Abstract
Whole-genome sequencing of serotype M3 group A streptococci (GAS) from oropharyngeal and invasive infections in Ontario recently showed that the gene encoding regulator of protease B (RopB) is highly polymorphic in this population. To test the hypothesis that ropB is under diversifying selective pressure among all serotype M3 GAS strains, we sequenced this gene in 1178 strains collected from different infection types, geographic regions, and time periods. The results confirmed our hypothesis and discovered a significant association between mutant ropB alleles, decreased activity of its major regulatory target SpeB, and pharyngitis. Additionally, isoallelic strains with ropB polymorphisms were significantly less virulent in a mouse model of necrotizing fasciitis. These studies provide a model strategy for applying whole-genome sequencing followed by deep single-gene sequencing to generate new insight to the rapid evolution and virulence regulation of human pathogens.
Group A Streptococcus (GAS) is a human-specific pathogen that causes infections ranging in severity from asymptomatic colonization and uncomplicated pharyngitis (“strep throat”) to life-threatening necrotizing fasciitis (“flesh-eating disease”) and pneumonia [1]. Despite decades of research, many aspects of the molecular basis for host-GAS interactions remain poorly understood. In particular, little information bearing on the ability of GAS to infect anatomically diverse sites or the evolution of GAS virulence during the course of human infection is available [1]. We have recently used an unbiased whole-genome sequencing strategy to investigate the relationship between GAS strain genotypes and human disease phenotypes among infections caused by serotype M3 strains in Ontario, Canada [2–5]. Serotype M3 strains are particularly interesting because they commonly cause both invasive and oropharyngeal infections, display epidemic behavior with rapid shifts in disease frequency, and are associated with a disproportionate risk of death compared with other GAS serotype strains [1, 2, 6].
Sequencing the genomes of ∼180 serotype M3 GAS strains recovered from patients with well-described disease manifestations in Ontario has generated several new leads for studying bacterial pathogenesis [2, 3, 7]. One noteworthy finding was an unexpectedly high frequency of polymorphisms in the regulator of protease B gene (ropB) among strains in this population. RopB positively regulates the expression of the gene encoding the secreted streptococcal cysteine protease B (SpeB), a potent extracellular protease virulence factor implicated in tissue destruction and bacterial dissemination in humans and animal models [7–11]. RopB also directly or indirectly regulates the expression of several other genes encoding proven or putative virulence factors [1, 12]. Furthermore, a truncating mutation in the ropB allele encoded by a single serotype M1 GAS strain was recently shown to significantly alter SpeB expression [12]. Herein, we tested the hypothesis that ropB is subject to diversifying selection in serotype M3 GAS and studied the effect of ropB polymorphisms on SpeB activity and strain virulence. The results confirmed our hypothesis and provide new understanding of the host-pathogen interactions that underlie human infections.
MATERIALS AND METHODS
Bacterial Strains
We studied 1178 serotype M3 GAS strains, including organisms from an ongoing 20-year study of invasive infections in Ontario (1990–2010) [2], recent outbreak of invasive infections in England (2008–2009) [13], ongoing 10-year United States Active Bacterial Core surveillance (ABCs) program for invasive infections (1998–2008, http://www.cdc.gov/abcs), pharyngitis strains from 6 public health and private diagnostic laboratories in Ontario (2002–2010) [3], invasive strains collected in the German Democratic Republic (1969–1990) [14, 15], and pharyngitis strains collected in Alberta, Canada (2010, strains courtesy of G. Tyrrell). The first 3 collections represent prospective, comprehensive, population-based studies. Genome-wide comparison of the Ontario pharyngitis and invasive strains results in superimposable phylogenetic trees with nearly identical topology [3], confirming that although they do not comprise a comprehensive collection, the Ontario pharyngitis strains are representative of the underlying GAS population. The latter 2 collections are convenience samples that are included because they expand the diversity of the disease types, geographic regions, and time points of the strains studied.
Gene Sequencing
GAS strains were grown overnight on Todd-Hewitt agar supplemented with 0.2% yeast extract, and genomic DNA was extracted by alkaline-boiling lysis [16]. The ropB gene allele was determined by Sanger sequencing using a 3730xL DNA analyzer (Applied Biosystems by Life Technologies), and the emm-type was confirmed (primers are listed in Table 1). Electropherograms were visually inspected using Sequencher4.7 (Gene Codes), ropB alleles were evaluated using MacVector11 (MacVector), and phylogeny was inferred using SplitsTree4 (University of Tubingen) software. Statistical analyses were performed using XLStat2010 software (Addinsoft) or Statistical Analysis Software (SAS Institute), with differences considered significant at P < .05 for all tests performed, and graphs were created using Prism4 (GraphPad Software).
Table 1.
Primers Used in This Study
| Primer | Sequence (5'–3') |
|---|---|
| ropB-3' | TTGAAAAAATCGCCCTGGACT |
| ropB-5' | CATAACCGACTATCATCCGAAC |
| emm-1 | TATTSGCTTAGAAAATTAA |
| emm-2 | GCAAGTTCTTCAGCTTGTTT |
Measurement of SpeB Expression and Protease Activity
SpeB expression and protease activity were evaluated by Western immunoblot and casein milk plate hydrolysis, respectively, as described elsewhere [7]. SpeB was semiquantitatively scored as 1 (present at near-wild-type levels), 0.5 (present at reduced levels), or 0 (absent expression/activity). SpeB was evaluated in every strain with a unique ropB allele; ≥2 strains for each allele that was identified in >1 strain, including 56 (∼50%) V7I strains and 9 (∼1%) wild-type strains and all 87 pharyngitis strains with a variant allele. Protease activity was confirmed in the isoallelic strains using a quantitative chromogenic azocasein hydrolysis assay as described elsewhere [17].
Modeling of ropB Polymorphisms
The structure of RopB was modeled as described elsewhere [18]. Functional domains were predicted by homology matching using the I-TASSER structure prediction server (http://zhanglab.ccmb.med.umich.edu/I-TASSER), and the RopB structure was visually inspected using the program PyMOL [19].
