301. doi: 10.1016/j.jvir.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 105.Kuo W T, Cupp J S. The excimer laser sheath technique for embedded inferior vena cava filter removal. J Vasc Interv Radiol. 2010;21(12):1896–1899. doi: 10.1016/j.jvir.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 106.Kuo W T, Odegaard J I, Louie J D. et al. Photothermal ablation with the excimer laser sheath technique for embedded inferior vena cava filter removal: initial results from a prospective study. J Vasc Interv Radiol. 2011;22(6):813–823. doi: 10.1016/j.jvir.2011.01.459. [DOI] [PubMed] [Google Scholar]
- 107.Kock H J, Pietsch M, Krause U, Wilke H, Eigler F W. Implantable vascular access systems: experience in 1500 patients with totally implanted central venous port systems. World J Surg. 1998;22(1):12–16. doi: 10.1007/s002689900342. [DOI] [PubMed] [Google Scholar]
- 108.Reddy A, Stangl A, Radbill B. Retained catheter fragment from a fractured tunneled catheter—a rare and potentially lethal complication. Semin Dial. 2010;23(5):536–539. doi: 10.1111/j.1525-139X.2010.00756.x. [DOI] [PubMed] [Google Scholar]
- 109.Fisher R G, Ferreyro R. Evaluation of current techniques for nonsurgical removal of intravascular iatrogenic foreign bodies. AJR Am J Roentgenol. 1978;130(3):541–548. doi: 10.2214/ajr.130.3.541. [DOI] [PubMed] [Google Scholar]
- 110.Choksy P, Zaidi S S, Kapoor D. Removal of intracardiac fractured port-A catheter utilizing an existing forearm peripheral intravenous access site in the cath lab. J Invasive Cardiol. 2014;26(2):75–76. [PubMed] [Google Scholar]
- 111.Michael T T, Banerjee S, Brilakis E S. Percutaneous retrieval of a fractured guide catheter using contralateral snaring. J Invasive Cardiol. 2012;24(8):E176–E178. [PubMed] [Google Scholar]