Mouse Virulence Studies Using ropB Isoallelic Strains
Strain MGAS10870 was recovered from a patient in Ontario with an invasive soft-tissue infection [2]. Its genome has been fully sequenced, is generally representative of serotype M3 strains that cause invasive infections, and has a wild-type allele for all major regulatory genes [2]. Isogenic mutant strains lacking the gene encoding either ropB (designated MGAS10870ΔropB) or speB (designated MGAS10870ΔspeB) were generated from wild-type strain MGAS10870 by insertional inactivation with a spectinomycin cassette, as described elsewhere [11, 18]. Isoallelic mutant strains with the ropB allele encoding the V7I (designated MGAS10870-V7I), R226Q (designated MGAS10870-R226Q) or V7I/R226Q (designated MGAS10870-V7I/R226Q) amino acid changes were also generated from wild-type strain MGAS10870, as described elsewhere [18]. Ten immunocompetent CD1 mice (Harlan Laboratories) were inoculated in the right hind limb muscle with 107 colony-forming units of each strain and monitored for near-mortality, as described elsewhere [7]. Visual and microscopic inspection of infected tissue was also performed. Lesions were excised and tissue was fixed in 10% phosphate-buffered formalin, decalcified, serially sectioned, and embedded in paraffin using automated standard instruments, as described elsewhere [7]. Hematoxylin-eosin– and Gram-stained sections were examined with a BX5 microscope and photographed with a DP70 camera (Olympus). Micrographs of tissue taken from the inoculation site that showed histopathology characteristic of each strain were selected for publication. The study was approved by the Institutional Animal Care and Use Committee of The Methodist Hospital Research Institute.
RESULTS
Polymorphism of ropB Among Serotype M3 GAS Strains
To test the hypothesis that ropB is highly polymorphic among all serotype M3 GAS, we sequenced the gene in 1178 strains from 6 collections that encompass a diverse array of infection types, geographic regions, and time periods. Among the 1178 strains studied (including strains from our whole-genome studies of serotype M3 GAS collected in Ontario [2, 3, 18]), 326 (28%) had a polymorphism in ropB (Figure 1A). In total, 84 distinct ropB alleles were identified, with the most common allele designated as the wild-type sequence (Table 2). Importantly, 64 of the 83 variant alleles have not been previously identified [2, 3, 18, 20]. Whereas only 1 of the previously identified ropB polymorphisms results in a protein alteration other than a single amino acid replacement, in this study we identified 17 polymorphisms that result in a major protein alteration by either premature termination or in-frame insertion (Figure 1B). We also identified 11 codons, which, compared with the wild-type sequence, were each affected by 2 distinct genetic changes resulting in different protein alterations in different strains (for example, independent R28K and R28G amino acid alterations occurred in 2 different strains) (Table 2). Additionally, we identified 5 alleles in which a single-nucleotide polymorphism (SNP) producing a V7I amino acid replacement occurred together with another SNP producing a second amino acid replacement (eg, the V7I and L61P amino acid alterations both occurred in the same strain) (Table 2). Of note, only 1 of these alleles with 2 changes relative to the wild-type sequence had been previously identified (V7I/R226Q) [2, 3, 18], and 2 of the involved amino acid alterations occurring together with the V7I replacement (R226Q and S103P) also occurred alone (Table 2).
Figure 1.
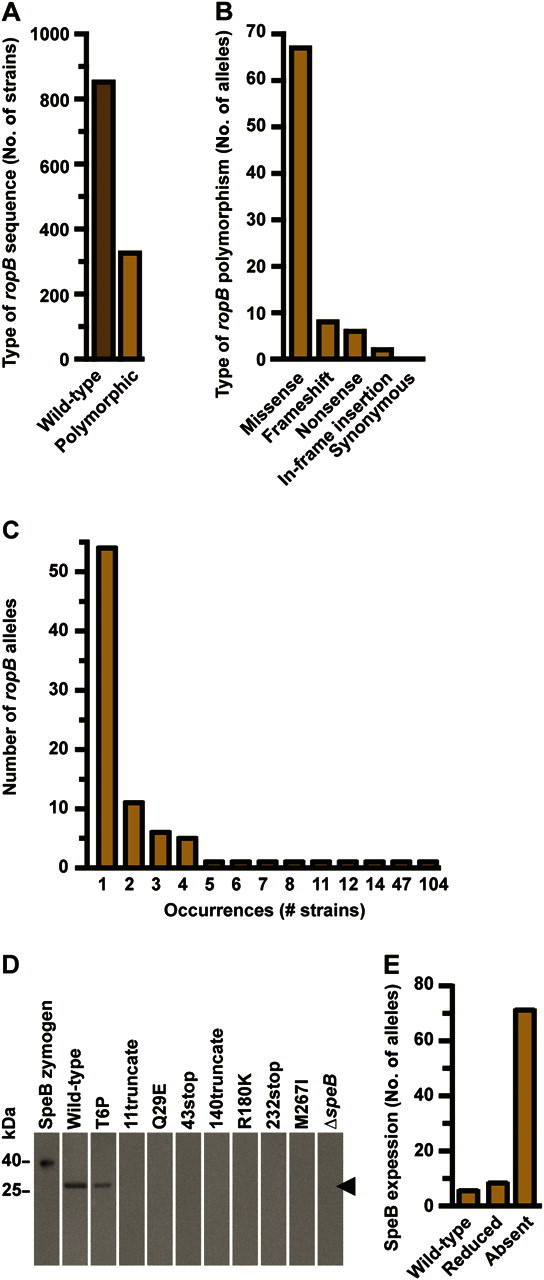
The ropB polymorphisms in serotype M3 group A streptococci (GAS) strains demonstrate a pattern of diversifying selection and significantly alter RopB regulatory activity. A, The ropB gene was sequenced in 1178 serotype M3 GAS strains collected from patients with different disease manifestations in diverse geographic regions and time periods. The numbers of strains with a wild-type (dark brown bar) or variant (light brown bar) ropB allele are shown. B, Gene sequencing identified 84 ropB alleles. Relative to the wild-type sequence, every polymorphism alters the RopB protein. The number of variant ropB alleles encoding each type of amino acid change is shown. C, Most of the 83 variant ropB alleles were present in only a few strains, with 54 alleles being specific to a single strain. D, RopB is the major regulator of secreted streptococcal cysteine protease B (SpeB), a proven virulence factor implicated in invasive disease. Most strains with a variant ropB allele demonstrated markedly reduced or absent SpeB expression as measured by Western immunoblot assay of culture supernatants. Representative strains are shown. Multiple immunoblots were performed simultaneously and processed identically, with lanes reordered such that ropB alleles were shown left to right with respect to the amino acid sequence. Recombinant SpeB zymogen and supernatant from a GAS strain with the gene encoding SpeB deleted (designated ΔspeB) were used as a positive and negative control, respectively. E, Similarly, most strains with a variant ropB allele demonstrated markedly reduced or absent SpeB secreted protease activity, as measured by casein hydrolysis in milk plates.
Table 2.
ropB Alleles Identified in 1178 Serotype M3 GAS Strains
| Allele No. | Codon | Nucleotide Change | Amino Acid Change | Occurrences, No. | Position in Codon | Strain Collections | SpeB Expression and Activity | Strains Tested for SpeB, No.a |
|---|---|---|---|---|---|---|---|---|
| 1 | Wild-type | None | None | 852 | … | All | Wild-typeb | 9 |
| 2 | 1 | G3A | M1I | 1 | 3 | OI | Absent | 1 |
| 3 | 1 | T2A | M1K | 1 | 2 | US | Absent | 1 |
| 4 | 6 | A16C | T6P | 1 | 1 | OI | Reduced | 1 |
| 5 | 7 | G19A | V7I | 104 | 1 | OI, US, UK, OP | Reducedc | 56 |
| 6 | 7, 61 | G19A, T182C | V7I L61P | 1 | 2 | OP | Absent | 1 |
| 7 | 7, 103 | G19A, T307C | V7I S103Pd | 1 | 1 | OP | Absent | 1 |
| 8 | 7, 104 | G19A, C311T | V7I T104I | 1 | 2 | OP | Absent | 1 |
| 9 | 7, 226 | G19A, G677A | V7I R226Qd | 47 | 2 | OI, OP | Absente | 28 |
| 10 | 7, 235 | G19A, T704C | V7I I235T | 1 | 2 | US | Absent | 1 |
| 11 | 11 | G33-1 | FS/truncation | 1 | 1 | US | Absent | 1 |
| 12 | 22 | G65A | C22Y | 8 | 2 | OI, US, OP | Absent | 7 |
| 13 | 28 | G83A | R28K | 1 | 2 | UK | Absent | 1 |
| 14 | 28 | A82G | R28G | 1 | 1 | GDR | Absent | 1 |
| 15 | 29 | C85G | Q29E | 1 | 1 | GDR | Absent | 1 |
| 16 | 31 | A92G | Y31C | 1 | 2 | US | Absent | 1 |
| 17 | 33 | C97 + 18 | 6 AA Insertion | 4 | In frame | US | Reduced | 2 |
| 18 | 33 | C97A | R33S | 1 | 1 | AP | Reduced | 1 |
| 19 | 34 | T100A | F34I | 1 | 1 | OI | Absent | 1 |
| 20 | 43 | C128A | Nonsense | 1 | 2 | OI | Absent | 1 |
| 21 | 55 | T164A | V55D | 1 | 2 | US | Absent | 1 |
| 22 | 57 | G169A | V57I | 3 | 1 | US | Wild-type | 3 |
| 23 | 58 | G172A | D58N | 1 | 1 | AP | Absent | 1 |
| 24 | 62 | T186 + 1 | FS/truncation | 7 | 1 | AP | Absentf | 7 |
| 25 | 63 | T188C | I63T | 12 | 2 | US | Absent | 2 |
| 26 | 68 | A204C | K68N | 2 | 3 | US | Wild-type | 1 |
| 27 | 72 | G214T | Nonsense | 1 | 1 | US | Absent | 1 |
| 28 | 73 | T218G | F73C | 1 | 2 | US | Absent | 1 |
| 29 | 75 | G223A | D75N | 1 | 1 | US | Wild-type | 1 |
| 30 | 76 | G228A | M76I | 1 | 3 | US | Absent | 1 |
| 31 | 80 | A240C | K80N | 14 | 3 | US, OP | Absent | 2 |
| 32 | 83 | T248C | F83S | 1 | 2 | OI | Absent | 1 |
| 33 | 85 | G254A | C85Y | 11 | 2 | OI, US | Reduced | 4 |
| 34 | 87 | C261 + 1 | FS/truncation | 2 | 1 | US | Absent | 2 |
| 35 | 90 | G268A | G90D | 1 | 1 | US | Absent | 1 |
| 36 | 91 | T272C | L91S | 1 | 2 | US | Absent | 1 |
| 37 | 94 | T281A | I94N | 1 | 2 | OI | Absent | 1 |
| 38 | 100 | G298 + 20 | FS/truncation | 1 | 2 | US | Absent | 1 |
| 39 | 103 | T307C | S103P | 5 | 1 | OI, reference | Reduced | 2 |
| 40 | 103 | C308A | Nonsense | 1 | 2 | US | Absent | 1 |
| 41 | 105 | A314T | K105M | 1 | 2 | US | Absent | 1 |
| 42 | 107 | G321T | K107N | 3 | 3 | US | Wild-type | 3 |
| 43 | 111 | G331A | A111T | 3 | 1 | US | Absent | 2 |
| 44 | 111 | C332A | A111D | 1 | 2 | UK | Absent | 1 |
| 45 | 134 | C402-8 | FS/truncation | 1 | 1 | US | Absent | 1 |
| 46 | 136 | C406A | L136I | 1 | 1 | US | Reduced | 1 |
| 47 | 140 | G418-1 | FS/truncation | 4 | 2 | OP | Absent | 4 |
| 48 | 140 | G418 + 6 | 2 AA insertion | 1 | In frame | US | Absent | 1 |
| 49 | 142 | G425A | Nonsense | 1 | 2 | US | Absent | 1 |
| 50 | 143 | G428A | S143N | 1 | 2 | OI | Absent | 1 |
| 51 | 150 | T448A | F150I | 1 | 3 | US | Absent | 1 |
| 52 | 151 | A451T | N151Y | 3 | 1 | OI | Absent | 2 |
| 53 | 151 | T453A | N151K | 1 | 3 | US | Absent | 1 |
| 54 | 152 | A455T | N152I | 1 | 3 | US | Absent | 1 |
| 55 | 154 | G462A | M154I | 1 | 3 | OI | Absent | 1 |
| 56 | 173 | C517T | L173F | 1 | 1 | US | Absent | 1 |
| 57 | 179 | T536A | L179Q | 1 | 2 | US | Absent | 1 |
| 58 | 180 | G539A | R180K | 1 | 2 | GDR | Absent | 1 |
| 59 | 184 | T552A | N184K | 2 | 3 | OI | Absent | 2 |
| 60 | 189 | G567A | M189I | 3 | 3 | OI, US | Absent | 2 |
| 61 | 196 | T587G | L196W | 2 | 2 | UK | Absent | 2 |
| 62 | 202 | A605G | E202G | 1 | 2 | US | Absent | 1 |
| 63 | 206 | C617A | A206D | 1 | 2 | OI | Absent | 1 |
| 64 | 206 | C617-1 | FS/truncation | 1 | 3 | UK | Absent | 1 |
| 65 | 220 | G658T | D220Y | 3 | 1 | UK | Absent | 2 |
| 66 | 222 | G665A | C222Y | 4 | 2 | OI, AP | Absent | 4 |
| 67 | 224 | T670C | Y224H | 4 | 1 | OI | Absent | 2 |
| 68 | 226 | G677A | R226Q | 1 | 2 | US | Absent | 1 |
| 69 | 227 | G680A | C227Y | 1 | 2 | OI | Absent | 1 |
| 70 | 232 | T696 + 1 | Nonsense | 2 | 1 | OI, UK | Absent | 1 |
| 71 | 232 | T696-1 | FS/truncation | 6 | 1 | US, GDR | Absent | 2 |
| 72 | 237 | G710T | G237V | 1 | 2 | OI | Absent | 1 |
| 73 | 238 | T713C | L238P | 2 | 2 | OP | Absent | 2 |
| 74 | 245 | G733A | A245T | 2 | 1 | OI, GDR | Absent | 2 |
| 75 | 245 | G733C | A245P | 2 | 1 | US | Absent | 2 |
| 76 | 247 | C739A | Nonsense | 4 | 1 | OI | Absent | 2 |
| 77 | 249 | G746A | C249Y | 2 | 2 | US | Absent | 2 |
| 78 | 252 | A754T | I252F | 1 | 1 | OI | Absent | 1 |
| 79 | 252 | C756-14 | FS/truncation | 2 | 1 | US | Absent | 2 |
| 80 | 256 | T767C | F256S | 1 | 2 | US | Absent | 1 |
| 81 | 260 | A779C | N260T | 1 | 2 | UK | Wild-type | 1 |
| 82 | 267 | G801A | M267I | 2 | 3 | OI, US | Absent | 2 |
| 83 | 268 | T803G | F268C | 1 | 2 | OI | Absent | 1 |
| 84 | 271 | T811C | Y271H | 1 | 1 | AP | Reduced | 1 |
Abbreviations: AA, amino acid; AP, Alberta pharyngitis strains; GDR, German Democratic Republic invasive strains; OI, Ontario invasive strains; OP, Ontario pharyngitis strains; SpeB, secreted streptococcal cysteine protease B; UK, United Kingdom invasive strains; US, United States Centers for Disease Control and Prevention invasive strains.
Randomly selected invasive strains and all pharyngitis strains with a regulator of protease B gene (ropB) polymorphism were tested for SpeB.
All strains tested secreted high levels of SpeB.
Of 56 strains tested, 48 secreted SpeB (10 at near wild-type levels, 38 at reduced levels; 8 lacked detectable SpeB).
The second single-nucleotide polymorphism occurring in the V7I genetic background of both these alleles also occurs alone.
Of 28 strains tested, 4 secreted SpeB (1 at near wild-type levels, 3 at reduced levels; 24 lacked detectable SpeB).
Of 7 strains tested, 1 secreted SpeB (at reduced levels; 6 lacked detectable SpeB).
Assuming that the background polymorphism rate across the genome of all serotype M3 GAS is similar to the rate calculated for Ontario strains used in our unbiased genome-wide study [2], our data demonstrate that polymorphisms in ropB are significantly overrepresented among the 1178 strains studied herein (χ2 test, P < .001, χ2 = 939, calculated using 83 polymorphisms observed and 6.27 polymorphisms expected if they were randomly distributed across the core serotype M3 genome [2]). The χ2 statistic was also significant when calculated for ropB polymorphisms identified in each individual strain collection (P < .001 for each collection; χ2 = 886 for UK invasive strains, 615 for US invasive strains, 327 for Ontario invasive strains, 169 for Alberta pharyngitis strains, 115 for Ontario pharyngitis strains, and 47 for German invasive strains). Thus, the significant overabundance of ropB polymorphisms in serotype M3 GAS strains is not restricted to a single disease phenotype, geographic region, or time period.
Diversifying Selection andropB
To test the hypothesis that ropB polymorphisms occur through diversifying selection, we evaluated the molecular consequence of each genetic change that defined the 83 variant alleles. Importantly, no synonymous (silent, not resulting in an amino acid replacement) polymorphisms were discovered (Figure 1B). That is, compared with the wild-type sequence, every allele encodes a protein with an altered RopB sequence. The nucleotide changes were significantly overrepresented in the first and second positions of the variant codons (Table 2), enabling us to reject the hypothesis of selective neutrality (χ2 test, P < .01, χ2 = 7.26, calculated using 68 nucleotide changes observed in the first 2 positions and 54 nucleotide changes expected if the 81 polymorphisms were randomly distributed across the ropB coding sequence; note that codon positions could not be unambiguously assigned for the 2 in-frame insertions). Most ropB alleles occurred in a single strain or very few strains (Figure 1C and Table 2). Only 4 of the 83 variant alleles occurred in >10% of the strains tested (Table 2). Strains with these 4 ropB alleles usually had the same emm3 allele and were present in a single location during a short time, suggesting that they share identity by descent rather than identity by independent evolutionary events (ie, convergence). Taken together, these genetic and epidemiologic data are consistent with a model of ropB evolution in which new alleles emerge through diversifying selection.
Effect of Amino Acid Alterations on Regulatory Activity of RopB
RopB is the major positive transcriptional regulator of secreted streptococcal cysteine protease B (SpeB) [1]. SpeB contributes to tissue destruction and mortality in animal models of necrotizing fasciitis and is believed to participate in the pathogenesis of some human invasive infections [7, 10, 11]. To test the hypothesis that amino acid replacements in RopB alter its regulatory activity, we assessed SpeB expression and protease activity in representative strains with each ropB allele. Strains with the wild-type allele secreted high levels of SpeB, whereas the majority of strains with a ropB polymorphism had either a marked reduction or complete absence of SpeB in vitro (Figure 1D and 1E). The results of the SpeB expression assays as measured by Western immunoblot, and protease activity assays, as measured by milk plate hydrolysis, were concordant for all strains tested (Table 2). Thus, the majority of ropB polymorphisms significantly decrease SpeB expression and secreted protease activity.
Domain Locations of Amino Acid Alterations in RopB
To investigate the potential molecular mechanism underlying ropB polymorphisms and decreased SpeB, we mapped the location of each genetic change to the ropB sequence (Figure 2A). Using a moving-average plot, we found that 6 regions within the ropB gene had a significant overabundance of polymorphisms (χ2 test, P < .05 for each region, calculated using the observed number of changes centered on each codon in a moving-average window of 5 codons and 1.45 polymorphisms per window expected if the 81 genetic changes were randomly distributed across the whole gene sequence; note that SNPs resulting in the S103P and R226Q amino acid replacements occurring both in wild-type and V7I genetic backgrounds were counted only once) (Figure 2B). Importantly, each of these significantly over represented regions corresponded to a key predicted functional domain of RopB, which if altered, could be expected to alter SpeB regulation. One occurred in the DNA-binding motif, 1 occurred in the linker helix, 3 occurred in the tetratricopeptide ligand-binding motif, and 1 occurred in the oligomerization motif (Figure 2A and 2B). Moreover, 5 of the 11 codons altered by different genetic changes in different strains (eg, R28K and R28G) are located within these significantly overrepresented regions.
Figure 2.
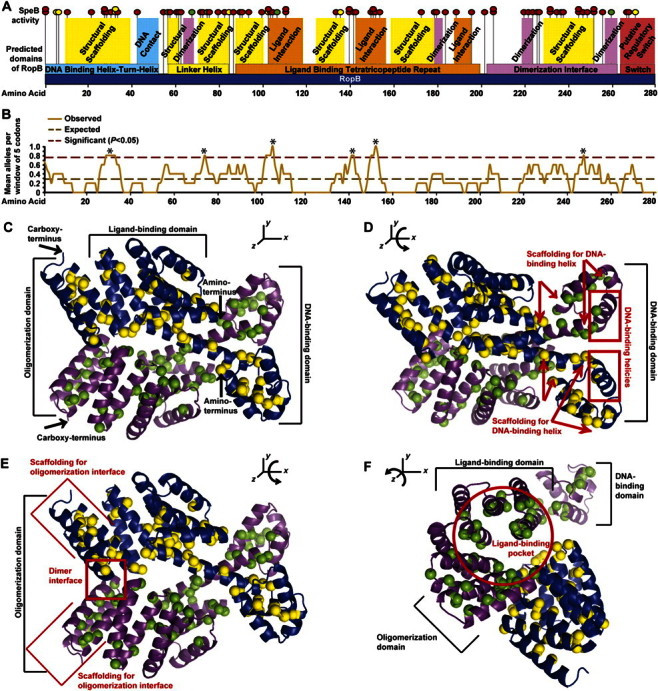
The ropB polymorphisms are predicted to alter RopB function. A, Location of each ropB polymorphism (vertical black lines with attached spheres) was mapped to the protein sequence (horizontal dark blue box with amino acid positions labeled). The DNA binding domain (horizontal light blue box), linker helix domain (horizontal yellow box), oligomerization domain (horizontal pink box), ligand interaction domain (horizontal orange box), and putative regulatory switch (horizontal red box) are shown. Protein regions predicted to form the DNA binding helix (vertical light blue box), ligand interaction interface (vertical orange box), oligomerization interface (vertical pink box) and structural scaffolding for these key functional domains (vertical yellow boxes) are also shown. The effect of each ropB polymoriphism on secreted streptococcal cysteine protease B (SpeB) expression and activity is also indicated (red, yellow, and green circles placed on top of each vertical line denoting the location of a particular polymorphism indicate absent, reduced or near wild-type levels of SpeB, respectively). The 11 codons altered by 2 different genetic changes in 2 different strains (eg, R28K and R28G) are indicated by partially overlapping stacked circles. B, Moving average plot of ropB polymorphisms per codon using a window of 5 amino acid residues (solid light brown line) was generated. Assuming a random distribution of altered codons, a rate of 0.29 polymorphisms per residue was expected (dashed dark brown line). Six regions with a significantly increased concentration of alterations were identified by the moving window analysis (asterisk above dashed maroon line indicates >0.77 polymorphisms per residue; χ2 test, P < .05). C, Structural model of the RopB dimer is shown, with the backbone of each monomer represented as a ribbon (violet and blue ribbons, respectively) and each amino acid altered by a polymorphism represented as a space filling sphere (green and yellow spheres, respectively). The DNA-binding domain, ligand-binding domain, oligomerization domain, and amino- and carboxy-terminus of each chain are labeled. D, Compared with view in C, the RopB structure has been rotated ∼45° around the x-axis to show the DNA-binding domain. Many amino acid replacements occur in the scaffolding helices (boundaries denoted by red arrows), but none occur in the DNA-binding helix (red box). E, Compared with view in C, the RopB structure has been rotated ∼25° around the x-axis to show the oligomerization domain. Many amino acid replacements occur in both the dimer interface (red box) and the scaffolding helices (boundaries denoted by red brackets). F, Compared with view in C, the RopB structure has been rotated ∼75° around the z-axis and ∼25° around the y-axis to show the ligand-binding domain. Note that many amino acid replacements occur along the interior surface of the ligand-binding pocket (red circle).
Next, we used molecular modeling to predict the consequence of these amino acid replacements to the structure of RopB (Figure 2C). Visual examination of the inferred RopB structure predicts that the amino acid replacements are highly represented in the scaffolding that supports the DNA-binding helix, but none are present in the DNA-binding helix itself (Figure 2D). Amino acid changes are also highly represented throughout the oligomerization domain (Figure 2E) and along the interior surface of the ligand-binding domain (Figure 2F). Thus, these data are consistent with the observation that the majority of ropB polymorphisms significantly alter the regulation of SpeB. Furthermore, the pattern of amino acid replacements affecting all key regulatory domains except the DNA-binding helix suggests an evolutionary model in which there is selection for mutations that change the global regulatory activity of RopB rather than interfere with its interaction with a single promoter sequence.
Effect ofropB Polymorphisms on Infection Type and GAS Strain Virulence
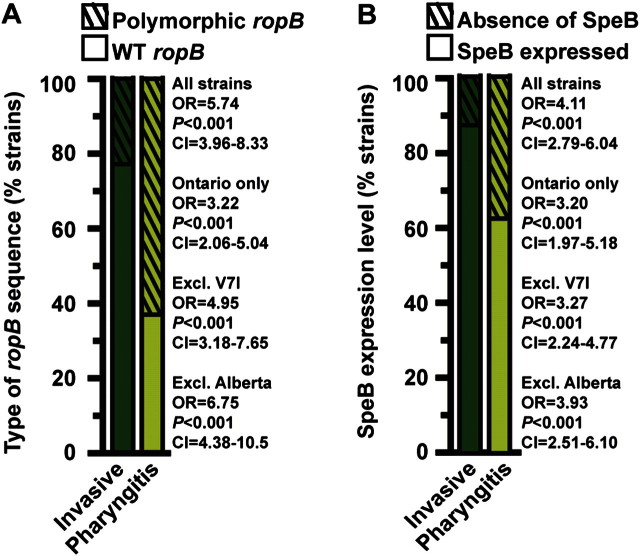
Next, to test the hypothesis that naturally occurring ropB polymorphisms contribute to disease-site specificity, the frequencies of ropB polymorphisms in strains isolated from different infection types were compared. By univariate analysis, we found that strains with ropB polymorphisms were significantly associated with pharyngitis compared with invasive infections (Figure 3A). Similarly, strains with decreased SpeB activity were also significantly associated with pharyngitis (Figure 3B). The association between ropB polymorphisms, decreased SpeB, and pharyngitis remained significant if univariate analysis was restricted to strains isolated in Ontario and was independent of the frequently identified SpeB-positive V7I strains (Figure 3A and 3B). Because Alberta pharyngitis strains comprise a possibly biased convenience sample, we also performed the univariate analysis excluding this collection and again found a significant association (Figure 3A and 3B). Thus, these data unambiguously demonstrate that ropB polymorphisms and decreased SpeB are significantly associated with pharyngitis.
Figure 3.
The ropB polymorphisms and decreased secreted streptococcal cysteine protease B (SpeB) are significantly associated with pharyngitis. A, Univariate analysis of the association between ropB alleles (wild type [solid bar] or variant [hatched bar]) and type of group A streptococci (GAS) infection (invasive [dark green bar] or oropharyngeal [light green bar]) is shown. B, Univariate analysis of the association between SpeB expression (SpeB expressed [solid bar] or absent [hatched bar]) and type of GAS infection (invasive [dark green bar] or oropharyngeal [light green bar]) is shown. Odds ratio (OR), probability (P), and confidence interval (CI) are shown for univariate analyses of all 1178 invasive and pharyngitis strains studied, only the Ontario invasive and pharyngitis strains, all strains except the frequently identified SpeB-positive V7I strains, and all strains except those from the Alberta collection.
Finally, to test the hypothesis that amino acid replacements in RopB alter GAS strain virulence, isoallelic mutant strains were assessed in a mouse model of necrotizing fasciitis. Compared with the wild-type strain, the isoallelic strain with the frequently identified SNP that results in a V7I amino acid replacement secreted an intermediate level of SpeB protease activity in vitro (Figure 4A) and had intermediate virulence in mice (Figure 4B and 4C). In contrast, isoallelic strains with a ropB sequence that encodes a R226Q or V7I/R226Q amino acid replacement lacked SpeB production (Figure 4A), killed significantly fewer mice (Figure 4B), and caused markedly smaller lesions with less tissue destruction (Figure 4C). As expected, these features closely mimicked the characteristics observed for the isogenic strain in which the speB gene was deleted (Figure 4B and 4C).
Figure 4.
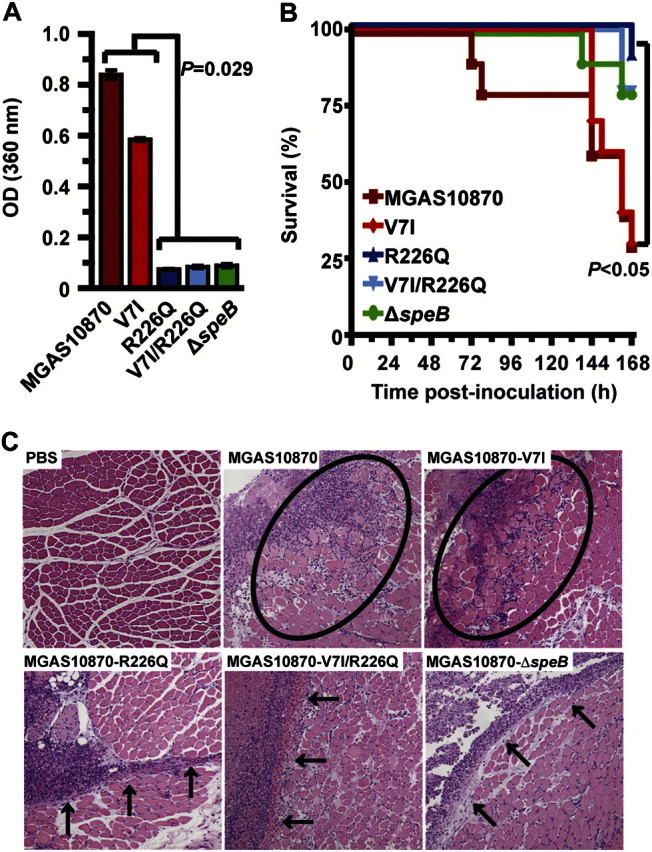
The ropB polymorphisms significantly alter group A streptococci (GAS) strain virulence. Isoallelic strains encoding a ropB sequence that results in the V7I, R226Q, and V7I/R226Q amino acid replacements or lacking the speB gene (designated ΔspeB) were created from representative wild-type strain MGAS10870. A, Level of SpeB secreted protease activity of each isoallelic strain was confirmed using a quantitative chromogenic azocasein hydrolysis assay. The mean ± standard error of the mean (SEM) from 4 replicate measurements is shown (Mann-Whitney test, P = .029 for either strain MGAS10870 or V7I compared with either strain R226Q, V7I/R226Q or ΔspeB). B, Virulence of these isoallelic strains was compared using a mouse model of necrotizing fasciitis. Results are graphically represented as a Kaplan-Meier survival curve (log-rank test, P < .05 for either strain MGAS10870 or V7I compared with either strain R226Q, V7I/R226Q or ΔspeB; difference not significant for strain MGAS10870 compared with strain V7I). C, Histologic analysis of infected limb tissue (representative micrographs are shown, hematoxylin and eosin stain, 40× original magnification). Wild-type strain MGAS10870 caused severe muscle necrosis (circled region), whereas the isoallelic R226Q, V7I/R226Q and SpeB-deficient strains caused markedly smaller and less destructive lesions that were restricted to the major fascial planes (black arrows). The isoallelic strain with a V7I amino acid replacement had an intermediate virulence phenotype (note the 72 h difference in the time to the first near-mortality event and the slightly less severe tissue destruction caused by strain V7I compared with wild-type strain MGAS10870).
DISCUSSION
The effect of SNPs and other minor gene mutations on human disease causation and susceptibility has been extensively investigated [21]. However, their consequence to bacterial virulence is poorly understood. We have recently used an unbiased whole-genome sequencing strategy to investigate strain genotype—disease phenotype relationships in infections caused by serotype M3 strains of GAS [22]. This strategy has been productive, leading to several new discoveries bearing on GAS virulence [22]. For example, we recently identified a naturally occurring SNP that disrupts the mtsR-prsA-SpeB virulence axis to significantly alter GAS necrotizing fasciitis capacity [4, 7]. Likewise, the gene encoding ropB, the major positive regulator of SpeB, was found to have the highest rate of nucleotide diversification among ∼180 pharyngitis and invasive GAS strains recovered in Ontario [3, 4, 18]. Herein, ropB was demonstrated to be highly polymorphic in a collection of 1178 serotype M3 GAS strains recovered from patients with different disease manifestations in diverse geographic regions and time periods. The excess of ropB alterations was statistically significant compared with random expectation, and no silent mutations were identified. Consistent with the observation that nearly every polymorphism markedly decreased RopB function, molecular modeling predicted that the amino acid alterations are concentrated within key functional domains. Similarly, Ikebe et al [20] recently identified 4 variant ropB alleles, 2 of which were not identified herein (SNPs encoding F161Y and I162F amino acid alterations), among 26 serotype M3 GAS strains collected in Japan. Together, these data lead us to conclude that ropB evolves under diversifying selective pressure in serotype M3 GAS [23].
This finding is particularly unusual because RopB is a cytosolic transcriptional regulator that would not be expected to undergo direct selective pressure from the host immune system to change its antigenic presentation. An alternative explanation for the high diversity found in ropB is that changing the RopB-mediated transcriptome confers a selective advantage to serotype M3 GAS strains in some, but not all, disease conditions; otherwise, the ropB gene would be inactivated in all serotype M3 GAS strains. In particular, variant ropB alleles were significantly associated with pharyngitis, suggesting that serotype M3 strains with altered RopB function have enhanced fitness in the host oropharynx compared with sites of invasive infection. This idea is consistent with SpeB being a proven virulence factor for invasive infections but not essential for growth in human saliva [7, 24]. During invasive infections, SpeB directly causes severe tissue destruction by degrading host extracellular matrix molecules, such as fibronectin and vitronectin [25], and it indirectly causes further tissue damage by activating host matrix metalloproteases and disrupting coagulation [26–28]. SpeB also activates host proinflammatory mediators such as interleukin-1β [25], inactivates host innate immune molecules such as complement factor C3b and the antimicrobial peptide LL37 [29], and stimulates the release of proapoptotic molecules from host macrophages and pneumocytes [27]. Importantly, SpeB is abundantly present in infected human tissue [10, 30]. This model of GAS evolution is fully supported by our genome-wide analysis of ∼180 serotype M3 strains recovered in Ontario, which also suggested that the oropharynx is the primary site of evolution for serotype M3 GAS strains, with invasive strains originating from lineages that cause pharyngitis [2, 3]. That is, most serotype M3 invasive strains are immediately descended from wild-type serotype M3 pharyngitis strains, not other invasive strains.
Inasmuch as RopB is best known for being the major positive regulator of SpeB [31], it also regulates the transcription of multiple other proven and putative virulence factors that may further contribute to decreased virulence of strains with variant ropB alleles. Additional targets of RopB regulation include the superantigens SpeK and SmeZ, the operon encoding the pilus structure that is critical to GAS adhesion to mucosal epithelium, the operon encoding the potent pore-forming cytotoxin streptolysin-S that inactivates host neutrophils, and the operon encoding the Opp oligopeptide transport system that is involved in nutrient acquisition [12, 18, 32]. Furthermore, RopB has also been implicated in the regulation of other GAS transcriptional regulators such as Mga (multiple gene activator), CcpA (catabolite control protein A), and the 2-component control systems Ihk/Irr and CovR/CovS [18, 33]. Because the RopB-mediated transcriptome varies considerably between strains of different GAS serotypes and even between strains of the same serotype [12, 18, 20, 32, 34], additional studies using our isoallelic strains are needed to fully define the transcriptome differences mediated by variant ropB alleles. In addition, genome-wide analysis of serotype M3 GAS strains serially collected from individual pharyngitis patients or asymptomatic carriers would enable a near-real-time determination of the sequential evolutionary events that occur in vivo and help elucidate how such events contribute to human disease.
In summary, we used whole-genome sequencing followed by deep single-gene sequencing to test a hypothesis bearing on bacterial virulence and host-pathogen interactions. The comprehensive evaluation of naturally occurring diversity in a single gene among a large number of strains without geographic or temporal restrictions gives new insight to the evolution of a pathogen. It also provides a partial genetic explanation for the difference in virulence phenotypes observed between closely related strains. Given the recent application of whole-genome sequencing to many pathogenic bacteria, this approach will have widespread utility [35–37].
Notes
Acknowledgments.We thank Dr Bernard W. Beall for assistance with the Active Bacterial Core surveillance (ABCs) strain collection.
Financial support.This work was supported in part by the American Heart Association (grant AHA0775045).
Potential conflicts of interest.All authors: no reported conflicts
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Olsen RJ, Shelburne SA, Musser JM. Molecular mechanisms underlying group A streptococcal pathogenesis. Cell Microbiol. 2009;11:1–12. doi: 10.1111/j.1462-5822.2008.01225.x. [DOI] [PubMed] [Google Scholar]
- 2.Beres SB, Carroll RK, Shea PR, et al. Molecular complexity of successive bacterial epidemics deconvoluted by comparative pathogenomics. Proc Natl Acad Sci U S A. 2010;107:4371–6. doi: 10.1073/pnas.0911295107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shea PR, Beres SB, Flores AR, et al. Distinct signatures of diversifying selection revealed by genome analysis of respiratory tract and invasive bacterial populations. Proc Natl Acad Sci U S A. 2011;108:5039–44. doi: 10.1073/pnas.1016282108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beres SB, Richter EW, Nagiec MJ, et al. Molecular genetic anatomy of inter- and intraserotype variation in the human bacterial pathogen group A Streptococcus. Proc Natl Acad Sci U S A. 2006;103:7059–64. doi: 10.1073/pnas.0510279103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beres SB, Sylva GL, Sturdevant DE, et al. Genome-wide molecular dissection of serotype M3 group A Streptococcus strains causing two epidemics of invasive infections. Proc Natl Acad Sci U S A. 2004;101:11833–8. doi: 10.1073/pnas.0404163101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Loughlin RE, Roberson A, Cieslak PR, et al. The epidemiology of invasive group A streptococcal infection and potential vaccine implications: United States, 2000–2004. Clin Infect Dis. 2007;45:853–62. doi: 10.1086/521264. [DOI] [PubMed] [Google Scholar]
- 7.Olsen RJ, Sitkiewicz I, Ayeras AA, et al. Decreased necrotizing fasciitis capacity caused by a single nucleotide mutation that alters a multiple gene virulence axis. Proc Natl Acad Sci U S A. 2010;107:888–93. doi: 10.1073/pnas.0911811107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lukomski S, Montgomery CA, Rurangirwa J, et al. Extracellular cysteine protease produced by Streptococcus pyogenes participates in the pathogenesis of invasive skin infection and dissemination in mice. Infect Immun. 1999;67:1779–88. doi: 10.1128/iai.67.4.1779-1788.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neely MN, Lyon WR, Runft DL, Caparon M. Role of RopB in growth phase expression of the SpeB cysteine protease of Streptococcus pyogenes. J Bacteriol. 2003;185:5166–74. doi: 10.1128/JB.185.17.5166-5174.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johansson L, Thulin P, Sendi P, et al. Cathelicidin LL-37 in severe Streptococcus pyogenes soft tissue infections in humans. Infect Immun. 2008;76:3399–404. doi: 10.1128/IAI.01392-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olsen RJ, Watkins ME, Cantu CC, Beres SB, Musser JM. Virulence of serotype M3 group A Streptococcus strains in wax worms (Galleria mellonella larvae) Virulence. 2011;2:111–9. doi: 10.4161/viru.2.2.14338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hollands A, Aziz RK, Kansal R, Kotb M, Nizet V, Walker MJ. A naturally occurring mutation in ropB suppresses SpeB expression and reduces M1T1 group A streptococcal systemic virulence. PLoS One. 2008;3:e4102. doi: 10.1371/journal.pone.0004102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lamagni TL, Efstratiou A, Dennis J, Nair P, Kearney J, George R. Increase in invasive group A streptococcal infections in England, Wales and Northern Ireland, 2008–9. Euro Surveill. 2009;14 doi: 10.2807/ese.14.05.19110-en. pii=19110. [DOI] [PubMed] [Google Scholar]
- 14.Kohler W, Gerlach D, Knoll H. Streptococcal outbreaks and erythrogenic toxin type A. Zentralbl Bakteriol Mikrobiol Hyg A. 1987;266:104–15. doi: 10.1016/s0176-6724(87)80024-x. [DOI] [PubMed] [Google Scholar]
- 15.Musser JM, Nelson K, Selander RK, et al. Temporal variation in bacterial disease frequency: molecular population genetic analysis of scarlet fever epidemics in Ottawa and in eastern Germany. J Infect Dis. 1993;167:759–62. doi: 10.1093/infdis/167.3.759. [DOI] [PubMed] [Google Scholar]
- 16.Hartas J, Hibble M, Sriprakash KS. Simplification of a locus-specific DNA typing method (Vir typing) for Streptococcus pyogenes. J Clin Microbiol. 1998;36:1428–9. doi: 10.1128/jcm.36.5.1428-1429.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lukomski S, Sreevatsan S, Amberg C, et al. Inactivation of Streptococcus pyogenes extracellular cysteine protease significantly decreases mouse lethality of serotype M3 and M49 strains. J Clin Invest. 1997;99:2574–80. doi: 10.1172/JCI119445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carroll RK, Shelburne SA, 3rd, Olsen RJ, et al. Naturally occurring single amino acid replacements in a regulatory protein alter streptococcal gene expression and virulence in mice. J Clin Invest. 2011;121:1956–68. doi: 10.1172/JCI45169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeLano WL. Unraveling hot spots in binding interfaces: progress and challenges. Curr Opin Struct Biol. 2002;12:14–20. doi: 10.1016/s0959-440x(02)00283-x. [DOI] [PubMed] [Google Scholar]
- 20.Ikebe T, Ato M, Matsumura T, et al. Highly frequent mutations in negative regulators of multiple virulence genes in group A streptococcal toxic shock syndrome isolates. PLoS Pathog. 2010;6:e1000832. doi: 10.1371/journal.ppat.1000832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jakobsson M, Scholz SW, Scheet P, et al. Genotype, haplotype and copy-number variation in worldwide human populations. Nature. 2008;451:998–1003. doi: 10.1038/nature06742. [DOI] [PubMed] [Google Scholar]
- 22.Musser JM, DeLeo FR. Toward a genome-wide systems biology analysis of host-pathogen interactions in group A Streptococcus. Am J Pathol. 2005;167:1461–72. doi: 10.1016/S0002-9440(10)61232-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hurst LD. The Ka/Ks ratio: diagnosing the form of sequence evolution. Trends Genet. 2002;18:486. doi: 10.1016/s0168-9525(02)02722-1. [DOI] [PubMed] [Google Scholar]
- 24.Shelburne SA, 3rd, Granville C, Tokuyama M, Sitkiewicz I, Patel P, Musser JM. Growth characteristics of and virulence factor production by group A Streptococcus during cultivation in human saliva. Infect Immun. 2005;73:4723–31. doi: 10.1128/IAI.73.8.4723-4731.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kapur V, Topouzis S, Majesky MW, et al. A conserved Streptococcus pyogenes extracellular cysteine protease cleaves human fibronectin and degrades vitronectin. Microb Pathog. 1993;15:327–46. doi: 10.1006/mpat.1993.1083. [DOI] [PubMed] [Google Scholar]
- 26.Matsuka YV, Pillai S, Gubba S, Musser JM, Olmsted SB. Fibrinogen cleavage by the Streptococcus pyogenes extracellular cysteine protease and generation of antibodies that inhibit enzyme proteolytic activity. Infect Immun. 1999;67:4326–3. doi: 10.1128/iai.67.9.4326-4333.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tamura F, Nakagawa R, Akuta T, et al. Proapoptotic effect of proteolytic activation of matrix metalloproteinases by Streptococcus pyogenes thiol proteinase (Streptococcus pyrogenic exotoxin B) Infect Immun. 2004;72:4836–47. doi: 10.1128/IAI.72.8.4836-4847.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meinert Niclasen L, Olsen JG, Dagil R, Qing Z, Sorensen OE, Kragelund BB. Streptococcal pyogenic exotoxin B (SpeB) boosts the contact system via binding of α-1 antitrypsin. Biochem J. 2011;434:123–32. doi: 10.1042/BJ20100984. [DOI] [PubMed] [Google Scholar]
- 29.Nyberg P, Rasmussen M, Bjorck L. alpha2-Macroglobulin-proteinase complexes protect Streptococcus pyogenes from killing by the antimicrobial peptide LL-37. J Biol Chem. 2004;279:52820–3. doi: 10.1074/jbc.C400485200. [DOI] [PubMed] [Google Scholar]
- 30.Thulin P, Johansson L, Low DE, et al. Viable group A streptococci in macrophages during acute soft tissue infection. PLoS Med. 2006;3:e53. doi: 10.1371/journal.pmed.0030053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lyon WR, Gibson CM, Caparon MG. A role for trigger factor and an Rgg-like regulator in the transcription, secretion and processing of the cysteine proteinase of Streptococcus pyogenes. Embo J. 1998;17:6263–75. doi: 10.1093/emboj/17.21.6263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dmitriev AV, McDowell EJ, Chaussee MS. Inter- and intraserotypic variation in the Streptococcus pyogenes Rgg regulon. FEMS Microbiol Lett. 2008;284:43–51. doi: 10.1111/j.1574-6968.2008.01171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chaussee MS, Watson RO, Smoot JC, Musser JM. Identification of Rgg-regulated exoproteins of Streptococcus pyogenes. Infect Immun. 2001;69:822–31. doi: 10.1128/IAI.69.2.822-831.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dmitriev AV, McDowell EJ, Kappeler KV, Chaussee MA, Rieck LD, Chaussee MS. The Rgg regulator of Streptococcus pyogenes influences utilization of nonglucose carbohydrates, prophage induction, and expression of the NAD-glycohydrolase virulence operon. J Bacteriol. 2006;188:7230–41. doi: 10.1128/JB.00877-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mwangi MM, Wu SW, Zhou Y, et al. Tracking the in vivo evolution of multidrug resistance in Staphylococcus aureus by whole-genome sequencing. Proc Natl Acad Sci U S A. 2007;104:9451–6. doi: 10.1073/pnas.0609839104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith EE, Buckley DG, Wu Z, et al. Genetic adaptation by Pseudomonas aeruginosa to the airways of cystic fibrosis patients. Proc Natl Acad Sci U S A. 2006;103:8487–92. doi: 10.1073/pnas.0602138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Croucher NJ, Harris SR, Fraser C, et al. Rapid pneumococcal evolution in response to clinical interventions. Science. 2011;331:430–4. doi: 10.1126/science.1198545. [DOI] [PMC free article] [PubMed] [Google Scholar]